Abstract
Purpose.
To investigate whether high glucose (HG) alters connexin 43 (Cx43) expression and gap junction intercellular communication (GJIC) activity in retinal Müller cells, and promotes Müller cell and pericyte loss.
Methods.
Retinal Müller cells (rMC-1) and cocultures of rMC-1 and retinal pericytes were grown in normal (N) or HG (30 mM glucose) medium. Additionally, rMC-1 transfected with Cx43 small interfering RNA (siRNA) were grown as cocultures with pericytes, and rMC-1 transfected with Cx43 plasmid were grown in HG. Expression of Cx43 was determined by Western blotting and immunostaining and GJIC was assessed by scrape-loading dye transfer (SLDT) technique. Apoptosis was analyzed by TUNEL or differential staining assay, and Akt activation by assessing Akt phosphorylation.
Results.
In monocultures of rMC-1 and cocultures of rMC-1 and pericytes, Cx43 protein level, number of Cx43 plaques, GJIC, and Akt phosphorylation were significantly reduced in HG medium. Number of TUNEL-positive cells was also significantly increased in rMC-1 monocultures and in rMC-1 and pericyte cocultures grown in HG medium. Importantly, when rMC-1 transfected with Cx43 siRNA were grown as cocultures with pericytes, a significant decrease in GJIC, and increase in TUNEL-positive cells was observed, concomitant with decreased Akt phosphorylation. Upregulation of Cx43 rescued rMC-1 from HG-induced apoptosis.
Conclusions.
Gap junction communication between Müller cells and pericytes is essential for their survival. Downregulation of Cx43 that is HG induced and impairment of GJIC activity in Müller cells contributes to loss of glial and vascular cells associated with the pathogenesis of diabetic retinopathy.
Keywords: connexin 43, gap junctions, high glucose, pericytes, Müller cells
This study examined the importance of cell-cell communication via gap junctions between Müller cells, and Müller cells and pericytes, related to cell survival. Reduced GJIC activity and HG-induced Cx43 downregulation in Müller cells can promote Müller cell and pericyte loss in diabetic retinopathy.
Introduction
Gap junctions are membrane channels1 that allow passage of ions and signaling molecules, less than 1 kD in size, between adjacent cells2–5 and thereby participate in the maintenance of cellular homeostasis.4–12 Connexins after oligomerizing into hexameric assemblies are transported to the plasma membrane where they act as gap junction channels.13 The Cx43 gap junction channels are abundantly expressed in the retina6,14–18 and play an essential role for maintenance of retinal homeostasis. High glucose or diabetes has been shown to reduce Cx43 expression in retinal vascular cells,6,14,19–21 and inhibit gap junction communication.6,14,19,21–23 Our recent studies indicate that inhibition of Cx43-mediated GJIC activity contributes to increased apoptosis in retinal vascular cells.14 However, the effects of HG-induced altered Cx43 expression and GJIC activity on Müller cells, and on the adjacent cells that are in contact with the Müller cell are unclear.
Müller cells are the principal glial cells in the retina that are located in the inner nuclear layer with processes extending in opposite directions, toward the outer limiting membrane and the inner limiting membrane.24–27 Some of the side branches from these processes wrap around retinal blood vessels and come in frequent contact with pericytes, located abluminally on the vessels.28–30 Studies have localized Cx43 gap junctions in retinal Müller cells using antibodies directed against Cx43 proteins,31–33 tracer coupling,31 and electron microscopy.34 The latter studies through electron microscopic images have revealed direct contact between Müller cells and pericytes.34 Müller to Müller cell communication through Cx43 channels has been shown to be necessary for their survival.35 Although studies have identified gap junctions between neurons and Müller cells, astrocytes and Müller cells, and between Müller cells and various other cell types, it is unclear whether gap junction activity is impacted by HG through the connexin channels between Müller cells and vascular cells.
Pericyte loss is a prominent characteristic of diabetic retinopathy; however, mechanisms underlying this abnormality remain unclear. Müller cells provide a functional link between retinal neurons and blood vessels.24 As such, changes induced by HG in Müller cell Cx43 level and GJIC activity may influence pericyte function and survival. Since frequent contacts between Müller cells and pericytes facilitate exchange of cell survival factors through gap junction,36 compromised GJIC activity between Müller cells and pericytes could have a profound effect. Moreover, Müller cells and pericytes are known to contribute to the maintenance of the inner blood retinal barrier (BRB) and play an important role in the maintenance of homeostasis of the retinal microenvironment.28 However, the role of HG-induced altered Cx43 expression in compromising GJIC activity between Müller cells, and between Müller cell and pericyte, and its impact on BRB breakdown is not well understood.37
Despite abundant histological and electrophysiological evidence for gap-junction coupling between vascular cells, and between Müller cells in the retina, the effect of HG on Cx43 expression, gap junction channel activity, and tissue homeostasis are only beginning to be understood in the context of diabetic retinopathy. In this study, we have investigated whether HG alters Cx43 expression and GJIC activity in Müller cells, compromises GJIC activity and induces apoptosis in Müller cells, and whether direct inhibition of Cx43 expression and GJIC activity in Müller cells inhibits Akt signaling and promotes the demise of retinal pericytes.
Methods
Cell Culture
Rat retinal Müller cells (rMC-1) used in this study were previously characterized as Müller cells on the basis of long and slender shape morphology, and expression of cellular retinaldehyde-binding (CRALBP) protein.38 Bovine retinal pericytes were isolated and cultured according to our previously described method.6 Retinal pericytes were characterized and identified on the basis of irregular, stellate morphology, lack of Von Willebrand factor antigen, presence of smooth muscle cell α-actin, and 3G5 pericyte specific antigen. To determine the effect of HG on Cx43 expression, localization, distribution, and GJIC activity, rMC-1 cells, and cocultures of rMC-1 and pericytes were grown for 7 days in N or HG medium or mannitol (30 mM) for osmotic control. Cocultures of rMC-1 and pericytes were grown at a ratio of 1:10 for studies on Cx43 expression, localization, GJIC activity, Akt activation, and TUNEL assay under the above conditions.
Cell Transfection With Cx43 siRNA or Cx43 Plasmid
To determine the effect of reduced Cx43 expression on communication between Müller cells and pericytes, rMC-1 was transfected with Cx43 siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or scramble siRNA (Qiagen, Valencia, CA, USA) as a negative control in the presence of 8 μM transfection reagent (Lipofectin; Invitrogen, Carlsbad, CA, USA). Transfected cells were washed and grown as rMC-1 monoculture and rMC-1/pericytes for 3 days. To confirm the effects of Cx43 downregulation and avoid “off target” effects, three Cx43 siRNAs targeted to different locations of the Cx43 transcript were independently transfected in rMC-1. Connexin 43 siRNA-1 (Santa Cruz Biotechnology), Cx43 siRNA-2 (Ambion, Austin, TX, USA), and Cx43 siRNA-3 (Invitrogen, Grand Island, NY, USA). Note, Cx43 siRNA-1 is referred to as Cx43 siRNA. The efficacy of each of the Cx43 siRNAs in reducing Cx43 expression was tested at different concentrations. In this study, we used 40-nM concentration of Cx43 siRNA to achieve approximately 30% reduction in Cx43 level to mimic the HG-induced Cx43 downregulation.
To determine whether Cx43 upregulation rescues cells from HG-induced apoptosis, rMC-1 were grown for 7 days in HG medium and transfected with plasmid pEGFPN1 containing full-length Cx43 cDNA or empty vector as control using transfection reagent (Lipofectamine 2000; Invitrogen) at a ratio of 1 μL of transfection reagent (Invitrogen) for every 1 μg plasmid DNA. In parallel, cells were grown in normal (N) or HG medium for 7 days to serve as control. Transfected cells were subsequently grown in the presence of G418 (Invitrogen) at 500 μg/mL until stable colonies formed.
Western Blot Analysis
Cells exposed to N or HG medium were washed with PBS and lysed in buffer containing 10 mmol/L Tris, pH 7.5 (Sigma-Aldrich Corp., St. Louis, MO, USA), 1 mmol/L EDTA, and 0.1% Triton X-100 (Sigma-Aldrich Corp.). Protein content in cell lysate was measured by bicinchoninic acid protein assay method (Pierce Chemical Co., Rockford, IL, USA); 20 μg of protein per lane was electrophoresed together with molecular weight standards (Bio-Rad Laboratories, Inc., Hercules, CA, USA) in separate lanes. After electrophoresis, the proteins were transferred onto polyvinylidene fluoride membranes (Millipore Corp., Billerica, MA, USA) according to Towbin's procedure39 using a semidry apparatus. Briefly, the membrane was blocked with 5% nonfat dry milk for 2 hours and incubated overnight at 4°C with rabbit polyclonal Cx43 antibody (1:1000; Cell Signaling, Danvers, MA, USA); rabbit monoclonal phospho-Akt (Ser473) antibody (1:2000; Cell Signaling); and Akt antibody (1:1000; Cell Signaling) solution in Tris-buffered saline containing 0.1% Tween-20 (TTBS) and 5% bovine serum albumin (BSA). The blot was washed with TTBS and then incubated with secondary antibody solution containing anti-rabbit IgG, AP-linked antibody (1:3000, Cell Signaling) for 1 hour. The membrane was washed as above, applied to the chemiluminescent substrate (Immun-Star; Bio-Rad Laboratories, Inc.), and exposed to X-ray film (Fujifilm, Tokyo, Japan). The amount of protein loaded in the gel lanes were confirmed through Ponceau-S staining after transfer, and by β-actin antibody (1:1000; Cell Signaling). To determine Cx43, phospho-Akt, Akt and β-actin protein expression, densitometric analysis of the Western blot signals was performed at nonsaturating exposures and analyzed using ImageJ software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA).
Immunostaining
To study the distribution pattern and relative amounts of Cx43, immunofluorescence staining for Cx43 was performed on rMC-1 cultures, and rMC-1 and pericytes cocultures grown in N or HG medium. Briefly, cells grown to confluency were plated on glass coverslips and fixed in ice-cold methanol, washed in PBS, and treated with 2% BSA to block nonspecific antibody binding. The cells were then incubated with a monoclonal human anti-mouse Cx43 antibody (Millipore Corp.) diluted 1:200 in PBS containing 2% BSA. After PBS wash, the cells were incubated with rhodamine red-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:600 in PBS containing 2% BSA. After PBS wash, cover slips were mounted in medium (Slow-Fade; Molecular Probes, Eugene, OR, USA). The cells were viewed and photographed using a fluorescence microscope (Diaphot; Nikon, Tokyo, Japan). The punctate rhodamine counts were assessed at the site of contact between adjacent cells within a different and random area. Connexin 43 plaque numbers were obtained by counting plaques located on adjacent cell.
Assessment of GJIC Activity
Scrape-loading dye transfer (SLDT) technique6,14,19 was used to assess GJIC activity. Briefly, cells were grown in N or HG medium to confluency on coverslips and rinsed three times with PBS containing 0.01% Ca2+ and 0.01% Mg2+ (Ca2+, Mg2+– PBS). An aliquot of 1.5 mL PBS containing 0.05% Lucifer yellow CH (Molecular Probes) was added to cover the coverslips, and several cuts were made on the monolayer using a razor. The cells were incubated for 5 minutes at room temperature in the dye solution and then rinsed with PBS containing Ca2+, Mg2+. The cells were then fixed with 1 mL of 4% paraformaldehyde and photographed using a fluorescence microscope (Diaphot; Nikon). The dye-coupled cells on either side of the scrape line were counted in at least 10 random areas to evaluate the GJIC activity.
TUNEL
To determine apoptosis in rMC-1 monocultures or rMC-1/pericytes cocultures, TUNEL assay was performed using an in situ apoptosis detection kit (ApopTag; Chemicon, Temecula, CA, USA) according to the manufacturer's instruction. Briefly, cells were fixed in 1% paraformaldehyde in PBS (pH: 7.4) and permeated with precooled mixture of ethanol and acetic acid mixed at 2:1 ratio. After wash with PBS, cells were incubated with equilibrium buffer and then incubated with TdT enzyme in a moist chamber at 37°C for 1 hour. Cells were then washed with PBS and 4′,6-diamidino-2-phenylindole (DAPI). The cells were viewed and images digitally recorded using a fluorescence microscope (Diaphot; Nikon). At least 10 random fields were scored for TUNEL-positive cells.
Differential Staining Assay to Detect Apoptotic Cells
Apoptotic cells were identified using the differential dye staining method,40 which relies on the uptake of fluorescent dyes, acridine orange, and ethidium bromide.41 The condition of the cell membrane integrity and the properties of the DNA binding dyes facilitate the distinction of viable versus early or late stage apoptotic cells.41 Retinal Müller cells grown on coverslips as specified in the experimental conditions were exposed to a dye mixture containing 25 μg/mL ethidium bromide (Cat. No. E-8751; Sigma-Aldrich Corp.) and 25 μg/mL acridine orange (Cat. No. A-6014; Sigma-Aldrich Corp.) for 10 minutes, washed with PBS, fixed and mounted in an antifade kit (SlowFade, Cat. No. S2828; Invitrogen). The cells were then visualized using a DAPI filter in at least 10 random fields, and photographed using a digital camera attached to a fluorescence microscope (Diaphot; Nikon). The number of apoptotic cells per field was expressed as a percentage of the total number of cells in the field.41
Statistical Analysis
Data are expressed as mean ± SD. Comparisons between groups were performed using ANOVA followed by the Student's t -test. A level of P < 0.05 was considered statistically significant.
Results
Effect of HG on Cx43 Expression in Müller Cells and Cocultures of Müller Cells and Retinal Pericytes
To determine the effects of HG on Cx43 protein expression in Müller cells and cocultures of Müller cells and pericytes, Western blot analyses were performed. Connexin 43 protein expression was significantly reduced in both rMC-1 monocultures and in cocultures of rMC-1 and retinal pericytes grown in HG compared with those grown in N medium (66.9 ± 18.7% of N, P < 0.05, n = 4; 64.0 ± 13.2% of N, P < 0.01, n = 4, respectively; Figs. 1A, 1B).
Figure 1.
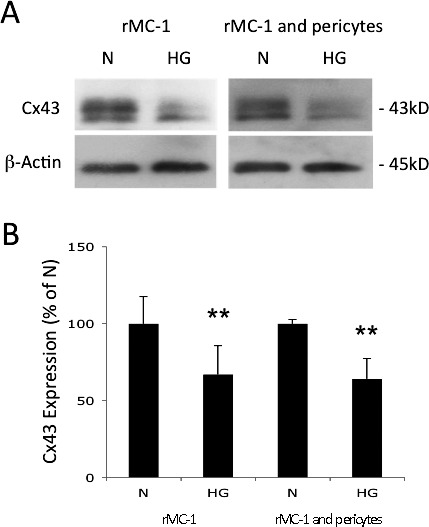
Effect of HG on Cx43 expression in Müller Cells and cocultures of Müller cells and retinal pericytes. (A) Representative Western blot shows HG significantly reduces Cx43 expression in rMC-1, and in rMC-1 and pericyte cocultures. (B) Graphical representation of Western blot data shows Cx43 protein levels were significantly reduced in rMC-1, and in cocultures of rMC-1 and pericytes grown in HG medium compared with those grown in N medium. Data are expressed as mean ± SD. **P < 0.01. n = 4.
Effect of HG on Cx43 Localization and Distribution in Müller Cells and Cocultures of Müller Cells and Retinal Pericytes
The localization and distribution of Cx43 in rMC-1 and in cocultures of rMC-1 and pericytes were determined using Cx43 specific antibodies and immunofluorescence microscopy. Localization of Cx43 was ascertained from punctate “dot-like” plaques at sites of contact between adjacent cells (Fig. 2A). The intensity of immunofluorescence associated with the Cx43 plaques was reduced in rMC-1 monocultures and in cocultures of rMC-1 and pericytes grown in HG medium compared with those in cells grown in N medium. Similarly, the number of Cx43 plaques was decreased in rMC-1 grown in HG medium, and in both cell types, when grown in HG as cocultures (Fig. 2A). Assessment of Cx43 gap junctions at cell-cell contacts, based on counts from plaques within random but defined areas, yielded a score showing significant decrease in the number of Cx43 plaques in rMC-1 grown as monocultures (Fig. 2B) and in rMC-1 and pericytes grown as cocultures (Figs. 2C, 2D; 66.4 ± 6.7% of N, P < 0.01; 61.9 ± 7.7% of N, P < 0.01; 60.8 ± 7.2% of N, P < 0.01, n = 4, respectively).
Figure 2.
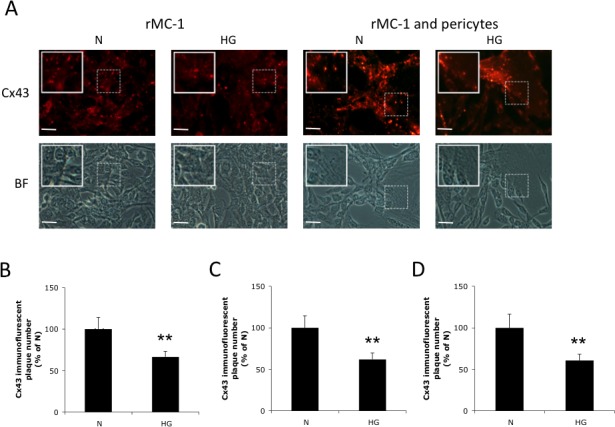
Effect of HG on Cx43 immunostaining in retinal Müller cells and in cocultures of rMC-1 and pericytes. (A) Representative images show significant decrease in the number of Cx43 plaques in rMC-1, and in rMC-1 and pericyte cocultures grown in HG. Scale bars: 50 μm. Graphical illustration of cumulative data showing number of Cx43 plaques was significantly reduced in (B) rMC-1, and in cocultures of (C) rMC-1 and (D) pericytes grown in HG medium compared with those grown in N medium. Insets: Enlarged images of dotted boxed areas showing Cx43 plaques between adjacent cells. Data are expressed as the mean ± SD; **P < 0.01. n = 4. BF, bright field.
Effect of HG on GJIC in Müller Cells and Cocultures of Müller Cells and Retinal Pericytes
To study the effect of HG on cell-cell communication between Müller cells and between Müller cells and pericytes, SLDT assay was performed in rMC-1 monocultures, and in cocultures of rMC-1 and pericytes. A significant decrease in the total number of dye-coupled cells was observed on either side of the scrape line in rMC-1 monocultures and in cocultures of rMC-1 and pericytes grown in HG medium compared with those grown in N medium (3.5 ± 0.2 vs. 2.1 ± 0.4, P < 0.01; 3.4 ± 0.5 vs. 2.1 ± 0.2; P < 0.01, n = 4, respectively; Figs. 3A, 3B).
Figure 3.
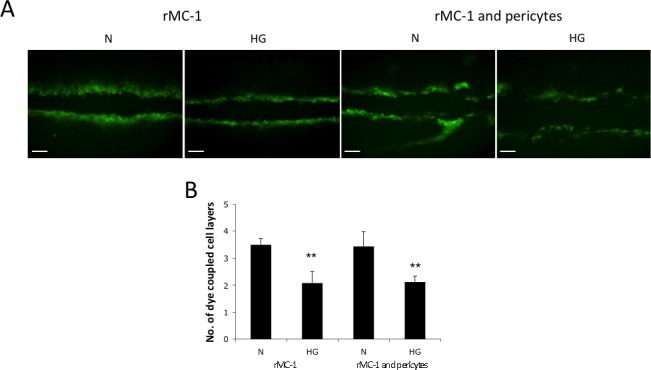
Effect of HG on GJIC activity in Müller cells and cocultures of Müller cells and retinal pericytes. (A) Representative SLDT images show HG significantly reduces the transfer of Lucifer yellow dye between contiguous cells in rMC-1, and between cocultures of rMC-1 and pericytes. Scale bars: 100 μm. (B) Graphical illustration of cumulative data showing GJIC was significantly reduced in rMC-1, and cocultures of rMC-1 and pericytes grown in HG medium compared with those grown in N medium. Data are expressed as the mean ± SD. **P < 0.01. n = 4.
Effects of HG-induced Cx43 Downregulation on Müller Cell and Retinal Pericyte Apoptosis
To determine whether HG-induced Cx43 downregulation contributes to apoptotic cell death, TUNEL assay was performed in monocultures of rMC-1 and cocultures of rMC-1 and pericytes grown in N or HG medium (Figs. 4A, 4B). The TUNEL assay indicated a significant increase in the number of TUNEL-positive cells in rMC-1 monocultures and in cocultures of rMC-1 and pericytes grown in HG compared with those grown in N medium (176.3 ± 23.3% of N, P < 0.01; 186.4 ± 35.2% of N, P < 0.01; respectively, n = 4; Figs. 4C, 4D). The number of TUNEL-positive cells showed no difference between cells exposed to 30 mM mannitol used as osmotic control versus cells grown in N medium.
Figure 4.
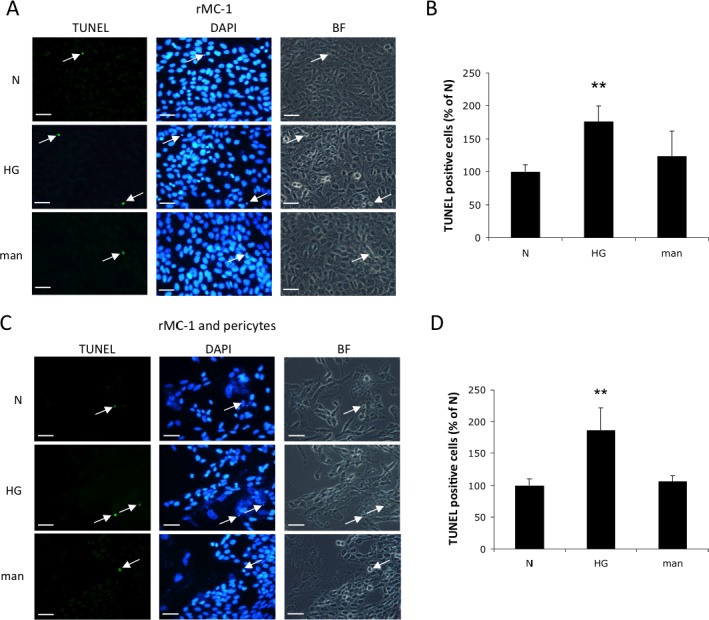
High glucose promotes apoptosis in retinal Müller cells and pericytes. (A) Representative images of TUNEL-positive cells (arrows) in rMC-1. Scale bars: 50 μm. (B) Graphical illustration of cumulative data showing TUNEL-positive cells were significantly increased in rMC-1 grown in HG medium compared with those grown in N or mannitol (man) medium. (C) Representative images of TUNEL-positive cells (arrows) in cocultures of rMC-1 and pericytes. Scale bars: 50 μm. (D) Graphical illustration of cumulative data showing TUNEL-positive cells were significantly increased in cocultures of rMC-1 and pericytes grown in HG medium compared with those grown in N or mannitol medium. Data are expressed as the mean ± SD. **P < 0.01. n = 4.
Cx43 Downregulation Reduces GJIC in Müller Cells and Cocultures of Müller Cells and Retinal Pericytes
To determine the effect of reduced Cx43 protein level on GJIC activity, Cx43 expression was decreased in rMC-1 using Cx43 siRNA, and assessed for the ability of Cx43 siRNA-transfected rMC-1 to transfer Lucifer yellow through gap junction into retinal pericytes. The total number of dye-coupled cells in Cx43 siRNA-transfected rMC-1 was significantly reduced compared with nontransfected rMC-1 (2.0 ± 0.2 vs. 3.5 ± 0.3; P < 0.01, n = 4) or cells transfected with scrambled siRNA or cells exposed to 30 mM mannitol (Fig. 5A). Similarly, in the cocultures, GJIC activity was significantly reduced in cells transfected with Cx43 siRNA compared with nontransfected cells or transfected with scrambled siRNA (2.2 ± 0.2 vs. 2.9 ± 0.5; P < 0.05; Fig. 5B). In the cocultures, GJIC activity between rMC-1 and pericytes were assessed (Fig. 5C). Importantly, in cocultures experiments, although only rMC-1 were transfected with Cx43 siRNA, dye-coupling was significantly reduced between the transfected MC-1 and the nontransfected pericytes compared with cocultures of nontransfected rMC-1 and nontransfected pericytes (2.4 ± 0.2 vs. 3.2 ± 0.3; P < 0.05; Fig. 5C). The GJIC activity in cells transfected with scrambled siRNA and cells exposed to 30 mM mannitol were similar to those of cells grown in N medium.
Figure 5.
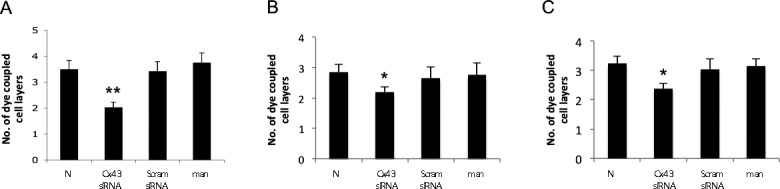
Downregulation of Cx43 reduces GJIC activity in retinal Müller cells and in cocultures of rMC-1 and pericytes. (A) Graphical illustration of cumulative data showing GJIC was significantly reduced in rMC-1 transfected with Cx43 siRNA compared with nontransfected rMC-1, rMC-1 transfected with scrambled (Scram) siRNA or rMC-1 exposed to mannitol. Graphical illustration of cumulative data showing significantly reduced dye-coupling in cocultures of rMC-1 and pericytes. In the cocultures, separate counts were assessed for (B) rMC-1, (C) rMC-1, and pericytes. Data are expressed as the mean ± SD, *P < 0.05. **P < 0.01. n = 4.
Cx43 Downregulation Induces Apoptosis in Müller Cells
To determine whether Cx43 downregulation promotes apoptosis in retinal Müller cells, rMC-1 was transfected with three different Cx43 siRNAs to avoid “off-target” effects, and TUNEL performed. The ability of each of the Cx43 siRNAs to reduce Cx43 protein level was confirmed through transfection and Western blot analyses (Fig. 6) prior to using them to determine whether apoptosis is triggered when Cx43 is downregulated.
Figure 6.
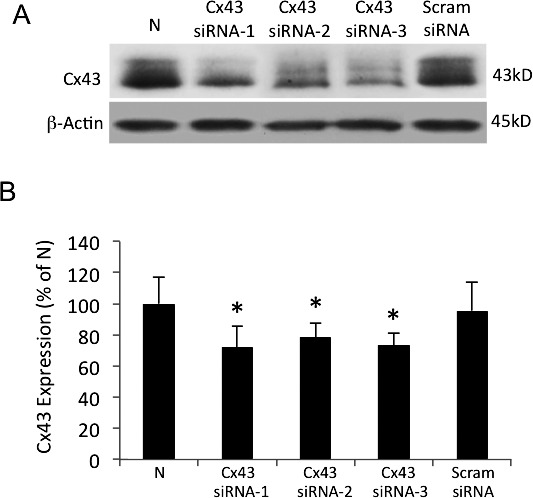
Connexin 43 downregulation using three different Cx43 siRNAs in rMC-1. (A) Representative Western blot shows significantly decreased Cx43 protein level in cells transfected independently with three different Cx43 siRNAs (Cx43 siRNA-1, Cx43 siRNA-2, Cx43 siRNA-3) compared with nontransfected rMC-1 or rMC-1 transfected with Scram siRNA. (B) Graphical illustration of cumulative data showing Cx43 protein levels were significantly reduced in rMC-1 transfected with each of the three Cx43 siRNAs. Data are expressed as mean ± SD. *P < 0.05. n = 5.
In rMC-1 transfected with the Cx43 siRNA, the number of TUNEL-positive cells were significantly increased compared with those of nontransfected rMC-1 or scrambled siRNA-transfected rMC-1 (185.4 ± 10.5% of N, P < 0.01, 181.6 ± 10.3% of scrambled siRNA, P < 0.01; n = 4, respectively; Figs. 7A, 7B). Transfection of rMC-1 with Cx43 siRNA-2 and Cx43 siRNA-3 also showed significant increase in the number of TUNEL-positive cells (220.6 ± 65.8% of N, P < 0.05; 221.4 ± 21.7% of N, P < 0.01; n = 4, respectively).
Figure 7.
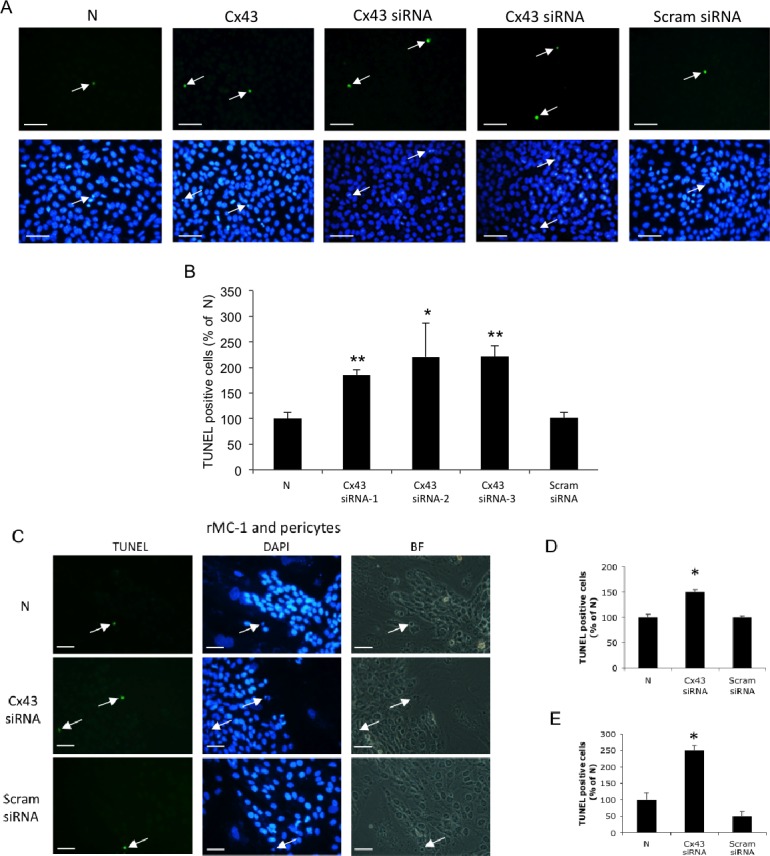
Downregulation of Cx43 promotes apoptosis in retinal Müller cells and in cocultures of rMC-1 and pericytes. (A) Representative images of TUNEL-positive cells (arrows) in Cx43 siRNA-transfected rMC-1 compared with nontransfected rMC-1 and Scram siRNA-transfected rMC-1. Scale bars: 50 μm. (B) Graphical illustration of cumulative data showing TUNEL-positive cells were significantly increased in Cx43 siRNA-transfected rMC-1, compared with nontransfected rMC-1 and scramble siRNA-transfected rMC-1. (C) Downregulation of Cx43 in Müller cells negatively influences retinal pericyte survival. Representative images of TUNEL-positive cells (arrows) in cocultures of Cx43 siRNA-transfected rMC-1 and pericytes compared with those of nontransfected rMC-1 and pericyte cocultures and scrambled siRNA-transfected rMC-1 and pericyte cocultures. Scale bars: 50 μm. (D) Graphical representations of TUNEL assay data. Number of TUNEL-positive rMC-1 was significantly increased in Cx43 siRNA-transfected rMC-1 and pericyte cocultures, compared with those of nontransfected rMC-1 and pericyte cocultures, or scrambled siRNA-transfected rMC-1 and pericyte cocultures. (E) Graphical representations of TUNEL assay data. Numbers of TUNEL-positive pericytes were significantly increased in Cx43 siRNA-transfected rMC-1 and pericyte cocultures, compared with those of nontransfected rMC-1 and pericyte cocultures, or scrambled siRNA-transfected rMC-1 and pericyte cocultures. Data are expressed as the mean ± SD. *P < 0.05. **P < 0.01. n = 4.
Cx43 Downregulation in Müller Cells Induces Apoptosis in Retinal Pericytes When Grown in Cocultures
To determine the effect of reduced Cx43 expression on Müller cell apoptosis, and on apoptosis of retinal pericytes when grown as cocultures, rMC-1 transfected with Cx43 siRNA were grown as monocultures, or cocultures with nontransfected pericytes. The number of TUNEL-positive cells was significantly increased in the Cx43 siRNA-transfected–rMC-1 compared with nontransfected rMC-1 (Figs. 7A, 7B). Interestingly, the number of TUNEL-positive pericytes was significantly increased in the cocultures. Assessment of apoptosis was based on independent counts of TUNEL-positive Müller cells and TUNEL-positive pericytes in cocultures. Number of TUNEL-positive cells for both cell types was increased in the cocultures of Cx43 siRNA-transfected rMC-1 and pericytes (rMC-1: 150.0 ± 4.4% of N, P < 0.05, pericyte: 250.0 ± 14.4% of N, P < 0.05; Figs. 7C–E).
Effects of HG and Cx43 siRNA Transfection on Akt Activity in Müller Cells
To determine the effects of HG or Cx43 downregulation by Cx43 siRNA transfection on Akt activity in rMC-1 monocultures or cocultures of rMC-1 and pericytes, Western blot analyses were performed (Figs. 8A–D). Western blot analysis indicated that the ratio of p-Akt to Akt was significantly decreased in cells grown in HG medium, and in cells transfected with Cx43 siRNA and grown in N medium, compared with those grown in N medium or in cells transfected with scrambled siRNA and grown in N medium (53.7 ± 15.0% of N, P < 0.01; 50.1 ± 12.6% of N, P < 0.01; 56.2 ± 15.7% of scrambled siRNA, P < 0.01; 52.5 ± 13.2% of scrambled siRNA, P < 0.01; n = 6; Fig. 8B). Western blot analysis revealed that Akt activity was significantly reduced based on decreased p-Akt level in cocultures of Cx43 siRNA-transfected rMC-1 and pericytes compared with nontransfected rMC-1 and pericyte cocultures (61.4 ± 4.7% of N, P < 0.01; 70.2 ± 5.2% of N, P < 0.01; 62.4 ± 4.8% of scrambled siRNA, P < 0.01; 71.4 ± 5.3% of scrambled siRNA, P < 0.01; n = 6; Fig. 8D).
Figure 8.
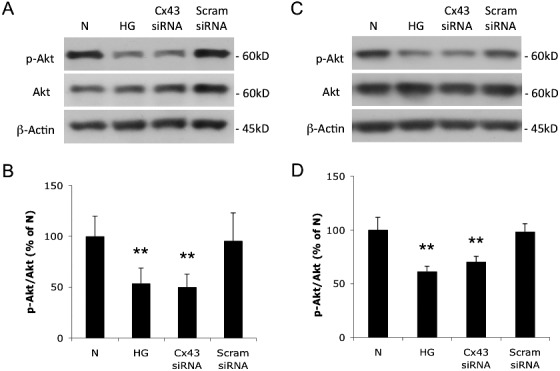
Effects of HG and Cx43 downregulation on Akt activity in Müller cells and cocultures of rMC-1 and pericytes. (A) Representative Western blot shows p-Akt and Akt expression in rMC-1. (B) Graphical representation of Western blot data. The ratios of p-Akt to Akt were significantly decreased in cells grown in HG medium and in cells grown in N medium transfected with Cx43 siRNA compared with nontransfected cells or cells transfected with Scram siRNA and grown in N medium. n = 6. (C) Representative Western blot shows p-Akt and Akt expression in cocultures of rMC-1 and pericytes. (D) Graphical representation of Western blot data shows ratios of p-Akt to Akt were significantly decreased in cells grown in HG medium and in cells grown in N medium transfected with Cx43 siRNA compared with nontransfected cells or cells transfected with scrambled siRNA and grown in N medium, n = 6. Data are expressed as mean ± SD. **P < 0.01.
Cx43 Upregulation Rescues Müller Cells From HG-Induced Apoptosis
To determine whether upregulation of Cx43 is sufficient to rescue HG-induced apoptosis in rMC-1, we transfected rMC-1 grown in HG with pEGFPN1 (Cx43p) plasmid and examined the cells after 4 days post transfection. Cx43 transfection and its effect on Cx43 protein level was verified using WB analysis (Figs. 9A, 9B). The number of apoptotic cells was significantly increased as expected in cells grown in HG medium (248% ± 45% of N, P < 0.01). Importantly, when cells grown in HG were transfected with Cx43p plasmid, a significant decrease in apoptotic cells was observed (142% ± 35% of N versus 248% ± 45% of N, P < 0.01). Empty vector used as control had no effect on the number of apoptotic cells (Figs. 9C–G).
Figure 9.

Upregulation of Cx43 rescues rMC-1 from HG-induced apoptosis. Western blot analysis shows Cx43 plasmid transfection upregulates Cx43 expression in rMC-1 grown in HG. (A) Representative WB image shows Cx43 expression is increased through Cx43p plasmid transfection. (B) Graphical illustration shows cumulative data from six experiments indicating Cx43 upregulation in plasmid transfected rMC-1. Differential staining assay shows increased number of apoptotic cells under HG condition, which was prevented through upregulation of Cx43 expression. *N versus HG. **H + Cx43p versus H + EV. (C–F) Representative images of cells undergoing apoptosis (arrow). (G) Graphical illustration of cumulative data showing upregulation of Cx43 expression rescues rMC-1 from HG-induced apoptosis; n = 6. H + Cx43p: cells grown in HG and transfected with Cx43 plasmid, H + EV: cells grown in HG and transfected with EV. Data are expressed as mean ± SD. *P < 0.05. Scale bars: 50 μm. EV, empty vector.
Discussion
The present study shows that: (1) retinal Müller cells grown in HG medium have reduced numbers of Cx43 gap junctions at the site of contact between adjacent Müller cells; (2) Cx43 protein expression and the number of Cx43 plaques are significantly decreased in both the Müller cell monoculture and in Müller cell-pericyte cocultures grown in HG condition; and (3) intercellular communication is significantly inhibited in monocultures of Müller cells as well as in cocultures of Müller cells and pericytes under HG condition. These results provide the first evidence that communication between Müller cells, and Müller cell to pericyte is compromised in HG condition. Furthermore, compromised intercellular communication resulted in increased apoptosis in these cells, in line with our previous observation in retinal vascular cells.14
We observed that HG impairs not only Cx43 expression in Müller cells, but also its functional activity that results in inhibiting GJIC and influencing pericyte survival. Furthermore, we have observed that reduced Cx43 level in Müller cells contributes to inactivation of Akt and promotes apoptosis. Taken together, these results indicate that Müller cell–pericyte communication is critical for maintenance of retinal homeostasis, and that HG-induced Cx43 downregulation plays a critical role in the demise of Müller cells and pericytes. These findings are in accord with studies, which show that HG-induced downregulation of Cx43 expression is an early trigger for inducing apoptosis in retinal vascular cells.14 Retinas from Cx43 knockout mice develop pericyte loss and acellular capillaries by apoptosis.20 It is currently unknown, however, whether reduced Cx43 in retinal Müller cells contribute to apoptosis. Findings from this study indicate that HG levels reduce Cx43 and GJIC activity in retinal Müller cells with concomitant increase in apoptosis. Interestingly, the effect of decreased Cx43 in Müller cells not only induces apoptosis among one another, but appears to induce apoptosis in pericytes that are in contact with the Müller cells expressing decreased levels of Cx43. Reduced GJIC contributes to diabetic vasculopathy19 by impairing diffusible transport of small molecules such as calcium ions, potassium ions, and other small molecules necessary for cell survival. Moreover, transfer of nitric oxide and glutathione through gap junctions may be negatively impacted as a result of reduced intercellular communication42–45 and thereby affect retinal vessel caliber and alter retinal blood flow. Furthermore, compromised GJIC activity in HG condition can affect cell proliferation, cell differentiation, and trigger apoptosis,6,14 resulting in the loss of glial and vascular cells in diabetic retinopathy.20
To evaluate the effect of HG on cell-cell communication, both monocultures and coculture experiments were studied at confluency. The coculture experiments were performed with Müller cells and pericytes present at approximately 1:1 ratio. Since overall GJIC was reduced by 35% in the coculture under HG condition, and the contribution of Müller cells was approximately 35% as determined from the monoculture data, the contribution of pericytes would be 35% to reach an overall 35% decrease in GJIC. This possibility is further supported by our previous study that showed HG reduces GJIC activity of pericytes by 39%.6 While our data suggests HG compromises cell-cell communication by equally affecting Müller cells and pericytes, further studies are needed to determine if one cell type influences the extent to which GJIC is affected in the other cell type in the context of Müller cell/pericyte coculture.
In the present study, we used morphological features to distinguish between Müller cells and pericytes in cocultures. As CRALBP and glial fibrillary acidic protein are expressed at low levels in rMC-1, we could not successfully use these markers for immunostaining Müller cells. Also, since these studies were performed in a cell culture system, it remains to be established whether Müller cell–pericyte interactions induce apoptosis in vivo under hyperglycemic conditions. Since connexins are highly conserved among mammals, species differences in a coculture system would have minimal impact, although some effect cannot be ruled out. Molecular mechanisms that link gap junction activity with apoptosis remain to be established. There is limited information available about the molecular mechanisms underlying altered gap junction activity and apoptosis. A few studies indicate that altered gap junction intercellular communication promotes apoptosis, and that gap junctional communication induces apoptosis in a connexin-type–dependent manner.46 Furthermore, downregulation of mitochondrial Cx43 can result in cytochrome-c release and induce apoptosis.47
Gap junctional coupling in retinal cells facilitates the propagation of intercellular signals and creates a functional syncytium. However, not all gap junctional coupling are symmetric. Studies have indicated asymmetric gap junctional coupling between astrocytes and Müller cells that allows a unidirectional transfer of intracellular molecules from astrocytes to Müller cells.48,49 In the current study, we have investigated whether reduced Cx43 expression in Müller cells influence apoptotic demise of retinal pericytes. Our finding that decreased Cx43 level in Müller cells contributes to apoptosis in pericytes suggests that the Müller cells are capable of influencing vascular cells. A recent study indicated Cx43 deficiency affects expression levels of glial glutamate transporters,50 which play an essential role in the transport of glutamate into Müller cells, and thereby participate in the regulation of apoptosis. In HG condition, glutamate uptake by Müller cells is known to be compromised with concomitant increase in ROS generation and apoptosis.51 Taken together, these findings suggest that the Müller cells influence vascular cells through GJIC and contribute to the functional stability and homeostasis of the retina.
Reduced GJIC in retinal Müller cells could have far-reaching effects on other cell types in the retina. Müller cells' long processes, which spread extensively from the inner limiting membrane to the outer limiting membrane,24–27 facilitate exchange of small molecules with various cell types in the retina including astrocytes and pericytes.28–30 Astrocytes are extensively coupled to one another and to Müller cells through Cx43 gap junctions.49,52,53 Additionally, studies have shown that the surface retinal vessels are in contact with the processes of Müller cells,28–30 and extensive Cx43 gap junctions present in the Müller cells.31–33,50,52,54 Findings from the current study suggest that HG inhibits GJIC between Müller cells and pericytes, and may contribute to the disruption of neurovascular homeostasis in diabetic retinopathy. Cell-cell communication between different cell types is as much essential as between one another of the same cell type. Further research in the area of neurovascular interactions mediated by GJIC activity between glial and vascular cells is necessary for better understanding of the basic processes underlying retinal dysfunction in diabetic retinopathy.
Acknowledgments
Supported by NEI, NIH EY018218 (SR), EY019325 (VS); in part by a departmental grant from the Massachusetts Lions Organization (SR); and unrestricted funds from Research to Prevent Blindness, Inc.
Disclosure: T. Muto, None; T. Tien, None; D. Kim, None; V.P. Sarthy, None; S. Roy, None
References
- 1. Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007; 293: C1112–C1119 [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y, Paul EM, Sathyendra V, et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS One. 2011; 6: e23516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He LQ, Cai F, Liu Y, et al. Cx31 is assembled and trafficked to cell surface by ER-Golgi pathway and degraded by proteasomal or lysosomal pathways. Cell Res. 2005; 15: 455–464 [DOI] [PubMed] [Google Scholar]
- 4. Wright JA, Richards T, Becker DL. Connexins and diabetes. Cardiol Res Pract. 2012; 2012: 496904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dbouk HA, Mroue RM, El-Sabban ME, Talhouk RS. Connexins: a myriad of functions extending beyond assembly of gap junction channels. Cell Commun Signal. 2009; 7: 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li AF, Sato T, Haimovici R, Okamoto T, Roy S. High glucose alters connexin 43 expression and gap junction intercellular communication activity in retinal pericytes. Invest Ophthalmol Vis Sci. 2003; 44: 5376–5382 [DOI] [PubMed] [Google Scholar]
- 7. Inoguchi T, Yu HY, Imamura M, et al. Altered gap junction activity in cardiovascular tissues of diabetes. Med Electron Microsc. 2001; 34: 86–91 [DOI] [PubMed] [Google Scholar]
- 8. Johnstone S, Isakson B, Biological Locke D. and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009; 278: 69–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Houghton FD. Role of gap junctions during early embryo development. Reproduction. 2005; 129: 129–135 [DOI] [PubMed] [Google Scholar]
- 10. Mathias RT, White TW, Gong X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol Rev. 2010; 90: 179–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alonso A, Reinz E, Jenne JW, et al. Reorganization of gap junctions after focused ultrasound blood-brain barrier opening in the rat brain. J Cereb Blood Flow Metab. 2010; 30: 1394–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liao Y, Day KH, Damon DN, Duling BR. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc Natl Acad Sci U S A. 2001; 98: 9989–9994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006; 397: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li AF, Roy S. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci. 2009; 50: 1400–1407 [DOI] [PubMed] [Google Scholar]
- 15. Danesh-Meyer HV, Kerr NM, Zhang J, et al. Connexin43 mimetic peptide reduces vascular leak and retinal ganglion cell death following retinal ischaemia. Brain. 2012; 135: 506–520 [DOI] [PubMed] [Google Scholar]
- 16. Pearson RA, Luneborg NL, Becker DL, Mobbs P. Gap junctions modulate interkinetic nuclear movement in retinal progenitor cells. J Neurosci. 2005; 25: 10803–10814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoang QV, Qian H, Ripps H. Functional analysis of hemichannels and gap-junctional channels formed by connexins 43 and 46. Mol Vis. 2010; 16: 1343–1352 [PMC free article] [PubMed] [Google Scholar]
- 18. Malfait M, Gomez P, van Veen TA, et al. Effects of hyperglycemia and protein kinase C on connexin43 expression in cultured rat retinal pigment epithelial cells. J Membr Biol. 2001; 181: 31–40 [DOI] [PubMed] [Google Scholar]
- 19. Sato T, Haimovici R, Kao R, Li AF, Roy S. Downregulation of connexin 43 expression by high glucose reduces gap junction activity in microvascular endothelial cells. Diabetes. 2002; 51: 1565–1571 [DOI] [PubMed] [Google Scholar]
- 20. Bobbie MW, Roy S, Trudeau K, Munger SJ, Simon AM. Reduced connexin 43 expression and its effect on the development of vascular lesions in retinas of diabetic mice. Invest Ophthalmol Vis Sci. 2010; 51: 3758–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandes R, Girao H, Pereira P. High glucose down-regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome-dependent mechanism. J Biol Chem. 2004; 279: 27219–27224 [DOI] [PubMed] [Google Scholar]
- 22. Kuroki T, Inoguchi T, Umeda F, Ueda F, Nawata H. High glucose induces alteration of gap junction permeability and phosphorylation of connexin-43 in cultured aortic smooth muscle cells. Diabetes. 1998; 47: 931–936 [DOI] [PubMed] [Google Scholar]
- 23. Oku H, Kodama T, Sakagami K, Puro DG. Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Invest Ophthalmol Vis Sci. 2001; 42: 1915–1920 [PubMed] [Google Scholar]
- 24. Bringmann A, Pannicke T, Grosche J, et al. Muller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006; 25: 397–424 [DOI] [PubMed] [Google Scholar]
- 25. Shen W, Li S, Chung SH, Gillies MC. Retinal vascular changes after glial disruption in rats. J Neurosci Res. 2010; 88: 1485–1499 [DOI] [PubMed] [Google Scholar]
- 26. Dyer MA, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000; 3: 873–880 [DOI] [PubMed] [Google Scholar]
- 27. Stuck MW, Conley SM, Naash MI. Defects in the outer limiting membrane are associated with rosette development in the Nrl-/- retina. PLoS One. 2012; 7: e32484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaur C, Foulds WS, Ling EA. Blood-retinal barrier in hypoxic ischaemic conditions: basic concepts, clinical features and management. Prog Retin Eye Res. 2008; 27: 622–647 [DOI] [PubMed] [Google Scholar]
- 29. Jones HC, Terasaki T. Fluids and barriers of the CNS: a new journal encompassing cerebrospinal fluid research. Fluids Barriers CNS. 2011; 8: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hosoya K, Tomi M. Advances in the cell biology of transport via the inner blood-retinal barrier: establishment of cell lines and transport functions. Biol Pharm Bull. 2005; 28: 1–8 [DOI] [PubMed] [Google Scholar]
- 31. Ball AK, McReynolds JS. Localization of gap junctions and tracer coupling in retinal Muller cells. J Comp Neurol. 1998; 393: 48–57 [DOI] [PubMed] [Google Scholar]
- 32. Kerr NM, Johnson CS, de Souza CF, et al. Immunolocalization of gap junction protein connexin43 (GJA1) in the human retina and optic nerve. Invest Ophthalmol Vis Sci. 2010; 51: 4028–4034 [DOI] [PubMed] [Google Scholar]
- 33. Zahs KR, Kofuji P, Meier C, Dermietzel R. Connexin immunoreactivity in glial cells of the rat retina. J Comp Neurol. 2003; 455: 531–546 [DOI] [PubMed] [Google Scholar]
- 34. Hollander H, Makarov F, Dreher Z, van Driel D, Chan-Ling TL, Stone J. Structure of the macroglia of the retina: sharing and division of labour between astrocytes and Muller cells. J Comp Neurol. 1991; 313: 587–603 [DOI] [PubMed] [Google Scholar]
- 35. Zahs KR, Ceelen PW. Gap junctional coupling and connexin immunoreactivity in rabbit retinal glia. Vis Neurosci. 2006; 23: 1–10 [DOI] [PubMed] [Google Scholar]
- 36. Cheng A, Tang H, Cai J, et al. Gap junctional communication is required to maintain mouse cortical neural progenitor cells in a proliferative state. Dev Biol. 2004; 272: 203–216 [DOI] [PubMed] [Google Scholar]
- 37. Rechtman E, Harris A, Garzozi HJ, Ciulla TA. Pharmacologic therapies for diabetic retinopathy and diabetic macular edema. Clin Ophthalmol. 2007; 1: 383–391 [PMC free article] [PubMed] [Google Scholar]
- 38. Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci. 1998; 39: 212–216 [PubMed] [Google Scholar]
- 39. Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979; 76: 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liegler TJ, Hyun W, Yen TS, Stites DP. Detection and quantification of live, apoptotic, and necrotic human peripheral lymphocytes by single-laser flow cytometry. Clin Diagn Lab Immunol. 1995; 2: 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McGahon AJ, Martin SJ, Bissonnette RP, et al. The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol. 1995; 46: 153–185 [DOI] [PubMed] [Google Scholar]
- 42. Yao J, Hiramatsu N, Zhu Y, et al. Nitric oxide-mediated regulation of connexin43 expression and gap junctional intercellular communication in mesangial cells. J Am Soc Nephrol. 2005; 16: 58–67 [DOI] [PubMed] [Google Scholar]
- 43. Guppy MJ, Wilton JC, Sharma R, Coleman R, Chipman JK. Modulation of phenobarbitone-induced loss of gap junctional intercellular communication in hepatocyte couplets. Carcinogenesis. 1994; 15: 1917–1921 [DOI] [PubMed] [Google Scholar]
- 44. Kameritsch P, Khandoga N, Nagel W, Hundhausen C, Lidington D, Pohl U. Nitric oxide specifically reduces the permeability of Cx37-containing gap junctions to small molecules. J Cell Physiol. 2005; 203: 233–242 [DOI] [PubMed] [Google Scholar]
- 45. Caruso RL, Upham BL, Harris C, Trosko JE. Biphasic lindane-induced oxidation of glutathione and inhibition of gap junctions in myometrial cells. Toxicol Sci. 2005; 86: 417–426 [DOI] [PubMed] [Google Scholar]
- 46. Kameritsch P, Khandoga N, Pohl U, Pogoda K. Gap junctional communication promotes apoptosis in a connexin-type-dependent manner. Cell Death Dis. 2013; 4: e584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trudeau K, Muto T, Roy S. Downregulation of mitochondrial connexin 43 by high glucose triggers mitochondrial shape change and cytochrome C release in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2012; 53: 6675–6681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Robinson SR, Hampson EC, Munro MN, Vaney DI. Unidirectional coupling of gap junctions between neuroglia. Science. 1993; 262: 1072–1074 [DOI] [PubMed] [Google Scholar]
- 49. Zahs KR, Newman EA. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia. 1997; 20: 10–22 [PubMed] [Google Scholar]
- 50. Unger T, Bette S, Zhang J, Theis M, Engele J. Connexin-deficiency affects expression levels of glial glutamate transporters within the cerebrum. Neurosci Lett. 2012; 506: 12–16 [DOI] [PubMed] [Google Scholar]
- 51. Xie B, Jiao Q, Cheng Y, Zhong Y, Shen X. Effect of pigment epithelium-derived factor on glutamate uptake in retinal Muller cells under high-glucose conditions. Invest Ophthalmol Vis Sci. 2012; 53: 1023–1032 [DOI] [PubMed] [Google Scholar]
- 52. Kerr NM, Johnson CS, Zhang J, Eady EK, Green CR, Danesh-Meyer HV. High pressure-induced retinal ischaemia reperfusion causes upregulation of gap junction protein connexin43 prior to retinal ganglion cell loss. Exp Neurol. 2012; 234: 144–152 [DOI] [PubMed] [Google Scholar]
- 53. Johansson K, Bruun A, Ehinger B. Gap junction protein connexin43 is heterogeneously expressed among glial cells in the adult rabbit retina. J Comp Neurol. 1999; 407: 395–403 [PubMed] [Google Scholar]
- 54. Kihara AH, Mantovani de Castro L, Belmonte MA, Yan CY, Moriscot AS, Hamassaki DE. Expression of connexins 36, 43, and 45 during postnatal development of the mouse retina. J Neurobiol. 2006; 66: 1397–1410 [DOI] [PubMed] [Google Scholar]


