Abstract
Rationale: Developmental patterns of lung function during childhood may have major implications for our understanding of the pathogenesis of respiratory disease throughout life.
Objectives: To explore longitudinal trajectories of lung function during childhood and factors associated with lung function decline.
Methods: In a population-based birth cohort, specific airway resistance (sRaw) was assessed at age 3 (n = 560), 5 (n = 829), 8 (n = 786), and 11 years (n = 644). Based on prospective data (questionnaires, skin tests, IgE), children were assigned to wheeze phenotypes (no wheezing, transient, late-onset, and persistent) and atopy phenotypes (no atopy, dust mite, non–dust mite, multiple early, and multiple late). We used longitudinal linear mixed models to determine predictors of change in sRaw over time.
Measurements and Main Results: Contrary to the assumption that sRaw is independent of age and sex, boys had higher sRaw than girls (mean difference, 0.080; 95% confidence interval [CI], 0.049–0.111; P < 0.001) and a higher rate of increase over time. For girls, sRaw increased by 0.017 kPa ⋅ s−1 per year (95% CI, 0.011–0.023). In boys this increase was significantly greater (P = 0.012; mean between-sex difference, 0.011 kPa ⋅ s−1; 95% CI, 0.003–0.019). Children with persistent wheeze (but not other wheeze phenotypes) had a significantly greater rate of deterioration in sRaw over time compared with never wheezers (P = 0.009). Similarly, children with multiple early, but not other atopy phenotypes had significantly poorer lung function than those without atopy (mean difference, 0.116 kPa ⋅ s−1; 95% CI, 0.065–0.168; P < 0.001). sRaw increased progressively with the increasing number of asthma exacerbations.
Conclusions: Children with persistent wheeze, frequent asthma exacerbations, and multiple early atopy have diminished lung function throughout childhood, and are at risk of a progressive loss of lung function from age 3 to 11 years. These effects are more marked in boys.
Keywords: lung function, childhood, longitudinal analysis, specific airway resistance, epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
Poor lung function shortly after birth has been established as one of the predisposing factors for early childhood wheezing. Furthermore, previous studies suggest that impaired lung function at school age may track from childhood into adulthood. However, there are little data on individual trajectories of lung function during early childhood, in part because of the difficulties in measuring lung function in early childhood.
What This Study Adds to the Field
This paper presents the first longitudinal study to investigate factors associated with the trajectories of lung function during childhood.
Developmental patterns of lung function during childhood may have major implications for the understanding of the pathogenesis of respiratory disease throughout life. Adult patients with persistent asthma have impaired lung function in school age, adolescence, and adulthood, suggesting that lung function tracks from childhood into adulthood (1). Studies in which lung function was measured soon after birth have provided invaluable insights into the relationship between early life lung function and subsequent respiratory disease. Diminished lung function in early infancy is one of the predisposing factors for early childhood wheezing (2, 3), and children with flow limitation in infancy are more likely to have reduced lung function at ages 6 and 11 years (4), and into early adulthood (5, 6). However, most such studies do not include lung function measures between infancy and age 6 years, in part because of the difficulties in measuring lung function in preschool-age children. The most common method of lung function assessment in older children is spirometry, which measures FEV1, FVC, and other parameters using forced expiratory maneuvers. However, methods that require active cooperation are often difficult for preschool children to perform consistently and reproducibly, because many cannot produce adequate forced breathing maneuvers (7). For younger children, the techniques that can be performed during tidal breathing are more practical (7). One such method is measurement of specific airway resistance (sRaw) using plethysmography (8–13), which can be a useful measure in clinical practice and research studies (11). We have previously shown that as early as age 3 years, sRaw differs between children with a history of wheezing and those without (9), and higher sRaw at age 3 years is associated with subsequent persistence of wheezing (14). In childhood, sRaw is considered to be independent of age, height, and sex, thus facilitating the interpretation of longitudinal measurements in individual subjects (5, 8, 12, 13). Previous data from our group indicated a possible increase in sRaw through early childhood (14). However, no longitudinal studies have as yet addressed this important issue.
Based predominantly on cross-sectional data, various factors have been associated with lung function impairment in childhood, including sex (15), low birth weight (16), maternal smoking during pregnancy (14, 17), maternal asthma (18), child’s atopic status (9), and among children with atopy high exposure to sensitizing allergens (19, 20). Genetic factors also have an important role; for example, we have recently reported significant associations between genetic variants in VEGF-A with lung function (including sRaw) measured during childhood, with the effect persisting into adulthood (21). Gene-by-environment interactions may also play a role in the developmental pathway of lung function (22). These studies have laid important foundations for the understanding of the physiologic, genetic, and environmental factors associated with diminished lung function. However, there are no existing studies investigating the developmental trajectories of lung function from early childhood, in which the same measure of lung function was assessed on more than two occasions.
We hypothesized that within a population of children, there are several different trajectories of lung function, each with differing predictors. We capitalized on the unique data collection in the Manchester Asthma and Allergy Study, in which measurement of sRaw was performed at four time points from preschool to mid-school age, to investigate factors associated with a change in lung function during childhood. We aimed to establish (1) whether sRaw increases, remains stable, or decreases over time; (2) the characteristics that predict the cross-sectional differences among children, and changes in sRaw over time; and (3) to identify a single multilevel model to describe the association between sRaw and these characteristics over time. We used multilevel longitudinal models to explore plausible relations between developmental trajectories of lung function and age, sex, height, weight, pet ownership, family history of asthma, atopy, and various phenotypes of respiratory disease during childhood to identify groups of children in whom lung function diminishes over time. Some of the results of these studies have been previously reported in the form of an abstract (23).
Methods
Study Population
Manchester Asthma and Allergy Study is a population-based birth cohort (24). Subjects were recruited prenatally and followed prospectively until age 11 years. Both parents were skin prick tested to ascertain atopic sensitization, and completed questionnaires to document personal history of asthma. The study was approved by the local ethics committee; parents gave written informed consent.
Data Sources
Clinical follow-up.
A series of risk factors including maternal smoking during pregnancy, pet ownership, gestational age, and birth weight were assessed by questionnaires at home visit in early infancy. Children attended follow-up clinics at ages 1, 3, 5, 8, and 11 years. Validated questionnaires were interviewer-administered to collect information on parentally reported symptoms and environmental exposures. At each review, we measured weight without shoes or outer clothing (to nearest 0.01 kg) and height (to nearest 0.1 cm) (25). We assessed atopy by skin prick tests. We also measured specific serum IgE to common inhalant and food allergens (ImmunoCAP; Phadia, Uppsala, Sweden).
Lung function measurement.
We measured sRaw at ages 3, 5, 8, and 11 years during tidal breathing using a constant volume whole-body plethysmography (9, 14, 19, 26). Children were symptom-free at the time of assessment of lung function. Short-acting β2-agonists were withheld for at least 4 hours and long-acting for at least 24 hours before testing. A detailed description of methods is provided in the online supplement.
Primary care data extraction.
We extracted all data from primary care medical records, including wheeze episodes, prescriptions of asthma medications, oral corticosteroid prescriptions, emergency department admissions, and asthma- and wheeze-related hospitalizations (27).
Definition of Predictive Phenotypes
Current wheeze.
A positive answer to the question “Has your child had wheezing or whistling in the chest in the last 12 months” defined current wheeze.
Wheeze phenotypes.
We used prospectively collected data to assign children into different wheeze phenotypes (14, 28). We used the following classification: No wheezing (no wheeze at any time point), Transient early wheezing (wheezing during the first 3 yr, with no wheezing after age 3 yr), Late-onset wheezing (no wheeze during the first 3 years, reported wheezing at age 5 yr or later), and Persistent wheezing (wheezing throughout the childhood).
Severe asthma and wheeze exacerbations.
This was defined by receipt of oral steroids for at least 3 days or admission to hospital or emergency department visit because of asthma requiring oral steroid use (ascertained from medical notes) (29). This definition was also applied to children younger than age 6 years.
Hospital admission.
This was defined as admission to hospital for asthma or wheeze (from medical notes).
Atopic sensitization.
This was defined by weal diameter 3 mm greater than negative control to at least one allergen.
Phenotypes of atopy.
In recent studies we used machine learning methods applied to longitudinal skin prick tests and IgE data to ascertain atopy phenotypes (30, 31), moving from a dichotomous (atopic/nonatopic) to a five-class model, where each class (phenotype) reflected distinct patterns of sensitization (32). We used these five classes as phenotypes of atopy: (1) nonatopic, (2) dust mite, (3) non–dust mite, (4) multiple early, and (5) multiple late (30).
Statistical Analysis
We used multilevel models to investigate factors associated with differences in sRaw between children, and changes in sRaw over time. We fitted random coefficient models to investigate both the time-varying and time-invariant (fixed) covariates (see Table E1 in the online supplement), which predicted differences in sRaw. By including an interaction term in the random coefficients model, we extended these models to investigate whether each of these factors were also predictors of change in sRaw over time. After fitting various submodels of predictors of interest individually, we combined them in a realistically complete mode of change in sRaw over time, selected on the basis of likelihood ratio tests of nested models. All statistical analysis was performed using Stata 12.2 (Stata Statistical Software; StataCorp LP, College Station, TX).
Missing data mechanisms were investigated using logistic regression. sRaw and the covariates were assumed to be missing at random. Based on this assumption, multiple imputation using chained equations was performed using the ice package in Stata (33, 34). Each of the 100 imputed datasets was then analyzed in the usual way, and the parameter estimates were combined using Rubin rule (35, 36). Similar results were obtained from analyses using data from complete cases compared with the imputed datasets (data not shown, available on request).
Results
Participant Flow and Demographics
We analyzed data from 1,051 of 1,184 children in the cohort (we excluded 113 who were randomized to the environmental intervention) (37, 38). sRaw was measured in 560 children at age 3, 829 at age 5, 786 at age 8, and 644 at age 11 years; 350 had data at all four time points, 668 at three or more, and 846 children had at least two sRaw measurements. Data were transcribed from primary care medical records of 814 of 1,051 (77.5%) children. Table E2 shows the proportion of observations for each variable investigated for association with sRaw at each time point. There were no significant differences in demographics, wheeze, and atopy for children with and without missing sRaw data (see Table E3).
Factors Affecting Mean Differences and Factors Affecting Rate of Change in sRaw
Although this study focuses on predictors of longitudinal change in sRaw over time, for completion we present cross-sectional associations at each time point in Table E4.
Age, sex, anthropometric characteristics, family history, and environmental exposures.
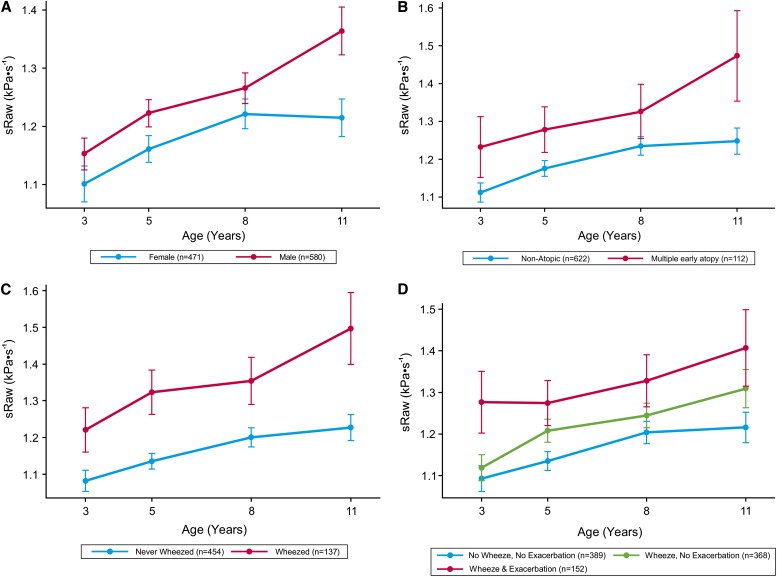
There was a significant increase in sRaw over time in the whole population (see Table E5, Model A). Longitudinal analysis revealed that boys had significantly higher sRaw during childhood than girls (mean difference, 0.08 kPa ⋅ s−1; 95% confidence interval [CI], 0.049–0.111; P < 0.001) (see Table E5, Model B), but also a faster rate of increase over time (Figure 1A; see Table E5, Model C). For girls, sRaw increased by 0.017 kPa ⋅ s−1 per year (95% CI, 0.011–0.023; P < 0.001), whereas in boys this increase was significantly greater (mean difference, +0.011 kPa ⋅ s−1; 95% CI, 0.003–0.019; P = 0.012), with a slope of 0.028 (0.017 + 0.011) kPa ⋅ s−1 per year.
Figure 1.
Trajectory and 95% confidence intervals of specific airway resistance (sRaw) measurements over time. (A) Sex; on average, boys had a higher sRaw value than girls, and a significantly higher rate of change in sRaw over time than girls. (B) Atopy phenotypes (no latent atopic vulnerability and multiple early atopic vulnerability); on average, children with multiple early atopic vulnerability had poorer lung function. (C) Wheeze phenotypes (no wheezing and persistent wheezing); children with persistent wheeze had consistently poorer lung function (higher sRaw) than children who never wheezed, and a significantly higher rate of deterioration in lung function (increase in sRaw). (D) Wheeze and asthma exacerbations; wheezers who experienced exacerbation had significantly poorer lung function than children who never wheezed. There was no difference in the rate of change in lung function over time. n = number of children in each group with at least one measurement at a single time point.
We calculated z scores for height, weight, and body mass index using published UK standards (39) to investigate their associations with lung function. As univariate predictors, weight and height were significantly associated with sRaw, but there was no significant association with body mass index standard deviation score (weight adjusted for height, age, and sex) (see Table E6).
The effect of parental atopic sensitization and asthma is presented in Table E7. Maternal asthma was significantly associated with higher sRaw in offspring (mean difference, 0.048 kPa ⋅ s−1; 95% CI, 0.005–0.091; P = 0.03) (see Table E7, Model C).
There was no evidence of the statistically significant association between sRaw and maternal smoking during pregnancy (P = 0.25), at birth (P = 0.44), or time-varying smoking exposure at ages 3–11 years (P = 0.43) (see Table E8). There was no significant association between dog or cat ownership and sRaw (P = 0.34 and P = 0.68, respectively) (see Table E9), and no significant interaction between pet sensitization (assessed using skin prick tests) and pet ownership (cat, P = 0.59; dog, P = 0.99).
Atopic sensitization.
Sensitized children had significantly higher sRaw than those who were not sensitized (mean difference, 0.052 kPa ⋅ s−1; 95% CI, 0.020–0.084; P = 0.002) (Table 1, Model A); however, the rate of change of sRaw over time did not differ between the groups (P = 0.16).
Table 1:
Association between sRaw and Sensitization, Atopy Phenotypes Defined on the Basis of Patterns of Sensitization over Time, Assuming That Atopy Phenotypes Have Different Rates of Change in sRaw over Time
| sRaw (kPa ⋅ s−1) | P Value | |
|---|---|---|
| Model A: Sensitization | ||
| Reference group (no sensitization, sRaw at age 3), mean (SE) | 1.129 (0.012) | |
| Sensitized (skin prick test), estimated difference (SE) | +0.052 (0.016) | 0.002 |
| Age (effect of time, per year), estimated difference (SE) | +0.022 (0.002) | <0.001 |
| Model B: Atopy phenotypes | ||
| Reference group (no sensitization, sRaw at age 3), mean (SE) | 1.057 (0.017) | |
| Non–dust mite, estimated difference (SE) | +0.013 (0.027) | 0.63 |
| Dust mite, estimated difference (SE) | +0.001 (0.037) | 0.98 |
| Multiple late, estimated difference (SE) | +0.028 (0.021) | 0.18 |
| Multiple early, estimated difference (SE) | +0.116 (0.026) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.023 (0.002) | <0.001 |
| Model C: Interaction of atopy phenotypes with time | ||
| Reference group (no sensitization, sRaw at age 3), mean (SE) | +1.130 (0.011) | |
| Non–dust mite, estimated difference (SE) | +0.013 (0.027) | 0.29 |
| Dust mite, estimated difference (SE) | +0.001 (0.037) | 0.65 |
| Multiple late, estimated difference (SE) | +0.003 (0.024) | 0.91 |
| Multiple early, estimated difference (SE) | +0.092 (0.029) | 0.002 |
| Age (effect of time, per year), estimated difference (SE) | +0.018 (0.002) | <0.001 |
| Interaction non–dust mite × age (effect of time, per year among children with non–dust mite atopy), estimated difference (SE) | −0.003 (0.005) | 0.52 |
| Interaction dust mite × age (effect of time, per year among children with dust mite atopy), estimated difference (SE) | +0.000 (0.007) | 0.97 |
| Interaction multiple late × age (effect of time, per year among children with multiple late atopy), estimated difference (SE) | +0.008 (0.004) | 0.047 |
| Interaction multiple early × age (effect of time, per year among children with multiple early atopy), estimated difference (SE) | +0.011 (0.005) | 0.032 |
Definition of abbreviation: sRaw = specific airway resistance.
sRaw (mean, SE) for children in the reference group (no sensitization, sRaw at age 3 yr) is presented in the first line. For each model we present the estimated difference in sRaw among children with a given characteristic (e.g., different atopy phenotypes in Model B) compared with children in the reference group. The estimate of age shows the expected increase in sRaw for every year increase in age.
When we investigated different phenotypes of atopy (30, 31), we found that only children with multiple early phenotype had significantly poorer lung function than those who were nonatopic (mean difference, 0.116 kPa ⋅ s−1; 95% CI, 0.065–0.168; P < 0.001) (Figure 1B), with no significant difference between other atopy phenotypes (Table 1, Model B; see Figure E1). Including a term for interaction with time (Table 1, Model C) revealed that the differences in sRaw increased with age between nonatopic compared with multiple early (Figure 1B, P = 0.032) and multiple late atopy phenotype (P = 0.047), with no differences in the rate of change of sRaw over time observed between other phenotypes of atopy (see Figure E1).
Wheeze and asthma.
Contemporaneous wheezing (i.e., current wheeze at the same time point when lung function was measured) was significantly associated with sRaw; children who wheezed had significantly higher sRaw compared with those who did not (mean difference, 0.068 kPa ⋅ s−1; 95% CI, 0.032–0.105; P < 0.001) (Table 2, Model A). The use of asthma medication and physician-diagnosed asthma were also significant indicators of poorer lung function (both P < 0.001) (Table 2, Models B and C). However, there was no significant difference in the rate of change of sRaw over time between those who do and do not wheeze (or between those with and without asthma).
Table 2:
Association between sRaw and Wheeze, Use of Asthma Medication, Doctor-diagnosed Asthma, Wheeze Phenotypes, and Assuming that Wheeze Phenotypes Have Different Rates of Change in sRaw over Time
| sRaw (kPa ⋅ s−1) | P Value | |
|---|---|---|
| Model A: Wheeze | ||
| Reference group (never wheezed, sRaw at age 3), mean (SE) | 1.128 (0.012) | |
| Wheeze, estimated difference (SE) | +0.068 (0.019) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.023 (0.002) | <0.001 |
| Model B: Use of asthma medication | ||
| Reference group (no asthma medication, sRaw at age 3), mean (SE) | 1.128 (0.012) | |
| Prescribed asthma medication, estimated difference (SE) | +0.089 (0.020) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.022 (0.002) | <0.001 |
| Model C: Doctor-diagnosed asthma | ||
| Reference group (no doctor-diagnosed asthma, sRaw at age 3), mean (SE) | 1.129 (0.012) | |
| Doctor-diagnosed asthma by age 11, estimated difference (SE) | +0.073 (0.024) | 0.002 |
| Age (effect of time, per year), estimated difference (SE) | +0.023 (0.002) | <0.001 |
| Model D: Wheeze phenotypes | ||
| Reference group (no wheezing, sRaw at age 3), mean (SE) | 1.019 (0.014) | |
| Transient early wheezing, estimated difference (SE) | +0.072 (0.016) | <0.001 |
| Late-onset wheezing, estimated difference (SE) | +0.076 (0.029) | 0.009 |
| Persistent wheezing, estimated difference (SE) | +0.194 (0.020) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.023 (0.002) | <0.001 |
| Model E: Interaction of wheeze phenotypes with time | ||
| Reference group (no wheezing, sRaw at age 3), mean (SE) | 1.033 (0.018) | |
| Transient early wheezing, estimated difference (SE) | +0.063 (0.029) | 0.031 |
| Late-onset wheezing, estimated difference (SE) | +0.075 (0.054) | 0.17 |
| Persistent wheezing, estimated difference (SE) | +0.116 (0.036) | 0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.019 (0.002) | <0.001 |
| Interaction transient wheeze × age (effect of time, per year among children with transient wheeze), estimated difference (SE) | +0.001 (0.004) | 0.71 |
| Interaction late-onset wheeze × age (effect of time, per year among children with late-onset wheeze), estimated difference (SE) | +0.000 (0.006) | 0.85 |
| Interaction persistent wheeze × age (effect of time, per year among children with persistent wheeze), estimated difference (SE) | +0.011 (0.004) | 0.009 |
Definition of abbreviation: sRaw = specific airway resistance.
sRaw (mean, SE) for children in the reference group (no symptoms, lung function at age 3 yr) is presented in the first line. For each model we present the estimated difference in sRaw among children with a given characteristic (e.g., current wheeze in Model A) compared with children in the reference group. The estimate of age shows the expected increase in sRaw for every year increase in age.
Different longitudinal patterns were observed when we used wheeze phenotypes as predictors (Table 2, Model D). Children with persistent wheeze had significantly higher sRaw than those who have never wheezed (mean difference, 0.194 kPa ⋅ s−1; 95% CI, 0.154–0.234; P < 0.001), and when including a term for interaction with time (Table 2, Model E), a significantly increased rate of change over time, reflecting a higher rate of deterioration in lung function (mean difference in rate of change over time, 0.011 kPa ⋅ s−1; 95% CI, 0.003–0.019; P = 0.009) (Figure 1C). Children with transient and late-onset wheeze had significantly poorer lung function than those who never wheezed, but the magnitude of difference was lower than for those with persistent wheeze, and the change over time was not significantly different (Table 2; see Figure E2).
Severe exacerbations and hospital admissions for asthma and wheeze.
Children with wheeze and asthma exacerbation had significantly poorer lung function than those with wheeze and no exacerbation (P < 0.001), and children who have never wheezed (P < 0.001) (Figure 1D, Table 3, Model A). Lung function was progressively worse with the increasing number of exacerbations (Table 3, Model B; see Figure E3). Similar trends were seen for asthma and wheeze hospital admissions (see Table E10, Figures E4 and E5). Children who within the first 3 years of life had severe exacerbation or were admitted to hospital had significantly poorer lung function throughout childhood (Table 3, Model C; see Figures E6 and E7).
Table 3:
Association between sRaw and Severe Wheeze/Asthma Exacerbations Ever, Exacerbations within the First 3 Years of Life, and Number of Exacerbations from Birth to Age 11 Years
| sRaw (kPa ⋅ s−1) | P Value | |
|---|---|---|
| Model A: Exacerbations ever | ||
| Reference group (never wheezed, sRaw at age 3), mean (SE) | 1.096 (0.013) | |
| Wheeze, no exacerbations, estimated difference (SE) | +0.057 (0.016) | 0.001 |
| Wheeze and exacerbations, estimated difference (SE) | +0.150 (0.022) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.019 (0.002) | <0.001 |
| Model B: Number of exacerbations from birth to age 11 yr | ||
| Reference group (no exacerbations, sRaw at age 3), mean (SE) | 1.124 (0.010) | |
| One exacerbation, estimated difference (SE) | +0.051 (0.027) | 0.059 |
| Two exacerbations, estimated difference (SE) | +0.133 (0.038) | 0.001 |
| Three or more exacerbations, estimated difference (SE) | +0.204 (0.037) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.019 (0.002) | <0.001 |
| Model C: Exacerbations within the first 3 yr of life | ||
| Reference group (never wheezed, sRaw at age 3), mean (SE) | 1.095 (0.013) | |
| Wheeze, no exacerbation by age 3, estimated difference (SE) | +0.062 (0.016) | <0.001 |
| Wheeze and exacerbation by age 3, estimated difference (SE) | +0.175 (0.025) | <0.001 |
| Age (effect of time, per year), estimated difference (SE) | +0.019 (0.002) | <0.001 |
Definition of abbreviation: sRaw = specific airway resistance.
sRaw (mean, SE) for children in the reference group (no wheeze, lung function at age 3 yr) is presented in the first line for each model. For each model we present the estimated difference in sRaw among children with a given characteristic (e.g., exacerbation ever in Model A) compared with children in the reference group. The estimate of age shows the expected increase in sRaw for every year increase in age.
Prototypical Trajectories for Change in Lung Function over Time
When putting predictors that we found to be significantly associated with sRaw in a multivariable longitudinal regression model, the set of predictors that best described the data were sex, wheeze phenotypes, and atopy phenotypes.
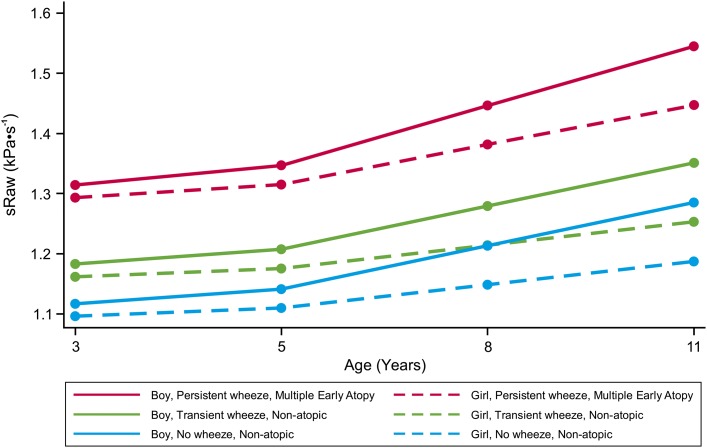
Persistent wheeze and male sex were significant predictors of increase in sRaw over time (Table 4). Height and weight were no longer significant independent predictors of sRaw in the multivariate regression model (see Table E11). Prototypical trajectories for change in sRaw over time for different hypothetical groups of children are shown in Figure 2, and are based on predicted values of the multivariate model with sex, wheeze phenotypes, and atopy phenotypes presented in Table 4. Girls without atopy who have never wheezed have the lowest sRaw (i.e., the best lung function) during childhood. Boys with persistent wheeze and multiple early atopy have the highest risk of diminished lung function during childhood.
Table 4:
Multivariable Longitudinal Regression Model for Characterizing Differences in sRaw
| sRaw (kPa ⋅ s−1) | P Value | |
|---|---|---|
| Predictors of sRaw | ||
| Reference group (female, no wheezing, no atopy, sRaw at age 3), mean (SE) | 1.083 (0.016) | |
| Age (effect of time, per year) in the reference group, estimated difference (SE) | 0.013 (0.003) | <0.001 |
| Sex (male), estimated difference (SE) | +0.010 (0.019) | 0.59 |
| Wheeze phenotype | ||
| Transient wheeze, estimated difference (SE) | +0.066 (0.023) | 0.004 |
| Late-onset wheeze, estimated difference (SE) | +0.051 (0.031) | 0.10 |
| Persistent wheeze, estimated difference (SE) | +0.122 (0.029) | <0.001 |
| Atopy phenotype | ||
| Non–dust mite, estimated difference (SE) | +0.011 (0.023) | 0.65 |
| Dust mite, estimated difference (SE) | −0.013 (0.032) | 0.69 |
| Multiple late, estimated difference (SE) | +0.007 (0.020) | 0.73 |
| Multiple early, estimated difference (SE) | +0.066 (0.024) | 0.007 |
| Predictors of rate of change in sRaw over
time | ||
| Sex (effect of time, per year among boys), estimated difference (SE) | +0.011 (0.003) | 0.001 |
| Persistent wheeze (effect of time, per year among persistent wheezers), estimated difference (SE) | +0.009 (0.005) | 0.05 |
Definition of abbreviation: sRaw = specific airway resistance.
sRaw (mean, SE) for the reference group (girls with no wheeze and no atopy) is presented in the first line. The estimate of age shows the expected increase in sRaw for every year increase in age in the reference group. We present estimated differences in sRaw in each of the wheeze and atopy phenotypes compared with the reference group. For predictors of rate of change over time (i.e., interactions with time), the estimated differences (SE) indicate mean differences in the rate of change over time for boys compared with girls and children with persistent wheeze compared with those with no wheeze.
Figure 2.
Prototypical trajectories for children with different groups of predictors of change in lung function development during childhood.
Discussion
Principal Findings
To the best of our knowledge, this is the first study to investigate factors associated with longitudinal change in sRaw during childhood. We set out to describe the patterns and predictors of lung function development from preschool to mid-school age, using sRaw measured at age 3 years, and repeated at ages 5, 8, and 11 years. Our results indicate that contrary to the commonly held view that sRaw is independent of age and sex (5, 8, 12, 13), this measure of lung function within individual subjects increases significantly with age during the prepubertal stage of life. Furthermore, there are clear differences in the profile of sRaw over time between males and females. Sex is not only a significant predictor of sRaw at each time point, but boys and girls also have a different rate of change in sRaw over time, with boys having significantly accelerated increase compared with girls from age 8 to 11 years. These differences in trajectory by sex are striking and are not caused by the higher prevalence of wheeze in boys than girls, because the trajectories for nonwheezing girls and nonwheezing boys show a similar divergence over time. Other independent predictors of sRaw trajectories were phenotypes of wheeze and atopy. There was an interaction between persistent wheeze and age, with trajectories of persistent wheezers differing from those of nonwheezers and indicating a loss in lung function over time. We observed similar interaction between one of the atopy phenotypes (multiple early atopy) and age, with trajectory that differed from those of children without atopy. In contrast, there was no significant effect of other wheeze or atopy phenotypes on the rate of change in lung function over time. The highest sRaw profiles (i.e., poorest lung function) were seen among children who had suffered severe asthma exacerbations.
Strengths and Limitations
One strength of our study is the large sample of children, representative of the local population, with a wealth of longitudinal measures available allowing us to model change in lung function throughout childhood. This enabled us to measure within-individual variation, contrasting with previous studies, which have been predominantly cross-sectional (11). Previous studies have indicated the difficultly in standardizing sRaw measurements in practice, and the large variability in measures introduced by differences in assessment tools for evaluating sRaw (9, 10). In the current study, sRaw was measured at each time point using the same equipment, the same methodology, and conducted by the same respiratory physiologist at each follow-up, reducing possible inconsistencies caused by methodologic and technical differences (11).
The limitation of our study is the lack of infant lung function data for most study participants (V’maxFRC was assessed in <5% of the study participants [3]), and we therefore made no attempt to use these data in the analysis. We were thus unable to track changes in lung function from earlier than age 3 years, and establish conclusively the causal mechanisms (6, 40). However, it is of note that the rapid thoracic compression technique that we (3) and others (2, 4, 6, 40) used to measure VmaxFRC in infancy may not be directly comparable with the measures of lung function during childhood using plethysmography.
We acknowledge that we have conducted multiple statistical tests. However, because the factors that we investigated as predicators of trajectories of lung function are biologically plausible and based on the published literature, we have not adjusted our analysis for multiples testing.
Interpretation
Wheezing illness is associated with poorer lung function; it is not entirely clear whether this deficit in lung function occurs as a consequence of wheeze, or predisposes to it, or both. A previous study from the Tucson cohort (with measurements of lung function available at ages 4 wk and 6 yr) suggested that for persistent wheezers the deficit in lung function is not present soon after birth, but is acquired by age 6 years, whereas children with transient early wheeze have diminished lung function shortly after birth (22). Although we are unable to investigate lung function in infancy in our analysis, we have shown a deficit in sRaw by age 3 years in those with transient and persistent wheeze (but not late-onset wheeze). This may give us a further indication that the important lung function deficits occur within the first 3 years of life. Furthermore, our longitudinal data demonstrate that not only do those with persistent wheeze have the poorest lung function, but their individual trajectories diverge from all other groups over time, suggesting a significant loss of lung function during the period from age 3 to 11 years.
The difference in lung function between children who wheeze, but have no exacerbations, and those who have exacerbations indicates that it is not merely wheeze, but persistent, severe wheeze that has an adverse effect on lung function. Lung function was progressively worse with the increasing number of exacerbations and hospital admissions. This is consistent with the results of our recent study in which children with persistent troublesome wheeze (defined using latent class analysis on both parental and primary-care reports of wheeze, and characterized by frequent exacerbations) had significantly greater increase in sRaw over time compared with persistent wheezers with controlled disease (27). Similar to the findings on wheeze phenotypes, our data demonstrate that it is not just atopy, but a specific atopy phenotype that is associated with poor lung function (multiple early atopy, comprising approximately one-quarter of children conventionally defined as being atopic [30]). The analysis indicated that there was no significant difference in lung function between the remaining three atopy phenotypes (i.e., approximately three-quarters of children with atopy), and children with no atopy. Children with both multiple early atopy and persistent wheeze are at an increased risk of poor lung function throughout childhood, with the effects more marked in boys than girls (Figure 2).
We found that cat and dog ownership did not have a significant effect on lung function at any time point, or on change in lung function over time. In contrast to the previous cross-sectional findings (19, 20), there was no evidence to indicate a significant interaction between allergen-specific sensitization and pet exposure on the development of lung function.
Conclusions
Our longitudinal study demonstrated that sRaw is not independent of age and sex, but that this measure of lung function within individual subjects increases significantly with age during the prepubertal period. Furthermore, there are differences in the trajectory of sRaw over time between boys and girls, with the accelerated increase in boys compared with girls. Continued follow-up during and after puberty would be important to ascertain whether this trend changes during this important developmental period in which sex switch in asthma prevalence occurs. We observed distinct associations of sRaw with different wheeze and atopy phenotypes, with children with multiple early atopy, persistent wheeze, and frequent severe exacerbations being at an increased risk of diminished lung function throughout childhood, and a progressive loss of lung function during the period from age 3 to 11 years. These effects were more marked in boys than girls.
Acknowledgments
Acknowledgment
The authors thank the children and their parents for their continued support and enthusiasm. They greatly appreciate the commitment they have given to the project. They also acknowledge the hard work and dedication of the study team (post-doctoral scientists, research fellows, nurses, physiologists, technicians, and clerical staff), and are grateful to Nailah Brown, M.Phil., for her contribution to the collection of lung function data.
Footnotes
Supported by JP Moulton Charitable Foundation, MRC Grants G0601361 and MR/K002449/1, National Institute for Health Research Clinical Research Facility at University Hospital of South Manchester NHS Foundation Trust, and Asthma UK Grant No 04/014.
Author Contributions: A.C. and A.S. conceived the idea. A.C. is a principal investigator of Manchester Asthma and Allergy Study. D.C.M.B. analyzed the data. L.L. performed the measurements of lung function. C.B. and I.B. provided input on the methodology for analyzing the data. All authors interpreted the data and wrote the report.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201309-1700OC on March 7, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sears MR, Greene JM, Willan AR, Wiecek EM, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Silva PA, Poulton R. A longitudinal, population-based, cohort study of childhood asthma followed to adulthood. N Engl J Med. 2003;349:1414–1422. doi: 10.1056/NEJMoa022363. [DOI] [PubMed] [Google Scholar]
- 2.Martinez FD, Morgan WJ, Wright AL, Holberg CJ, Taussig LM. Diminished lung function as a predisposing factor for wheezing respiratory illness in infants. N Engl J Med. 1988;319:1112–1117. doi: 10.1056/NEJM198810273191702. [DOI] [PubMed] [Google Scholar]
- 3.Murray CS, Pipis SD, McArdle EC, Lowe LA, Custovic A, Woodcock A National Asthma Campaign-Manchester Asthma and Allergy Study Group. Lung function at one month of age as a risk factor for infant respiratory symptoms in a high risk population. Thorax. 2002;57:388–392. doi: 10.1136/thorax.57.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner SW, Palmer LJ, Rye PJ, Gibson NA, Judge PK, Young S, Landau LI, Le Souëf PN. Infants with flow limitation at 4 weeks: outcome at 6 and 11 years. Am J Respir Crit Care Med. 2002;165:1294–1298. doi: 10.1164/rccm.200110-018OC. [DOI] [PubMed] [Google Scholar]
- 5.Doershuk CF, Fisher BJ, Matthews LW. Specific airway resistance from the perinatal period into adulthood. Alterations in childhood pulmonary disease. Am Rev Respir Dis. 1974;109:452–457. doi: 10.1164/arrd.1974.109.4.452. [DOI] [PubMed] [Google Scholar]
- 6.Stern DA, Morgan WJ, Wright AL, Guerra S, Martinez FD. Poor airway function in early infancy and lung function by age 22 years: a non-selective longitudinal cohort study. Lancet. 2007;370:758–764. doi: 10.1016/S0140-6736(07)61379-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beydon N, Davis SD, Lombardi E, Allen JL, Arets HG, Aurora P, Bisgaard H, Davis GM, Ducharme FM, Eigen H, et al. American Thoracic Society/European Respiratory Society Working Group on Infant and Young Children Pulmonary Function Testing. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med. 2007;175:1304–1345. doi: 10.1164/rccm.200605-642ST. [DOI] [PubMed] [Google Scholar]
- 8.Klug B, Bisgaard H. Measurement of the specific airway resistance by plethysmography in young children accompanied by an adult. Eur Respir J. 1997;10:1599–1605. doi: 10.1183/09031936.97.10071599. [DOI] [PubMed] [Google Scholar]
- 9.Lowe L, Murray CS, Custovic A, Simpson BM, Kissen PM, Woodcock A NAC Manchester Asthma and Allergy Study Group. Specific airway resistance in 3-year-old children: a prospective cohort study. Lancet. 2002;359:1904–1908. doi: 10.1016/S0140-6736(02)08781-0. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen KG. Plethysmographic specific airway resistance. Paediatr Respir Rev. 2006;7:S17–S19. doi: 10.1016/j.prrv.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 11.Kirkby J, Stanojevic S, Welsh L, Lum S, Badier M, Beardsmore C, Custovic A, Nielsen K, Paton J, Tomalak W, et al. Asthma UK. Reference equations for specific airway resistance in children: the Asthma UK initiative. Eur Respir J. 2010;36:622–629. doi: 10.1183/09031936.00135909. [DOI] [PubMed] [Google Scholar]
- 12.Bisgaard H, Nielsen KG. Plethysmographic measurements of specific airway resistance in young children. Chest. 2005;128:355–362. doi: 10.1378/chest.128.1.355. [DOI] [PubMed] [Google Scholar]
- 13.Kaminsky DA.What does airway resistance tell us about lung function? Respir Care 20125785–96.discussion 96–99 [DOI] [PubMed] [Google Scholar]
- 14.Lowe LA, Simpson A, Woodcock A, Morris J, Murray CS, Custovic A NAC Manchester Asthma and Allergy Study Group. Wheeze phenotypes and lung function in preschool children. Am J Respir Crit Care Med. 2005;171:231–237. doi: 10.1164/rccm.200406-695OC. [DOI] [PubMed] [Google Scholar]
- 15.Gold DR, Wypij D, Wang X, Speizer FE, Pugh M, Ware JH, Ferris BG, Jr, Dockery DW. Gender- and race-specific effects of asthma and wheeze on level and growth of lung function in children in six U.S. cities. Am J Respir Crit Care Med. 1994;149:1198–1208. doi: 10.1164/ajrccm.149.5.8173760. [DOI] [PubMed] [Google Scholar]
- 16.Lawlor DA, Ebrahim S, Davey Smith G. Association of birth weight with adult lung function: findings from the British Women’s Heart and Health Study and a meta-analysis. Thorax. 2005;60:851–858. doi: 10.1136/thx.2005.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, Avol E, Peters JM. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55:271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young S, Le Souëf PN, Geelhoed GC, Stick SM, Turner KJ, Landau LI. The influence of a family history of asthma and parental smoking on airway responsiveness in early infancy. N Engl J Med. 1991;324:1168–1173. doi: 10.1056/NEJM199104253241704. [DOI] [PubMed] [Google Scholar]
- 19.Lowe LA, Woodcock A, Murray CS, Morris J, Simpson A, Custovic A. Lung function at age 3 years: effect of pet ownership and exposure to indoor allergens. Arch Pediatr Adolesc Med. 2004;158:996–1001. doi: 10.1001/archpedi.158.10.996. [DOI] [PubMed] [Google Scholar]
- 20.Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–770. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 21.Simpson A, Custovic A, Tepper R, Graves P, Stern DA, Jones M, Hankinson J, Curtin JA, Wu J, Blekic M, et al. Genetic variation in vascular endothelial growth factor-a and lung function. Am J Respir Crit Care Med. 2012;185:1197–1204. doi: 10.1164/rccm.201112-2191OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blekic M, Kljaic Bukvic B, Aberle N, Marinho S, Hankinson J, Custovic A, Simpson A.17q12–21 and asthma: interactions with early-life environmental exposures Ann Allergy Asthma Immunol 2013110347–353.e342 [DOI] [PubMed] [Google Scholar]
- 23.Belgrave D, Lowe L, Simpson A, Custovic A. A longitudinal study investigating factors associated with changes in lung function over time in early life (age 3 to 11) [abstract] Eur Respir J. 2011;38:571S. [Google Scholar]
- 24.Custovic A, Simpson BM, Murray CS, Lowe L, Woodcock A NAC Manchester Asthma and Allergy Study Group. The National Asthma Campaign Manchester Asthma and Allergy study. Pediatr Allergy Immunol. 2002;13:32–37. doi: 10.1034/j.1399-3038.13.s.15.3.x. [DOI] [PubMed] [Google Scholar]
- 25.Murray CS, Canoy D, Buchan I, Woodcock A, Simpson A, Custovic A. Body mass index in young children and allergic disease: gender differences in a longitudinal study. Clin Exp Allergy. 2011;41:78–85. doi: 10.1111/j.1365-2222.2010.03598.x. [DOI] [PubMed] [Google Scholar]
- 26.Lowe L, Murray CS, Martin L, Deas J, Cashin E, Poletti G, Simpson A, Woodcock A, Custovic A. Reported versus confirmed wheeze and lung function in early life. Arch Dis Child. 2004;89:540–543. doi: 10.1136/adc.2003.038539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belgrave DC, Simpson A, Semic-Jusufagic A, Murray CS, Buchan I, Pickles A, Custovic A.Joint modeling of parentally reported and physician-confirmed wheeze identifies children with persistent troublesome wheezing J Allergy Clin Immunol 2013132575–583.e512 [DOI] [PubMed] [Google Scholar]
- 28.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ The Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 29.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, Casale TB, Chanez P, Enright PL, Gibson PG, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 30.Simpson A, Tan VY, Winn J, Svensén M, Bishop CM, Heckerman DE, Buchan I, Custovic A. Beyond atopy: multiple patterns of sensitization in relation to asthma in a birth cohort study. Am J Respir Crit Care Med. 2010;181:1200–1206. doi: 10.1164/rccm.200907-1101OC. [DOI] [PubMed] [Google Scholar]
- 31.Lazic N, Roberts G, Custovic A, Belgrave D, Bishop CM, Winn J, Curtin JA, Hasan Arshad S, Simpson A. Multiple atopy phenotypes and their associations with asthma: similar findings from two birth cohorts. Allergy. 2013;68:764–770. doi: 10.1111/all.12134. [DOI] [PubMed] [Google Scholar]
- 32.Custovic A, Lazic N, Simpson A. Pediatric asthma and development of atopy. Curr Opin Allergy Clin Immunol. 2013;13:173–180. doi: 10.1097/ACI.0b013e32835e82b6. [DOI] [PubMed] [Google Scholar]
- 33.Royston P. Multiple imputation of missing values. Stata J. 2004;4:227–241. [Google Scholar]
- 34.Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J. 2009;9:466–477. [Google Scholar]
- 35.Little RJA, Rubin DB. New York: Wiley; 1987. Statistical analysis with missing data. [Google Scholar]
- 36.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–489. [Google Scholar]
- 37.Custovic A, Simpson BM, Simpson A, Hallam C, Craven M, Brutsche M, Woodcock A. Manchester Asthma and Allergy Study: low-allergen environment can be achieved and maintained during pregnancy and in early life. J Allergy Clin Immunol. 2000;105:252–258. doi: 10.1016/s0091-6749(00)90073-3. [DOI] [PubMed] [Google Scholar]
- 38.Simpson A, Simpson B, Custovic A, Craven M, Woodcock A. Stringent environmental control in pregnancy and early life: the long-term effects on mite, cat and dog allergen. Clin Exp Allergy. 2003;33:1183–1189. doi: 10.1046/j.1365-2745.2003.01679.x. [DOI] [PubMed] [Google Scholar]
- 39.Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–29. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bisgaard H, Jensen SM, Bønnelykke K. Interaction between asthma and lung function growth in early life. Am J Respir Crit Care Med. 2012;185:1183–1189. doi: 10.1164/rccm.201110-1922OC. [DOI] [PubMed] [Google Scholar]