To the Editor:
The systemic capillary leak syndrome (SCLS) is a rare disease of unknown etiology characterized by transient episodes of distributive shock (1). SCLS is difficult to diagnose prospectively because the clinical criteria—hypotension, hemoconcentration, low serum albumin—are nonspecific and not present in all cases. Correct diagnosis is critical, because this debilitating disease can now be effectively treated with oral theophylline and terbutaline or monthly infusions of high-dose intravenous immunoglobulin (IVIG) (2, 3).
Recently, a 59-year-old man with a history of hypertension and asthma was referred to our center with a diagnosis of SCLS, made in 2003 during an acute episode characterized by hypotension (systolic blood pressure 50–70 mm Hg, baseline 130–150 mm Hg), hemoconcentration (Hb, 17.8 g/dl, baseline 14–16 g/dl), and hypoalbuminemia (3.0 g/dl, baseline 3.7–4 g/dl). However, for the past several years, he reported less-severe, self-limited episodes of symptomatic hypotension (systolic blood pressure, 60–80s mm Hg) on a regular basis every weekend, in stark contrast to the infrequent, sporadic, and catastrophic attacks of hypotensive shock and anasarca that occur in the majority of subjects with SCLS. These episodes were also characterized by the virtual absence of peripheral, periorbital, or facial edema, myalgias, generalized body pain, or dyspnea—symptoms that were associated with acute SCLS episodes on presentation in a large case series (4). No clear environmental trigger for attacks was identified from a detailed history. Systemic anaphylaxis and primary endocrine or cardiovascular abnormalities were excluded by appropriate tests. A monoclonal gammopathy of unknown significance was present in 84% of subjects with SCLS (93 of 111 patients described in three published case series from 1960–2011), and the serum “paraprotein” was of the IgG isotype in all but 4 patients (96%) (4–6). However, we detected IgA monoclonal gammopathy of unknown significance in our patient, which was observed in only one other subject with SCLS. Furthermore, he has not responded clinically to several therapies for SCLS, including theophylline and terbutaline, lisinopril, montelukast, and IVIG.
Based on recent evidence that humoral factors promote vascular permeability during flares of SCLS (6), we sought to develop molecular biomarkers to aid diagnosis. We analyzed circulating mediators of vascular permeability and proinflammatory cytokines in acute episodic sera from 14 patients with SCLS (including the aforementioned patient) and sera from 37 healthy control subjects. We monitored barrier function of human microvascular endothelial cells (HMVEC) after treatment with SCLS sera using transendothelial electrical resistance assays.
Consistent with our previous study, the permeability factor vascular endothelial growth factor (VEGF) was increased in sera from acutely ill subjects with SCLS (including the atypical patient) when compared with sera from healthy control subjects (mean ± SEM, 311 ± 86 vs. 67 ± 14 pg/ml; P = 0.002, Kruskal-Wallis). Sera from three patients experiencing mild episodes (i.e., not requiring intravenous fluid and/or hospital admission, patients 2, 5, 13 in Table 1) and another specimen collected mid-episode (patient 4) contained relatively low amounts of VEGF. These results support our previous hypothesis that increased VEGF levels mark the onset of acute SCLS flares and correlate with episode severity (6). When we limited our analysis to sera from patients with classic acute SCLS (13 patients), we found that IL-1β, IL-6, CCL2, VEGF, and CXCL10 levels were significantly higher in acute SCLS sera than in sera from healthy control subjects. In contrast, our highlighted subject’s acute serum cytokine profile included very high levels of VEGF, IL-6, IL-8, and tumor necrosis factor (TNF)-α but low concentrations of CXCL10 (patient 14 in Table 1). Because CXCL10 levels were markedly increased in acute sera from nearly all subjects with classical SCLS (11 of 13), this cytokine may differentiate patients with classic acute SCLS from those with atypical disease features.
Table 1:
Cytokine Profiles of Acute Sera from Individual Subjects with Systemic Capillary Leak Syndrome
| Patient No. | IL-1β | IL-6 | IL-8 | IFN-γ | CXCL10 | CCL2 | TNF-α | VEGF |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 51 | 1,933 | 248 | 6,030 | 766 | 27 | 633 |
| 2 | 2 | 11 | 14 | 107 | 2,932 | 276 | 13 | 50 |
| 3 | 2 | 20 | 26 | 145 | 401 | 32 | 23 | 50 |
| 4 | 2 | 7 | 12 | 58 | 15,479 | 199 | 9 | 157 |
| 5 | 0 | 1 | 0 | 0 | 493 | 9 | 0 | 58 |
| 6 | 8 | 20 | 333 | 159 | 4,620 | 446 | 27 | 996 |
| 7 | 2 | 32 | 13 | 83 | 7,157 | 416 | 15 | 229 |
| 8 | 2 | 8 | 15 | 99 | 4,104 | 146 | 21 | 392 |
| 9 | 3 | 7 | 47 | 66 | 2,514 | 126 | 2 | 237 |
| 10 | 0 | 2 | 2 | 0 | 2,792 | 82 | 2 | 162 |
| 11 | 2 | 5 | 4 | 6 | 1,275 | 112 | 0.2 | 175 |
| 12 | 0 | 2 | 12 | 6 | 2,886 | 78 | 2 | 195 |
| 13 | 0 | 1.5 | 3 | 0 | 662 | 26 | 0 | 53 |
| 14 (atypical patient) | 651 | 1,600 | 26,902 | 160 | 759 | 5454 | 585 | 959 |
| Classical SCLS, mean ± SEM (n = 13) | 2 ± 0.7 | 13 ± 4 | 186 ± 147 | 75 ± 21 | 3,950 ± 1,121 | 209 ± 60 | 11 ± 3 | 261 ± 76 |
| Control, mean ± SEM (n = 37) | 1 ± 0.2 | 3 ± 0.8 | 11 ± 6 | 24 ± 8 | 521 ± 120 | 50 ± 9 | 3 ± 1 | 67 ± 14 |
| P value | 0.03 | 0.01 | n.s. | n.s. | 0.0001 | 0.02 | n.s. | 0.006 |
n.s. = not significant; SCLS = systemic capillary leak syndrome; TNF = tumor necrosis factor; VEGF = vascular endothelial growth factor.
Application of the atypical patient’s acute sera to HMVEC monolayers elicited striking hyperpermeability (decreased resistance) compared with treatment with his remission sera or that from healthy control subjects (Figure 1A), supporting the clinical diagnosis of SCLS. In parallel with its use in preventing SCLS episodes, IVIG pretreatment of HMVECs did not significantly affect baseline resistance values (data not shown) yet markedly reduced hyperpermeability induced by exposure to acute sera from several patients with classical SCLS compared with bovine serum albumin pretreatment (Figure 1B). In contrast, application of IVIG to HMVECs in the transendothelial electrical resistance assay had no impact on permeability evoked by our atypical patient’s acute serum, which was consistent with the clinical history (Figure 1C). As might be predicted by his cytokine profile, neutralizing antibodies against IL-8 or TNF-α (infliximab) did significantly reduce permeability induced by his episodic sera under similar conditions (Figure 1D).
Figure 1.
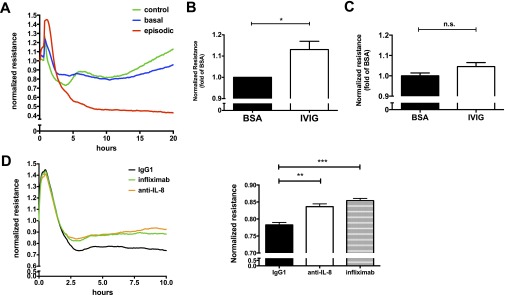
Molecular classification of systemic capillary leak syndrome (SCLS) using transendothelial electrical resistance (TER) assays. Human microvascular endothelial cells (HMVEC) were plated to confluence on TER assay wells. Cells were serum starved in 1% fetal bovine serum followed by addition of sera at time zero, and resistance was measured over time. (A) Normalized resistance values (absolute resistance at each time point divided by resistance at time zero) after addition of patient 14’s basal (blue) or episodic (red) sera or sera pooled from eight healthy control subjects (green). Plot is from a single experiment representative of 3 to 5 replicates per condition. (B, C) The effect of intravenous immunoglobulin (IVIG) on HMVEC permeability induced by acute sera from patients with classical SCLS (n = 5) (B) or atypical patient 14 (C). Cells were pretreated with bovine serum albumin (BSA) or IVIG at a final concentration of 1.25 mg/ml for 1 hour followed by TER measurements. Bar graph shows end point resistance values at 2.5 hours after serum addition (mean ± SEM of 3–5 replicates per condition; *P = 0.01, t test). n.s. = not significant. (D) Anti-IL8 (5 μg/ml), infliximab (5 μg/ml), or equivalent concentrations of control IgG1 (Thermo Fisher Scientific, Waltham, MA) was applied to HMVECs 1 hour before addition of acute SCLS sera from patient 14. Bar graph shows end point resistance values at 2.5 hours after serum addition (mean ± SEM of 3–5 replicates per condition; **P = 0.003, ***P = 0.0005, t test). All patients in this study gave informed consent for the publication of this letter.
In summary, we present the case of a patient with atypical symptoms that nonetheless met the existing clinical criteria for SCLS. However, he did not respond clinically to IVIG, which is uncommon. In the largest case series published to date describing the use of IVIG (2), 11 of 13 patients who received the recommended dose (2 g/kg monthly) responded favorably (i.e., experienced no severe episodes) for periods of up to 6 years after initiation of IVIG therapy. In our cohort, 23 of 25 patients (92%) who have received the full monthly dose of IVIG have experienced complete or near-complete remissions for periods of up to 8 years (unpublished data).
In this case, the microvascular barrier function assay corroborated our atypical patient’s clinical resistance to IVIG and suggested that his episodes were driven by acute phase cytokines IL-8 and TNF-α. Thus, our data suggest that SCLS may have clinically varying forms of presentation and that within the group of patients with SCLS, different cytokines may mediate capillary leak. We propose that the current clinical criteria for SCLS are limited and may contribute to ongoing morbidity and inappropriate use of expensive and ineffective medication. Quantitative molecular and humanized cell-based assays for humoral mediators of permeability should improve diagnostic specificity for SCLS and enable clinicians to screen for effective therapies ex vivo.
Footnotes
Supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Project AI001830 (K.M.D); the National Institutes of Health grants R01-HL093234 and R01-DK095072 (S.M.P.); and the American Diabetes Association grant 1-13-BS-1-41 (S.M.P.).
Author Contributions: Conception and design: Z.X., C.C.G., S.M.P., K.M.D.; analysis and interpretation: Z.X., C.C.G., S.M.P., K.M.D.; drafting the manuscript for important intellectual content: S.M.P., K.M.D.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Druey KM, Greipp PR. Narrative review: the systemic capillary leak syndrome. Ann Intern Med. 2010;153:90–98. doi: 10.1059/0003-4819-153-2-201007200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gousseff M, Arnaud L, Lambert M, Hot A, Hamidou M, Duhaut P, Papo T, Soubrier M, Ruivard M, Malizia G, et al. The systemic capillary leak syndrome: a case series of 28 patients from a European registry. Ann Intern Med. 2011;154:464–471. doi: 10.7326/0003-4819-154-7-201104050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Tahirkheli NK, Greipp PR. Treatment of the systemic capillary leak syndrome with terbutaline and theophylline: a case series. Ann Intern Med. 1999;130:905–909. doi: 10.7326/0003-4819-130-11-199906010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor P, Greipp PT, Schaefer EW, Mandrekar SJ, Kamal AH, Gonzalez-Paz NC, Kumar S, Greipp PR. Idiopathic systemic capillary leak syndrome (Clarkson’s disease): the Mayo Clinic experience. Mayo Clin Proc. 2010;85:905–912. doi: 10.4065/mcp.2010.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawabe S, Saeki T, Yamazaki H, Nagai M, Aoyagi R, Miyamura S. Systemic capillary leak syndrome. Intern Med. 2002;41:211–215. doi: 10.2169/internalmedicine.41.211. [DOI] [PubMed] [Google Scholar]
- 6.Xie Z, Ghosh CC, Patel R, Iwaki S, Gaskins D, Nelson C, Jones N, Greipp PR, Parikh SM, Druey KM. Vascular endothelial hyperpermeability induces the clinical symptoms of Clarkson disease (the systemic capillary leak syndrome) Blood. 2012;119:4321–4332. doi: 10.1182/blood-2011-08-375816. [DOI] [PMC free article] [PubMed] [Google Scholar]


