Abstract
There is evidence that persistent psychiatric disorders lead to age-related disease and premature mortality. Telomere length has emerged as a promising biomarker in studies that test the hypothesis that internalizing psychiatric disorders are associated with accumulating cellular damage. We tested the association between the persistence of internalizing disorders (depression, generalized anxiety disorder and post-traumatic stress disorder) and leukocyte telomere length (LTL) in the prospective-longitudinal Dunedin Study (N=1037). Analyses showed that the persistence of internalizing disorders across repeated assessments from ages 11 to 38 years predicted shorter LTL at age 38 years in a dose-response manner, specifically in men (β= −.137, 95% CI: −.232, −.042, p=.005). This association was not accounted for by alternative explanatory factors, including childhood maltreatment, tobacco smoking, substance dependence, psychiatric medication use, poor physical health, or low socioeconomic status. Additional analyses using DNA from blood collected at two time points (ages 26 and 38 years) showed that LTL erosion was accelerated among men who were diagnosed with internalizing disorder in the interim (β= −.111, 95% CI: −.184, −.037, p=.003). No significant associations were found among women in any analysis, highlighting potential sex differences in internalizing-related telomere biology. These findings point to a potential mechanism linking internalizing disorders to accelerated biological aging in the first half of the life course, particularly in men. Because internalizing disorders are treatable, the findings suggest the hypothesis that treating psychiatric disorders in the first half of the life course may reduce the population burden of age-related disease, and extend health expectancy.
Keywords: Internalizing disorders, telomere length, depression, generalized anxiety disorder, post-traumatic stress disorder, longitudinal
Introduction
Human life expectancy is increasing1, and policy makers and citizens alike are concerned that these extra years of life should be healthy, productive, and enjoyable, not extra years of prolonged disease and disability. The science of age-related diseases has recently turned to a life-course developmental view, based on evidence that the underlying pathogenesis of age-related diseases involves gradually accumulating physiological damage to organ systems, beginning in the first half of the life course2–4. This developmental view raises the possibility that primary prevention could reverse disease-causing processes while people are still healthy5.
The hope of preventing age-related diseases is fostering efforts to identify candidate risk targets that can be treated in the first half of the life course6. Internalizing psychiatric disorder is a promising novel candidate to investigate for several reasons. First, compared to the general population, patients with internalizing disorders such as depression, generalized anxiety disorder (GAD) and post-traumatic stress disorder (PTSD) have higher mortality rates, but die of the same age-related diseases as the population, such as heart disease, cerebrovascular disease, and cancer7–10. Second, internalizing disorders are together sufficiently common to be a public-health prevention target11, and the timing is right because internalizing disorders peak during the first half of the life course, whereas age-related diseases onset in the second half11. Third, internalizing disorders are themselves treatable. If the hypothesis that internalizing disorders accelerate progression toward age-related disease were true, this would imply that the population burden of age-related disease could be reduced by screening for and treating internalizing psychiatric conditions early in life. Further, we studied internalizing disorders instead of life stressors for several reasons: (a) there is an implicit assumption that if stress is consequential enough, it will generate symptoms of mental disorder, (b) persistent disorder is measured more reliably than stress events, and (c) disorders can be treated, and thus have translational relevance, whereas stress is generally not a treatment target.
We report a test of the association between internalizing disorders and leukocyte telomere length (LTL). The length of telomeres has been proposed as a biomarker for studying accumulating cellular damage throughout the life course12. Telomeres, the protective caps at the end of linear chromosomes, erode in somatic tissues with each division of a cell. Ultimately, telomere shortening in cells leads to mitochondrial and metabolic dysfunction, and cell cycle arrest13. Experimental and longitudinal studies have implicated shorter telomere length in disease susceptibility and greater risk of age-related disease and early mortality14–16.
Previous studies have provided mixed support for the association between internalizing disorders and shorter telomere length17–23. In the only study to date of internalizing disorder with repeated telomere length measurements, depression was associated with shorter LTL among elderly patients with coronary heart disease, but did not predict 5-year change in LTL24. Thus, it remains unclear whether internalizing disorder is associated with shortening of telomeres, that is, actual erosion.
Our study aimed to elaborate current knowledge by using a prospective-longitudinal design to test the link between lifetime assessment of internalizing disorders and LTL in the Dunedin birth cohort. Given that persistent depression, GAD and PTSD have high comorbidity11, 25, shared etiological mechanisms26, and overlap in symptomatology, we examined all three internalizing disorders. Since longer illness duration has previously correlated with shorter LTL19, we used data from our four-decade prospective study to test the association between persistence of internalizing disorder diagnoses and LTL. Further, given reports of sex differences in the (1) prevalence of internalizing disorders27, 28, (2) telomere length29, and (3) mortality risk associated with mental disorders9, 30, we tested for sex differences in all analyses.
We carried out two sets of analyses. The first set (Figure 1, analysis plan 1) tested the hypothesis that persistence of internalizing disorders across repeated assessments from ages 11 to 38 years would predict shorter LTL at age 38 years. This analysis considered alternative explanatory variables (including childhood maltreatment, cigarette consumption, substance dependence, psychiatric medication use, poor physical health and low socioeconomic status (SES)) thought to be associated with both internalizing disorders and LTL. The second set of analyses (Figure 1, analysis plan 2) took advantage of our two LTL measurements to test the hypothesis that LTL erosion between ages 26 to 38 years would be associated with internalizing disorder diagnosed in the interim. This hypothesis was also tested for depression, GAD and PTSD separately.
Figure 1.

Analysis plan flow chart to test the hypothesis of association between internalizing disorder and leukocyte telomere length.
Methods
Participants
Participants are members of the Dunedin Multidisciplinary Health and Development Study, a longitudinal investigation of health and behavior in a complete birth cohort. Study members (N=1037; 91% of eligible births; 52% male) were all individuals born between April 1972 and March 1973 in Dunedin, New Zealand, who were eligible for the longitudinal study based on residence in the Otago province at age 3 and who participated in the first follow-up assessment at age 3. The cohort represents the full range of SES in the general population of New Zealand’s South Island and is primarily White. Assessments were carried out at birth and at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, and, most recently, 38 years, when 95% of the 1 007 living study members underwent assessment in 2010–2012. At each assessment wave, each Study member is brought to the Dunedin Research Unit for a full day of interviews and examinations.
Assessments of internalizing disorders
The Dunedin Study longitudinally ascertains psychiatric disorders using a strategy akin to experience sampling; every two to six years, we interview participants about past-year symptoms. Past-year reports maximize reliability and validity, because recall of symptoms over longer periods has been shown to be inaccurate. Of course it is possible that past-year reports separated by one to five years miss episodes of mental disorder occurring only in gaps between assessments. We tested this by using life-history calendar interviews to ascertain indicators of mental disorder occurring in the gaps, including inpatient treatment, outpatient treatment, or spells taking prescribed psychiatric medication (these indicators are salient and recalled more reliably than individual symptoms)31. Life-history calendar data indicated that virtually all participants having disorder consequential enough to be associated with treatment have been detected in our net of past-year diagnoses made at ages 18, 21, 26, 32 and 38. Specifically, we identified only 11 people who reported treatment but had not been captured in our net of diagnoses from ages 18–38.
At ages 11, 13, and 15 years, the Diagnostic Interview Schedule for Children (DISC-C)32 was used to assess past-year depression and overanxious disorder of childhood (which in adulthood is subsumed by GAD), according to DSM-III. At ages 18, 21, 26, 32, and 38 years the Diagnostic Interview Schedule (DIS)33 was used to assess past-year major depression and GAD, according to the then-current versions of DSM-III-R and DSM-IV. PTSD was assessed for the first time at age 26, when lifetime reports were obtained, and subsequently at ages 32 and 38 years past-six-years PTSD was assessed. Interviewers were health professionals. All disorders were diagnosed regardless of the presence of other disorders. We included GAD and not phobias because GAD entails distress comparable to depression and PTSD, whereas most phobias include avoidance and thus are not accompanied by ongoing distress. The Dunedin cohort 12-month prevalence rates of internalizing disorders match rates from US and New-Zealand national surveys34.
For this study, given high comorbidity between internalizing disorders, we summed the number of assessments during which each Study member met diagnostic criteria for any internalizing disorder at each phase/age: 372 Study members (45.0%) had no history of internalizing disorder from 11 to 38 years; 210 (25.4%) met diagnostic criteria for an internalizing disorder at one assessment phase/age, 124 (15.0%) met criteria at two assessment phases/ages, 68 (8.2%) at three, 32 (3.9%) at four, and 21 (2.5%) at five or more assessment phases/ages.
Measurement of mean relative leukocyte telomere length
Leukocyte DNA was extracted from blood using standard procedures35, 36. Age-26 and age-38 DNA was stored at −80°C until assayed, to prevent degradation of the samples. All DNA samples were assayed for LTL at the same time, independently of caseness, and all operations were carried out by a laboratory technician blind to cases or controls. LTL was measured using a validated quantitative PCR method37, as previously described38, which determines mean telomere length across all chromosomes for all cells sampled. The method involves two quantitative PCR reactions for each subject; one for a single-copy gene (S) and the other in the telomeric repeat region (T). All DNA samples were run in triplicate for telomere and single-copy reactions at both ages 26 and 38, i.e., 12 reactions per Study member. Measurement artifacts (e.g., differences in plate conditions) may lead to spurious results when comparing LTL measured on the same individual at different ages. To eliminate such artifacts, we assayed DNA triplicates from the same individual, from both ages 26 and 38, on the same plate (see Supplementary Figure S1). The average coefficient of variation (CV) for the triplicate Ct values was 0.81% for the telomere (T) and 0.48% for the single-copy gene (S), indicating low measurement error. LTL, as measured by T/S ratio, was normally distributed (Kolomogorov-Smirnov tests of normality), with a skew of 0.90 and kurtosis 1.59 at age 26, and a skew of 0.48 and kurtosis 0.38 at age 38.
Alternative explanatory variables
We tested for alternative explanatory variables known to be associated with both internalizing disorders and LTL. These variables have been previously published, and have good reliability and validity in this cohort. They included: childhood maltreatment, lifetime cigarette consumption, substance dependence disorders between ages 18–38 years, psychiatric medication use between ages 20–38 years, poor physical health and low adult SES at age 38 years. All alternative explanatory variables are described in Table 1.
Table 1.
Description of alternative explanatory variables that may explain the association between internalizing disorder and leukocyte telomere length.
| Alternative explanatory variable | Description | Assessment Age(s) |
|---|---|---|
| Childhood maltreatment | Childhood maltreatment is a risk factor for internalizing disorders in adulthood73, and has also been associated with shorter telomere length (reviewed in56, 57). As previously described74, adverse childhood experiences during the first decade of life (ages 3–11) were ascertained using behavioral observations, parental reports, and retrospective reports by Study members once they reached adulthood. Measures of childhood maltreatment in the Dunedin study included (1) maternal rejection assessed at age 3 years by observational ratings of mothers’ interaction with the study children, (2) harsh discipline assessed at ages 7 and 9 years by parental report of disciplinary behaviors, (3) exposure to disruptive caregiver changes assessed through age 11 and defined by two or more changes of the child’s primary caregiver, and (4) exposure to physical abuse and (5) exposure to sexual abuse assessed retrospectively at age 26 years. The number of indicators was summed to a scale of 0 to 2+. 65% of children experienced no maltreatment, 27% experienced 1 indicator of maltreatment, and 8% experienced 2 or more indicators of maltreatment. | 3 to 11 |
| Lifetime cigarette consumption (pack years) | Higher cigarette consumption is associated with both internalizing disorders75 and shorter telomere length76. Here, we defined lifetime cigarette consumption as: pack-years = the number of cigarettes smoked per day, divided by 20 and multiplied by the number of years smoked at that rate through age 38 (M = 5.77, SD = 8.36). | Lifetime up to 38 |
| Substance dependence disorders lifetime | Substance dependence and internalizing disorders tend to co-occur in the same individuals 77, and there is some evidence that substance-use disorders may be associated with shorter telomere length78, 79. Past-year substance dependence in the Dunedin Study was assessed at ages 18, 21, 26, 32 and 38 years. Diagnoses included; cannabis, alcohol and hard-drug dependence. Diagnoses at each age followed the then-current version of the DSM (M = 0.76 diagnoses, SD = 1.21). | 18 to 38 |
| Psychiatric medication use | There is concern about potential adverse effects of psychiatric medications on health and physiological processes80. Use of psychiatric medications in the Dunedin Study was assessed using successive life history calendars31 covering the period from ages 20 to 38 years. 28.8% reported taking medication for a psychiatric problem. | 20 to 38 |
| Physical health problem index | Poor physical health has been associated with both internalizing disorders81 and shorter telomere length61. Here, physical health at age 38 years was measured by 9 clinical indicators of poor adult health, including metabolic abnormalities (waist circumference, high-density lipoprotein level, triglyceride level, blood pressure, and glycated hemoglobin), cardiorespiratory fitness, pulmonary function, periodontal disease, and systemic inflammation (high-sensitivity C-reactive protein). Pregnant women were excluded from the reported analyses. Descriptions for each clinical indicator and clinical cutoffs are provided in Supplementary Table S1. We summed the number of clinical indicators on which Study members exceeded clinical cutoffs. 24.7% had 0 clinical indicators, 23.8% had 1 clinical indicator, 20.8% had 2 clinical indicators, 14.3% had 3, 7.7% had 4, 4.9% had 5, and 3.9% had 6 clinical indicators or more. | 38 |
| Adult socioeconomic status (SES) | Low SES has been associated with psychiatric morbidity82, and weakly associated with telomere length83. Adult SES at age 38 years was determined based on the Study members’ occupation following the New Zealand socio-economic index, and coded to a six-point scale84 (M = 3.79, SD = 1.41). | 38 |
Statistical analysis
In analysis plan 1 (Figure 1), we tested the hypothesis that persistence of internalizing disorders would predict LTL, by regressing age-38 LTL on a predictor variable indicating the number of phases/ages during which Study members met criteria for an internalizing disorder. To test for alternative explanations of the association between internalizing disorder and LTL, we statistically controlled for alternative explanatory variables in ordinary least squares multiple regression analyses. Supplementary analyses ruled out potential effects of white blood cell counts on LTL at age 38 years (see Supplementary Table S3).
In analysis plan 2 (Figure 1), we tested the hypothesis that LTL erosion between ages 26 and 38 years would be associated with internalizing disorder in the interim. We compared Study members who met diagnostic criteria for internalizing disorders after the age-26 assessment but before the age-38 assessment (N=234) to controls who did not experience internalizing disorders during this time period (N=524). To estimate the statistical contribution of internalizing disorders to change in LTL, we regressed age-38 LTL on age-26 LTL (generating an estimate of LTL change) and a dummy variable indicating whether a Study member met criteria for an internalizing disorder between ages 26 and 38 years (an estimate of the association between internalizing disorder and greater LTL change).
Given the possibility of sex differences, we relaxed our significance criterion (to α =0.20) in order to reduce the risk of making type II error when testing for sex interaction, as previously recommended 39 (p. 208). Given that in both analyses (plan 1 and 2) the sex interaction was p ≤ .20, we present results separately for men and women. Age was not controlled statistically, as all study members were the same age (1972–73 births).
Results
Analysis plan 1: Does persistence of internalizing disorder predict leukocyte telomere length at age 38 years?
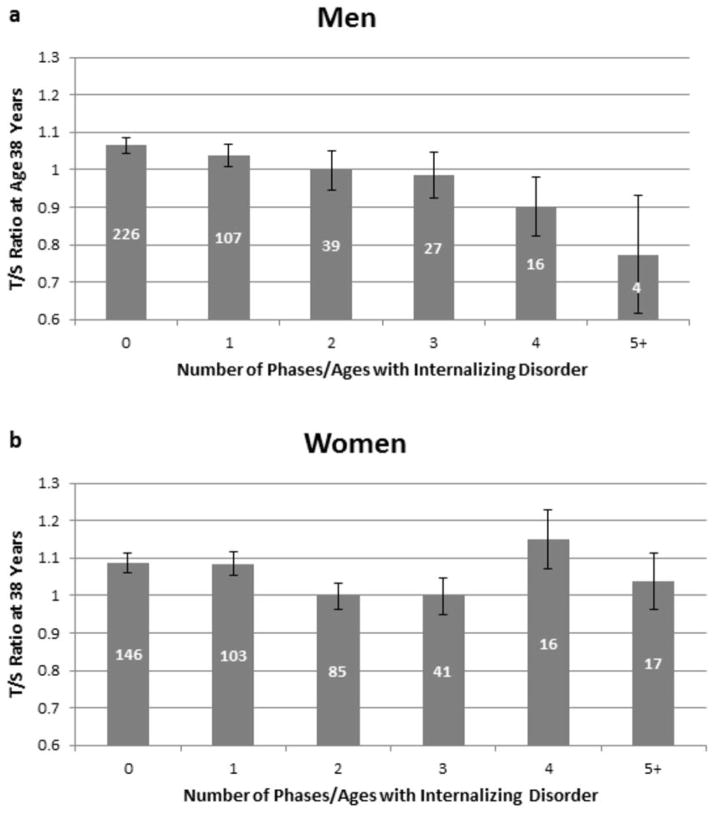
Persistence of internalizing disorder from 11 to 38 years was significantly associated with shorter LTL at age 38 years among men, in a dose-response manner (n=419; β= −.137, 95% CI: −.232, −.042, p=.005) (Figure 2a). In contrast, there was no significant association between internalizing disorder and shorter LTL among women, and no evidence of a dose-response association (n=408; β= −.066, 95% CI: −.163, .032, p=.185) (Figure 2b). To be certain that the association between internalizing disorder and shorter LTL among men was not driven by the four men who had internalizing disorders at five or more phases/ages, we repeated the analysis removing these men. The association remained significant (n=415; β= −.116, 95% CI: −.212, −.020, p=.018).
Figure 2. Telomere Length.
Association between internalizing disorder from age 11 to 38 years and LTL at 38 years, for men (a) and women (b). Error bars reflect standard errors. Numbers within bars represent the number of Study members in each group.
We next tested if factors that may be associated with both internalizing disorders and telomere length can account for the association between internalizing disorder and shorter LTL. Given that the positive association between internalizing disorders and LTL was limited to men, the following analyses are presented for men only. Table 2 shows that all alternative explanatory variables were significantly correlated with internalizing disorder from 11 to 38 years. In addition, lifetime cigarette consumption, life-time substance dependence and poorer physical health were significantly correlated with shorter age-38 LTL (p ≤ .05). Controlling for each of the alternative explanatory variables individually did not alter the initial finding, nor did controlling for all variables simultaneously (n=419; β= −.124, 95% CI: −.232, −.016, p=.025) (Table 2).
Table 2.
Pearson correlations and multivariate linear regression analyses of internalizing disorder from 11 to 38 years, predicting LTL at 38 years, controlling for alternative explanatory variables. Results are presented for men only (N=419).
| Bivariate Pearson correlation | Association between internalizing disorder 11–38 and LTL at age 38 years | |||
|---|---|---|---|---|
| Internalizing disorder age 11 to 38 years | LTL at age 38 years | β (95% CI) | p value | |
| Internalizing disorder from age 11 to 38 years | - | - | −.137 ( −.232, −.042) | .005 |
| Controlling for alternative explanatory variables: | ||||
| Childhood maltreatment | .173** | −.032 | −.135 (−.232, −.039) | .006 |
| Lifetime cigarette consumption (pack year) up to age 38 years | .245** | −.105* | −.118 (−.217, −.020) | .018 |
| Substance dependence disorders from age 18 to 38 years | .335** | −.092 | −.119 (−.221, −.018) | .021 |
| Psychiatric medication use from age 20 to 38 years | .413** | −.019 | −.156 (−.260, −.051) | .004 |
| Physical health problem index at age 38 years1 | .129** | −.135** | −.121 (−.217, −.026) | .013 |
| Adult SES at age 38 years2 | −.138** | .051 | −.132 (−.229, −.036) | .007 |
| All alternative explanatory variables | - | - | −.124 (−.232, −.016) | .025 |
Analyses for the different physical health indicators are provided in Supplementary Table S2.
Higher scores on the scale indicate higher SES at age 38 years. Significant p values are highlighted in boldface;
p < .05;
p < .005.
Analysis plan 2: Do internalizing disorders predict accelerated leukocyte telomere length erosion from ages 26 to 38 years?
The correlation between age-26 LTL and age-38 LTL was significant, among both men (r=.676, p< .001) and women (r=.678, p< .001), indicating stability of individual differences in LTL over time; individuals with long telomeres at age 26 continued to have relatively long telomeres at age 38. In addition, there was significant decline in mean LTL from age 26 to 38 years. The average LTL declined from 1.182 T/S (SD=0.40) to 1.028 T/S (SD=0.32) among men (General Linear Model repeated measures: F(388,1)=102.59, p< 0.001), and from 1.196 T/S (SD=0.40) to 1.046 T/S (SD=0.30) among women (F(368,1)=97.25, p< 0.001).
Against this background, men who experienced internalizing disorder between ages 26 to 38 years showed significantly more LTL erosion than men with no internalizing disorder (n=389; β= −.111, 95% CI: −.184, −.037, p=.003) (Figure 3a). In contrast, there was no significant association between internalizing disorder and accelerated LTL erosion among women (n=369; β= −.031, 95% CI: −.106, .045, p=.427) (Figure 3b).
Figure 3. Telomere Erosion.

Association between GAD, depression and PTSD between ages 26 to 38 years and LTL at age 38 years (after controlling for baseline LTL at age-26 years) for men (a) and women (b). Error bars reflect standard errors. Numbers within bars represent the number of Study members in each group. The sum of each disorder (GAD, depression and PTSD) exceeds the total for any diagnosis due to comorbidity across internalizing disorders. Because fewer than half of individuals in each of the three disorder groups had only one type of internalizing disorder, we lacked statistical power for testing pure cases.
Figure 3a shows a similar effect for each type of disorder on accelerated LTL erosion, among men. There was significantly accelerated LTL erosion among men who experienced depression (β= −.114, 95% CI: −.190, −.039, p=.003) and GAD (β= −.100, 95% CI: −.181, −.020, p=.015). The association for PTSD among men was in the same direction, but did not reach significance (β= −.065, 95% CI: −.147, .017, p=.120), in part because of lack of power. In contrast, women who experienced depression (β= −.010, 95% CI: −.088, .067, p=.795), GAD (β= −.056, 95% CI: −.140, .029, p=.194), or PTSD (β= −.007, 95% CI: −.095, .081, p=.879) did not show significantly more LTL erosion (Figure 3b). Because fewer than half of individuals in each of the three disorder groups had only one type of internalizing disorder, we lacked statistical power for testing pure cases. However, among them, the pattern of mean LTL differences decreased somewhat, suggesting more LTL erosion among comorbid cases.
Finally, given uncertainty about the interpretation of telomere lengthening (is it measurement error40, regression to the mean41 or a real biological phenomenon?42), we retested the association between internalizing disorder and LTL erosion after excluding Study members whose telomeres lengthened. As previously described38, lengthening was defined as >15% increase in LTL between measurements (12.8% of this cohort, 12.9% of men and 12.8% of women). Internalizing disorder between ages 26 to 38 years remained significantly associated with accelerated LTL erosion in men (n=339; β= −.075, 95% CI: −.142, −.009, p=.026), and remained unassociated in women (n=322; β= −.037, 95% CI: −.106, .033, p=.302).
Discussion
The present study tested the hypothesis that internalizing disorders are associated with shorter telomere length and accelerated telomere erosion. After accounting for multiple alternative explanatory factors, depression, GAD, and PTSD were associated with shorter LTL at age 38 years and with accelerated LTL erosion across a 12-year period, among men. No significant associations were observed between internalizing disorder and LTL among women.
This study has several strengths. While most previous telomere studies have assessed internalizing disorders at a single time-point, the four-decade prospective nature of our cohort allows reliable repeated assessments of Study members’ psychiatric histories covering a period of more than 25 years. Moreover, the two DNA collection phases enabled us to test for association between internalizing disorder and LTL change over a 12-year period. Further, given the numerous factors assumed to affect both internalizing disorder and LTL, it was useful to test multiple alternative explanations of the association between internalizing disorder and LTL. We did so using measures that were both lifelong (e.g., smoking) and contemporaneous (physical health). Finally, most previous studies have focused on depression. We took the opportunity to study GAD and PTSD, as well as depression. Our study extends previous knowledge to suggest a potential common mechanism of accelerated cellular aging linked with all three internalizing disorders.
Our findings suggest that the association between internalizing disorder and LTL may be stronger in men, or even absent in women. Despite the higher prevalence of internalizing disorder among women, there is some evidence that men who suffer from mental disorder are at higher risk of mortality than women9, 30, which is consistent with the sex difference we observed in this study. Several physiological and biochemical processes implicated in internalizing disorders have been shown, in some studies, to be more affected among men than women, including dysregulation of the hypothalamic-pituitary-adrenal axis43, elevated proinflammatory cytokines44 and elevated oxidative stress markers45. These sex-varying processes are also implicated in the regulation of telomere length46–48 and could provide a basis for the stronger associations we observed here among men. Further, our study period covered the cohort womens’ child-bearing years; evolutionary theory might allow sex-specific stress-protection factors for women of reproductive age. For example, animal studies suggest mitochondrial antioxidant protection among females, specifically during reproductive age49, 50, a difference thought to be partially explained by estrogens49. Notably, mitochondrial dysfunction is considered a mechanism by which telomeres erode as a result of higher oxidative stress46, 51. Moreover, the expression and activity of telomerase is increased in the presence of estrogens52, 53. If true, women may be less susceptible to disorder-linked telomere erosion during reproductive years. Although plausible, our sex-specific finding must be replicated (prior studies have not systematically tested sex differences). Future studies may also test whether the non-association we observed is limited to women of reproductive age.
Our findings should be interpreted in light of limitations and caveats. First, as the Dunedin cohort is primarily White, future studies need to test whether the association between internalizing disorder and LTL generalizes to other populations. Second, although the Dunedin Study was one of the first cohort studies to extract DNA in the 1990s, our first LTL measure represented age 26 years and we have no earlier LTL baseline in childhood. Third, we used an experience-sampling approach, ascertaining mental disorder in eight one-year windows spaced across 27 years. Contiguous annual assessments would be better, but neither funders nor research participants favor this approach. Fourth, our finding of within-individual LTL change is consistent with the hypothesis that internalizing disorders affect LTL, but it is also possible that telomere erosion can be a cause of disorder, or the association between them might be brought about by an unmeasured third variable54. For example, theoretically individuals genetically predisposed to accelerated telomere erosion may also be at risk for disorders. Fifth, our study was ill-equipped to test if mental health treatment use can prevent telomere erosion, because we had inadequate information about treatment type, quality, duration, compliance, or response. Finally, a noteworthy caveat is that we did not find an association between childhood maltreatment and LTL at age 38 years. Although another study has reported null association55, a null finding contradicts our positive finding from a cohort of young children38, as well as other previous studies (reviewed in56, 57). More research is needed to understand why the link between maltreatment and LTL has emerged in some studies, but not all.
The present findings have implications for basic and translational research. With regard to basic research, long-term follow-up studies are needed to test whether accelerated telomere erosion indeed mediates the link between internalizing disorders and later age-related disease outcomes. The use of LTL as a biomarker of aging remains controversial58. If future research shows that LTL mediates the association between internalizing disorders and age-related diseases, telomeres might become an eventual therapeutic target59. Moreover, research should test whether telomere erosion accompanies other persistent mental disorders, such as psychoses. Furthermore, research should elucidate the molecular pathways by which the connection between internalizing disorder and LTL damage occurs. Proposed mechanisms include elevated oxidative stress, mitochondrial dysfunction and telomerase regulation. Immune-system changes are considered one potential mechanism19, 47, as can be observed in this study with telomere length measured from immune cells. However, an indicator of elevated systemic inflammation (C-reactive protein) was unrelated to LTL at age 38 years in this cohort (see Supplementary Table S2), and thus could not mediate the association we observed. Previous research has shown that certain inflammatory markers are associated with shorter LTL47, 60, 61. However, the association between high levels of CRP and short telomere length is ambiguous. While several studies have reported positive associations61–64, others have reported no association47, 65–68. It is also worth noting that these studies have used different methods to measure telomere length (e.g., Southern blot versus qPCR) and CRP (e.g., high-sensitivity versus not) that may have contributed to the mixed findings. Moreover, the majority of studies were of older, and mostly clinical, populations. More studies are needed to determine whether CRP is associated with telomere length in younger age groups.
With regard to translational research, our findings suggest that randomized clinical trials of treatments for internalizing disorders could incorporate before-and-after-treatment telomere length69, 70 and/or telomerase71 measurement, to ascertain whether treatments that ameliorate psychiatric disorder might also prevent or decelerate telomere erosion72. If yes, this would raise the possibility that effective screening and treatment for internalizing disorders in the first half of the life course might prevent or reverse processes underlying age-related disease, and enhance population health expectancy.
Supplementary Material
Acknowledgments
We thank the Dunedin Study members, Unit research staff, Bob Hancox, Murray Thomson, and Study founder Phil Silva. This research received support from the U.S. National Institute of Aging (AG032282) and the U.K. Medical Research Council grant (MR/K00381X). The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council. Additional support was provided by the Klaus-Grawe Foundation and the Jacobs Foundation. The study protocol was approved by the institutional ethical review boards of the participating universities. Study members gave informed consent before participating.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Vaupel JW. Biodemography of human ageing. Nature. 2010;464(7288):536–542. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305(5691):1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408(6809):233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 4.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Wellcome Trust. Ageing: Can We Stop the Clock? Wellcome Trust; 2006. [Google Scholar]
- 6.Brayne C. The elephant in the room - healthy brains in later life, epidemiology and public health. Nat Rev Neurosci. 2007;8(3):233–239. doi: 10.1038/nrn2091. [DOI] [PubMed] [Google Scholar]
- 7.Colton CW, Manderscheid RW. Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis. 2006;3(2):A42. [PMC free article] [PubMed] [Google Scholar]
- 8.Druss BG, Zhao L, Von Esenwein S, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness 17-year follow up of a nationally representative US Survey. Med Care. 2011;49(6):599–604. doi: 10.1097/MLR.0b013e31820bf86e. [DOI] [PubMed] [Google Scholar]
- 9.Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72(3):227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108(1):29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 12.Epel ES. Telomeres in a life-span perspective: a new “psychobiomarker”? Curr Dir Psychol Sci. 2009;18(1):6–10. [Google Scholar]
- 13.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470(7334):359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armanios M, Blackburn EH. The telomere syndromes. Nature Rev Genet. 2012;13 (10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen S, Janicki-Deverts D, Turner RB, Casselbrant ML, Li-Korotky HS, Epel ES, et al. Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults. JAMA. 2013;309(7):699–705. doi: 10.1001/jama.2013.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiecolt-Glaser JK, Jaremka LM, Derry HM, Glaser R. Telomere length: a marker of disease susceptibility? Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, et al. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27(12):1111–1116. doi: 10.1002/da.20749. [DOI] [PubMed] [Google Scholar]
- 19.Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su YL, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress - preliminary findings. PloS One. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladwig K-H, Brockhaus AC, Baumert J, Lukaschek K, Emeny RT, Kruse J, et al. Posttraumatic stress disorder and not depression is associated with shorter leukocyte telomere length: findings from 3,000 participants in the population-based KORA F4 Study. PloS One. 2013;8(7):e64762. doi: 10.1371/journal.pone.0064762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okereke OI, Prescott J, Wong JYY, Han JL, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PloS One. 2012;7(7):e40516. doi: 10.1371/journal.pone.0040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L, et al. Do symptoms of depression predict telomere length? evidence from the West of Scotland Twenty-07 Study. Psychosom Med. 2013;75(3):288–296. doi: 10.1097/PSY.0b013e318289e6b5. [DOI] [PubMed] [Google Scholar]
- 24.Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, et al. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med. 2011;73(7):541–547. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brady KT, Killeen TK, Brewerton T, Lucerini S. Comorbidity of psychiatric disorders and posttraumatic stress disorder. J Clin Psychiatry. 2000;61 (Suppl 7):22–32. [PubMed] [Google Scholar]
- 26.Sartor CE, Grant JD, Lynskey MT, McCutcheon VV, Waldron M, Statham DJ, et al. Common heritable contributions to low-risk trauma, high-risk trauma, posttraumatic stress disorder, and major depression. Arch Gen Psychiatry. 2012;69 (3):293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton NR, Keyes KM, Krueger RF, Balsis S, Skodol AE, Markon KE, et al. An invariant dimensional liability model of gender differences in mental disorder prevalence: evidence from a national sample. J Abnorm Psychol. 2012;121(1):282–288. doi: 10.1037/a0024780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133(2):183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 29.Barrett EL, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. 2011;10(6):913–921. doi: 10.1111/j.1474-9726.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang CK, Hayes RD, Broadbent M, Fernandes AC, Lee W, Hotopf M, et al. All-cause mortality among people with serious mental illness (SMI), substance use disorders, and depressive disorders in southeast London: a cohort study. BMC Psychiatry. 2010;10(77) doi: 10.1186/1471-244X-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caspi A, Moffitt TE, Thornton A, Freedman D, Amell JW, Harrington H, et al. The life history calendar: a research and clinical assessment method for collecting retrospective event-history data. Int J Method Psych. 1996;6(2):101–114. [Google Scholar]
- 32.Costello A, Edelbrock C, Kalas R, Kessler M, Klaric S. NIMH Diagnostic Interview for Children: Child Version. Rockville, Md: National Institute of Mental Health; 1982. [Google Scholar]
- 33.Robins L, Cottler L, Bucholz K, Compton W. Diagnostic Interview Schedule for DSM-IV (DIS-IV) 1995. [DOI] [PubMed] [Google Scholar]
- 34.Moffitt TE, Caspi A, Taylor A, Kokaua J, Milne BJ, Polanczyk G, et al. How common are common mental disorders? evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychol Med. 2010;40(6):899–909. doi: 10.1017/S0033291709991036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bowtell DD. Rapid isolation of eukaryotic DNA. Anal Biochem. 1987;162(2):463–465. doi: 10.1016/0003-2697(87)90421-0. [DOI] [PubMed] [Google Scholar]
- 36.Jeanpierre M. A rapid method for the purification of DNA from blood. Nucleic Acids Res. 1987;15(22):9611. doi: 10.1093/nar/15.22.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2013;18(5):576–81. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvin S. Statistical analysis of epidemiologic data. Oxford University Press; 2004. [Google Scholar]
- 40.Steenstrup T, Hjelmborg JV, Kark JD, Christensen K, Aviv A. The telomere lengthening conundrum--artifact or biology? Nucleic Acids Res. 2013;41(13):e131. doi: 10.1093/nar/gkt370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verhulst S, Aviv A, Benetos A, Berenson GS, Kark JD. Do leukocyte telomere length dynamics depend on baseline telomere length? an analysis that corrects for ‘regression to the mean’. Eur J Epidemiol. 2013 doi: 10.1007/s10654-013-9845-4. [DOI] [PubMed] [Google Scholar]
- 42.Epel E. How “reversible” is telomeric aging? Cancer Prev Res. 2012;5(10):1163–1168. doi: 10.1158/1940-6207.CAPR-12-0370. [DOI] [PubMed] [Google Scholar]
- 43.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychology. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 44.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 45.Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22(3):438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- 46.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27 (7):339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 47.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, et al. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PloS One. 2011;6(5):e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J, Fauce SR, Effros RB. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun. 2008;22(4):600–605. doi: 10.1016/j.bbi.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vina J, Sastre J, Pallardo’ F, Borras C. Mitochondrial theory of aging: importance to explain why females live longer than males. Antioxid Redox Sign. 2003;5(5):549–556. doi: 10.1089/152308603770310194. [DOI] [PubMed] [Google Scholar]
- 50.Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radical Bio Med. 2003;34(5):546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Misiti S, Nanni S, Fontemaggi G, Cong YS, Wen JP, Hirte HW, et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20(11):3764–3771. doi: 10.1128/mcb.20.11.3764-3771.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bayne S, Jones MEE, Ll H, Liu JP. Potential roles for estrogen regulation of telomerase activity in aging. Ann Ny Acad Sci. 2007;1114:48–55. doi: 10.1196/annals.1396.023. [DOI] [PubMed] [Google Scholar]
- 54.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Glass D, Parts L, Knowles D, Aviv A, Spector TD. No correlation between childhood maltreatment and telomere length. Biol Psychiatry. 2010;68(6):e21. doi: 10.1016/j.biopsych.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shalev I. Early life stress and telomere length: investigating the connection and possible mechanisms. BioEssays. 2012;34(11):943–952. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mather KA, Jorm AF, Parslow RA, Christensen H. Is Telomere Length a Biomarker of Aging? A Review. J Gerontol A Biol Sci Med Sci. 2011;66 (2):202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 59.de Jesus BB, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4(8):691–704. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damjanovic AK, Yang YH, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carrero J, Stenvinkel P, Fellström B, Qureshi A, Lamb K, Heimbürger O, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med. 2008;263(3):302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 62.Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6(5):639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 63.Solorio S, Murillo-Ortiz B, Hernandez-Gonzalez M, Guillen-Contreras J, Arenas-Aranda D, Solorzano-Zepeda FJ, et al. Association between telomere length and C-reactive protein and the development of coronary collateral circulation in patients with coronary artery disease. Angiology. 2011;62(6):467–472. doi: 10.1177/0003319710398007. [DOI] [PubMed] [Google Scholar]
- 64.Masi S, Nightingale CM, Day IN, Guthrie P, Rumley A, Lowe GD, et al. Inflammation and not cardiovascular risk factors is associated with short leukocyte telomere length in 13-to 16-year-old adolescents. Arterioscler Thromb Vasc Biol. 2012;32(8):2029–2034. doi: 10.1161/ATVBAHA.112.250589. [DOI] [PubMed] [Google Scholar]
- 65.Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29(2):283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- 66.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PloS One. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiol Aging. 2012;33(7):1486, e1483–1488. doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 68.Sanders JL, Fitzpatrick AL, Boudreau RM, Arnold AM, Aviv A, Kimura M, et al. Leukocyte telomere length is associated with noninvasively measured age-related disease: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2012;67 (4):409–416. doi: 10.1093/gerona/glr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Biegler KA, Anderson A, Wenzel LB, Osann K, Nelson EL. Longitudinal changes in telomere length and chronic stress response: implications for cancer prevention from a randomized biobehavioral clinical study. Cancer Prev Res (Phila) 2012;5(10):1173–1182. doi: 10.1158/1940-6207.CAPR-12-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ornish D, Lin J, Chan JM, Epel E, Kemp C, Weidner G, et al. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 2013;14(11):1112–1120. doi: 10.1016/S1470-2045(13)70366-8. [DOI] [PubMed] [Google Scholar]
- 71.Daubenmier J, Lin J, Blackburn E, Hecht FM, Kristeller J, Maninger N, et al. Changes in stress, eating, and metabolic factors are related to changes in telomerase activity in a randomized mindfulness intervention pilot study. Psychoneuroendocrinology. 2012;37(7):917–928. doi: 10.1016/j.psyneuen.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moffitt TE, Klaus-Grawe Think Tank. Childhood exposure to violence and lifelong health: clinical intervention science and stress biology research join forces. Dev Psychopathol. 2012 doi: 10.1017/S0954579413000801. (25th Anniversary Special issue) (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, et al. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry. 2010;197(5):378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- 75.Breslau N, Novak SP, Kessler RC. Psychiatric disorders and stages of smoking. Biol Psychiatry. 2004;55(1):69–76. doi: 10.1016/s0006-3223(03)00317-2. [DOI] [PubMed] [Google Scholar]
- 76.McGrath M, Wong JYY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidem Biomar. 2007;16(4):815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- 77.Kessler RC, Walters EE, Aguilar-Gaxiola S, Andrade L, Borges LG, Caraveo-Anduaga JJ, et al. Handbook of Drug Abuse Prevention. Springer; 2006. Cross-national comparisons of co-morbidities between substance use disorders and mental disorders; pp. 447–472. [Google Scholar]
- 78.Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, et al. Shortened telomeres in individuals with abuse in alcohol consumption. Int J Cancer. 2011;129(4):983–992. doi: 10.1002/ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang ZY, Ye JY, Li CD, Zhou DZ, Shen Q, Wu J, et al. Drug addiction is associated with leukocyte telomere length. Sci Rep. 2013;3:1542. doi: 10.1038/srep01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mackin P. Cardiac side effects of psychiatric drugs. Hum Psychopharmacol. 2008;23 (Suppl 1):3–14. doi: 10.1002/hup.915. [DOI] [PubMed] [Google Scholar]
- 81.Scott KM, Bruffaerts R, Tsang A, Ormel J, Alonso J, Angermeyer MC, et al. Depression-anxiety relationships with chronic physical conditions: results from the World Mental Health Surveys. J Affect Disord. 2007;103(1–3):113–120. doi: 10.1016/j.jad.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 82.Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 83.Robertson T, Batty GD, Der G, Fenton C, Shiels PG, Benzeval M. Is socioeconomic status associated with biological aging as measured by telomere length? Epidemiol Rev. 2013;35:98–111. doi: 10.1093/epirev/mxs001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milne B, Byun U, Lee A. New Zealand socio-economic index 2006. Wellington: Statistics New Zealand; 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.