Abstract
Objective
The objective of this study was to evaluate peri-operative and survival outcomes of ovarian cancer patients undergoing percutaneous upper gastrointestinal decompression for malignant bowel obstruction (MBO).
Methods
Retrospective chart review was used to identify patients with ovarian, peritoneal, or fallopian tube cancer who underwent palliative decompressive treatment for MBO from 1/2002–12/2010. Kaplan-Meier methods were used to estimate the median survival (MS) and multivariate analysis used to determine if any variables were associated with the hazard of death.
Results
Fifty-three patients met inclusion criteria. Median length of diagnosis prior to intervention was 21 months. Fifteen (28.3%) patients experienced complications and 9 required revision. Forty-nine (92.5%) experienced relief of symptoms after placement, and 91% tolerated some form of oral intake. Following placement, 19 (36%) patients received additional chemotherapy and 21(41%) patients received total parental nutrition (TPN). Thirty-five patients were discharged home/outpatient facility, 16 to hospice care, and 2 died prior to discharge. MS for all patients was 46 days. Patients who received chemotherapy had a MS of 169 days compared to 33 days (p<0.001). We failed to find an association between survival and TPN or performance status.
Conclusions
Malignant bowel obstruction is a common complication of ovarian cancer. Management is palliative; risks and benefits of any therapy must be considered. Percutaneous decompressive therapy provides relief from associated symptoms, and allows patients to be discharged home. Median survival in this group is limited, and decisions regarding aggressive therapy should be individualized.
Introduction
Over 22,000 estimated new cases of ovarian cancer will be diagnosed in the United States this year [1]. The majority of women are diagnosed at an advanced stage, and over 75% will develop a recurrence and eventually succumb to their disease [2]. One of the commonly experienced complications of recurrence is malignant bowel obstruction (MBO), which is reported to affect 25–50% of these patients [3, 4]. When diagnosed, management usually begins with conservative measures such as bowel rest, intravenous fluids, pharmacologic agents, and nasogastric tube placement [2]. However, if symptoms persist despite this therapy, additional measures, such as surgical intervention, must be considered. Many patient and disease-related factors contribute to this complicated decision making process. Most patients in this situation are not candidates or do not desire major surgery and therefore placement of a percutaneous upper gastrointestinal decompressive tube (PDT) may be considered.
Though reports of PDT placement demonstrate that it is a safe and feasible option to palliate the symptoms of malignant bowel obstruction details regarding length and type of therapy after PDT placement are not available [5–9]. Furthermore, outcomes such as discharge location, readmission rates, and code status discussion are limited. Therefore, the objective of this study was to evaluate the peri-operative and survival outcomes relating to palliative percutaneous decompression in our patient population.
Methods
Approval to conduct this study was obtained from the Institutional Review Board at The Ohio State University Wexner Medical Center. All patients who underwent placement of a PDT for a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer at our institution from 1/2002 to 12/2010 were retrospectively identified from hospital databases using ICD-9 codes for ovarian, fallopian tube, or peritoneal carcinoma and CPT code for percutaneous gastrostomy tube placement. Patients were excluded from the study for the following: non-epithelial carcinoma, incomplete medical records, or placement for indication other than MBO. Primary outcome was median survival after placement of PDT tube.
Individual subject data were collected retrospectively from inpatient and outpatient medical records. This included patient demographics, patient age at diagnosis, clinical performance status, date of initial surgery, surgical procedures performed, International Federation of Gynecology and Obstetrics (FIGO) stage, tumor histology and grade, adjuvant therapy(s) received, and number of prior admissions for bowel obstruction. Surgical reports at the time of PDT placement were reviewed in all cases. The following peri-operative information was abstracted: patient age at time of procedure, code status (before and after procedure), length of stay, complications related to the procedure, symptom relief (defined as resolution of nausea and vomiting), post PDT placement cancer treatments, use of total parenteral nutrition (TPN), diet tolerance, hospital readmissions, hospice referral, and date of last follow-up/death.
Kaplan-Meier methods were used to estimate the median survival in recurrent ovarian cancer patients that had a PDT placed. Log-rank methods were used to test for survival differences across categorical variables while a Cox proportional hazard regression was used to determine if any of the patient demographics or clinical characteristics was associated with the hazard of death. The patient population was characterized by means, standard deviations, and medians for continuous variables and by frequencies and percent for categorical variables. All analyses were run using Stata 11.1, Stata Corporation, College Station, TX.
Results
A total of 73 patients met study inclusion criteria. Twenty patients were excluded for the following reasons: PDT placed for alternate (non-MBO) indications (11), incomplete medical records (5), and primary disease site not consistent with ovarian, peritoneal, or fallopian tube cancer (4); leaving 53 evaluable patients. Patient characteristics are shown in Table 1. The median age for the entire study group was 60 years (range 38–78 years). The mean follow up time for study patients was 3.3 months (range <1–31 months). A majority of patients had a Eastern Cooperative Oncology Group performance status of 0–3, and the median time from initial diagnosis to PDT placement was 26 months (<1–96 months). Prior to PDT placement, 87% of patients received multiple chemotherapy regimens (median of 3 regimens) and the median time since last cycle of chemotherapy prior to PDT placement was 1.4 months. Preoperative albumin level was available for 45 patients with a mean value of 2.6 g/dL. Only 17.8% (8/45) were noted to have an albumin level within the institutional normal range. Over half (55%) had other admissions for MBO in the 6 months prior to the admission for PDT placement. In this time frame, only four patients had undergone surgery for MBO. Of these four, one was successfully palliated for 5 months prior to PDT placement and the other three had failed operations and required PDT during that hospital stay. The median length of stay prior to placement of PDT was 6 days (range 1–27).
Table 1.
Baseline patient characteristics.
| N=53 | Chemotherapy after PGT N=19 | No Chemotherapy after PGT N=34 | P-value1 | |
|---|---|---|---|---|
| Mean age in years, (range) | 60 (37–89) | 57 (37–74) | 61 (39–89) | 0.24 |
| Stage | ||||
| I | 4 (7.5%) | 2 (10.5%) | 2 (5.9%) | 0.59 |
| II | 2 (3.7%) | 0 (0%) | 2 (5.9%) | |
| III | 35 (66%) | 14 (73.7%) | 21 (61.8%) | |
| IV | 10 (18.9%) | 2 (10.5%) | 8 (23.5%) | |
| Unknown | 2 (3.8%) | 1 (5.3%) | 1 (2.9%) | |
| Grade | ||||
| 1 | 3 (5.7%) | 1 (5.3%) | 2 (5.9%) | > 0.99 |
| 2 | 2 (3.8%) | 1 (5.3%) | 1 (2.9%) | |
| 3 | 46 (86.8%) | 16 (84.2%) | 30 (88.2%) | |
| Unknown | 2 (3.8%) | 1 (5.3%) | 1 (2.9%) | |
| Median time since initial diagnosis in months, (range) | 26 (0–96) | 27 (6–80) | 23.5 (0–96) | 0.0.93 |
| Median number of chemotherapy regimens prior to PGT placement, (range) | 3 (0–8) | 3 (2–8) | 3 (0–8) | 0.54 |
| Median days from last chemotherapy treatment at time of PGT placement, (range) | 22 (4–936) | 18 (9–500) | 23 (0–936) | 0.44 |
| Mean albumin, (range) | 2.6 (1.5–3.7) | 2.8 (1.6–3.7) | 2.5 (1.5–3.7) | 0.091 |
| Median number of admissions for MBO in last 6 months, (range) | 1 (0–4) | 1 (0–3) | 0 (0–4) | 0.14 |
p-value based on two-sided t-test for means, Wilcoxon rank-sum for medians, and Fisher’s exact test for categorical variables
All patients were able to have a tube placed for decompression of the upper GI tract. Prior to intervention, 38 (74.5%) patients had ascites and/or 41 (80.3%) had carcinomatosis on imaging. Though drainage of ascites prior to PDT placement was not routinely required, 3 patients (5.7%) underwent a preoperative paracentesis. Thirty-three (62%) patients had their procedure completed by a general surgeon, 13 (25%) by an interventional radiologist, and 6 (11%) by a gastroenterologist. Eight (15%) procedures were completed under general anesthesia and the remainder were completed under conscious sedation. Four patients failed an initial attempt at endoscopic placement; failure to trans-illuminate was cited as the reason for failure in each of these cases, and was attributed to the presence of both ascites and carcinomatosis on imaging. Among these patients two underwent successful endoscopic placement under general anesthesia, one required an open gastrostomy tube, and one underwent successful placement of a jejunostomy tube by interventional radiology. Two patients underwent primary jejunostomy placement, without attempt at gastrostomy, by interventional radiology.
Forty-nine patients (92.5%) experienced control of symptoms (nausea and vomiting) post-PDT, defined as resolution of symptoms prior to discharge; however, 46 patients required supplemental anti-emetic medication. Following PDT placement, 48 (91%) patients were able to tolerate some form of oral intake: regular diet (8), soft diet (6) and liquid diet (34). Two patients tolerated tube feeds only and 2 patients were unable to tolerate any form of dietary intake. Additionally, twenty-one patients (40%) received total parental nutrition (TPN) due to inadequate oral intake, which was administered for a median of 30 days (range 6–180).
Median length of stay after PDT placement was 3 days (range 1–41). Following hospital admission, 71.7% were discharged home; 9.4% to an inpatient hospice facility, 15.1% to a nursing facility, and there were two inpatient deaths. Sixteen patients (30.2%) opted for hospice services. Code status at the time of PDT placement was also assessed from hospital charts. Prior to PDT placement, code status was listed as full, do not resuscitate (DNR), and unknown in 35, 0, and 18 patients, respectively. Following PDT placement, 22 patients were full code status, 19 were DNR, and 12 cases were unknown.
There was no associated mortality directly related to PDT placement, however, post-operative complications occurred in 15 (28.3%) patients. The most common complication, which occurred in nine (17%) patients, was obstruction of the PDT. Of these patients four (7.5%) were found to have mechanical obstruction, which were successfully controlled with re-education regarding liquid or pureed low residue diet. Four experienced leakage of enteric contents around their PDT, and five patients developed PDT site infections. Nine (17%) patients required replacement of their PDT and two patients developed fistulas. Following PDT placement, 25 patients required a median of 1 readmission (range 1–13). Accounting for 60% of readmissions, PDT complication was the most common indication, followed by dehydration, sepsis, and failure to thrive. After discharge to hospice, patients did not have any reported complications or readmissions related to PDT placement.
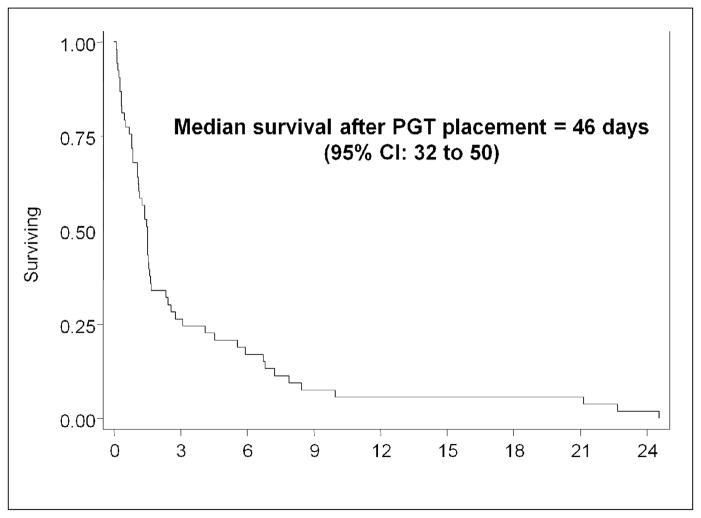
Following PDT placement, median survival for all patients was 46 days (range 2–736 days). (Figure 1) Nineteen (36%) patients received chemotherapy following PDT placement. The median number of cycles was 3 (range 0–13) and only 5 patients received 5 or more cycles of chemotherapy (Table 2). Patients who received chemotherapy had a significantly longer median overall survival compared to those who did not, 169 days versus to 33 days (p < 0.001), respectively. Those who received TPN, with or without chemotherapy, had similar survival to those who did not receive TPN. However, there was a significant survival difference in patients who were able to tolerate a regular diet, 220 days versus 35 days in those who could not (p = 0.007). We failed to find an association between performance status and survival (Table 3). Among the 45 patients with evaluable pre-operative levels, lower albumin was associated with poorer survival (HR=0.34; 95% CI: 0.19, 0.6, p < 0.0001). Older age was also associated with shorter survival (HR=1.04; 95% CI: 1.01, 1.07, p=0.018). There was no association between disease extent (ascites, carcinomatosis, hepatic or gastric involvement) and overall survival.
Figure 1.
Median survival after PDT placement.
Table 2.
Clinical outcomes following PDT placement.
| N (%) | |
|---|---|
| Successful symptom management post PDT | 49 (92.5%) |
| Complication post PDT | 15 (28.3%) |
| Leak | 4 (7.5%) |
| Infection | 5 (9.4%) |
| Blocked | 9 (17.0%) |
| Fistula | 2 (3.7%) |
| PDT revision | 9 (16.9%) |
| TPN after PDT Placement | 21 (39.6%) |
| Length of TPN, days | |
| 0 | 34 (64.1%) |
| 4 – 27 | 7 (13.2%) |
| 30 – 60 | 9 (16.9%) |
| >120 | 3 (5.7%) |
| Chemotherapy after PDT Placement | 19 (35.9%) |
| Number of chemotherapy cycles after PDT | |
| 0 | 34 (64.2%) |
| 1 | 7 (13.2%) |
| 2 – 3 | 3 (5.7%) |
| 4 – 6 | 6 (11.3%) |
| >8 | 3 (5.7%) |
| Diet Tolerated | |
| None | 2 (3.4%) |
| Liquid | 34 (64.2%) |
| Soft | 6 (11.3%) |
| Regular | 8 (15.0%) |
| Tube Feeding | 2 (3.4%) |
| Discharge location | |
| Home | 27 (50.9%) |
| Nursing Facility | 8 (15.1%) |
| Inpatient hospice | 5 (9.4%) |
| Home hospice | 11 (20.8%) |
| Death in hospital | 2 (3.8%) |
Table 3.
Multivariate survival analysis for discrete variables after PDT placement.
| Median survival, days (95% Confidence Interval) | P value | |
|---|---|---|
| Chemotherapy | ||
| No | 33 (16, 45) | < 0.001 |
| Yes | 169 (74, 240) | |
| TPN | ||
| No | 35 (16, 46) | 0.076 |
| Yes | 49 (32, 169) | |
| TPN, no chemotherapy | ||
| No | 33 (10, 46) | 0.933 |
| Yes | 38 (7, 49) | |
| Chemotherapy, no TPN | ||
| No | 33 (10, 46) | < 0.001 |
| Yes | 138 (32, 303) | |
| Diet | ||
| Soft/liquid | 35 (26, 46) | 0.007 |
| Regular | 220 (42, 644) | |
Discussion
Malignant bowel obstruction is common among patients with recurrent ovarian cancer and the goal of intervention often shifts to one of symptom management. Although surgical correction may be possible, it should be restricted to highly selected patients due to high morbidity and mortality, as well as limited survival outcomes [10, 11]. In patients who are not candidates due to obstruction that is multifocal and/or not amenable to surgical correction, performance status, or personal preferences, PDT is a viable option. Therefore, we sought to evaluate the use of PDT and its outcomes.
In the present study, PDT resulted in control of symptoms (nausea and vomiting) in 92.5% of the patients with MBO secondary to recurrent ovarian cancer. These findings are comparable to other reports that report relief of these symptoms with PDT in 82–91% of patients [5, 6, 8]. Although palliative in most cases, 91% of patients in our series were able to tolerate oral intake, with over a quarter tolerating a soft or regular diet. Pothuri and colleagues found similar success with 96% of patients tolerating an oral diet and 42% a soft/regular diet [8]. However, success rates in other series, though often a variety of disease sites, report the ability to tolerate an oral diet ranging from 27–42% [5, 12]. These findings suggest that PDT can be used to palliate the most significant symptoms of MBO, and afford the possibility of resuming a limited oral intake.
PDT is quite effective for short-term symptom relief related to MBO, however there is little information regarding long-term outcomes after PDT [5, 6, 8]. Reported complication rates vary depending on indication, however, in general, major complications, which require readmission or surgical revision, were reported in 2–23% and minor complications in 8–65% [13–15]. Our complication rates fall within these ranges and, interestingly, half may have been prevented with proper diet and maintenance by patients/caregivers. For example, diet after PDT placement should be limited to liquids/foods that can pass through the tube without resulting in mechanical obstruction. In addition, patients should be instructed to avoid traction to prevent dislodgement and perform routine flushing to maintain patency of the tube. The readmission rate in our series was 47%, which was comparable to the limited data in the literature [12]. Furthermore, our results demonstrated that following PDT placement, a majority of patients were discharged home and/or to hospice care, consistent with the palliative intent of the procedure. [7].
Historically ascites had been considered a relative contraindication to placement of a PDT [16]. However, more recently PDT placement has been reported to be possible in patients with ascites, carcinomatosis, and tumor encasing the stomach [6, 8, 13, 17]. We found similar results. All patients were ultimately able to undergo decompressive therapy, with only one requiring placement of an open gastrostomy tube. Interesting, three patients were able to have a jejunostomy tube placed for decompression when gastrostomy tube was not possible, providing an additional palliative option to patients and physicians.
In similar populations with MBO managed with conservative or decompressive measures only, median survival of ranges from 28–84 days, which is comparable to our results of 46 days [6, 8, 9, 18, 19]. Consistent with other reports, overall survival was significantly longer in patients who received chemotherapy following PDT placement compared to those who did not [8, 12]. Similar to Pothuri and colleagues our results showed that the use of TPN may not independently impact survival outcomes [8]. Though Brard et al found that survival was improved with the addition of TPN, this factor did not impart a statically significant survival benefit when accounting for chemotherapy [19]. In contrast, a series of 21 patients demonstrated that the combination of TPN and chemotherapy provided a statistically, but questionable clinically significant, survival benefit of 18 days over chemotherapy alone[20]. Therefore, utilization of chemotherapy may result in improved survival after PDT placement in carefully selected patients, but since TPN has not been found to provide significant benefit we do not recommend its use this population.
In reviewing this retrospective study, there are limitations that should be addressed. First, though effort was made to include all eligible patients, our sample size is small and is restricted to a single institution. Additionally, missing data and failed PDT placement may have resulted in the exclusion of patients, all of which make it difficult to draw definitive conclusions. Secondly, the data obtained regarding complications and readmission rates was limited to our institutions and care received at other institutions may have resulted in under-reporting of outcomes. Lastly, we were unable to address quality of life outcomes after PDT placement. Despite these limitations, we were able to provide comprehensive overview on outcomes including survival, complications, and discharge disposition. However, our findings showed that almost a quarter of patients did not have code status documented in their medical record, thus, areas for continued improvement exists.
In conclusion, for women with MBO related to ovarian cancer, PDT placement provided relief from associated symptoms and allowed for discharge to home care in most cases. Patients and caregivers should be educated on the prevention and signs of common complications as well as routine PDT maintenance. Lastly, the need for PDT placement may also provide an opportunity to discuss goals of care, including code status and prognosis. Given the limited median survival in this group, any decision regarding aggressive therapy after MBO requiring PDT placement should be individualized.
Footnotes
No author has a conflict of interest with the content of this manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Barakat RR, Markman M, Randall M. Principles and practice of gynecologic oncology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 3.Tunca JC, Buchler DA, Mack EA, Ruzicka FF, Crowley JJ, Carr WF. The management of ovarian-cancer caused bowel obstruction. Gynecol Oncol. 1981;12(2):186–92. doi: 10.1016/0090-8258(81)90148-7. [DOI] [PubMed] [Google Scholar]
- 4.Dvoretsky PM, Richards KA, Angel C, Rabinowitz L, Beecham JB, Bonfiglio TA. Survival time, causes of death, and tumor/treatment-related morbidity in 100 women with ovarian cancer. Hum Pathol. 1988;19(11):1273–9. doi: 10.1016/s0046-8177(88)80281-8. [DOI] [PubMed] [Google Scholar]
- 5.Dalal KM, Gollub MJ, Miner TJ, Wong WD, Gerdes H, Schattner MA, et al. Management of Patients with Malignant Bowel Obstruction and Stage IV Colorectal Cancer. J Palliat Med. 2011;14(7):822–8. doi: 10.1089/jpm.2010.0506. [DOI] [PubMed] [Google Scholar]
- 6.Campagnutta E, Cannizzaro R, Gallo A, Zarrelli A, Valentini M, De Cicco M, et al. Palliative treatment of upper intestinal obstruction by gynecological malignancy: the usefulness of percutaneous endoscopic gastrostomy. Gynecol Oncol. 1996;62(1):103–5. doi: 10.1006/gyno.1996.0197. [DOI] [PubMed] [Google Scholar]
- 7.Meyer L, Pothuri B. Decompressive percutaneous gastrostomy tube use in gynecologic malignancies. Curr Treat Options Oncol. 2006;7(2):111–20. doi: 10.1007/s11864-006-0046-1. [DOI] [PubMed] [Google Scholar]
- 8.Pothuri B, Montemarano M, Gerardi M, Shike M, Ben-Porat L, Sabbatini P, et al. Percutaneous endoscopic gastrostomy tube placement in patients with malignant bowel obstruction due to ovarian carcinoma. Gynecol Oncol. 2005;96(2):330–4. doi: 10.1016/j.ygyno.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham MJ, Bromberg C, Kredentser DC, Collins MB, Malfetano JH. Percutaneous gastrostomy for decompression in patients with advanced gynecologic malignancies. Gynecol Oncol. 1995;59(2):273–6. doi: 10.1006/gyno.1995.0021. [DOI] [PubMed] [Google Scholar]
- 10.Kolomainen DF, Daponte A, Barton DP, Pennert K, Ind TE, Bridges JE, et al. Outcomes of surgical management of bowel obstruction in relapsed epithelial ovarian cancer (EOC) Gynecol Oncol. 2012;125(1):31–6. doi: 10.1016/j.ygyno.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Pothuri B, Vaidya A, Aghajanian C, Venkatraman E, Barakat RR, Chi DS. Palliative surgery for bowel obstruction in recurrent ovarian cancer:an updated series. Gynecol Oncol. 2003;89(2):306–13. doi: 10.1016/s0090-8258(03)00073-8. [DOI] [PubMed] [Google Scholar]
- 12.Keung EZ, Liu X, Nuzhad A, Rabinowits G, Patel V. In-hospital and long-term outcomes after percutaneous endoscopic gastrostomy in patients with malignancy. J Am Coll Surg. 2012;215(6):777–86. doi: 10.1016/j.jamcollsurg.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Shaw C, Bassett RL, Fox PS, Schmeler KM, Overman MJ, Wallace MJ, et al. Palliative venting gastrostomy in patients with malignant bowel obstruction and ascites. Ann Surg Oncol. 2012 doi: 10.1245/s10434-012-2643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, Manganiello WD, et al. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: a comparison of indications, complications and outcomes in 370 patients. Eur J Radiol. 2005;56(1):84–90. doi: 10.1016/j.ejrad.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Mori M, Bruera E, Dev R. Complications of a gastrostomy tube used for decompression of an inoperable bowel obstruction in a patient with advanced cancer. J Pain Symptom Manage. 2009;38(3):466–72. doi: 10.1016/j.jpainsymman.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Wills JS, Oglesby JT. Percutaneous gastrostomy. Radiology. 1983;149(2):449–53. doi: 10.1148/radiology.149.2.6414043. [DOI] [PubMed] [Google Scholar]
- 17.Lee MJ, Saini S, Brink JA, Morrison MC, Hahn PF, Mueller PR. Malignant small bowel obstruction and ascites: not a contraindication to percutaneous gastrostomy. Clin Radiol. 1991;44(5):332–4. doi: 10.1016/s0009-9260(05)81270-x. [DOI] [PubMed] [Google Scholar]
- 18.Chi DS, Phaeton R, Miner TJ, Kardos SV, Diaz JP, Leitao MM, Jr, et al. A prospective outcomes analysis of palliative procedures performed for malignant intestinal obstruction due to recurrent ovarian cancer. Oncologist. 2009;14(8):835–9. doi: 10.1634/theoncologist.2009-0057. [DOI] [PubMed] [Google Scholar]
- 19.Brard L, Weitzen S, Strubel-Lagan SL, Swamy N, Gordinier ME, Moore RG, et al. The effect of total parenteral nutrition on the survival of terminally ill ovarian cancer patients. Gynecol Oncol. 2006;103(1):176–80. doi: 10.1016/j.ygyno.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Rustum NR, Barakat RR, Venkatraman E, Spriggs D. Chemotherapy and total parenteral nutrition for advanced ovarian cancer with bowel obstruction. Gynecol Oncol. 1997;64(3):493–5. doi: 10.1006/gyno.1996.4605. [DOI] [PubMed] [Google Scholar]