Abstract
Rationale: The risk of cardiovascular events after severe sepsis is not known, and these events may explain increased long-term mortality in survivors of severe sepsis.
Objectives: To determine whether survivors of severe sepsis hospitalization have high long-term risk of cardiovascular events. We examined whether higher risk is due to severe sepsis hospitalization or poor prehospitalization health status, and if the higher risk is also observed in patients hospitalized for infectious and noninfectious reasons, and in other critically ill patients.
Methods: Unmatched and matched-cohort analyses of Medicare beneficiaries. For unmatched analysis, we compared patients with severe sepsis admitted to the intensive care unit (ICU) and survived hospitalization (n = 4,179) to unmatched population control subjects (n = 819,283). For matched analysis, we propensity-score-matched each patient with severe sepsis to four control subjects (population, hospitalized, non–severe sepsis ICU control subjects, and infection hospitalization). Primary outcome was 1-year incidence rate of hospitalization for cardiovascular events.
Measurements and Main Results: Cardiovascular events were common among patients discharged alive after severe sepsis hospitalization (29.5%; 498.2 events/1,000 person-years). Survivors of severe sepsis had a 13-fold higher risk of cardiovascular events compared with unmatched control subjects (498.2 vs. 36 events/1,000 person-years; P < 0.0001), and a 1.9-fold higher risk compared with matched-population control subjects (P < 0.0001). Survivors of severe sepsis had 1.1-fold higher risk compared with matched hospitalized patients and infection hospitalizations (P = 0.002 and 0.001) and similar risk compared with matched-ICU control subjects.
Conclusions: Survivors of severe sepsis have high risk of cardiovascular events. The higher risk is mainly due to poor prehospitalization health status, and is also seen in a broader population of acutely ill patients.
Keywords: sepsis, severe sepsis, cardiovascular disease, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
Cardiovascular events may be an important reason for increased long-term mortality in survivors of sepsis, but the long-term risk of cardiovascular events after severe sepsis is not known.
What This Study Added to the Field
This study shows that survivors of severe sepsis have high risk of cardiovascular events, but the higher risk is mainly due to poor health status before occurrence of severe sepsis, and is also seen in a broad group of patients requiring acute care.
Severe sepsis occurs in over 750,000 individuals in the United States, up to 19 million individuals worldwide annually, and accounts for 10% of all intensive care unit (ICU) admissions (1–3). Survivors of severe sepsis have high long-term mortality (4), and these consequences cannot be solely explained by poor health status before the infection (5, 6). A potential mechanism to explain increased long-term mortality is worsening of pre-existing chronic diseases or emergence of new chronic diseases. Although prior studies have examined whether severe sepsis increases the risk of chronic diseases, such as dementia (7), whether severe sepsis increases long-term risk of cardiovascular disease (CVD) is not known.
We analyzed the relationship between severe sepsis and subsequent cardiovascular events in over 1.6 million Medicare beneficiaries. We focused on cardiovascular events, including myocardial infarction, stroke, transient ischemic attack (TIA), and coronary artery revascularization, because prior studies suggest that the short-term risk of these events is high after less severe infections (6, 8–11), cardiovascular events are a leading cause of death in survivors of infection hospitalization and the general population (12), and prevention strategies are readily available. We sought to address three questions. First, we determined whether patients with severe sepsis who required ICU care and survived the hospitalization had high risk of subsequent cardiovascular events. Second, we examined whether the higher risk is attributed to poor prehospitalization health status or severe sepsis hospitalization itself. Third, we examined whether the higher risk of cardiovascular events also occurs in similar patients hospitalized for infectious or noninfectious reasons or required ICU care, but did not develop severe sepsis.
Methods
See the online supplement for additional details.
Study Design
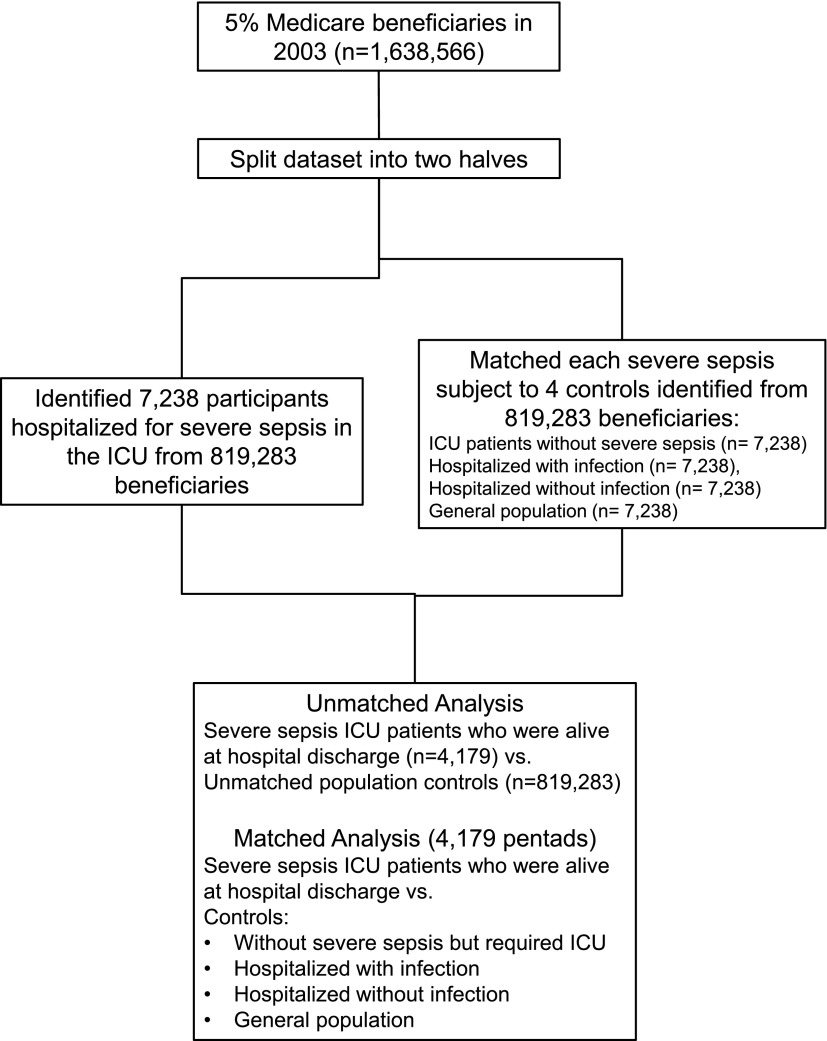
We conducted a retrospective matched-cohort analysis where patients with severe sepsis requiring ICU care were matched to several control subjects. The patients with severe sepsis and control subjects were selected from a 5% random sample of fee-for-service Medicare beneficiaries over 65 years of age from 2002–2006 (see the online supplement). We identified patients with severe sepsis and control subjects in 2003, used data from 2002 with a 1-year lookback approach to determine health status before the index hospitalization (13), and used data from the index hospitalization until 2006 or death to examine outcomes. We randomly split the 2003 dataset into two halves and used two approaches to identify patients with severe sepsis and control subjects (Figure 1). First, we identified patients with severe sepsis who were admitted to the ICU and survived the hospitalization in one half, and compared their risk of cardiovascular events to all Medicare beneficiaries in the other half (unmatched population control subjects). Second, we matched each patient with severe sepsis admitted to the ICU from one half and matched them to four propensity-matched control subjects with similar health status before the index hospitalization from the other half. We used an unbiased approach similar to incidence density sampling to match patients with severe sepsis and control subjects (14), where each patient with severe sepsis from one half of the dataset is matched to control subjects from the other half based on whether they were at risk for severe sepsis during that quarter in 2002, regardless of whether they developed severe sepsis in later quarters (15). This research was considered exempt from human subject review by the University of Pittsburgh Institutional Review Board, because it involved secondary analyses of deidentified data.
Figure 1.
Flowchart describing selection of subjects for unmatched and matched analyses. ICU = intensive care unit.
Severe Sepsis and Control Subjects
We identified patients with severe sepsis using previously validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9 CM) codes (16). For each patient with severe sepsis admitted to the ICU, we matched four control subjects to form a pentad. Control subjects were matched population control subjects, those who were hospitalized, but never had an infection (matched hospitalized control subjects), individuals admitted to the ICU, but not with severe sepsis (matched ICU control subjects), and those hospitalized with infection, but who did not incur organ dysfunction (matched infection hospitalizations). Reasons for each control group are described in Table 1 and in brief subsequently here.
Table 1:
Incidence of Cardiovascular Events Was Compared among Patients with Severe Sepsis Who Required Intensive Care Unit Care and Survived and Control Subjects
| Control Subjects | n | Reasons for Choosing Control Group |
|---|---|---|
| Unmatched population control subjects* | 819,283 | To compare incidence of cardiovascular events among survivors of severe sepsis to the average annual risk of cardiovascular events among all Medicare beneficiaries* |
| Matched population control subjects* | 4,179 | To compare incidence of cardiovascular events among survivors of severe sepsis to individuals whose health status was similar to patients with severe sepsis |
| Matched hospital control subjects | 4,179 | To compare incidence of cardiovascular events among survivors of severe sepsis to patients who require acute care. Inclusion of these control subjects allowed for better adjustment for prehospitalization health status (e.g., worsening physical function) immediately before the hospitalization, which may not be captured in claims data, and to account for diagnostic (selection) bias. Diagnostic bias may occur because events during the hospitalization may lead to additional investigations during the hospitalization or recovery to uncover coronary artery disease and subsequently require revascularization. |
| Matched infection hospitalization | 4,179 | To determine whether higher risk is unique to patients with severe sepsis (infection and organ dysfunction) or occurs in similar patients with infection alone |
| Matched ICU control subjects | 4,179 | To determine whether higher risk is unique to patients with severe sepsis who require ICU care and survive hospitalization or occurs in a broader population of ICU patients without severe sepsis. |
Definition of abbreviation: ICU = intensive care unit.
Incidence of cardiovascular events was compared among unmatched and matched control subjects to determine the risk attributable to poor health status among patients with severe sepsis.
We compared the risk of cardiovascular events among patients with severe sepsis to unmatched and matched population control subjects to determine whether the higher risk of cardiovascular events after severe sepsis is largely attributable to poor health status before the index hospitalization or due to severe sepsis itself. We compared risk of CVD among ICU patients with severe sepsis and three control subjects, including matched hospitalized control subjects, infection hospitalizations, and ICU control subjects. We chose matched hospitalized control subjects to better account for changes in prehospitalization health status during the weeks before the index hospitalization that may not be captured using claims data, and to determine whether the higher risk of CVD is observed in a broader population of patients requiring acute care (Table 1). We included infection hospitalization to determine whether risk of CVD varies by presence of organ dysfunction. We included ICU control subjects without severe sepsis to determine whether higher risk is observed in similar patients who required ICU care.
All three hospitalized controls were constructed using methods similar to the incidence density sampling approach and selecting index hospitalizations that occurred in the same quarter as that of the corresponding severe sepsis case. Population control subjects were individuals who were not hospitalized during the same quarter, regardless of whether they were hospitalized at later time points, and were randomly chosen individuals from the Medicare dataset who had at least one claim filed during 2003.
Propensity Score and Control Subjects
We matched cases to control subjects based on age, sex, admission for medical or surgical reasons, CVD before the index hospitalization, and propensity to be hospitalized with severe sepsis. The propensity score (see the online supplement) was developed in one half of the cohort and validated in the other half. The propensity score included age, sex, and detailed measures of different chronic diseases, including CVD, and infection hospitalization (Tables E3 and E4 in the online supplement) (17–21). Although age, sex, and prior history of CVD were included in the propensity score, we additionally matched for these variables, because they are important confounders, and were not matched well in some pentads using propensity score alone. We matched for CVD before the index hospitalization by assigning subjects to four hierarchical groups based on previously validated ICD-9 CM codes (Tables E1 and E2): those with cardiovascular events, presence of chronic CVD, and presence of two or more or less than two risk factors based on the Framingham score (22). Details of the 1-year lookback approach to compare health status (13), the ICD-9 CM codes to identify different conditions, and model performance are included in the online supplement.
The Medicare 5% dataset included 1,638,566 eligible subjects (Figure 1). For the matched analyses, we identified 4,179 patients with severe sepsis who were admitted to the ICU and survived hospitalization, and the four sets of matched control subjects, for a total cohort of 20,895. For the unmatched analyses, we compared the 4,179 patients with severe sepsis who survived severe sepsis hospitalization to 819,283 unmatched population control subjects.
Outcomes
The primary outcome was hospitalization for cardiovascular events (stroke, TIA, myocardial infarction, or coronary revascularization, including percutaneous coronary intervention and coronary artery bypass graft surgery) during the first year after hospital discharge, assigned based on previously validated ICD-9 CM code-based approaches (22–24).
Statistical Analysis
We constructed failure plots to compare number of patients in each group that incurred a new cardiovascular event after the index hospitalization. A limitation of time to event analysis is that it cannot account for multiple events. We therefore compared the incidence of cardiovascular events in ICU patients with severe sepsis to unmatched population control subjects and matched control subjects. Patients with severe sepsis had higher long-term mortality, and comparing incidence rates allowed us to account for differences in exposure time between patients with severe sepsis and control subjects. Because we conducted five comparisons, we used a P value of less than 0.01 to indicate statistical significance. We used a negative binomial regression model to compare the risk of cardiovascular events (25). Details of statistical approaches and sensitivity analyses are included in the online supplement.
Results
Participant Characteristics
Table 2 shows clinical characteristics before the index hospitalization. The average age of all subjects was 77 years, 44% were men, and 11% were black. Both ICU patients with severe sepsis and matched control subjects had a high burden of chronic diseases. For instance, approximately 26% had chronic CVD and 30% had cardiovascular events within the preceding year. Approximately 37% had diabetes, 31% had chronic lung disease and cancer, 24% were hospitalized with infection, and 10% had chronic kidney disease. In comparison to patients with severe sepsis, unmatched control subjects had a lower burden of chronic diseases, including diabetes (37.5 vs. 18%), chronic lung disease (31.7 vs. 19.2%), CVD (26.6 vs. 15.7%), and kidney disease (10.2 vs. 3.4%). In addition, acute health conditions were also less frequent among unmatched control subjects compared with patients with severe sepsis in the prior year, such as acute exacerbation of chronic lung disease (12.7 vs. 2.9%), cardiovascular events (29.4 vs. 13.1%), and infection hospitalization (24.5 vs. 5.8%).
Table 2:
Clinical Characteristics of Patients Hospitalized with Severe Sepsis in the Intensive Care Unit
| Propensity-matched Cohort |
Unmatched Cohort |
||||||
|---|---|---|---|---|---|---|---|
| |
Developed Severe Sepsis and Required ICU Care |
Required ICU Care but Did Not Develop Severe Sepsis |
Hospitalized with Infection |
Hospitalized Patients |
Population Control Subjects |
Developed Severe Sepsis and Required ICU Care |
Population Control Subjects |
| Variables | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 819,283) |
| Demographics | |||||||
| Age in years, mean (SD, median) | 77.4 (7, 77) | 75.7 (13, 77) | 77.2 (8, 77) | 77.4 (7, 77) | 77.4 (7, 77) | 77.4 (7, 77) | 75.9 (7.3, 75) |
| Race, % black | 11.6 | 10.9 | 9.8 | 11.2 | 11.3 | 11.6 | 8 |
| Sex, % male | 44.2 | 44.2 | 44.2 | 44.2 | 44.2 | 44.2 | 41.3 |
| Propensity score, mean (SD, median) | 14.3 (8.4, 13) | 13.9 (8.5, 13) | 14.1 (8.3, 13) | 14.2 (8.2, 13) | 14.1 (8.2, 13) | 14.3 (8.4, 13) | 7.5 (6.3, 6) |
| Health conditions during the year before index admission | |||||||
| Diabetes, % | 37.5 | 37.1 | 36.9 | 37.3 | 36.6 | 37.5 | 18 |
| Lung disease, % | |||||||
| Chronic lung disease | 31.7 | 31.8 | 31.2 | 31.4 | 30.9 | 31.7 | 19.2 |
| Acute exacerbation of chronic lung disease | 12.7 | 13.1 | 14.5 | 10.7 | 13.7 | 12.7 | 2.9 |
| CVD, % | |||||||
| <Two CVD risk factors* | 28.3 | 28.2 | 28.4 | 28.3 | 28.3 | 28.3 | 53.6 |
| ≥Two CVD risk factors* | 15.7 | 15.1 | 15.7 | 15.7 | 15.7 | 15.7 | 17.6 |
| Chronic CVD | 26.6 | 26.7 | 26.4 | 26.6 | 26.6 | 26.6 | 15.7 |
| Cardiovascular events | 29.4 | 30.0 | 29.5 | 29.4 | 29.4 | 29.4 | 13.1 |
| Dementia, % | 1.6 | 1.0 | 1.4 | 1.0 | 1.3 | 1.6 | 3.2 |
| Infection, % | |||||||
| As an outpatient | 41.0 | 40.1 | 39.3 | 44.3 | 43.5 | 41.0 | 26.2 |
| Hospitalized | 24.5 | 24.9 | 27.7 | 22.9 | 24.1 | 24.5 | 5.8 |
| Kidney disease, % | |||||||
| Chronic kidney disease | 10.2 | 9.8 | 8.3 | 10.1 | 10.4 | 10.2 | 3.4 |
| Dialyses or acute kidney injury | 8.5 | 7.7 | 5.8 | 8.2 | 6.4 | 8.5 | 1.2 |
| Cancer, % | |||||||
| Nonhematologic cancer | 29.1 | 32.7 | 31.0 | 35.5 | 30.8 | 29.1 | 20.7 |
| Hematologic cancer | 3.1 | 3.0 | 3.2 | 3.5 | 3.5 | 3.1 | 1.3 |
| Reason for admission for hospitalized subjects, % | |||||||
| Medical | 63.7 | 63.8 | 65 | 63.7 | — | 63.7 | — |
| Surgical | 36.3 | 36.2 | 35 | 36.3 | — | 36.3 | — |
| Length of stay in days, median (interquartile range) | |||||||
| Intensive care unit | 6 (3–11) | 2 (1–5) | — | — | — | 6 (3–11) | — |
| Hospital stay | 11 (7–18) | 6 (3–10) | 6 (3–10) | 4 (2–6) | — | 11 (7–18) | — |
Definition of abbreviations: CVD = cardiovascular disease; ICU = intensive care unit.
Matched control subjects were matched based on age, sex, admission for medical or surgical reasons, and propensity score based on health conditions during the year before index admission (score assigned as follows: 9 points for hospitalization for infection, dialysis or acute kidney injury, and respiratory category 2; 6 points for age over 85 years; 5 points for chronic renal disease; 4 points for chronic pulmonary disease, leukemia, outpatient visit for infection; 3 points for infection, chronic CVD, or unstable angina, black race; 2 points for men; 2 points for hospitalizations for conditions other than infections; and 1 point for any other chronic disease (up to a maximum of 2 points). These subjects were in the ICU, hospitalized with infection, hospitalized for other reasons, and general population. Only patients with severe sepsis and hospitalized control subjects who were alive at hospital discharge are shown.
CVD risk factors include smoking, hypertension, diabetes, and hyperlipidemia, and are based on the Framingham risk score.
As expected, all ICU patients with severe sepsis incurred organ failure, and none of the patients hospitalized with infection incurred organ failure. Of the patients with severe sepsis, 449 (10.7%) had shock. A small proportion of hospitalized and ICU control subjects also incurred organ dysfunction (13.5 and 9.5% of ICU and hospitalized control subjects). As expected, the cases and control subjects had similar characteristics for variables used for matching, such as demographic characteristics (age and sex), propensity score, and chronic diseases (Table 2).
Mortality
Patients with severe sepsis who required ICU admission and survived had higher mortality at 1, 2, and 3 years compared with the control groups (Table 3). For instance, the 1-year mortality for patients with severe sepsis requiring ICU admission and surviving was sevenfold higher compared with unmatched population control subjects (40.8 vs. 5.3%; P < 0.0001). Patients with severe sepsis had threefold higher mortality at 1 year compared with matched population control subjects, twofold higher compared with hospitalized control subjects, and 1.5-fold higher compared with those infection hospitalization and ICU control subjects with nonsevere sepsis (1-yr mortality for severe sepsis requiring ICU, nonsevere sepsis ICU control subjects, infection hospitalization, hospital control subjects, and population control subjects were 40.8, 25.4, 27.9, 20.5, and 12.8%, respectively; P < 0.0001). For the subset that was discharged home, the mortality rates were lower for the different groups, but the magnitude of differences was similar (Table 3).
Table 3:
Mortality during the Subsequent Year for Patients Hospitalized with Severe Sepsis in the Intensive Care Unit, Matched Intensive Care Unit Control Subjects, Matched Hospitalized with Infection, Matched Hospital Control Subjects, and Matched and Unmatched Population Control Subjects
| |
Developed Severe Sepsis and Required ICU Care |
Required ICU Care but Did Not Develop Severe Sepsis |
Hospitalized with Infection but Did Not Require ICU Care |
Hospitalized Patients |
Matched Population Control Subjects |
Unmatched Population Control Subjects |
|---|---|---|---|---|---|---|
| Variables | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 4,179) | (n = 819,283) |
| Mortality, %* | ||||||
| 1-yr mortality | 40.8 | 25.4 | 27.9 | 20.5 | 12.8 | 5.3 |
| 2-yr mortality | 51.2 | 36.5 | 38.9 | 30.7 | 21.3 | 10.3 |
| 3-yr mortality | 58.9 | 44.3 | 48.2 | 39.1 | 28.5 | 15.3 |
| Mortality for those discharged home, % | ||||||
| 1-yr mortality | 27.4 | 17.83 | 21.4 | 16.1 | — | — |
| 2-yr mortality | 40.1 | 29.2 | 31.6 | 25.5 | — | — |
| 3-yr mortality | 47.2 | 36.9 | 40.6 | 33.7 | — | — |
For definition of abbreviation see Table 1.
All hospitalized patients survived to hospital discharge during the index admission.
P < 0.0001 for comparisons between patients with severe sepsis and control subjects.
Cardiovascular Events
A cardiovascular event was common among survivors of severe sepsis hospitalization. For example, 29.5% incurred a cardiovascular event over 1 year (498.2 events/1,000 person-years). Stroke was the most common event, occurring in 18.3%, TIA and acute myocardial infarction occurred in 7.8 and 8.8%, respectively, whereas 1.3 and 0.4% underwent percutaneous coronary intervention and coronary artery bypass graft surgery. Of the 29.5% who incurred a cardiovascular event, most patients incurred a single event (23.1%), whereas 5.6, 0.7, and 0.1% incurred two, three, and four cardiovascular events, respectively. A total of 45% of survivors of severe sepsis did not have CVD before the hospitalization, and even this subgroup had a high risk of subsequent cardiovascular events (25.9%, 364.4 events/1,000 person-years). The incidence was high even when subjects who incurred a cardiovascular event during severe sepsis hospitalization were excluded (445.2 events/1,000 person-years; n = 2,562).
The incidence of cardiovascular events in unmatched population control subjects (n = 819,283) was consistent with prior studies (10.3 and 11 cases per 1,000 person-years for myocardial infarction and stroke, respectively) (23, 24, 26). Patients with severe sepsis had a 13-fold higher risk of cardiovascular events compared with unmatched control subjects (incidence: 498.2 versus 36 events/1,000 person-years; P < 0.0001).
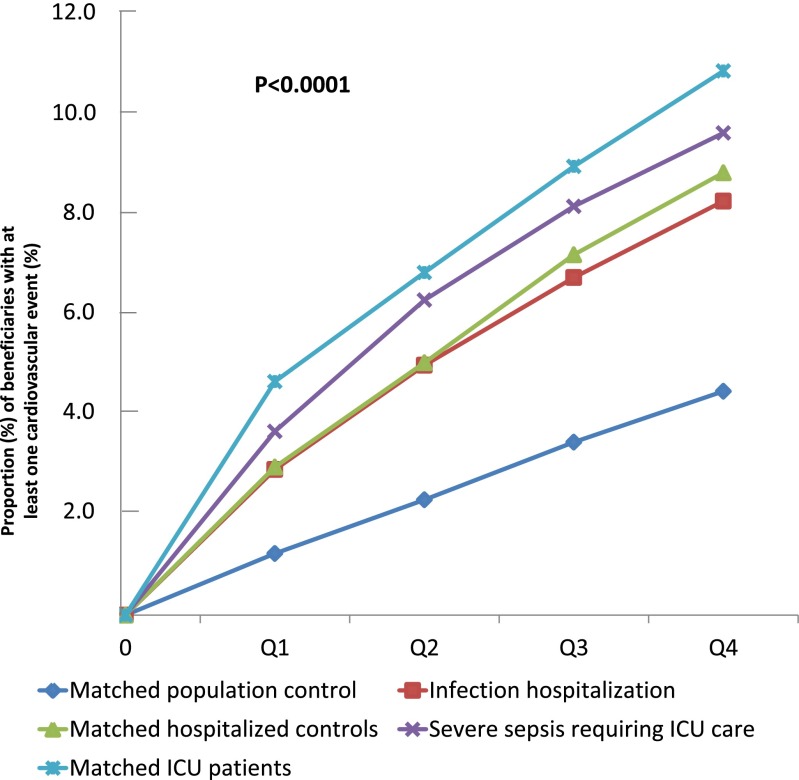
Figure 2 shows the failure plots for patients with severe sepsis and control subjects who incurred a cardiovascular event over the four quarters. In general, patients with severe sepsis and matched ICU, hospitalized, and infection hospitalization control subjects had similar 1-year risk of having at least one cardiovascular event (9.7, 10.9, 8.9, and 8.3%, respectively), and the risk was lower for the matched general population (4.5%).
Figure 2.
Failure plots showing proportion of beneficiaries with at least one cardiovascular event after the index hospitalization. Data are shown by quarter in the calendar year, because exact hospitalization dates are not available. The first, second, third, and fourth quarter after the index hospitalizations are labeled as Q1, Q2, Q3, and Q4, respectively. ICU = intensive care unit.
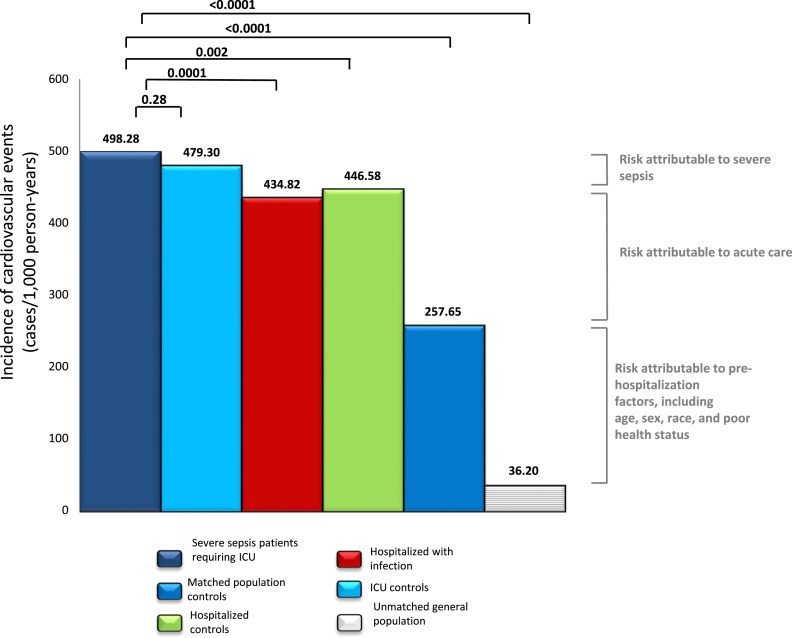
Severe sepsis was associated with a 1.9-fold higher risk of cardiovascular events when compared with matched population control subjects (incidence rates: 498.2 vs. 257.6 events/1,000 person-years; P < 0.01; Figure 3). However, the risk of cardiovascular events was only 1.1-fold higher for patients with severe sepsis compared with matched hospitalized control subjects (incidence rates: 498.2 vs. 446.5 events/1,000 person-years; P = 0.002; Figure 3) and similar among patients with severe sepsis and ICU control subjects without severe sepsis (498.2 vs. 479.3 events/1,000 person-years; P = 0.28). Thus, the higher risk of CVD among patients with severe sepsis was observed in a broad group of patients hospitalized for infections and noninfectious reasons.
Figure 3.
Incidence rates of cardiovascular events among patients with severe sepsis who required the intensive care unit (ICU) and survived hospitalization and matched and unmatched control subjects. Matched control subjects include ICU control subjects, infection hospitalizations, hospitalized patients, and general population.
A small increase in risk was observed with organ dysfunction. For instance, the risk of CVD was 1.1-fold higher for patients with severe sepsis compared with matched infection hospitalization control subjects (incidence rates: 498.2 vs. 434.8 events/1,000 person-years; P = 0.0001; Figure 3). In addition, in exploratory analyses, the risk of CVD was slightly higher among those with organ dysfunction compared with those without organ dysfunction in hospitalized control subjects and ICU control subjects, but results were not statistically significant (incidence rates: 514 vs. 446 events/1,000 person-years and 493 vs. 480 events/1,000 person-years, respectively; P > 0.05). Similarly, the risk of CVD was 520 and 495 events/1,000 person-years in ICU patients with severe sepsis with and without shock (P = 0.5).
Sensitivity Analyses
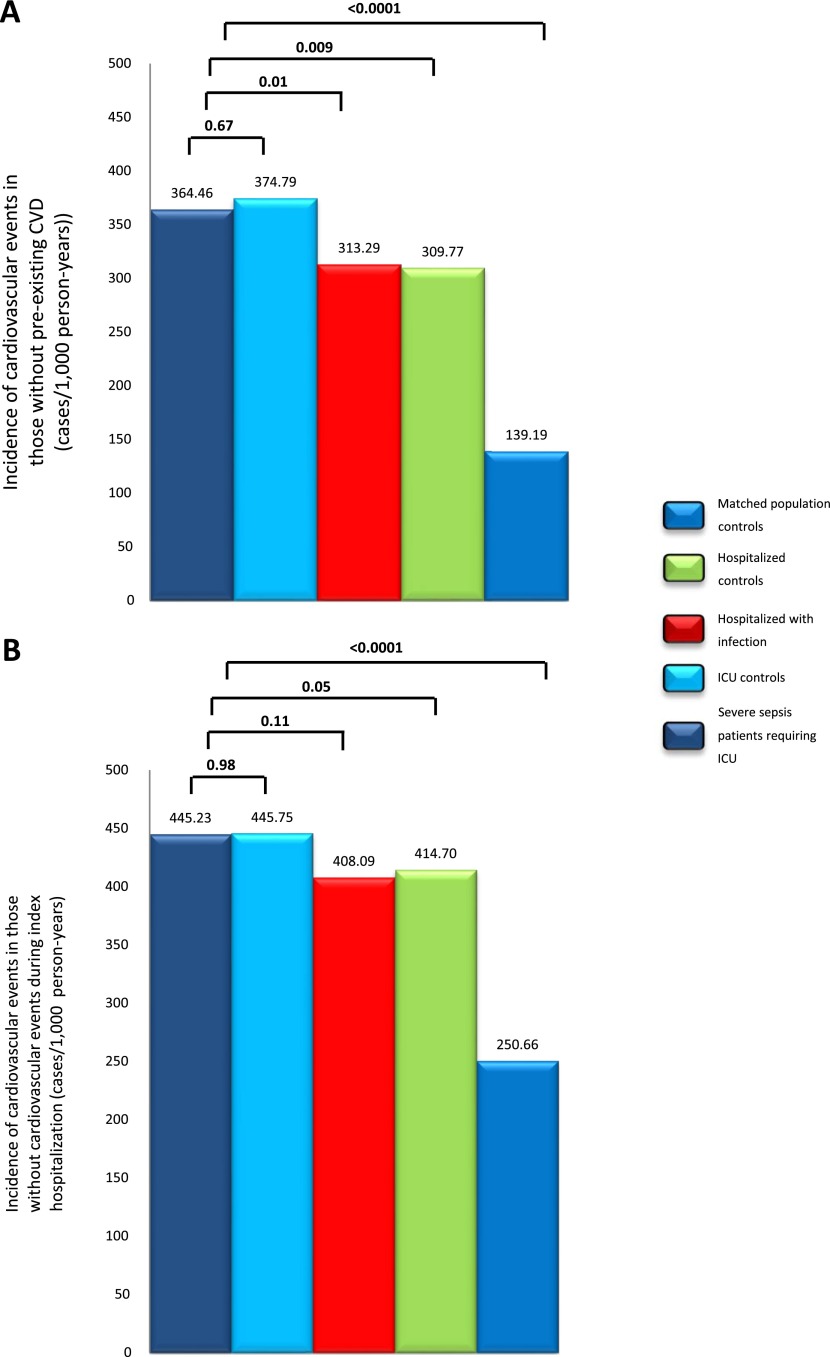
Sensitivity analyses showed that the magnitude of differences between patients with severe sepsis and matched control subjects were similar for myocardial infarction and stroke (Table 4). The magnitude of differences were similar when analyses were restricted to the pentads without pre-existing CVD (Figure 4A). The magnitude of differences were also similar when pentads where at least one subject with a cardiovascular event during the index hospitalization were excluded (2,562 pentads; Figure 4B), although the results did not reach statistical significance.
Table 4:
Incidence Rates (Events per 1,000 Person-Years) of Cardiovascular Events, Myocardial Infarction, Stroke, and Transient Ischemic Attack during the First Year after Hospital Discharge for Patients Who Survived Hospitalization with Severe Sepsis in the Intensive Care Unit, Matched Control Subjects, and for Unmatched Control Subjects
| Variables | Cardiovascular Events | Acute Myocardial Infarction | Stroke and Transient Ischemic Attack |
|---|---|---|---|
| Severe sepsis | 498.2 | 119.7 | 353.7 |
| Matched ICU control subjects | 479.3 | 113 | 326.5 |
| Matched infection hospitalization | 434.8 | 83.2 | 331.2 |
| Matched hospitalized control subjects | 446.5 | 83.7 | 331.1 |
| Matched population control subjects | 257.6 | 36 | 203 |
| Unmatched population control subjects | 36.0 | 12.3 | 11.0 |
For definition of abbreviation see Table 1.
Matched control subjects were in the ICU, hospitalized for noninfectious conditions, hospitalized with infection, and general population.
Figure 4.
Incidence rates of cardiovascular events for matched analyses comparing patients with severe sepsis requiring intensive care unit (ICU) and surviving hospitalization, and control subjects. Each case was matched to four control subjects to form a pentad. Incidence rates are shown for those without preillness cardiovascular disease ([A] includes 1,840 pentads; n = 9,200), and those who did not incur a cardiovascular event during the index hospitalization ([B] includes 2,562 pentads; n = 12,810). CVD = cardiovascular disease.
Discussion
We have shown that survivors of severe sepsis who require ICU care had a high risk of subsequent cardiovascular events. The high risk is largely explained by poor health status before the severe sepsis hospitalization. We observed a 1.9-fold higher risk for CVD for patients with severe sepsis compared with matched population control subjects. However, we also observed a similar or slightly lower risk of CVD for matched ICU control subjects or those hospitalized for infectious or noninfectious reasons. Thus, the higher risk of CVD may be seen in a broad group of patients requiring acute care. The 1.9-fold increased risk is similar to the risk of cardiovascular events observed for smoking cigarettes, diabetes, and hypercholesterolemia (23, 24, 26). Our findings have important implications to understand why survivors of severe sepsis, critical illness, or acute care have high long-term mortality.
The higher risk of cardiovascular events after severe sepsis can be due to the acute episode of severe sepsis or to factors that were present before the severe sepsis hospitalization. For example, severe sepsis occurs more often in older individuals, African-American men, and those with chronic diseases, such as diabetes and kidney disease. Not surprisingly, the higher risk of cardiovascular events was largely attributable to factors that were present before severe sepsis hospitalization. Acute care, including severe sepsis hospitalization, also independently increased risk of cardiovascular events. We used multiple control subjects to determine whether the higher risk of severe sepsis was observed in similar patients who required ICU care and hospitalized for infectious and noninfectious reasons. We observed similar or small differences in risk between these control subjects, suggesting that hospitalized patients with health status similar to patients with severe sepsis may increase risk for CVD.
The association between acute care and long-term risk of cardiovascular events may be due to several mechanisms. First, organ dysfunction occurred in patients with severe sepsis and some of the control groups. The organ dysfunction may persist during recovery and increase risk of CVD. For example, acute kidney injury may increase risk of chronic kidney disease and subsequent cardiovascular events (27). Second, a dysregulated immune response, particularly increased systemic inflammatory markers (28), may fail to resolve (29), and may persist during recovery (12, 30). Persistently increased vascular inflammation may convert stable atherosclerotic plaques to vulnerable plaques and increase risk of subsequent cardiovascular events (31–33). Similarly, a procoagulant response may persist during recovery and increase risk of subsequent cardiovascular events (34). Finally, reduced physical function associated with or unintentional discontinuation of medication during hospitalization may also play a role (35).
Regardless of whether the higher risk of cardiovascular events after acute care is due to prehospitalization risk factors or to an acute episode itself, hospitalization may be an important opportunity to initiate primary prevention strategies, such as statins or aspirin (36). For example, approximately half of patients with severe sepsis did not have pre-existing CVD, and prior studies suggest that many of these patients are not receiving statin therapy (37). Future studies should test initiation of statins and aspirin, and continue this intervention after hospital discharge to improve long-term outcomes in these patients.
Our findings extend results of prior studies that showed higher risk of acute cardiovascular events after infection in two areas (8–11). First, our results suggest that the higher risk of CVD may be seen in other acute conditions. Second, prior studies largely focused on events occurring over a few weeks to months after the infection, whereas our results suggest that the higher risk may persist for up to a year. Strengths of our study include inclusion of a nationally representative sample of patients, large sample size, careful matching of cases and control subjects based on preillness characteristics, an unbiased approach to selecting control subjects, and inclusion of several control groups and sensitivity analyses to account for potential confounders.
Our study has limitations. First, we were unable to account for the effect of competing risk of death. The negative binomial models accounted for different exposure times, and the higher mortality among patients with severe sepsis would suggest that we may have underestimated the risk of cardiovascular events after severe sepsis. Second, our results apply only to older patients. The relationship between pre-existing health status, severe sepsis, and subsequent cardiovascular events may be different in younger patients.
In conclusion, survivors of severe sepsis have a high long-term risk of cardiovascular events. The higher risk among survivors of severe sepsis may be seen in a broader population of patients requiring acute care. Future studies should test preventive strategies, such as statins or aspirin (36), to improve long-term outcomes of these patients.
Footnotes
Supported in part by National Institutes of Health grants K23GM083215 and R01GM097471.
Author Contributions: S.Y., W.L.-Z., and D.C.A. contributed to conception and design of the study. W.L.-Z. acquired the data. S.Y., W.L.-Z., and L.A.W. performed the analyses. All authors contributed to interpretation of the data. S.Y. prepared the initial draft, and all authors provided critical revisions to the drafts.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201307-1321OC on January 23, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Adhikari NKJ, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti C, Brun-Buisson C, Burchardi H, Martin C, Goodman S, Artigas A, Sicignano A, Palazzo M, Moreno R, Boulmé R, et al. Epidemiology of sepsis and infection in ICU patients from an international multicentre cohort study. Intensive Care Med. 2002;28:108–121. doi: 10.1007/s00134-001-1143-z. [DOI] [PubMed] [Google Scholar]
- 4.Winters BD, Eberlein M, Leung J, Needham DM, Pronovost PJ, Sevransky JE. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]
- 5.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 6.Yende S, Angus DC, Ali IS, Somes G, Newman AB, Bauer D, Garcia M, Harris TB, Kritchevsky SB. Influence of comorbid conditions on long-term mortality after pneumonia in older people. J Am Geriatr Soc. 2007;55:518–525. doi: 10.1111/j.1532-5415.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrales-Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, Fergusson DA. Cardiac complications in patients with community-acquired pneumonia: a systematic review and meta-analysis of observational studies. PLoS Med. 2011;8:e1001048. doi: 10.1371/journal.pmed.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smeeth L, Cook C, Thomas S, Hall AJ, Hubbard R, Vallance P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet. 2006;367:1075–1079. doi: 10.1016/S0140-6736(06)68474-2. [DOI] [PubMed] [Google Scholar]
- 10.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351:2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 11.Clayton TC, Capps NE, Stephens NG, Wedzicha JA, Meade TW. Recent respiratory infection and the risk of myocardial infarction. Heart. 2005;91:1601–1602. doi: 10.1136/hrt.2004.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yende S, D’Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, Kong L, Carter M, Angus DC GenIMS Investigators. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang JX, Iwashyna TJ, Christakis NA. The performance of different lookback periods and sources of information for Charlson comorbidity adjustment in Medicare claims. Med Care. 1999;37:1128–1139. doi: 10.1097/00005650-199911000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Wang M-H, Shugart YY, Cole SR, Platz EA. A simulation study of control sampling methods for nested case–control studies of genetic and molecular biomarkers and prostate cancer progression. Cancer Epidemiol Biomarkers Prev. 2009;18:706–711. doi: 10.1158/1055-9965.EPI-08-0839. [DOI] [PubMed] [Google Scholar]
- 15.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 16.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60:1070–1077. doi: 10.1111/j.1532-5415.2012.03989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jokinen C, Heiskanen L, Juvonen H, Kallinen S, Karkola K, Korppi M, Kurki S, Rönnberg PR, Seppä A, Soimakallio S, et al. Incidence of community-acquired pneumonia in the population of four municipalities in eastern Finland. Am J Epidemiol. 1993;137:977–988. doi: 10.1093/oxfordjournals.aje.a116770. [DOI] [PubMed] [Google Scholar]
- 18.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of U.S. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989;104:350–360. [PMC free article] [PubMed] [Google Scholar]
- 19.Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–320. doi: 10.1016/0002-9343(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 20.O’Meara ES, White M, Siscovick DS, Lyles MF, Kuller LH. Hospitalization for pneumonia in the Cardiovascular Health Study: incidence, mortality, and influence on longer-term survival. J Am Geriatr Soc. 2005;53:1108–1116. doi: 10.1111/j.1532-5415.2005.53352.x. [DOI] [PubMed] [Google Scholar]
- 21.Yende S, Alvarez K, Loehr L, Folsom AR, Newman AB, Weissfeld LA, Wunderink RG, Kritchevsky SB, Mukamal KJ, London SJ, et al. Atherosclerosis Risk in Communities Study; Cardiovascular Health Study; Health, Aging, and Body Composition Study. Epidemiology and long-term clinical and biologic risk factors for pneumonia in community-dwelling older Americans: analysis of three cohorts. Chest. 2013;144:1008–1017. doi: 10.1378/chest.12-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 23.May DS, Kittner SJ. Use of Medicare claims data to estimate national trends in stroke incidence, 1985–1991. Stroke. 1994;25:2343–2347. doi: 10.1161/01.str.25.12.2343. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Normand S-LT, Wang Y, Drye EE, Schreiner GC, Krumholz HM. Recent declines in hospitalizations for acute myocardial infarction for Medicare fee-for-service beneficiaries: progress and continuing challenges. Circulation. 2010;121:1322–1328. doi: 10.1161/CIRCULATIONAHA.109.862094. [DOI] [PubMed] [Google Scholar]
- 25.Allison PD, Waterman RP. Fixed-effects negative binomial regression models. Sociol Methodol. 2002;32:247–265. [Google Scholar]
- 26.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 27.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol. 2007;18:1292–1298. doi: 10.1681/ASN.2006070756. [DOI] [PubMed] [Google Scholar]
- 28.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC GenIMS Investigators. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116:793–802. doi: 10.1161/CIRCULATIONAHA.106.678359. [DOI] [PubMed] [Google Scholar]
- 31.Naghavi M, Wyde P, Litovsky S, Madjid M, Akhtar A, Naguib S, Siadaty MS, Sanati S, Casscells W. Influenza infection exerts prominent inflammatory and thrombotic effects on the atherosclerotic plaques of apolipoprotein E–deficient mice. Circulation. 2003;107:762–768. doi: 10.1161/01.cir.0000048190.68071.2b. [DOI] [PubMed] [Google Scholar]
- 32.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 33.Epstein SE, Zhu J, Najafi AH, Burnett MS. Insights into the role of infection in atherogenesis and in plaque rupture. Circulation. 2009;119:3133–3141. doi: 10.1161/CIRCULATIONAHA.109.849455. [DOI] [PubMed] [Google Scholar]
- 34.Yende S, D’Angelo G, Mayr F, Kellum JA, Weissfeld L, Kaynar AM, Young T, Irani K, Angus DC GenIMS Investigators. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One. 2011;6:e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Care. 2006;15:122–126. doi: 10.1136/qshc.2005.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tleyjeh IM, Kashour T, Hakim FA, Zimmerman VA, Erwin PJ, Sutton AJ, Ibrahim T. Statins for the prevention and treatment of infections: a systematic review and meta-analysis. Arch Intern Med. 2009;169:1658–1667. doi: 10.1001/archinternmed.2009.286. [DOI] [PubMed] [Google Scholar]
- 37.Kruger PS, Harward ML, Jones MA, Joyce CJ, Kostner KM, Roberts MS, Venkatesh B. Continuation of statin therapy in patients with presumed infection: a randomized controlled trial. Am J Respir Crit Care Med. 2011;183:774–781. doi: 10.1164/rccm.201006-0955OC. [DOI] [PubMed] [Google Scholar]