Abstract
Background
Theophylline is often used to treat chronic obstructive pulmonary disease (COPD). Current evidence leaves the effectiveness and safety of this drug open to question. Thus, we evaluated the effectiveness of theophylline on the rate of hospitalizations and disease exacerbations by examining routine data from the ambulatory disease management program for COPD in the German state of Bavaria.
Methods
Data sets from a total of 30 330 patients were examined. Logistic regression models were used to calculate propensity scores that controlled for baseline characteristics. These propensity scores, in turn, were used to create comparable patient groups, which were observed for a median follow-up time of 9 quarters (the theophylline group) and 10 quarters (the control group).
Results
1496 patients with first prescription of theophylline were matched with 1496 patients with no record of theophylline treatment. 1. The probability of suffering an exacerbation during the period of observation, was 33.5% for the control group and 43.4% for the theophylline group [hazard ratio (HR) 1.41; 95% confidence interval (CI) 1.24 to 1.60], yielding a number needed to harm (NNH) of 11 (95% CI 7.7 to 20.9). The probability for hospitalization was 11.4% for the control group and 17.4% of the theophylline group (HR 1.61; 95% CI 1.29 to 2.01), yielding a NNH of 17 (95%CI 11.0–34.5).
Conclusion
Treatment with theophylline is associated with an elevated incidence of exacerbations and hospitalizations. The therapeutic value of this drug should be reconsidered and investigated in further studies.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of death worldwide and has major economic and social impact (1). The prevalence of COPD at GOLD stage II or higher (GOLD, Global Initiative for Chronic Obstructive Lung Disease) is estimated at more than 10% (2). Exacerbation of COPD has been demonstrated to go hand in hand with rapid deterioration of health (3).
The currently valid guidelines recommend beta-2 agonists and anticholinergics for the primary treatment of COPD, with inhaled corticosteroids as a possibility for patients in later stages of the disease (4).
From the group of bronchodilatory substances, methylxanthines represent a further option for the treatment of COPD. The most commonly used derivative is theophylline. Although theophylline is still relatively often prescribed, the most recently issued guidelines recommend it only as a third-line treatment. The reasons given are (a) that more effective bronchodilators are available (4) and (b) that theophylline has a very narrow therapeutic spectrum but carries a high risk of adverse effects such as headache, nausea, generalized tonic–clonic seizures; and cardiac arrhythmia (4– 6). Two reviews showed a moderately positive influence of theophylline on forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) (7, 8), but these effects were accompanied by an elevated risk of nausea (8).
The potential interactions of theophylline with other active substances render it problematic in the treatment of patients with COPD (9). The risk is particularly great in the case of outpatient care, where it is far more difficult to organize regular monitoring of the serum concentration of theophylline and treatment of any adverse effects that occur. Roberts et al. showed that for patients with an acute exacerbation of COPD, the presence of five or more medications at the time of hospital admission can be viewed as a predictor of readmission in the future (10). However, there is little information on the effect of theophylline with regard to exacerbations and hospitalizations.
A systematic review failed to find any positive impact of theophylline in the treatment of acute exacerbations of COPD—no bronchodilatory effect was detected, and at the same time adverse effects increased (11). In a large placebo-controlled study, Niewoehner et al. showed that initial treatment with theophylline constituted a risk factor for further exacerbations (12). A German primary care study demonstrated that asthma patients who received theophylline had to be admitted to hospital more frequently than those who did not (13). However, this was a secondary outcome of a study on quality improvement in the treatment of asthma, so a random effect cannot be absolutely excluded. Therefore, it needs to be established whether patients actually profit from treatment with theophylline or whether, in the outpatient scenario, the benefits of the drug are counterbalanced by the adverse effects.
Using data from a group of COPD patients registered in a Bavarian disease management program (DMP) who were treated on an outpatient basis, we investigated the connection between theophylline and the risk of exacerbations and emergency hospital treatment. According to a recently published expert report from the Institute for Quality and Efficiency in Health Care (IQWiG), the recommendations of the current international guidelines essentially conform to the requirements of the DMP for COPD (14).
Methods
Study population and design
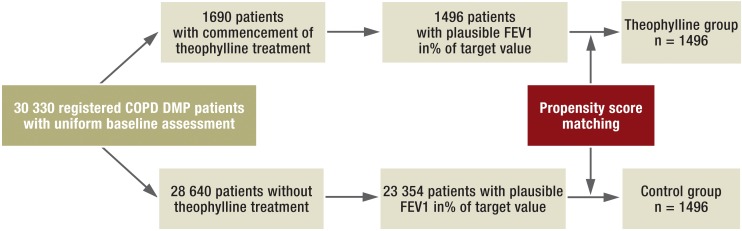
We analyzed anonymous patient data that had been collected in the framework of the DMP for COPD under the aegis of the National Association of Statutory Health Insurance Physicians of Bavaria. A precondition for registration of a patient in the COPD DMP was establishment and documentation of the diagnosis according to standardized criteria. Figure 1 shows the study design. The potential study population comprised 30 330 patients who were registered in the DMP for COPD between July 2006 and the beginning of the observation period (from January 2009). Two separate groups of patients were formed retrospectively: a theophylline group and a control group. For inclusion in the theophylline group, the first prescription of theophylline had to follow a period of at least 6 months during which no treatment with theophylline was documented. A total of 1690 patients fulfilled this criterion. The potential control group consisted of all patients who received no theophylline at any time during their participation in the DMP. Thus 28 640 patients were identified who could be considered as potential control group participants. Because theophylline is used most frequently in severe COPD, FEV1 (in% of target value, [15]) is rated the most important confounding factor, affecting the propensity score as well as the occurrence of exacerbations and hospitalization.
Figure 1.
Study design. FEV1, forced expiratory volume in 1 second; DMP, disease management program; COPD, chronic obstructive pulmonary disease
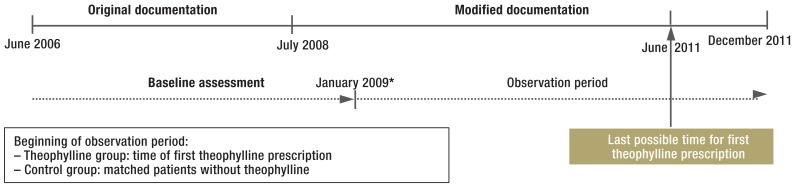
Patients with absent or implausible data on height, age, sex, and FEV1 were excluded from analysis. Following data cleansing, 1496 patients were assigned to the theophylline group and 23 354 patients to the potential control group. The timeline of the study is shown in Figure 2.
Figure 2.
Timeline of the study
*Observation only possible from January 2009, to ensure uniform and complete baseline assessment
Matching
We assume that the severity of the disease has a causal effect both on the endpoints and on the prescription of theophylline. Failure to take account of this dependency would lead to spurious associations. We therefore used Rosenbaum and Rubin’s propensity score method to enable meaningful conclusions regarding the action of theophylline.
In this scenario, the propensity score expresses the probability that a patient has received treatment with theophylline, in light of all relevant variables (16). Under perfect conditions such a quasi-experiment can be considered equivalent to a randomized controlled trial (RCT). In practice, however, such equivalence with observational data can only be approximated. Nevertheless, the danger of a distorted result is greatly reduced by targeted modeling and verification of the causal assumptions. For this reason the propensity score method is being used increasingly often in medicine and other fields of science (17).
The propensity scores are calculated by performing logistic regression on the baseline variables from the assessment phase. The following variables were included:
Sex
Age
Body mass index
FEV1
Smoking
-
Medication
Short-acting beta-2 agonists or anticholinergics
Long-acting beta-2 agonists
Long-acting anticholinergics
Inhaled corticosteroids
Systemic corticosteroids
Other COPD-specific medications.
Furthermore, the status of the coordinating physician (primary care physician or pulmonologist) was considered.
The endpoint variables (numbers of hospitalizations and exacerbations) documented during the 9-month assessment phase were also taken into account during matching. Differences between the groups were analyzed on the basis of the respective absolute standardized differences. A difference of less than 10% was considered inconsequential (18– 20). The methods and the underlying quality of observation are described in detail in the eBox.
eBox. Detailed description of method and follow-up quality.
Methods
Study population and design
We analyzed anonymized patient data that had been collected in the framework of the disease management program (DMP) for COPD of the National Association of Statutory Health Insurance Physicians of Bavaria. The DMP for COPD encourages participating patients to visit their family physician (pulmonologist) every 3 months (6 months) so that their disease status can be better monitored and an individualized treatment plan drawn up and modified if necessary. At each of these consultations the physician completes a standardized documentation sheet that records disease-specific medications, relevant events such as exacerbations or emergency inpatient treatment, patient training, FEV1, and other disease- and treatment related details. These data were originally collected for purposes of quality assurance and evaluation of the participating physicians. The participating physicians receive financial compensation for every documentation sheet handed in. However, collection of the data is not coupled with a pay-for-performance system, so no sanctions are implied in the case of poor treatment or documentation quality. The standardized documentation sheet was revised in 2008, resulting in a certain discontinuity of particular variables. The study design therefore had to be selected such that the study questions rested on a uniform basis. For a patient to be registered in the DMP, the diagnosis has to be confirmed and documented according to the following criteria: apart from a typical COPD history and FEV1 less than 80% of the target value, at least one of the following three criteria must be met (German National Care Guideline for COPD, long version, version 1.9 January 2012; www.copd.versorgungsleitlinien.de):
Demonstration of obstruction and reversibility with beta-2 agonists or anticholinergics and demonstration of FEV1/VC ≤ 70% and an increase in FEV1 of less than 15% and/or less than 200 mL
Demonstration of obstruction and reversibility with glucocorticosteroids (oral 14 days, inhaled 28 days) in a stable phase of disease and demonstration of FEV1/VC ≤ 70% and an increase in FEV1 of less than 15% and/or less than 200 mL
If FEV1/VC < 70% with radiologic exclusion of other disease: diagnosis confirmed by elevated airway resistance or lung hyperinflation or gas exchange disorder.
These diagnostic criteria were recently assessed by the Institute for Quality and Efficiency in Health Care (IQWiG) as essentially concordant with the recommendations of currently valid international guidelines (14).
Because of the revision of the documentation sheet in July 2008, only patients registered in the DMP before July 2008 could be included in our study. This ensured that important covariables which were included only in the first version of the documentation sheet were available for the subsequent matching process. To produce a data set comparable with the theophylline group, the time between registration and the beginning of the observation period was established with the aid of randomized assignment according to the theophylline group.
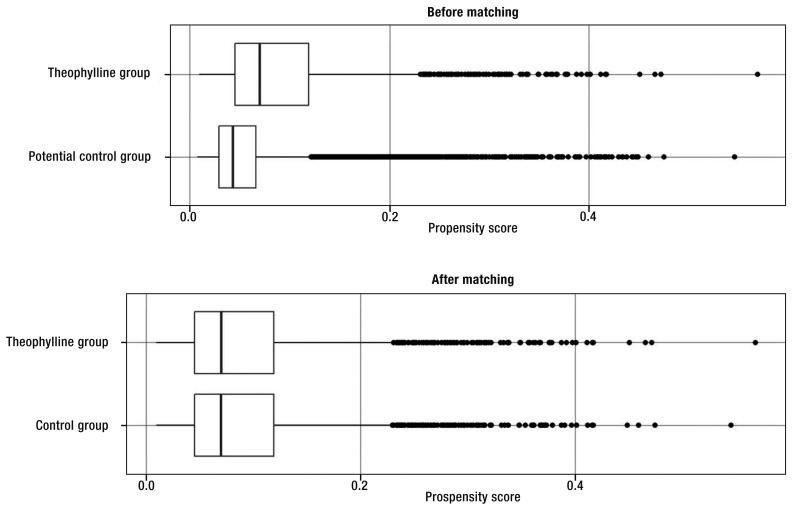
The endpoint variables (number of hospitalizations and exacerbations) documented within the 9-month assessment phase were also taken into account during matching, because the occurrence of a severe exacerbation increases the risk of a renewed exacerbation and hospitalization (3). In addition the following characteristics were considered, provided they were documented within the assessment phase: comorbidities that also cause dyspnea (bronchial asthma, other lung diseases, cardiovascular diseases), participation in training for COPD patients, oxygen therapy, and noninvasive ventilation. The year of diagnosis and the method of ascertaining the diagnosis were taken from the registration documentation. The c-statistic of the underlying logistic regression model was 0.70; the distributions of the propensity scores before and after matching are shown in the eFigure. The success of the procedure, however, was measured primarily on the basis of the standardized differences, because the aim of the modeling was not to optimize prediction of group affiliation (18) but to increase the comparability of the groups with regard to the endpoints. Owing to the high number of potential control patients, the matching took place without replacement (i.e., no patients were used twice in the course of the matching process) to find a suitable proband from the control group for each member of the theophylline group. Allowance was made for possible biases after calculation and comparison of the respective standardized absolute differences, with a difference of less than 10% rated as insignificant (18, 19).
Follow-up quality
One potential source of error is the uncertainty of the follow-up documentation in the framework of routine care. A central component of the DMP is the quarter- or half-yearly visit including standardized documentation. Reminder systems are implemented to help make sure these regular consultations take place. Participation in the program is voluntary, however, and the patients can leave the program at any time without giving reasons. It is therefore highly important at the outset to ensure that the quality of the data collected permits sufficiently stable and undistorted results. The eTable classifies the patients according to the completeness of the observed data. The observation was viewed as complete when documentation had taken place at least once every 6 months. Patients for whom the most recent documentation was more than 6 months before the end of the observation period were defined as drop-outs. The drop-outs were right-censored and the documentation from the period before their withdrawal was included in the analysis. A further group of patients comprised those with gaps in documentation of more than 6 months during the observation period, e.g., because of temporary withdrawal. As shown in the eTable, complete documentation was available for the majority of patients (66 to 69%). Around a fifth of the patients dropped out prematurely, and about 10% had documentation gaps of more than 6 months during the observation period. The proportion of drop-outs was circa 2% higher in the theophylline group than in the control group. This difference turned out not to be significant, however (chi-square test, p = 0.2), and cannot explain the differences between the groups of circa 7% for emergency inpatient events and circa 10% for exacerbations (figure 4).
Follow-up of theophylline and control groups
The participants in the theophylline and control groups were retrospectively observed until June 2012. The individual observation time varied, because the beginning of the observation period was determined by the first prescription of theophylline (theophylline group) or a corresponding selected time point (control group) to match the groups.
The documentation of the participants over the whole observation period was checked for regularity and completeness. The time to the first exacerbation of COPD or the first COPD-related hospital admission was ascertained. The generated data were analyzed for both endpoints by means of Kaplan–Meier curves (21) and Cox regression models (22). The Kaplan–Meier curves provide a nonparametric visual summary of the results, while the regression models yield the hazard ratio (HR) for the effect of theophylline, adjusted for the matching variables (23). The Kaplan–Meier method enables estimation of the proportion of participants who display no events within the observation period. In contrast, the number needed to harm (NNH) is defined as the inverse of the proportional difference of the groups. This value can be interpreted as the mean number of patients who have to be treated with theophylline in order to observe a theophylline-induced event in the course of the observation period (24). The NNH was calculated to improve comprehension of the meaning of the results. However, the 3.5-year observation period must always be borne in mind when interpreting this value. Statistical analysis was carried out by means of the statistics software R with the additional packages “matching” and “survival” (25– 27).
Results
Baseline characteristics
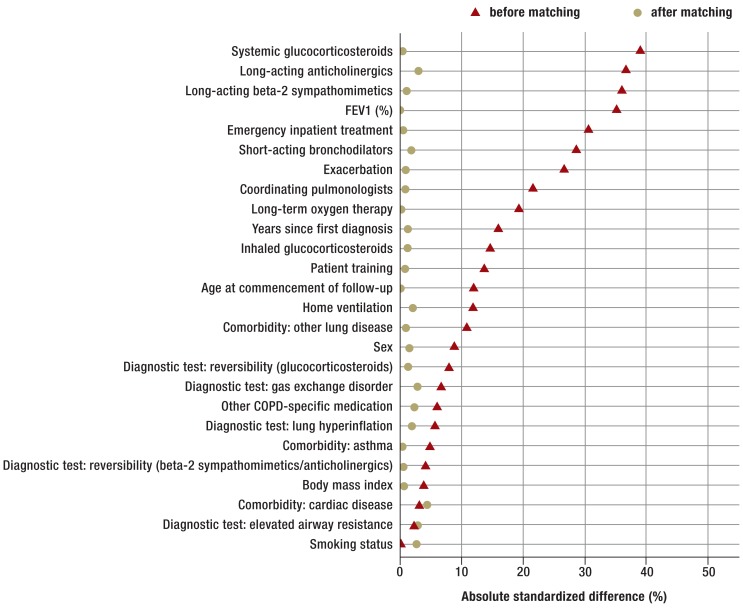
Comparable patients from the potential control group were matched to the 1496 patients of the theophylline group on the basis of their baseline characteristics. Table 1 and Figure 3 show the distribution of the matching variables in both groups before and after matching.
Table. Baseline characteristics before and after matching.
| Theophylline group | (Potential) control group | ||||
|---|---|---|---|---|---|
| After matching | Absolute STD (%) | Before matching | Absolute STD (%) | ||
| Number of patients (n) | 1496 | 1496 | – | 23354 | – |
| Sex: male (%) | 58 | 59 | 1.6 | 54 | 8.8 |
| Age (mean and SD) | 68 (10.6) | 68 (10.5) | 0.1 | 67 (11.5) | 12.0 |
| BMI (mean and SD) | 28 (5.6) | 28 (5.5) | 0.7 | 28 (5.3) | 3.8 |
| FEV1 in % of target value (mean and SD) | 65 (28.3) | 65 (25.7) | 0.3 | 74 (27.0) | 35.2 |
| Smokers (%) | 24.5 | 25.7 | 0.1 | 24.5 | 1.2 |
| Coordinating pulmonologist (%) | 11.9 | 11.6 | 0.8 | 5.8 | 21.6 |
| Emergency inpatient treatment in preceding 9 months (%) | 11.7 | 11.9 | 0.6 | 3.7 | 30.6 |
| Exacerbations in preceding 9 months (%) | 69.7 | 69.3 | 0.9 | 56.9 | 26.6 |
| Medication | |||||
| Short-acting bronchodilators (%) | 68.9 | 69.8 | 2.0 | 55.1 | 28.5 |
| Long-acting beta-2 sympathomimetics (%) | 71.1 | 70.5 | 1.2 | 53.9 | 36.0 |
| Long-acting anticholinergics (%) | 50.8 | 49.3 | 2.9 | 33.0 | 36.6 |
| Inhaled corticosteroids (%) | 44.1 | 44.8 | 1.3 | 36.9 | 14.7 |
| Other COPD-specific medication (%) | 5.0 | 5.5 | 2.4 | 3.8 | 6.1 |
| Oral corticosteroids | 18.4 | 18.2 | 0.5 | 5.9 | 39.0 |
| Other comorbidity causing dyspnea | |||||
| Bronchial asthma (%) | 11.0 | 10.9 | 0.4 | 9.6 | 4.8 |
| Other lung diseases (%) | 13.4 | 13.7 | 1.0 | 9.9 | 10.8 |
| Cardiac disease (%) | 30.3 | 28.3 | 4.4 | 28.8 | 3.2 |
| Structured training of COPD patients (%) | 19.7 | 19.3 | 0.8 | 14.5 | 13.7 |
| Long-term oxygen therapy (%) | 7.6 | 7.5 | 0.3 | 3.2 | 19.2 |
| Home ventilation (%) | 3.4 | 3.8 | 2.2 | 1.6 | 11.9 |
| Time to diagnosis in years (SD) | 10.2 (6.9) | 10.3 (7.1) | 1.3 | 9.1 (6.6) | 15.9 |
| Diagnostic test | |||||
| Reversibility with beta-2 sympathomimetics or anticholinergics*1, *2 (%) | 71.6 | 7 1.3 | 0.6 | 69.7 | 4.1 |
| Reversibility with glucocorticoids*1, *2 (%) | 13.3 | 12.8 | 1.4 | 10.7 | 7.9 |
| Elevated airway resistance *1, *3 (%) | 29.4 | 30.5 | 2.9 | 28.1 | 2.4 |
| Lung hyperinflation *1, *3 (%) | 21.2 | 22.0 | 1.9 | 18.9 | 5.7 |
| Gas exchange disorder *1, *3 (%) | 8.7 | 7.9 | 2.9 | 6.9 | 6.7 |
*1COPD-typical history and FEV1 < 80%; *2FEV1/VC ≤ 70% and increase in FEV1 < 15% and/or < 200 mL; *3if FEV1/VC > 70%
COPD, chronic obstructive pulmonary disease; SD, standard deviation; absolute STD, absolute standardized differences; FEV1, forced expiratory volume in 1 second; VC, vital capacity
Figure 3.
Absolute standardized differences of all covariables before and after matching between the theophylline and control groups. FEV1, forced expiratory volume in 1 second; COPD, chronic obstructive pulmonary disease
Table 1 shows that patients with a prescription of theophylline already tended to suffer from advanced COPD. Moreover, most covariables differed strikingly between the groups (Figure 3), the greatest differences being seen for the parameters medication, FEV1, exacerbations, and emergency admissions.
Medication with corticosteroids was surprisingly high in the theophylline group, where inhaled corticosteroids were prescribed to 44% and oral corticosteroids to 18% of the patients. The average FEV1 was far higher in the potential control group than in the theophylline group, which could indicate a higher proportion of healthy patients in the control group. After matching, all 26 baseline characteristics were comparable in the two groups. The absolute standardized differences were reduced to less than 10%, so the observed confounders can be regarded as adequately cleansed (18, 19).
Principal endpoints
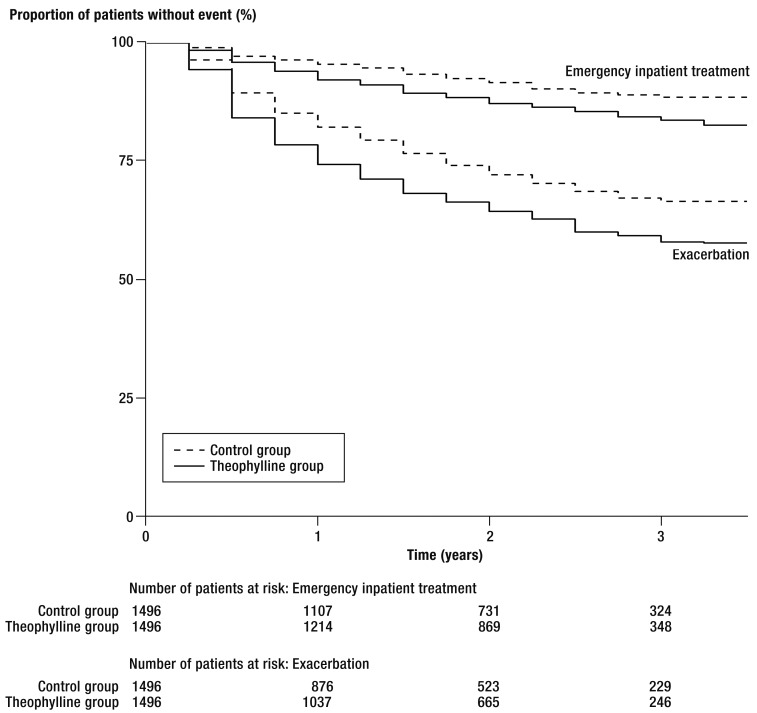
The time to occurrence of the first exacerbation or hospitalization is depicted by the Kaplan–Meier curves in Figure 4. These endpoints were observed over a maximal period of 3.5 years. The median observation period was 9 quarters in the theophylline group and 10 quarters in the control group. For both endpoints, a significantly higher risk was identified in the members of the theophylline group.
Figure 4.
Kaplan–Meier curves for exacerbations and emergency hospital admissions
The Cox regression models revealed a HR of 1.41 (95% confidence interval [CI] 1.24 to 1.60) for time to first exacerbation and 1.61 (95% CI 1.29 to 2.01) for time to first hospitalization. Kaplan–Meier estimation showed a probability of exacerbation of 33.5% (95% CI 30.7 to 36.3) for the control group and 42.4% (95% CI 39.2 to 45.3) for the theophylline group. This yielded a NNH of 11 (95% CI 7.7 to 20.9) for an exacerbation within the 42-month observation period. For hospitalization the probability was 11.4% (95% CI 9.5 to 13.3) in the control group and 17.4% (95% CI 14.9 to 19.8) in the theophylline group, resulting in a NNH of 17 (95% CI 11.0 to 34.5).
Discussion
Our analysis suggests that theophylline should be regarded as problematic in the treatment of patients with COPD. Although the observed effects are moderate, the size of the study population renders the results robust and clinically significant, with NNH of 11 (HR 1.41) for exacerbations and 17 (HR 1.61) for hospitalizations.
The current guidelines regard theophylline as less effective and less well tolerated even in comparison with long-acting bronchodilators. It should thus be recommended only if “other long-term treatment bronchodilators are unavailable or unaffordable” (4). Nevertheless, there is no clear consensus regarding the role of theophylline in the outpatient treatment of patients with COPD. Evaluating data on a large cohort of 36 492 COPD patients from the databases of the health care authority of the province of Quebec in Canada, Cyr et al. found that over an observation period of 7 years patients treated with theophylline showed a lower likelihood of exacerbations than patients who received long-acting beta-2 sympathomimetics (rate ratio 0.89, 95% CI 0.84 to 0.95) (28). However, the findings of Cyr and colleagues differ considerably from those in the present study, in which we used a causally oriented method in the attempt to differentiate the effects of theophylline and confounders.
In a systematic review, Ram et al. reported no significant difference between the placebo group and the theophylline group in two studies investigating the occurrence of acute exacerbations during treatment with theophylline (8). However, the total number of patients (n = 244) may have been too small, or the short observation period of only 2 to 4 weeks may have been responsible for the lack of significance of the differences between the groups.
Rossi et al. showed that salmeterol was more effective than theophylline in the treatment of COPD as assessed by FEV1. Furthermore, adverse effects were more frequent in patients who received theophylline (29). ZuWallack and colleagues also found far more adverse effects with theophylline than with salmeterol (30). In comparison with the HR of 1.41 for exacerbations in the present study, Niewoehner et al., in a multicenter secondary analysis, found that treatment with theophylline represented a risk factor for exacerbations, with a HR of 1.26 (95% CI 1.02 to 1.57).
Limitations
One major limitation of our study is that the data were derived exclusively from standardized reports by physicians. There was no systematic external control of diagnoses or validation of the medical documentation. Moreover, the data contained no details of the precise time of drug intake or of dosage.
With regard to the spirometric parameters, only a pathologic Tiffeneau ratio and the FEV1 in liters were recorded. This permits calculation of FEV1 as % of the predicted value for sex and age.
These limitations mean that the data quality of the DMP documentation is not comparable with a clinical trial carried out under optimal circumstances.
The data from the DMP for COPD, though standardized, were not generated exclusively for medical research purposes. This has advantages and disadvantages. However, our analysis confirms that the reality of primary care is reflected more accurately than would be possible in a specific survey of relevant details for study purposes.
Some patients were registered in the DMP despite FEV1 ratings of more than 80%. This cannot be fully explained by the additional examinations or other data recorded—such as total airway resistance, hyperinflation of the lungs (determined by whole-body plethysmography) or reduced gas exchange. It is therefore possible that despite their inclusion in the DMP for COPD, some of these patients could have suffered from bronchial asthma. However, FEV1 was one of the parameters taken into account in propensity score matching.
There was a tendency for mainly patients with advanced disease to be compared (Table). The causal interpretation of routine data from observational studies is limited by the lack of randomization and clinical trial planning. As a consequence, even when a regression model takes all potential confounders into account, the effect one wants to measure cannot be identified with sufficient confidence (31– 35). The use of propensity score matching is thus an important strength of our study in that it yields reliable separation of the effects. Despite the known weaknesses, we view the regularly collected data as a sufficiently solid basis for the longitudinal study described here.
Summary
While analysis of large data sets permits generalization to broad primary care, the underlying pharmacotherapeutic effects cannot be clarified conclusively on the basis of the available data and results. To this end, further studies are required to investigate the role of theophylline in primary management, especially with regard to the amount of drug prescribed and consumed.
The true effectiveness of theophylline and its potential adverse effects in comparison with other drugs could only be conclusively assessed in RCTs. It is hard to imagine, however, that ethics committees would approve such studies. Therefore, further broad-based studies of routine data are needed, e.g., the databases of individual health insurance providers, the German Central Institute for Outpatient Care Provision, or national associations of statutory health insurance physicians from other federal states of Germany.
Key Messages.
The prescription of theophylline is associated with an elevated risk of exacerbations.
The prescription of theophylline is associated with an elevated risk of COPD-related hospitalization.
The probability of an exacerbation within 3.5 years was 33.5% for the control group and 43.4% for the theophylline group, resulting in a number needed to harm of 11 (95% CI 7.7 to 20.9).
The probability of hospitalization within 3.5 years was 11.4% in the control group and 17.4% in the theophylline group; resulting in a number needed to harm of 17 (95% CI 11.0 to 34.5).
The therapeutic role of theophylline should be reconsidered, because the potential harm may outweigh the benefit.
eFigure.
Distribution of propensity scores before and after matching
eTable. Completeness of observed cases.
| Theophylline group | Control group | |||
|---|---|---|---|---|
| n | % | n | % | |
| Complete observation | 1025 | 68.5 | 985 | 65.8 |
| Drop-outs | 327 | 21.9 | 355 | 23.7 |
| Incomplete observation | 144 | 9.6 | 156 | 10.4 |
| Total | 1496 | 100.0 | 1496 | 100.0 |
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
Professor Schneider is external assessor for the disease management program COPD in the Federal Joint Committee. He has received honoraria from the National Association of Statutory Health Insurance Physicians of Bavaria for educational lectures in the framework of the disease management program Asthma/COPD.
The remaining authors declare that no conflict of interest exists.
References
- 1.Murray CJL LA. Injuries and risk factors in 1990 and projected to 202. Cambridge: Harvard University Press; 1996. The global burden of disease: A comprehensive assessment of mortality and disability from diseases. [Google Scholar]
- 2.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 3.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for the diagnosis, management and prevention of COPD, 2013. www.goldcopd.org. (last accessed on 11 March 2014)
- 5.Gaudreault P, Guay J. Theophylline poisoning. Pharmacological considerations and clinical management. Medical toxicology. 1986;1:169–191. doi: 10.1007/BF03259836. [DOI] [PubMed] [Google Scholar]
- 6.Barnes PJ. Theophylline: new perspectives for an old drug. American journal of respiratory and critical care medicine. 2003;167:813–818. doi: 10.1164/rccm.200210-1142PP. [DOI] [PubMed] [Google Scholar]
- 7.Molfino NA, Zhang P. A meta-analysis on the efficacy of oral theophylline in patients with stable COPD. International journal of chronic obstructive pulmonary disease. 2006;1:261–266. doi: 10.2147/copd.2006.1.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ram FS, Jardin JR, Atallah A, et al. Efficacy of theophylline in people with stable chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respiratory medicine. 2005;99:135–144. doi: 10.1016/j.rmed.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Antoniou T, Gomes T, Mamdani MM, Juurlink DN. Ciprofloxacin-induced theophylline toxicity: a population-based study. European Journal of Clinical Pharmacology. 2011;67:521–526. doi: 10.1007/s00228-010-0985-0. [DOI] [PubMed] [Google Scholar]
- 10.Roberts CM, Lowe D, Bucknall CE, Ryland I, Kelly Y, Pearson MG. Clinical audit indicators of outcome following admission to hospital with acute exacerbation of chronic obstructive pulmonary disease. Thorax. 2002;57:137–141. doi: 10.1136/thorax.57.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr RG, Rowe BH, Camargo CA., Jr. Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta-analysis of randomised trials. BMJ. 2003;327 doi: 10.1136/bmj.327.7416.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131:20–28. doi: 10.1378/chest.06-1316. [DOI] [PubMed] [Google Scholar]
- 13.Schneider A, Wensing M, Biessecker K, Quinzler R, Kaufmann-Kolle P, Szecsenyi J. Impact of quality circles for improvement of asthma care: results of a randomized controlled trial. Journal of evaluation in clinical practice. 2008;14:185–190. doi: 10.1111/j.1365-2753.2007.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.IQWiG. Systematische Leitlinienrecherche und -bewertung sowie Extraktion neuer und relevanter Empfehlungen für das DMP COPD. www.iqwig.de/download/V12-01_Abschlussbericht_Kurzfassung_Leitlinienrecherche-und-bewertung-fuer-das-DMP-COPD.pdf) (last accessed on 11 March 2014)
- 15.Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. The European respiratory journal Supplement. 1993;16:5–40. [PubMed] [Google Scholar]
- 16.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 17.D’Agostino RB., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. 2nd edition. Hillsdale, New Jersey: Erlbaum; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 22.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society Series B (Methodological) 1972;34:187–220. [Google Scholar]
- 23.Hill J. Discussion of research using propensity-score matching: comments on ’A critical appraisal of propensity-score matching in the medical literature between 1996 and 2003’ by Peter Austin, Statistics in Medicine. Stat Med. 2008;27:2055–2061. doi: 10.1002/sim.3245. discussion 2066-59. [DOI] [PubMed] [Google Scholar]
- 24.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.R Development Core Team R. A language and environment for statistical computing. Vienna, Austria: the R Foundation for Statistical Computing 2013. www.r-project.org. (last accessed on 11 March 2014)
- 26.Jasjeet S Sekhon. Multivariate and propensity score matching software with automated balance Ooptimization: the matching package for R. Journal of Statistical Software. 2011;42:1–52. [Google Scholar]
- 27.Therneau T. A package for survival analysis in S. R package version 2.37-7. www.cran.r-project.org/web/packages/survival/index. (last accessed on 11 March 2014) [Google Scholar]
- 28.Cyr MC, Beauchesne MF, Lemiere C, Blais L. Effect of theophylline on the rate of moderate to severe exacerbations among patients with chronic obstructive pulmonary disease. British journal of clinical pharmacology. 2008;65:40–50. doi: 10.1111/j.1365-2125.2007.02977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossi A, Kristufek P, Levine BE, Thomson MH, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow-release theophylline in the treatment of COPD. Chest. 2002;121:1058–1069. doi: 10.1378/chest.121.4.1058. [DOI] [PubMed] [Google Scholar]
- 30.ZuWallack RL, Mahler DA, Reilly D, et al. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest. 2001;119:1661–1670. doi: 10.1378/chest.119.6.1661. [DOI] [PubMed] [Google Scholar]
- 31.Cochran W, Chambers S. The planning of observational studies of human populations. Journal of the Royal Statistical Society Series A (General) 1965;128:234–266. [Google Scholar]
- 32.Cox D, Wermuth N. Causality: a statistical view. International Statistics Review. 2004;72:285–305. [Google Scholar]
- 33.Pearl J. 2nd edition. New York: Cambridge University Press; 2000. Causality: Models, reasoning, and interference. [Google Scholar]
- 34.Rosenbaum PR. 2nd edition. New York: Springer; 2002. Observational Studies. [Google Scholar]
- 35.Box GEP. Use and abuse of regression. Technometrics. 1966;8:625–629. [Google Scholar]