Abstract
Rationale: Xpert MTB/RIF cycle threshold values are a measure of sputum mycobacterial burden. Data on the impact of HIV infection and immunosuppression on this measure are limited.
Objectives: Examine the impact of HIV status and level of immunosuppression on the distribution of mean cycle threshold values, and the correlation of cycle threshold values and smear microscopy grade with time to culture positivity.
Methods: Paired sputum samples from 2,406 individuals with suspected pulmonary tuberculosis in South Africa were tested by Xpert MTB/RIF, concentrated smear microscopy, and liquid culture to quantify bacterial burden using cycle threshold values, smear grading, and time to culture positivity.
Measurements and Main Results: Cycle threshold values were lower in HIV-uninfected versus HIV-infected individuals (22.9 vs. 26.6; P < 0.001). Among HIV-infected, CD4 count was an independent predictor of cycle threshold value, with an average increase of 1.50 cycles for CD4 count greater than or equal to 200 (P 0.071) and 3.66 cycles for CD4 count less than 200 (P < 0.001) compared with HIV-uninfected individuals. Correlation between cycle threshold value and time to culture positivity was similar to that between smear status and time to culture positivity (both Spearman ρ 0.58). The strength of correlation between measures decreased as the level of immunosuppression increased. A cycle threshold value cutoff of 28 had good predictive value for smear positivity.
Conclusions: We observed decreasing bacillary burden with increasing level of immunosuppression as measured by Xpert MTB/RIF cycle threshold values. A cycle threshold value of 28 can be used as a measure of bacterial burden and smear status in a high HIV burden setting.
Keywords: tuberculosis, diagnostics, South Africa
At a Glance Commentary
Scientific Knowledge on the Subject
Xpert MTB/RIF cycle threshold (CT) values are a measure of sputum mycobacterial burden. Data on the impact of HIV infection and immunosuppression on this measure are limited.
What This Study Adds to the Field
Using Xpert MTB/RIF CT value as a measure of sputum bacillary burden at diagnosis, this study finds that HIV and level of immunosuppression are independent predictors of bacillary burden: HIV-infected individuals with CD4 greater than 200 cells/mm3 had approximately a 60% lower sputum mycobacterial burden compared with HIV-uninfected individuals, and those with CD4 less than 200 cells/mm3 had approximately 92% lower bacterial burden compared with HIV-uninfected individuals. An important new finding is that the correlation between CT value and time to culture positivity was similar to that between smear microscopy status and time to culture positivity, and that this correlation decreases as the level of immunosuppression increases. These data show that the Xpert CT value is a suitable replacement to smear microscopy as a rapid measure of bacillary burden, with moderate correlation to time to culture positivity and superior diagnostic accuracy. A CT value cutoff of 28 cycles offers good predictive value for smear positivity. Xpert MTB/RIF CT value could replace smear microscopy status to judge the level of infectiousness of individuals diagnosed with tuberculosis and allocate resources to public health interventions aimed at reducing ongoing community transmission from infectious individuals.
Measurement of mycobacterial burden in sputum is valuable for identifying those individuals with active tuberculosis (TB) who pose the highest risk for transmission of Mycobacterium tuberculosis (MTB). Traditionally, smear microscopy positivity has been used as a proxy of infectiousness (1, 2). Two other measures of mycobacterial burden, time to culture positivity (TTCP) and CFU, are culture-based methods that are routinely used to quantify the bacillary burden in sputum in research studies (3). Bacillary burden measured by culture at diagnosis has been consistently shown to be a strong predictor of long-term outcome (4–6). Among patients with culture-confirmed TB, TTCP, but not smear microscopy grading, has been found to be an independent predictor of culturable cough aerosols, indicating that smear status may not be a good proxy for level of infectiousness (7). Culture is, however, frequently not available in high-burden countries, and even if available can take up to 6 weeks.
The Xpert MTB/RIF assay (Xpert; Cepheid, Sunnyvale, CA) uses a real-time polymerase chain reaction to amplify mycobacterial DNA, and has been endorsed by the World Health Organization as the primary diagnostic test for HIV-associated pulmonary TB (8). The assay’s cycle threshold value (CT), which is the number of polymerase chain reaction cycles after which each of the five probes is considered positive, provides a semiquantitative measure of bacillary burden in sputum (9, 10). An increase in CT value of one cycle represents a 50% decrease in the amount of MTB DNA present in a sample (10). Xpert CT values have been demonstrated to correlate with both microscopy and culture-based methods of quantifying MTB in sputum. Using serial dilutions of MTB colonies, van Zyl-Smit and coauthors showed a strong correlation between the mean Xpert CT value of all five probes and CFU, with a detection limit of approximately 100 bacilli (10, 11). Using clinical samples, Xpert CT values have been shown to correlate with CFU, TTCP, and smear microscopy grade (9, 12, 13).
The bacillary burden in sputum is lower in HIV-infected individuals (14–16), rendering smear microscopy of diminished utility in this population (17–19). The impact of HIV infection on Xpert CT values is unknown. As the global rollout of Xpert increases exponentially (20), it is important to understand the full utility of this technology, including the ability to measure mycobacterial burden, particularly among those with HIV infection and low CD4 counts. The ability to reliably measure bacillary burden in this subgroup at the highest risk for poor outcomes and mortality could be a key advantage of this test. Using data from individuals evaluated for TB in South Africa, a high HIV coinfection setting, we aimed to examine the impact of HIV status and level of immunosuppression on the distribution of mean CT values, and the correlation of Xpert CT values and smear microscopy grade with TTCP.
Some of the results of these studies have been previously reported in the form of an abstract (21).
Methods
Study Design and Populations
Participants in this cross-sectional study were evaluated for TB at three sites in Johannesburg and Cape Town, South Africa. Those from Johannesburg were consecutive individuals (≥15 yr) suspected of TB presenting for care from July 2011 to July 2012 at a primary care clinic. Clinical data were collected through chart review and electronic patient records. Cape Town participants were consecutive adults (≥18 yr) suspected of TB at two primary care clinics from February 2007 to April 2010. No participants were on TB treatment at the time of enrollment and sample collection. Clinical data were recorded on standardized case report forms, as previously described (22). All patients were offered provider-initiated HIV counseling and testing.
Microbiology and Measures of Bacillary Burden
Paired spot sputum samples were collected from all participants. After decontamination with NALC-NaOH, concentrated fluorescence sputum smear microscopy and liquid culture using BACTEC MGIT 960 (BD Diagnostics, Franklin Lakes, NJ) were performed on one arbitrarily chosen sample. The other sputum sample was tested using Xpert (G3 cartridge) in Johannesburg. In Cape Town, the second sputum was frozen at −20°C for later testing using Xpert (G2 cartridge).
Samples for smear were concentrated by centrifugation at 3,200 rpm and decontaminated with 1% NaOH before fixation (heat fixation in Cape Town and Mycohold Cell Adhesive [Wescor Inc., Logan, UT] in Johannesburg). Smears were graded according to the International Union Against Tuberculosis and Lung Disease scale (23). TTCP was recorded as the days elapsed between culture inoculation and MGIT result. The mean CT value of probes A–E for each participant was used as the primary measure of bacillary burden by Xpert. In a sensitivity analysis, we used the CT value of the earliest positive probe as an alternative.
Valid Xpert results were those providing a usable result (i.e., not an invalid or error result), and valid culture results were those samples that were not contaminated and were positive for MTB complex DNA. Xpert results with internal control CT values greater than 34 were excluded from analysis. These samples were excluded because an internal control value greater than 34 indicates polymerase chain reaction inhibition, which results in less accurate quantitation than tests with internal control CT values less than or equal to 34 (9).
Statistical Analysis
Standard descriptive statistics were used to characterize the cohort. The population median of mean CT values and TTCP were compared between groups using a nonparametric equality of medians test. Mean CT values and the geometric mean TTCP among those positive by each test were compared among groups using t tests. Univariate and multivariate linear regression were used to examine the association of patient characteristics with CT values among Xpert-positive samples and log-transformed TTCP among culture positives. Growth of MTB in liquid culture was the reference standard for calculating sensitivity and specificity. A two-sample test of proportions was used to compare estimates of accuracy among subgroups. Optimal CT value cutpoints for prediction of smear positivity were determined in two ways: the CT value, which maximized correct classification of smear status; and that which had a sensitivity of at least 95% (a “rule out” cutpoint) (12). Correlation between pairs of measures of bacterial burden (smear and TTCP, Xpert and TTCP, smear and Xpert) was assessed using Spearman ρ in two ways: among all culture positives, and among those with a positive test result. Confidence intervals (CI) for Spearman ρ, and the optimal CT cutoff, were constructed from the distribution of 2,000 bootstrapped samples (24). Spearman ρ for subgroups were compared using Fisher z-transformation (25). All statistical analyses were conducted using Stata 12 (StataCorp, College Station, TX) and the R statistical package version 3.0.1 (Vienna, Austria).
Ethics Approval
This study was approved by institutional review boards at the University of North Carolina, Chapel Hill; the University of the Witwatersrand, Johannesburg, South Africa; and the University of Cape Town, Cape Town, South Africa. All participants provided written consent for research use of clinical data.
Results
Study Population
In total, 2,482 participants had a valid Xpert result and accompanying valid culture and/or smear microscopy result. Seventy-six individuals (3.4%) were excluded (n = 66 because of internal control CT values >34, and n = 10 with rifampicin resistance on Xpert). Among the remaining 2,406 individuals, 1,629 (68%) were evaluated at the Johannesburg site and 777 (32%) at the Cape Town sites (Table 1). The median age was 37 years (interquartile range [IQR], 29–46), and 62% were female. HIV coinfection in the cohort was common (58%), and was higher at the Johannesburg site (71%) compared with the Cape Town sites (30%; P < 0.001). Overall, among HIV-infected individuals evaluated for TB, the median CD4 cell count was 273 cells/mm3 (IQR, 131–449), and was lower at the Cape Town site (median CD4 count, 221 cells/mm3; IQR, 120–380) compared with the Johannesburg site (285 cells/mm3; IQR, 136–459; P 0.001). Among the 406 culture-positive individuals, the proportion positive by smear microscopy varied by level of immune suppression: 67% of HIV-uninfected individuals (n = 92 of 137), 40% of HIV-infected individuals with CD4 greater than or equal to 200 cells/mm3 (n = 35 of 87), and 34% of those with CD4 less than 200 were smear microscopy positive (n = 38 of 112) (P < 0.001).
Table 1:
Characteristics by Xpert Status of 2,406 Individuals with Presumptive Tuberculosis at Two Sites in South Africa
| Xpert Positive (n = 373) |
Xpert Negative (n = 2,033) |
All Presumptive TB (n = 2,406) |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Site | ||||||
| Johannesburg | 205 | 55 | 1,424 | 70 | 1,629 | 68 |
| Cape Town | 168 | 45 | 609 | 3 | 777 | 32 |
| Median age (IQR) | 35 (29–42) |
37 (30–46) |
37 (29–45) |
|||
| Sex | ||||||
| Female | 206 | 55 | 1,293 | 64 | 1,499 | 62 |
| Male | 167 | 45 | 740 | 36 | 907 | 38 |
| HIV | ||||||
| Positive | 222 | 60 | 1,172 | 58 | 1,394 | 58 |
| Negative | 138 | 37 | 792 | 39 | 930 | 39 |
| Unknown | 13 | 3 | 69 | 3 | 82 | 3 |
| CD4 count (cells/mm3), median (IQR) | 132 (56–265) |
303 (163–461) |
272 (131–439) |
|||
| CD4 category | ||||||
| <200 cells/mm3 | 126 | 57 | 338 | 29 | 464 | 33 |
| ≥200 cells/mm3 | 68 | 31 | 723 | 62 | 791 | 57 |
| Missing* | 28 | 13 | 111 | 9 | 139 | 10 |
| Culture | ||||||
| Positive | 299 | 83 | 107 | 5 | 406 | 17 |
| Negative | 38 | 10 | 1,638 | 81 | 1,676 | 70 |
| Contaminated | 15 | 4 | 106 | 6 | 121 | 5 |
| Error/missing† | 21 | 6 | 173 | 9 | 194 | 8 |
| NTM | 0 | 0 | 9 | 0.3 | 9 | 0.3 |
| Time to culture positive (d), median (IQR) | 12 (8–17) |
20 (14–27) |
14 (9–20) |
|||
| Smear microscopy | ||||||
| Positive | 193 | 52 | 17 | 1 | 210 | 8 |
| Scanty | 22 | 12 | 4 | 27 | 28 | 14 |
| 1+ | 44 | 24 | 5 | 33 | 49 | 25 |
| 2+ | 47 | 26 | 6 | 40 | 53 | 27 |
| 3+ | 69 | 38 | 0 | 0 | 69 | 35 |
| Negative | 153 | 41 | 1,817 | 89 | 1,970 | 82 |
| Error/missing† | 27 | 8 | 199 | 10 | 226 | 9 |
Definition of abbreviations: CI = confidence interval; IQR = interquartile range; NTM = nontuberculous mycobacteria; TB = tuberculosis; Xpert = Xpert MTB/RIF.
CD4 count was considered missing if a CD4 count was not available within 6 months of TB suspect visit date.
Errors on culture and smear microscopy were those where the sample was reported to have leaked in transit.
Diagnostic Accuracy of Xpert and Smear
Among all individuals evaluated for TB, 2,082 (87%) had a valid culture result: 406 positive, 1,676 negative, and an additional 9 grew nontuberculous mycobacteria. The overall sensitivity of Xpert was 74% (95% CI, 69–78%; n = 299 of 406), and the specificity was 98% (95% CI, 97–99%; n = 1,638 of 1,685). The sensitivity of Xpert was higher among those with smear-positive (96%; 95% CI, 92–98%; n = 178 of 186) compared with smear-negative TB (52%; 95% CI, 45–59%; P < 0.001; n = 97 of 188), but did not vary significantly by HIV status among those with smear-positive TB (HIV+, 94%, 95% CI, 87–98%; HIV−, 98%, 95% CI, 92–99%; P 0.171) or smear-negative TB (HIV+, 56%, 95% CI, 48–64%; HIV−, 40%, 95% CI, 26–54%; P 0.062). Among HIV-infected persons, the sensitivity of Xpert was higher among those with CD4 less than 200 (76%; 95% CI, 67–83%; n = 93 of 123) compared with those with CD4 greater than or equal to 200 (60%; 95% CI, 49–70%; n = 56 of 94; P 0.012). The overall sensitivity of smear microscopy was 50% (95% CI, 45–55%; n = 186 of 374), and the specificity was 99% (95% CI, 98–99%; n = 1,472 of 1,482). The sensitivity of smear microscopy was higher among HIV-uninfected (67%; 95% CI, 59–75%; n = 92 of 137) compared with HIV-infected individuals (38%; 95% CI, 28–48%; P < 0.001; n = 85 of 223). Among HIV-infected participants, the sensitivity of smear microscopy did not differ by CD4 cell count category (P 0.384).
Distribution of CT Value and TTCP
The mean CT value among Xpert-positive participants was 25.2 (95% CI, 24.6–25.8), the median was 25.1 (IQR, 20.9–30.2), and the range was 10.0–38.0 (Figure 1A). The mean TTCP among culture-positive participants was 15.4 days (95% CI, 14.6–16.3), the median was 14 days (IQR, 9–20), and the range was 6–54 days (Figure 1B). Mean CT value was similar by sex and age, whereas mean TTCP was similar by age, but slightly higher among women (Table 2). Mean CT value and mean TTCP were lower at the Cape Town sites compared with the Johannesburg site (mean CT value, 23.3 vs. 26.7, P < 0.001; mean TTCP, 9.9 vs. 17.2 d, P < 0.001). Mean CT value and mean TTCP varied by HIV status, with lower CT values and TTCP among HIV-uninfected compared with HIV-infected individuals (CT value, 22.9 vs. 26.6, P < 0.001; TTP, 10.6 vs. 15.8 d, P < 0.001) (Figure 2 and Table 2). Among HIV-infected individuals, those with a CD4 count greater than or equal to 200 cells/mm3 had a lower mean CT value (25.2; 95% CI, 24.0–26.4) compared with those with a CD4 count less than 200 cells/mm3 (27.6; P 0.002). These differences in mean CT values correspond to HIV-infected individuals with CD4 less than 200 cells/mm3 having approximately a 75% lower sputum mycobacterial burden compared with HIV-uninfected individuals, and those with CD4 less than 200 cells/mm3 having approximately 94% lower bacterial burden compared with HIV-uninfected individuals. In contrast, mean TTCP was similar by CD4 count category (P 0.529). Mean CT value and mean TTCP did not differ by antiretroviral treatment use (P 0.436 and P 0.422, respectively).
Figure 1.
(A) Histogram of individuals’ mean CT values among those testing positive by Xpert MTB/RIF. (B) Histogram of the time to culture positivity among those testing positive by culture.
Table 2:
Mean CT Value and Mean Time to Culture Positivity by Cohort Characteristics
| Mean CT Value (95% CI) | P Value | Mean Days to Culture Positivity (95% CI)* | P Value | |
|---|---|---|---|---|
| Sex | 0.301 | 0.014 | ||
| Male | 24.9 (24.0–25.7) | 12.6 (11.7–13.6) | ||
| Female | 25.5 (24.7–26.4) | 14.3 (13.3–15.6) | ||
| Age category | 0.750 | 0.844 | ||
| ≤35 | 25.2 (24.3–25.9) | 14.3 (12.4–14.4) | ||
| >35 | 25.2 (24.2–26.1) | 13.3 (12.3–14.4) | ||
| Site | <0.001 | <0.001 | ||
| Johannesburg | 26.7 (26.0–27.4) | 17.2 (16.4–18.4) | ||
| Cape Town | 23.3 (22.3–24.3) | 9.9 (9.0–10.6) | ||
| HIV | <0.001 | <0.001 | ||
| Negative | 22.9 (22.0–23.9) | 10.6 (9.8–11.6) | ||
| Positive | 26.6 (25.8–27.3) | 15.8 (14.7–16.8) | ||
| CD4 category | 0.002 | 0.549 | ||
| <200 | 27.6 (26.7–28.5) | 16.6 (14.9–17.3) | ||
| ≥200 | 25.2 (24.0–26.4) | 15.5 (14.0–16.9) | ||
| ART usage | 0.199 | 0.422 | ||
| No ART | 20.4 (18.5–22.4) | 16.1 (13.6–17.8) | ||
| ART | 23.3 (18.99–27.8) | 15.8 (14.3–17.1) |
Definition of abbreviations: ART = antiretroviral treatment; CI = confidence interval; CT = cycle threshold.
Geometric mean.
Figure 2.
(A) Box plots of individuals’ mean CT values among those testing Xpert MTB/RIF positive, by level of immunosuppression. (B) Box plots of time to culture positivity among those testing culture positive, by level of immunosuppression. P values in each panel are the result of a nonparametric test for equality of medians.
In sensitivity analysis, we found similar inferences when using the CT value of the first positive probe as an alternative to the mean CT value (data not shown).
Individual Level Predictors of CT Value and TTCP
In a multivariate linear regression model, level of immunosuppression was the only significant independent predictor of CT value and log TTCP (Table 3). After controlling for age, sex, and site, the mean CT value was 1.50 cycles (95% CI, −0.13 to 3.14; P 0.071) higher among HIV-infected individuals with CD4 count greater than or equal to 200 cells/mm3 and 4.62 cycles (95% CI, 3.28–5.97; P < 0.001) higher in those with a CD4 count less than 200 cells/mm3 compared with the reference group of Xpert-positive, HIV-uninfected individuals. Similarly, compared with culture-positive HIV-uninfected individuals, those with HIV infection and a CD4 count greater than or equal to 200 cells/mm3 had a 21% (95% CI, 8–33%; P 0.001) increase in TTCP, and those with a CD4 count less than 200 cells/mm3 had a 22% (95% CI, 10–34%; P < 0.001) increase.
Table 3:
Univariate and Multivariate Linear Regression Models for Mean CT Value among Xpert Positives and Log Time to Culture Positivity among Individuals with Culture-Positive Tuberculosis
| Outcome: Mean CT Value |
Outcome: Log Time to Culture Positivity |
|||||||
|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||
| β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | |
| Sex | ||||||||
| Male | REF | REF | REF | REF | ||||
| Female | 0.64 (−0.57 to 1.86) | 0.300 | −0.81 (−2.03 to 0.40) | 0.186 | 0.13 (0.028 to 0.24) | 0.014 | −0.20 (−0.12 to 0.79) | 0.691 |
| Age category | ||||||||
| ≤35 | REF | REF | REF | REF | ||||
| >35 | 0.20 (−1.02 to 1.41) | 0.750 | 0.47 (−0.72 to 1.65) | 0.4 | −0.010 (−0.11 to 0.096) | 0.844 | 0.01 (−0.08 to 0.11) | 0.776 |
| Site | ||||||||
| Johannesburg | REF | REF | REF | REF | ||||
| Cape Town | −3.42 (−4.59 to −2.25) | <0.001 | −1.24 (−2.57 to 0.60) | 0.086 | −0.57 (−0.66 to −0.47) | <0.001 | −0.27 (−0.46 to 0.36) | 0.0.98 |
| HIV | ||||||||
| Negative | REF | REF | REF | REF | ||||
| HIV+, CD4 ≥ 200 | 1.99 (0.38 to 3.59) | 0.015 | 1.50 (−0.13 to 3.14) | 0.071 | 0.35 (0.22 to 0.48) | <0.001 | 0.21 (0.08 to 0.33) | 0.001 |
| HIV+, CD4 < 200 | 4.62 (3.28 to 5.97) | <0.001 | 3.66 (2.17 to 5.16) | <0.001 | 0.41 (0.29 to 0.53) | <0.001 | 0.22 (0.10 to 0.34) | <0.001 |
| ART use | ||||||||
| Not on ART | REF | REF | ||||||
| On ART | 1.90 (−3.49 to 7.29) | 0.473 | 0.28 (−0.10 to 0.65) | 0.141 | ||||
Definition of abbreviations: ART = antiretroviral treatment; CI = confidence interval; CT = cycle threshold; REF = reference; Xpert = Xpert MTB/RIF.
CT Value Cutpoint Predicting Smear Positivity
The optimal CT value cutpoint to correctly classify smear status was 27.5 (95% CI, 24.9–32.3). This cutpoint correctly classified 81.8% of all culture-positive participants (95% CI, 76.7–86.1%), and had a sensitivity of 88.8% (95% CI, 83.4–93.0) (Table 4). The CT value cutpoint that could be used to rule out smear positivity was higher, at 30.5 (95% CI, 27.2–34.7), with a sensitivity of 95.5% (95% CI, 91.3–98.0). Using a cutoff of 28 cycles (“very low” level of bacilli according to manufacturer’s insert), 81.8% (95% CI, 76.7–86.2%) of all participants were correctly classified, with a sensitivity of 89.9% (95% CI, 84.5–93.9%) and specificity of 67.0% (95% CI, 56.3–75.9%). This cutoff correctly classified significantly more HIV-uninfected participants (88.0%; 95% CI, 80.3–93.4) compared with HIV-infected (76.4%; 95% CI, 69.0–82.8; P 0.018).
Table 4:
Optimal CT Value Cutoffs for Predicting Smear Positivity among Culture-Positive Participants
| Optimal CT Value Cutoff (95% CI) | Correctly Classified % (95% CI) | Sensitivity % (95% CI) | Specificity % (95% CI) | PPV % (95% CI) | NPV % (95% CI) | Median (IQR) TTCP if CT ≥ Cutoff (d) | Median (IQR) TTCP if CT < Cutoff (d) | |
|---|---|---|---|---|---|---|---|---|
| Maximizing correct classification |
||||||||
| Overall (n = 275) | 27.5 (24.9–32.3) | 81.8 (76.7–86.1) | 88.9 (83.4–93.0) | 69.1 (58.8–78.1) | 84.0 (78.0–89.0) | 77.0 (67.0–85.4) | 18 (12–24) | 10 (7–14) |
| HIV infected (n = 157) | 26.7 (23.5–32.6) | 78.3 (71.1–84.5) | 77.5 (66.8–86.1) | 79.2 (68.5–87.6) | 79.5 (68.8–87.8) | 77.2 (66.4–85.9) | 18 (13–23) | 12 (9–15) |
| HIV uninfected (n = 108) | 28.8 (24.9–35.8) | 89.8 (82.5–94.8) | 96.6 (90.6–99.3) | 55.6 (30.8–78.5) | 91.6 (84.0–96.3) | 76.9 (46.2–95.0) | 14 (8–22) | 9 (6–12) |
| “Rule out” (sensitivity of at least 95%) |
||||||||
| Overall (n = 275) | 30.5 (27.2–34.7) | 78.9 (73.6–83.6) | 95.5 (91.3–98.0) | 48.4 (38.2–58.8) | 77.3 (71.1–82.6) | 85.4 (73.3–93.5) | 19 (13–25) | 11 (7–15) |
| HIV infected (n = 157) | 31.6 (27.9–36.1) | 66.9 (58.9–74.1) | 95.0 (87.7–98.6) | 38.7 (27.6–50.6) | 62.2 (53.0–70.9) | 87.9 (71.8–96.6) | 19 (14–26) | 11 (7–15) |
| HIV uninfected (n = 108) | 28.8 (24.9–35.8) | 89.8 (82.5–94.8) | 96.6 (90.6–99.3) | 55.6 (30.8–78.5) | 91.6 (84.0–96.3) | 76.9 (46.2–95.0) | 14 (8–22) | 9 (6–12) |
Definition of abbreviations: CI = confidence interval; CT = cycle threshold; IQR = interquartile range; NPV = negative predictive value; PCR = polymerase chain reaction; PPV = positive predictive value; TTCP = time to culture positivity in days.
Correlation of Measures of Mycobacterial Burden in Sputum
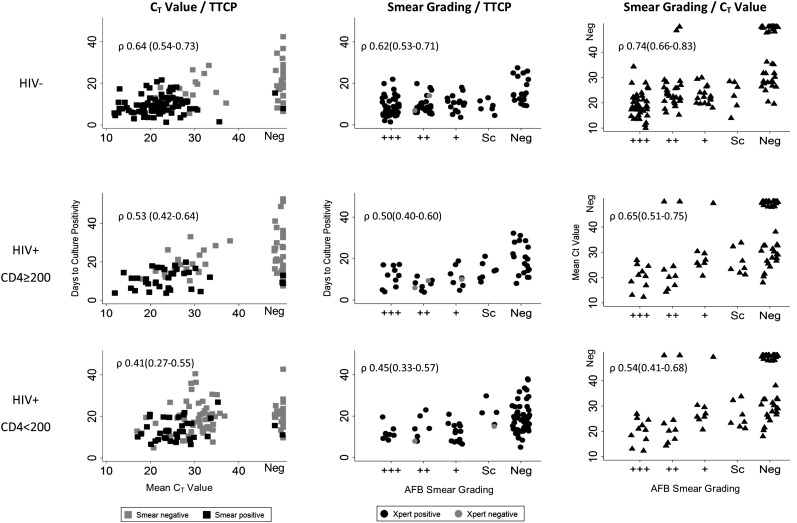
Figure 3 displays the correlation between pairwise measures of bacillary burden (by Xpert, culture, smear microscopy) by HIV status and level of immunosuppression among culture positives. Overall, Xpert CT value and smear grading had similar correlation to TTCP: ρ 0.58 (95% CI, 0.53–0.63) for Xpert CT and TTCP and ρ 0.58 (95% CI, 0.53–0.64; P 1.00) for smear grading and TTCP (Table 5). For Xpert and TTCP, the correlation tended to be strongest among HIV-negative individuals (ρ 0.64; 95% CI, 0.53–0.63), weaker among HIV-infected individuals with CD4 count greater than or equal to 200 (ρ 0.53; 95% CI, 0.42–0.64; P 0.289), and weakest among HIV-infected individuals with CD4 count less than 200 (ρ 0.41; 95% CI, 0.27–0.55; P 0.247). A similar pattern was observed for correlation between smear and TTCP and Xpert and TTCP. Correlation between Xpert and smear (ρ 0.75; 95% CI, 0.71–0.78) tended to be stronger than that between Xpert and TTCP (P 0.119) and smear and TTCP (P 0.147) (Figure 3).
Figure 3.
Scatter plots of measures of bacillary burden among culture positives. Left column, Xpert CT value and TTCP. Middle column, AFB smear grading and TTCP. Right column, Xpert CT value and AFB smear grading. Rows represent levels of immunosuppression. CT = cycle threshold; neg = negative; Sc = scanty positive; TTCP = time to culture positivity; Xpert = Xpert MTB/RIF; ρ = Spearman correlation coefficient.
Table 5:
Correlation between Pairs of Measures of Bacillary Burden among Culture-Positives Only and Test-Positives Only
| All Culture-Positive Samples |
Culture-Positive Samples with Positive Xpert/Smear |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Xpert CT/TTCP |
Smear/TTCP |
Xpert CT/TTCP |
Smear/TTCP |
|||||||
| N | Spearman ρ | N | Spearman ρ | P Value | N | Spearman ρ | N | Spearman ρ | P Value | |
| Overall | 406 | 0.58 (0.53 to 0.63) | 302 | 0.58 (0.53 to 0.64) | 1.00 | 299 | 0.57 (0.50 to 0.64) | 179 | 0.35 (0.21 to 0.48) | 0.003 |
| HIV− | 133 | 0.64 (0.54 to 0.73) | 119 | 0.62 (0.53 to 0.71) | 0.795 | 120 | 0.44 (0.28 to 0.59) | 90 | 0.15 (−0.03 to 0.32) | 0.023 |
| HIV+, CD4 ≥200 | 61 | 0.53 (0.42 to 0.64) | 52 | 0.50 (0.40 to 0.60) | 0.834 | 56 | 0.59 (0.41 to 0.76) | 34 | 0.21 (0.12 to 0.55) | 0.040 |
| HIV+, CD4 <200 | 106 | 0.41 (0.27 to 0.55) | 96 | 0.45 (0.33 to 0.57) | 0.734 | 93 | 0.45 (0.31 to 0.59) | 35 | 0.44 (0.15 to 0.72) | 0.952 |
Definition of abbreviations: ρ = Spearman correlation coefficient; CT = cycle threshold; TTCP = time to culture positivity; Xpert = Xpert MTB/RIF.
When considering correlations among those with a positive result (i.e., among those with a positive culture and a positive Xpert result, or a positive culture and a positive smear result) the correlation between Xpert CT value and TTCP was stronger than that between smear grading and TTCP (ρ 0.57, 95% CI, 0.50–0.64 vs. ρ 0.35, 95% CI, 0.21–0.48; P 0.003).
False-positive Xpert and smear microscopy results occurred predominantly in samples with low bacillary burden compared with true-positives. The median CT value of false-positive Xpert results was 29.9 (IQR, 27.3–33.2), higher than the median CT value of true-positives (24.2; IQR, 20.3–28.7; P 0.001). Most (75%) false-positive smear results were graded + or scanty. The median TTCP of samples with a false-negative Xpert was higher than that of true-positives (20 d [IQR, 14–27] vs. 12 d [IQR, 8–17]; P < 0.001). Likewise, the median TTCP of samples with false-negative smear microscopy results was higher than that of true-positives (19 d [IQR, 14–25] vs. 10 d [IQR, 7–14]; P < 0.001).
Discussion
When using Xpert CT values as a measure of bacillary burden at TB diagnosis, we observed decreasing bacillary burden with increasing level of immunosuppression, with the lowest CT values (i.e., highest bacillary burden) among HIV-uninfected persons, higher CT values among HIV-infected with CD4 greater than or equal to 200 cells/mm3, and the highest CT values (i.e., lowest bacillary load) among HIV-infected with CD4 less than 200 cells/mm3. This association persisted after accounting for age, sex, and study site, suggesting an independent effect of level of immunosuppression, with HIV-infected individuals with CD4 greater than 200 cells/mm3 having approximately a 60% lower sputum mycobacterial burden compared with HIV-uninfected individuals, and those with CD4 less than 200 cells/mm3 having approximately 92% lower bacterial burden compared with HIV-uninfected individuals. Additionally, we found similar correlation between TTCP and CT value as compared with TTCP and smear grading among those with culture-proved TB. Combined with the proved superior diagnostic accuracy of Xpert compared with smear microscopy (26), these results suggest that Xpert is a suitable replacement for smear not only for diagnosis of TB, but also as a correlate of bacterial burden.
Our data build on earlier findings suggesting that the bacillary burden in sputum is lower among HIV-infected individuals compared with uninfected. In two cohort studies of TB patients, Joloba and coworkers (16) found that HIV-infected patients had longer TTCP using solid media compared with uninfected patients, and Elliott and coworkers (15) showed that lower-grade sputum smears, lower CFUs, and longer TTCP were associated with HIV infection. This difference in sputum bacillary burden by HIV status may be caused by the lower incidence of cavitating disease in those with HIV coinfection (14), and the concurrent increase in “atypical” TB presentation characterized by lower lung field involvement and diffuse pulmonary infiltrates (16), resulting in fewer MTB concentrated in the sputum. Because bacterial burden is an important predictor of TB treatment outcome (4–6), and those with HIV and low CD4 counts may be at the highest risk for poor outcomes, accurate and reliable measure of mycobacterial burden in this group is a key advantage of Xpert.
Among culture-positive TB cases, the correlation between measures was similar for CT value and TTCP compared with that between smear grading and TTCP, suggesting that the ability of Xpert CT to quantify bacterial burden present in sputum is similar to that of smear positivity. The correlation among measures varied by HIV status, with correlation decreasing as the level of immunosuppression increased. The ability of Xpert CT to reliably quantify bacterial burden in highly immunosuppressed people is thus diminished, similar to smear, suggesting that Xpert would be less useful for quantifying bacterial burden in this group. This pattern fits with our findings of decreased bacterial burden in those with advanced immunosuppression (CD4 <200). Among test-positives only (excluding culture-positive samples that were Xpert or smear negative), we found that Xpert CT value had superior correlation to TTCP compared with smear grading (scanty, +, ++, or +++), which is in keeping with known limitations of smear microscopy (27). To date, only one other study has performed an in-depth interrogation of the correlation between CT value, TTCP, and smear grading in clinical specimens (9). We found similar correlation between Xpert and TTCP as Blakemore and coworkers (9). However, Blakemore and coworkers did not assess the effect of HIV status or level of immunosuppression, an important determinant of sputum bacterial load. The ability of Xpert to reliably quantify mycobacterial burden is significantly diminished when used to monitor early bactericidal activity after treatment, as demonstrated by Kayigire and coworkers (28), limiting the utility of this technology to samples before treatment initiation.
A CT value cutoff of 30.5 performed best to rule out smear positivity. However, a cutoff of 28 cycles indicating “very low” levels of bacilli as per the manufacturer insert and easily available from the standard Xpert assay report performed similarly, suggesting that classifying those with low, medium, or high levels of bacilli as reported on by the Xpert software could replace smear microscopy status for infection control and contact tracing efforts. If validated in transmission studies, this cutoff could be a suitable surrogate for infectivity among new TB cases in settings where smear microscopy is replaced by Xpert as the initial diagnostic.
The inclusion of multiple clinical sites in this study is a strength, because it increases the generalizability of the findings. This, however, also resulted in several limitations caused by differences between the sites including the use of the earlier Xpert cartridge version, use of frozen versus fresh sputum samples, and differences in the prevalence of HIV coinfection and distribution of CD4 cell count. An unadjusted model of CT value showed a difference between the sites, with lower values at the Cape Town sites. We believe that this was caused by the effect of HIV rather than differences in Xpert testing methods given that, after controlling for HIV status and CD4 count, this difference was no longer statistically significant. A systematic review found that Xpert sensitivity does not vary between frozen or fresh samples, or between cartridge version (26). When the data were analyzed on a per site basis, the conclusions remained unchanged. Another limitation of our study is that we did not measure or account for other factors that determine an individual’s ability to transmit MTB, including the presence of lung cavitation, cough frequency, sputum consistency, or TB strain properties (29). Sputum bacillary burden is, however, the easiest of these to measure in a routine clinical context, and our findings suggest that Xpert CT value could provide a rapid and accurate measure of infectivity, even in real time at point of care. Finally, smears were done using concentrated samples in this study, and our conclusions regarding correlation of smear and TTCP may not be generalizable or comparable with settings where unconcentrated samples are used.
In conclusion, although smear microscopy has been traditionally used as a measure of infectiousness, with smear-negative TB considered less infectious but not an insignificant source of transmission (2, 29–31), our data illustrate that the Xpert CT value provides a rapid measure of sputum bacillary burden that is comparable with that of smear microscopy positivity, with moderate correlation to TTCP and superior diagnostic accuracy. National laboratory providers should consider reporting CT values to clinicians. The use of Xpert CT value could thus help prioritize resources for infection control, including contact tracing. Before such recommendation can be made, studies are required to assess the association between CT value, bacillary load in respirable cough aerosol droplets, and infection in close contacts.
Acknowledgments
Acknowledgment
The authors are grateful to all patients who participated in this study. The authors also sincerely thank Brad Cunningham at the University of the Witwatersrand for assisting in extraction of Xpert MTB/RIF cycle threshold value data at the Johannesburg site, Michael Hudgens at the University of North Carolina for statistical advice, and Isabel Rodriguez-Barraquer at the Johns Hopkins Bloomberg School of Public Health for assistance with R programming.
Footnotes
Supported by PEPFAR and the National Institutes of Health grant UM1 AI069463; United States Agency for International Development grants 674-A-00-08-00007, 674-A-12-00020, and 674-A-12-00033; European and Developing Countries Clinical Trials Partnership (TB-NEAT and TESA); and Wellcome Trust (G.T.).
Author Contributions: Conceived of and designed the study, C.F.H. and A.V.R. Data collection, C.F.H. Analysis and interpretation, C.F.H., G.T., A.V.R., W.S., and L.S. Drafting the manuscript, all authors.
Originally Published in Press as DOI: 10.1164/rccm.201312-2140OC on May 1, 2014
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc. 1954;69:724–732. doi: 10.1164/art.1954.69.5.724. [DOI] [PubMed] [Google Scholar]
- 2.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 3.Jindani A, Doré CJ, Mitchison DA. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am J Respir Crit Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- 4.Pheiffer C, Carroll NM, Beyers N, Donald P, Duncan K, Uys P, van Helden P. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis. 2008;12:792–798. [PubMed] [Google Scholar]
- 5.Perrin FM, Woodward N, Phillips PP, McHugh TD, Nunn AJ, Lipman MC, Gillespie SH. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14:1596–1602. [PubMed] [Google Scholar]
- 6.Diacon AH, Maritz JS, Venter A, van Helden PD, Andries K, McNeeley DF, Donald PR. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur J Clin Microbiol Infect Dis. 2010;29:1561–1565. doi: 10.1007/s10096-010-1043-7. [DOI] [PubMed] [Google Scholar]
- 7.Fennelly KP, Jones-López EC, Ayakaka I, Kim S, Menyha H, Kirenga B, Muchwa C, Joloba M, Dryden-Peterson S, Reilly N, et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2012;186:450–457. doi: 10.1164/rccm.201203-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health OrganizationPolicy statement: automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance. Xpert MTB/RIF system. Geneva, Switzerland: World Health Organization; 2011 [PubMed] [Google Scholar]
- 9.Blakemore R, Nabeta P, Davidow AL, Vadwai V, Tahirli R, Munsamy V, Nicol M, Jones M, Persing DH, Hillemann D, et al. A multisite assessment of the quantitative capabilities of the Xpert MTB/RIF assay. Am J Respir Crit Care Med. 2011;184:1076–1084. doi: 10.1164/rccm.201103-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K, Kop J, Owens MR, Rodgers R, Banada P, et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010;48:229–237. doi: 10.1128/JCM.01463-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Zyl-Smit RN, Binder A, Meldau R, Mishra H, Semple PL, Theron G, Peter J, Whitelaw A, Sharma SK, Warren R, et al. Comparison of quantitative techniques including Xpert MTB/RIF to evaluate mycobacterial burden. PLoS ONE. 2011;6:e28815. doi: 10.1371/journal.pone.0028815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theron G, Pinto L, Peter J, Mishra HK, Mishra HK, van Zyl-Smit R, Sharma SK, Dheda K. The use of an automated quantitative polymerase chain reaction (Xpert MTB/RIF) to predict the sputum smear status of tuberculosis patients. Clin Infect Dis. 2012;54:384–388. doi: 10.1093/cid/cir824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rachow A, Zumla A, Heinrich N, Rojas-Ponce G, Mtafya B, Reither K, Ntinginya EN, O’Grady J, Huggett J, Dheda K, et al. Rapid and accurate detection of Mycobacterium tuberculosis in sputum samples by Cepheid Xpert MTB/RIF assay: a clinical validation study. PLoS ONE. 2011;6:e20458. doi: 10.1371/journal.pone.0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chamie G, Luetkemeyer A, Walusimbi-Nanteza M, Okwera A, Whalen CC, Mugerwa RD, Havlir DV, Charlebois ED. Significant variation in presentation of pulmonary tuberculosis across a high resolution of CD4 strata. Int J Tuberc Lung Dis. 2010;14:1295–1302. [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott AM, Namaambo K, Allen BW, Luo N, Hayes RJ, Pobee JO, McAdam KP. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuber Lung Dis. 1993;74:191–194. doi: 10.1016/0962-8479(93)90010-U. [DOI] [PubMed] [Google Scholar]
- 16.Joloba ML, Johnson JL, Namale A, Morrissey A, Assegghai AE, Mugerwa RD, Ellner JJ, Eisenach KD. Quantitative sputum bacillary load during rifampin-containing short course chemotherapy in human immunodeficiency virus-infected and non-infected adults with pulmonary tuberculosis. Int J Tuberc Lung Dis. 2000;4:528–536. [PubMed] [Google Scholar]
- 17.Bruchfeld J, Aderaye G, Palme IB, Bjorvatn B, Britton S, Feleke Y, Källenius G, Lindquist L. Evaluation of outpatients with suspected pulmonary tuberculosis in a high HIV prevalence setting in Ethiopia: clinical, diagnostic and epidemiological characteristics. Scand J Infect Dis. 2002;34:331–337. doi: 10.1080/00365540110080025. [DOI] [PubMed] [Google Scholar]
- 18.Chaidir L, Parwati I, Annisa J, Muhsinin S, Meilana I, Alisjahbana B, van Crevel R. Implementation of LED fluorescence microscopy for diagnosis of pulmonary and HIV-associated tuberculosis in a hospital setting in Indonesia. PLoS ONE. 2013;8:e61727. doi: 10.1371/journal.pone.0061727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harries AD, Maher D, Nunn P. An approach to the problems of diagnosing and treating adult smear-negative pulmonary tuberculosis in high-HIV-prevalence settings in sub-Saharan Africa. Bull World Health Organ. 1998;76:651–662. [PMC free article] [PubMed] [Google Scholar]
- 20.World Health OrganizationWHO monitoring of Xpert MTB/RIF roll-out. 2013[accessed 2013 Aug 1]. Available from: http://www.who.int/tb/laboratory/mtbrifrollout/en/
- 21.Hanrahan C, Selibas K, Cunningham B, Deery CB, Scott L, Stevens W, Sanne I, Van Rie A.Quantitative Xpert MTB/RIF and the role of HIV among a cohort of TB suspects: South Africa. Presented at the Conference on Retroviruses and Opportunistic Infections. March 3–6, 2013. Atlanta, GA [Google Scholar]
- 22.Theron G, Peter J, van Zyl-Smit R, Mishra H, Streicher E, Murray S, Dawson R, Whitelaw A, Hoelscher M, Sharma S, et al. Evaluation of the Xpert MTB/RIF assay for the diagnosis of pulmonary tuberculosis in a high HIV prevalence setting. Am J Respir Crit Care Med. 2011;184:132–140. doi: 10.1164/rccm.201101-0056OC. [DOI] [PubMed] [Google Scholar]
- 23.Enarson DA, Rieder HL, Arnadottir TA.Management of tuberculosis: a guide for low income countries. Paris, France: IUATLD; 2000 [Google Scholar]
- 24.Haukoos JS, Lewis RJ. Advanced statistics: bootstrapping confidence intervals for statistics with “difficult” distributions. Acad Emerg Med. 2005;12:360–365. doi: 10.1197/j.aem.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Myers L, Sirois MJ.Differences between Spearman correlation coefficientsKotz S, Read CB, Balakrishnan N, Vidakovic B, editors. Encyclopedia of statistical sciences. Hoboken, NJ: John Wiley and Sons; 2004. pp. 1–2. [Google Scholar]
- 26.Steingart KR, Sohn H, Schiller I, Kloda LA, Boehme CC, Pai M, Dendukuri N. Xpert MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2013;1:CD009593. doi: 10.1002/14651858.CD009593.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz M.External quality assessment for AFB smear microscopy. Washington, DC: Association of Public Health Laboratories; 2002 [Google Scholar]
- 28.Kayigire XA, Friedrich SO, Venter A, Dawson R, Gillespie SH, Boeree MJ, Heinrich N, Hoelscher M, Diacon AH Pan African Consortium for the Evaluation of Anti-tuberculosis Antibiotics. Direct comparison of Xpert MTB/RIF assay with liquid and solid mycobacterial culture for quantification of early bactericidal activity. J Clin Microbiol. 2013;51:1894–1898. doi: 10.1128/JCM.03290-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Escombe AR, Moore DA, Gilman RH, Pan W, Navincopa M, Ticona E, Martínez C, Caviedes L, Sheen P, Gonzalez A, et al. The infectiousness of tuberculosis patients coinfected with HIV. PLoS Med. 2008;5:e188. doi: 10.1371/journal.pmed.0050188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernández-Garduño E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59:286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, van Soolingen D. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47:1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]