Abstract
The aim of this study was to clarify the efficacy of procyanidin C1 (Pro C1) for modulating vascular tone. Pro C1 induced a potent vasorelaxant effect on phenylephrine-constricted endothelium-intact thoracic aortic rings, but had no effect on denuded thoracic aortic rings. Moreover, Pro C1 caused a significant increase in nitric oxide (NO) production in endothelial cells. Pro C1-induced vasorelaxation and Pro C1-induced NO production were significantly decreased in the presence of a nonspecific potassium channel blocker (tetraethylammonium chloride [TEA]), an endothelial NO synthase inhibitor (NG-monomethyl-L-arginine [L-NMMA]), and a store-operated calcium entry inhibitor (2-aminoethyl diphenylborinate [2-APB]). Pro C1-induced vasorelaxation was also completely abolished by an inhibitor of soluble guanyl cyclase, which suggests that the Pro C1 effects observed involved cyclic guanosine monophosphate (cGMP) production. Interestingly, Pro C1 significantly enhanced basal cGMP levels. Taken together, these results indicate that Pro C1-induced vasorelaxation is associated with the activation of the calcium-dependent NO/cGMP pathway, involving potassium channel activation. Thus, Pro C1 may represent a novel and potentially therapeutically relevant compound for the treatment of cardiovascular diseases.
Key Words: K+ channel, NO/cGMP, procyanidin C1, store-operated Ca2+ entry, vasorelaxation
Introduction
Cardiovascular diseases (CVD) remain the leading cause of mortality in both men and women worldwide. Blood vessels consist of an endothelial layer and a smooth muscle layer. The vascular endothelium is probably the most extensive tissue in the body. It is composed of a single layer of cells located between the circulating blood and the vessel wall.1 In addition to being a physical barrier, the endothelium plays an important role in many physiological functions, such as angiogenesis, metabolism, inflammatory cell adhesion, platelet aggregation, vascular tone, and vascular permeability,2,3 The vascular endothelium is responsible for maintaining the balance between vasorelaxation and vasoconstriction. Disturbance of this balance leads to endothelial dysfunction, which in turn affects the vascular tone and causes damage to the arterial wall,4 leading to CVD, morbidity, and mortality.5 Human behavioral habits considered protective against chronic cardiovascular pathologies have gained considerable attention. In particular, the role of a polyphenol-rich diet in preventing CVD associated with endothelial dysfunction has been evaluated.6 Several epidemiological studies indicate that the intake of polyphenol-rich foods, including various fruits and vegetables, is associated with a lower risk of coronary heart disease.7–9 In particular, many flavonoids, including procyanidins, are well known as the most potent endothelium-dependent vasorelaxant compounds,10,11 and can be found in a variety of foods (e.g., apples, wine, tea, peanuts, almonds, and cocoa).
Procyanidin-rich fractions from Parkia biglobosa (Mimosaceae) leaves and Guazuma ulmifolia barks have been shown to cause endothelium-dependent relaxation in porcine coronary arteries.12 Giancarlo et al. reported that procyanidins from Vitis vinifera L. seeds protected endothelial cells from peroxynitrite damage and enhanced endothelium-dependent vasorelaxation in human arteries.13 Furthermore, procyanidins in the apple Malus pumila induced a potent vasorelaxatant effect through an endothelium-dependent pathway.14 It has been proposed that the vasorelaxant properties of procyanidins result from their ability to stimulate the rapid formation of nitric oxide (NO) through endothelial nitric oxide synthases (eNOS), leading in turn to increased accumulation of cyclic guanosine monophosphate (cGMP).15,16 Interestingly, our previous study demonstrated that procyanidin C1 (Pro C1), an epicatechin trimer, induced NO production in endothelial cells through both hyperpolarization and PI3K/Akt pathways.17 Although the protective effects of endothelium-derived relaxing factors, such as NO and prostacyclin, and of endothelium-derived hyperpolarizing factor, against CVD have been attributed, at least in part, to polyphenolic compounds,18 the efficacy of Pro C1 for modulating vascular function ex vivo remains unclear and its mechanisms of action need to be mechanistically clarified. The present study was conducted to validate our previous findings based on vascular endothelial cell signaling.17
We therefore investigated the potential role of Pro C1 (Fig. 1) in regulating (1) vessel functions by using thoracic aortic rings from Sprague-Dawley (SD) rats and (2) the vessel signaling pathways involved in Pro C1-induced vasorelaxation.
FIG. 1.
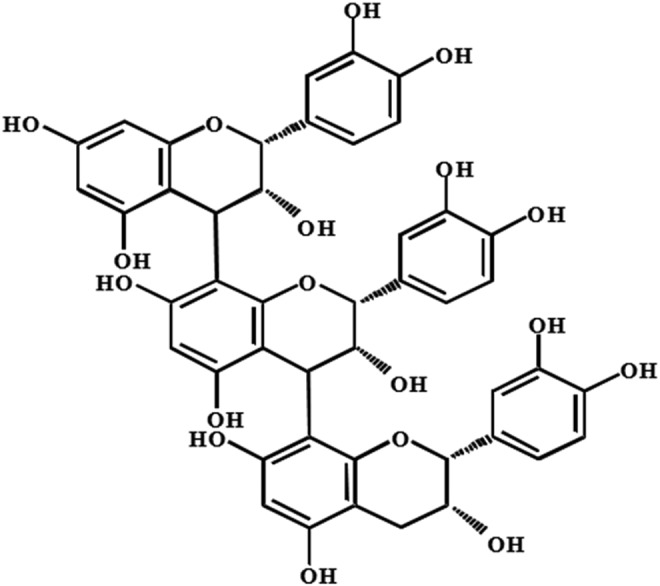
Chemical structure of Pro C1 [epicatechin-(4β→8)-epicatechin-(4β→8)-epicatechin]. Pro C1, procyanidin C1.
Materials and Methods
Materials
Pro C1 was purchased from Sigma (San Diego, CA, USA). Phenylephrine (PE), NG-monomethyl-L-arginine (L-NMMA), tetraethylammonium chloride (TEA), 1-H-[1,2,4]oxadiazolo-[4,3]quinoxalin-1-one (ODQ), 2-aminoethyl diphenylborinate (2-APB), and acetylcholine (ACh) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical reagent grade and used without further purification in this study.
Preparation of isolated thoracic aortic rings
The animal procedures were in strict accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1996) and were approved by the Institutional Animal Care and Utilization Committee of Wonkwang University. Male SD rats (weight, 250–300 g) were purchased from the Korean Experimental Animals Co. (Daejeon, Korea). After the animals were killed by decapitation, the thoracic aorta was rapidly and carefully dissected and placed in an ice-cold Krebs solution (118 mM NaCl, 4.7 mM KCl, 1.1 mM MgSO4, 1.2 mM KH2PO4, 1.5 mM CaCl2, 25 mM NaHCO3, and 10 mM glucose, pH 7.4). The aorta, free of connective tissue and fat, was then cut into rings of approximately 3 mm width. All dissecting procedures were performed with extreme care to protect the endothelium from inadvertent damage. To examine the endothelium-independent vascular responses, the aorta was denuded by gently rubbing the luminal surface with a fine needle. The effectiveness of the endothelium removal procedure was confirmed by the absence of vasorelaxation, induced by 100 μM Ach, in constricted thoracic aortic rings.
Recording isometric vascular tone
First, two L-shaped stainless steel wires were inserted into the lumen of the aortic rings, which were then placed in a tissue bath containing the Krebs solution (pH 7.4) at 37°C. The solution was continuously bubbled with 95% O2 and 5% CO2 and then equilibrated for 60 min. The baseline load placed on the aortic rings was 3 g, and the changes in isometric tension were recorded with a transducer (Grass FT 03; Grass Instrument Co., Quincy, MA, USA) connected to a Grass Polygraph recording system (Model 7E; Grass Instrument Co.). In the first series of experiments, to determine the vasorelaxant effects of Pro C1, the aortic rings were exposed to PE (1 μM) treatment for maximal contraction. Once the maximal response to PE was obtained, the aortic rings were exposed to increasing doses of the testing agent. The responses were recorded and then stopped by washing the aortic rings with a fresh Krebs solution. In all experiments aimed at measuring endothelium-dependent vascular responses, special care was taken to avoid damaging the luminal surface of the endothelium. For constricted studies using inhibitors, thoracic aortic rings were pretreated with 10 μM L-NMMA, 10 μM ODQ, 100 μM 2-APB, or 10 mM TEA for 15 min before the addition of 1 μM PE.
Measurement of cGMP in thoracic aortic rings
Vascular cGMP levels were evaluated in prepared thoracic aortic segments. Thoracic aortic rings (20 mg) were incubated with 1 μM PE, alone or in the presence of 50 μg/mL Pro C1 or 100 μM ACh. After incubation for 15 min, the thoracic aortic rings were homogenized in 0.1 M HCl. The homogenate was centrifuged for 10 min at 1500 g. The total concentration of proteins in the supernatant was assayed using a protein assay (Bio-Rad, Tokyo, Japan) and bovine serum albumin as the standard. The cGMP concentration in the supernatant was assayed using a cGMP enzyme immunoassay (cGMP EIA; Assay Designs, Ann Arbor, MI, USA) and expressed in picomole of cGMP/mg protein from thoracic aortic rings.
Cell culture
Rat aortic endothelial cells (RAECs) were purchased from Cell Applications, Inc. (San Diego, CA, USA). RAECs were cultured in a rat endothelial cell medium (RECM, San Diego, CA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen Corporation's GIBCO, Carlsbad, CA, USA). RAECs were cultured in a 25-cm2 flask (Corning, Corning, NY, USA) and then incubated at 37°C in 5% CO2 incubator up to 95% confluence. RAECs of passages 7 were used in all experiments.
Measurement of cell cytotoxicity
Cytotoxicity measurements for RAECs were conducted using a WST-8 assay. Briefly, RAECs (5×103 cells/well) were cultured in 96-well plates in the DMEM containing 10% FBS and then incubated at 37°C in a 5% CO2 incubator up to 95% confluence. The DMEM was changed to the FBS-free medium for 24 h before experimental testing. The RAECs were then incubated with Pro C1 (6.25–100 μg/mL) in a volume of 100 μL. After incubation for 24 h, 10 μL of the WST-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added to each well and incubated for 4 h. Then, absorbance was measured at 450 nm using a Wallac 1420-microplate reader.
Measurement of NO production
The concentration of NO in the culture medium was determined by measuring its oxidation product, nitrite, using the Griess method. Briefly, RAECs (1.5×104 cells/well) were cultured in 96-well plates in the DMEM containing 10% FBS and then incubated at 37°C in a 5% CO2 incubator up to 95% confluence. Pro C1 (6.25–50 μg/mL) was added to each well and incubated at 37°C for 24 h. To investigate the signaling pathways involved in Pro C1-induced NO production, RAECs were pretreated with L-NMMA (100 μM), 2-APB (100 μM), or TEA (1 mM) for 10 min, before Pro C1 application (50 μg/mL). The culture medium was then collected, mixed with the Griess reagent (1:1), and incubated at room temperature for 15 min. The absorbance of the solution at 517 nm was then measured using a microplate reader (Zenyth 3100; Anthos Labtec Instruments GmbH). Solutions of NaNO2 (0–100 μM), freshly prepared in deionized water, were used to generate a standard curve and to calculate the corresponding nitrite concentration in the cell culture medium.
Statistical analyses
All experiments were repeated at least three times, and the results are expressed as mean±SEM. A two-way analysis of variance (ANOVA) test and a Student's t-test were performed to examine the statistical difference between two groups. Statistical significance was defined as P<.05. All analyses were conducted with Stat View J5.0 (SAS Institute, Inc., Cary, NC, USA).
Results and Discussion
Effect of Pro C1 on vasorelaxation in the presence or absence of endothelium
Epidemiological studies have shown a significant inverse correlation between cardiovascular risks and consumption of polyphenols such as those from fruits and vegetables.19 In particular, polyphenolic compounds have been reported to modulate the vascular tone (e.g., through anti-arteriosclerotic activity) and to prevent lifestyle-related diseases, such as obesity.11,20,21 Nevertheless, the effects of Pro C1 on vasorelaxation are still unclear. We first tested the effect of Pro C1 on the vasorelaxation of thoracic aortic rings in the presence or absence of endothelium. Pro C1 had a potent dose-dependent vasorelaxant effect on 1 μM PE-constricted endothelium-intact thoracic aortic rings, whereas it had no effect on denuded thoracic aortic rings (Fig. 2). These results suggest that Pro C1 causes an endothelium-dependent vasorelaxation, which is in agreement with previous findings.13
FIG. 2.
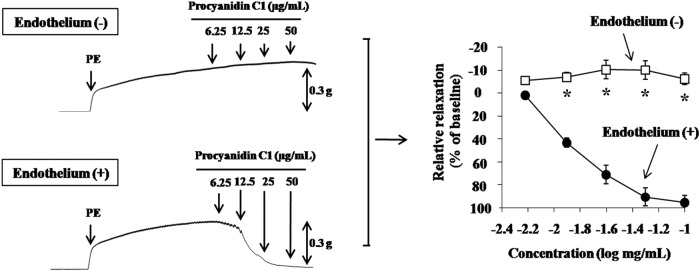
Vasorelaxation profiles of endothelium-intact (+) or endothelium-denuded (−) thoracic aortic rings by Pro C1. Pro C1 was added in a cumulative manner (6.25–50 μg/mL) to 1.0 μM PE-constricted thoracic aortic rings. Results are expressed as mean±SEM (n=4). *P<.01 versus vehicle. PE, phenylephrine.
Effect of NOS and K+ channel inhibition on the Pro C1-induced vasorelaxation
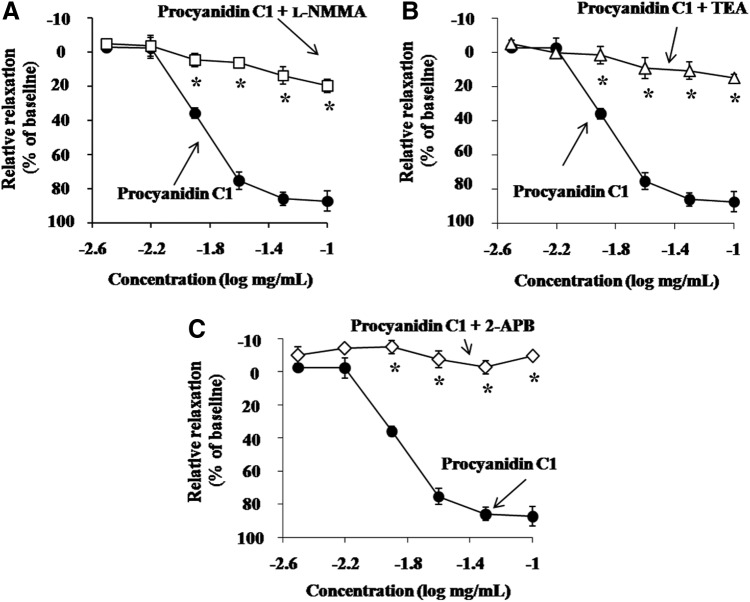
One of the most important pathways involved in the control of vascular tone is the NO/cGMP signaling pathway. NO-induced vasorelaxation is generally associated with the activation of soluble guanyl cyclase (sGC) and the subsequent accumulation of cGMP.22,23 Moreover, the activation of K+ channels is known to be involved in vasorelaxation by natural products of plant origin.24 Therefore, we analyzed the involvement of the NO/cGMP system and the effects of K+ channel inhibitors on Pro C1-induced vasorelaxation. To verify the involvement of NO/K+ channel pathways in Pro C1-induced vasorelaxation, we pretreated endothelium-intact thoracic aortic rings with the eNOS inhibitor L-NMMA (10 μM) and the nonspecific K+ channel inhibitor TEA (10 mM) followed by Pro C1. We observed that the addition of L-NMMA and TEA caused a significant reduction in Pro C1-induced vasorelaxation (Fig. 3A, B). Taken together, these results strongly indicate that Pro C1-induced vasorelaxation is mediated by the eNOS pathway in combination with K+ channel activation, which confirms the findings of our previous report demonstrating that Pro C1 promoted NO production and hyperpolarization in RAECs.17 These results are also in agreement with those of other studies showing the involvement of EDHF, acting on K+ channels, in the vasorelaxant effects of plant extracts in coronary arteries.10
FIG. 3.
Vasorelaxation effect of Pro C1 on 1.0 μM PE-constricted thoracic aortic rings in the presence of inhibitors. (A) 10 μM L-NMMA, (B) 10 mM TEA, and (C) 100 μM 2-APB were added before 1.0 μM PE constriction. Constricted tension was recorded after the addition of Pro C1. Results are expressed as mean±SEM (n=4). *P<.01 versus vehicle. 2-APB, 2-aminoethyl diphenylborinate; L-NMMA, NG-monomethyl-L-arginine; TEA, tetraethylammonium chloride.
Effect of store-operated Ca2+ entry inhibition on the Pro C1-induced vasorelaxation
Store-operated Ca2+ entry (SOCE) is an important molecular mechanism involved in the regulation of Ca2+ entry in the vascular endothelium.25,26 We investigated the involvement of Ca2+ influx in the Pro C1-induced vasorelaxation by using modulators of SOCE. To further define the nature of Ca2+ entry involved in the Pro C1-induced vasorelaxation, we tested an inhibitor of SOCE. Figure 3C shows that 100 μM of a SOCE inhibitor, 2-APB, attenuated the Pro C1-induced vasorelaxation, suggesting that SOCE mechanisms are involved in the Pro C1-induced vasorelaxation. These results are consistent with previous findings showing the importance of SOCE in vasorelaxation and eNOS activation.27,28
Effect of sGC inhibition on Pro C1-induced vasorelaxation
It has been shown that activation of sGC by NO production facilitates the conversion of guanosine-5′-triphosphate to the intracellular second messenger cGMP, which mediates smooth muscle relaxation.29,30 Therefore, we investigated the involvement of cGMP in Pro C1-induced vasorelaxation by pretreating thoracic aortic rings with the sGC inhibitor (ODQ) and then analyzed the effects of Pro C1. In the presence of ODQ (10 μM), we observed a complete abolishment of the Pro C1-induced vasorelaxation of PE-constricted thoracic aortic rings (Fig. 4). These results indicate that Pro C1-induced vasorelaxation is associated with cGMP accumulation. To further assess the direct involvement of cGMP in Pro C1-induced vasorelaxation, we incubated PE-stimulated thoracic aortic rings with Pro C1. In PE-constricted thoracic aortic rings, Pro C1 significantly enhanced the basal cGMP level, similar to the positive control (100 μM ACh) (Fig. 6). Taken together, these results suggest that Pro C1-induced vasorelaxation is mediated by sGC signaling and subsequent cGMP accumulation.
FIG. 4.
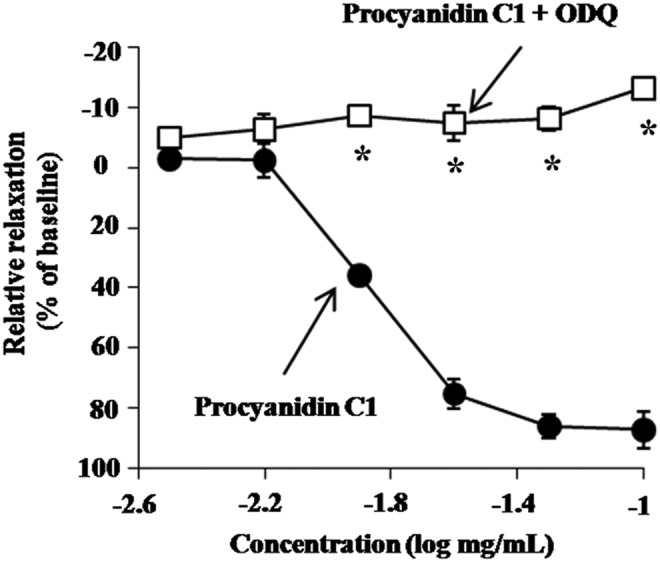
Vasorelaxation effect of Pro C1 on 1.0 μM PE-constricted thoracic aortic rings in the presence of inhibitors. ODQ (10 μM) was added before 1.0 μM PE constriction. Constricted tension was recorded after the addition of Pro C1. Results are expressed as mean±SEM (n=4). *P<.01 versus vehicle. ODQ, 1-H-[1,2,4]oxadiazolo-[4,3]quinoxalin-1-one.
FIG. 6.
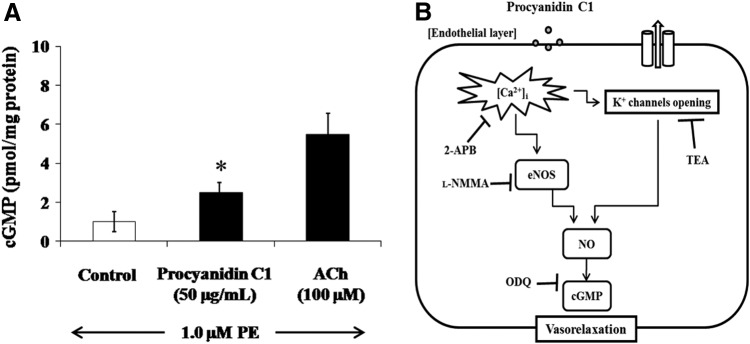
Effect of Pro C1 on cGMP level in 1.0 μM PE-stimulated intact thoracic aortic rings. Pro C1 (50 μg/mL) was added to 1.0 μM PE-stimulated thoracic aortic rings (about 20 mg). (A) The cGMP level (pmol/mg protein) was determined by a cGMP enzyme immunoassay. The results are expressed as mean±SEM (n=4). *P<.05 was considered to be significant. 100 μM ACh was used as a positive control to confirm the vascular tone of the thoracic aortic rings in this experiment. (B) Proposed mechanism(s) of Pro C1-induced vasorelaxation. ACh, acetylcholine; cGMP, cyclic guanosine monophosphate.
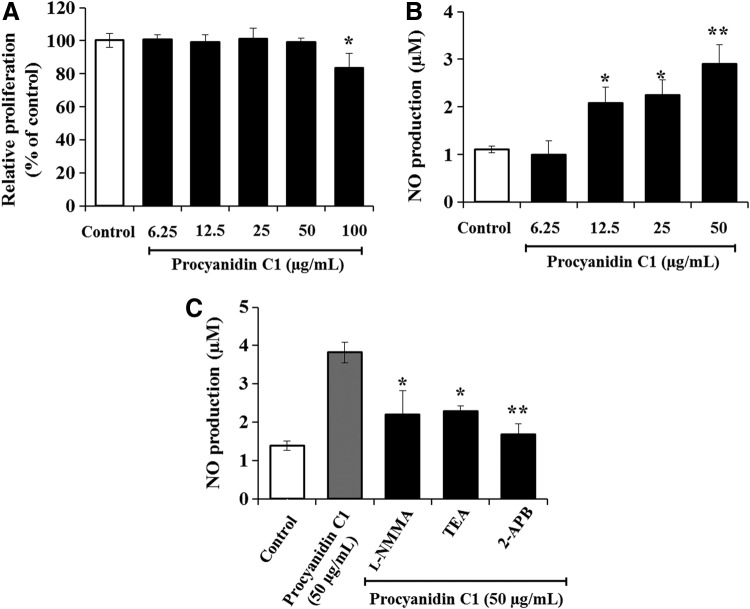
Effect of Pro C1 on the metabolic activity
eNOS/NO activation is an important pathway mediating vasorelaxation, through endothelial cell hyperpolarization, as well as Ca2+ influx.31 To determine the optimal concentration of Pro C1 for use in in vitro studies, the cytotoxic effect of Pro C1 was examined in RAECs. The administration of 6.25–50 μg/mL Pro C1 did not affect cell proliferation, whereas higher concentrations of Pro C1 (100 μg/mL) showed significant cell cytotoxicity compared to the corresponding control group finding (Fig. 5A). For this reason, 50 μg/mL Pro C1 was used as the maximum dose throughout the subsequent experiments. As shown in Figure 5B and C, Pro C1 significantly enhanced the level of NO production in a concentration-dependent manner. Interestingly, Pro C1-induced NO production was significantly decreased in the presence of TEA (1 mM), 2-APB (100 μM), or L-NMMA (100 μM). These findings, in concert with several previous reports, confirm that endothelial NO production leading to vasorelaxation is mediated by K+ channel activation as well as by a decrease in intracellular calcium concentrations.14,32,33
FIG. 5.
Effect of Pro C1 on the metabolic activity of rat aortic endothelial cells. (A) Dose-dependent effect of Pro C1 (6.25–100 μg/mL) on proliferation was analyzed using the EZ-Cytox Cell Viability Kit. (B) Dose-dependent effect of Pro C1 (6.25–50 μg/mL) on NO production was analyzed by a Griess reagent assay. (C) Effect of Pro C1 (50 μM)-induced NO production in the presence of 100 μM L-NMMA, 100 μM 2-APB, or 1 mM TEA (n=4). The results are expressed as mean±SEM (n=4). **P<.01, *P<.05 versus Pro C1. NO, nitric oxide.
Summary
In this study, we clarified the efficacy of Pro C1 for vascular tone modulation. Pro C1 is a powerful endothelium-dependent vasodilator, stimulating endothelial NO production, including hyperpolarization, and regulating Ca2+ entry in the vascular endothelium. Pro C1 can facilitate vasorelaxation and, therefore, represents a novel and effective therapeutically relevant compound for the treatment of CVD associated with endothelial dysfunction.
Acknowledgments
This study was supported by the Basic Research Support Program of the Korea Atomic Energy Research Institute and the Nuclear Research & Development Program of the National Research Foundation of Korea. The authors thank Prof. Dae Gill Kang for her helpful support in the animal experiments.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Vane JR, Anggard EE, Botting RM: Regulatory functions of the vascular endothelium. N Engl J Med 1990;323:27–36 [DOI] [PubMed] [Google Scholar]
- 2.Aird WC: The endothelium in health and disease. In: Endothelial Biomedicine (Aird WC, ed.) Cambridge University Press, New York, 2007, p. 1111 [Google Scholar]
- 3.Luscher TF, Barton M: Biology of the endothelium. Clin Cardiol 1997;20Suppl. 2:3–10 [PubMed] [Google Scholar]
- 4.Kinlay S, Libby P, Ganz P: Endothelial function and coronary artery disease. Curr Opin Lipidol 2001;12:383–389 [DOI] [PubMed] [Google Scholar]
- 5.Sanders M: Molecular and cellular concepts in atherosclerosis. Pharmacol Ther 1994;61:109–153 [DOI] [PubMed] [Google Scholar]
- 6.Mario DA, Alessandra B, Enrica B: Vascular effects of wine polyphenols. Cardiovasc Res 2004;63:593–602 [DOI] [PubMed] [Google Scholar]
- 7.Hertog MG, Hollman PC, Katan MB, et al. : Intake of potentially anticarcinogenic flavonoids and their determinants in adults in the Netherlands. Nutr Cancer 1993;20:21–29 [DOI] [PubMed] [Google Scholar]
- 8.Keli SO, Hertog MG, Feskens EJ, et al. : Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Int Med 1996;156:637–642 [PubMed] [Google Scholar]
- 9.Renaud S, Lorgeril MD: Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992;339:1523–1526 [DOI] [PubMed] [Google Scholar]
- 10.Fitzpatrick DF, Hirschfield SL, Coffey RG: Endothelium–dependent vasorelaxing activity of wine and other grape products. Am J Physiol 1993;265:774–778 [DOI] [PubMed] [Google Scholar]
- 11.Adamson GE, Lazarus SA, Mitchell AE: HPLC method for the quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J Agric Food Chem 1999;47:4184–4188 [DOI] [PubMed] [Google Scholar]
- 12.Magos GA, Mateos JC, Paez E, et al. : Hypotensive and vasorelaxant effects of the procyanidin fraction from Guazuma ulmifolia bark in normotensive and hypertensive rats. J Ethnopharmacol 2008;117:58–68 [DOI] [PubMed] [Google Scholar]
- 13.Giancarlo A, Marina C, Angela P, et al. : Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium–dependent relaxation in human artery: new evidences for cardio–protection. Life Sci 2003;73:2883–2898 [DOI] [PubMed] [Google Scholar]
- 14.Matsui T, Korematsu S, Byun EB, et al. : Apple procyanidins induced vascular relaxation in isolated rat aorta through NO/cGMP pathway in combination with hyperpolarization by multiple K+ channel activations. Biosci Biotechnol Biochem 2009;73:2246–2251 [DOI] [PubMed] [Google Scholar]
- 15.Andriambeloson E, Kleschyov AL, Muller B, et al. : Nitric oxide production and endothelium–dependent vasorelaxation induced by wine polyphenols in rat aorta. Br J Pharmacol 1997;120:1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwan CY, Zhang WB, Nishibe S, et al. : A novel in vitro endothelium–dependent vascular relaxant effect of Apocynum venetum leaf extract. Clin Exp Pharmcol Physiol 2005;32:789–795 [DOI] [PubMed] [Google Scholar]
- 17.Byun EB, Ishikawa T, Suyama A, et al. : A procyanidin trimer, C1, promotes NO production in rat aortic endothelial cells via both hyperpolarization and PI3K/Akt pathways. Eur J Pharmcol 2012;692:52–60 [DOI] [PubMed] [Google Scholar]
- 18.Knekt P, Jarvinen R, Reunanen A, et al. : Flavonoid intake and coronary mortality in Finland: a cohort study. Br Med J 1996;312:478–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakachi K, Matsuyama S, Miyake S, et al. : Preventive effects of drinking green tea on cancer and cardiovascular disease: epidemiological evidence for multiple targeting prevention. BioFactors 2000;13:49–54 [DOI] [PubMed] [Google Scholar]
- 20.Sugiyama H, Akazome Y, Shoji T, et al. : Oligomeric procyanidins in apple polyphenol are main active components for inhibition of pancreatic lipase and triglyceride absorption. J Agric Food Chem 2007;55:4604–4609 [DOI] [PubMed] [Google Scholar]
- 21.Pearson DA, Tan CH, German JB, et al. : Apple juice inhibits human low density lipoprotein oxidation. Life Sci 1999;64:1913–1920 [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Palmer RJM, Higgs EA: Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991;43:109–142 [PubMed] [Google Scholar]
- 23.DalBo S, Goulart S, Horst H, et al. : Activation of endothelial nitric oxide synthase by proanthocyanidin–rich fraction from Croton celtidifolius (Euphorbiaceae): involvement of extracellular calcium influx in rat thoracic aorta. J Pharmacol Sci 2008;107:181–189 [DOI] [PubMed] [Google Scholar]
- 24.McNeill JR, Jurgens TM: A systematic review of mechanisms by which natural products of plant origin evoke vasodilation. Can J Physiol Pharmacol 2006;84:803–821 [DOI] [PubMed] [Google Scholar]
- 25.Parekh AB, Penner R: Store depletion and calcium influx. Physiol Rev 1997;77:901–930 [DOI] [PubMed] [Google Scholar]
- 26.Nilius B, Droogmans G: Ion channels and their functional role in vascular endothelium. Physiol Rev 2001;81:1415–1459 [DOI] [PubMed] [Google Scholar]
- 27.Lin S, Fagan KA, Li KX, et al. : Sustained endothelial nitric–oxide synthase activation requires capacitative Ca2+ entry. J Biol Chem 2000;275:17979–17985 [DOI] [PubMed] [Google Scholar]
- 28.Parekh AB, Putney JJW: Store–operated calcium channels. Physiol Rev 2005;85:757–810 [DOI] [PubMed] [Google Scholar]
- 29.Hobbs AJ: Soluble guanylate cyclase: the forgotten sibling. Trends Pharmacol Sci 1997;18:484–491 [DOI] [PubMed] [Google Scholar]
- 30.Hussai MB, Hobbs AJ, MacAllister RJ: Autoregulation of nitric oxide–soluble guanylate cyclase–cyclic GMP signalling in mouse thoracic aorta. Br J Pharmacol 1999;128:1082–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feletou M: Calcium-activated potassium channels and endothelial dysfunction: therapeutic options? Br J Pharmacol 2009;156:545–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly RA, Balligand JL, Smith TW: Nitric oxide and cardiac function. Circ Res 1996;79:363–380 [DOI] [PubMed] [Google Scholar]
- 33.Martin S, Andriambeloson E, Takeda K, Andriantsitohaina R: Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. Br J Pharmacol 2002;135:1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]