Abstract
Background:
To explore the origin of Propionibacterium in surgical wounds and to suggest an optimized strategy for culturing this organism at the time of revision surgery, we studied the presence of this organism on the skin and in the surgical wounds of patients who underwent revision arthroplasty for reasons other than apparent infection.
Methods:
Specimens were cultured in broth and on aerobic and anaerobic media. The presence and degree of positivity of Propionibacterium cultures were correlated with sex. The results of dermal and deep cultures were correlated. Times to positivity and the yields of each media type and specimen source were investigated.
Results:
Propionibacterium grew in twenty-three of thirty cultures of specimens obtained preoperatively from the unprepared epidermis over the area where a skin incision was going to be made for a shoulder arthroplasty; males had a greater average degree of positivity than females (p < 0.002). Twelve of twenty-one male subjects and zero of twenty female subjects who had cultures of dermal specimens obtained during revision shoulder arthroplasty had positive findings for Propionibacterium (p = 0.0001). Twelve of twenty male subjects and only one of twenty female subjects had positive deep cultures (p = 0.0004). The positivity of dermal cultures for Propionibacterium was significantly associated with the positivity of deep cultures for this organism (p = 0.0001). If Propionibacterium was present in deep tissues, it was likely that it would be recovered by culture if four different specimens were obtained and cultured for a minimum of seventeen days on three different media: aerobic, anaerobic, and broth.
Conclusions:
Because the surgical incision of dermal sebaceous glands may be a source of Propionibacterium in deep wounds, strategies for minimizing the risk of Propionibacterium infections may need to be directed at minimizing the contamination of surgical wounds from these bacteria residing in rather than on the skin. Obtaining at least four specimens, observing them for seventeen days, and using three types of culture media optimize the recovery of Propionibacterium at the time of revision surgery.
Propionibacterium species are often grown on culture of specimens obtained at the time of orthopaedic revision surgery. The pathogenicity of this organism has been demonstrated for patients who have undergone shoulder implant surgery1-14, hip or knee arthroplasty15-22, or spine surgery23-37. While many cases of Propionibacterium infections are attributed to Propionibacterium acnes, other species of Propionibacterium have been recovered from revision surgical sites38. It is commonly assumed that the patient’s epidermal skin surface is the source of the Propionibacterium grown on culture of specimens from infected orthopaedic implant sites. Unfortunately, presurgical skin surface preparations do not completely eliminate Propionibacterium39-42, perhaps because the organism is sheltered in dermal sebaceous glands, each of which may harbor as many as 105 Propionibacterium organisms per follicle43. Surgical incisions transect the sebaceous glands, after which these organisms may begin to leak into the surgical wound. A better understanding of the source of these organisms may help us to design strategies to reduce the risk of contamination of surgical implants with Propionibacterium.
It is likely that the published rates of positive cultures for Propionibacterium in revision orthopaedic surgery are falsely low because (1) the clinical manifestations of the infection may be subtle and be delayed by months or years after the index surgery, reducing the suspicion of infection; (2) the surgical site may not appear to be infected and thus culture specimens are not obtained; and (3) the most sensitive strategies for culturing this organism are not used14. Although a variety of approaches have been proposed for culturing Propionibacterium from surgical wounds44, the optimal sources of the culture specimens, the optimal number of specimens, the optimal culture media, and the optimal duration of culture observation have not been determined.
We analyzed our experience in culturing Propionibacterium from the skin surface of normal shoulders prior to elective arthroplasty and from dermal and deep specimens from shoulders undergoing revision arthroplasty with the goal of answering the following questions:
How did the results of cultures of unprepared epidermis from male subjects compare with those of female subjects?
How did the results of dermal cultures from male patients undergoing revision arthroplasty compare with those of female patients?
Which sources of deep specimens had the highest rate of positive cultures?
What was the optimal number of deep specimens used for culture?
Which culture media had the highest yield of positive cultures?
For how long was it necessary to maintain the cultures before they became positive?
On the basis of these observations we sought to better understand the possible sources of Propionibacterium in surgical wounds and to develop an evidence-based approach for optimizing the culturing of Propionibacterium from shoulders undergoing revision arthroplasty.
Materials and Methods
Epidermal Cultures
We sought to characterize the epidermal microbiome along the planned skin incision in healthy patients immediately prior to elective primary shoulder arthroplasty and before the administration of prophylactic antibiotics. Thirty adults (mean age [and standard deviation], 63 ± 15 years; eighteen male and twelve female) consented to undergo culture of a single specimen collected with a Copan ESwab (Copan Diagnostics, Murrieta, California) from their unprepared skin surface. None of the subjects were taking antibiotics at the time of specimen collection. All subjects had used bactericidal soap (4% chlorhexidine) for showers the night before and the morning of the surgery.
Dermal and Deep Cultures
In the second part of the study, forty-one patients (mean age, 61 ± 12 years; twenty-one male and twenty female) consented to have Copan ESwab culture-specimen collection from the incised dermal edges of the skin incision along with removal of deep culture specimens (arthroplasty explants, membranes, other tissues, and fluid) during revision shoulder arthroplasty. Our standard practice in revision surgery is to prepare the skin with chlorhexidine and adhesive iodine-impregnated plastic drape but withhold systemic antibiotics until all culture specimens are obtained. Dermal culture specimens were obtained through the skin immediately after the skin incision was made (initial dermal culture) and then again after deep culture specimens had been obtained (final dermal culture) just prior to antibiotic administration. Three to seven deep culture specimens were taken according to the degree of clinical suspicion. When taking the dermal and deep culture specimens we tried to avoid contact of one with the other and used separate instruments to harvest the specimens to minimize the risk of cross contamination.
Culture Methods
All specimens were processed within one hour after surgery in a class-2 laminar-flow biological safety cabinet. Specimens were directly inoculated onto the following culture media with use of a sterile disposable Pasteur pipette and struck for single-colony isolation with sterile disposable loops: blood agar (trypticase soy agar with 5% sheep blood), chocolate agar, Brucella agar (with blood, hemin, and vitamin K), and brain-heart infusion broth (Remel, Lenexa, Kansas)38,44. Tissue specimens were homogenized in 3 mL of sterile saline solution with use of a Seward Stomacher 80 (Seward, Port Saint Lucie, Florida) prior to culture media inoculation, and the sterility of each vial of saline solution was confirmed at the time of use by bacterial culture. Explants were vortexed for one minute in 3 mL of sterile saline solution, which was then used for inoculation. MycoSeal sealant (Hardy Diagnostics, Santa Maria, California) was applied to the exterior of all agar plates to prevent desiccation during culture incubation. All media, with the exception of the Brucella agar, were incubated aerobically at 37°C with 5% CO2 for twenty-eight days. Brucella agar plates were incubated anaerobically at 37°C for twenty-eight days. Media were visually examined daily for growth and were opened only if growth was noted. Cultures were observed up to twenty-eight days after inoculation.
Analysis of Culture Results
In our microbiology laboratory, growth of microorganisms on culture media is reported semiquantitatively as one colony only, growth in broth only, 1+, 2+, 3+, or 4+ (i.e., light-to-heavy growth). In the absence of an established method by which these semiquantitative results could be combined and compared, we assigned numerical values to the results of each specimen culture in consultation with the head of our microbiology laboratory. Values of 0, 0.1, and 0.2 were assigned to “no growth,” “one colony only,” and “growth in broth only,” and values of 1, 2, 3, and 4 were assigned to 1+, 2+, 3+, and 4+, respectively. We refer to this number as the “degree of positivity” for each specimen in that it roughly reflects the amount of bacterial growth. When multiple deep cultures of specimens from a shoulder were positive for Propionibacterium, we summed the degrees of positivity for that shoulder as the “Propionibacterium score.” We recognize that these numerical scores are not a precise indication of the number of bacteria present; for example, a 3+ culture does not necessarily have three times the bacteria as a 1+ culture.
Exploration of an Evidence-Based Approach to Culturing Propionibacterium
In the third part of our study, we identified positive deep cultures (Propionibacterium score > 0.1) for seventy-four patients who had undergone revision shoulder arthroplasty, from May 10, 2005 to January 31, 2012, for shoulders stiffness, pain, or component loosening but had not had fever, chills, local swelling, erythema, drainage, tenderness, or abnormal results of laboratory studies. Specimens from each of these shoulders were cultured on aerobic (blood agar and chocolate agar), anaerobic (Brucella agar with blood, hemin, and vitamin K), and broth (brain-heart infusion) media as described above. For each shoulder we documented (1) the number of specimens taken for culture, (2) the source of each specimen, (3) which of the three media yielded a positive culture, (4) the time for each culture to become positive, and (5) the degree of positivity. We then determined the positivity rates of the different media and the different specimen sources, and the average time to positivity for each.
To obtain an indication of the sensitivity of the results with regard to the amount of Propionibacterium growth in the cultures performed for each shoulder, we repeated the analysis for the thirty-eight shoulders with Propionibacterium scores of >1.0.
This study was approved by our Human Subjects Review Committee (Approval #42232).
Source of Funding
Intramural support for this research was received from the University of Washington’s DePuy/Douglas T. Harryman II Endowed Chair for Shoulder Research.
A portion of R. Bumgarner’s effort on this project was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000423. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Results
Epidermal Cultures
In the first part of the study, sixteen of the eighteen epidermal cultures performed for male subjects were positive for Propionibacterium, whereas seven of the twelve cultures performed for female subjects were positive for this organism (Fisher exact test, p = 0.0837). The average degree of positivity was significantly greater for the males (1.62) than for the females (0.59) (unpaired t test, p = 0.0018) (Fig. 1).
Fig. 1.
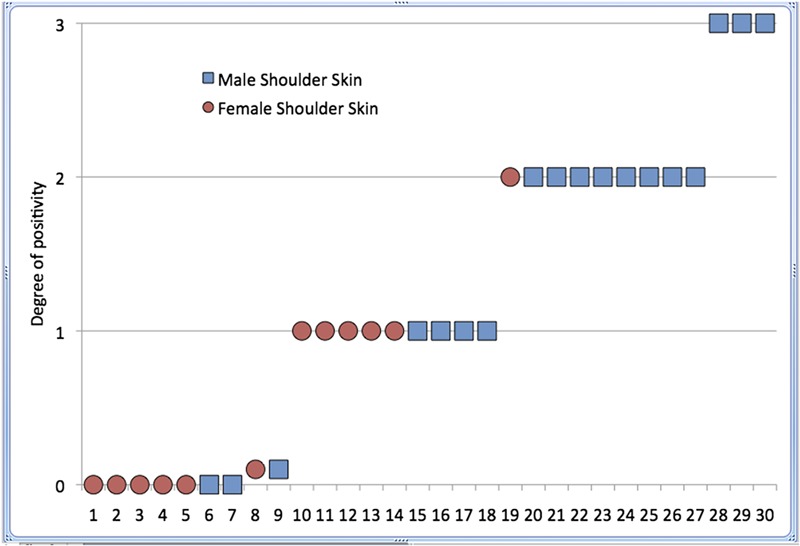
Results of culture of specimens from the surface of the unprepared epidermis of eighteen healthy male volunteers (square symbols) and twelve healthy female volunteers (round symbols). The degree of positivity (vertical axis) is a numerical value assigned to the quasi-quantitative result of the culture (no growth = 0, one colony = 0.1, broth only = 0.2, 1+ = 1, 2+ = 2, and 3+ = 3). The epidermis of the male subjects had a higher rate and degree of positivity than the female subjects.
Dermal and Deep Cultures
In the second part of the study, initial dermal, final dermal, and deep culture results were available for seventeen female patients; three additional female patients had final dermal and deep cultures but not initial dermal cultures (Fig. 2). Initial dermal, final dermal, and deep culture results were available for seventeen male patients. Initial and final dermal culture results but not deep culture results were available for one male patient; initial dermal and deep culture results only, for two; and final dermal and deep culture results only, for one. None of the initial or final dermal cultures for the twenty female subjects were positive for Propionibacterium, while five of the twenty initial dermal cultures and eleven of the nineteen final dermal cultures were positive for the male subjects (Fisher exact test, p = 0.001 and 0.0001, respectively) (Fig. 2). One of the twenty deep cultures for the females was culture-positive, while eleven of the twenty deep cultures for the males were positive (Fisher exact test, p = 0.0004). Thirty-five shoulders had both initial and final dermal cultures; both cultures were negative for twenty-four of these shoulders and both were positive for five (Fisher exact test, p = 0.001). Thirty-six shoulders had both deep and initial dermal cultures; both were negative for twenty-three and both were positive for five (Fisher exact test, p = 0.003). Thirty-eight shoulders had both deep and final dermal cultures; both were negative for twenty-five and both were positive for eleven (Fisher exact test, p < 0.0001). The degrees of positivity were correlated among the three different specimen sources: final dermal versus deep (Pearson r = 0.66), initial dermal versus final dermal (Pearson r = 0.62), and initial dermal versus deep (Pearson r = 0.53).
Fig. 2.
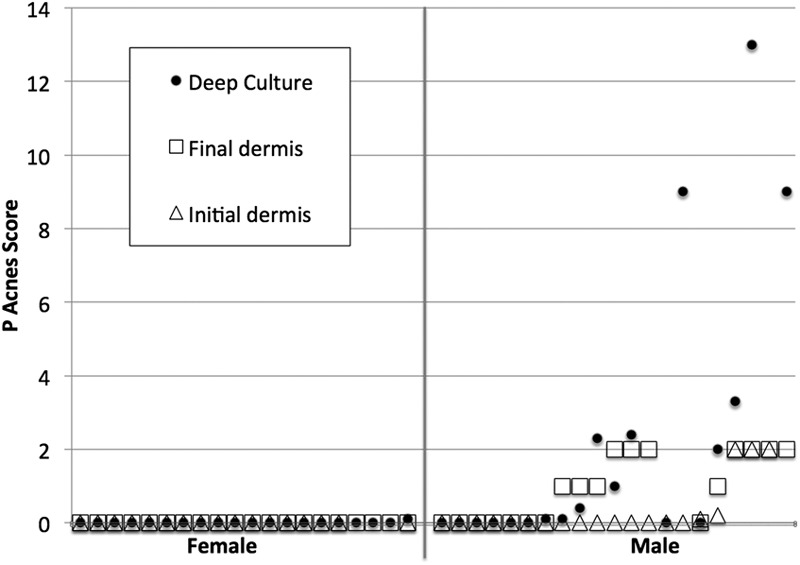
Culture results for twenty female patients (left of the vertical line) and twenty-one male patients (right of the vertical line) who underwent revision shoulder arthroplasty for issues other than clinically apparent infection (i.e., stiffness, pain, or component loosening). Triangles indicate the result of dermal cultures obtained immediately after the skin incision (Initial dermis). Squares indicate the result of dermal cultures obtained immediately prior to the administration of antibiotics (Final dermis). Solid dots indicate the results of the deep cultures. The vertical axis indicates the total degree of positivity for each of the specimens obtained with use of the system described for Figure 1. For the deep cultures, the Propionibacterium score indicates the sum of the degrees of positivity for all deep cultures of specimens obtained from the shoulder. In contrast to what was found for the male patients, the dermal and deep cultures for the female patients were consistently negative. Male patients with positive dermal cultures often had positive deep cultures as well.
Exploration of an Evidence-Based Approach to Culturing Propionibacterium
In the third part of the study, data were available for seventy-four shoulders that had a Propionibacterium score of >0.1. A total of 372 cultures (average five per shoulder) were obtained for these seventy-four shoulders, and 203 (55%) of them were positive (Table I). More than half of the cultures of component-explant and soft-tissue specimens were positive, whereas only 40% of the cultures of fluid were positive (Table I). Of the 203 positive cultures, 142 (70%) were positive in anaerobic (Brucella) media and 105 and 106 (52%) were positive in aerobic and broth media, respectively. If only the anaerobic media had been used, 30% (sixty-one) of the 203 positive cultures would have been missed. The average time for the cultures to turn positive was 8.0 ± 4.7 days (range, one to twenty-five days). Anaerobic media cultures had a shorter average time to positivity (7.2 ± 3.3 days) than aerobic (9.0 ± 5.1 days) or broth (12.9 ± 7.3 days) cultures.
TABLE I.
Times to Positivity and Fractions (Percents) of Cultures Positive for Propionibacterium for All Shoulders and for Three Different Culture Media for the Different Specimen Sources
| Average Time for First Culture to Become
Positive (days) |
|||||||||
| All Media | Aerobic | Broth | Anaerobic | Shoulders with Positive Cultures* | Cultures Positive for Propionibacterium † | Aerobic Positive‡ | Broth Positive‡ | Anaerobic Positive‡ | |
| Shoulders with Propionibacterium score >0.1 (n = 74) | |||||||||
| Head explant | 6.0 | 10.0 | 9.0 | 6.4 | 21/34 (62%) | 21/34 (62%) | 12/21 (57%) | 6/21 (29%) | 20/21 (95%) |
| Body explant | 8.3 | 7.8 | 11.0 | 6.4 | 8/13 (62%) | 8/13 (62%) | 4/8 (50%) | 2/8 (25%) | 5/8 (63%) |
| Glenoid explant | 5.7 | 7.0 | 8.7 | 6.0 | 6/10 (60%) | 6/10 (60%) | 4/6 (67%) | 2/6 (33%) | 5/6 (83%) |
| Glenoid membrane | 7.0 | 8.4 | 11.6 | 6.7 | 19/30 (63%) | 26/47 (55%) | 15/26 (58%) | 20/26 (77%) | 18/26 (69%) |
| Humeral membrane | 7.9 | 8.6 | 13.8 | 7.2 | 47/61 (77%) | 71/115 (62%) | 37/71 (52%) | 38/71 (54%) | 48/71 (68%) |
| Tissue | 9.9 | 10.4 | 13.0 | 8.3 | 30/39 (77%) | 40/75 (53%) | 14/40 (35%) | 26/40 (65%) | 22/40 (55%) |
| Fluid | 8.0 | 9.0 | 14.1 | 7.8 | 20/53 (38%) | 31/78 (40%) | 19/31 (61%) | 12/31 (30%) | 24/31 (77%) |
| All sources | 8.0 ± 4.7 | 9.0 ± 5.1 | 12.9 ± 7.3 | 7.2 ± 3.3 | 74/74 (100%) | 203/372 (55%) | 105/203 (52%) | 106/203 (52%) | 142/203 (70%) |
| Shoulders with Propionibacterium score >1.0 (n = 38) | |||||||||
| All sources | 6.9 ± 3.9 | 8.8 ± 5.4 | 12.4 ± 7.7 | 6.8 ± 3.0 | 38/38 (100%) | 150/203 (74%) | 89/150 (59%) | 76/150 (51%) | 113/150 (75%) |
No. of shoulders with positive cultures/total no. of shoulders (%).
No. of positive cultures/total no. of cultures (%).
No. of positive cultures in indicated culture medium/total no. of positive cultures (%).
When the analysis was repeated for the thirty-eight shoulders with Propionibacterium scores of >1.0, 203 cultures (average, five per shoulder) were obtained, and 150 (74%) of them were positive. Of 150 positive cultures, 113 (75%) were positive in anaerobic (Brucella) media and eighty-nine (59%) and seventy-six (51%) were positive in aerobic media and broth, respectively. If only the anaerobic media had been used, thirty-seven (25%) of the 150 positive cultures would have been missed. As expected, the higher Propionibacterium scores (>1.0) for this subset of shoulders had a somewhat shorter overall average time to positivity overall (6.9 ± 3.9 days), although the time to positivity for the individual media were essentially unchanged (anaerobic: 6.8 ± 3.0 days, aerobic: 8.8 ± 5.4 days, and broth: 12.4 ± 7.7 days).
Discussion
Our goal was to provide some evidence bearing on clinically relevant questions regarding the sources of Propionibacterium in surgical wounds and practical strategies for culturing this organism—i.e., what is the likely source of these organisms in surgical wounds and what are some practical guidelines for culturing specimens from surgical wounds so that the presence of this organism is not overlooked? The important features of this study were that (1) it described cultures not only in terms of whether or not they were positive, but also with regard to the degree of positivity; (2) we investigated cultures of the cut dermal surface at the time of revision surgery and correlated the results with those of deep cultures of specimens from the same shoulders; (3) we investigated the effect of the patient’s sex on the results of epidermal, dermal, and deep cultures; and (4) we investigated the yield of positive cultures in terms of the specimen source, different culture media, time of observation, and number of specimens cultured.
Our study revealed that Propionibacterium is common on the epidermal surface of unprepared skin of normal subjects and is found more frequently and in greater numbers in male subjects. This result is consistent with previous observations in the shoulder10,40,45,46 and in the spine41.
Propionibacterium usually did not grow on culture of dermal specimens from female subjects whereas dermal cultures were commonly positive for male patients. Deep cultures of specimens from the shoulders of females were rarely positive, but they were commonly positive for males. There was a strong positive relationship (Pearson r > 0.6) between the degree of positivity of the initial and final dermal cultures, that of the initial dermal cultures and the deep cultures, and that of the final dermal cultures and the deep cultures. These observations support the concept that the dermis (which cannot be sterilized by conventional means), rather than the surgically prepared epidermis, is a potential source of the Propionibacterium found in deep cultures. Because surgical skin preparation applied to the epidermis cannot reach the organisms harbored in the dermal sebaceous glands, the incised surface of the dermis may seed the surgical wound with these organisms throughout the surgical procedure and perhaps after the wound is closed.
Our study also provided evidence on strategies for culturing Propionibacterium. Cultures of component-explant and soft-tissue specimens, especially humeral membranes, had the highest yield of positive results. Fluid cultures had the lowest yield, possibly suggesting why aspiration is relatively ineffective for the identification of this organism. The next question that we sought to answer was how many specimens should be submitted for culture. Applying the probability of a geometric distribution47 the chances of detecting a positive culture are 1 − (1 − p)k, where p is the chance of each sample being positive and k is the number of samples obtained. If we use 53% (see Table I) as a conservative estimate of the chance of each tissue specimen being positive, four different specimens would need to be cultured to have a 95% chance of detecting the organism. Another question was which media should be used to culture these specimens? Even though the anaerobic medium had the highest yield of positive results, sixty-one (30%) of the positive cultures would have been missed if the aerobic media and broth had not been included; use of all three types of media (aerobic, anaerobic, and broth) contributes to the identification of Propionibacterium. Finally, we asked: how long should the cultures be maintained? To achieve 95% of the positive cultures, a laboratory needs to maintain the cultures for the average time to a positive culture (eight days) plus two standard deviations (4.7 days times two), or 17.4 days.
These results need to be viewed in light of certain limitations. First, they reflect only one practice and one microbiology laboratory with a specific culture protocol; thus, they may lack generalizability. Second, during the time over which the culture specimens were obtained, our practice of obtaining cultures evolved, so that not all shoulders had the same number and types of specimens analyzed. Third, while we sought to avoid the potential for cross contamination of the different specimens, it is possible that one deep specimen came into contact with another or that a deep specimen came into contact with the dermis. Hence, the positive relationship among the positivities of the initial dermal, final dermal, and deep specimen cultures suggests the edge of the incision as a potential source of Propionibacterium in deep infections. It is possible that some of the shoulders with low Propionibacterium scores (0.2 to 1.0) were culture-positive because of specimen contamination; however, the results were not substantially different when the analysis was repeated for shoulders with higher Propionibacterium scores (>1.0). Finally, we applied numerical values to the different culture results, including 0 for no growth, 0.1 for one colony only, 0.2 for broth only, and 1, 2, 3, and 4 for reports of 1+, 2+, 3+, and 4+. While these numerical values reflect the degree of positivity and the ordinal arrangement of the results, they incorrectly suggest that, for example, a 3+ result indicates three times the number of organisms indicated by a 1+ result. The results that we presented must be interpreted with this limitation in mind. We used this system in the absence of an established way to represent the degree of positivity of culture results. In spite of these limitations, the results of this investigation provide evidence for the conclusions listed below.
In summary, males are more likely to have positive Propionibacterium cultures of unprepared shoulder epidermis than females. Cultures of specimens from the cut surface of the incised skin (dermal cultures) obtained after skin preparation but before the administration of antibiotics in patients undergoing revision shoulder arthroplasty are commonly positive for Propionibacterium in males but not females. Positivity of dermal cultures for Propionibacterium is significantly associated with positivity of deep cultures for this organism. This observation suggests that strategies for minimizing the risk of Propionibacterium infections may need to be directed at managing contamination of surgical wounds from these bacteria residing in rather than on the skin.
If Propionibacterium is present in deep tissues, its recovery by culture is very likely if four different specimens are obtained and cultured for a minimum of seventeen days on three different media: aerobic, anaerobic, and broth. Taking fewer specimens, observing them for less time, and using fewer types of media may risk overlooking the presence of Propionibacterium.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Deep infection after rotator cuff repair. J Shoulder Elbow Surg. 2007 May-Jun;16(3):306-11. Epub 2007 Feb 22. [DOI] [PubMed] [Google Scholar]
- 2.Athwal GS, Sperling JW, Rispoli DM, Cofield RH. Acute deep infection after surgical fixation of proximal humeral fractures. J Shoulder Elbow Surg. 2007 Jul-Aug;16(4):408-12. Epub 2007 Apr 19. [DOI] [PubMed] [Google Scholar]
- 3.Cheung EV, Sperling JW, Cofield RH. Revision shoulder arthroplasty for glenoid component loosening. J Shoulder Elbow Surg. 2008 May-Jun;17(3):371-5. Epub 2008 Feb 20. [DOI] [PubMed] [Google Scholar]
- 4.Cheung EV, Sperling JW, Cofield RH. Infection associated with hematoma formation after shoulder arthroplasty. Clin Orthop Relat Res. 2008 Jun;466(6):1363-7. Epub 2008 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foruria AM, Fox TJ, Sperling JW, Cofield RH. Clinical meaning of unexpected positive cultures (UPC) in revision shoulder arthroplasty. J Shoulder Elbow Surg. 2013 May;22(5):620-7. Epub 2012 Sep 13. [DOI] [PubMed] [Google Scholar]
- 6.Franta AK, Lenters TR, Mounce D, Neradilek B, Matsen FA., 3rd The complex characteristics of 282 unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2007 Sep-Oct;16(5):555-62. Epub 2007 May 16. [DOI] [PubMed] [Google Scholar]
- 7.Hasan SS, Leith JM, Campbell B, Kapil R, Smith KL, Matsen FA., 3rd Characteristics of unsatisfactory shoulder arthroplasties. J Shoulder Elbow Surg. 2002 Sep-Oct;11(5):431-41. [DOI] [PubMed] [Google Scholar]
- 8.Herrera MF, Bauer G, Reynolds F, Wilk RM, Bigliani LU, Levine WN. Infection after mini-open rotator cuff repair. J Shoulder Elbow Surg. 2002 Nov-Dec;11(6):605-8. [DOI] [PubMed] [Google Scholar]
- 9.Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication. J Clin Microbiol. 2009 Jun;47(6):1878-84. Epub 2009 Mar 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saltzman MD, Marecek GS, Edwards SL, Kalainov DM. Infection after shoulder surgery. J Am Acad Orthop Surg. 2011 Apr;19(4):208-18. [DOI] [PubMed] [Google Scholar]
- 11.Singh JA, Sperling JW, Schleck C, Harmsen W, Cofield RH. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg. 2012 Oct;21(10):1304-9. Epub 2011 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh JA, Sperling JW, Schleck C, Harmsen WS, Cofield RH. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012 Nov;21(11):1534-41. Epub 2012 Apr 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topolski MS, Chin PY, Sperling JW, Cofield RH. Revision shoulder arthroplasty with positive intraoperative cultures: the value of preoperative studies and intraoperative histology. J Shoulder Elbow Surg. 2006 Jul-Aug;15(4):402-6. [DOI] [PubMed] [Google Scholar]
- 14.Pottinger P, Butler-Wu S, Neradilek MB, Merritt A, Bertelsen A, Jette JL, Warme WJ, Matsen FA., 3rd Prognostic factors for bacterial cultures positive for Propionibacterium acnes and other organisms in a large series of revision shoulder arthroplasties performed for stiffness, pain, or loosening. J Bone Joint Surg Am. 2012 Nov 21;94(22):2075-83. [DOI] [PubMed] [Google Scholar]
- 15.Kamme C, Lindberg L. Aerobic and anaerobic bacteria in deep infections after total hip arthroplasty: differential diagnosis between infectious and non-infectious loosening. Clin Orthop Relat Res. 1981 Jan-Feb;(154):201-7. [PubMed] [Google Scholar]
- 16.Launder WJ, Hungerford DS. Late infection of total hip arthroplasty with Propionibacterium acnes: a case and review of the literature. Clin Orthop Relat Res. 1981 Jun;(157):170-7. [PubMed] [Google Scholar]
- 17.McDowell A, Valanne S, Ramage G, Tunney MM, Glenn JV, McLorinan GC, Bhatia A, Maisonneuve JF, Lodes M, Persing DH, Patrick S. Propionibacterium acnes types I and II represent phylogenetically distinct groups. J Clin Microbiol. 2005 Jan;43(1):326-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mutnal A, Patel P, Cardona L, Suarez J. Periprosthetic Propionibacterium granulosum joint infection after direct anterior total hip arthroplasty: a case report. JBJS Case Connector, 2011 Nov 23; 1(2):e10 1-4. [DOI] [PubMed] [Google Scholar]
- 19.Namdari S, Voleti PB, Baldwin KD, Lee GC. Primary total joint arthroplasty performed in operating rooms following cases of known infection. Orthopedics. 2011 Sep;34(9):e541-5. Epub 2011 Sep 09. [DOI] [PubMed] [Google Scholar]
- 20.Stoll T, Stucki G, Brühlmann P, Vogt M, Gschwend N, Michel BA. Infection of a total knee joint prosthesis by peptostreptococcus micros and propionibacterium acnes in an elderly RA patient: implant salvage with longterm antibiotics and needle aspiration/irrigation. Clin Rheumatol. 1996 Jul;15(4):399-402. [DOI] [PubMed] [Google Scholar]
- 21.Tunney MM, Dunne N, Einarsson G, McDowell A, Kerr A, Patrick S. Biofilm formation by bacteria isolated from retrieved failed prosthetic hip implants in an in vitro model of hip arthroplasty antibiotic prophylaxis. J Orthop Res. 2007 Jan;25(1):2-10. [DOI] [PubMed] [Google Scholar]
- 22.Tunney MM, Patrick S, Curran MD, Ramage G, Hanna D, Nixon JR, Gorman SP, Davis RI, Anderson N. Detection of prosthetic hip infection at revision arthroplasty by immunofluorescence microscopy and PCR amplification of the bacterial 16S rRNA gene. J Clin Microbiol. 1999 Oct;37(10):3281-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aleissa S, Parsons D, Grant J, Harder J, Howard J. Deep wound infection following pediatric scoliosis surgery: incidence and analysis of risk factors. Can J Surg. 2011 Aug;54(4):263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beguiristain J, del Río J, Duart J, Barroso J, Silva A, Villas C. Corrosion and late infection causing delayed paraparesis after spinal instrumentation. J Pediatr Orthop B. 2006 Sep;15(5):320-3. [DOI] [PubMed] [Google Scholar]
- 25.Bémer P, Corvec S, Tariel S, Asseray N, Boutoille D, Langlois C, Tequi B, Drugeon H, Passuti N, Touchais S. Significance of Propionibacterium acnes-positive samples in spinal instrumentation. Spine (Phila Pa 1976). 2008 Dec 15;33(26):E971-6. [DOI] [PubMed] [Google Scholar]
- 26.Carricajo A, Nuti C, Aubert E, Hatem O, Fonsale N, Mallaval FO, Vautrin AC, Brunon J, Aubert G. Propionibacterium acnes contamination in lumbar disc surgery. J Hosp Infect. 2007 Jul;66(3):275-7. Epub 2007 Jun 18. [DOI] [PubMed] [Google Scholar]
- 27.Di Silvestre M, Bakaloudis G, Lolli F, Giacomini S. Late-developing infection following posterior fusion for adolescent idiopathic scoliosis. Eur Spine J. 2011 May;20(Suppl 1):S121-7. Epub 2011 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn F, Zbinden R, Min K. Late implant infections caused by Propionibacterium acnes in scoliosis surgery. Eur Spine J. 2005 Oct;14(8):783-8. Epub 2005 Apr 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haidar R, Najjar M, Der Boghossian A, Tabbarah Z. Propionibacterium acnes causing delayed postoperative spine infection: review. Scand J Infect Dis. 2010 Jul;42(6-7):405-11. [DOI] [PubMed] [Google Scholar]
- 30.Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Osmon DR. Propionibacterium acnes vertebral osteomyelitis: seek and ye shall find? Clin Orthop Relat Res. 2007 Aug;461:25-30. [DOI] [PubMed] [Google Scholar]
- 31.McLorinan GC, Glenn JV, McMullan MG, Patrick S. Propionibacterium acnes wound contamination at the time of spinal surgery. Clin Orthop Relat Res. 2005 Aug;(437):67-73. [DOI] [PubMed] [Google Scholar]
- 32.Richards BR, Emara KM. Delayed infections after posterior TSRH spinal instrumentation for idiopathic scoliosis: revisited. Spine (Phila Pa 1976). 2001 Sep 15;26(18):1990-6. [DOI] [PubMed] [Google Scholar]
- 33.Rihn JA, Lee JY, Ward WT. Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine (Phila Pa 1976). 2008 Feb 1;33(3):289-94. [DOI] [PubMed] [Google Scholar]
- 34.Sampedro MF, Huddleston PM, Piper KE, Karau MJ, Dekutoski MB, Yaszemski MJ, Currier BL, Mandrekar JN, Osmon DR, McDowell A, Patrick S, Steckelberg JM, Patel R. A biofilm approach to detect bacteria on removed spinal implants. Spine (Phila Pa 1976). 2010 May 20;35(12):1218-24. [DOI] [PubMed] [Google Scholar]
- 35.Tribus CB, Garvey KE. Full-thickness thoracic laminar erosion after posterior spinal fusion associated with late-presenting infection. Spine (Phila Pa 1976). 2003 May 15;28(10):E194-7. [DOI] [PubMed] [Google Scholar]
- 36.Uçkay I, Dinh A, Vauthey L, Asseray N, Passuti N, Rottman M, Biziragusenyuka J, Riché A, Rohner P, Wendling D, Mammou S, Stern R, Hoffmeyer P, Bernard L. Spondylodiscitis due to Propionibacterium acnes: report of twenty-nine cases and a review of the literature. Clin Microbiol Infect. 2010 Apr;16(4):353-8. Epub 2009 Jun 06. [DOI] [PubMed] [Google Scholar]
- 37.Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine (Phila Pa 1976). 1997 Oct 15;22(20):2444-50; discussion 2450-1. [DOI] [PubMed] [Google Scholar]
- 38.Butler-Wu SM, Sengupta DJ, Kittichotirat W, Matsen FA, 3rd, Bumgarner RE. Genome sequence of a novel species, Propionibacterium humerusii. J Bacteriol. 2011 Jul;193(14):3678 Epub 2011 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murray MR, Saltzman MD, Gryzlo SM, Terry MA, Woodward CC, Nuber GW. Efficacy of preoperative home use of 2% chlorhexidine gluconate cloth before shoulder surgery. J Shoulder Elbow Surg. 2011 Sep;20(6):928-33. Epub 2011 May 25. [DOI] [PubMed] [Google Scholar]
- 40.Saltzman MD, Nuber GW, Gryzlo SM, Marecek GS, Koh JL. Efficacy of surgical preparation solutions in shoulder surgery. J Bone Joint Surg Am. 2009 Aug;91(8):1949-53. [DOI] [PubMed] [Google Scholar]
- 41.Savage JW, Weatherford BM, Sugrue PA, Nolden MT, Liu JC, Song JK, Haak MH. Efficacy of surgical preparation solutions in lumbar spine surgery. J Bone Joint Surg Am. 2012 Mar 21;94(6):490-4. [DOI] [PubMed] [Google Scholar]
- 42.Shiono Y, Watanabe K, Hosogane N, Tsuji T, Ishii K, Nakamura M, Toyama Y, Chiba K, Matsumoto M. Sterility of posterior elements of the spine in posterior correction surgery. Spine (Phila Pa 1976). 2012 Mar 15;37(6):523-6. [DOI] [PubMed] [Google Scholar]
- 43.Leeming JP, Holland KT, Cunliffe WJ. The microbial ecology of pilosebaceous units isolated from human skin. J Gen Microbiol. 1984 Apr;130(4):803-7. [DOI] [PubMed] [Google Scholar]
- 44.Butler-Wu SM, Burns EM, Pottinger PS, Magaret AS, Rakeman JL, Matsen FA, 3rd, Cookson BT. Optimization of periprosthetic culture for diagnosis of Propionibacterium acnes prosthetic joint infection. J Clin Microbiol. 2011 Jul;49(7):2490-5. Epub 2011 May 04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel A, Calfee RP, Plante M, Fischer SA, Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. 2009 Nov-Dec;18(6):897-902. Epub 2009 Apr 11. [DOI] [PubMed] [Google Scholar]
- 46.Perry A, Lambert PA. Propionibacterium acnes: infection beyond the skin. Expert Rev Anti Infect Ther. 2011 Dec;9(12):1149-56. [DOI] [PubMed] [Google Scholar]
- 47.Wikipedia. Geometric distribution. http://en.wikipedia.org/wiki/Geometric_distribution. Accessed 2012 Nov 14.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest


