Abstract
Abundant evidence indicates a pivotal role of prostaglandin F2α (PGF2α) in human parturition. Both the fetal and maternal sides of the fetal membranes synthesize PGF2α. In addition to the synthesis of PGF2α from PGH2 by PGF synthase (PGFS), PGF2α can also be converted from PGE2 by carbonyl reductase 1 (CBR1). Here, we showed that there was concurrent increased production of cortisol and PGF2α in association with the elevation of CBR1 in human amnion obtained at term with labor versus term without labor. In cultured primary human amnion fibroblasts, cortisol (0.01–1μM) increased PGF2α production in a concentration-dependent manner, in parallel with elevation of CBR1 levels. Either siRNA-mediated knockdown of glucocorticoid receptor (GR) expression or GR antagonist RU486 attenuated the induction of CBR1 by cortisol. Chromatin immunoprecipitation (ChIP) showed an increased enrichment of both GR and RNA polymerase II to CBR1 promoter. Knockdown of CBR1 expression with siRNA or inhibition of CBR1 activity with rutin decreased both basal and cortisol-stimulated PGF2α production in human amnion fibroblasts. In conclusion, CBR1 may play a critical role in PGF2α synthesis in human amnion fibroblasts, and cortisol promotes the conversion of PGE2 into PGF2α via GR-mediated induction of CBR1 in human amnion fibroblasts. This stimulatory effect of cortisol on CBR1 expression may partly explain the concurrent increases of cortisol and PGF2α in human amnion tissue with labor, and these findings may account for the increased production of PGF2α in the fetal membranes prior to the onset of labor.
Abundant evidence indicates a critical role of prostaglandin F2α (PGF2α) in parturition (1–3). PGF2α levels are increased in the amniotic fluid as well as on the maternal side of the fetal membranes, while PGF2α receptor (FP) expression is enhanced in the myometrium toward the end of pregnancy (4–6). Moreover, injection of PGF2α into the amniotic fluid has been shown to induce human labor (1, 7), whereas FP knockout mice never succeed in labor (8). In addition to the stimulation of myometrial contractility and cervical dilation, PGF2α also plays important roles in the fetal–maternal interface at the onset of human parturition by inducing the expression of matrix metalloproteinases (MMPs), such as MMP-2 and MMP-9 in decidua and cyclooxygenase-2 (COX-2) in amnion fibroblasts (9, 10).
In the fetal membranes, the amnion, particularly amnion fibroblasts, is widely considered as a major source of prostaglandin E2 (PGE2) whereas the choriodecidua is regarded as a major source of PGF2α toward the end of pregnancy (5, 11). However in contrast to this dogma, the amnion, when separated from choriodecidua, is able to produce PGF2α although less than the choriodecidua (5). In addition to the formation of PGF2α from PGH2 by PGF synthase, PGF2α can also be converted from PGE2 by the enzyme carbonyl reductase 1 (CBR1) (12). However, the expression pattern of CBR1 in the fetal membranes remains largely unknown.
In most mammalian species including humans, there is an increase in glucocorticoid concentration in the maternal and fetal circulations as well as in the amniotic fluid toward the end of gestation and at the onset of labor (3, 13–15). This surge of glucocorticoids is believed to be crucial to the maturation of fetal organs as well as to the cascade of events in the initiation and maintenance of labor (3, 16). In addition to the de novo synthesis of glucocorticoids in the adrenal glands, cortisol can be regenerated from cortisone, its biologically inactive counterpart, and by 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) in the fetal membranes in pregnancy (17, 18). Cortisol has been shown to promote the expression and activity of 11β-HSD1 in the fetal membranes thus forming a local feed-forward loop of cortisol regeneration in these tissues toward the end of gestation (18–21). Interestingly, we and others have demonstrated that glucocorticoids stimulate PGE2 synthesis in the fetal membranes (22, 23), which is in marked contrast to the inhibition of PGE2 synthesis by glucocorticoids in most other tissues in the body (24). This stimulation of PGE2 synthesis by glucocorticoids was found to be mediated by the induction of cytosolic phospholipase A2α (cPLA2α) and COX-2, the key enzymes involved in prostaglandin synthesis, in human amnion fibroblasts (22, 25–28). Under the influence of feed-forward regeneration of cortisol, the production of PGE2 may consequently be increased towardthe end of gestation in the fetal membranes, which may be an integral part of the feed-forward mechanisms of human parturition (3, 16, 29). However, it remains unclear whether cortisol can also affect the expression of CBR1 thereby increasing the production of PGF2α from PGE2 in the fetal membranes. In this study, we examined the distribution of CBR1 in the fetal membranes and the regulation of its expression by cortisol with respect to PGF2α production in cultured primary human amnion fibroblasts.
Materials and Methods
Collection of human fetal membranes and preparation of amnion fibroblast cells
Human fetal membranes were collected at term from pregnant women after spontaneous labor (term labor, TL) or at elective cesarean section without labor (term nonlabor; TNL) under a protocol approved by the Institutional Review Board of the University of Texas Health Science Center San Antonio. Pregnancies with complications such as preeclampsia, fetal growth restriction, gestational diabetes, etc, were excluded from the study. To study the effect of labor, amnion was separated from the choriodecidua in both TL and TNL groups and frozen at −80 C for later measurements of PGF2α, cortisol, cPLA2α, COX-2, and CBR1. For the study of the regulation of CBR1 and PGF2α synthesis by cortisol, human amnion fibroblast cells were isolated from TNL amnion. Briefly, amnion was digested with 0.125% trypsin twice (Sigma) and the epithelial cells were discarded by centrifugation. The remaining amnion tissue was washed to remove the residual epithelial cells and then digested with 0.1% collagenase (Roche). The fibroblasts were collected after separation with centrifugation in Percoll (Sigma) gradients (5, 20, 40, and 60%) and cultured in Dulbecco's Modified Eagle Medium (DMEM) (Life Technologies, Inc) containing 10% fetal calf serum (FCS) (Life Technologies) and antibiotic-antimycotic (Life Technologies). The identity of cells has been previously verified, the fibroblasts were more than 90% pure (23). Cell culture was maintained at 37°C with a water saturated atmosphere of 5% CO2 in air.
Immunohistochemical staining of human fetal membranes and amnion fibroblast cells for CBR1
Immunohistochemical staining for CBR1 was carried out on sections cut from paraffin-embedded fetal membranes collected from TNL patients after fixing in 10% formalin. Immunocytochemical staining of cultured amnion fibroblasts as described above for CBR1 was performed after fixing the cells in 1% formaldehyde. Immunohistochemical and immunocytochemical staining were conducted using the avidin-biotin-peroxidase method after a protocol provided by the manufacturer (Vector Laboratories Inc.). CBR1 antibody at 1:100 dilution was applied as the primary antibody. Red color reaction was developed using the substrate 3-amino-9-ethyl carbazole. Cells were counterstained with Carazzi's hematoxylin. To test the specificity of immunocytochemical staining, tissue sections and cells were also stained with preimmune serum instead of the specific primary antibody.
Measurements of PGF2α and cortisol in human amnion tissue and PGF2α in the culture medium of human amnion fibroblast cells
Cortisol and PGF2α in amnion tissue collected from pregnant women at TNL and TL were extracted with ethyl acetate after homogenization in PBS and measured with enzyme immunoassay (EIA) kits (Cayman Chemical Company) following protocols provided by the manufacturer. PGF2α in human amnion fibroblast cell culture medium with or without cortisol (1 μM) treatment for 24 hours in the presence or absence of siRNA-mediated knockdown of CBR1 expression or rutin (100 μM; Sigma), an inhibitor of CBR1 activity (30) was measured directly without ethyl acetate extraction.
Treatment of human amnion fibroblast cells
On the third day of cell culture, the culture medium was changed to FCS-free medium and the cells were treated with increasing concentrations of cortisol (0.01–1 μM; Sigma) for 24 hours. To examine whether the effect was mediated by glucocorticoid receptor (GR), cells were treated with cortisol (1 μM) for 24 hours in the presence or absence of GR antagonist RU486 (1 μM) as well as with or without siRNA-mediated knockdown of GR expression. Small interfering RNA (siRNA) (100 nM) against GR was transfected into cells on the second day of cell culture with LTX lipofectamine (Invitrogen). Forty-eight hours after transfection, the culture medium was switched to FCS-free medium in the presence or absence of cortisol (1 μM). Total RNA and protein were isolated for the analysis of GR, cPLA2α, COX-2, CBR1, AKR1B1, and AKR1C3 mRNA and protein levels with qRT-PCR and Western blotting.
To study whether the effect of cortisol on PGF2α secretion was dependent on CBR1, cells were treated with cortisol (1 μM) for 24 hours in the presence or absence of siRNA-mediated knockdown of CBR1 expression as well as in the presence or absence of rutin (100 μM, Sigma), an inhibitor of CBR1 activity. Cells were transfected with CBR1 siRNA (100 nM) on the second day of culture and were treated with cortisol (1 μM, 24 h) in an FCS-free medium 48 hours after transfection. Culture medium was collected for the measurement of PGF2α with EIA.
Detection of cPLA2α, COX-2, and CBR1 levels with quantitative real time PCR and Western blotting
Total RNA and protein were extracted from homogenized amnion tissue or cultured amnion fibroblast cells using a RNeasy mini extraction kit (QIAGEN) and radio-immunoprecipitation assay lysis buffer (Millipore) respectively. To measure mRNA levels, quantitative real time PCR (qRT-PCR) was performed on the cDNA transcribed from the extracted total RNA with the primers as illustrated in Table 1. To control for sampling errors, qRT-PCR for 18S RNA was performed on each sample. Protein levels were examined with Western blotting. Briefly, 25 μg denatured protein of each sample were electrophoresed in 4–20% SDS-polyacrylamide gel (Bio-Rad) and transferred to the nitrocellulose blot (Bio-Rad). After blocking with milk, the blot was probed with 1:500 dilution of mouse or rabbit antibodies against human cPLA2α (Santa Cruz Biotechnology), COX-2 (Santa Cruz), CBR1 (Santa Cruz), AKR1B1 (Abgent), AKR1C3 (Abgent), and GR (Santa Cruz). The blot was also probed with 1:5000 dilution of mouse antibody against human β-actin (Sigma) for loading control. After incubation with anti-rabbit or anti-mouse IgG antibody conjugated with horseradish peroxidase (Sigma), the enhanced chemiluminescence detection system (Millipore) was used to detect the bands. The ratio of target mRNA and protein over 18S RNA and β-actin were obtained respectively to represent the target mRNA and protein levels.
Table 1.
Primer Sequences Used in qRT-PCR
| Genes | Primer Sequences | Accession No. | Product Size |
|---|---|---|---|
| cPLA2α | F: 5′ATGGCCTTGGTGAGTGATTC3′ | NM_024420 | 178bp |
| R: 5′TCAGGATCTGCTACAGCTGC3′ | |||
| COX-2 | F: 5′ TGTGCAACACTTGAGTGGCT3′ | NM_000963 | 297bp |
| R: 5′ ACTTTCTGTACTGCGGGTG3′ | |||
| CBR1 | F: 5′CCTGGACGTGCTGGTCAACA3′ | NM_001757 | 197bp |
| R: 5′ACGTTCACCACTCTCCCTTG3′ | |||
| AKR1B1 | F: 5′ACGTGGGCGGCCATGGAAGA3′ | NM_001628.2 | 219bp |
| R: 5′GAGGGGGCTGTAGGCGGTCA3′ | |||
| AKR1C3 | F: 5′ CTCTGAGGAGAAGCAGCAGCAAACA3′ | NM_003739.4 | 251bp |
| R: 5′ GCTTCGGATGGCCAGTCCAACC 3′ | |||
| GRα | F: 5′ AGGTTGCTGAGGCTCTGACCCA3′ | NM_000176.2 | 236bp |
| R: 5′ AACTGCTGGGGGAGGGCTGT3′ | |||
| 18S rRNA | F: 5′ GTAACCCGTTGAACCCCATT3′ | NR_003286.2 | 150bp |
| R: 5′CCATCCAATCGGTAGTAGCG3′ |
F indicates forward; and R, reverse.
Chromatin immunoprecipitation assay to demonstrate the enrichment of GR and Pol II on the CBR1 promoter in human amnion fibroblast cells
Chromatin immunoprecipitation (ChIP) assay was conducted using a kit from Upstate Biotechnology (Millipore). Briefly, on the third day of cell culture, amnion fibroblasts were treated with cortisol (1 μM) for 12 hours and then fixed with 1% formaldehyde to cross-link the proteins bound to the chromatin DNA on ice, which was then terminated with 1 M glycine. The lysed cells were sonicated to shear the chromatin DNA to an optimal size around 500 bp. After precleaning the sheared DNA with protein A agarose/Salmon Sperm DNA (Millipore), sheared chromatin DNA was immunoprecipitated with 4 μg of antibodies against human RNA polymerase II (Pol II) (Millipore) or GR (Santa Cruz). Nonimmune IgG served as negative control. The immunoprecipitate was then incubated with Magna ChIP™ Protein A agarose Magnetic Beads (Millipore) and pulled down on a magnetic stand. After washing, reverse cross-linking was performed in 5 M NaCl at 65°C for overnight. Contaminating RNA was cleaned with RNase A for 30 minutes at 37°C and protein was digested with proteinase K for 1 hour at 45°C. Finally, the sheared DNA recovered from reverse cross-linking was extracted with DNA extraction kit for further qRT-PCR analysis. Sheared DNA without immunoprecipitation served as input control. The aligning positions and sequences of the primers used for qRT-PCR are shown in Figure 4B. For qRT-PCR, the absolute DNA levels in each sample were calculated according to a standard curve set-up using serial dilutions of known amounts of specific templates against corresponding cycle threshold values. The ratio of DNA precipitated by specific antibodies over input control was obtained.
Figure 4.
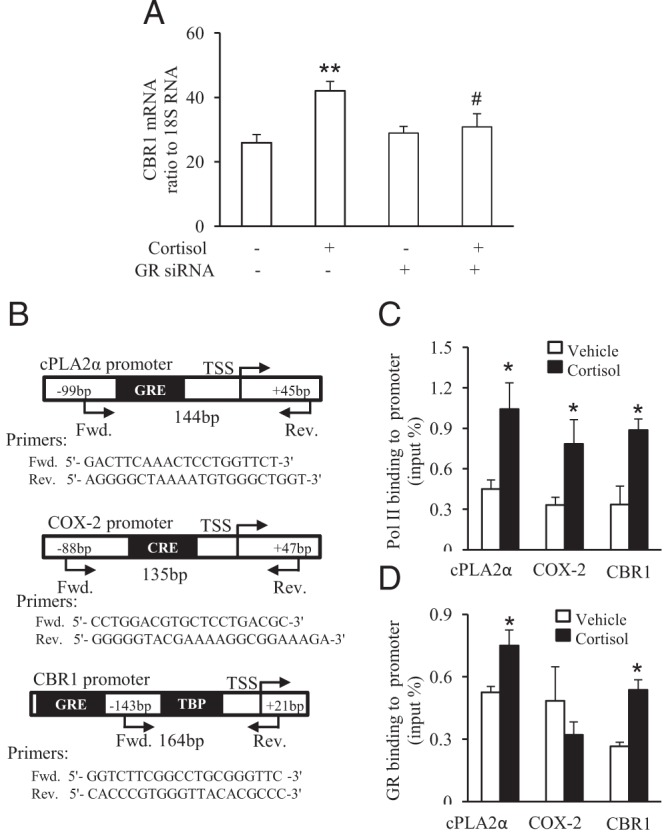
GR-dependent up-regulation of CBR1 transcription by cortisol. A, siRNA-mediated knockdown of GR attenuated cortisol-induced CBR1 mRNA expression. B, Chromatin immunoprecipitation (ChIP) assay revealed increased enrichment of GR and RNA polymerase II (Pol II) to CBR1 promoter upon cortisol (1 μM) stimulation of human amnion fibroblasts. A, Primer sequences and schematic illustration of their aligning sites on cPLA2α, COX-2, and CBR1 promoters. C, The enrichment of RNA polymerase II to cPLA2α, COX-2, and CBR1 promoters. D, The enrichment of GR to cPLA2α, COX-2, and CBR1 promoters. n = 3–4; *, P < .05 versus vehicle control.
Statistical analysis
All data are reported as mean ± SEM. After examination of normal distribution, paired Student's t test or 1-way ANOVA test followed by the Student-Newman-Keuls test was used to assess significant differences where appropriate using SPSS software version 12.0. Significance was set at P < .05.
Results
Immunohistochemical staining of human fetal membranes and amnion fibroblast cells for CBR1
Immunohistochemical staining of human fetal membranes revealed strong staining of CBR1 in amnion epithelial and fibroblast cells as well as in chorionic trophoblast cells (Figure 1B). Immunocytochemical staining of cultured human amnion fibroblast cells confirmed the expression of CBR1 in this cell type (Figure 1D).
Figure 1.
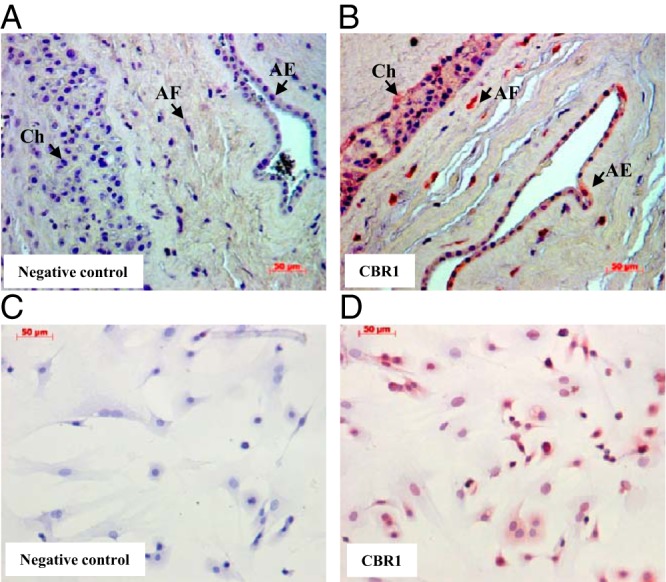
Predominant expression of CBR1 in amnion fibroblasts. A and B, Immunohistochemical staining of CBR1 in human fetal membranes. AE indicates amnion epithelium; AF, amnion fibroblasts; and Ch, chorion trophoblasts. C and D, Immunocytochemical staining of CBR1 in cultured human amnion fibroblasts.
Effect of labor on the levels of cortisol, PGF2α, cPLA2α, COX-2, and CBR1 in human amnion tissue
Enzyme immunoassay demonstrated that the process of labor significantly increased the levels of cortisol from 1550 ± 217 pg/mg to 4501 ± 663 pg/mg and PGF2α from 88 ± 23 pg/mg to 398 ± 138 pg/mg respectively in human amnion tissue (Figure 2A). Analysis with Western blotting showed that the protein levels of cPLA2α, COX-2, and CBR1 were significantly increased with labor (Figure 2B).
Figure 2.
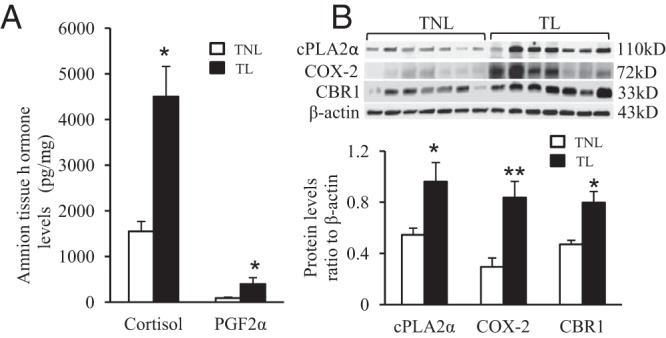
Concurrent increases of cortisol and PGF2α levels and cPLA2α, COX2, and CBR1 protein levels in labor. A, Increased cortisol and PGF2α levels in human amnion tissues with term labor (TL) versus term nonlabor (TNL). B. Elevated cPLA2α, COX-2, and CBR1 protein levels in human amnion tissues TL versus TNL. n = 7; *, P < .05; **, P < .01 versus TNL.
Induction of PGF2α, cPLA2α, COX-2, and CBR1 but not AKR1B1 and AKR1C3 by cortisol in human amnion fibroblast cells
Enzyme immunoassay and Western blotting analysis demonstrated that cortisol (0.01–1 μM) increased PGF2α production (Figure 3A) as well as cPLA2α, COX-2, and CBR1 protein levels (Figure 3B) in human amnion fibroblasts in a concentration-dependent manner, but cortisol (1 μM) treatment did not induce AKR1B1 and AKR1C3 expression (Figure 3C).
Figure 3.
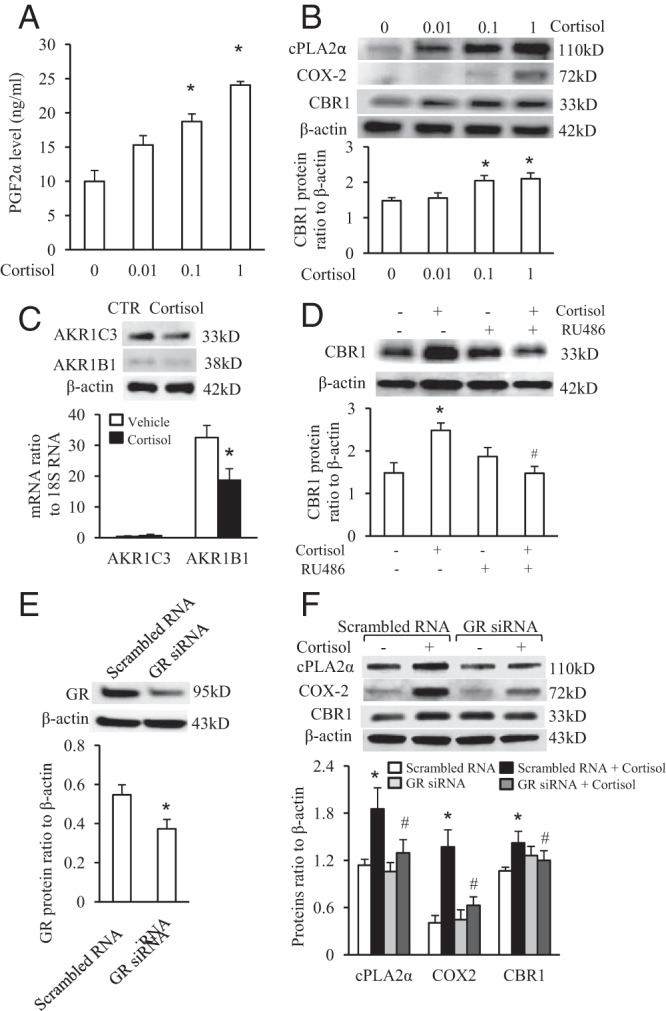
Induction of PGF2α production, cPLA2α, COX-2, and CBR1 protein by cortisol via GR in cultured human amnion fibroblasts. A, Cortisol (0.01–1 μM) dose-dependently increased PGF2α production. B, Cortisol (0.01–1 μM) dose-dependently increased cPLA2α, COX-2, and CBR1 protein levels. C, Cortisol (1 μM) does not induce AKR1B1 and AKR1C3 expression. D, GR antagonist RU486 (1 μM) blocked induction of CBR1 by cortisol. E, siRNA-mediated knockdown of GR protein level. F, siRNA-mediated knockdown of GR blocked the induction of cPLA2α, COX-2, and CBR1 protein by cortisol (1 μM). n = 4;*, P < .05; **, P < .01 versus vehicle or scrambled RNA control; #, P < .05; ##, P < .01 versus cortisol.
GR mediated-induction of cPLA2α, COX-2, and CBR1 by cortisol in human amnion fibroblast cells
GR antagonist RU486 (1 μM) blocked cortisol (1 μM)-stimulated CBR1 protein expression in human amnion fibroblasts (Figure 3D). The induction of cPLA2α, COX-2, and CBR1 protein levels by cortisol (1 μM) was also attenuated by siRNA-mediated knockdown of GR expression (Figure 3, E and F).
Detection of GR and Pol II on CBR1 promoter upon cortisol stimulation
In addition to the induction of CBR1 protein expression, cortisol (1 μM) also increased CBR1 mRNA level which was attenuated by siRNA-mediated knockdown of GR (Figure 4A), suggesting that GR may mediate the induction of CBR1 transcription by cortisol. Chromatin immunoprecipitation (ChIP) assay revealed that the binding of both GR and Pol II to CBR1 promoters was significantly increased by cortisol (1 μM) treatment (Figure 4, C and D). Our previous studies have demonstrated that cortisol induces cPLA2α expression via activation of binding of GR directly to its promoter while it increases COX-2 expression indirectly by GR via enhancing cAMP response element binding protein enrichment to its promoter (26, 27). Therefore, we used cPLA2α and COX-2 promoter as positive and negative controls respectively in the ChIP assay of this study. By assaying the same samples, we found that cortisol increased the binding of GR to cPLA2α promoter but not to COX-2 promoter despite an increased enrichment of Pol II to both cPLA2α and COX-2 promoters. These data indicate that the binding of GR to CBR1 promoter upon cortisol stimulation is specific.
Inhibition of CBR1 decreases basal and blocked cortisol-induced PGF2α production in human amnion fibroblast cells
Small interfering RNA-mediated knockdown of CBR1 not only significantly decreases basal but also blocked cortisol-stimulated PGF2α production in human amnion fibroblasts (Figure 5, A and B). Consistently treatment of human amnion fibroblasts with rutin (100 μM), an inhibitor of CBR1 activity, significantly decreases both basal and cortisol-induced PGF2α production in human amnion fibroblasts (Figure 5C).
Figure 5.
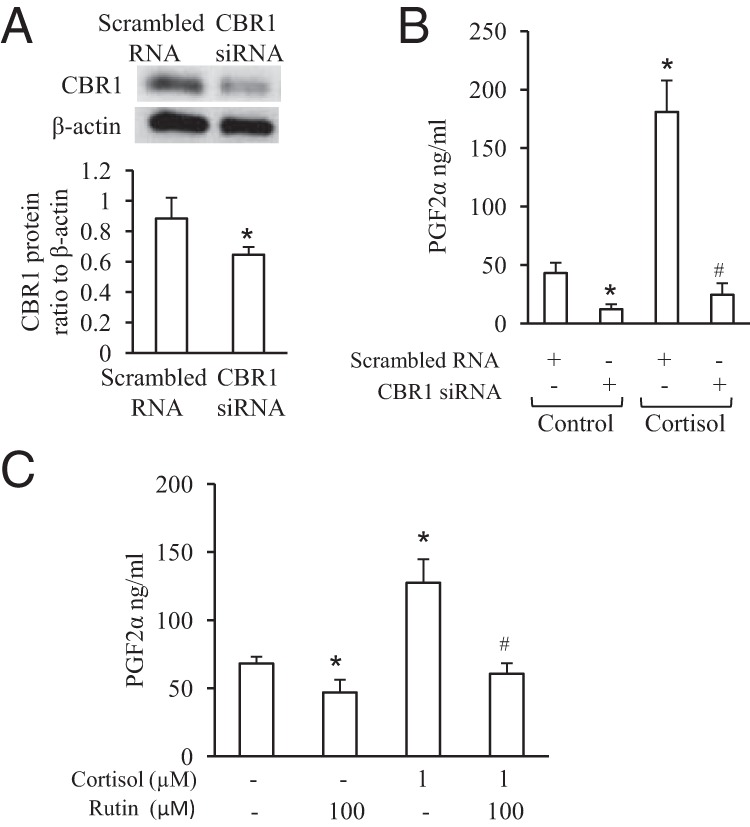
CBR1 is involved in basal and cortisol-induced PGF2α production in human amnion fibroblasts. A, siRNA-mediated knockdown of CBR1 expression. B, siRNA-mediated knockdown of CBR1 expression decreased both basal and cortisol-stimulated PGF2α production. C, Inhibition CBR1 activity by Rutin (100 μM) decreased both basal and cortisol-stimulated PGF2α production. n = 4–5; *, P < .05 versus scrambled RNA control or vehicle control; #, P < .05 versus scrambled RNA with cortisol treatment or with cortisol treatment without rutin.
Discussion
This study confirmed the findings by others that human amnion is a source of PGF2α in human pregnancy (5, 22). Of importance, we found that there were concurrent increases of PGF2α and cortisol levels in the amnion tissue with labor. Unlike the inhibitory effect of cortisol on prostaglandin synthesis in most other tissues in the body (24), cortisol has been shown to stimulate the expression of cPLA2α and COX-2, the two crucial enzymes involved in prostaglandin synthesis thereby increasing the production of PGE2 in human amnion fibroblasts (22, 23, 25–28). The parallel increases of PGF2α and cortisol levels seen in the amnion tissue with labor strongly indicate a possible stimulatory effect of cortisol on PGF2α synthesis in human amnion fibroblast cells. The findings that cortisol stimulated PGF2α production in human amnion fibroblast cells provide direct evidence for this notion. The concurrent increases of CBR1, cPLA2α, and COX-2 levels found in the amnion tissue with labor suggest that the enhanced conversion of PGE2 to PGF2α by CBR1 is one of the pathways accounting for the stimulatory effect of cortisol on PGF2α production in human amnion fibroblast cells. Indeed the findings that either siRNA-mediated knockdown of CBR1 expression or inhibition of CBR1 activity attenuated both basal and cortisol-induced PGF2α production indicate that the conversion of PGE2 to PGF2α by CBR1 indeed constitutes a significant pathway of both basal and cortisol-induced PGF2α production in human amnion fibroblasts. In addition, we have demonstrated that cortisol also stimulates H6PD in amnion fibroblasts (20), which generates NADPH, a cofactor for NADPH-dependent enzymes including CBR1. Because cortisol increases CBR1 mRNA as well as protein levels and ChIP assay revealed GR and RNA polymerase II bindings to CBR1 promoter, we believe that the induction of CBR1 by cortisol is a GR-mediated transcriptional event, which is supported by the findings that either siRNA-mediated knockdown of GR expression or GR antagonist RU486 attenuated the induction of CBR1 by cortisol. In addition to the CBR1 pathway of conversion of PGE2 to PGF2α, the amnion has been reported to express the aldo-keto reductase (AKR) family 1 members C3 and B1 (AKR1C3, AKR1B1), which are enzymes responsible for the formation of PGF2α from PGH2 (31, 32). However, cortisol has no induction effect on AKR1B1 and AKR1C3 expression, so it is unlikely that AKR1B1 and AKR1C3 mediate the induction of PGF2α production by cortisol in human amnion fibroblasts. However, at the current stage we could not rule out the contribution of the pathway of cPLA2, COX2, and PGF2α isomerases to PGF2α production upon cortisol stimulation of human amnion fibroblasts because cPLA2 and COX2 are also cortisol-sensitive (23), although PGF2α isomerases (AKR1B1 and AKRC3) are not sensitive.
Compared with other animals, cortisol concentrations in the human fetus rise more slowly and to a more modest extent at the end of gestation (33). In contrast, the cortisol level in maternal circulation rises dramatically at late gestation (15, 34). However, the rise of free cortisol level, the biologically active form, in maternal circulation is limited by the increasing corticoid binding globulin level with gestation (35). Therefore cortisol regenerated locally in the intrauterine tissues might be more effective and crucial with respect to its participation in the onset of parturition. The earlier findings that the ratio of cortisol/cortisone in human amniotic fluid increases steadily with gestational age and is considerably higher than that of cord serum (36) suggest that the fetal membranes might be an extra-adrenal source of cortisol during gestation. It has been reported that human amnion increasingly converts cortisone to cortisol during pregnancy, thereby potentially contributing to the increasing cortisol concentration in the amniotic fluid (37). Murphy (13) observed that chorion with adherent decidua carefully scraped off retained a high degree of conversion of cortisone to cortisol and that the rise in chorionic conversion of cortisone to cortisol in early pregnancy corresponds to the rise at 15–20 weeks in amniotic fluid cortisol. A high degree of reductive glucocorticoid metabolism has been demonstrated in the decidua attached to chorion (38). Together these observations indicate a role of fetal membranes and attached decidua in regeneration of cortisol from cortisone during pregnancy. These findings were conclusively supported by studies showing 11β-HSD1 mRNA activity and were detectable in cultured human amnion epithelial cells, amnion fibroblasts, and chorionic trophoblasts (18, 39). Studies have shown that the expression level and reductive activity of 11β-HSD1 in the fetal membranes increases with gestational age and labor (37, 40), which coincides with the increase of cortisol level in the amniotic fluid and circulation (13, 15, 36). Cortisol levels in the amniotic fluid and maternal circulation increased with gestational age and further increased with labor reaching concentrations of 29.9 ng/ml and 437 ng/ml respectively (13, 15). As blood plasma has a density of approximately 1.025, the cortisol concentration in maternal circulation at labor is thus around 0.426ng/mg. In this study we found that cortisol level increased by about 3-fold in the amnion tissue with labor reaching a concentration about 4.5ng/mg, which is about 10 times of the concentration of cortisol in the maternal blood and even greater than that of the amniotic fluid at labor (13, 15, 36), further indicating a major role of human fetal membranes in the regeneration of cortisol in parturition. Thus the stimulatory effects of cortisol on CBR1 expression and PGF2α production observed with the concentration range of cortisol in this study are physiologically relevant.
In addition to the well-documented effects of PGF2α in human parturition such as contracting the myometrium, ripening the cervix and rupturing the fetal membranes, PGF2α has also been found to stimulate COX-2 expression and enhance the regeneration of cortisol via stimulation of 11β-HSD1 reductase activity in the fetal membranes (10, 41). Taken together with the previous demonstration that cortisol, as a product of 11β-HSD1, has a positive feedback on the expression and reductase activity of 11β-HSD1 in the fetal membranes (18–21, 29), we propose a feed-forward loop among PGE2, PGF2α, and cortisol in human fetal membranes toward the end of gestation which mutually potentiates production, which might be of importance in the production of sufficient amounts of PGE2, PGF2α, and cortisol at the onset of labor (3, 29).
In conclusion, we have demonstrated that CBR1 is involved in both basal and cortisol-stimulated PGF2α production in human amnion fibroblasts. The transcriptional stimulation of CBR1 expression by cortisol is mediated by GR binding to the CBR1 promoter. This stimulatory effect of cortisol on CBR1 expression may explain at least in part the concurrent increases of cortisol and PGF2α in human amnion tissue with labor and how PGE2 induces labor. These findings may account for the increased production of PGF2α in the fetal membranes prior to the onset of labor. Overall, this and our previous data provide increasing evidence of a local glucocorticoid-mediated mechanism involved in the initiation of labor in humans.
Acknowledgments
This work was supported by NIH number R01 HD 31514, Natural Science Foundation of China 81330018 and 81270704, and National Key Basic Research Program of China 2011CB944403.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AKR
- aldo-keto reductase
- CBR1
- carbonyl reductase 1
- ChIP
- chromatin immunoprecipitation
- FCS
- fetal calf serum
- FP
- PGF2α receptor
- GR
- glucocorticoid receptor
- MMP
- matrix metalloproteinase
- PGE2
- prostaglandin E2
- PGF2α
- prostaglandin F2α.
References
- 1. Lindberg B. The induction of labour by the intravenous infusion of prostaglandin F2alpha. Prostaglandins. 1977;14:993–1004 [DOI] [PubMed] [Google Scholar]
- 2. Bygdeman M. The use of prostaglandins and their analogues for abortion. Clin Obstet Gynaecol. 1984;11:573–584 [PubMed] [Google Scholar]
- 3. Challis JRG, Matthews SG, Gibb W, Lye SJ. Endocrine and paracrine regulation of birth at term and preterm. Endocr Rev. 2000;21:514–550 [DOI] [PubMed] [Google Scholar]
- 4. Romero R, Munoz H, Gomez R, et al. Increase in prostaglandin bioavailability precedes the onset of human parturition. Prostaglandins Leukot Essent Fatty Acids. 1996;54:187–191 [DOI] [PubMed] [Google Scholar]
- 5. Kredentser JV, Embree JE, McCoshen JA. Prostaglandin F2 alpha output by amnion-chorion-decidua: relationship with labor and prostaglandin E2 concentration at the amniotic surface. Am J Obstet Gynecol. 1995;173:199–204 [DOI] [PubMed] [Google Scholar]
- 6. Brodt-Eppley J, Myatt L. Prostaglandin receptors in lower segment myometrium during gestation and labor. Obstet Gynecol. 1999;93:89–93 [DOI] [PubMed] [Google Scholar]
- 7. Korda A, Shearman RP, Smith ID. Termination of pregnancy by intra-uterine prostaglandin F 2. Aust N Z J Obstet Gynaecol. 1972;12:166–169 [DOI] [PubMed] [Google Scholar]
- 8. Sugimoto Y, Yamasaki A, Segi E, et al. Failure of parturition in mice lacking the prostaglandin F receptor. Science. 1997;277:681–683 [DOI] [PubMed] [Google Scholar]
- 9. Ulug U, Goldman S, Ben-Shlomo I, Shalev E. Matrix metalloproteinase (MMP)-2 and MMP-9 and their inhibitor, TIMP-1, in human term decidua and fetal membranes: the effect of prostaglandin F(2alpha) and indomethacin. Mol Hum Reprod. 2001;7:1187–1193 [DOI] [PubMed] [Google Scholar]
- 10. Guo CM, Kasaraneni N, Sun K, Myatt L. Cross talk between PKC and CREB in the induction of COX-2 by PGF2α in human amnion fibroblasts. Endocrinology. 2012;153:4938–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rehnstrm J, Ishikawa M, Fuchs F, Fuchs AR. Stimulation of myometrial and decidual prostaglandin production by amniotic fluid from term, but not midtrimester pregnancies. Prostaglandins. 1983;26:973–981 [DOI] [PubMed] [Google Scholar]
- 12. Ziboh VA, Lord JT, Penneys NS. Alterations of prostaglandin E2–9-ketoreductase activity in proliferating skin. J Lipid Res. 1977;18:37–43 [PubMed] [Google Scholar]
- 13. Murphy BE. Chorionic membrane as an extra-adrenal source of foetal cortisol in human amniotic fluid. Nature. 1977;266:179–181 [DOI] [PubMed] [Google Scholar]
- 14. Sippell WG. Unconjugated cortisol and corticosterone levels in amniotic fluid. Am J Obstet Gynecol. 1981;140:353–354 [DOI] [PubMed] [Google Scholar]
- 15. Goldkrand JW, Schulte RL, Messer RH. Maternal and fetal plasma cortisol levels at parturition. Obstet Gynecol. 1976;47:41–45 [PubMed] [Google Scholar]
- 16. Smith R. Parturition. N Engl J Med. 2007;356:271–283 [DOI] [PubMed] [Google Scholar]
- 17. Sun K, Yang K, Challis JR. Differential expression of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 in human placenta and fetal membranes. J Clin Endocrinol Metab. 1997;82:300–305 [DOI] [PubMed] [Google Scholar]
- 18. Sun K, Myatt L. Enhancement of glucocorticoid-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by proinflammatory cytokines in cultured human amnion fibroblasts. Endocrinology. 2003;144:5568–5577 [DOI] [PubMed] [Google Scholar]
- 19. Yang Z, Guo C, Zhu P, Li W, Myatt L, Sun K. Role of glucocorticoid receptor and CCAAT/enhancer-binding protein alpha in the feed-forward induction of 11beta-hydroxysteroid dehydrogenase type 1 expression by cortisol in human amnion fibroblasts. J Endocrinol. 2007;195:241–253 [DOI] [PubMed] [Google Scholar]
- 20. Wang W, Guo C, Li W, et al. Involvement of GR and p300 in the induction of H6PD by cortisol in human amnion fibroblasts. Endocrinology. 2012;153:5993–6002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun K, He P, Yang K. Intracrine induction of 11beta-hydroxysteroid dehydrogenase type 1 expression by glucocorticoid potentiates prostaglandin production in the human chorionic trophoblast. Biol Reprod. 2002;67:1450–1455 [DOI] [PubMed] [Google Scholar]
- 22. Casey ML, MacDonald PC, Mitchell MD. Despite a massive increase in cortisol secretion in women during parturition, there is an equally massive increase in prostaglandin synthesis. A paradox? J Clin Invest. 1985;75:1852–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun K, Ma R, Cui X, et al. Glucocorticoids induce cytosolic phospholipase A2 and prostaglandin H synthase type 2 but not microsomal prostaglandin E synthase (PGES) and cytosolic PGES expression in cultured primary human amnion cells. J Clin Endocrinol Metab. 2003;88:5564–5571 [DOI] [PubMed] [Google Scholar]
- 24. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723 [DOI] [PubMed] [Google Scholar]
- 25. Guo C, Yang Z, Li W, Zhu P, Myatt L, Sun K. Paradox of glucocorticoid-induced cytosolic phospholipase A2 group IVA messenger RNA expression involves glucocorticoid receptor binding to the promoter in human amnion fibroblasts. Biol Reprod. 2008;78:193–197 [DOI] [PubMed] [Google Scholar]
- 26. Zhu XO, Yang Z, Guo CM, et al. Paradoxical stimulation of cyclooxygenase-2 expression by glucocorticoids via a cyclic AMP response element in human amnion fibroblasts. Mol Endocrinol. 2009;23:1839–1849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo C, Li J, Myatt L, Zhu X, Sun K. Induction of Galphas contributes to the paradoxical stimulation of cytosolic phospholipase A2alpha expression by cortisol in human amnion fibroblasts. Mol Endocrinol. 2010;24:1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Potestio FA, Zakar T, Olson DM. Glucocorticoids stimulate prostaglandin synthesis in human amnion cells by a receptor-mediated mechanism. J Clin Endocrinol Metab. 1988;67:1205–1210 [DOI] [PubMed] [Google Scholar]
- 29. Myatt L, Sun K. Role of fetal membranes in signaling of fetal maturation and parturition. Int J Dev Biol. 2010;54:545–553 [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez-Covarrubias V, Ghosh D, Lakhman SS, Pendyala L, Blanco JG. A functional genetic polymorphism on human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic activity and NADPH binding affinity. Drug Metab Dispos. 2007;35:973–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Breuiller-Fouché M, Leroy MJ, Dubois O, et al. Differential expression of the enzymatic system controlling synthesis, metabolism, and transport of PGF2 alpha in human fetal membranes. Biol Reprod. 2010;83:155–162 [DOI] [PubMed] [Google Scholar]
- 32. Byrns MC. Role of aldo-keto reductase enzymes in mediating the timing of parturition. Front Pharmacol. 2012;2:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jenkin G, Young IR. Mechanisms responsible for parturition; the use of experimental models. Anim Reprod Sci. 2004;82–83:567–581 [DOI] [PubMed] [Google Scholar]
- 34. Lippincott J. Mean concentrations of cortisol, aldosterone, and deoxycorticosterone in sera of women throughout normal pregnancy. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia, PA: JB Lippincott; 1990:887 [Google Scholar]
- 35. Rosenthal HE, Slaunwhite WR, Jr, Sandberg AA. Transcortin: a corticosteroid-binding protein of plasma. X. Cortisol and progesterone interplay and unbound levels of these steroids in pregnancy. J Clin Endocrinol Metab. 1969;29:352–367 [DOI] [PubMed] [Google Scholar]
- 36. Blankstein J, Fujieda K, Reyes FI, Faiman C, Winter JS. Aldosterone and corticosterone in amniotic fluid during various stages of pregnancy. Steroids. 1980;36:161–165 [DOI] [PubMed] [Google Scholar]
- 37. Tanswell AK, Worthington D, Smith BT. Human amniotic membrane corticosteroid 11-oxidoreductase activity. J Clin Endocrinol Metab. 1977;45:721–725 [DOI] [PubMed] [Google Scholar]
- 38. Giannopoulos G, Jackson K, Tulchinsky D. Glucocorticoid metabolism in human placenta, decidua, myometrium and fetal membranes. J Steroid Biochem. 1982;17:371–374 [DOI] [PubMed] [Google Scholar]
- 39. Li W, Gao L, Wang Y, Duan T, Myatt L, Sun K. Enhancement of cortisol-induced 11beta-hydroxysteroid dehydrogenase type 1 expression by interleukin 1beta in cultured human chorionic trophoblast cells. Endocrinology. 2006;147:2490–2495 [DOI] [PubMed] [Google Scholar]
- 40. Alfaidy N, Li W, MacIntosh T, Yang K, Challis J. Late gestation increase in 11beta-hydroxysteroid dehydrogenase 1 expression in human fetal membranes: a novel intrauterine source of cortisol. J Clin Endocrinol Metab. 2003;88:5033–5038 [DOI] [PubMed] [Google Scholar]
- 41. Alfaidy N, Xiong ZG, Myatt L, Lye SJ, MacDonald JF, Challis JR. Prostaglandin F2alpha potentiates cortisol production by stimulating 11beta-hydroxysteroid dehydrogenase 1: a novel feedback loop that may contribute to human labor. J Clin Endocrinol Metab. 2001;86:5585–5592 [DOI] [PubMed] [Google Scholar]


