Abstract
Background
Acne is the most common skin disease in adolescence, with a prevalence of nearly 100%. About 60% of affected adolescents have mild acne for which they use non-prescription preparations without consulting a physician. The remaining 40% constitute the population of acne patients seen in medical practice. The course of acne can be either acute or chronic; its manifestations can appear in waves, sometimes with dramatically severe inflammation leading rapidly to scarring. Acne often has adverse emotional consequences. Its treatment is markedly better than in the past because of new pharmacological and physicochemical approaches and because evidence-based guidelines are now available.
Methods
This article is based on a selective review of the literature and also incorporates the authors’ own clinical and scientific experience.
Results
Acne vulgaris of grade I or II in an adolescent is generally not hard to treat. In contrast, the more severe grades III and IV and conglobate acne often present a therapeutic challenge, as they are associated with varying constellations of acute lesions, scarring, inflammation, and emotional disturbances. These conditions often require systemic treatment with tetracyclines, which are especially useful because of their para-antibiotic anti-inflammatory effect. Severe cases must be treated with isotretinoin. Women can benefit from anti-androgenic contraceptive drugs. Retinoids or azelaic acid are used in maintenance therapy to suppress the formation of microcomedones, the precursor stage of acne lesions.
Conclusion
A variety of effective treatments for acne are available, depending on the severity of the condition.
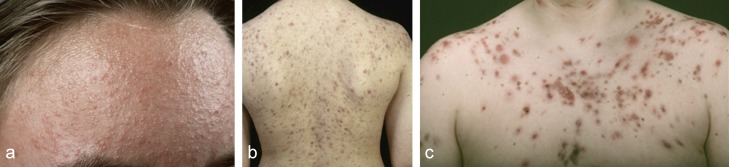
Apolymorphic clinical appearance (Figure 1), varying degrees of severity, acute as well as chronic forms, numerous subtypes and wide spectrum of topical and systemic treatment modalities all help define the group of diseases known as acne. The general public and many non-dermatologists consider acne a mild disease which resolves spontaneously in a matter of months to two years. A survey of 504 customers in 48 pharmacies across Germany showed that in choosing products to treat acne, 35% of patients with mild acne treated themselves, following the advice of a pharmacist (22.5%), another individual (3.3%) or relying on their own judgment (9.2%) (e1). This mild form of acne, sometimes called “physiologic acne” with relatively few “pimples” and “blackheads”, accounts for 60% of the cases, responds well to over-the-counter products and will not be further discussed in this article. Instead we will concentrate on the 40% of patients with more severe “clinical acne” who require medical treatment; they primarily are managed by dermatologists (93%) but also by general practitioners (6.3%) and pediatricians (0.6%) (e1). In addition, the complexity of acne is reflected less by acne vulgaris which refers to papulo-pustular acne of grades I–II after Plewig and Kligman, but instead by the more severe courses including nodular acne (conglobate acne), infantile acne, juvenile acne and acne tarda that occurs after the classic age range (>25 years of age) (1, 2). In addition, there are complex cases presenting as part of syndromes, secondary to hormonal disturbances, caused by medications, or as a result of provocation or continuous promotion by exogenous factors (3, e2, e3). In addition, one must separate out a wide range of acneiform dermatoses including the recently described PRIDE syndrome secondary to kinase inhibitors as well as classical rosacea, perioral dermatitis and gram-negative folliculitis.
Figure 1.
The various clinical appearances of acne vulgaris [from (e1)]
Acne.
A polymorphic clinical appearance, varying degrees of severity, acute as well as chronic forms, numerous subtypes and a wide spectrum of topical and systemic treatment modalities all characterize acne.
Patients with clinical acne require medical therapy, either because of the severity or duration of their disease. Acne is the most common dermatologic diagnosis accounting for 22–32% of cases and is one of the most common reasons for visiting a physician (1.1%) (3).
In addition, acne is a socio-economic problem. In 1995 acne was the most common dermatologic diagnosis in the USA with 10.2 million cases, accounting for 25.4% of the dermatologic diagnoses of all physicians (4). The patients received 6.5 million prescriptions for a systemic acne therapy (either antibiotics or isotretinoin) yearly, costing more than 1 billion US dollars. In 2001, 2.l billion euros were spent world-wide on acne medications; this is 18.3% of the annual expenditures for treating dermatologic diseases. In 2004, the direct costs in the USA had climbed to over 2.2 billion US dollars (e4).
Scientific advances in the past 20 years have greatly contributed to a better understanding of the pathogenesis of acne and to optimizing the therapeutic approach (4– 6). Numerous epidemiologic, socio-economic and psychological aspects of acne have at the same time been extensively evaluated (7– 12).
Frequency.
Acne is the most common dermatologic diagnosis accounting for 22–32% of cases and is one of the most common reasons for visiting a physician (1.1%).
Learning objectives
Classification of clinical acne according to degree of severity as prerequisite for choice of therapy
Using topical and systemic agents and combinations thereof according to guideline recommendations
Awareness of the psychosomatic and psychiatric complications of acne, the special variants and the problems young acne patients have with compliance.
Cost of therapy.
According to a health service study, acne was in 1995 the most common dermatologic diagnosis in the USA with 10.2 million cases, accounting for 25.4% of the dermatologic diagnoses of all physicians.
Methods
This article reflects the experience of the authors, the German S2k and the European S3 guidelines for acne, numerous personal experimental and clinical studies and as well as an extensive selective review of the national and international literature.
Epidemiology
Acne is an almost universal disease (8, 10, 13); nonetheless, its prevalence has been estimated as 0% in rural Brazil (e5) and 100% among children and adolescents in the UK (e6). Acne’s prevalence peaks between age 14 and the start of the 3rd decade (8, 9).
Some 20–40% of the cases persist past the age of 25 years. In a survey of 48 665 employees in Germany, the over-all prevalence of acne was 4.2% (9); in the sub-analysis the prevalence in the age group 16–20 years was 24.8%. Other studies show a prevalence in adolescents between 9 and 20 years of up to 95%, without separating clinical from “physiologic” acne. As long ago as 1931, a Swiss study reported a prevalence of 88–99% in young men and 88–97% in young women (e7). An English study in 1971 yielded similar results (e6); in Sweden large studies in school children showed a prevalence of clinical acne among 15–16 year olds of 50–54% in boys and 37–39% in girls (e8, e9). The prevalence of moderately severe acne among 15–24 year olds was about 14% (e10).
In a population-based epidemiological study in Hamburg, the prevalence among men was 29.9% and among women 23.7% for a group with a median age of 42 years (e11). Additional studies indicate that up to 40% of women over 25 years of age still have acne, even though the natural course of acne is to exhibit regression by this age (e12). Three well-documented studies show a prevalence of late-type acne especially in patients with a genetic predisposition to be as high as 50–53% (e13– e15). Prospective cohort studies in China (e16), France (e17) and Great Britain (e18) show that familial predisposition is associated with severe forms of acne and persistence into the 3rd decade (odds ratio 3.5), Familial predisposition and especially acne in the mother are significantly associated with a more severe course (10).
Prevalence.
In a population-based epidemiological study in Hamburg, the prevalence among men was 29.9% and among women 23.7% for a group with a median age of 42 years.
Twin studies show identical sebum production in monozygotic twins, of up to 98% (e18). In a study involving 458 monozygotic and 1099 dizygotic twins, 81% (95% confidence interval [CI], 73–87%) of the variability in disease severity was attributed to genetic factors and 19% to environmental or epigenetic factors (e19). Exogenous factors such as androgenic hormones, competitive sports, nicotine and diet with a high hyperglycemic index make the natural course of acne more severe and persistent (4, 5, 8). Well-documented large cohort studies show no association between acne and obesity, instead suggesting that the content of the diet is more important. In the Glasgow alumni cohort study, students with and without a history of acne were compared; a positive acne history correlated with a clearly reduced risk of coronary artery disease but a higher risk for prostate cancer (e20).
Clinical course.
A wide variety of clinical patterns can be observed.
Clinical course
A wide variety of clinical patterns can be observed. The most common course is a gradual development of clinically apparent disease, which can last months up to a year, then a continuous phase with occasional flares, and finally a slow regression at the end of the 2nd decade or beginning of 3rd decade. This classical pattern has different variants with more rapid onset and greater severity which reach a plateau, then wax and wane depending on the topical and systemic therapy, and finally undergo natural regression (1, 7). The most disastrous forms are those associated with dramatic progression, maximal inflammation, and a tendency to early scarring; they involve papulo-pustular nodular acne (grade IV) and/or conglobate acne. Each clinical course is unique and influenced by the type of therapeutic intervention. Other patients follow the typical course of papulo-pustular acne grade I–II or chronic inflammatory acne grade III after Plewig and Kligman. The former group often responds well to long-term continuous topical therapy. Finally there are some patients, usually young men, who after a chronic mild course flare dramatically at age 17–18 years.
Treatment of mild to moderate papulo-pustular acne.
There is strong evidence for the effectiveness of adapalene + benzoyl peroxide (BPO) (fixed combination) or BPO + clindamycin (fixed combination) in treating mild to moderate papulo-pustular acne.
Therapeutic options for women.
Hormonal anti-androgens and systemic antibiotics (in princial together with topical combination products) are an alternative for women with severe nodular acne and conglobate acne.
Even though all forms that start at puberty are associated with elevated androgen [dehydroepiandrosterone sulfate [DHEA-S)] levels, there is no long-term study that correlates androgen levels prior to, at the start of, and throughout puberty with clinical features of acne. Elevation of DHEA-S levels is best documented in women in the 3rd decade. In addition, it is becoming clearer that acne is not a short-term problem but a chronic disease with persistent inflammation over years (7). Many different courses may be observed—clear improvement and then severe relapse, sudden flares, or gradual re-occurrence. Finally the psycho-social aspects of the disease are of great importance for the patients in dealing with their family, partner and friends, as well as coping at school, when starting college or entering the job market (11). For this reason acne has been re-classified in recent years as a chronic inflammatory disease, similar to atopic dermatitis (7) (Table 1). The course of acne confirms to both the older and the most recent definitions of chronicity from the World Health Organization (WHO) (7). In particular, the number of acne patients outside the classic age range, as in acne tarda (14, e11), is increasing. Here one may encounter cases that after naturally regressing flare again in the second half of the 3rd decade, others that never clear, and still others that first become apparent during the 3rd decade. Frequently the patient is a woman who has used hormonal contraception since puberty and then in the 3rd decade stops contraception because of a desire to have children, but develops both increasing amount of sebum and acne of varying degrees of severity (e11).
Table 1. Acne as chronic disease using the WHO criteria*.
| Aspect | Acne | Atopic dermatitis |
|---|---|---|
| Major feature | Inflammation | Inflammation |
| Duration | >3 months to 10−30 years |
>3 months to 5−40 years |
| Genetic background | +, long course, polygenic | +, polygenic |
| Age of onset | circa 10 years | circa 1 year |
| Spontaneous remission | > 80% (3rd decade) | > 80% (2nd–3rd decade) |
| Recurrences | Common | Common |
| Follow-up | At intervals/over years | At intervals/over years |
| Therapy | Long-term/ with pauses | Long-term/ with pauses |
| Social counselling | + | + |
| Psychological counselling | Important | Important |
| Scarring Anatomic Psychological |
+ + |
+ + |
*from Gollnick et al. (7)
Chronic course.
Acne is not a short-term illness, but instead shows a chronic course with persistence over years.
Pathogenesis
The classic concept is that acne results from the combination of increased sebaceous gland activity with seborrhea, abnormal follicular differentiation with increased keratinization, microbial hyper-colonization of the follicular canal and increased inflammation primarily through activation of the adaptive immune system. New research results have led to a modification of this classical explanation as more primary pathophysiologic factors have been identified.
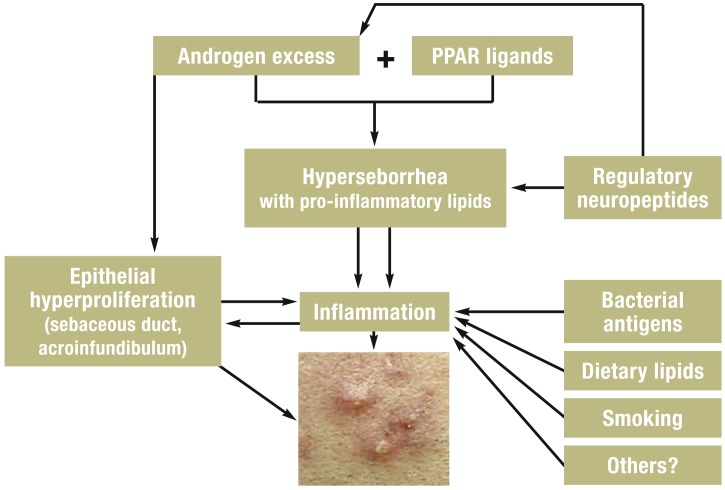
Along with a genetic predisposition, other major factors include androgens, pro-inflammatory lipids as ligands of sebocyte peroxisome proliferator-activated receptors (PPAR), and other inflammatory pathways. In addition, neuroendocrine regulatory mechanisms, diet and exogenous factors all may contribute to this multi-factorial process (3– 5) (Figure 2).
Figure 2.
Modern aspects of the pathogenesis of acne. Androgens, lipid ligands of the peroxisome proliferation-activating receptor (PPAR), regulatory neuropeptides with hormonal and non-hormonal activity and environmental factors led to hyperseborrhea, epithelial hyperproliferation in the sebaceous duct and acroinfundibulum and to expression of pro-inflammatory chemokines/cytokines, which stimulate the development of comedones and inflammatory acne lesions (from [4])
Pathogenesis.
Along with a genetic predisposition, other major factors include androgens, pro-inflammatory lipids as ligands of sebocyte peroxisome proliferator-activated receptors (PPAR), and other inflammatory pathways.
Cyclic growth of sebaceous follicles and triggers of inflammatory cascade
The hyperproliferation of the follicular epithelium leads to the development of microcomedones. Although clinically not visible, these are the initial lesions in acne and can be found in apparently normal skin of acne patients (e21). It has been proposed that the sebaceous follicles go through a cyclic pattern leading to spontaneous resolution of the microcomedones (e22– e25). Bioactive interleukin (IL)-1 is found in open comedones of untreated patients (e26). There is no correlation between the cytokine level and the number of follicular microbes. Overstimulation of subclinical inflammatory process or lack of negative feedback regulation could be the reasons for the interruption of the normal cyclic process and for the development of clinically relevant follicular inflammation in acne (4, 6).
Etiologic factors
Androgens—especially DHEA-S—play an important role in the early pathogenesis of acne, leading to larger sebaceous glands with increased sebum production (14, e27, e28). In addition, androgens stimulate the keratinocytes of the sebaceous duct (progenitor cells) and infundibulum (e29– e31). Excessive androgen production leads to increased sebum production and acne of varying severity (e32). Skin not responsive to androgens produces no sebum and develops no acne (e33, e34).When follicles lack androgen receptors, they can develop seborrhea but no comedones or inflammatory acne lesions (for example, familial nevoid sebaceous gland hyperplasia).
Etiologic factors.
Androgens—primarily DHEA-S—play an important role in initiating acne, leading to both increased sebaceous gland volume and increased sebum production.
Lipid synthesis requires androgens and peroxisome proliferator-activated receptors (PPAR) (e35). Neuropeptides (with hormonal and non-hormonal actions) can control the development of clinical inflammation in acne (neurogenic co-control) (15). Substance P can be identified in numerous immune-reactive nerve fibers of acne skin (e36) and sebaceous glands respond to it with the synthesis of the inactivating neutral endopeptidase.
There are elevated levels of corticotrophin releasing hormone (CRH) (16), while adrenal dehydroepiandrosterone can initiate inflammation in sebaceous follicles (e37– e39).
In recent years a dietary hypothesis for the pathogenesis of acne has been brought forward (e40– e44). The relationship between hyperinsulinemia and elevated IGF-1 and reduced IGF-BP has been increasingly supported by controlled cohort studies. The relationship between the number of cigarettes smoked daily and the severity of acne is controversial (e11, e20, e45, e46).
Synthesis of chemokines and cytokines by keratinocytes can be stimulated by certain Propionibacterium acnes (P. acnes) strains through the activation of toll-like receptor 2 (e47– e49). P. acnes must first develop a biofilm to become a relevant pathogen, then playing an important role in maintaining inflammation (e50, e51).
Hormone levels.
There are elevated levels of corticotrophin releasing hormone, while adrenal dehydroepiandrosterone can initiate inflammation in sebaceous follicles.
Therapy
The treatment of acne was slow to profit from the development of new topical and systemic medications. (17, e52– e54). After tretinoin in 1962, two additional topical retinoids became available in the 1980s and 1990s, namely isotretinoin and adapalene. The latter is a rexinoid (RAR/RXR/VDR) whose development was made possible by a better understanding of the cytoplasmic and nuclear retinoid receptors and their control of gene expression (18). In the mid-1980s, azelaic acid, a dicarboxylic acid, was introduced into topical therapy. The use of systemic antibiotics in acne therapy was originally based on the knowledge that oral tetracycline could inhibit experimentally induced pustules (19). Initially the goal was to suppress P. acnes. Today antibiotic treatment of acne is undergoing a paradigm change, as one shifts to a sub-anti-microbial dose of 40 mg daily in a delayed release formulation, using the para-antibiotic, anti-inflammatory action of these substances (doxycycline, minocycline) (e55). Oral erythromycin is almost exclusively reserved for severe acne during pregnancy (1, 2). With the development of the anti-androgenic substances cyproterone acetate and chlormadinone acetate, additional options for women with severe acne became available (14, 20). The development of the spironolactone derivative drospirenone as a component of 4th generation oral contraceptives was the last advance in this area, but enthusiasm has been dampened because of the apparent increased risk of thrombosis.
History of acne therapy.
Acne therapy has been slow to profit from the development of new topical and systemic medications.
Oral erythromycin.
Oral erythromycin is almost exclusively reserved for severe acne during pregnancy.
Because of increasing resistance of P. acnes, there are worldwide efforts to replace topical antibiotics with BPO, topical retinoids and azelaic acid, or combinations thereof (17, 19). Since acne is a disease that tends to recur, maintenance therapy to suppress the development of the precursor microcomedones that lead to both inflammatory and non-inflammatory acne lesions is desirable; numerous evidence-based studies in recent years have shown that topical retinoids can accomplish this (1, 2, 18). Physical and chemical treatment methods for acne have not been adequately studied with evidence-based methods (21). Irradiation with blue or blue-red light destroys P. acnes via an endogenous porphyrin activation leading to cell death. UVA irradiation is comedogenic, while treatment with UVB irradiation is obsolete. The effects of interventional therapy with lasers, chemical peels, fillers or photodynamic therapy have not been sufficiently studied (21– 23) (Table 2). The latter are especially suitable for treating the late manifestations of acne, such as hypertrophic and atrophic scars, as well as post-inflammatory hyperpigmentation. The Global Alliance for Better Outcomes in Acne, formed in 2001, organizes regional conferences in Asia, Europe, South America and North America, as well as national acne study groups, in order to develop guidelines and enhance evidence-based studies. The S2k acne guidelines published in Germany in 2010 (1) were the basis for the 2012 European S3 guidelines which are now being advanced to global guidelines (2). They are summarized in (Table 3; these are only general recommendations which can be modified and personalized by the treating physicians taking into account the complex nature and variable course of acne.
Table 2. Summary of treatment recommendations from the European S3 guideline.
| Grade of recommendation | Comedonal acne | Mild to moderate papulo-pustular acne | Severe papulo-pustular acne/papulo-pustular nodular acne | Severe nodular acne/conglobate acne*4 |
|---|---|---|---|---|
| Strong recommendation (A) |
No evidence | Adapalene plus BPO (f. c.) or BPO plus clindamycin (f. c.) [1a] |
Isotretinoin*1 [3b] |
Isotretinoin*1 [1b] |
| Moderate recommendation (B) |
Topical retinoids*3 [2b] |
Azelaic acid or BPO or topical retinoids *3 or systemic antibiotics*2 plus adapalene*5 [2b] |
Systemic antibiotics plus adapalene*5 or systemic antibiotics plus azelaic acid*8 or systemic antibiotics plus adapalene plus BPO (f. c.) [2b] |
Systemic antibiotics*10 plus azelaic acid [2b] |
| Weak recommendation (C) |
Azelaic acid or BPO [3b] |
Blue light or oral zinc or topical erythromycin plus topical isotretinoin (f. c.) or topical erythromycin plus tretinoin (f. c.) or systemic antibiotics*2 , plus BPO *7 or systemic antibiotics*2, *10 plus azelaic acid or systemic antibiotics*2, *10 plus adapalene plus BPO (f. c.) *9 [3b] |
Systemic antibiotics*5 plus BPO*7 [3b] |
Systemic antibiotics*10 plus BPO*7 or systemic antibiotics*10 plus adapalene*5, *9 or systemic antibiotics*10 plus adapalene plus BPO (f. c.)*9 [3b] |
| Alternatives for female patients |
See above | See above | Hormonal anti-androgens plus topical therapy or hormonal anti-androgens plus systemic antibiotics*6 [3b] |
Hormonal anti-androgens plus systemic antibiotics*6 [3b] |
*1Conditions which could make the use of therapy with a low level of evidence as first line treatment necessary (for example, costs/reimbursement/regional legal requirements/availability or approval/drug interactions/associated diseases/allergies/intolerance reactions).
*2When there is extensive involvement including extending beyond the face, moderately severe acne may require systemic antibiotics initially.
*3Adapalene is preferred over tretinoin/isotretinoin
*4Systemic corticosteroids can be considered.
*5Doxycycline and lymecycline
*6Low level of evidence
*7Indirect evidence from a study also including chlorhexidine on the basis of expert recommendation
*8Indirect evidence for nodular acne and conglobate acne on the basis of expert recommendation
*9Indirect evidence for severe papulo-pustular acne
*10Only studies with systemic antibiotics + adapalene, isotretinoin and tretinoin (combination therapy) were considered relevant on the basis of expert recommendation.
f.c.: fixed combination; BPO, benzoyl peroxide. [] indicates the evidence level
Adapted from Nast et al. (2)
Table 3. Side effects of topical acne therapy.
| Erythema | Stinging/burning | Desquamation | Xerosis | Bacterial resistance | |
|---|---|---|---|---|---|
| Tretinoin 0.05%*1 |
+++ | ++ | ++ | + + | – |
| Tretinoin 0.025%*1 |
++ | ++ | + | + | – |
| Isotretinoin | + | + | + | + | – |
| Adapalene | + | + | + | + | – |
| Azelaic acid | (+) | ++ | – | + | – |
| Benzoyl peroxide*3 2.5% |
+ | + | + | + + | – |
| Benzoyl peroxide*3 5% |
++ | ++ | + | + + | – |
| Erythromycin*2/ Clindamycin*2 |
– – |
– – |
– – |
– – |
+ ++ ++(+) |
*1New delayed formulations cause much less irritation
*2Resistance occurs with monotherapy
*3New formulations cause less irritation. All BPO (benzoyl peroxide) preparations can cause bleaching.
() uncommon
Indications for isotretinoin.
Isotretinoin should be used in severe nodular and papulo-pustular acne at grade IV and when systemic antibiotics fail.
Length of remission following isotretinoin.
About 20–30% of patients experience a recurrence after one year. Age and dose are important factors. A low dose is associated with a higher recurrence rate.
Based on these guidelines (1, 2) topical monotherapy with antibiotics is not recommended. Instead, topical combination therapy with BPO is recommended for mild papulo-pustular acne. Topical retinoids are recommended as monotherapy for comedonal acne or in combination with BPO or topical antibiotics for milder papulo-pustular acne (Table 2). Topical azelaic acid therapy is the second-line recommendation for comedonal and mild papulo-pustular acne. Topical corticosteroids should not be used in treating acne (Table 3).
Combination therapy with topical drugs and oral antibiotics (doxycycline > minocycline, tetracycline) is recommended for moderate to severe acne, as well as less severe inflammatory acne which fails to respond to topical therapy alone. The systemic antibiotics should not be used alone but instead in combination with topical retinoids, BPO or azelaic acid. For women, oral contraceptives containing anti-androgens are another option. The recommended antibiotic dosages are doxycycline 50 mg daily–b.i.d. to 100 mg b.i.d., minocycline 50 mg b.i.d., tetracycline 500 mg b.i.d., and erythromycin 500 mg b.i.d.; therapy can be continued for three months (Table 4).
Table 4. Side effects of systemic antibiotics commonly used to treat acne.
| Tetracycline | Doxycycline | Minocycline | Erythromycin | |
|---|---|---|---|---|
| Gastrointestinal symptoms | + | + | + | + |
| Vaginal candidiasis | + | + | + | + |
| Esophagitis | (+) | (+) | − | − |
| Phototoxicity | + | (+) | (−) | − |
| Hyperpigmentation | − | − | + | − |
| Drug-induced lupus erythematosus | − | − | + | − |
| Cholestatic hepatitis, pancreatitis, pseudomembranous colitis | (+) | (+) | + | ((+)) |
| Exanthema | (+) | (+) | (+) | (+) |
| Headache | + | + | + | ((+)) |
() uncommon, (()) very uncommon
Hormonal anti-androgen therapy is not recommended as a first-line monotherapy for uncomplicated acne. Ethinylestradiol in combination with cyproterone acetate, chlormadinone acetate, dienogest or drospirenone is recommended only as a combination therapy for women with moderate papulo-pustular acne to conglobate acne (14, 19).
Systemic combination therapy.
Topical and systemic combination therapy with oral antibiotics is recommended for moderately severe inflammatory acne as standard therapy; it should only be used for milder inflammatory acne that fails to respond to topical therapy.
Systemic corticosteroids can play a role in the initial therapy of severe inflammatory acne, in patients with systemic inflammatory manifestations (such as acne fulminans), or to control exacerbations during systemic isotretinoin therapy.
Maintenance therapy with topical retinoids (adapalene > isotretinoin, tretinoin) or azelaic acid is also recommended.
During pregnancy and nursing, possible treatment options include topical therapy with BPO, azelaic acid, erythromycin and chemical peels, as well as lasers and phototherapy. Systemic erythromycin, zinc and corticosteroids can be used in adjusted dosages in severe flares. Topical retinoids and clindamycin are contraindicated during pregnancy.
Therapy with oral isotretinoin: comments
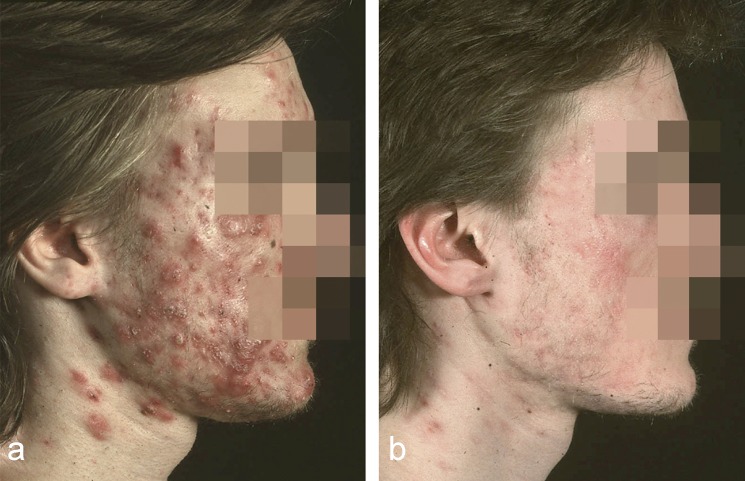
Isotretinoin is without question the most effective oral agent for severe acne (papulo-pustular nodular acne, conglobate acne) (24, e56– e58) (Figure 3). Since its introduction in the 1980s and the detailed knowledge of its side effects, there have been repeated controversies over the indications for its use. Until the beginning of 2002, the above indications were well-accepted and it was understood among physicians experienced in treating acne that isotretinoin—including low-dose therapy—was not indicated in patients with mild acne. The EMA (European Medicines Agency) altered the recommendations at that time, requiring a trial of oral and topical medications in combination; if this failed, then isotretinoin could be employed. The registered indications were correspondingly altered. Physicians worldwide reacted differently then and continue to do so, basing their actions on many years of experience. The authors presented the arguments for isotretinoin as first choice treatment for severe acne to the EMA (25). Dermatologists in private practice and other physicians are in a position of conflict since the publication of the European S3 guidelines, as this document once again recommends isotretinoin as the drug of choice for severe acne (papulo-pustular nodular acne and conglobate acne). The feeling is that the sooner isotretinoin is employed for acne with marked inflammation and a tendency to early scarring, the better it is for the patient. The recommended doses are 0.3–0.5 mg/kg body weight (BW) for severe papulo-pustular nodular acne and ≥0.5 mg/kg BW for conglobate acne; therapy should be continued for 6 months, sometimes even longer (24) (Table 5).
Figure 3.
Conglobate acne before (left) and after one month of a total of six months of treatment with isotretinoin (right) [from (e74)]
Table 5. Efficacy of isotretinoin therapy for severe acne*1.
| Source | Patients (n) | Acne type | Dose | Duration of treatment (months) | Results | Grade of recommendation/ level of evidence |
|---|---|---|---|---|---|---|
| Goldstein et al., 1982 (e59) |
7 | Severe nodular acne |
1 mg/kg BW daily | 4 | Overall 47.2% reduction in lesions; 2/7patients failed to improve |
C/4 |
| Jones et al., 1983 (e60) |
76 | Severe nodular acne |
0.5 mg/kg BW daily | 4 | 90% improvement; 70% reduction in degree of severity; 66.6% no recurrence |
C/4 |
| Hennes et al., 1984 (e61) |
87 | Conglobate acne |
1 mg/kg BW daily 0.2 mg/kg BW daily |
3 3 |
96% remission after 6 months, 81% remission after 12 months |
B/3b |
| 0.5 mg/kg BW daily; 0.2 mg/kg BW daily |
4 4 |
84%remission after 6 months, 47% remission after 12 months |
C/4 | |||
| 0.2 mg/kg BW daily | 6 | 74% remission after 6 months, 37% remission after 12 months |
C/4 | |||
| Harms et al., 1986 (e62) |
89 | Severe acne | Not given | Not given | 85% remission after 6-47 months (83% men, 91% women); recurrences with low total dose and young age |
C/4 |
| Cunliffe und Norris, 1987 (e63) |
Severe acne | 1 mg/kg BW daily | 4 | Persistence or recurrence more common with younger patients (14–19 years), shorter duration of illness, acne on trunk, recurrent seborrhea after therapy |
C/4 | |
| Chivot und Midoun, 1990 (e64) |
172 | Severe acne | Not given | Not given | 79% remission after 12–41 months, recurrence rate depends on severity of acne, age of patient, duration of illness after treatment, total dosage, daily dose, duration of therapy |
C/4 |
| Layton et al., 1993 (e65) |
88 | Acne (chronic) |
0.5–1 mg/kg BW daily |
4 | 61% remission after 36 months | C/4 |
| Lehucher- Ceyrac und Weber-Buisset, 1993 (e66) |
237 | Nodulocystic acne |
0.5–1 mg/kg BW daily |
Up to 108 | 71% remissions—86% after 1 year, 60% after 3 years and 52% after 5 years |
C/4 |
| Lehucher- Ceyrac et al., 1999 (e67) | C/4 | |||||
| Shahidullah et al., 1994 (e68) |
250 | Nodulocystic acne |
0.33–1 mg/kg BW daily |
1–12 | 94% remission after 6 months | C/4 |
| Goulden et al., 1997 (e69) |
80 | Acne resistant to systemic antibiotics |
0.5 mg/kg BW daily | 6 (1 week of every 4) |
61% remission after 12 months; recurrence rate depended on sebum excretion rate, patient age and disease duration |
C/4 |
| Quereux et al., 2006 (e70) |
52 | Moderate to severe acne |
0.3–1 mg/kg BW daily |
Prospective study (2 years); 48% remission; recurrence rate depended on seborrhea after therapy, numerous superficial inflammatory lesions in young patients, family history, prepubertal acne |
B/3b |
*1from Zouboulis (e56). BW, body weight
Use of isotretinoin.
Isotretinoin is without question the most effective oral agent for severe acne (papulo-pustular nodular acne, conglobate acne).
In addition to the risk of teratogenicity with systemic retinoid therapy—just as with high-dose vitamin A administration—one must pay careful attention to the possible induction of depression and suicidal ideation. This problem has only been reported when isotretinoin was used to treat acne, not when it has been employed to treat ichthyosis or psoriasis (it was used extensively for the latter indication in the USA prior to the introduction of etretinate and acitretin). Prospective cohort studies have shown that acne patients tend to have suicidal ideation as well as depression because of their altered appearance and psychosocial complications (26, e71). During treatment with oral antibiotics, however, suicide and depression were found to be just as common as under treatment with oral antibiotics and in controls (e72). Nonetheless every patient starting on isotretinoin must be assessed for a risk of depression, counselled and then followed closely. Recently, it has been noted that patients treated with high doses of isotretinoin and with repeated cycles have a greater risk of inflammatory colitis but not Crohn disease (27, e73– e75). The risk of rhabdomyolysis has been known for 30 years and is small, but every patient should be warned not to engage in over-strenuous activity at work or sports. Clinical observation and laboratory monitoring are recommended.
Risks after repeated courses.
Recently is has been reported that after treatment with isotretinoin in higher doses and over repeated cycles, there is an increased risk of inflammatory colitis but not of Crohn disease.
Risk of suicide.
In addition to the risk of teratogenicity with systemic retinoid therapy—just as with high-dose vitamin A administration—one must pay careful attention to the possible induction of depression and suicidal ideation.
Further information on CME.
This article has been certified by the North Rhine Academy for Postgraduate and Continuing Medical Education. Deutsches Ärzteblatt provides certified continuing medical education (CME) in accordance with the requirements of the Medical Associations of the German federal states (Länder). CME points of the Medical Associations can be acquired only through the Internet, not by mail or fax, by the use of the German version of the CME questionnaire. See the following website: cme.aerzteblatt.de.
Participants in the CME program can manage their CME points with their 15-digit “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be entered in the appropriate field in the cme.aerzteblatt.de website under “meine Daten” (“my data”), or upon registration. The EFN appears on each participant’s CME certificate.
This CME unit can be accessed until 20 July 2014.
The CME unit “The Differential Diagnosis and Treatment of Tremor“
(Issue 13/2014) can be accessed until 22 June 2014.
The CME unit “Structured Management of Otitis Media” (Issue 9/2014) can be accessed until 25 May 2014.
The CME unit “The Treatment of Type 2 Diabetes” (Issue 5/2014) can be accessed until 27 April 2014.
Please answer the following questions to participate in our certified Continuing Medical Education program. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the prevalence of acne in the general population in Germany?
>1%
<5%
>10%
Around 4%
25%
Question 2
Which factors are statistically significantly associated with a more severe disease course in late-onset acne?
Spicy foods and atopic dermatitis
Obesity and carbohydrate-rich diet
Familial predisposition and acne in the mother
Gender and ethnicity
Biofilm formation and composition of sebum
Question 3
Regarding P. acnes, which of the following plays an especially important role in determining the severity of acne?
Number of P. acnes per cm2 skin surface
Biofilm formation
O2 saturation in follicular canal
Composition of sebum
Interfollicular distribution of P. acnes
Question 4
Repeated cycles of isotretinoin increase the risk of developing which disease?
Hypertension
Inflammatory colitis
Crohn disease
Cardiac arrhythmias
Psoriasis
Question 5
Which type of acne or degree of severity requires systemic therapy?
Acne keloidalis
Acne neonatorum
Physiologic acne
Conglobate acne
Acne comedonica
Question 6
Which side effects may develop during monotherapy with erythromycin?
Tingling
Burning
Scaling
Xerosis
Bacterial resistance
Question 7
What is the recommended second choice therapy for acne comedonica and mild papulo-pustular acne?
Systemic hormone therapy
Systemic glucocorticosteroids
Topical monotherapy with antibiotics
Oral combination therapy with doxycycline and benzoyl peroxide
Topical therapy with azelaic acid
Question 8
Which treatment for conglobate acne is best supported by evidence (Level A)?
Azelaic acid
Doxycycline
Topical antibiotics
Isotretinoin
Erythromycin
Question 9
Which maintenance therapy for acne is appropriate?
Low-dose doxycycline
Topical erythromycin
Benzoyl peroxide
Topical retinoids
Low-dose oral isotretinoin
Question 10
Which medication for acne is contraindicated during pregnancy?
Oral zinc gluconate
Topical erythromycin
Azelaic acid
Benzoyl peroxide
Topical retinoids
Acknowledgments
Translated from the original German by Walter H.C. Burgdorf, MD
Footnotes
Conflict of interest statement
Prof. Gollnick has participated in acne-relevant clinical and experimental studies, symposia and advisory boards of Galderma, Stiefel/GSK, Intendis, Meda, Merz, Hoffmann-LaRoche, Novartis, Schering, Pierre Fabre, IMTM, and Vichy.
Prof. Zouboulis has patents and receives royalties for “Sebocytes, sebocyte cell lines and applications thereof” DE19991003920 as well as “Acne treatment with lipoxygenase inhibitors” DE20011021252. He has served as a paid advisor to Bayer-Schering, Boehringer-Ingelheim, Dermira, Galderma, Intendis, Leo, Merz, and Stiefel/GSK. He has received honoraria for publications from BASF. He has received reimbursement for travel and accomodation costs from Bioderma, General Topics, Glenmark, and Stiefel/GSK. He has received honoraria for organizing scientific continuing medical education symposia from Bayer/Schering, Bioderma, General Topics, Glenmark, and Stiefel/GSK. He has received honoraria for clinical studies from Galderma, Merz, and Stiefel/GSK. He has received honoraria for experimental research projects which he initiated from Galderma, Hoffmann-LaRoche, and MSD.
References
- 1.Nast A, Bayerl C, Borelli C, et al. S2k-Leitlinie zur Therapie der Akne. J Dtsch Dermatol Ges. 2010;8(Suppl 2):1–59. doi: 10.1111/j.1610-0387.2010.07466.x. [DOI] [PubMed] [Google Scholar]
- 2.Nast A, Dréno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26(Suppl 1):1–29. doi: 10.1111/j.1468-3083.2011.04374.x. [DOI] [PubMed] [Google Scholar]
- 3.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Eady A, Philpott M, et al. What is the pathogenesis of acne? Exp Dermatol. 2005;14:143–152. doi: 10.1111/j.0906-6705.2005.0285a.x. [DOI] [PubMed] [Google Scholar]
- 5.Kurokawa I, Danby FW, Ju Q, et al. New developments in our understanding of acne pathogenesis and treatment. Exp Dermatol. 2009;18:821–832. doi: 10.1111/j.1600-0625.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 6.Zouboulis CC. Moderne Aspekte der Aknepathogenese. J Dtsch Dermatol Ges. 2010;8(Suppl 1):7–14. doi: 10.1111/j.1610-0387.2009.07168.x. [DOI] [PubMed] [Google Scholar]
- 7.Gollnick HP, Finlay AY, Shear N. Global Alliance to Improve Outcomes in Acne: Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol. 2008;9:279–284. doi: 10.2165/00128071-200809050-00001. [DOI] [PubMed] [Google Scholar]
- 8.Schäfer T, Kahl C, Rzany B. Epidemiologie der Akne. J Dtsch Dermatol Ges. 2010;8(Suppl 1):4–6. doi: 10.1111/j.1610-0387.2009.07167.x. [DOI] [PubMed] [Google Scholar]
- 9.Schaefer I, Rustenbach SJ, Zimmer L, Augustin M. Prevalence of skin diseases in a cohort of 48,665 employees in Germany. Dermatology. 2008;217:169–172. doi: 10.1159/000136656. [DOI] [PubMed] [Google Scholar]
- 10.Ghodsi SZ, Orawa H, Zouboulis CC. Prevalence, severity and severity risk factors of acne in high school pupils: A community-based study. J Invest Dermatol. 2009;129:2136–2141. doi: 10.1038/jid.2009.47. [DOI] [PubMed] [Google Scholar]
- 11.Niemeier V, Kupfer J, Gieler U. Akne vulgaris - Psychosomatische Aspekte. J Dtsch Dermatol Ges. 2010;8(Suppl 1):95–104. doi: 10.1111/j.1610-0387.2006.06110_suppx.x. [DOI] [PubMed] [Google Scholar]
- 12.Radtke MA, Schäfer I, Augustin M. Pharmakoökonomie der Akne - Bewertung von Nutzen und Wirtschaftlichkeit. J Dtsch Dermatol Ges. 2010 8;(Suppl 1):1055–1114. doi: 10.1111/j.1610-0387.2009.07175.x. [DOI] [PubMed] [Google Scholar]
- 13.Jemec GB, Linneberg A, Nielsen NH, Frolund L, Madsen F, Jorgensen T. Have oral contraceptives reduced the prevalence of acne? A population-based study of acne vulgaris, tobacco smoking and oral contraceptives. Dermatology. 2002;204:179–184. doi: 10.1159/000057878. [DOI] [PubMed] [Google Scholar]
- 14.Zouboulis CC. Acne vulgaris - Rolle der Hormone. Hautarzt. 2010;61:107–114. doi: 10.1007/s00105-009-1830-1. [DOI] [PubMed] [Google Scholar]
- 15.Thielitz A, Gollnick H. Overview of new therapeutic developments for acne. Expert Rev Dermatol. 2009;4:55–65. [Google Scholar]
- 16.Ganceviciene R, Graziene V, Fimmel S, Zouboulis CC. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345–352. doi: 10.1111/j.1365-2133.2008.08959.x. [DOI] [PubMed] [Google Scholar]
- 17.Fluhr JW, Degitz K. Antibiotika, Azelainsäure und Benzoylperoxid in der topische Aknetherapie. J Dtsch Dermatol Ges. 2010;8(Suppl 1):24–30. doi: 10.1111/j.1610-0387.2009.07169.x. [DOI] [PubMed] [Google Scholar]
- 18.Thielitz A, Abdel-Naser MB, Fluhr JW, Zouboulis CC, Gollnick H. Topische Retinoide bei Akne - eine evidenzbasierte Übersicht. J Dtsch Dermatol Ges. 2010;8(Suppl 1):15–23. doi: 10.1111/j.1610-0387.2008.06741_suppx.x. [DOI] [PubMed] [Google Scholar]
- 19.Ochsendorf F. Systemische Antibiotika zur Behandlung der Acne vulgaris. J Dtsch Dermatol Ges. 2010;8(Suppl 1):31–46. doi: 10.1111/j.1610-0387.2006.06053_suppx.x. [DOI] [PubMed] [Google Scholar]
- 20.Zouboulis CC, Rabe T. Hormonelle Antiandrogene in der Aknetherapie. J Dtsch Dermatol Ges. 2010;8(Suppl 1):60–74. doi: 10.1111/j.1610-0387.2009.07171.x. [DOI] [PubMed] [Google Scholar]
- 21.Degitz K, Plewig G, Gollnick H. Ergänzende Verfahren in der Aknetherapie - Aktualisierung 2010. J Dtsch Dermatol Ges. 2010;8(Suppl 1):75–80. doi: 10.1111/j.1610-0387.2005.04087_suppx.x. [DOI] [PubMed] [Google Scholar]
- 22.Bayerl C, Degitz K, Meigel E, Kerscher M. Adjuvante dermatokosmetische Aknetherapie. J Dtsch Dermatol Ges. 2010;8(Suppl 1):89–94. doi: 10.1111/j.1610-0387.2009.07174.x. [DOI] [PubMed] [Google Scholar]
- 23.Jansen T, Podda M. Therapie der Aknenarben. J Dtsch Dermatol Ges. 2010;8(Suppl 1):81–88. doi: 10.1111/j.1610-0387.2009.07173.x. [DOI] [PubMed] [Google Scholar]
- 24.Ganceviciene R, Zouboulis CC. Isotretinoin: state of the art treatment for acne vulgaris. Expert Rev Dermatol. 2007;2:693–706. doi: 10.1111/j.1610-0387.2009.07238.x. [DOI] [PubMed] [Google Scholar]
- 25.Layton AM, Dreno B, Gollnick HPM, Zouboulis CC. A review of the European directive for prescribing systemic isotretinoin for acne vulgaris. J Eur Acad Dermatol Venereol. 2006;20:773–776. doi: 10.1111/j.1468-3083.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- 26.Marqueling AL, Zane LT. Depression and suicidal behavior in acne patients treated with isotretinoin: a systematic review. Semin Cutan Med Surg. 2007;26:210–220. doi: 10.1016/j.sder.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Arzneimittelkommission der Deutschen Ärzteschaft. Isotretinoin und chronisch-entzündliche Darmerkrankung. Dtsch Arztebl. 2012;109 [Google Scholar]
- e1.Franzke N, Zimmer L, Schäfer I, Radermacher C, Kresken J, Augustin M. Quality of medical care of patients with acne vulgaris in Germany - nationwide survey of pharmacy clients. J Dtsch Dermatol Ges. 2009;7:1060–1063. doi: 10.1111/j.1610-0387.2009.07155.x. [DOI] [PubMed] [Google Scholar]
- e2.Zouboulis CC. Moderne Aspekte der Aknepathogenese. Akt Dermatol. 2006;32:296–302. doi: 10.1111/j.1610-0387.2009.07168.x. [DOI] [PubMed] [Google Scholar]
- e3.Chen W, Obermayer-Pietsch B, Hong J-B, et al. Acne-associated syndromes: Models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol. 2011;25:637–646. doi: 10.1111/j.1468-3083.2010.03937.x. [DOI] [PubMed] [Google Scholar]
- e4.Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490–500. doi: 10.1016/j.jaad.2006.05.048. [DOI] [PubMed] [Google Scholar]
- e5.Bechelli L, Haddad N, Pimenta W, et al. Epidemiological survey of skin diseases in schoolchildren living in the Purus Valley (Acre State, Amazonia, Brazil) Dermatologica. 1981;163:78–93. doi: 10.1159/000250144. [DOI] [PubMed] [Google Scholar]
- e6.Burton JL, Cunliffe WJ, Stafford I, et al. The prevalence of acne vulgaris in adolescence. Br J Dermatol. 1971;85:119–126. doi: 10.1111/j.1365-2133.1971.tb07195.x. [DOI] [PubMed] [Google Scholar]
- e7.Bloch B. Metabolism, endocrine glands and skin diseases, with special reference to acne vulgaris and xanthoma. Br J Dermatol. 1931;43:77–87. [Google Scholar]
- e8.Hellgren L. A preliminary report of the prevalence of seborrhoeic dermatitis, acne vulgaris and warts in the county of Skaraborg in Sweden. Acta Derm Venerel (Stockh) 1963;43:502–509. [Google Scholar]
- e9.Larsson PA, Liden S. Prevalence of skin diseases among adolescents 12-16 years of age. Acta Derm Venereol. 1980;60:415–423. [PubMed] [Google Scholar]
- e10.Rea J, Newhouse M, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med. 1976;30:107–114. doi: 10.1136/jech.30.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Schäfer T, Nienhaus A, Vieluf D, Berger J, Ring J. Epidemiology of acne in the general population: the risk of smoking. Br J Dermatol. 2001;145:100–104. doi: 10.1046/j.1365-2133.2001.04290.x. [DOI] [PubMed] [Google Scholar]
- e12.Dréno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013 Sep 27;:1063–1070. doi: 10.1111/jdv.12061. [DOI] [PubMed] [Google Scholar]
- e13.Knaggs HE, Wood EJ, Rizer RL, Mills OH. Post-adolescent acne. Int J Cosmet Sci. 2004;26:129–138. doi: 10.1111/j.1467-2494.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- e14.Goulden V, McGeown CH, Cunliffe WJ. The familial risk of adult acne: a comparison between first-degree relatives of affected and unaffected individuals. Br J Dermatol. 1999;14 doi: 10.1046/j.1365-2133.1999.02979.x. [DOI] [PubMed] [Google Scholar]
- e15.Dumont-Wallon G, Dréno B. Acné de la femme de plus de 25 ans: spécifique par sa clinique et les facteurs favorisants: Étude rétrospective de 79 femmes. Presse Med. 2008;37:585–591. doi: 10.1016/j.lpm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- e16.Shen Y, Wang T, Zhou C, et al. Prevalence of acne vulgaris in Chinese adolescents and adults: a community-based study of 17,345 subjects in six cities. Acta Derm Venereol. 2012;92:40–44. doi: 10.2340/00015555-1164. [DOI] [PubMed] [Google Scholar]
- e17.Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56–59. doi: 10.1016/j.jaad.2007.06.045. [DOI] [PubMed] [Google Scholar]
- e18.Walton S, Wyatt EH, Cunliffe WJ. Genetic control of sebum excretion and acne—a twin study. Br J Dermatol. 1988;118:393–396. doi: 10.1111/j.1365-2133.1988.tb02433.x. [DOI] [PubMed] [Google Scholar]
- e19.Bataille V, Snieder H, MacGregor AJ, Sasieni P, Spector TD. The influence of genetics and environmental factors in the pathogenesis of acne: a twin study of acne in women. J Invest Dermatol. 2002;119:1317–1322. doi: 10.1046/j.1523-1747.2002.19621.x. [DOI] [PubMed] [Google Scholar]
- e20.Galobardes B, Smith GD, Jeffreys M, Kinra S, McCarron P. Acne in adolescence and cause-specific mortality: Lower coronary heart disease but higher prostate cancer mortality. The Glasgow Alumni Cohort Study. Am J Epidemiol. 2005;161:1094–1101. doi: 10.1093/aje/kwi147. [DOI] [PubMed] [Google Scholar]
- e21.Norris JF, Cunliffe WJ. A histological and immunocytochemical study of early acne lesions. Br J Dermatol. 1988;118:651–659. doi: 10.1111/j.1365-2133.1988.tb02566.x. [DOI] [PubMed] [Google Scholar]
- e22.Cunliffe WJ, Holland DB, Clark SM, Stables GI. Comedogenesis: some new aetiological, clinical and therapeutic strategies. Br J Dermatol. 2000;142:1084–1091. doi: 10.1046/j.1365-2133.2000.03531.x. [DOI] [PubMed] [Google Scholar]
- e23.Xu Y, Yang L, Yang T, Xiang M, Huang E, Lian X. Expression pattern of cyclooxygenase-2 in normal rat epidermis and pilosebaceous unit during hair cycle. Acta Histochem Cytochem. 2008;41:157–163. doi: 10.1267/ahc.08024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e24.Jeremy AHT, Holland DB, Roberts SG, Thomson KF, Cunliffe WJ. Inflammatory events are involved in acne lesion initiation. J Invest Dermatol. 2003;121:20–27. doi: 10.1046/j.1523-1747.2003.12321.x. [DOI] [PubMed] [Google Scholar]
- e25.Zouboulis CC. Is acne vulgaris a genuine inflammatory disease? Dermatology. 2001;203:277–279. doi: 10.1159/000051771. [DOI] [PubMed] [Google Scholar]
- e26.Ingham E, Eady EA, Goodwin CE, Cove JH, Cunliffe WJ. Pro-inflammatory levels of interleukin-1 alpha-like bioactivity are present in the majority of open comedones in acne vulgaris. J Invest Dermatol. 1992;98:895–901. doi: 10.1111/1523-1747.ep12460324. [DOI] [PubMed] [Google Scholar]
- e27.Pochi PE, Strauss JS. Sebaceous gland response in man to the administration of testosterone, δ4-androstenedione, and dehydroisoandrosterone. J Invest Dermatol. 1969;52:32–36. doi: 10.1038/jid.1969.4. [DOI] [PubMed] [Google Scholar]
- e28.Chen W, Yang CC, Liao CY, et al. Expression of sex-determining genes in human sebaceous glands and their possible roles in pathogenesis of acne. J Eur Acad Dermatol Venereol. 2006;20:846–852. doi: 10.1111/j.1468-3083.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- e29.Cunliffe W, Forster R. Androgen control of the pilosebaceous duct? Brit J Dermatol. 1987;116 [Google Scholar]
- e30.Zouboulis CC. The sebaceous gland in adolescent age. Eur J Ped Dermatol. 2008;18:150–154. [Google Scholar]
- e31.Lucky AW, Biro FM, Huster GA, Leach AD, Morrison JA, Ratterman J. Acne vulgaris in premenarchal girls. Arch Dermatol. 1994;130:308–314. doi: 10.1001/archderm.130.3.308. [DOI] [PubMed] [Google Scholar]
- e32.Stewart ME, Downing DT, Cook JS, Hansen JR, Strauss JS. Sebaceous gland activity and serum dehydroepiandrosterone sulfate levels in boys and girls. Arch Dermatol. 1992;128:1345–1348. [PubMed] [Google Scholar]
- e33.Marynick SP, Chakmajian ZH, McCaffree DL, Herdon JH. Androgen excess in cystic acne. N Engl J Med. 1983;308:981–986. doi: 10.1056/NEJM198304283081701. [DOI] [PubMed] [Google Scholar]
- e34.Imperato-McGinley J, Gautier T, Cai LQ, Yee B, Epstein J, Pochi P. The androgen control of sebum production. Studies of subjects with dihydrotestosterone deficiency and complete androgen insensitivity. J Clin Endocrinol Metabol. 1993;76:524–528. doi: 10.1210/jcem.76.2.8381804. [DOI] [PubMed] [Google Scholar]
- e35.Alestas T, Ganceviciene R, Fimmel S, Müller-Decker K, Zouboulis CC. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75–87. doi: 10.1007/s00109-005-0715-8. [DOI] [PubMed] [Google Scholar]
- e36.Toyoda M, Nakamura M, Makino T, Kagoura M, Morohashi M. Sebaceous glands in acne patients express high levels of neutral endopeptidase. Exp Dermatol. 2002;11:241–247. doi: 10.1034/j.1600-0625.2002.110307.x. [DOI] [PubMed] [Google Scholar]
- e37.Alesci S, Bornstein SR. Neuroimmunoregulation of androgens in the adrenal gland and the skin. Horm Res. 2000;54:281–286. doi: 10.1159/000053272. [DOI] [PubMed] [Google Scholar]
- e38.Slominski AT, Botchkarev V, Choudhry M, et al. Cutaneous expression of CRH and CRH-R. Is there a „skin stress response system?“. Ann N Y Acad Sci. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- e39.Zouboulis CC. The human skin as a hormone target and an endocrine gland. Hormones. 2004;3:9–26. doi: 10.14310/horm.2002.11109. [DOI] [PubMed] [Google Scholar]
- e40.Letawe C, Boone M, Pierard GE. Digital image analysis of the effect of topically applied linoleic acid on acne microcomedones. Clin Exp Dermatol. 1998;23:56–58. doi: 10.1046/j.1365-2230.1998.00315.x. [DOI] [PubMed] [Google Scholar]
- e41.Melnik BC. Diet in acne: further evidence for the role of nutrient signalling in acne pathogenesis. Acta Derm Venereol. 2012;92:228–231. doi: 10.2340/00015555-1358. [DOI] [PubMed] [Google Scholar]
- e42.Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584–1590. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- e43.Smith RN, Mann NJ, Braue A, Mäkeläinen H, Varigos GA. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107–115. doi: 10.1093/ajcn/86.1.107. [DOI] [PubMed] [Google Scholar]
- e44.Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787–793. doi: 10.1016/j.jaad.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e45.Firooz A, Sarhangnejad R, Davoudi SM, Nassiri-Kashani M. Acne and smoking: is there a relationship? BMC Dermatol. 2005;5 doi: 10.1186/1471-5945-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e46.Klaz I, Kochba I, Shohat T, Zarka S, Brenner S. Severe acne vulgaris and tobacco smoking in young men. J Invest Dermatol. 2006;126:1749–1752. doi: 10.1038/sj.jid.5700326. [DOI] [PubMed] [Google Scholar]
- e47.Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e48.Pivarcsi A, Bodai L, Rethi B, et al. Expression and function of Toll-like receptors 2 and 4 in human keratinocytes. Int Immunol. 2003;15:721–730. doi: 10.1093/intimm/dxg068. [DOI] [PubMed] [Google Scholar]
- e49.Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemény L. Distinct strains of Propionibacterium acnes induces selective humanβ-defensin-2 and interleukin-8 expression in human keratinocytes through Toll-like receptors. J Invest Dermatol. 2005;124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- e50.Alexeyev O, Lundskog B, Ganceviciene R, et al. Pattern of tissue invasion by Propionibacterium acnes in acne vulgaris. J Derm Sci. 2012;67:63–66. doi: 10.1016/j.jdermsci.2012.03.004. [DOI] [PubMed] [Google Scholar]
- e51.Jahns AC, Lundskog B, Ganceviciene R, et al. An increased incidence of Propionibacterium acnes biofilms in acne vulgaris: a case control study. Br J Dermatol. 2012;167:50–58. doi: 10.1111/j.1365-2133.2012.10897.x. [DOI] [PubMed] [Google Scholar]
- e52.Stüttgen G. Zur Lokalbehandlung von Keratosen mit Vitamin A-Säure. Dermatologica. 1962;124:65–80. [PubMed] [Google Scholar]
- e53.Schumacher A, Stüttgen G. Vitamin-A-Säure bei Hyerkeratosen, epithelialen Tumoren und Akne. Deutsch Med Wschr. 1971;96:1547–1551. doi: 10.1055/s-0028-1110177. [DOI] [PubMed] [Google Scholar]
- e54.Peck GL, Olsen TG, Yoder FW, et al. Prolonged remissions of cystic and conglobate acne with 13-cis retinoic acid. N Engl J Med. 1979;300:329–333. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- e55.Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–265. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- e56.Zouboulis CC. The truth behind this undeniable efficacy—recurrence rates and relapse risk factors of acne treatment with oral isotretinoin. Dermatology. 2006;212:99–100. doi: 10.1159/000090646. [DOI] [PubMed] [Google Scholar]
- e57.Orfanos CE, Zouboulis CC. Oral retinoids in the treatment of seborrhoea and acne. Dermatology. 1998;196:140–147. doi: 10.1159/000017848. [DOI] [PubMed] [Google Scholar]
- e58.Zouboulis CC, Piquero-Martin J. Update and future of systemic acne treatment. Dermatology. 2003;206:37–53. doi: 10.1159/000067821. e74. [DOI] [PubMed] [Google Scholar]
- e59.Goldstein JA, Comite H, Mescon H, Pochi PE. Isotretinoin in the treatment of acne: histologic changes, sebum production, and clinical observations. Arch Dermatol. 1982;118:555–558. [PubMed] [Google Scholar]
- e60.Jones DH, King K, Miller AJ, Cunliffe WJ. A dose-response study of 13-cis-retinoic acid in acne vulgaris. Br J Dermatol. 1983;1083:33–43. doi: 10.1111/j.1365-2133.1983.tb03973.x. [DOI] [PubMed] [Google Scholar]
- e61.Hennes R, Mack A, Schell H, Vogt HJ. 13-cis-retinoic acid in conglobate acne. A follow-up study of 14 trial centers. Arch Dermatol Res. 1984;276:209–215. doi: 10.1007/BF00414230. [DOI] [PubMed] [Google Scholar]
- e62.Harms M, Masouye I, Radeff B. The relapses of cystic acne after isotretinoin treatment are age-related: a long-term follow-up study. Dermatologica. 1986;172:148–153. doi: 10.1159/000249320. [DOI] [PubMed] [Google Scholar]
- e63.Cunliffe WJ, Norris JF. Isotretinoin - an explanation for its long-term benefit. Dermatologica. 1987;175(suppl 1):133–137. doi: 10.1159/000248869. [DOI] [PubMed] [Google Scholar]
- e64.Chivot M, Midoun H. Isotretinoin and acne—a study of relapses. Dermatologica. 1990;180:240–243. doi: 10.1159/000248038. [DOI] [PubMed] [Google Scholar]
- e65.Layton AM, Knaggs H, Taylor J, Cunliffe WJ. Isotretinoin for acne vulgaris—10 years later: A safe and successful treatment. Br J Dermatol. 1993;129:292–296. doi: 10.1111/j.1365-2133.1993.tb11849.x. [DOI] [PubMed] [Google Scholar]
- e66.Lehucher-Ceyrac D, Weber-Buisset MJ. Isotretinoin and acne in practice: a prospective analysis of 188 cases over 9 years. Dermatology. 1993;186:123–128. doi: 10.1159/000247322. [DOI] [PubMed] [Google Scholar]
- e67.Lehucher-Ceyrac D, de La Salmoniere P, Chastang C, Morel P. Predictive factors for failure of isotretinoin treatment in acne patients: results from a cohort of 237 patients. Dermatology. 1999;198:278–283. doi: 10.1159/000018130. [DOI] [PubMed] [Google Scholar]
- e68.Shahidullah M, Tham SN, Goh CL. Isotretinoin therapy in acne vulgaris: a 10-year retrospective study in Singapore. Int J Dermatol. 1994;33:60–63. doi: 10.1111/j.1365-4362.1994.tb01500.x. [DOI] [PubMed] [Google Scholar]
- e69.Goulden V, Clark SM, McGeown C, Cunliffe WJ. Treatment of acne with intermittent isotretinoin. Br J Dermatol. 1997;137:106–108. [PubMed] [Google Scholar]
- e70.Quéreux G, Volteau C, N’Guyen JM, Dréno B. Prospective study of risk factors of relapse after treatment of acne with oral isotretinoin. Dermatology. 2006;212:168–176. doi: 10.1159/000090658. [DOI] [PubMed] [Google Scholar]
- e71.Karadag AS, Bilgili SG, Selvi Y, et al. Effects of isotretinoin treatment on general psychiatric symptoms, quality of life and social phobia in acne vulgaris patients. J Eur Acad Dermatol Venereol. 2013;27:260–261. doi: 10.1111/j.1468-3083.2011.04439.x. [DOI] [PubMed] [Google Scholar]
- e72.Rubinow DR, Peck GL, Squillace KM, Gantt GG. Reduced anxiety and depression in cystic acne patients after successful treatment with oral isotretinoin. J Am Acad Dermatol. 1987;17:25–32. doi: 10.1016/s0190-9622(87)70166-2. [DOI] [PubMed] [Google Scholar]
- e73.Bernstein CN, Nugent Z, Longobardi T, Blanchard JF. Isotretinoin is not associated with inflammatory bowel disease: a population-based case-control study. Am J Gastroenterol. 2009;104:2774–2778. doi: 10.1038/ajg.2009.417. [DOI] [PubMed] [Google Scholar]
- e74.Zouboulis CC. Acne: Current aspects on pathology and treatment. Dermatol Experiences. 1999;1:6–37. [Google Scholar]
- e75.Crockett SD, Porter CQ, Martin CF, et al. Isotretinoin use and the risk of inflammatory bowel disease: a case-control study. Am J Gastroenterol. 2010;105:1986–1993. doi: 10.1038/ajg.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]