To the Editor:
Intelectin-1 (ITLN-1) is an epithelial cell protein that is up-regulated in asthma (1). ITLN-1 is a pleotropic adipokine (also known as omentin-1) with roles in the gut ranging from host defense against pathogenic bacteria to promotion of insulin-stimulated glucose uptake (2–4). The host defense roles of ITLN-1 may result from its ability to bind structures expressed by microorganisms in a carbohydrate-dependent manner (5). ITLN-1 is also a binding partner for lactoferrin (6), and ITLN-1 may cooperate with lactoferrin in host defense (6, 7).
Little is known about the function of ITLN-1 in human asthma. One possibility is that it participates in pathways of inflammation downstream of IL-13 (1). Indeed, studies in a mouse model of asthma suggest that ITLN-1 mediates IL-13–induced monocyte chemotactic protein-1 and -3 production in epithelial cells (8). Another possibility is that ITLN-1 is a component of airway mucus and contributes to pathologic mucus gel formation in disease. Supporting this possibility are studies in the gastrointestinal tract showing that ITLN-1 is a goblet cell protein that is secreted with mucus into the intestinal lumen (9). In addition, other studies in the intestine have suggested mucin–intelectin interactions that could alter the biophysical properties of mucus (10). Some of the results of these studies have been previously reported in the form of an abstract (11)
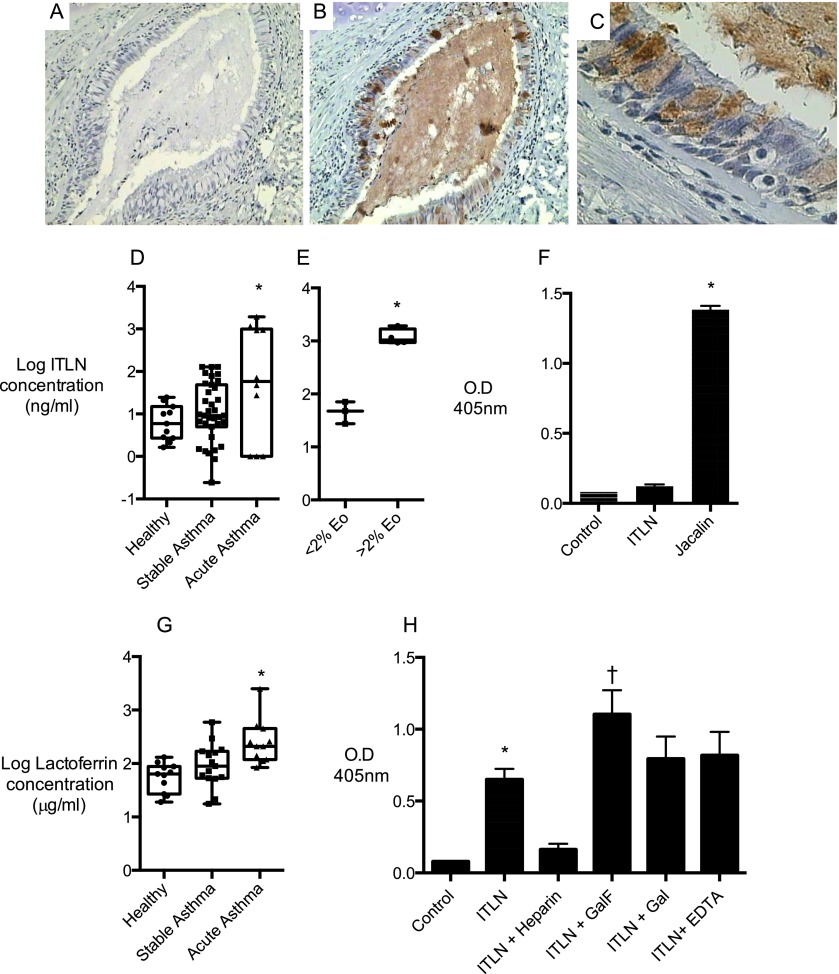
Because mucus pathology causes mucus plugging and airway occlusion (12, 13), especially in fatal asthma (14), we set out to determine if ITLN-1 is a component of pathologic mucus in acute asthma. We first immunostained lung tissue sections from cases of fatal asthma and found prominent ITLN-1 immunostaining in the pathologic mucus plugs that occlude the airways (Figures 1A–1C). The cellular source of the ITLN-1 appears to be goblet cells (Figure 1C). We next measured ITLN-1 protein in sputum from 11 patients with acute severe asthma and two control groups (35 subjects with chronic stable asthma and 11 healthy control subjects) (Table 1). We found that ITLN-1 protein levels in the subgroup of patients with asthma in exacerbation were significantly higher than in stable asthma and in healthy control subjects (Figure 1D). We also noted that the increase in ITLN-1 in acute asthma was driven by the subgroup with increased sputum eosinophils (>2%) (Figure 1E), a finding that is consistent with ITLN-1’s regulation by IL-13 in airway epithelial cells (1). ITLN-1 up-regulation is thus a feature of “Th2-high” asthma, and the known pathologic characteristics of this disease endotype can be extended to include high ITLN-1 protein concentrations in mucus forming during disease exacerbations.
Figure 1.
Intelectin-1 (ITLN-1) protein in airway biospecimens and binding of ITLN-1 to airway mucins and lactoferrin. Sections of lung tissue from lungs of patients with fatal asthma were stained with an anti–ITLN-1 antibody or peptide blocking control. (A) The peptide blocking control showed no staining. (B) The anti–ITLN-1 antibody showed prominent immunostaining in occlusive airway mucus plugs. (C) Higher magnification images show expression of ITLN-1 is prominent in goblet cells. The data are representative of findings in lung sections from four fatal asthma cases; additional methods details are provided in the online supplement. (D) ITLN-1 concentrations in spontaneously expectorated sputum from patients experiencing an acute exacerbation (n = 11) are higher than those in induced sputum from patients with chronic stable asthma (n = 35) or healthy nonasthmatic control subjects (n = 11). (E) In sputum from patients with acute asthma exacerbation, the concentrations of ITLN-1 are higher in sputum with eosinophilia (n = 4) than without (n = 6). (F) Biotinylated ITLN-1 does not bind to mucins purified from asthmatic-induced sputum and immobilized on a microtiter plate; biotinylated jacalin binds well. *Significantly different at P < 0.05. Additional details about human subjects, biospecimens, the ITLN-1 assay, and the mucin binding assay are provided in the online supplement. (G) Lactoferrin concentrations in spontaneously expectorated sputum from patients experiencing an acute exacerbation (n = 11) are higher than those in induced sputum from patients with chronic stable asthma (n = 15) or healthy nonasthmatic control subjects (n = 11). (H) Biotinylated ITLN-1 binding to human milk lactoferrin is inhibited by heparin and increased by methyl galactofuranose. Galactose and EDTA do not influence ITLN-1 binding to lactoferrin. *Significantly different at P < 0.05. Additional details about the lactoferrin assay and the binding assay are provided in the online supplement.
Table 1:
Subject Characteristics
| Healthy | Asthma | |
|---|---|---|
| Induced sputum study | ||
| n | 11 | 35 |
| Age, yr | 37 ± 12 | 36 ± 13 |
| Female, n (%) | 5 (45) | 23 (66) |
| BMI, kg/m2 | 26.6 ± 4.9 | 28.7 ± 7.1 |
| FEV1, L | 4.01 ± 1.00 | 2.73 ± 0.90 |
| FEV1, % predicted | 108.1 ± 11.0 | 79.1 ± 17.4 |
| PC20 (n = 31) | N/A | 0.69 (0.12–12.60) |
| On ICS, n (%) | N/A | 19 (54) |
| Smoking history | ||
| Never, n (%) | 8 (73) | 17 (49) |
| Former, n (%) | 3 (27) | 18 (51) |
| Pack-years | 0 (0–1.36) | 0 (0–10.00) |
| Acute asthma study | ||
| n | 11 | |
| Age, yr | 43 ± 11 | |
| Female, n (%) | 8 (73) | |
| On ICS, n (%) | 9 (82) | |
| On LABA, n (%) | 6 (55) | |
| On anticholinergic, n (%) | 3 (27) | |
| % admitted | 2 (18) | |
| Smoking history | ||
| Never, n (%) | 3 (27) | |
| Former, n (%) | 7 (64) | |
| Current, n (%) | 1 (9) | |
| Pack-years | 1 (0–8) | |
| On ICS, n (%) | 3 (60) | |
| Mucin study | ||
| n | 5 | |
| Age, yr | 39.4 ± 14.5 | |
| Female, n (%) | 3 (60) | |
| BMI, kg/m2 | 29.7 ± 7.4 | |
| FEV1, L | 2.55 ± 0.86 | |
| FEV1, % predicted | 73.6 ± 17.4 | |
| Smoking history | ||
| Never, n (%) | 3 (60) | |
| Former, n (%) | 2 (40) | |
| Pack-years | 0 (0–8.5) | |
| PC20 | 0.39 (0.15–1.19) | |
| On ICS, n (%) | 3 (60) |
Definition of abbreviations: BMI = body mass index; ICS = inhaled corticosteroids; LABA = long-acting β-agonists; PC20 = provocative concentration of methacholine (mg/ml) that results in a 20% fall in FEV1.
Values are presented as mean ± SD or median (range), unless otherwise noted.
The prominent immunostaining for ITLN-1 in mucus plugs in fatal asthma and the high concentrations of ITLN-1 in sputum in acute severe asthma prompted us to explore if ITLN-1 can bind to human airway mucins. ITLN-1 is a lectin with known specificity for galactosyl structures, especially the galactofuranosyl sugars expressed by microorganisms (5). To determine if ITLN-1 binds to human airway mucin glycans, we developed a plate-based binding assay using high-molecular-weight mucin preparations that we purified from induced sputum samples from subjects with chronic stable asthma (“mucin study”; Table 1). Specifically, we used biotinylated recombinant ITLN-1 to probe mucin coated on microtiter plates (see online supplement). Biotinylated jacalin, a tetrameric plant seed lectin with specificity for galactose, was used as a positive control. Although jacalin showed binding to mucin, ITLN-1 did not (Figure 1F). It is possible that ITLN-1 cannot recognize the pyranosyl forms of galactose in human mucin, but another possibility is that mucin glycans prevent binding through steric hindrance. It could also be that the plate assay is suboptimal for measuring ITLN-1 binding to mucin because other proteins or cofactors involved in an ITLN-1–mucin interaction in vivo are not represented in vitro.
Because ITLN-1 has been characterized as the lactoferrin receptor (6, 7), we considered if ITLN-1 interacts with lactoferrin in airway mucus in acute asthma. We found that lactoferrin levels in sputum from patients with acute severe asthma are significantly higher than in control samples (Figure 1G). Notably, the concentration of lactoferrin ranged from 500 to 1,000 μg/ml in some of these sputum samples, a 1,000-fold higher concentration than ITLN-1. This large amount of lactoferrin in asthmatic mucus may bind and concentrate ITLN-1 in mucus. To examine the binding of ITLN-1 to lactoferrin, we used a plate-binding assay similar to the one we used for mucin-ITLN-1 binding. In this way, we found that biotinylated ITLN-1 binds avidly to immobilized lactoferrin (Figure 1H). This binding was inhibited by heparin, suggesting a charge-based interaction between ITLN-1 and lactoferrin’s basic N-terminal region (15), and increased by methyl galactofuranoside. Galactofuranoside is found in many microbial polysaccharides and is recognized as a preferred glycan ligand for ITLN-1 (7, 16). Our data suggest that ITLN-1 binding to galactofuranosyl residues on microorganisms might improve its ability to bind lactoferrin and target it to regions of high microorganism burden.
We conclude that ITLN-1 is a prominent protein component of pathologic mucus in fatal asthma and in acute severe asthma, especially in the context of eosinophilic airway inflammation. The binding of ITLN-1 to lactoferrin is increased by galactofuranoside providing a mechanism by which ITLN-1 can cooperate with lactoferrin to defend against microbes.
Footnotes
This work was supported by National Institutes of Health grant HL080414 and HL107201 and by a research grant from Genentech.
This letter has an online supplement, which is accessible from this issue's table of contents online at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM, Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functional genomics. J Allergy Clin Immunol. 2005;116:305–311. doi: 10.1016/j.jaci.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochem Biophys Res Commun. 1998;251:759–762. doi: 10.1006/bbrc.1998.9513. [DOI] [PubMed] [Google Scholar]
- 3.Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. 2010;20:143–148. doi: 10.1016/j.tcm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, Shuldiner AR, Fried SK, McLenithan JC, Gong DW. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, Seya T. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276:23456–23463. doi: 10.1074/jbc.M103162200. [DOI] [PubMed] [Google Scholar]
- 6.Suzuki YA, Shin K, Lönnerdal B. Molecular cloning and functional expression of a human intestinal lactoferrin receptor. Biochemistry. 2001;40:15771–15779. doi: 10.1021/bi0155899. [DOI] [PubMed] [Google Scholar]
- 7.Shin K, Wakabayashi H, Yamauchi K, Yaeshima T, Iwatsuki K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol Pharm Bull. 2008;31:1605–1608. doi: 10.1248/bpb.31.1605. [DOI] [PubMed] [Google Scholar]
- 8.Gu N, Kang G, Jin C, Xu Y, Zhang Z, Erle DJ, Zhen G. Intelectin is required for IL-13-induced monocyte chemotactic protein-1 and -3 expression in lung epithelial cells and promotes allergic airway inflammation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L290–L296. doi: 10.1152/ajplung.90612.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Washimi K, Yokose T, Yamashita M, Kageyama T, Suzuki K, Yoshihara M, Miyagi Y, Hayashi H, Tsuji S. Specific expression of human intelectin-1 in malignant pleural mesothelioma and gastrointestinal goblet cells. PLoS ONE. 2012;7:e39889. doi: 10.1371/journal.pone.0039889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pemberton AD, Verdon B, Inglis NF, Pearson JP. Sheep intelectin-2 co-purifies with the mucin Muc5ac from gastric mucus. Res Vet Sci. 2011;91:e53–e57. doi: 10.1016/j.rvsc.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Kerr SC, Carrington SD, Yuan S, Solon M, Fahy JV. Galactose binding lectins cross-link airway mucins and are a novel mediator of mucus plug formation in acute asthma. Am J Respir Crit Care Med. 2010;181:A5510. [Google Scholar]
- 12.Dougherty RH, Fahy JV. Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy. 2009;39:193–202. doi: 10.1111/j.1365-2222.2008.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med. 2010;363:2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hays SR, Fahy JV. The role of mucus in fatal asthma. Am J Med. 2003;115:68–69. doi: 10.1016/s0002-9343(03)00260-2. [DOI] [PubMed] [Google Scholar]
- 15.Mann DM, Romm E, Migliorini M. Delineation of the glycosaminoglycan-binding site in the human inflammatory response protein lactoferrin. J Biol Chem. 1994;269:23661–23667. [PubMed] [Google Scholar]
- 16.Tsuji S, Yamashita M, Hoffman DR, Nishiyama A, Shinohara T, Ohtsu T, Shibata Y. Capture of heat-killed Mycobacterium bovis bacillus Calmette-Guérin by intelectin-1 deposited on cell surfaces. Glycobiology. 2009;19:518–526. doi: 10.1093/glycob/cwp013. [DOI] [PMC free article] [PubMed] [Google Scholar]