Abstract
Background: Limb muscle dysfunction is prevalent in chronic obstructive pulmonary disease (COPD) and it has important clinical implications, such as reduced exercise tolerance, quality of life, and even survival. Since the previous American Thoracic Society/European Respiratory Society (ATS/ERS) statement on limb muscle dysfunction, important progress has been made on the characterization of this problem and on our understanding of its pathophysiology and clinical implications.
Purpose: The purpose of this document is to update the 1999 ATS/ERS statement on limb muscle dysfunction in COPD.
Methods: An interdisciplinary committee of experts from the ATS and ERS Pulmonary Rehabilitation and Clinical Problems assemblies determined that the scope of this document should be limited to limb muscles. Committee members conducted focused reviews of the literature on several topics. A librarian also performed a literature search. An ATS methodologist provided advice to the committee, ensuring that the methodological approach was consistent with ATS standards.
Results: We identified important advances in our understanding of the extent and nature of the structural alterations in limb muscles in patients with COPD. Since the last update, landmark studies were published on the mechanisms of development of limb muscle dysfunction in COPD and on the treatment of this condition. We now have a better understanding of the clinical implications of limb muscle dysfunction. Although exercise training is the most potent intervention to address this condition, other therapies, such as neuromuscular electrical stimulation, are emerging. Assessment of limb muscle function can identify patients who are at increased risk of poor clinical outcomes, such as exercise intolerance and premature mortality.
Conclusions: Limb muscle dysfunction is a key systemic consequence of COPD. However, there are still important gaps in our knowledge about the mechanisms of development of this problem. Strategies for early detection and specific treatments for this condition are also needed.
Executive Summary
Contents
Overview
Introduction
Methodology
Scope and Definition
Normal Muscle Structure and Function
Normal Motor Structure
Determinants of Muscle Strength
Normal Mitochondrial Function
Determinants of Muscle Endurance
Regulation of Muscle Mass
Limb Muscles in Clinically Stable COPD
Muscle Atrophy in COPD
Structural Alterations of Limb Muscle in COPD
Mitochondrial Function of Limb Muscle in COPD and Bioenergetics
Oxidative Damage in COPD
Limb Muscle Function in COPD
Limb Muscle Function and Exacerbation of COPD
Consequences of Limb Muscle Dysfunction in COPD
Implications for Exercise Intolerance
Etiology of Limb Muscle Dysfunction in COPD
Disuse versus Myopathy
Mechanisms of Limb Muscle Dysfunction in COPD
Mechanisms of Muscle Susceptibility to Fatigue in COPD
Assessment of Limb Muscle Function in COPD
Assessment of Muscle Mass
Assessment of Limb Muscle Strength
Assessment of Limb Muscle Endurance
Assessment of Muscle Oxygenation Using Near-Infrared and Magnetic Resonance Spectroscopy
Effects of Interventions on Limb Muscle Function in COPD
Exercise Training
Neuromuscular Stimulation
Oxygen Therapy
Nutritional Supplementation
Testosterone and Other Anabolic Steroids
Growth Hormone and its Secretagogues
Other Anabolic Drugs and Bioactive Nutrients
Antioxidants
Vitamin D Supplementation
Suggestions for Future Research
Conclusions
Overview
Limb muscle dysfunction is an important systemic consequence of chronic obstructive pulmonary disease (COPD) because of its impact on physical activity, exercise tolerance, quality of life, and even survival in this disease. Although some mechanisms underlying the development of limb muscle dysfunction have been identified (e.g., deconditioning), much needs to be learned about the impact of other potential contributors to this clinical manifestation in COPD. Limb muscle dysfunction can be prevented and improved, in part, with exercise training, but it is clear that novel therapies are needed to better address this problem.
The purpose of this document is to update the 1999 American Thoracic Society/European Respiratory Society (ATS/ERS) statement on limb muscle dysfunction. We intend to provide researchers and clinicians with the recent advances in this field, with emphasis on the following areas: (1) structural and metabolic alterations found in limb muscles, (2) consequences and clinical evaluation of limb muscle dysfunction, (3) mechanisms of development of this comorbidity, and (4) treatment approaches of limb muscle dysfunction in COPD. Future research directions are also discussed. To be consistent with the 1999 statement, this document focuses specifically on limb muscles, recognizing that the issues related to respiratory muscles should be treated separately.
Major conclusions of the statement include:
-
•
Limb muscle dysfunction is prevalent in COPD. Muscle atrophy and weakness carry important consequences, such as difficulties in engaging in physical activity, exercise intolerance, poor quality of life, and premature mortality. Metabolic alterations in relation to lower limb muscle structural changes within the lower limb muscle are also involved in exercise limitation.
-
•
Lower limb muscle function is further compromised during episodes of COPD exacerbations. Patients experiencing exacerbations may be targeted for rehabilitative interventions aiming at preserving limb muscle function.
-
•
Assessment of limb muscle function should be encouraged.
-
•
Knowledge of the biochemical regulation of muscle mass will likely lead to the development of specific therapy for muscle atrophy in COPD.
-
•
Although physical inactivity is involved in the development of limb muscle dysfunction development in COPD, other mechanisms, such as inflammation, oxidative stress, nutritional imbalance, and hypoxemia, likely play a role.
-
•
The most potent currently available treatment option for limb muscle dysfunction in COPD is exercise training, a key component of integrated management of COPD.
-
•
Neuromuscular electrical stimulation is emerging as a useful training modality in patients severely impaired by COPD and during exacerbations.
We hope that this statement will raise further awareness toward this important problem in COPD and that research in this area will result in the development of specific therapies and in better care for patients with COPD.
Introduction
Limb muscle dysfunction is a major systemic consequence of COPD. Strong scientific and clinical evidence support a role of limb muscle in exercise intolerance in this disease. Furthermore, limb muscle dysfunction may be associated with increased mortality, poor quality of life, and increased health care use. Although some mechanisms underlying the development of limb muscle dysfunction have been identified (e.g., deconditioning), much needs to be learned about the impact of other potential contributors to limb muscle dysfunction in COPD (e.g., inflammation, malnutrition, oxidative stress, hypoxemia). Limb muscle dysfunction can be improved in part with exercise training, but it is obvious that novel therapies will have to be developed to better address this problem.
In 1999, Drs. Richard Casaburi and Rik Gosselink led a group of scientists to produce a Statement of the ATS and ERS on limb muscle dysfunction in COPD (1). This document was most useful in establishing the state-of-the art knowledge on this topic and in increasing the awareness of the scientific and medical community about its importance. However, this document was produced at a time when our understanding of limb muscle dysfunction was in its infancy. Since then, the amount of science related to limb muscle dysfunction in COPD has exploded, thanks to the effort of several research groups throughout the world. Members of the Pulmonary Rehabilitation and Clinical Problems assemblies of the ATS and ERS believe that it is timely to update this Statement to incorporate the large amount of knowledge that has been gained in the intervening years.
The primary objective of this document is to update the current scientific and clinical knowledge on this topic and to provide guidance on future research directions. As such, our document will be useful not only to scientists involved in the area but also to clinicians, for whom we wish to raise the level of awareness regarding the clinical relevance of limb muscle dysfunction in patients with COPD. It can be legitimately believed that a more thorough understanding and treatment of limb muscle dysfunction in COPD will improve the outcome of patients with COPD.
Methodology
The present document is intended to belong to the Clinical Statement category. The chair and the co-chair initially identified a group of 25 scientists on the basis of their specific expertise and with a variety of academic backgrounds (clinicians, physiologists, basic scientists, nutritionists, exercise specialists). We met on three occasions from December 2011 to September 2012. Each member was responsible for the review of the literature and for writing the first draft of his/her attributed section(s). Each specific section was circulated and discussed among all members of the committee to produce a preliminary version of the document. A librarian was consulted to perform a search of the literature using PubMed, Embase, and CINAHL according to the following strategy: “Pulmonary disease, Chronic Obstuctive”[Majr] or “Pulmonary emphysema”[Majr:NoExp] and (((((“Muscle, Limb”[Mesh]) or “Muscle Strength”[Mesh:NoExp]) or “Muscle Tonus”[Mesh]) or “Muscle Fatigue”[Mesh]) or “Muscle Weakness”[Mesh]) or “Muscular Atrophy”[Mesh] and “Oxidative Stress”[Mesh] and (muscle or muscles or muscular) and “Myositis”[Mesh:NoExp] OR (“Inflammation”[Mesh] and (muscle or muscles or muscular)). We also received advice from a methodologist to ensure that the methodological process was consistent with the approved methodology of the ATS documents development and implementation committee. A working draft of the document was circulated among members in the spring of 2012 and, based on their comments, it was extensively revised by the co-chairs and submitted again for agreement on the scientific content. The document was then fully edited by one committee member (R.C.). All members of the committee agreed with the content of the final document, which was intended for online publication only. This online document provides a thorough review of limb muscle dysfunction in COPD, whereas the published document summarizes the most critical aspects of the entire document.
Scope and Definition
This document focuses on limb muscles, as the committee members believed that respiratory muscles are a separate topic. The term limb muscle was preferred over peripheral muscles, which is less specific and may have a different interpretation to various individuals. Limb muscle dysfunction is used to reflect the morphological and functional changes that are seen in limb muscles in patients with COPD, with no implications as to the underlying mechanisms. The most commonly studied limb muscle is the quadriceps, because of its role in ambulation and because it is easily accessible. Other limb muscles from the upper extremity or the distal lower limb have also received some attention. They will be specifically mentioned when appropriate. The reader should also be aware that most studies on limb muscle function in patients with COPD have involved patients with severe to very severe COPD (Global Initiative for Obstructive Lung Disease [GOLD] spirometry classes 3–4). Muscle atrophy indicates a small muscle mass in comparison with healthy standards. This situation is different from cachexia, which implies an ongoing and dynamic loss of muscle mass (2). Very few longitudinal studies exist on the evolution of muscle mass in COPD (3, 4) and, in most cases, patients with low muscle mass instead of “true” cachectic patients were studied. Whether or not patients in these studies were actively losing muscle mass is uncertain.
Normal Muscle Structure and Function
Normal Motor Structure
Limb muscles are composed of functional units (motor units) consisting of a motoneuron and the muscle fibers it innervates. The motor unit is the final functional element that produces force. Based on contractile speed, motor units are classified as either slow-twitch (S) or fast-twitch (F) (5). The fast-twitch motor units are further subdivided into fast-twitch fatigue-resistant (FR), fast-twitch fatigue-intermediate (Fint), and fast-twitch fatigable (FF) (6–8). Data on motor unit organization and characteristics in human muscles are scarce. Enoka was the first to report data on motor unit properties of human muscles (9). He estimated that the extensor hallucis brevis muscle contains approximately 56 motor units and at least two types of motor units. In comparison, the human medial gastrocnemius contains about 550 motor units (10) and three types of motor units (S, FR, FF) (11), similar to cat gastrocnemius.
Within a muscle, each motor unit is composed of muscle fibers of a given type with the classification of S, FR, FInt, and FF motor unit types corresponding well with the muscle fiber classification based either on myofibrillar ATPase staining (I, IIa, IIx, and IIb) or on myosin heavy chain (MHC) immunoreactivity (MHCslow, MHC2A, MHC2X, and MHC2B). This has been shown for the human medial gastrocnemius, where the physiological characteristics of the motor unit types were in agreement with the histological typing of their constituent muscle fibers (11). Because human muscle fibers formerly identified as type IIb fibers by histochemistry express the IIx MHC isoform rather than the IIb isoform, we will refer to type IIx fibers for the remainder of the document (12).
In limb muscles, graded contractions are achieved by either changes in the firing rate of the individual motor units or by recruitment of additional motor units within the same muscle. In addition, recruitment order follows the size principle, with S units being recruited first followed by FR, FInt, and FF units (13). This size principle has been confirmed in humans during voluntary isometric contractions for several muscles, including the first dorsal interosseus (14, 15), the masseter and temporalis (16), and the tibialis anterior (17). Finally, motor units of lower limb muscles in humans are also recruited in a task-dependent manner (18). In the standing position, the soleus motor units are continuously active (19), whereas the medial gastrocnemius has an irregular pattern of activation that is mainly due to recruitment of motor units (20).
Motor unit characteristics can be altered by several processes, such as aging and reduced physical activity and training. In humans, aging is associated with a loss of motoneurons, especially the larger ones with higher recruitment thresholds (21). This results in a decreased number of muscle fibers (21), with many of them losing their innervation, beginning to atrophy, and ceasing to function together (22). In fact, significantly reduced cross-sectional areas of both type I and II fibers are seen in the elderly compared with younger adults (23), although type II fibers appear to be preferentially affected by age-related atrophying process (24, 25). Some fibers are reinnervated by neighboring motoneurons (26), but the capacity for motoneuron sprouting is limited with age (27). The size principle of motor unit recruitment seems to be preserved in older adults (28). Reduced physical activity leads to a decline in median frequency of vastus lateralis muscle stimulation that is in line with the concurrent reduction of muscle fiber conduction velocity (29). Interestingly, short-term (14 d) bed rest reduces the muscle fiber conduction velocity of individual motor units from the vastus lateralis, vastus medialis, and tibialis anterior muscles without a significant effect on muscle force (30). On the other hand, 6 to 8 weeks of hand or limb immobilization is associated with a decrease in the motoneuron firing rate of the adductor pollicis and first dorsal interosseous muscles (31–33). This effect is already present after 1 week of immobilization (34), and its magnitude is much greater after 3 weeks of immobilization (33). The size order recruitment of the motor unit is maintained during immobilization (32). Finally, exercise training also alters motor unit characteristics. Thus, integrated and root mean square surface EMG values increase significantly with strength training, particularly during the first 3 to 4 weeks, and motor unit firing frequency is enhanced after high-resistance strength training (35). With resistance training, motor unit recruitment threshold decreases and motor unit discharge rates increase (36).
Determinants of Muscle Strength
Because limb muscles have the capacity to generate force, they are essential to the ability to move the body during daily activities, exercise, and sports. Basically, the amount of force developed is determined by velocity of shortening and the type of contraction as well as by number, size, rate, and type of motor units activated.
The force–velocity and the length–tension curves illustrate the importance of the velocity of the contraction and the length of sarcomeres in determining muscle strength. There is an optimal length of each fiber for which there is optimal overlap of actin and myosin filaments, thus maximizing cross-bridge interaction (37). When a sarcomere is fully stretched or shortened, little force can be developed because there is little cross-bridge interaction. Muscle force generation also depends on the velocity of muscle contraction. During concentric (shortening) contractions, maximal force development decreases progressively with the velocity. In contrast, during eccentric (lengthening) contractions, maximal force development increases progressively with the velocity (38).
The force generated by a motor unit depends on three interrelated factors: (1) the innervation ratio of the unit (i.e., the number of muscle fibers innervated by a motoneuron), (2) total functional cross-sectional area of all muscle fibers within the unit, and (3) specific force of the muscle fibers (i.e., the force per cross-sectional area).
The number and type of motor units recruited as well as the rate and synchronization of firing grade the intensity of a muscle contraction (39). The number of motor units varies according the muscle. For example, in the large muscles of the lower limb, motor units range in size from approximately 500 to 1,000 fibers. This gives the muscles their capacity for very forceful and rapid contractions. Importantly, all the muscle fibers of a motor unit are of the same fiber type, determining the mechanical and fatigue properties of the muscles (6). In most muscles, the Fint and FF motor units generate greater force per unit of area compared with S and FR units (40). The importance of the neural activation to muscle strength is elegantly demonstrated during deconditioning. During bed rest experiments, only 50% of the reduction of muscle strength is explained by reduction in muscle mass, the other part being due to impaired neural activation, as demonstrated by EMG (41). Alternatively, during the first weeks of muscle training most of the progression in strength is explained by enhanced muscle activation.
The force generated by single limb muscle fibers is determined primarily by the level of activation, the intracellular Ca2+ concentration (iCa2+), and the force per cross-sectional area of muscle (specific force). As the frequency of neural activation increases, the force generated by muscle fibers increases in a sigmoidal fashion. In motor unit studies, it has been shown that the force/frequency relationship of S motor units is shifted leftward compared with FF motor units (42). Thus, at a given frequency of submaximal neural activation, S motor units generate a greater percentage of their maximal force. This difference in the force/frequency relationship of motor units could relate to the amount of Ca2+ released from the sarcoplasmic reticulum at a given frequency of activation, to differences in excitation–contraction coupling (43–46), to differences in sarcoplasmic reticulum Ca2+ reuptake (47), or to differences in the Ca2+ sensitivity of myofibrillar proteins (48–52).
A number of studies have examined the force/Ca2+ relationship in single permeabilized limb muscle fibers, where iCa2+ can be clamped at different levels. Generally, muscle fibers expressing the MHCslow isoform have greater Ca2+ sensitivity than do fibers expressing fast MHC isoforms, so that slow fibers generate a greater fraction of their maximal force for a given iCa2+. Accordingly, the force/Ca2+ relationship of slow muscle fibers is shifted leftward compared with fast fibers (50–54).
Although other variables, such as pennation, neuromuscular recruitment contraction velocity, and angular position may also account for differences in strength over that of size alone (55), muscle cross-sectional area has a strong relationship with muscle strength (56–58). Maximum specific force in single limb muscle fibers is dependent on the number of cross bridges per half sarcomere, the average force per cross bridge, and the fraction of cross bridges in the force-generating state. Despite some controversies (59), fiber type differences in specific force have been reported in human limb muscles (60–62), and it was suggested that the lower force produced by slow fibers may be due to less force per cross bridge compared with fast fibers (63). In addition, differences in mitochondrial volume densities may contribute to fiber type differences in specific force (54). The higher mitochondrial volume densities of fibers expressing the MHCslow and MHC2A isoforms would presumably be at the expense of a correspondingly lower myofibrillar volume density, lower MHC content, and, hence, fewer cross bridges in parallel for a given fiber cross-sectional area.
In cross-sectional studies, muscle mass has been found to correlate with sex, age, and training status. Compared with women, men have more muscle mass both in absolute values and as percentage of the total body weight (64, 65). Although absolute muscle strength is therefore larger in men, relative muscle strength (force per cm2) is not different between men and women (57).
Generally beginning at age 30 years (64), the rate of decline in muscular strength appears greater in the lower body than the upper body (66). Both strength and speed of contraction were found to decline with age (64). Aging affects muscle function, and, after the age of 50 years, muscle mass (65), fiber area (mainly type II [24, 25]), and the number of muscle fibers decrease (21, 67). The proportion of type I fiber increases with age (25). Additionally, there is a slowing of motor unit firing rates, a decrease in the pennation angle of the muscle fibers, and reduced tendon stiffness (68).
In the elderly, higher levels of customary physical activity have been found to be associated with significantly higher muscle strength (69), but it is still unclear whether men and women derive similar benefits (70, 71).
Normal Mitochondrial Function
Maintenance of mitochondrial integrity is crucial to the preservation of cellular homeostasis. Mitochondria play a central role in ATP production through oxidative phosphorylation, particularly in energy-demanding tissues, including limb muscle. They actively participate in cellular Ca2+ dynamics through their capacity to take up and release Ca2+ (72–74). They generate metabolic outputs, which can modulate multiple signaling cascades, and nuclear gene expression programs through genetic and epigenetic (relevant modifications to the genome not involving changes in the nucleotide sequence) mechanisms (75). They constitute one of the main sources of reactive oxygen species (ROS), which can participate in cell signaling or cell dysfunction/death under physiological and pathological conditions, respectively (see section on oxidative damage for further discussion of this cascade) (76, 77). Finally, in response to stress-induced signaling events converging on the mitochondria, or to intrinsic dysfunctions within mitochondria caused by acute or chronic pathological conditions, these organelles can trigger apoptotic and necrotic cell death through permeabilization of their double-membrane system (78, 79). This event can occur through opening of the permeability transition pore (PTP) and/or formation of channels by proapoptotic members of the B-cell lymphoma 2 family of proteins (78, 80).
In healthy limb muscle, the volume–density and the functions of mitochondria can change according to fiber type and in response to physiological cues such as exercise or inactivity (81, 82). In addition, defective organelles are the primary cause of numerous mitochondrial genetic disorders (83) and may also play a role in the pathogenesis of chronic conditions affecting multiple physiological systems (84).
Determinants of Muscle Endurance
Endurance can be defined as the ability to sustain a specific physical task. The determinants of endurance performance depend on whether a whole body or local muscle task is considered. In healthy humans, oxygen delivery and extraction rather than ventilatory function limit maximal whole-body exercise performance. For submaximal performance (where exercise is performed below critical power and a steady state exists where o2 is constant and meets the energy requirements of the task), the constraints to continuing work will depend on substrate availability, thermoregulation, and motivation (85–87). At the muscle level, this means that the energy requirements of muscle contraction can be met from oxidative sources without significant lactate accumulation or adenine nucleotide loss.
Fatigue, in a physiological sense, is defined as a failure of force generation after loaded muscle contractions that is reversible by rest (88). Undertaking a task to the point of failure may be associated with fatigue, or even cause fatigue, but the reverse is not true; physical performance may continue in the presence of low-frequency fatigue (88). Two types of fatigue are generally recognized: central and peripheral. Central fatigue occurs when task failure is manifest but additional force can be generated by nerve stimulation; this implies a contractile reserve of the muscle. In peripheral muscle fatigue, there are at least two different mechanisms by which repeated contractions may cause impairment: the “transmission mechanism” involving the neuromuscular junction, muscle membrane, and/or endoplasmic reticulum and the “contractile mechanism” involving the muscle filaments (89–93). Although high-frequency fatigue is a recognized physiological entity, its clinical significance remains uncertain and is not considered further here (94). In normal humans there is a complex interplay between peripheral and central fatigue, so that the presence of peripheral fatigue leads to central inhibition in limb muscles (95), preventing the development of further peripheral fatigue.
Muscle endurance performance involves a complex interplay between the availability and extraction of oxygen and the incorporation of substrate into mitochondria. Adequate muscle oxygen supply is determined by cardiac output, local muscle perfusion, and blood oxygen content. In turn, muscle capillarity, mitochondrial density, and muscle enzyme concentration influence oxygen extraction.
The energy for muscle contraction is released by the dephosphorylation of ATP by adenylate kinase. Intramuscular ATP stores are sufficient to sustain contraction for only a few seconds and, if work is to continue, ATP stores must be replenished from other sources. ATP can be formed by the breakdown of phosphocreatine (PCr) to creatine and phosphate. Although this system provides energy for high-intensity exercise and during the early stages of contraction, it is rapidly exhausted. Glycolysis leads to the formation of pyruvate and oxidative phosphorylation, allowing the products of carbohydrate, protein, or fat metabolism to enter the mitochondria, where they are metabolized to water and carbon dioxide and thereby provide most of the ATP required for sustained muscle contractions. Once formed, pyruvate is metabolized to lactate by glycolysis or enters the mitochondria to form acetyl coenzyme A and fuel oxidative phosphorylation. At higher exercise intensities, pyruvate accumulation exceeds its uptake by the mitochondria, and it must be broken down to form lactate. It has long been assumed that lactate formation is the result of inadequate mitochondrial oxygen concentrations. However, although oxygen delivery is a requirement for oxidative energy production, significant lactate can be produced despite the presence of adequate oxygen supplies, and factors other than oxygen are crucial to the integration of oxidative and glycolytic metabolism (96). Lactate accumulation is likely to be determined by the balance of pyruvate production and oxidation in the mitochondria. In this respect, the role of the pyruvate dehydrogenase complex (PDC) appears to be pivotal (97). This enzyme is situated on the mitochondrial membrane and regulates the irreversible entry of pyruvate into mitochondria, where the Krebs cycle operates. PDC can be activated pharmacologically by infusing dichloroacetate (98), and this has been shown to attenuate lactate accumulation and increase maximal work rates in healthy subjects (99). The expansion of intermediates of the Krebs cycle (known as anapleurosis) is another potential stimulator of oxidative phosphorylation. However, artificial expansion of the Krebs cycle intermediate pool by the infusion of glutamine does not result in an increase in mitochondrial oxidative phosphorylation. These findings suggest that pyruvate availability through the activity of PDC is the principal regulator of mitochondrial oxidative metabolism rather than anapleurosis (100).
Regulation of Muscle Mass
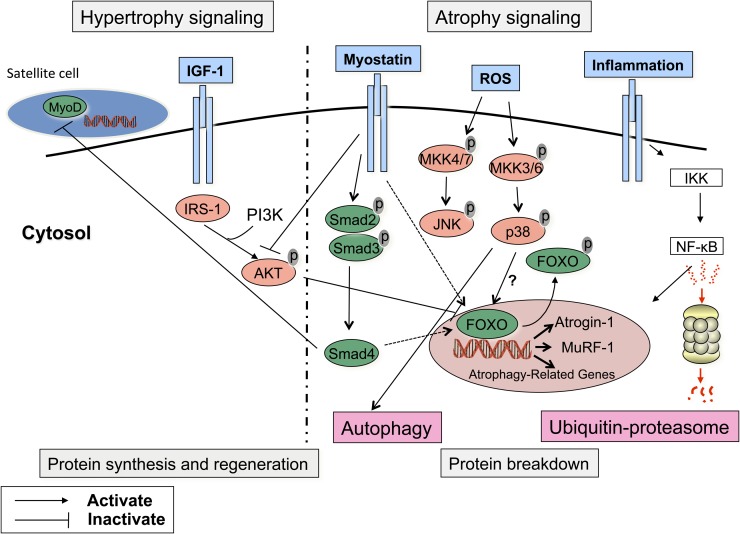
Homeostasis in muscle tissue is ensured by a tight and complex balance between protein synthesis and degradation (Figure 1). The regenerative capacity of muscle tissue is also involved in this equation (101–103). At the molecular level, cachexia is characterized by an increased muscle proteolysis with the activation of the ubiquitin proteasome (UbP) pathway (104). In this pathway, proteins are initially marked for degradation by ubiquitination and are subsequently recognized and processed by the proteasome, the catalytic core of the pathway. An increase in messenger ribonucleic acid (mRNA) encoding for key enzymes and proteins of this pathway is a hallmark of cachexia in several animal models (104–108). A major advance in the understanding of the regulation of muscle proteolysis was the identification of two muscle-specific E3 ligases, muscle ring finger protein 1 (MuRF1) and atrogin-1, which are directly involved in several atrophying conditions (109, 110). These E3 ligases act as the substrate recognition component of the ubiquitination system, therefore preventing nonspecific protein degradation by the proteasome complex. Of note, the UbP pathway is unable to degrade native and intact contractile structures (111). Preliminary steps aimed at disrupting the myofibrillar assembly are necessary before contractile protein degradation can be initiated. Calcium-dependent pathways (m- and u-calpains) (112), autophagy/lysosomal pathways (113), and cysteine proteases (caspase-3) (114) have all been demonstrated to be able to disrupt myofiber organization, thus providing substrates for the UbP system. Among them, the autophagy/lysosomal pathway is receiving a great deal of attention, because it may be the most important proteolytic pathway in some experimental models of muscle atrophy (115), although this issue is still disputed (116). Autophagy, which involves the formation of vesicles (autophagosomes) that transport their content for degradation by lysosomes, is also constitutively active in human limb muscle (117). There is insufficient information to make conclusions regarding the relative contribution of the UbP pathway and of autophagy to the muscle atrophying process in humans and more specifically in COPD.
Figure 1.
Regulation of muscle mass. The maintenance of muscle mass is the result of a tight equilibrium between hypertrophic and atrophic signaling pathways. A major advance in the understanding of the regulation of muscle proteolysis was the identification of two muscle-specific E3 ligases, atrogin-1 and Muscle Ring Finger protein 1 (MuRF1), that are directly involved in several atrophic conditions. These E3 ligases act as the substrate recognition component of the ubiquitination system, therefore preventing nonspecific protein degradation by the proteasome complex. Of note, the ubiquitin-proteosome (UbP) is unable to degrade native and intact contractile structures. Preliminary steps aimed at disrupting the myofibrillar assembly are necessary before contractile protein degradation can be initiated. Among them, the autophagy/lysosomal pathway is receiving a great deal of attention, because it may be the most important proteolytic pathway in some experimental models of muscle atrophy. The activation of the muscle-specific E3 ligases is under the control of various pathways: (1) the Forkhead box O (FOXO) class of transcription factors enhances the nuclear transcription of MuRF1 and atrogin-1 unless they are phosphorylated and inactivated by AKT. Conversely, depressed AKT activity and reduced FOXOs phosphorylation will allow FOXOs nuclear translocation and the induction of MuRF1 and atrogin-1; (2) proinflammatory cytokines can activate the nuclear factor (NF)-κB, which in turn can also induce atrophy through the activation of MuRF1; (3) the mitogen-activated protein kinases (MAPK) pathway can be triggered by reactive oxygen species (ROS) and has been implicated in the activation of the UbP pathway and in the initiation of the cachectic process in rodent and cell models of muscle atrophy. Among the various members of the MAPK family, p38 MAPK has received considerable attention because it stimulates the expression of atrogin-1, whereas its inhibition prevents muscle atrophy. JNK MAPK has also been implicated in the atrophying process in some experimental models, although its role is less convincing compared with p38. Myostatin, a negative regulator of muscle mass, is able to halt muscle growth by direct inhibition of the kinase activity of AKT or, through the SMAD signaling pathway, by inhibiting satellite cell replication and differentiation by blocking the activity of myogenic differentiation factor-D (MyoD). Myostatin is also able to enhance the proteasomal-dependent degradation of contractile protein by increasing the transcriptional activity of FOXO-1. Activation of the atrophic cascade is opposed by the hypertrophic response. In this regard, the importance of the insulin-like growth factor-1 (IGF-1) pathway to promote muscle growth has been appreciated for some years. The protein synthesis response to IGF-1 is mediated through AKT. On phosphorylation, AKT phosphorylates several proteins whose activation (mammalian target of rapamycin [mTOR] and 70-kD ribosomal S6 protein [p70S6] kinase) or inhibition (glycogen synthase kinase-3β [GSK3β]) will enhance protein synthesis. IGF-1 may also suppress protein degradation by down-regulating atrogin-1 again via the PI3K/AKT pathway as well as FOXOs phosphorylation and entrapment in the cytoplasm.
The activation of the muscle-specific E3 ligases is under the control of various pathways: (1) the forkhead box O (FOXO) class of transcription factors enhances the nuclear transcription of MuRF1 and atrogin-1 unless they are phosphorylated and inactivated by AKT (118). Conversely, depressed AKT activity and reduced FOXOs phosphorylation will allow FOXOs nuclear translocation and the induction of MuRF1 and atrogin-1. The role of the FOXOs in the protein degradation process is reinforced by the involvement of FOXO-3 in the regulation of autophagy (113, 117); (2) proinflammatory cytokines can activate the nuclear factor-κB (NF-κB), which, in turn, can activate MuRF1 (116, 119); (3) the mitogen-activated protein kinase (MAPK) pathway has been implicated in the activation of the UbP pathway and in the initiation of the cachectic process in rodent and cell models of muscle atrophy (115, 120). Among the various members of the MAPK family, p38 MAPK is of interest because it stimulates the expression of muscle-specific E3 ligases, whereas its inhibition prevents muscle atrophy (115). JNK MAPK has also been implicated in the atrophying process in some experimental models of hind limb suspension (121) and sepsis-induced diaphragmatic dysfunction (122), although its role is less convincing in comparison to p38 (120). Myostatin, a negative regulator of muscle mass, is able to halt muscle growth by direct inhibition of the kinase activity of AKT (123, 124) or by inhibiting satellite cell replication and differentiation by blocking the activity of myogenic differentiation factor D (MyoD) (125), a key protein involved in regulating muscle differentiation. Myostatin is also able to enhance the proteasomal-dependent degradation of contractile protein by increasing the transcriptional activity of FOXO-1 (126).
Activation of the atrophic cascade is opposed by the hypertrophic response. In this regard, the importance of the insulin-like growth factor 1 (IGF-1) pathway to promote muscle growth has been appreciated for some years (127). The protein synthesis response to IGF-1 is mediated through AKT. After being phosphorylated itself, AKT phosphorylates several proteins whose activation (mammalian target of rapamycin [mTOR] [128] and 70-kD ribosomal S6 protein [p70S6] kinase) or inhibition (glycogen synthase kinase-3β [GSK3β]) will enhance protein synthesis (116, 118, 128). IGF-1 may also suppress protein degradation by down-regulating atrogin-1 and by promoting FOXOs phosphorylation and entrapment in the cytoplasm, where it cannot enhance the nuclear transcription of E3-ligases (118).
Limb Muscles in Clinically Stable COPD
Several structural changes of the limb muscles have been reported in patients with COPD. They are described in this section and summarized in Figure 2. Most of these structural changes have been reported in the quadriceps, although some abnormalities have also been found in distal lower limb muscles. The upper limb muscles are relatively preserved from these structural changes.
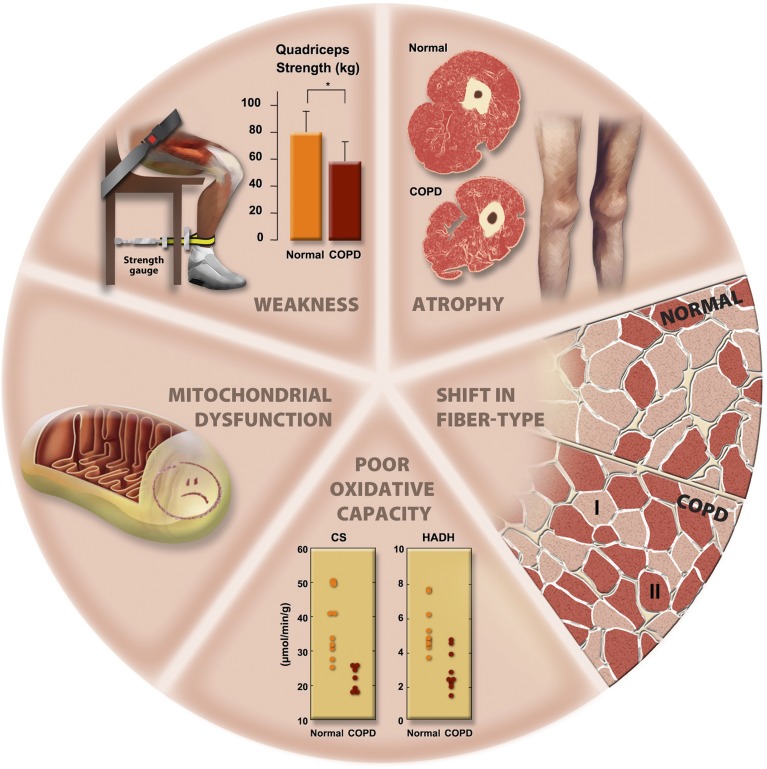
Figure 2.
Morphological and structural alterations reported in limb muscles in patients with chronic obstructive pulmonary disease (COPD). CS = citrate synthase; HADH = 3-hydroxyacyl CoA dehydrogenase.
Muscle Atrophy in COPD
Although the World Health Organization states that a body mass index (BMI) less than 18.5 kg/m2 defines underweight, a cut-off value of 21 kg/m2 is often used in COPD, as it corresponded to less than 90% ideal body weight in the Metropolitan Life Insurance Tables (129, 130). Using the World Health Organization criteria, the prevalence of underweight in COPD was found to increase with disease severity, especially in women, up to 30% in patients with GOLD class 4 disease (131). A key message is that the BMI classification does not consider body composition, such as fat mass (FM), fat-free mass (FFM), and bone mass or bone mineral density. Moreover, changes or shifts in body composition—which are frequently associated with COPD—remain unrevealed by BMI alone, and additional methods are required, as discussed elsewhere in this statement (see Assessment of Muscle Mass). This makes it important to consider the assessment of body composition in chronic diseases such as COPD. Along those lines, several criteria have been proposed to define low muscle mass (see Assessment of Muscle Mass). For example, an FFM index (FFMI) less than 16 kg/m2 for men and less than 15 kg/m2 for women (132), or an FFMI less than the 10th (131) or 25th (133) percentile of the general population, have all been used. Irrespective of the criteria, muscle atrophy is common in COPD, with a prevalence rate of 4 to 35% (131, 134, 135). Using the 10th percentile criterion, the prevalence of low FFMI in COPD increases with disease severity, especially in women, amounting to 50% in patients with GOLD 4 COPD (131). Moreover, a low FFMI has been reported in 26% of patients with COPD with a normal BMI, underscoring the importance of assessing body composition to precisely quantify muscle atrophy (131). Even in the COPD population with a normal BMI, a low FFMI is a strong predictor of mortality, as strong as in the underweight COPD population (132). Another important notion is that the lower limb muscles are particularly vulnerable to the atrophying process in COPD (3, 136, 137). The observation that the magnitude of loss of thigh muscle mass is relatively greater than that of whole body weight indicates a preferential loss of muscle tissue over other body tissues in patients with COPD (138).
The prevalence of overweight and even obesity is increasing in COPD. In a U.S. cohort, the prevalence of obesity was 54% in mild COPD versus 22% in the general population (139). In a Dutch population, the prevalence of obesity amounted to 24% in mild COPD versus 11% in the general population (140). The prevalence of overweight is higher in chronic bronchitis (blue bloater) patients, whereas underweight is typically associated with emphysema (pink puffer) (141). The distribution of fat accumulation is also important to consider. The accumulation of abdominal fat, a highly active tissue involved in the production of several proinflammatory mediators, is a strong risk factor for cardiovascular and metabolic diseases (142–146). COPD has been associated with increased risk of abdominal obesity (147, 148). Although a high BMI has been paradoxically associated with improved survival in advanced COPD (131, 149, 150), overweight and obesity, particularly in their visceral forms, may not be beneficial in milder forms of COPD, as they may mask muscle wasting and be associated with cardiovascular and metabolic complications (151, 152).
The prevalence of osteoporosis and osteopenia is also increased in COPD, amounting to 9 to 69% and 27 to 67%, respectively (153–155). Disease severity and systemic corticosteroids are associated with the risk of osteoporosis (156). Because the prevalence of osteoporosis is associated with a low FFM (157), it can be speculated that loss of bone and muscle mass share common mechanisms (158–160).
To our knowledge, only one longitudinal study has assessed the changes in body composition in COPD (4). During the 7-year follow-up, FFM declined faster than the FM, indicating the progressive occurrence of sarcopenia in this population. However, these changes were comparable between patients with COPD and healthy subjects (4). This study also showed that baseline BMI and FFM were similarly low in patients with COPD and smokers with normal lung function as compared with lifetime nonsmokers, suggesting that a common insult, occurring earlier in life and related to smoking, may contribute to muscle atrophy.
Structural Alterations of Limb Muscle in COPD
Muscle fiber shift and atrophy.
A shift in fiber type distribution of the quadriceps, from type I fibers in favor of type IIx fibers, is a typical feature of advanced COPD (137, 161–165). This finding is inconsistent with normal aging, which is not associated with a shift toward type II fibers (67, 166). The proportion of type I fibers correlates inversely with disease severity and proportionally with BMI (161, 164), a finding further supported by the absence of modification in the different fiber types in milder COPD (167). The shift in fiber type distribution reported in the quadriceps and tibialis anterior (101) muscles is not observed in upper extremity muscles such as the deltoid (168), indicating that muscle structural abnormalities are not homogeneously distributed among different muscle groups. All fiber types of the quadriceps are affected by the atrophying process (163), although some authors argue that the type IIx fibers are more specifically affected (169–171).
Changes in capillarization.
Capillary density (i.e., the number of capillaries per mm2 of muscle fibers) and the number of capillaries per muscle fiber are reduced in limb muscles of patients with COPD (163, 164). This is not a universal finding (172), perhaps due to the fact that, in some studies, patients were involved in exercise training, which could improve muscle capillarization (163, 173). The capillary to muscle fiber cross-sectional area is similar in subjects with COPD and healthy subjects (174). This may indicate that the oxygen diffusion distance is maintained in COPD (174).
Mitochondrial Function of Limb Muscle in COPD and Bioenergetics
Mitochondrial function is altered in COPD muscle, although it remains difficult to discern whether these abnormalities are indicative of a myopathic process specific to COPD or whether they reflect muscle inactivity in this population. Locomotor muscle oxidative capacity is reduced in COPD (44, 45, 175–179, 185–191). This has been demonstrated by direct measures of mitochondrial density by electron microscopy (175); by spectrophotometric determination of mitochondrial enzyme activities including citrate synthase (CS), succinate dehydrogenase (SDH), 3-hydroxyacyl-coenzyme A dehydrogenase (HAD), and cytochrome oxidase (COX) (176–178); and by measurements of respiration in permeabilized muscle fibers (179). In line with these observations, the mRNA and/or protein expression of key mitochondrial transcriptional factors and coactivators, including peroxisome proliferator-activated receptor γ coactivator-1 (PGC1), peroxisome proliferator-activated receptors (PPAR), and mitochondrial transcription factor A (Tfam), are/is also reduced (180), suggesting a lower drive for mitochondrial biogenesis in COPD muscle. Overall, the reduction in muscle oxidative capacity in COPD is consistent with the type I to type IIx fiber shift typically reported in this population (161, 163).
When compared with healthy control subjects, mitochondrial density and mitochondrial function are reduced in the lower limb muscle of patients with COPD (175, 176, 179, 181–183). In addition to this, the presence of specific mitochondrial impairments in COPD may affect energy conversion efficiency and selected respiratory chain complexes. However, this finding currently remains controversial. Regarding coupling efficiency, studies reported lower respiratory control ratios in isolated mitochondria from COPD muscle (176, 181), which could reflect reduced coupling of oxidation to phosphorylation. However, it is important to consider that greater fragility of mitochondria within diseased muscle can result in reduced respiratory control ratios caused by isolation-induced damage to organelles, as it does for other indices of mitochondrial function (179). Furthermore, a reduction of uncoupling protein 3 (UCP3) expression has been reported in patients with COPD (181, 184, 185), possibly representing a specific adaptation to modulate the efficiency of oxidative phosphorylation (184). Finally, two studies reported an increase in COX activity in the quadriceps in patients with COPD compared with healthy control subjects (176, 186). In both studies, up-regulation of COX activity was inversely correlated to PaO2, leading to the suggestion that hypoxia could specifically modulate this enzyme (176, 186). These data are, however, at odds with results from other studies showing a reduction in COX activity (178), mitochondrial content (175), and biogenic signaling (180) in COPD. Nevertheless, considering the complexity of COX regulation, it is possible that yet unidentified post-translational mechanisms underlie the reported enhanced COX activity. Overall, more work is required to establish the presence of specific alterations of mitochondrial energy metabolism in COPD muscle.
Thus far, only two studies have assessed mitochondrial ROS release in COPD muscle. Picard and colleagues (179) reported the net release of hydrogen peroxide (H2O2) per mitochondrion to be higher in permeabilized fibers of patients with COPD compared with control subjects. This was observed during baseline respiration and under active phosphorylation, indicating that mitochondria from COPD muscle display properties that potentiate H2O2 production. Similar data have been reported in isolated mitochondria (176). Currently, the mechanisms underlying this difference are unknown but could involve enhanced production of H2O2 and/or lower endogenous H2O2 scavenging capacities in mitochondria (176, 179). Although enhanced H2O2 release could reflect pathological alterations of mitochondrial ROS handling (176), it may also represent a signature of the fiber type switch present in COPD muscle (179). This is based on findings that healthy muscle mitochondria within type II fibers release significantly greater amounts of H2O2 than their counterparts in type I fibers (187, 188).
Evidence of increased apoptosis is reported in wasted COPD muscle (189), but the role of mitochondria as a triggering factor remains debated. A recent study by Puente-Maestu and colleagues (182) reported greater susceptibility to typical triggers of PTP opening, including Ca2+ and H2O2, in isolated mitochondria from COPD muscle. In contrast, Picard and colleagues (179) reported greater resistance to Ca2+-induced opening of the PTP in permeabilized fibers from patients with COPD compared with healthy control subjects and attributed this to the greater predominance of type II fibers, which are intrinsically more resistant to Ca2+-induced PTP opening (187). Factors underlying the discrepancy between the two studies could be related to the assessment technique whereby permeabilized muscle fibers allow characterization of the entire population of mitochondria in a more preserved cytoarchitectural environment compared with isolated mitochondria. Another difference is the fact that, in the studies by Puente-Maestu and colleagues (176, 182), patients with COPD and control subjects had lung cancer. Additional investigations are warranted.
Abnormal limb muscle bioenergetics.
Aerobic capacity is decreased and glycolytic activity preserved in lower limb muscles of patients with COPD (178, 190–196). Consistent with altered oxidative profile, the limb muscle metabolic profile exhibits, at rest, low concentrations of high-energy phosphates such as ATP and creatine phosphate as well as lower aerobic enzyme activity compared with age-matched healthy control subjects (197, 198). In addition, intermediate markers of glycolysis, namely glucose-6-phosphate, glucose-1-phosphate, and fructose-6-phosphate as well as phosphofructokinase, and lactate dehydrogenase activities are elevated in resting COPD muscles (198, 199).
The limb muscle transcriptomics (200) (the RNA that are transcripted in the muscle), proteomics (201) (the proteins that are expressed in the muscle), and metabolomics (202) (metabolic profile in the muscle) are altered in patients with COPD compared with age-matched healthy sedentary subjects. These abnormalities are more evident in patients with muscle atrophy. Moreover, a recent systems biology approach to the problem (200) suggested that patients with COPD show a failure to coordinately activate relevant limb muscle pathways, such as bioenergetics, inflammation, and tissue remodeling, that may lead to the abnormal structural changes seen in these patients. In this study, a significant association was observed between a number of histone modifiers and peak oxygen uptake, lending to the hypothesis that cell hypoxia, facilitated by sedentarity, might play a role in muscle dysfunction through epigenetic mechanisms (200).
Low mechanical efficiency and high resting energy expenditure.
Increased limb muscle O2 requirements at a given submaximal work rate has been reported (172, 192). The change in fiber type profile in COPD muscle may explain low mechanical efficiency in these patients (203). This finding is supported by a study demonstrating higher ATP consumption for a given mechanical work rate in patients with COPD (204). Measurements of resting and total whole-body energy expenditure also support higher energy requirements of patients with COPD (205, 206). Enhanced muscle protein turnover could also contribute to this phenomenon (207, 208).
Oxidative Damage in COPD
In resting and contracting limb muscle fibers, superoxide anion and nitric oxide (NO) are the primary free radicals generated. Superoxide anion gives rise to hydrogen peroxide, hydroxyl radicals, and other oxidants that form the ROS cascade. NO targets sulfhydryl groups in various proteins through the process of S-nitrosylation but can also react with superoxide anion to form highly reactive nitrogen species (RNS), such as peroxynitrite and nitrogen dioxide. In resting muscles, ROS and RNS are generated at low levels, and they promote physiological functions including regulation of contractile process, glucose uptake, and blood flow. During strong contractions or under pathophysiological conditions (209), ROS and RNS are synthesized at higher rates, which may overcome tissue antioxidant capacity, thereby leading to the development of oxidative stress. Oxidative damage may alter the structure and function of membrane lipids, proteins, and DNA, eventually leading to cell injury and death.
In limb muscles, ROS are mainly produced by the mitochondrial respiratory chain, especially during contractile activity. ROS can also be derived from enzymes such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (210), xanthine oxidase, and from chemical reactions with transition metals (211–213). NO is continuously produced by nitric oxide synthases (NOS) in limb muscle fibers, and its generation is enhanced during contraction. Three isoforms of NOS have been identified so far: constitutive endothelial (eNOS) and neuronal (nNOS), which are calcium dependent, and inducible (iNOS), which is calcium independent (214). Several RNS are formed inside limb muscle fibers, including the highly reactive peroxynitrite, which triggers post-translational modifications of proteins, including nitration of tyrosine residues leading to the formation of nitrotyrosine (215). Peroxynitrite also exerts direct oxidative effects on various proteins or other structures within the muscle fibers. Contractile proteins, key metabolic enzymes, sarcoplasmic reticulum, and calcium sensitivity are potential cellular targets of ROS and RNS within the muscle fibers. The generation of free radicals in COPD muscles under resting and contracting conditions has been demonstrated by the identification of oxidation of proteins, lipids, and DNA in systemic compartments (216, 217).
Oxidative stress is emerging as a major contributor to muscle dysfunction in patients with COPD, especially in those with severe disease. Under resting and exercise conditions, patients with COPD exhibit higher levels of lipid peroxidation, oxidized glutathione, and protein oxidation and nitration in the blood and limb muscles (167, 171, 177, 201, 217–232). Interestingly, chronic exposure to cigarette smoke increases several limb muscle oxidative stress markers in healthy smokers (233) and animals (233, 234). In the latter models, oxidation of muscle proteins anteceded the pathological features induced by cigarette smoke in the lungs of the animals (233).
The development of oxidative stress has strong functional implications for the contractile performance of limb muscles. For instance, Koechlin and colleagues (227) reported that systemic oxidative stress levels were directly related to quadriceps endurance time in patients with severe COPD. These authors also demonstrated that hypoxemic patients exhibited greater levels of oxidative stress in limb muscles, both at rest and after exercise, while showing a poorer quadriceps performance (229). Quadriceps muscle force is inversely related to the levels of protein oxidation being generated within the muscle (201, 223, 233). Importantly, exercise capacity is inversely related to protein oxidation levels within the quadriceps of patients with severe COPD (201).
One possible mechanism through which excessive ROS generation may adversely influence muscle contractile performance is by inducing post-translational modifications that may inhibit key muscle enzymes and proteins while enhancing their degradation. In this regard, contractile proteins such as MHC (171, 230) and enzymes such as creatine kinase undergo severe oxidation within the limb muscles of patients with COPD (171, 222, 230, 233, 235). Oxidation of these proteins induces a significant decrease in the content of contractile MHC as well as a reduction in the activity of creatine kinase (171, 222, 230, 233). The functional implication of these changes in MHC and creatine kinase regarding poor contractile performance of limb muscles in patients with COPD remains unclear. Another issue that remains under investigation is whether oxidative stress triggers proteolysis, which may partly account for muscle atrophy seen in COPD. In one study, however, oxidative stress did not correlate with muscle protein loss in the limb muscles of these patients (171).
The development of oxidative stress in limb muscles of patients with COPD may be the result of enhanced inflammatory cell infiltration and cytokine production. Nevertheless, there is no strong relationship between muscle oxidative stress and local inflammation in patients with COPD (171, 223, 233, 236). In fact, although local and systemic levels of inflammatory mediators are relatively low in patients with COPD regardless of their body composition, evidence of strong oxidative stress is consistently found in limb muscles and in the blood of these patients (171, 217, 223, 232, 233, 236). This specific issue is covered in more detail in a section below (Mechanisms of Limb Muscle Dysfunction in COPD).
Limb Muscle Function in COPD
Limb muscle function is altered in patients with COPD as evidenced by muscle weakness and reduced endurance. Lower limb muscle strength has been widely assessed in patients with COPD, and most of the data have been obtained in the quadriceps muscle. This muscle is readily accessible, and it represents a typical example of a primary locomotor muscle that is underused in patients with COPD. Quadriceps strength is usually assessed by volitional tests while measuring maximal isometric voluntary contraction (MVC), and studies consistently show that this measure is reduced by 20 to 30% in patients with COPD (3, 138, 224, 237–248). This has been confirmed by nonvolitional assessment tests based on evoked twitch tension in response to magnetic stimulation of the femoral nerve (246, 247, 249, 250). In one study, the annual decline in quadriceps strength was accelerated in patients with COPD, averaging 4.3% per year (3), in comparison with about 1 to 2% per year in the healthy aging population. However, another study reported a similar rate of decline in quadriceps strength in COPD versus the healthy aging population (4). The potential for a differential decline in limb muscle strength between COPD and the healthy elderly requires further investigation.
One of the major characteristics of muscle dysfunction in COPD is its interindividual heterogeneity, a fact leading to the concept of a limb muscle dysfunction phenotype in COPD. Seymour and colleagues (248) reported muscle weakness in 20% of patients with mild to moderate COPD, a population in which muscle weakness would not be expected as a major issue. By contrast, more than 50% of patients with severe COPD in whom muscle weakness was expected did not show reduced quadriceps strength. These data show that unexpected phenotypes regarding muscle weakness may exist in patients with COPD.
In one study, no alteration in contractile apparatus was found in COPD, as reflected by preserved in vitro contractile properties of vastus lateralis muscle bundles (239). In addition, when quadriceps strength is normalized by thigh muscle cross-sectional area or by muscle mass, no significant difference between patients with COPD and healthy control subjects was seen (3, 138, 224, 244). Therefore, the reduced quadriceps strength is mostly a reflection of the loss in muscle mass (136, 188, 242, 388, 389). However, in some patients, the loss in strength may be disproportional to the reduction in muscle mass (239, 248). This may occur in patients frequently exposed to systemic corticosteroids (138, 251). This finding may only apply to chronically treated patients, as no further decrease in quadriceps strength was reported in short-term corticosteroid-treated patients with COPD compared with untreated patients (252).
Muscle weakness is not homogeneously distributed among muscle groups. Although muscle weakness can be found in the upper extremities, the strength of these muscles is better preserved than that of lower limb muscles (136, 138, 168, 220, 237, 238, 244, 249, 253). In addition, the force of the distal upper limb muscles was better preserved than that of the proximal upper limb muscles.
Muscle endurance and fatigue.
Muscle endurance in patients with COPD has been mainly assessed in lower limb muscles. Volitional (227, 229, 242, 243, 254–257) and nonvolitional (258) assessments of muscle endurance have shown that quadriceps endurance is decreased in COPD. The magnitude of this decrease is, however, highly variable (range, 32–77%), probably because of differences in test procedures. The reduction in quadriceps endurance seems to be of similar magnitude in men and women with COPD (255, 257) and in patients with and without depleted FFM (244). Impaired quadriceps endurance is also present in patients with mild to moderate COPD and is only poorly associated with the degree of physical activity (243, 256, 259, 260). Endurance is more severely reduced in the presence of hypoxemia (229). The endurance of the elbow flexor muscle (261), the biceps (244), and the triceps and posterior deltoid (237) is preserved in patients with COPD, whereas that of the adductor pollicis muscle is slightly reduced in the presence of chronic hypoxemia (262), providing additional information about the heterogeneity of the muscle abnormalities in COPD.
Many patients with COPD stop exercise primarily because of leg fatigue complaints before they become ventilatory limited (263). The perception of fatigue has to be differentiated with objective measurements of fatigue. Most of the studies examining muscle fatigue after exercise in COPD have used a nonvolitional technique: twitch measurement after magnetic stimulation (240, 250, 264–268). Additionally, the EMG median frequency represents a valuable indirect marker to predict contractile fatigue (269). Objective contractile leg fatigue, as evidenced by a temporary reduction in quadriceps strength occurring after exercise, has been reported in 48 to 58% of patients with COPD (240, 250, 265, 266). However, the incidence increases to 58 to 81% when potentiated twitch instead of nonpotentiated twitch measurement was used to assess muscle fatigue (265). The occurrence of muscle fatigue is not an abnormal phenomenon in itself, but the key observation is that, for the same absolute oxygen uptake and the same duration of cycle exercise, the degree of contractile fatigue elicited by exercise is greater in patients with COPD than in healthy individuals (240). In addition, an inverse relationship between the degree of contractile muscle fatigue and dynamic hyperinflation after exercise has been reported in COPD, indicating that those patients with greater hyperinflation tend to be more limited by ventilatory constraint than by leg fatigue (264). Interestingly, quadriceps fatigue is infrequent after exhaustive walking in patients with COPD, suggesting that the mechanisms of limitation in COPD are exercise specific (250, 270). Nevertheless, the gastrocnemius and the tibialis anterior may also be susceptible to fatigue during walking (271, 272).
Limb Muscle Function and Exacerbation of COPD
Acute COPD exacerbations are common in the course of the disease and are associated with systemic events, including effects on limb muscle function. Quadriceps strength often decreases during hospitalization for a COPD exacerbation (241, 273, 274). The reduction in quadriceps force during hospitalization is significantly correlated to a smaller improvement in walking time 1 month after discharge (273). Importantly, quadriceps force only partially recovered 3 months after discharge from the hospital (241). Upper limb muscle function is also affected, as documented by a reduced handgrip force in patients hospitalized for a COPD exacerbation (241). Reduced handgrip force is also associated with an increased risk of hospital readmission due to acute exacerbation (275). The maintenance of muscle mass is compromised during an exacerbation, with multiple atrophying pathways being up-regulated (274, 276) while markers of the mitochondrial respiration pathway are down-regulated (276). The cause of muscle dysfunction during exacerbations is probably multifactorial, involving inflammation, nutritional imbalance, physical inactivity, and the use of systemic corticosteroids. Enhanced systemic inflammation may potentially contribute to deterioration of muscle function. Supporting this contention is the inverse correlation between IL-8 systemic levels and isometric quadriceps strength during exacerbations (241), whereas the presence of inflammatory markers within the muscle is not evident (274). During exacerbation, dyspnea and fatigue compromise dietary intake (277). In addition, resting energy expenditure is acutely increased during the first days of hospitalization (278). The resulting negative energy balance may contribute to physical inactivity in patients experiencing an exacerbation who may choose to preserve their energy. Indeed, patients with COPD are very inactive during hospitalization for an acute exacerbation and remain inactive even 1 month after discharge (273, 279). Patients with frequent exacerbations recover their physical activity level to a lesser extent than patients without frequent exacerbations (273). Patients not improving their walking distance within 1 month after exacerbation are at higher risk for hospital readmission (273). The use of systemic corticosteroids could also be involved in the worsening in muscle function in the course of a COPD exacerbation (251).
Consequences of Limb Muscle Dysfunction in COPD
Limb muscle dysfunction contributes to COPD morbidity. One major impact of COPD is the incapacity to perform daily activities, and muscle dysfunction may contribute to this problem. Arguably, the most troublesome consequence of muscle dysfunction is its negative effect on life expectancy (Figure 3), although a causal relationship has not been proven. Parameters such as reduced midthigh muscle cross-sectional area (280, 281) and lower quadriceps strength (282, 391) predict mortality in COPD, independently of lung function impairment: (1) a midthigh muscle cross-sectional area less than 70 cm2 as assessed by computed tomography scanning is associated with a fourfold increase in mortality after adjusting for age, sex and FEV1 (280); (2) an FFMI less than 16 kg/m2 in men and less than 15 kg/m2 in women is associated with a 1.9-fold increase in mortality after adjusting for age, sex, and FEV1 (132); and (3) a quadriceps strength (kg) to BMI (kg/m2) ratio less than 120% is associated with increased mortality; each 10% increment of this ratio is associated with a 9% reduction in mortality (282). This highlights the importance for clinicians to monitor body composition and muscle strength when evaluating a patient with COPD.
Figure 3.
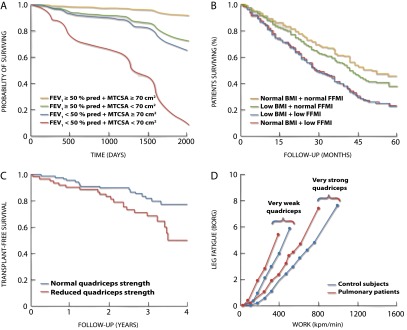
Relationships between muscle mass and strength and clinical outcomes in patients with chronic obstructive pulmonary disease (COPD). A midthigh muscle cross-sectional area (MTCSA) < 70 cm2 (A) (280), a low fat-free mass index (FFMI) defined as an FFMI < 16 kg/m2 in men and < 15 kg/m2 in women (B) (132), and a reduced quadriceps strength defined as a quadriceps strength (kg) to body mass index (BMI, kg/m2) ratio < 120% (C) (282) are predictors of mortality in COPD after adjusting for traditional mortality risk factor such as age and FEV1. The strength of the quadriceps is a significant contributor to exercise capacity in COPD (D) (285). All panels adapted by permission from the indicated references.
Implications for Exercise Intolerance
Exercise intolerance is a major consequence of COPD, and it cannot be explained solely on the basis of limitations in ventilation and gas exchange. For instance, the degree of impairment in lung function is a poor predictor of exercise capacity (283). Quadriceps strength correlates with poor exercise tolerance in this disease (284). The strength of the quadriceps is a strong predictor of exercise capacity in patients with chronic pulmonary diseases: a twofold increase in muscle strength is associated with a 1.4- to 1.6-fold increase in work capacity (285) (Figure 3). This may be related to the influence of muscle strength on the perception of leg effort during exercise (285), the main limiting symptom in 40 to 45% of patients with COPD (263). Perhaps the most striking clinical observation pointing to a peripheral component of exercise limitation in COPD is that exercise capacity remains abnormally low in most lung transplant recipients despite normalization of lung function (286). In patients with COPD, exercise termination usually occurs before a true plateau in o2 is reached. In many patients, psychological factors such as anxiety, fear of dyspnea, and poor motivation may contribute to exercise intolerance. As a result, the physiologic contributions of individual factors to reduced peak o2 is difficult to assess. The contribution of limb muscle abnormalities to exercise limitation in COPD has been challenged by Richardson and colleagues (203). These authors showed that the aerobic capacity of the lower limb muscles was not reached during cycling exercise (when a large muscle mass was involved), because of the early occurrence of central limitation to exercise. The implication of this is that the aerobic capacity of the exercising muscles of the lower limbs, even if reduced, is not overwhelmed during whole-body exercise in patients with COPD.
Patients with COPD stop exercising because of exertional discomfort and not necessarily because of physiologic constraints. Although dyspnea is undoubtedly the primary limiting symptom in many patients, a significant portion stops exercise because of leg fatigue (263). This could be related to the fact that limb muscle alterations, such as poor oxidative capacity, atrophy, and weakness, increase susceptibility to contractile fatigue. The impact of leg fatigue on the exercise response to acute bronchodilatation was evaluated to test the hypothesis that the improvement in airflow obstruction would not translate into better exercise capacity in patients with higher susceptibility to leg fatigue (266). In this study, bronchodilation provided by the administration of ipratropium bromide did not enhance exercise tolerance in patients who developed contractile fatigue after cycling exercise as it did in those who did not develop contractile leg fatigue (266). This study highlights how contractile fatigue of the quadriceps may influence the exercise response to bronchodilation in COPD, a concept that has been confirmed in subsequent studies (287).
Lower capacity for muscle aerobic metabolism may influence exercise tolerance. Increased lactic acidosis for a given exercise work rate, which is a common finding in COPD (177, 190, 288, 289), increases ventilatory needs by increasing nonaerobic carbon dioxide production (288). This imposes an additional burden on the respiratory muscles, which are already facing increased impedance to breathing. In addition, the resulting acidemia may act as a breathing stimulus through the carotid bodies. Premature muscle acidosis, a contributory factor to muscle fatigue and early exercise termination in healthy subjects, may be an important mechanism contributing to exercise intolerance in COPD (290). This may be exacerbated by a tendency to retain carbon dioxide (respiratory acidosis) during exercise (288). Perturbation in muscle energy metabolism is another potential contributor to exercise intolerance in COPD. Direct measurements of muscle metabolites in biopsy samples taken immediately after a standardized bout of constant work rate exercise (at 80% peak work rate) showed a significant accumulation of muscle lactate, degradation of muscle PCr, and loss of muscle ATP in COPD. The magnitude of these metabolite modifications was similar to that seen in healthy control subjects, but they occurred at substantially lower absolute exercise work rates (196, 291). This observation was replicated when plasma ammonia concentration was measured as a surrogate for adenine nucleotide loss (292). Moreover, when mitochondrial energy delivery was increased by pharmacological activation of PDC, both blood lactate and ammonia accumulation were reduced during maximal exercise in a cohort of patients with COPD (293). Maximal exercise work rates were increased after this intervention, suggesting that mitochondrial ATP delivery is relevant to whole-body exercise performance in COPD.
The degree of limb muscle fatigue reached during exercise appears to be highly regulated in healthy subjects (95, 294) and in COPD (196, 295). This is viewed as a protective mechanism (95) whereby feedback signals originating from the fatigued muscles inhibit motor cortical output via the stimulation of group III and IV muscle afferents, thus preventing subsequent locomotor recruitment and the development of dangerous and potentially irreversible fatigue (294, 296). This concept is in line with the notion that the central nervous system integrates information originating from the limb muscles to determine the duration of exercise and the degree of muscle fatigue (294, 297). Preinduction of muscle fatigue by neuromuscular electrical stimulation contributed to limitation of exercise tolerance in patients with COPD (298). Spinal anesthesia, presumably blocking group III/IV sensory afferent signals from the lower limb muscles, was shown to improve exercise duration to constant work rate exercise in patients with COPD (295). Spinal anesthesia was associated with less leg fatigue perception and allowed patients to reach a more profound degree of fatigue and greater central motor output to the exercising muscles. The reduced ventilatory response with spinal anesthesia was also important in explaining the enhanced exercise duration with this intervention. Based on studies showing early reliance on glycolytic metabolism and increased lactate production in exercising patients with COPD as compared with healthy subjects (196, 290), it is conceivable that the afferent signal originating from the contracting muscle may even be greater in patients with COPD than in healthy individuals and that this mechanism might have a larger effect in the former individuals. Thus, limb muscle dysfunction could, by enhancing the level of sensory afferent signalization, directly contribute to exercise intolerance.
The impact of lower limb muscle dysfunction on exercise tolerance in COPD has been mostly assessed using the cycling exercise modality. Gagnon and colleagues (272) reported recently that, after walking exercise standardized to induce similar energy expenditure, dorsi and plantar flexors were much more prone to the development of fatigue in patients with COPD than in healthy control subjects. These data are in line with another study in which the electrical activity of five different lower limb muscles behaved in a similar fashion in patients with COPD and in healthy control subjects despite a markedly lower walking distance during a 6-minute walk (271). Together, these studies highlight the potential contribution of the distal limb muscle fatigue in daily physical activities in patients with COPD. The occurrence of muscle fatigue may also modulate the response to exercise training programs, as illustrated by the observation that patients with COPD developing quadriceps fatigue during exercise training have larger training effects compared with those who did not fatigue during the same session (299).
Other likely consequences of muscle atrophy and poor limb muscle function in COPD include reduction in quality of life (300) and greater use of health care resources (301).
Etiology of Limb Muscle Dysfunction in COPD
The physiopathological interaction between chronic lung disease and alterations in limb muscle tissue is still poorly understood and constitutes an important research area. Several factors have been hypothesized to initiate and/or promote changes reported in limb muscles of patients with COPD (Table 1). Multiple factors are likely to interact in a given individual, and the relative contribution of each individual factor is likely to vary from one patient to another. The different factors that are reviewed in this section all have the ability to activate various cascades that could initiate or enhance, alone or most probably in combination, changes in fiber type phenotypic expression, contractile proteolysis, metabolic alterations, and regenerative defects in limb muscles of patients with COPD.
Table 1:
Etiologies of Limb Muscle Atrophy, Weakness, and Susceptibility to Fatigue
| Mechanisms Involved | |
|---|---|
| Factors leading to muscle atrophy and weakness | |
| Disuse | Associated with weakness, atrophy, changes in fiber type distribution, and metabolic alterations (303–306, 310) |
| Inflammation | Triggering of the muscle proteolysis cascade (102, 116, 322, 325) |
| Oxidative stress | Triggering of the muscle proteolysis cascade (336, 339, 340) |
| Associated with reduced muscle endurance (222, 227, 229) | |
| Protein carbonylation possibly involved in exercise intolerance and weakness (201) | |
| Hypoxemia | Decreased muscle protein synthesis |
| Activation of muscle degradation through hypoxia-inducible factor/von Hippel–Lindau signaling cascade (347–350) | |
| Hypercapnia | Intracellular acidosis/alterations in contractile protein synthesis/degradation (105, 362) |
| Low levels of anabolic hormones and growth factors | Associated with reduced muscle protein synthesis (371, 372) |
| Impaired energy balance | Associated with reduced muscle protein synthesis (381, 383) |
| Corticosteroids | Reduced muscle protein synthesis and enhanced proteolysis through increased myostatin levels and reduced insulin-like growth factor-1 levels (385) |
| Vitamin D deficiency | Associated with muscle weakness, type II atrophy impaired calcium metabolism (392, 400, 405) |
| Factors leading to muscle susceptibility to fatigue | |
| Central fatigue—afferent feedback from limb muscles | Reduced motor output to the contracting muscles (295) |
| Reduced O2 delivery (impaired cardiac output, blood flow competition between the respiratory and limb muscle, reduced capillarity) | Changes in muscle metabolism in favor of glycolysis; accumulation of muscle metabolites associated with muscle fatigue |
| Muscle metabolic alteration (reduced oxidative enzyme activity, reduced mitochondrial function) | Preferential use of glycolysis and accumulation of muscle metabolites associated with muscle fatigue (179, 180, 190, 199, 443) |
Disuse versus Myopathy
One important question is whether limb muscle dysfunction in COPD simply reflects years of physical inactivity or whether some factors specifically related to COPD could be invoked. Limb muscle dysfunction in COPD has been attributed in part to reduced physical activity (“detraining” or “deconditioning”) that characterizes this disease (302). Muscle disuse in general can lead to many of the features seen in limb muscles of patients with COPD: muscle weakness, overall muscle atrophy, decreased cross-sectional area of muscle fibers, loss of type I fibers, reduced oxidative enzyme activity, reduced capillary-to-fiber ratio, early lactate release, reduced rate of phosphocreatine resynthesis after exercise, and altered redox status (303–307). In healthy adults, these muscle alterations are usually reversible after a period of training.
Whether limb muscle dysfunction and cellular alterations in COPD are further compounded by a myopathy (that is, intrinsic alterations in limb muscle over and above the changes due to inactivity, and which are not fully reversible by exercise training) has been the subject of debate and controversy in last decade and is still not resolved (307–311). This may be in part because of failure to identify and separately study and discuss findings in different phenotypic (and in the future genotypic) subgroups of COPD. The obvious example is in COPD cachexia. Nobody would dispute that muscle mass loss taking a patient to below the 95% confidence limit of normal is a pathological process. But for patients with normal muscle mass, the question remains.
This question is not a semantic one, as identification of the mechanisms of muscle dysfunction in COPD will potentially result in rational and efficient therapeutics. If dysfunction is only due to deconditioning and inactivity, appropriate exercise training would be the logical way to improve patients and should be sufficient. If, however, myopathy occurs in COPD, other therapeutic approaches might be necessary to target the identified pathways. However, it can be argued that the inability to reach a sufficiently intense training stimulus for an adequate period of time during whole-body exercise in patients with COPD prevents proper testing of the hypothesis that exercise can restore structure and function to normal.
Although we cannot close the debate, we can expose some of the main positions. Against the hypothesis of a disease-specific myopathy are the following: (1) many functional and cellular findings in COPD are identical to those of disuse; (2) limb muscle is trainable, because training has been shown to improve muscle strength and endurance, oxidative enzyme activities, fiber cross-sectional area, and muscle capillarization, at least in some patients (307, 310); (3) in a small study, high-intensity aerobic interval training was shown to restore work performance and oxidative capacity of the quadriceps to a level similar to that found in sedentary individuals (312); and (4) the fact that full recovery of muscle function is unusual does not necessarily indicate a myopathy, because the duration of the training program may be too short to normalize alterations that have developed over several decades (313, 314), or early ventilatory limitation may compromise the ability to tolerate sufficient muscle stimulus to improve global or cellular function (311).
On the other hand, several observations are in favor of the existence of a myopathy in patients with COPD: (1) muscle function (strength or endurance) is not (or is poorly) related to the degree of physical activity in COPD, as assessed by questionnaires or actual measurements (302), suggesting that mechanisms other than inactivity may exist; (2) when patients with COPD are compared with healthy subjects with consistent and comparable low physical activity, differences in muscle function and/or structure remain (259, 308); (3) although exercise training improves some muscle features in COPD, muscle typology does not improve, (i.e., increase in type I fiber proportion [163, 315, 316]) to the same extent in COPD in comparison to what is seen in healthy subjects (317–319); (4) quadriceps weakness occurs in patients with COPD across all severity stages (310).
In summary, a consensus exists for a significant role of muscle disuse to explain limb muscle dysfunction in COPD, although it is not the only mechanism. The debate remains open on the concept of a separate myopathy in this disease. In this debate, one limit is the lack of clear and consensual definition of what is a COPD-related myopathy. Similar debates are also taking place in other chronic diseases, such as chronic heart failure and diabetes (320, 321).
Mechanisms of Limb Muscle Dysfunction in COPD
Inflammation.
The close relationship between the systemic inflammatory response in several acute and chronic conditions and the development of muscle atrophy is acknowledged in the literature (for review, see [116, 322]). Production of key cytokines can generate an array of cellular responses, including the induction of the UbP system through the transcriptional activities of NF-κB and FOXOs (119), apoptosis (323), and macroautophagy (324), which have all been linked to the development of muscle atrophy. In agreement with other atrophying models in which inflammatory response is reported, increased expression of Atrogin-1, MuRF-1, and neural precursor cell expressed developmentally down-regulated protein 4 (Nedd4), all E3-ligases, are observed in the quadriceps of patients with COPD (102, 325). Increased Nedd4 expression compromises satellite cell proliferation and muscle regeneration by favoring ubiquitination and degradation of Notch (326), an important transcription factor involved in the regulation of these processes (327). Conflictual or negative results regarding apoptosis and macroautophagy in the muscles of COPD demand further studies to explore the role of these mechanisms (102, 189, 236). Although increased inflammatory response is seen during exacerbated COPD (328, 329), similar observation is controversial in stable patients (201, 232, 330–332). One relevant negative observation is the lack of evidence of muscle inflammation in this human model of muscle atrophy (332, 333). A critical appraisal of the literature reveals only scant and unconvincing data supporting the presence of quadriceps inflammation in COPD (331, 334, 335), even in the presence of muscle atrophy (223, 333) or during periods of acute exacerbation when inflammatory bursts should be expected (276). As such, a clear role of muscle inflammation as a key event in the development of limb muscle dysfunction in COPD has yet to be confirmed.
Oxidative stress.
In several chronic atrophying conditions, including COPD, unbalanced oxidative stress could alter integrity of muscle proteins, enhancing their degradation (336). In COPD, systemic (226) and local (219, 220) oxidative stress have been reported. Acute bouts of exercise (224, 226) as well as acute exacerbation (329, 337) increase the level of oxidative stress. Direct oxidative stress exposure (338) or indirect production of ROS through inflammatory response (339, 340) induces proteolysis and increases expression of UbP components. Although precise mechanisms are still unknown, induction of NF-κB (339) and FOXOs transcriptional activities (341) or p38 kinase activity (120, 342) by oxidative stress is believed to play a role in the proteolytic signaling.
Hypoxia.
Under hypoxic conditions, muscle mass in animals (343) and humans (344) decreases. Moreover, under low oxygen content, cultured myoblasts have defective myogenic processes (slower proliferation and differentiation) (345). Patients with COPD having a low arterial O2 level (330) or a reduction in O2 delivery (346) tend to have a lower body mass than those with a normal arterial O2 level or a sufficient O2 delivery. During episodes of low O2 availability, a coordinated response for cell survival is accomplished by hypoxia-inducible factor-1 (HIF; for review, see [347]). In COPD, hypoxia is a likely factor driving changes in limb muscle tissue (200). As a correlate, increased expression of von Hippel–Lindau (VHL) protein, an E3 ligase involved in the proteolytic degradation of HIF-1α subunit, has been reported in muscle samples of patients with severe COPD (mean PaO2 of 69 mm Hg) compared with healthy subjects (348). Under such circumstances, the altered HIF signalization may further compromise muscle mass. In addition, regulated in development and DNA damage responses-1 (REDD1) gene activation was recently reported in muscle samples from hypoxemic patients with COPD (349). In response to several cellular stresses, REDD1 inactivates the kinase activity of mTOR, a key player in the transduction of the AKT signal during protein synthesis. At the tissue level, increased proteolysis through activation of the UbP system and halted synthesis have been demonstrated in cultured myotubes (350).
In patients with COPD, chronically altered oxygen transport (351, 352) and impaired oxygen use (176, 181–183, 353) facilitate limb muscle oxidative stress (201, 217, 226, 227, 229, 354) leading to nitroso-redox imbalance (355–357) and explaining post-transcriptional alterations (inhibition of S-nitrosylation) contributing to limb muscle dysfunction (201). Hypoxemia may also potentiate the inflammatory response (358), providing an additional mechanism linking hypoxia to specific cellular responses predisposing to muscle atrophy. Hypoxemia may also compromise muscle oxidative capacity and capillarization (359) and predispose to muscle fatigue (360, 361).
Hypercapnia.
Increase in cellular CO2 content is present in chronic hypercapnic patients and will develop or worsen during COPD exacerbation. Consequently, tissue pH decreases (197), and acidosis can alter contractile protein synthesis and degradation (362). In muscle tissue, acidosis increases the expression of genes encoding proteins of the UbP pathway (105) and impairs the AKT signaling (363), thereby reducing protein synthesis and further promoting activity of the UbP pathway (118, 364).
Low levels of anabolic hormones and growth factors.
Low testosterone levels have been observed in patients with COPD (365–369). Chronic hypoxia and corticosteroid therapy may all contribute to this observation. Indeed, current endocrine society guidelines suggest active case finding in populations at high risk of symptomatic hypogonadism, including those with COPD (370). Cross-sectional studies have demonstrated inconsistent association of indices of limb muscle function with serum testosterone levels in COPD. Laghi and colleagues observed a higher prevalence of hypogonadism in men with COPD but no association with limb or respiratory muscle performance or whole-body exercise capacity (368). In contrast, Van Vliet and colleagues observed that quadriceps strength, but not whole-body exercise tolerance, was related to low circulating testosterone levels (365).
Growth factors have an essential role in muscle tissue homeostasis. Although growth hormone (GH)/IGF-1 axis (reviewed in [371]) or testosterone (372) increase muscle content, myostatin is a strong negative regulator of muscle growth (373). Myostatin is a transforming growth factor-β family member that acts as a negative regulator of limb muscle growth. Its global role in whole-body homeostasis has recently been reviewed (374). The primary means by which myostatin negatively regulates muscle growth is by suppressing myoblast proliferation through the inhibition of cell cycle progression (375). Increased expression of myostatin mRNA transcripts (102, 376) and protein expression (377) in the quadriceps and elevated serum myostatin (378) were reported in patients with COPD. Myostatin mRNA transcript level are also correlated with muscle strength in patients with COPD (376). In turn, resistance training during an acute exacerbation may reduce quadriceps myostatin mRNA expression in patients with COPD (379). Based on the increased expression of Nedd4 (102) and on the repressive activity of myostatin on satellite cell proliferation and differentiation (373), it is tempting to speculate that muscle repair is likely altered in COPD.
Impaired energy balance.
Impaired energy balance, due to elevated energy requirements, reduced dietary intake, or both, may lead to limb muscle atrophy (380, 381). The energy balance may be compromised in COPD due to the complex interaction of a number of factors, including anorexia, elevated whole-body energy requirements, imbalance between muscle protein synthesis and breakdown, enhanced lipolysis, and an increase in pulmonary and systemic inflammatory mediators (382). Impairment in energy balance and protein balance may occur simultaneously, but these processes can also be dissociated, as reflected in the different COPD body composition phenotypes. Patients with a negative energy balance and a negative protein balance will deplete both body fat and protein stores, as reflected in weight loss, loss of FM, and muscle wasting. Patients with a normal energy balance but a negative protein balance will experience loss of muscle mass despite normal and stable weight (383).
Corticosteroids.
Although a short course of systemic corticosteroids may not alter limb muscle function in COPD (252), these antiinflammatory agents have a trophism for the muscles, and their chronic or repeated use can potentiate muscle atrophy and weakness in patients with COPD (251, 384). Morphological changes have been reported in the quadriceps in patients with COPD presenting with a corticosteroids-related myopathy (384). These include increased variations in muscle fiber size, increased amount of connective tissue, increased number of central and subsarcolemmal nuclei and diffuse necrotic fibers. Corticosteroids, particularly in their fluorinated form, appear to preferentially affect type IIx fibers; these fibers can become severely atrophic when exposed to these medications (384). Mechanisms by which corticosteroids may impact muscle function are related to the ability of these agents to compromise the production of contractile proteins and down-regulate the IGF-1 pathway. Corticosteroids may also enhance proteolysis by increasing myostatin levels (385).
Vitamin D deficiency.
The vitamin D receptor (VDR) is abundantly expressed in limb muscles, where it mediates several gene promoters resulting in differential gene expression (386). Nongenomic or more rapid effects of vitamin D through intracellular VDR have also been documented in the muscle (387). As such, the vitamin D pathway may play an important role in the maintenance of limb muscle health. Rickets often presents with severe muscle weakness, and low vitamin D serum (25-OH vitamin D) levels are associated with reduced limb muscle strength and increased risk of falls (388–390). In elderly individuals, vitamin D status predicts physical performance and subsequent functional decline during long-term follow-up (391). Yet, the underlying mechanisms by which these potential effects of vitamin D are mediated are not fully understood. Adults with severe vitamin D deficiency show predominantly type II muscle fiber atrophy (392), with interfibrillar spaces infiltrated with fat, fibrocytes, and glycogen granules (393). Conversely, increases in relative fiber composition and type II fiber dimensions have been reported in the elderly after treatment with vitamin D (394, 395). 1,25(OH)2 vitamin D also influences active calcium transport into the sarcoplasmatic reticulum (396), as it regulates Ca-ATPase by phosphorylation (392). 1,25(OH)2D, through VDR-mediated gene transcription, (397) affects calmodulin (398), actin, and troponin C content (399) and can also up-regulate the expression of IGF-1 (386).
In the context of COPD, vitamin D deficiency may contribute to limb muscle dysfunction (400, 401). Vitamin D deficiency is highly prevalent in patients with COPD compared with age-matched smoking control subjects, with 60 to 70% of nonsupplemented patients with severe disease having 25-OHD levels less than 20 ng/ml or 50 nmol/L (248, 402), which is typically accompanied by a fiber type shift to type II fibers (161, 169, 190). Vitamin D deficiency preferentially reduces the size of type II fibers that are important in fall prevention (389), a problem that can be tackled by vitamin D supplementation (403). However, one study failed to find a significant relationship between vitamin D status, muscle MHC protein expression, and limb muscle strength in patients with COPD (404). Yet, in another study, genetic polymorphisms in the VDR correlated with measures of strength (405). Together, these studies indicate that, to a certain extent, alterations in the vitamin D pathway may be present in COPD and that this pathway may be further compromised during disease progression, thereby affecting the muscle independently of serum levels.
Renin-angiotensin system.
The renin-angiotensin system may have implications for the development of limb muscle dysfunction in COPD (406). This system is expressed in skeletal muscle and produces angiotensin II that inhibits the IGF-1 signalization cascade (407), stimulates NF-κB and, as a result, the UbP pathway (408), and interacts positively with myostatin (409). Polymorphism of the angiotensin-converting enzyme (ACE) gene, with the deletion of a pair base sequence on chromosome 17, is associated with higher tissue level of ACE activity and consequently in angiotensin II tissue levels (410). This has been associated with a less oxidative muscle phenotype with lower proportion of type I fibers (411–413). In contrast, the same polymorphism is associated with better-preserved muscle strength in COPD (414) and possibly with a better strength response after resistance training in healthy subjects (415). As a result of these apparently conflicting effects of ACE muscle activity on the muscle phenotype, the effect of interventions on this pathway is difficult to predict; this question is currently being addressed (see clinicaltrials.gov: NCT01014338).
Smoking.
Cigarette smoking is unlikely to be the main mechanism involved in limb muscle dysfunction in COPD because, in several studies, patients and control subjects were matched for smoking history. However, smoking by itself has some effects on muscle biology (416–419), and it is possible that it may predispose patients to the development of limb muscle dysfunction. Smoking by itself may be associated with muscle atrophy and weakness in otherwise healthy subjects (4, 236, 248). Smoking is also associated with a decrease in type I fiber cross-sectional area, reduced type I fiber proportion, reduced cytochrome oxidase activity, increased lactate dehydrogenase activity, and a higher level of protein oxidation in the quadriceps (420–422).
Mechanisms of Muscle Susceptibility to Fatigue in COPD
Impaired quadriceps endurance is not explained by reduced muscle mass (193), but reduced muscle aerobic capacity and oxidative stress have been implicated (196, 224, 227, 242, 423).
Neural drive and muscle afferents.
In healthy young subjects, the interaction between central motor command and limb muscle afferent feedback plays a key role in modulating the physiologic response to exercise by affecting the cardiorespiratory response (267), development of muscle fatigue (294), and endurance performance (424). The interplay between central motor drive and the muscle feedback during exercise confines limb muscle fatigue within a critical threshold (294). Despite potential relevance in COPD, few researchers have studied the neural drive and muscle afferents in this context. However, the available evidence supports the idea that increased afferent feedback from the limb muscles during exercise is involved in the regulation of muscle fatigue during exercise in COPD (295, 298).
Blood flow, oxygen delivery, and extraction.
In recent years, the influence of increased work of breathing on limb muscle fatigue during exercise has been documented in healthy subjects. During strenuous exercise, peripheral vasoconstriction associated with the high demand for respiratory muscle blood flow appears to compromise limb muscle perfusion (261, 425, 426) and consequently enhance muscle fatigue (427). A blood redistribution phenomenon in favor of the respiratory muscles may also occur in COPD (428), and it has been hypothesized that strategies that reduce respiratory muscle work may alleviate limb muscle fatigue by restoring blood flow to the limb muscles (267, 429). This hypothesis was supported by a study from Amann and coworkers, who gave a range of interventions designed to improve oxygenation (by supplemental oxygen) and/or reduce work of breathing (proportional-assist ventilation or helium or both) (267). Interestingly, quadriceps fatigue was reduced partially and to similar extent with each intervention. These data were interpreted as showing that only 30 to 40% of quadriceps fatigue could be attributed to muscle oxygen delivery issues.
Enhanced muscle fatigability can also occur when the left ventricular function is compromised (267, 430). This may be relevant in the subset of patients with COPD (431), including those with cardiac comorbidities (432) and those with pulmonary hypertension (433), in whom altered cardiac output and oxygen delivery have been reported.
Reduced muscle capillarity could contribute to limit blood and oxygen delivery to the muscle and increase susceptibility to development of muscle fatigue, as indicated by the positive relationship between muscle capillarity and exercise performance (174). Saey and coworkers confirmed that capillarity was reduced in patients with COPD who developed quadriceps fatigue after cycling exercise (423). Vogiatzis and colleagues showed that exercise training improved capillarity in COPD (434). Thus, augmenting capillarity may offer a route to reduce fatigability; this issue requires further exploration.
Mechanisms within the motor unit.
Mechanisms involved in limb muscle fatigue include the conduction of the action potential along the muscle fiber membrane into the transverse tubule system, the release of calcium into the myoplasm, the binding of calcium to troponin, the interaction between myosin and actin during cross-bridge cycling, and the active reuptake of calcium into the sarcoplasmic reticulum (92). Also, an impaired excitation–contraction coupling may be a contributing common factor to fatigue induced by diverse forms of exercise (90, 91). Metabolic changes in the muscle, such as lactate accumulation and phosphocreatine depletion (435), limitations in muscle energy supply (436–438), and structural and metabolic disorganization of contractile proteins (91, 92), can all be involved in the development of contractile muscle fatigue. The accumulation of lactic acid in muscle has historically been suggested to be the major cause of muscle fatigue (439) (see review by Westerblad and colleagues [92] and by Fitts [440]). Although lactic acid is dissociated into lactate and hydrogen ions leading to a significant decrease in pH, lactate itself has no effect on muscular contraction, but the increased concentration of hydrogen ions, which affects the excitation–contraction coupling by decreasing the quantity of calcium released, may contribute to the development of contractile fatigue. Other factors, such as redox status (209) and muscle inflammation (441, 442), must also be considered as potential contributors to muscle fatigue in patients with COPD.
In COPD, changes in muscle metabolism favor glycolysis, and phosphorus-31 nuclear magnetic resonance (31P-NMR) studies suggest early depletion and prolonged recovery of phosphocreatine in the quadriceps after exercise (443). Changes in muscle enzymatic profile and capillarization leading to a greater reliance on glycolytic metabolism during exercise are also associated with muscle fatigue in patients with COPD (423). During exercise, the extent of changes in muscle metabolism and muscle fatigue after a cycle exercise performed until exhaustion were similar in patients with COPD and age-matched control subjects despite a much lower total work performed by the COPD group (196). Moreover, taking into account the amount of work performed, use of glycogen and accumulation of lactate and the key intermediate markers of glycolysis show significant increases after exercise in patients with COPD (196). Together with the known reduction in oxidative enzymes in COPD (199), these findings suggest that an impaired muscle oxidative capacity, with a reduction in phosphorylation potential and a greater reliance on glycolysis that may well contribute to the muscle fatigue in COPD. The greater reliance on glycolysis can, in part, be explained by the reduced proportion of type I fibers (444, 445) and the lower oxidative enzyme capacities observed in the quadriceps compared with age-matched healthy subjects (190, 199) as well as by the higher phosphofructokinase activity observed in COPD (199). Moreover, an additional component of glycolytic reliance might be related to lactate and glucose transporters, whose activity was found be altered in COPD (198). Because these transporters are crucial for lactate removal and glucose entry in limb muscle, they could influence the relative contribution of the glycolytic pathway to the total energy expenditure during exercise. With the reduced capillarity and decreased muscle mitochondria activities and density, patients with COPD show electron transport chain blockade and excessively increased levels of systemic and local oxidative stress (201, 446). Couillard and colleagues demonstrated that exhaustive quadriceps exercise induces local oxidative stress in patients with COPD. They also found a reduction in antioxidant activity 48 hours post exercise in patients with COPD compared with healthy control subjects (224). Although muscle fatigue was not assessed in this study, muscle oxidative stress was associated with reduced quadriceps endurance, suggesting a relationship between muscle fatigue and oxidative stress in patients with COPD. This relationship is further supported by the finding that antioxidant therapy may alleviate muscle fatigue in COPD (227).
Thus, there are several interrelated mechanisms by which muscle fatigue can contribute to exercise limitation in patients with COPD. Some act directly on the muscle contraction process and others through their effects on both the cardiorespiratory and nervous systems.
Assessment of Limb Muscle Function in COPD
The existing relationships between limb muscle mass and strength and important clinical outcomes in COPD (see previous section, Consequences of Limb Muscle Dysfunction in COPD) suggest that assessing body composition and limb muscle strength in the clinical evaluation of COPD can identify patients who are at increased risk for exercise intolerance and premature mortality. One strategy could be to evaluate body composition and quadriceps strength at the time of referral to the exercise laboratory for the assessment of dyspnea and/or exercise intolerance. Some exercise laboratories implemented this practice several years ago (285). Isometric maximal volitional limb muscle strength can easily be assessed using strain gauges (Figure 4). Body composition can be assessed in clinical practice using either bioelectrical impedance (BIA) or dual-energy X-ray absorptiometry (DEXA).
Figure 4.
Standard operating procedure for isometric quadriceps strength assessment. During the maneuver, vigorous encouragement of the patient is needed. Patient is positioned in a standardized fashion (typically sitting with knees and hips in 90° flexion or, less often, supine [248]). Maximal voluntary contraction force (reported in kilograms or newtons) can be reliably assessed as the best of three reproducible maneuvers. Maximal voluntary contraction is recorded as the maximal force that can be maintained for 1 full second.
Assessment of Muscle Mass
Assessing only BMI is clearly insufficient to quantify the impact of COPD on the different body compartments in general and on muscle mass in particular (132). Several techniques are available to assess the mass of functioning muscles (281, 447), including both direct and indirect approaches. The two-compartment model of body composition divides body mass into FM and FFM. FFM can be further divided in an intracellular compartment, which includes muscle mass, bone mineral mass, and other metabolizing tissues, and an extracellular fluid compartment. FFM is commonly used as a surrogate for muscle mass in clinically stable patients with COPD.
BIA is based on the higher conductivity of an electric current through FFM than FM (448). The measurement is noninvasive, is inexpensive, takes only a few minutes, and requires no active collaboration. The technique has been validated using total body water assessed by deuterium dilution space as a reference method in patients with COPD (281). It is, however, important to use equations to estimate body composition that have been validated in COPD and to be cautious with built-in equations provided by the BIA systems (447, 449).
DEXA enables differentiation of body composition into three compartments by the assessment of FM, lean tissue, and bone mineral content. Although more expensive than BIA, DEXA is accepted as a safe, convenient, and noninvasive method with a low radiation dose (<0.02 mSv). In patients with COPD, DEXA also appears to be a suitable alternative method to deuterium dilution (450) for assessing FFM. In addition, DEXA enables identification of bone mineral loss and of trunk and appendicular muscle mass.
There are significant intermethod differences for measuring body composition (447). The current gold standard is considered by many to be the deuterium dilution technique, but its use is restricted to highly specialized hospital or research centers. Although simple and cheap, skinfold anthropometry generally results in overestimation of FFM compared with other methods (281, 447, 451). Two reasonable compromises are BIA and DEXA. The amount of FFM assessed by BIA was shown to be lower than with DEXA in patients with COPD (449, 451), especially in men (447, 449). However, DEXA is not without potential limitation, because it provides systematically higher values for FFM compared with deuterium dilution (450). Furthermore, DEXA results differ between the different commercial devices. This issue is less important for longitudinal research purposes, as long as the same method is used throughout the follow-up of patients. Obviously, the cut-off thresholds to define normality will influence the prevalence of FFM depletion. Several COPD-specific cut-offs were reported in the last decades and are presented in Table 2.
Table 2:
Cut-offs for Depletion of Whole-Body Fat-Free Mass and Fat-Free Mass Index in Patients with Chronic Obstructive Pulmonary Disease
| Study | Rationale | Men (kg/m2) | Women (kg/m2) |
|---|---|---|---|
| Schols et al. (134) | FFM corresponding to a weight of <90% of ideal body weight according to Metropolitan Health Insurance Tables (129, 130) | <16 | <15 |
| Vestbo et al. (131) | 10th percentile of FFM in Copenhagen City Heart Study | <17.1 | <14.6 |
| Coin et al. (661) | 10th percentile of FFM in the age range 60–69 yr | <17.8 | <14.6 |
Definition of abbreviations: FFM = fat-free mass.
In addition to the previously described techniques assessing whole-body FFM, several measures of regional limb muscle mass are available. Although measurement of thigh circumference is simple and cheap, it may not accurately reflect local muscle mass (280). Alternatively, computed tomography (280), magnetic resonance imaging (MRI) (452), and ultrasonography (247) have been used to assess quadriceps size in COPD. Using MRI-based three-dimensional shape analysis, regional shape anomalies of individual muscles were demonstrated in patients with COPD (453). The amount of local muscle mass measured with these techniques has also been related to relevant outcomes in COPD, such as muscle strength (247) and mortality (280). Correlation between regional and whole-body muscle mass is poor in COPD, suggesting that direct lower limb muscle assessment indeed has additional value (247). These methods are useful in research, because they may be more responsive to specific lower limb interventions (e.g., strengthening exercise) than the assessment of whole-body muscle mass.
Finally, muscle biopsy is a valuable tool to assess morphologic and biochemical properties of limb muscle (454). The Bergström needle is the most commonly used (455, 456), but open surgical techniques have also been applied (168, 239). Despite useful information obtained from muscle biopsy, this technique is invasive and has the potential for complications, including discomfort and minor scarring. Bleeding and infection can also occur, but these are rare events (<1%) (457). To reduce the discomfort associated with this procedure, a microbiopsy technique has been developed (458). Although the quantity of muscle obtained through this technique is less than with the regular Bergström biopsy, it can still provide sufficient tissue for biochemical and molecular analyses with a reasonable degree of agreement with the Bergström biopsy (458). This technique would seem particularly suited when several muscle biopsies are required, for example when evaluating the time course of response of a given protein or molecule to a specific intervention. Finally, the choice of muscle for biopsy and the representation of only a tiny fraction of the entire muscle should be taken into consideration.
Assessment of Limb Muscle Strength
Although strength is not the most sensitive measure to assess muscle function, it is an accessible way to investigate to which extent the limb muscles are affected in COPD and to prescribe adequate loads for resistance training. Learning about the status of the limb muscles provides clinicians with important information for understanding the mechanisms of exercise limitation in a given patient.
Volitional assessment.
Several studies have measured limb muscle strength in patients with COPD and healthy elderly control subjects. Quadriceps muscle force is typically reduced compared with age-matched control subjects (248). Much less is known about limb muscle function in community or primary care settings (459, 460). Technical aspects of assessing volitional muscle force are reported in detail elsewhere (461). Because limb muscle strength can vary in individual patients from very low to supranormal, techniques to assess limb muscle strength need to be able to deal with a large range of force development. Some techniques, such as manual muscle testing, in which limb muscle strength is qualitatively assessed from “none at all” to “normal,” may therefore be less preferred in COPD. These techniques may be useful in the critical care setting (462), particularly if sum scores are calculated over several muscle groups (463). Hand-held dynamometry, which provides more quantitative information than the former measurement, is promising, but even in frail elderly the limits of agreement are wide (464), and results depend on the skills and strength of the tester. Nevertheless, at a group level, using this technique, muscle weakness has been shown in patients with COPD (238). Muscle strength can also be measured by assessing the maximum weight that a patient can move once over the full range of motion without compensatory movements (i.e., the one-repetition measurement [465]). This technique, although used in clinical trials in COPD to assess the effect of interventions (466), does not allow easy comparison of limb muscle force across different settings, as the result obtained depends on the technique of the patient, the machine used (e.g., number of pulleys), and starting position. Hydraulic resistances can also be applied to assess limb muscle strength dynamically in patients (138, 467). The obtained strength values will depend on the speed of the contraction, which may be difficult to standardize across equipment.
Although various methodologies exist to measure muscle strength, isometric maximal volitional limb muscle strength assessment is one methodology that could be implemented in clinical practice to provide reliable and reproducible measurements (468, 469). A standard operating procedure for this measure is provided in Figure 4. The maximal voluntary contraction force has historically been reported in kilograms, because weights are used to calibrate the apparatus. Because force should technically be reported in newtons, some investigators prefer to do this by multiplying the measured force in kilograms by the gravitational pull (9.81 m/s2). MVC force is typically assessed as the best of three reproducible maneuvers. During the maneuvers, patients need to be vigorously encouraged. Patients are positioned in a standardized position (typically sitting with knees and hips in 90° flexion or, less often, supine [248]). Isometric muscle force can also be assessed on specifically built computerized dynamometers (e.g., Cybex or Biodex). In this case, the peak torque is assessed at fixed joint angle speeds (470, 471). Higher angular speeds do result in lower peak torque in subjects with COPD (472), as in control subjects. The outcome is normally reported as torque in newton-meters (Nm), rather than newtons or kilograms, which are only appropriate for isometric efforts.
Limb muscle strength is negatively related to age and positively related to body weight. Male patients have superior strength compared with female patients (248). Because COPD occurs in both sexes over a wide range of ages, it is best to report values as percent of the predicted normal value (238, 248), although we acknowledge that there are no universal reference values for this variable. Alternatively, for muscles working against gravity (knee extensors, ankle dorsiflexors), a correction for body weight, which is the main factor associated to limb muscle strength, has been used (471).
Nonvolitional strength.
One limitation of volitional techniques to assess limb muscle strength is their dependence on cooperation of the patients. The assessment is also dependent on all central and peripheral factors linked to contraction (motor cortex, central command, peripheral nerve conduction).
Nonvolitional assessment of limb muscle strength can be used for research or diagnostic purposes. In this method, electrical or magnetic stimulation of a peripheral nerve is conducted by the application of a single stimulus. This technique is practiced by only a handful of research laboratories but has provided important insight in the involvement of different muscle groups in COPD. Using this technique, investigators have shown that quadriceps twitch force was proportionally more reduced than the abductor pollicis (thumb opposition) twitch force in COPD, suggesting either that distal muscles are less impaired than more proximal muscles or the upper-limb muscles are less impaired than lower-limb muscles (249).
Electrical stimulation of the peripheral motor nerve allows the construction of force–frequency curves in vivo, where force is reported at different stimulation frequencies. In a small study, Degens and colleagues were unable to show differences between patients with severe COPD and control subjects matched for FFM (473). This study also reported a similar involvement of hand and quadriceps muscles.
Stimulation of the muscle can be done in a rested muscle to obtain an unpotentiated twitch stimulation, or it may be performed seconds after a vigorous voluntary contraction to obtain a potentiated twitch stimulation. Clearly, the latter demands cooperation of the patient. None of these can be considered physiologic maneuvers.
Twitch stimulations are obtained by supramaximal stimulation of the conducting nerve. To ensure supramaximality, the stimulator output is slowly increased until the obtained twitch force or the EMG signal accompanying the muscle fiber discharge does not further increase. More recent studies tend to use a plateau in force output to ensure supramaximality (266, 299, 474, 475). The assessment of twitch quadriceps force has less day-to-day variability compared with MVC (474). Twitch muscle force has been used to measure limb muscle fatigue (applying the twitch before and after limb muscle loading). Methodological aspects, such as the choice of the coil, influence the outcome of the measurement (476). Potentiated and unpotentiated twitch muscle force can be used as nonvolitional evoked contractions on stimulation of the femoral nerve. Unpotentiated twitches are obtained by stimulating at 1 Hz the rested/relaxed quadriceps. Potentiated twitches are obtained a few seconds after muscle potentiation, using an MVC preceding the 1-Hz twitch stimulation of the femoral nerve. Although theoretically the unpotentiated twitch may be the preferred stimulation, it is difficult for some patients to keep the quadriceps completely relaxed for the 20 minutes required to achieve depotentiation. Also, the potentiated twitch may exhibit bigger changes after exercise or multiple efforts and therefore may be more reliable and more sensitive to detect signals of muscle fatigue (475). Normal expected values are reported in relatively small sets of healthy control subjects (247, 477). The twitch force of the quadriceps is reduced in COPD, whereas the twitch/MVC ratio is unchanged (247).
Transcranial magnetic stimulation has been used in COPD (478, 479). With this technique, the stimulation is applied on the motor cortex through the skull of the patient, allowing the stimulus to travel through the whole path of neuronal discharge. Outcomes of this test are based on EMG recordings: latency time in milliseconds and motor evoked potentials in microvolts assessed by surface EMG (430).
In vitro contractile properties can be studied in muscle fibers harvested from patients with COPD and control subjects, and results suggest that contractile properties of limb muscle fiber bundles were relatively preserved in patients with COPD compared with control subjects (239). To the best of our knowledge, single-skinned fiber contractile properties have only been studied in the diaphragm (480) and not in the limb muscle.
Assessment of Limb Muscle Endurance
Endurance of specific limb muscles is generally not used as outcome in clinical studies of COPD (481, 482). Nevertheless, the evaluation of limb muscle endurance may be more sensitive and useful to study pharmacological and nonpharmacological interventions specifically targeting the limb muscles. So far, no direct comparisons between different endurance protocols, no standardization, and no reference values are available, explaining why a broader application of assessing limb muscle endurance has not yet found its way to clinical practice. One protocol that could be recommended for implementation consists of repeated leg extension maneuvers at a predetermined rate of 12 contractions per minute performed at 10% MVC with a duty cycle of 0.4. The outcome of interest with this protocol is time to task failure, defined as the time at which leg extension falls below 80% targeted value for three successive contractions (243).
According to the definition of endurance, time to task failure should be the favored outcome of the test. Tests that directly record endurance time are conceptually superior to measurements that measure fatigue by reporting alternative outcomes, such as drops in muscular strength or changes in EMG signaling after a time-fixed contractile maneuver (483–485). Although muscle fatigue may strongly correlate with endurance, it should not be interpreted as a direct assessment of endurance. Measurement of endurance requires a muscle or muscle group to contract against a load, producing a time-dependent loss of mechanical function. Over recent decades, a broad variety of tests have been developed to assess muscle endurance, which may differ on three general components: nature of the contractions (volitional or nonvolitional), exercise conditions, and intensity (484, 486).
Muscle stimulation can be exogenously imposed by direct nerve or muscle electromagnetic stimulation (258, 269, 483), but most endurance protocols rely on a volitional contractions. The latter method is easier and less invasive than the former but also more difficult to control, as it relies on patient collaboration. The exercise condition can be based on isometric contractions with maintenance of muscle length (258, 298, 483), on a dynamic contractions to shorten the muscle against a fixed load (concentric contractions) (243, 256, 257), or on variable loads but with constant angular speed (isokinetic) (193, 487). Isometric and concentric contractions are physiological maneuvers that can be easily implemented at low cost but with a drawback of between-test variability and reduced sensitivity. Isokinetic contractions are nonphysiological maneuvers that require more sophisticated and costly devices, in addition to an appropriate training of the patient. However, this method has the clear advantage of being very reliable.
The intensity of the endurance maneuver may be maximal or submaximal and can be imposed via a single or a repeated number of contractions. Although maximal maneuvers are more representative of the maximal anaerobic capacity of the muscle, and they reflect maximal strength, repeated submaximal contractions are probably the method of choice for assessing endurance. It should be stressed that before a series of submaximal contractions, a similar but maximal type of contraction is needed in the same individual to determine patient-specific optimal loads (243, 256). Submaximal loads are then imposed at certain pace with fixed duty cycle and may range from 10 to 80% of the maximal load according to the protocol used (243, 488). For isokinetic measurements, repeated maneuvers at higher angular speeds (90–300°/s) have been used (193, 486).
Assessment of Muscle Oxygenation Using Near-Infrared and Magnetic Resonance Spectroscopy
Near-infrared spectroscopy (NIRS) constitutes a simple in vivo method to quantify muscle oxygenation, oxygen dynamics, and oxidative energy metabolism at rest and during exercise. NIRS is advantageous because it is noninvasive, exhibits low movement artifacts, and provides good temporal and spatial resolution (489). The use of NIRS may be limited in the presence of subcutaneous fat accumulation, which impairs the transmission of the signal at the muscle level. NIRS technology has been applied to the study of a number of chronic health conditions, including patients with COPD. Such studies have investigated the impact of the pulmonary system limitations on locomotor muscle fatigue in patients with COPD (490–492). Collectively, these studies (491–493) have shown that respiratory muscle unloading (via oxygen and/or heliox breathing, bronchodilation, and proportional-assist ventilation) improves leg muscle oxygen availability during exercise in patients with COPD. Recently, NIRS has been used in combination with the light-absorbing tracer indocyanine green dye to quantify regional blood flow in muscle and connective tissue during dynamic exercise in patients with COPD (352). Subsequent studies showed that respiratory muscle unloading improves locomotor muscle blood flow and oxygen delivery during exercise in patients with different patterns and degrees of dynamic hyperinflation (429, 494). 31P-NMR spectroscopy is a noninvasive method to evaluate high-energy compounds ATP, PCr, inorganic phosphate (Pi), and intracellular pH of single muscle groups during exercise and recovery (495).
Effects of Interventions on Limb Muscle Function in COPD
Several interventions have been used in an attempt to improve muscle function in patients with COPD. These and their respective effects on limb muscles are summarized in Table 3.
Table 3:
Effects of Treatments for Limb Muscle Dysfunction in Chronic Obstructive Pulmonary Disease
| Treatment | Mass | Strength | Exercise Tolerance | Survival |
|---|---|---|---|---|
| Exercise | √ (497) | √ (496, 497) | √ (662) | ? |
| Oxygen | ? | ? | √ (539–541) | √ |
| Nutrition alone | No (663) | No (663) | No (663) | ? |
| Nutrition with exercise training | √ (552, 554, 567) | √ (554, 567) | √ (552, 554, 567) | ? |
| Nutrition with exercise training and anabolic hormone supplementation | √ (558) | √ (558) | √ (558) | ? |
| Testosterone | √ (466) | √ (466) | No (466, 575) | ? |
| Growth hormones | √ (596) | No (596) | No (596) | ? |
| Ghrelin | ? | ? | ? | ? |
| Megestrol | No (630) | ? | No (630) | ? |
| Creatine | ? | ? | No (633–635) | ? |
| Antioxidants | ? | ? | ? | ? |
| Vitamin D alone | ? | ? | ? | ? |
| Vitamin D with exercise training | ? | ? | ? | ? |
√: Studies support that the treatment has a favorable effect on the outcome; No: studies support that the treatment has no favorable effect on the outcome; ?: there are no supporting data for a treatment effect on the outcome.
Exercise Training
Rehabilitative exercise training improves limb muscle function and morphology in patients with COPD (313, 314, 496–498). As such, quadriceps muscle strength, endurance, and fatigability all improve significantly after exercise training (265, 314, 496–499), with smaller, albeit significant, increases in midthigh muscle cross-sectional area (466) and FFM (497). Typically, longer exercise programs produce greater physiological training effects, with a recommended minimum of 8 weeks to achieve a substantial effect (500). The number of sessions per week also varies; although outpatient programs commonly include 2 to 3 days a week, inpatient programs are usually planned for 5 days a week. The session length per day is generally 1 to 4 hours (501). The optimal exercise intensity is unknown. Nevertheless, high level of intensity of exercise (>60% maximal work rate) for 20 to 60 minutes per session is more potent in terms of inducing a physiological response to training than less intense exercise (<40% maximal work rate) (288). However, many individuals with chronic lung disease are unable to train at that level, and substantially lower intensity of exercise is an alternative (500). One option is interval exercise training, in which the long constant-load exercise session is replaced by several smaller bouts (typically lasting 30 to 180 s) of high-intensity exercise (80–120% peak capacity) separated either by periods of rest (lasting 30 to 60 s) or by lower-intensity exercise bouts (50–80%), thereby allowing intense loads to be applied to locomotor muscles with lower sensations of dyspnea and/or leg discomfort compared with continuous or constant-load exercise training (502). Despite these interesting features of interval training, the outcome is very similar to constant-load modality (502). As such, interval training should be viewed as another available option, among the several strategies available, to train patients with COPD, the goal being to find ways to retain patients in the training program. Single-leg cycling exercise training uses the concept of training a smaller muscle mass at a time, thereby reducing the demand on the respiratory system while allowing training of the targeted muscles at an intensity that would not otherwise be possible with two-legged cycling (503, 504). Proof-of-concept of this training modality has been reported in a small number of patients suggesting physiological superiority of single-leg cycling over two-legged cycling (503, 504). Whether these observations impact on clinical outcomes, such as quality of life, or on long-term outcome is unknown.
Endurance exercise training (either of interval or constant-load modality) increases the cross-sectional area of all fiber types within the quadriceps muscle (163, 165, 505, 506). In addition, endurance training reduces in the proportion of type IIx fibers in the quadriceps (434, 505, 506). Metabolic improvements observed after training include: (1) increased oxidative capacity (documented by the increase in both the capillary-to-fiber ratio and the activity of oxidative enzymes) (434, 505–507), (2) a reduction in exercise-induced lactic acid production (288, 507), and (3) normalization of the decline in the PCr/Pi ratio during exercise (192). These morphological and typological adaptations in limb muscles are not different across GOLD classes 2 to 4 (165). In addition, interval training yields muscle adaptations that are similar to those obtained by constant-load training (505).
The phenotypical adaptations within the limb muscles are accompanied by the up-regulation of key factors governing muscle hypertrophy and regeneration, namely the IGF-1 and MyoD (434, 466, 506). Furthermore, endurance training reduces the expression of myostatin (316, 434). Patients with COPD with normal lower limb muscle mass exhibit substantial capacity for muscle adaptation in response to exercise training, irrespective of the magnitude and direction of changes in the expression of local muscle inflammatory factors (334, 434, 506) or the induction of exercise-induced oxidative and nitrosative stress (201, 334). There is also emerging evidence to suggest that, in patients with COPD with either normal or low muscle mass, exercise training seems to reduce the activity of protein breakdown pathways, in particular NF-κB–activated UbP pathway (434). Most exercise training studies performed have involved patients with GOLD spirometry classes 3 to 4 COPD; more needs to be learned about the milder form of the disease (GOLD 1–2).
The magnitude of response to exercise training in COPD is highly variable, with some patients showing little or no benefit (508). This interindividual variability in the response to training is not unique to COPD, because similar observations have also been made in healthy individuals. There seems to be a genetic component to this phenomenon (415, 509). The lack of response to exercise training may also be related to the inability to tolerate sufficient intensity and/or duration of training and/or to poor compliance to the training intervention (288, 510). Patients who develop quadriceps contractile fatigue during the training sessions show greater training effects in terms of functional exercise capacity and health-related quality of life (299). This would argue in favor of the intensity of the training stimulus at the muscle level being an important determinant of the outcome of exercise training.
The available evidence also suggests that the muscle response to training differs to some extent between COPD and healthy control subjects (511). The muscle angiogenic and molecular response to training may be blunted in patients with COPD (512, 513); whether this can be entirely explained by a lower training intensity in COPD is uncertain. Patients with COPD and healthy sedentary subjects present a distinct gene expression response after endurance training, from a quantitative and qualitative perspective. Fewer genes are significantly changed in patients with COPD compared with healthy control subjects (511). In turn, genes associated with oxidative stress, UbP, and cyclooxygenase pathways are distinctly induced only in patients with COPD, potentially reflecting the specific molecular response of the muscle to exercise and suggesting additional mechanisms for exercise limitation in COPD (511). In some patients with COPD, the occurrence of oxidative (231) and nitrosative (196) stress at the muscle level has been reported after exercise training; cachectic patients seem to be more prone to this effect (514). Some studies have reported an enhancement in antioxidant activity with exercise training (515, 516), whereas others have consistently shown no significant changes or even a reduction in the antioxidant potential (reduced glutathione) of the limb muscles of patients with severe COPD after exercise training (201, 217, 231, 514, 517, 518), which was not observed among the healthy control subjects. However, the clinical relevance of these observations is unclear (201, 334), and it should be stressed that exercise training is a very safe and effective intervention for the vast majority of patients with COPD.
Resistance training is accomplished when muscle contractions are performed against a specific opposing force generated by a resistance (519). In a recent systematic review, increases in muscle strength and mass after short-term resistance exercises were demonstrated in patients with COPD (520). The key features of resistance exercise protocols used in these trials included, on average, 12 weeks of progressive resistance exercise training consisting of two to three sessions per week, each session including two to four sets of 8 to 12 repetitions of each exercise, at loads ranging and progressing from ∼30 to ∼90% of one repetition maximum. The improvement in muscle function translated into better performance of some daily activities (520). Combination of resistance training with endurance training in COPD has revealed a greater improvement in quadriceps muscle strength and thigh muscle cross-sectional area compared with endurance training alone (497). Although short-term effects of resistance training on muscle mass and strength are well known in COPD, long-term effects remain to be determined (521).
The effects of resistance training on intrinsic muscle changes have been scarcely studied in COPD (481). In patients with moderately severe COPD and normal whole-body muscle mass, resistance exercise training enhances the expression of muscle IGF-1 and other components of the muscle IGF system and of myogenic regulatory factors (316).
Exercise training early after an exacerbation.
Keeping in mind the deleterious effects of exacerbations on limb muscles, several interventions to prevent or counteract muscle impairment have been considered (522). These interventions, mainly based on exercise training, have been used during, immediately after, or late after the exacerbation (523). A Cochrane Collaboration metaanalysis revealed that pulmonary rehabilitation immediately after an exacerbation significantly improved exercise capacity in addition to reducing hospital admissions and mortality (524). Postexacerbation pulmonary rehabilitation was also associated with an improvement of quadriceps strength (525, 526). Moreover, 1 month after discharge, the functional status and muscle force remained better in the group that underwent training during the exacerbation (379), suggesting that this modality may facilitate functional recovery after an acute exacerbation.
To date, only a few interventions have taken place during the hospitalization period. Resistance training initiated during the second day of hospitalization counteracted limb muscle dysfunction, as quadriceps force increased by 10% and 6-minute walking distance improved at discharge (379). This was associated with an increase in anabolic markers in the muscle (379). In that study, trained patients with COPD exhibited a lower mRNA expression level of myostatin (an indication of muscle growth) with a higher myogenin/MyoD ratio (reflecting the regenerative capacity of the muscle) in comparison to nontrained patients. On the other hand, no between-group differences were detected for IGF-1, myogenic growth factor, MURF-1, MAFbx, and NEDD4 mRNA expression levels. Therefore, the suggested positive effect of high-intensity resistance training on muscle regenerative regulatory factors deserves further exploration.
Neuromuscular Stimulation
Transcutaneous neuromuscular electrical stimulation (NMES) involves the application of an electrical current through electrodes placed on the skin over the targeted muscles, thereby depolarizing motor neurons and, in turn, inducing muscle contractions (527). NMES is a promising training modality to improve lower limb muscle function that can be particularly useful for severely disabled patients with COPD in whom the tolerance to whole-body training is compromised (528). Indeed, NEMS may even be applicable for home use (529) and in unstable patients (530). Nonetheless, larger well-designed trials are needed to improve our understanding of NMES and to clarify how it can be optimally used. The effects of NMES on intrinsic muscular changes have not been extensively explored. Dal Corso and colleagues reported an increase in type II fiber cross-sectional area with a decrease in type I fiber cross-sectional area of the quadriceps after a 6-week home-based NMES program in moderately impaired patients with COPD (531). In another study, the application of a 6-week NMES program in patients with COPD recovering from an acute exacerbation decreased muscle oxidative stress and improved MHC content and the proportion of type I muscle fibers in the quadriceps (530). In patients with advanced COPD, NMES improved midthigh and calf muscle cross-sectional area. This was associated with a more favorable muscle anabolic to catabolic balance (532). In this study, the improvement in walking distance after NMES training was associated with gains in muscle strength, reduced ventilation during walking, and the ability to tolerate higher NMES stimulation intensity (532).
An interesting feature of NMES is its small impact on ventilation, heart rate, and dyspnea so that it can be applied during periods of exacerbation, during admission to the intensive care unit for acute COPD exacerbation (530), and in bed-bound patients. In these settings, NMES may improve muscle function and decrease the number of days needed to transfer from bed to chair (462).
Oxygen Therapy
Long-term oxygen therapy (LTOT) enhances survival in hypoxemic patients with COPD (533–535). Presumably, this is due to effects of oxygen at the tissue level, although the mechanisms by which LTOT enhances survival were not determined in these early studies. Since then, it would be considered unethical to conduct a clinical trial in which hypoxemic patients with COPD would not be provided with LTOT; for this reason, the possibilities for investigating the effects of LTOT at the limb muscle level are very limited. Furthermore, the investigation of the effects of LTOT on the muscle is confounded by several methodological considerations. For example, the therapeutic goal of LTOT is the correction of arterial hypoxemia, but, in the context of muscle function, the goal might be to prevent cell hypoxia (536). The characteristics of oxygen transport from the atmosphere to the mitochondria (351) are well known, but regional competition for blood flow between respiratory and limb muscles during exercise (352) and resistance of oxygen transport at the peripheral level may be of major importance in patients with COPD (351). Those phenomena may result in cell hypoxia without apparent arterial hypoxemia. Moreover, difficulties in assessing cell hypoxia in the clinical arena seem to have stalled progress in this area despite the fact that LTOT, muscle training, and active lifestyle should be key relevant interventions with potentially synergistic effects.
Despite the physiological rationale supporting the deleterious effects of chronic cell hypoxia on limb muscle function and a beneficial effect of oxygen administration, little is known about the effects of oxygen therapy on limb muscle function in patients with COPD. There is little doubt that oxygen therapy administered acutely improves muscle energy metabolism during exercise (360, 361). Consistent with these acute improvements, two muscle biopsy studies highlighted that correction of hypoxemia during an episode of acute respiratory failure or chronically with LTOT was associated with improved quadriceps bioenergetics status in hypoxemic patients with COPD (537, 538). A recent study by Amann and colleagues (267) clearly shows that increasing oxygen transport by different types of interventions reduces muscle susceptibility to fatigue by approximately 30 to 40%. Acute oxygen supplementation also improves whole-body exercise performance by reducing ventilation, dynamic hyperinflation, and the perception of dyspnea (539–542). Oxygen supplementation during the training sessions may thus help patients to reach higher training intensities (543) and possibly enhance limb muscle adaptation. However, this hypothesis has yet to be directly tested. In addition, reports on the effectiveness of oxygen supplementation as an adjunct to exercise training have been equivocal (544); for this reason, oxygen supplementation cannot be widely recommended in this setting.
Nutritional Supplementation
Muscle atrophy is often associated with muscle weakness and exercise limitation (545, 546), and it worsens the prognosis of many chronic diseases, including COPD (131, 547), and impairs health-related quality of life (548, 549). Moreover, underweight smokers appear to be more susceptible to develop COPD (550). As body weight gain is associated with an improvement in the prognosis of patients with COPD (149), efforts should be developed to establish appropriate treatments for the underweight patients. A caveat of this intervention is that restoration of energy and protein balance by nutritional intervention will predominantly result in gain of FM, with little effect on limb muscle mass and function (551, 552).
Depending on the body composition abnormality, intervention strategies will be oriented toward restoring energy and protein balance (weight loss), restoring protein balance (hidden muscle atrophy), or decreasing energy balance while maintaining protein balance (obesity and visceral fat expansion). Furthermore, specific nutrients may be considered as ergogenic aids to enhance efficacy of pulmonary rehabilitation.
One of the first nutritional intervention studies in COPD showed that high-caloric oral liquid dietary supplementation during 3 months was able to restore energy balance and increase body weight by more than 4 kg in underweight patients with COPD (553). The study also illustrated that discontinuation of the intervention for the next 3 months led to a rapid and parallel decline in weight, midarm muscle circumference, and limb muscle function. In weight-losing patients with COPD, even dietary counseling and food fortification resulted in significant body weight gain (2 kg) and FM with maintenance of FFM during the 6-month intervention and 6-month follow-up period (551). In this study, the absence of an anabolic stimulus in association with the nutritional intervention may have prevented a gain in FFM and limb muscle strength. During the same period, the control group lost weight and FFM.
Nutritional interventions have been coupled with exercise training in malnourished patients aiming to provide an adequate anabolic stimulus and a positive effect on FFM. The effects of 7 weeks of nutritional supplementation on top of a rehabilitation program were investigated in a randomized controlled trial (552). The treatment group showed weight gain that predominantly consisted of FM. Absence of an enhancing effect of nutrition on FFM could be related to the low anabolic stimulus of the exercise program that consisted of walking and low-impact conditioning exercise. A similar nutritional intervention strategy when combined with a more intensive exercise program (5 d/wk, strength and endurance exercise) did show positive effects on FFM and limb muscle function after 8 weeks (554).
To judge efficacy of nutritional intervention on muscle protein synthesis and muscle mass, it is important to have information of the quantity and quality of protein in the diet. It appears that supplementation of dietary protein (>1.5 g/kg/d) is able to increase muscle mass (555). Baldi and colleagues (556) randomized patients with COPD experiencing an ongoing weight loss to a 12-week rehabilitation program alone or in conjunction with a nutritional intervention consisting of an amino acid mixture with high branched chain amino acid concentrations (total protein intake > 1.5 g/kg). Body weight and FFM significantly increased in the exercise and amino acid supplementation group but not in the control group. In an acute experiment in normal body weight patients with COPD and mild muscle atrophy, Engelen and colleagues (557) showed that branched chain amino acid supplementation with soy protein enhanced whole-body protein synthesis and altered interorgan protein metabolism in favor of the limb muscles. The effect was more pronounced in COPD than in a healthy age-matched control group.
Pison and colleagues (558) investigated the efficacy of a multimodal intervention approach including nutrition, anabolic steroids, and exercise versus education only in patients with chronic respiratory failure. The combined approach was indeed successful in improving body weight, FFM, exercise tolerance, and even survival in patients compliant with the protocol. One limitation with this study is that it is not possible to tease out the respective benefit that could be attributed to each individual component of the intervention.
In general, the response to nutritional supplementation is highly variable. This may be related to patient characteristics, type of intervention, or adherence to the treatment. Steiner and colleagues (552) reported a better response to nutritional rehabilitation in less-wasted patients. Creutzberg and colleagues (559) attempted to identify determinants of poor response to nutritional rehabilitation in a controlled clinical setting. Poor responders were characterized by higher levels of systemic inflammatory markers and relative expansion of FM despite a similar degree of weight loss as the responders.
N-3 polyunsaturated fatty acids (PUFA) may improve muscle maintenance by modulating systemic inflammation. Three months of nutritional supplementation enriched with PUFA as adjunct to exercise training decreased systemic inflammatory markers, including C-reactive protein, tumor necrosis factor-α, and IL-8 in elderly patients with COPD (560). This was confirmed by the same group in a recent study investigating the effects of nutritional supplementation enriched with PUFA and vitamins A, C, and E incorporated into a 12-week home-based pulmonary rehabilitation program; the intervention also yielded improved exercise performance (561). However, the effect of a mixture of n-3/n-6 PUFA as a supplement to pulmonary rehabilitation on systemic inflammation could not be subsequently confirmed (562). This negative finding was possibly related to the duration of the intervention. In the same study (562), a significantly enhanced exercise capacity was also shown in the treated group. This effect might be related to positive effects of PUFA on muscle oxidative metabolism, because PUFA are natural ligands of the PPARs, which are important regulators of oxidative metabolism (180). Other studies on nutritional modulation of muscle oxidative metabolism showed inconclusive results, as recently reviewed (563). Decreased muscle glutamate concentration in COPD is consistently reported and is associated with decreased muscle glutathione concentration and early lactic acidosis (225). Continuous oral glutamate ingestion for 80 minutes, however, did not lead to an acute effect on limb muscle substrate metabolism and muscle performance in patients with COPD as well as in healthy age-matched control subjects (564). In line, with this negative finding, glutamine supplementation did not enhance o2 peak, o2 at lactate threshold, or speed pulmonary oxygen uptake kinetics in COPD (565).
Patients with COPD are vulnerable to denutrition during an acute exacerbation of the disease. Adequate nutritional support, especially in patients with already impaired energy balance, is also important. As such, nutritional interventions during hospitalization with protein intake exceeding 1.5 g/kg body weight was shown to result in protein and total energy intake improvement without a drop in normal dietary intake, although this did not result in any improvement in muscle strength (278, 566).
Most studies so far have focused on wasted patients with advanced COPD. In this population, therapeutic efficacy and feasibility are hampered by the severity of respiratory impairment, persistent low-grade systemic inflammation, and limb muscle pathology, including active muscle protein degradation and intrinsic abnormalities in muscle oxidative metabolism. Because muscle atrophy is not limited to advanced disease, one could argue that early intervention may be indicated to improve or maintain physical functioning. Furthermore it is important to maintain long-term beneficial effects also from a cost-effectiveness point of view. A prescheduled post hoc analysis of muscle-wasted patients with moderate COPD participating in a clinical trial showed that 4 months of intervention consisting of exercise and standardized nutritional supplements followed by a 20-month maintenance program including nutritional counseling and supplements on indication resulted in significant long-term effects on FFM, limb muscle function, and 6-minute walking distance compared with usual care trial (567). Cost analysis furthermore revealed significantly lower hospital admission costs in the intervention group. One limitation of this study is that the nutritional intervention was embedded within a rehabilitation program, making it difficult to pinpoint the role of nutrition in the observed improvement.
In conclusion, some improvement in FFM and in muscle strength can be achieved with nutritional intervention, particularly when coupled with an anabolic stimulus. However, nutritional interventions are not widely used, because the magnitude of the beneficial effects in enhancing exercise performance on top of exercise training is of uncertain clinical importance. Nevertheless, some patients, particularly those who are actively losing body weight and in whom nutritional intake is deficient, should be targeted for this intervention.
Testosterone and Other Anabolic Steroids
Testosterone and its analogs are potent anabolic agents acting to increase muscle protein synthesis and reduce muscle protein breakdown, yielding muscle mass increases. These drugs have lipolytic effects, so that FM is decreased. Testosterone levels decline with age (around 1–2% per year), and a significant number of older men have levels below the lower limit of normal for young men. There is debate among endocrinologists about whether this is representative of normal ageing or an indication of a pathologic state. Current endocrine society guidance suggests testosterone supplementation for older men with symptomatic hypogonadism (370). Reduced muscle mass and function is a common feature of hypogonadism in addition to loss of libido, erectile dysfunction, loss of energy and vitality, and reduced bone mineral density and muscle mass.
Testosterone is not effective when administered orally. It can be given by intramuscular injection or absorbed transcutaneously via a patch or in a gel. Some testosterone analogs can be administered orally (e.g., oxandrolone). Testosterone has a number of toxicities that limit its use. Prominent among them are stimulation of the prostate in men, with the attendant risk of accelerating the growth of prostate cancer foci. In women, testosterone in high doses yields virilization. A new class of molecules, dubbed selective androgen receptor modulators, has the potential to yield muscle hypertrophy while avoiding prostate stimulation and virilization (568, 569); these molecules are now being tested in clinical trials.
Testosterone yields muscle fiber hypertrophy, an adaptation similar to that seen with a resistance training program. Muscle mass and strength increases are to be expected. Most studies in healthy younger and older subjects have detected increases in muscle strength but not muscle endurance. Healthy eugonadal men respond to supraphysiologic doses of testosterone with increased muscle mass and strength but decreased FM (372). The dose–response relationship for increase in muscle mass and strength and decrease in FM in both healthy young men (570) and older men (571) is linear. In women, randomized trials have been reported in which testosterone administration in lower doses than generally given to men has induced increases in both muscle mass (481) and strength (572).
Only a few investigators have studied testosterone supplementation in COPD. Casaburi and colleagues conducted a trial of testosterone supplementation or placebo with or without resistance training in 47 men with COPD who had low circulating testosterone levels at recruitment (466). A weekly injection of 100 mg of testosterone enanthate for 10 weeks increased testosterone levels to the middle of the range seen in healthy young men. Improvements were seen in leg muscle strength (assessed as one repetition maximum testing of leg extension) and mass (assessed by DEXA scanning) with testosterone alone that were similar to those receiving resistance training alone. Gains in muscle strength and mass in supplemented patients who undertook resistance training were even greater, suggesting a synergistic effect. Svartberg and colleagues compared testosterone supplementation with placebo in 29 men with COPD who were not selected or screened for biochemical hypogonadism at recruitment and did not present with low muscle mass (573). Testosterone was administered intramuscularly every 4 weeks for 26 weeks. There were increases in FFM and concomitant reductions in FM in the active treatment group.
The effects of testosterone analog supplementation in COPD have been investigated in few studies. Schols and colleagues administered a relatively low dose of nandrolone every 2 weeks for 8 weeks to men and women with COPD (574); small increases in lean body mass and respiratory muscle strength were observed. Six months of stanozolol administration to 10 men with COPD resulted in increased body weight and lean body mass but no endurance exercise changes (575). Forty-nine male and female patients with COPD completed a 4-month observational study of oxandrolone; body weight increased, but 6-minute walking distance did not (576). In a randomized placebo-controlled multicenter trial of oxandrolone involving 142 underweight men and women with COPD, an increase in lean mass was discerned, but FM was decreased; 6-minute walking distance was unchanged (577). Creutzberg and colleagues administered nandrolone to 33 men with COPD for 8 weeks; lean body mass increased, but changes in muscle strength and endurance did not differ significantly from those in a matched control group (578). Interestingly, patients on maintenance low dose of systemic corticosteroids benefited from the intervention by showing improvements in respiratory muscle strength and exercise capacity (578). Sharma and colleagues randomized 16 men and women with COPD to receive biweekly injections of nandrolone or placebo for 16 weeks (579). No differences between groups in body composition, muscle function, or quality-of-life measures were discerned.
There are limited mechanistic data on the effect of testosterone on limb muscle function in COPD. Muscle fiber cross-sectional area and IGF signaling were assessed in the intervention study of Lewis and colleagues (316) and Casaburi and colleagues (466). In patients receiving testosterone during resistance training, a global increase in MHC, IGF, and myogenin mRNA expression was observed, suggesting the intervention had a significant anabolic effect at a muscle level. Also, endothelial and neuronal NOS protein levels increased in subjects receiving testosterone supplementation, which might be expected to improve muscle vasodilatory capabilities (466, 580).
In conclusion, testosterone is a powerful anabolic agent that has a potential therapeutic role in the management of older patients with chronic diseases such as COPD, wherein muscle atrophy is a prevalent and clinically significant problem. Benefits are likely to be maximized if combined with resistance training. The role of therapy in patients with preserved serum testosterone levels is uncertain. Low-dose supplementation may also be tolerable in women, although the optimal dose that improves functionality without inducing virilization may be hard to define. Apparently, testosterone supplementation is more effective than its analogs. Despite this, testosterone supplementation cannot be currently recommended as routine treatment of muscle atrophy in COPD because of the possibility of adverse effects. Another point is that the available studies are only short term, and the clinical impact of this supplementation on important clinical outcomes such as exercise tolerance, quality of life, and survival is unknown. Novel selective androgen receptor modulators are undergoing clinical trials and may provide additional benefits.
Growth Hormone and Its Secretagogues
GH is a 191–amino acid peptide hormone secreted mainly by the anterior pituitary gland. Despite the continuous hormone release, most of the GH production is secreted in a pulsatile manner (581). GH synthesis and release is directly controlled by GH-releasing hormone (GHRH; also known as GH-releasing factor, GRF) and peripheral feedback signals (582). Exercise itself is a peripheral stimulus for GH production.
GH is an anabolic agent that stimulates growth through an increased production of IGF-1. This factor enhances protein synthesis and lipolysis, promoting the preferential use of lipids rather than carbohydrates for energy generation (583). It also inhibits protein oxidation and degradation, stimulates calcium retention and bone mineralization, promotes myoblast differentiation and muscle growth, and improves the immune response (583). Overall, the effect of the GH–IGF-1 axis is to increase the baseline metabolic rate, and it will affect body composition by improving lean body mass (584). GH has also cardiovascular effects, inducing an increase in heart rate and improving endothelial function in patients with arteriosclerosis. Although the plasma levels of this hormone can be increased in patients with COPD (585–587), the function of the GH axis may be impaired in this disease (588). Moreover, some conditions and treatments frequently associated with COPD, such as aging, reduction in physical activity, and the use of systemic steroids may also be associated with reduced levels of GH in plasma (589–591). Although exogenous GH does not seem to improve limb muscle strength in athletes, an increase in maximal respiratory pressures was shown (584, 592). However, its effects on exercise capacity are not clear, because different studies have reported similar work rate and oxygen uptake but higher levels of lactate after GH administration (584). This is in line with studies showing an improvement in anaerobic performance after GH administration (593).
Although effects of GH are not very clear in athletes, they seem to be more promising in patients with cachexia. In this regard, GH has demonstrated clear anabolic effects in such patients and is currently approved for use in HIV/AIDS-associated wasting syndrome as well as in other conditions, such as chronic renal failure and some cases of short bowel syndrome. GH has also been used in patients with COPD and malnutrition, inducing substantial weight improvement and lean body mass gain (594, 595). Nonetheless, results on respiratory and limb muscle strength and exercise capacity are still controversial (587, 594, 596, 597). Therapy with GH is currently administered through subcutaneous injections of recombinant GH (0.05–0.06 mg/kg daily), several times per week, and side effects include paresthesias, arthralgias, insulin resistance–glucose intolerance, sodium retention, peripheral edema, and arterial hypertension (598). In addition, it still remains debatable whether GH treatment may induce carcinogenic effects (599). All these considerations, along with its relatively expensive price, do not support the use of GH in the treatment of COPD muscle dysfunction. The adverse effects of GH therapy have led to the development of GH secretagogues that have some potential advantages.
GRF is a C-terminal peptide of 40 to 44 amino acids (600). Administration of human GRF might be able to recover GH secretion pulses (601) and, therefore, the physiological actions of this pituitary hormone. However, the clinical usefulness of human GRF therapy is limited, because there is a wide intersubject variability in the GH response, and the peptide is rapidly degraded in plasma. Therefore, its effect is limited to roughly 2 hours, even at high doses (602).
Ghrelin is a 28–amino acid peptide hormone that can circulate in two distinct forms: acylated and unacylated molecules. Although acylation is essential for binding to its receptor, unacylated ghrelin is also an active peptide. Ghrelin was originally isolated from the stomach (603), but it has also been isolated in pancreas, brain, pituitary gland, kidneys, placenta, heart, and lungs (604). Interestingly, ghrelin receptor is also expressed in different tissues, including the lungs (605). The main function of ghrelin is to induce the release of GH from the anterior pituitary (603, 604). Interestingly, ghrelin has a synergistic relationship with GRF. However, ghrelin has many other functions, including the regulation of appetite (potent orexigenic) (606) and gut motility. It may also play a role in the immune and cardiovascular systems. In this regard, ghrelin is a potent antiinflammatory mediator, reducing the levels of different proinflammatory cytokines. Therefore, it is considered as a promising therapeutic agent for the treatment of different chronic inflammatory diseases. As previously mentioned, ghrelin is also expressed in the myocardium, inhibiting cell apoptosis while improving ventricular function during ischemia-reperfusion injury (607). Although still controversial, this peptide also seems to be a vasodilator agent, through the induction of nitric oxide production, and a stimulator of angiogenesis. Finally, ghrelin influences fat metabolism by reducing lipid oxidation and increasing adiposity (608). Pharmacological doses of ghrelin successfully reverse muscle wasting in different disorders that are associated with cachexia (609, 610). However, both ghrelin and its receptor are also expressed in different cancers and cancer cell lines. Therefore, it has been suggested that it might play a role in the progression of the disease, and more specifically in cell proliferation, invasion, and migration (611).
Plasma levels of endogenous ghrelin increase both in normal subjects with weight loss induced by a low caloric intake (612, 613) and in underweight patients with COPD (614, 615). This increase is attributed to a compensatory mechanism. Nevertheless, exogenous ghrelin has been used in patients with COPD with nutritional abnormalities, inducing similar effects to GH: it increases food intake and muscle mass (616). It seems to be more effective than GH in improving the strength of respiratory and limb muscles (610). Ghrelin has also exhibited antiinflammatory effects in patients with COPD, reducing the number of neutrophils in the sputum (617). Current therapeutic doses of ghrelin are established as 2 μg/kg administered intravenously twice a day for at least 3 consecutive weeks.
Tesamorelin is a GRF analog composed of a 44–amino acid sequence (618, 619). Its advantage over that of classical GRF is that it has been stabilized, and therefore it maintains its biological activity in plasma (600). Tesamorelin markedly increases levels of both GH and IGF-1 (618, 620, 621), thus resulting in the physiologic effects of these two anabolic agents. Moreover, tesamorelin seems to increase both baseline and pulse GH secretion (622). Tesamorelin is already in use to treat lipodystrophy in HIV-infected patients (619). Moreover, some studies have been performed in other muscle wasting disorders, such as COPD (623). In a multicenter, double-blind, randomized placebo-controlled study, tesamorelin improved body composition by increasing lean mass and improved limb and respiratory muscle strength (623–625). Current doses are 2 mg administered daily via subcutaneous injection. This ensures an 8-hour period of GH release, and side effects appear to be minor (618). However, the high price of this therapy is still a limiting factor for its widespread use (620). Unfortunately, a recent multicenter large phase II study using tesamorelin in patients with COPD was interrupted, precluding the recommendation of the use of this drug in clinical settings (626).
Sermorelin is a GRF-like compound that can be administered subcutaneously. However, its physiological effects, potential advantages over that of the other secretagogues, and possible therapeutic indications still need more extensive studies to be precisely determined (602). Examorelin is another synthetic GRF analog, of only six amino acids, which might have some advantages over previous secretagogues because it can be released using subcutaneous, oral, or intranasal administration (627). Another promising GRF analog is LAB GHRH, which can also be administered either orally, by injection, or by inhalation. However, as with sermorelin, conclusive studies are lacking for examorelin and LAB GHRH. Furthermore, they have not as yet been used in underweight patients with COPD.
In conclusion, GH, ghrelin, and GRF analogs are anabolic agents that improve body weight and muscle mass, being logical treatments for different chronic conditions associated with cachexia. However, their actions on muscle function are not yet clear. Because larger-scale trials are still awaited, especially for new secretagogues, the role of these peptides in the treatment of COPD needs to be further clarified. In view of present data, we can speculate that they should probably be restricted to patients with severe muscle atrophy.
Other Anabolic Drugs and Bioactive Nutrients
Anabolism may be defined as the synthesis of complex molecules in living organisms from simpler ones together with the storage of energy. Using this definition, several pharmacologic agents have been described that have anabolic potential. Some, however, have not been evaluated in COPD at this time.
Megestrol acetate.
Megestrol acetate (MA) is a progestational appetite stimulant that also has antiinflammatory effects (628, 629). A prospective, double-blind, randomized, multicenter, placebo-controlled outpatient trial involving patients with COPD was published in 2002 (630). Body weight increased by 3.2 kg in the MA group and 0.7 kg in the placebo group, but unfortunately this was mainly fat. Spirometry and maximum inspiratory pressure did not change significantly. Changes in 6-minute walking distance did not differ statistically between groups at Weeks 2 and 4 but were greater in the placebo group at Week 8. Consistent with its known ability to stimulate ventilation, PaCO2 decreased in the MA group. Questionnaires revealed that body image and appetite improved in the MA but not the placebo group. Cortisol decreased substantially, and, in male subjects, testosterone levels decreased by 85% in the MA group. Therefore, this study showed that MA increased body weight and stimulated pulmonary ventilation in underweight patients with COPD but did not improve muscle function. It may be concluded that megestrol acetate is anabolic to fat, but not to muscle, likely because this drug lowers testosterone levels substantially.
Creatine.
Creatine monohydrate is a nutritional supplement in wide use as an aid to exercise performance. In limb muscle, creatine undergoes phosphorylation to form PCr, a source of high-energy phosphate that supports ATP resynthesis during exercise. This mechanism of action suggests that creatine supplementation would be expected to enhance exercise endurance capacity. A substantial number of studies have evaluated creatine supplementation in athletes and healthy elderly subjects. Two metaanalyses have concluded that, in general, creatine supplementation enhances exercise performance (631, 632).
Three randomized placebo-controlled studies in patients with moderate to severe COPD have evaluated the additive benefit of creatine supplementation in conjunction with pulmonary rehabilitation. Fuld and colleagues (633) randomized 38 subjects to undergo a 2-week “loading period” with creatine versus placebo followed by a 10-week pulmonary rehabilitation program. The creatine group had a greater increase in FFM (about 1 kg) as well as evidence of greater improvement in leg muscle strength and endurance. But whole-body exercise capacity (incremental and endurance shuttle walk distance, incremental and constant work rate cycle ergometer testing) increase was no greater than with rehabilitation alone. Faager and colleagues (634) randomized 23 patients with COPD to oral creatine supplementation versus placebo in combination with 8 weeks of exercise training. Those randomized to creatine showed no augmentation of improvement in endurance shuttle walk distance compared with those receiving placebo. Deacon and colleagues (635) studied 100 patients with COPD who were randomized to creatine or placebo during 7 weeks of exercise training. Quadriceps muscle biopsies, performed in a subset of participants, showed creatine uptake in the group receiving creatine. However, the two groups showed no difference in improvement in incremental shuttle walk distance or knee extensor strength. A recent metaanalysis (636) concluded that creatine supplementation when added to pulmonary rehabilitation does not improve exercise capacity, muscle strength, or quality of life in patients with COPD.
l-Carnitine.
Carnitine is required for optimal mitochondrial fatty acid oxidation and is a critical source of energy (637). Studies in the sports medicine literature have tended to demonstrate a performance-improving effect of l-carnitine, whereas studies in untrained healthy individuals have tended not to show this benefit (638).
The effects of adding l-carnitine to a whole-body and respiratory training program were determined in patients with moderate to severe COPD (639). Sixteen patients with COPD were studied; they were randomly assigned to oral l-carnitine or placebo for 6 weeks. Both groups participated in thrice weekly 30-minute treadmill and threshold inspiratory muscle training sessions. Anthropometry detected no change in body composition in either group. Peak work rate in the incremental treadmill test was significantly improved in both groups, with no significant difference between groups. However, blood lactate, blood pressure, and heart rate at identical exercise levels during the incremental test were lower in the carnitine group after training. Inspiratory muscle strength and 6-mintue walk distance were improved in both groups, but the gains of the carnitine group were significantly greater. This small study suggests that carnitine may be able to improve exercise tolerance in patients with COPD in the context of a pulmonary rehabilitation program, but larger, longer-term studies will be required to confirm this.
Myostatin inhibitor.
No studies exploring the effect of myostatin inhibitors have been performed in patients with COPD. A safety trial of a neutralizing antibody to myostatin, MYO-029, in 116 patients with adult muscular dystrophy was reported in 2008 (640). The drug was well tolerated; further studies may be forthcoming.
Antioxidants
Limb muscle fibers possess strong antioxidant systems that protect the myocytes from potential deleterious effects of ROS. For instance, the antioxidants CuZn-superoxide dismutase (SOD), catalase, and glutathione peroxidase are present in the sarcoplasm, whereas Mn-SOD and glutathione peroxidase are localized within the mitochondrial matrix. Other thiol-based antioxidant proteins such as thioredoxins and peroxiredoxins are also abundantly expressed inside the myocytes. Moreover, nonenzymatic antioxidant systems complement the action of the antioxidant enzymes, such as the lipid-soluble compounds vitamin E, carotenes, and ubiquinol, which are localized to cell membranes. Ascorbic acid, urate, lipoate, and glutathione, the most abundant nonprotein thiols, are water soluble and widely distributed within the muscle fibers. The ratio of reduced to oxidized glutathione is an indicator of the redox tissue potential. In this regard, thiol oxidation is a sensitive marker of oxidative stress that has been clearly implicated in muscle fatigue (641).
In resting muscles of patients with COPD, the levels of antioxidant enzymes are significantly increased (171, 201, 217, 222, 642). Specifically, both mitochondrial SOD content and total SOD activity were consistently shown to be greater in the quadriceps of patients with severe COPD (171, 201, 217, 222). A significant rise in Mn-SOD content was also detected in the quadriceps of healthy smokers as well as in respiratory and limb muscles of mice chronically exposed to cigarette smoke (233). Nonetheless, tissue antioxidant potential as measured by reduced glutathione was not different between patients with COPD and control subjects in their limb muscles (217, 231, 514). Similar findings were reported in muscles of guinea pigs chronically exposed to cigarette smoke, in whom antioxidant content levels did not differ from those encountered in the control animals (233).
The issue of whether patients with COPD should be treated with antioxidants to delay the development of limb muscle fatigue is still debatable. Previous studies in humans (643, 644) have demonstrated that nutritional antioxidants (vitamins C and E and carotenes) do not attenuate fatigue, despite reducing biochemical markers of oxidative stress. However, treatment of animals with actual antioxidant enzymes such as SOD and catalase, which selectively scavenge ROS, was shown to diminish fatigue during repetitive muscle contractions (645, 646). Allopurinol, which directly inhibits the activity of the ROS-producing enzyme xanthine oxidase, was shown to significantly decrease systemic lipid peroxidation and oxidized glutathione, with no changes in quadriceps endurance, after strenuous exercise in patients with severe COPD (226). Furthermore, inhibitors of cyclooxygenase and antioxidants may reduce the perception of muscle fatigue in patients with cancer cachexia (647). Nonetheless, current knowledge on whether antioxidant enzyme promoters and inhibitors of ROS-producing enzymes may lessen fatigue and improve muscle performance is at its infancy, and further research, probably conducted in animal experiments at an initial stage, is warranted.
An alternative approach to the use of antioxidants has been the administration of compounds such as N-acetyl cysteine (NAC), glutathione, and whey-based cysteine donors that prevent thiol oxidation. These compounds improve muscle performance and attenuate oxidative stress markers in experimental models of resistive loading and in patients with COPD (227, 518, 641, 648). Moreover, inhibition of glutathione levels worsened muscle fatigue (641), suggesting that glutathione redox status is an important determinant of muscle fatigue. NAC, an acetyl derivative of the amino acid cysteine, neutralizes ROS by reducing disulfide bonds. NAC may also enhance intracellular levels of glutathione in vivo in several organs, including the lungs (649). Importantly, investigations conducted in healthy subjects have shown that treatment with NAC delays fatigue and increases glutathione availability in response to chronic endurance training (650, 651). These are relevant findings that may have implications in the management of the COPD-associated muscle dysfunction, especially when targeting improvement of muscle performance.
In conclusion, increasing antioxidant potential seems to improve muscle performance while attenuating fatigue. These findings have important implications in the design of endurance exercise training programs in patients with COPD. Future research is warranted to advance current knowledge on the effects of nutritional and pharmacological agents tailored to enhancing antioxidant enzyme activity and/or inhibition of ROS production in muscles of patients with COPD.
Vitamin D Supplementation
Several randomized trials and metaanalyses in elderly subjects systematically demonstrate that vitamin D supplementation improves balance and reduces falls by approximately 20% (403, 652). It is still debated whether these effects are obtained because of improved neuromuscular control and better neural signaling rather than through optimization of limb muscle strength. Indeed, data on strength are less consistent, with one metaanalysis revealing efficacy of supplementation only when baseline 25-OHD levels are below 10 ng/ml (653). However, a large cross-sectional study shows that muscle strength continues to increase from 25-OHD levels of 9 ng/ml to 37 ng/ml (388), which made some experts suggest that, for obtaining beneficial effects on the muscle, higher doses of supplementation are necessary (654). It should be mentioned that none of these studies has reported on patients with COPD.
Surprisingly, few studies have explored vitamin D supplementation in the context of training (655). Two intervention studies evaluated the effect of a daily dose of vitamin D (400–800 IU) and calcium (1 g) in combination with strength or resistance training in an elderly population (656, 657). They found no effect, perhaps because both the supplementation dosage and the load of the training program were too low to generate positive results (654, 655). When focusing on COPD, vitamin D deficiency has been linked to more drop-out and worse outcome after pulmonary rehabilitation (658). A randomized controlled trial (659) in 50 patients with COPD referred for rehabilitation showed mild additional benefits of high-dose vitamin D supplements (3,000 U/d) on limb muscle strength and exercise tolerance (659, 660). A larger prospective randomized controlled study is currently ongoing (clinicaltrials.gov: NCT01416701). Additional studies are needed to evaluate clinical and long-term benefits in patients or subgroups of patients with COPD.
Suggestions for Future Research
Despite the progress made since the first limb muscle dysfunction statement in COPD, much remains to be learned about this important systemic consequence of this disease. For example, we do not know whether limb muscle dysfunction in COPD is the mere reflection of years of disuse or whether there is a specific form of myopathy in this condition or in specific COPD phenotypes. Along these lines, it is unclear whether the development of muscle weakness parallels the development of COPD or whether different causal factors are influencing the trajectory of decline of muscle strength and lung function. Body composition abnormalities in COPD are rapidly changing in the context of a worldwide epidemic of obesity. Clinicians are now faced with many more obese patients with COPD than lean ones. In this situation, the diagnosis of muscle atrophy may become more difficult, as clinicians are likely to be misled by the presence of a normal or increased BMI, believing that this implies normal muscle mass. Although muscle atrophy is less prevalent when BMI is normal or increased, there is still a portion of patients in this category exhibiting muscle atrophy and weakness. As such, the incorporation of body composition analysis in the clinical arena will become even more relevant.
There are some examples/suggestions for future research that should help advance our understanding of the development and treatment of limb muscle dysfunction in COPD.
-
1.
The choice of the appropriate comparator in studies evaluating muscle function in COPD is critical. If the question about the presence of disuse versus myopathy is to be answered, it will be important to match patients with COPD and control subjects with similar degree of physical activities.
-
2.
Studies should be done to determine widely accepted normative values for quadriceps muscle strength.
-
3.
There is a need to know more about the onset of limb muscle dysfunction in COPD. As such, the focus on patients with mild disease would be of interest. Longitudinal studies looking at the changes in limb muscle function over time will be important to understand when the pathological processes within the muscles start and how they evolve over time.
-
4.
The investigation of the basic/molecular mechanisms of limb muscle dysfunction is a key issue for the development of specific and safe strategies to target this problem.
-
5.
Clinical trials are warranted to evaluate to which extent treatment of limb muscle function affects clinical outcomes (exercise capacity, quality of life, and survival) in COPD.
-
6.
Large and multicenter studies in thoroughly phenotyped patients will be instrumental in understanding specific risk factors for developing limb muscle dysfunction and evaluating treatment for this condition.
-
7.
Whether muscle abnormalities can be completely normalized with exercise training is a question that should be addressed specifically in a clinical trial.
-
8.
In the context of increasing prevalence of (abdominal) obesity in COPD, emphasis should be placed on the possible cross-talk between fat and muscle. For example, it may be that limb muscle dysfunction could influence the prevalence of the metabolic syndrome.
Conclusions
The limb muscles have been the topic of several publications since the first ATS/ERS statement on this topic. Limb muscle dysfunction is a clinically relevant systemic manifestation of COPD because it influences important clinical outcomes in this disease. This comorbid condition associated with COPD can be treated with exercise training. Future research should allow a better understanding of the mechanisms involved in the development of skeletal muscle dysfunction with the hope for specific therapies for this problem.
Acknowledgments
This statement was prepared by an ad hoc subcommittee of the ATS Assembly on Pulmonary Rehabilitation and the ERS Scientific Group 01.02 “Rehabilitation and Chronic Care.”
Members of this committee were as follows:
François Maltais, M.D. (Chair)
Marc Decramer, Ph.D., M.D. (Co-Chair)
Esther Barreiro, M.D., Ph.D.
Yan Burelle, Ph.D.
Richard Casaburi, Ph.D, M.D.
Richard Debigaré, Ph.D.
P. N. Richard Dekhuijzen, M.D., Ph.D.
Frits Franssen, M.D., Ph.D.
Ghislaine Gayan-Ramirez, Ph.D.
Joaquim Gea, M.D.
Harry R. Gosker, Ph.D.
Rik Gosselink, Ph.D.
Maurice Hayot, Ph.D., M.D.
Sabah N. A. Hussain, Ph.D., M.D.
Wim Janssens, Ph.D., M.D.
Michael I. Polkey, Ph.D.
Josep Roca, M.D.
Didier Saey, P.T., Ph.D.
Annemie M. W. J. Schols, Ph.D.
Martijn A. Spruit, Ph.D.
Michael Steiner, M.D.
Tanja Taivassalo, Ph.D.
Thierry Troosters, Ph.D.
Ioannis Vogiatzis, Ph.D.
Peter D. Wagner, M.D.
Acknowledgment
The authors thank Annette De Bruyne and Eline Lahousse for their secretarial assistance and Hélène Trudel for drawing the figures. They also thank Kevin Wilson and Guy Brusselle for their guidance during the preparation of this document.
Footnotes
This official statement of the American Thoracic Society (ATS) and the European Respiratory Society (ERS) was approved by the ATS Board of Directors, November 2013, and by the ERS Executive Committee, September 2013
F.M. holds a CIHR/GSK Research Chair on COPD at Université Laval. R.C. holds the Grancell/Burns Chair in the Rehabilitative Sciences. E.B.’s contribution to this statement was supported by CIBERES, FIS 11/02029, 2009-SGR-393, SEPAR 2010, FUCAP 2011, FUCAP 2012, and Marató TV3 (MTV3-07-1010) (Spain) and by the ERS COPD Research Award 2008. M.I.P.’s contribution to this statement was supported by the NIHR Respiratory Disease Biomedical Research Unit at the Royal Brompton and Harefield NHS Foundation Trust and Imperial College London, who partially fund his salary.
Author Disclosures: F.M. was on an advisory committee and received speaker fees from GlaxoSmithKline ($5,000–24,999 combined); he received research support from GlaxoSmithKline ($50,000–99,999). R.C. received research support from Novartis ($100,000–249,999). J.G. holds a patent for a training valve, with proceeds paid to his institution. W.J. received speaker fees from AstraZeneca ($1–4,999), was on an advisory committee and received speaker fees from Boehringer Ingelheim ($1–4,999 combined), and received research support from Boehringer Ingelheim ($5,000–24,999); he was on an advisory committee of Chiesi ($1–4,999), and on an advisory committee and received speaker fees from GlaxoSmithKline ($1–4,999 combined); he was on an advisory committee and received speaker fees from Novartis ($1–4,999 combined). M.D., E.B., Y.B., R.D., P.N.R.D., F.F., G.G-R., H.R.G., R.G., M.H., S.N.A.H., M.I.P., J.R., D.S., A.M.W.J.S., M.A.S., M.S., T. Taivassalo, T. Troosters., I.V., and P.D.W. reported no relevant commercial interests.
References
- 1.Skeletal muscle dysfunction in chronic obstructive pulmonary disease: a statement of the American Thoracic Society and European Respiratory Society. Am J Respir Crit Care Med. 1999;159:S1–S40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Hopkinson NS, Tennant RC, Dayer MJ, Swallow EB, Hansel TT, Moxham J, Polkey MI. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res. 2007;8:25. doi: 10.1186/1465-9921-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Borst B, Koster A, Yu B, Gosker HR, Meibohm B, Bauer DC, Kritchevsky SB, Liu Y, Newman AB, Harris TB, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66:961–969. doi: 10.1136/thoraxjnl-2011-200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burke RE. Revisiting the notion of ‘motor unit types.’. Prog Brain Res. 1999;123:167–175. [PubMed] [Google Scholar]
- 6.Burke RE, Levine DN, Zajac FE., III Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- 7.Burke RE. Motor unit types of cat triceps surae muscle. J Physiol. 1967;193:141–160. doi: 10.1113/jphysiol.1967.sp008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sieck GC, Prakash YS. Morphological adaptations of neuromuscular junctions depend on fiber type. Can J Appl Physiol. 1997;22:197–230. doi: 10.1139/h97-014. [DOI] [PubMed] [Google Scholar]
- 9.Sica RE, McComas AJ. Fast and slow twitch units in a human muscle. J Neurol Neurosurg Psychiatry. 1971;34:113–120. doi: 10.1136/jnnp.34.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feinstein B, Lindegard B, Nyman E, Wohlfart G. Morphologic studies of motor units in normal human muscles. Acta Anat (Basel) 1955;23:127–142. doi: 10.1159/000140989. [DOI] [PubMed] [Google Scholar]
- 11.Garnett RA, O’Donovan MJ, Stephens JA, Taylor A. Motor unit organization of human medial gastrocnemius. J Physiol. 1979;287:33–43. doi: 10.1113/jphysiol.1979.sp012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira Sant’Ana JA, Ennion S, Sargeant AJ, Moorman AF, Goldspink G. Comparison of the molecular, antigenic and ATPase determinants of fast myosin heavy chains in rat and human: a single-fibre study. Pflugers Arch. 1997;435:151–163. doi: 10.1007/s004240050495. [DOI] [PubMed] [Google Scholar]
- 13.Henneman E. Relation between size of neurons and their susceptibility to discharge. Science. 1957;126:1345–1347. doi: 10.1126/science.126.3287.1345. [DOI] [PubMed] [Google Scholar]
- 14.Milner-Brown HS, Stein RB, Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973;230:359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens JA, Usherwood TP. The mechanical properties of human motor units with special reference to their fatiguability and recruitment threshold. Brain Res. 1977;125:91–97. doi: 10.1016/0006-8993(77)90361-4. [DOI] [PubMed] [Google Scholar]
- 16.Yemm R. The orderly recruitment of motor units of the masseter and temporal muscles during voluntary isometric contraction in man. J Physiol. 1977;265:163–174. doi: 10.1113/jphysiol.1977.sp011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desmedt JE, Godaux E. Ballistic contractions in man: characteristic recruitment pattern of single motor units of the tibialis anterior muscle. J Physiol. 1977;264:673–693. doi: 10.1113/jphysiol.1977.sp011689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wakeling JM. Motor units are recruited in a task-dependent fashion during locomotion. J Exp Biol. 2004;207:3883–3890. doi: 10.1242/jeb.01223. [DOI] [PubMed] [Google Scholar]
- 19.Mori S. Discharge patterns of soleus motor units with associated changes in force exerted by foot during quiet stance in man. J Neurophysiol. 1973;36:458–471. doi: 10.1152/jn.1973.36.3.458. [DOI] [PubMed] [Google Scholar]
- 20.Vieira TM, Loram ID, Muceli S, Merletti R, Farina D. Recruitment of motor units in the medial gastrocnemius muscle during human quiet standing: is recruitment intermittent? What triggers recruitment? J Neurophysiol. 2012;107:666–676. doi: 10.1152/jn.00659.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 22.Faulkner JA, Larkin LM, Claflin DR, Brooks SV. Age-related changes in the structure and function of skeletal muscles. Clin Exp Pharmacol Physiol. 2007;34:1091–1096. doi: 10.1111/j.1440-1681.2007.04752.x. [DOI] [PubMed] [Google Scholar]
- 23.Essén-Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126:107–114. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- 24.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 25.Korhonen MT, Cristea A, Alén M, Häkkinen K, Sipilä S, Mero A, Viitasalo JT, Larsson L, Suominen H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J Appl Physiol (1985) 2006;101:906–917. doi: 10.1152/japplphysiol.00299.2006. [DOI] [PubMed] [Google Scholar]
- 26.Brown MC, Holland RL, Hopkins WG. Motor nerve sprouting. Annu Rev Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- 27.Luff AR. Age-associated changes in the innervation of muscle fibers and changes in the mechanical properties of motor units. Ann N Y Acad Sci. 1998;854:92–101. doi: 10.1111/j.1749-6632.1998.tb09895.x. [DOI] [PubMed] [Google Scholar]
- 28.Fling BW, Knight CA, Kamen G. Relationships between motor unit size and recruitment threshold in older adults: implications for size principle. Exp Brain Res. 2009;197:125–133. doi: 10.1007/s00221-009-1898-y. [DOI] [PubMed] [Google Scholar]
- 29.Mulder ER, Gerrits KH, Kleine BU, Rittweger J, Felsenberg D, de Haan A, Stegeman DF. High-density surface EMG study on the time course of central nervous and peripheral neuromuscular changes during 8 weeks of bed rest with or without resistive vibration exercise. J Electromyogr Kinesiol. 2009;19:208–218. doi: 10.1016/j.jelekin.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Cescon C, Gazzoni M. Short term bed-rest reduces conduction velocity of individual motor units in leg muscles. J Electromyogr Kinesiol. 2010;20:860–867. doi: 10.1016/j.jelekin.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Duchateau J, Hainaut K. Electrical and mechanical changes in immobilized human muscle. J Appl Physiol (1985) 1987;62:2168–2173. doi: 10.1152/jappl.1987.62.6.2168. [DOI] [PubMed] [Google Scholar]
- 32.Duchateau J, Hainaut K. Effects of immobilization on contractile properties, recruitment and firing rates of human motor units. J Physiol. 1990;422:55–65. doi: 10.1113/jphysiol.1990.sp017972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seki K, Taniguchi Y, Narusawa M. Effects of joint immobilization on firing rate modulation of human motor units. J Physiol. 2001;530:507–519. doi: 10.1111/j.1469-7793.2001.0507k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seki K, Kizuka T, Yamada H. Reduction in maximal firing rate of motoneurons after 1-week immobilization of finger muscle in human subjects. J Electromyogr Kinesiol. 2007;17:113–120. doi: 10.1016/j.jelekin.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 35.Folland JP, Williams AG. The adaptations to strength training : morphological and neurological contributions to increased strength. Sports Med. 2007;37:145–168. doi: 10.2165/00007256-200737020-00004. [DOI] [PubMed] [Google Scholar]
- 36.Griffin L, Cafarelli E. Resistance training: cortical, spinal, and motor unit adaptations. Can J Appl Physiol. 2005;30:328–340. doi: 10.1139/h05-125. [DOI] [PubMed] [Google Scholar]
- 37.Gonyea WJ. Role of exercise in inducing increases in skeletal muscle fiber number. J Appl Physiol. 1980;48:421–426. doi: 10.1152/jappl.1980.48.3.421. [DOI] [PubMed] [Google Scholar]
- 38.Edman KA. Double-hyperbolic force-velocity relation in frog muscle fibres. J Physiol. 1988;404:301–321. doi: 10.1113/jphysiol.1988.sp017291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Enoka RM. Muscle fatigue—from motor units to clinical symptoms. J Biomech. 2012;45:427–433. doi: 10.1016/j.jbiomech.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain S, Lewis DM. Contractile characteristics and innervation ratio of rat soleus motor units. J Physiol. 1989;412:1–21. doi: 10.1113/jphysiol.1989.sp017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol (1985) 1997;82:182–188. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- 42.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol (1985) 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 43.Fryer MW, Neering IR. Actions of caffeine on fast- and slow-twitch muscles of the rat. J Physiol. 1989;416:435–454. doi: 10.1113/jphysiol.1989.sp017770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salviati G, Volpe P. Ca2+ release from sarcoplasmic reticulum of skinned fast- and slow-twitch muscle fibers. Am J Physiol. 1988;254:C459–C465. doi: 10.1152/ajpcell.1988.254.3.C459. [DOI] [PubMed] [Google Scholar]
- 46.Delbono O, Meissner G. Sarcoplasmic reticulum Ca2+ release in rat slow- and fast-twitch muscles. J Membr Biol. 1996;151:123–130. doi: 10.1007/s002329900063. [DOI] [PubMed] [Google Scholar]
- 47.Ferguson DG, Franzini-Armstrong C. The Ca2+ ATPase content of slow and fast twitch fibers of guinea pig. Muscle Nerve. 1988;11:561–570. doi: 10.1002/mus.880110607. [DOI] [PubMed] [Google Scholar]
- 48.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 49.Laszewski-Williams B, Ruff RL, Gordon AM. Influence of fiber type and muscle source on Ca2+ sensitivity of rat fibers. Am J Physiol. 1989;256:C420–C427. doi: 10.1152/ajpcell.1989.256.2.C420. [DOI] [PubMed] [Google Scholar]
- 50.Mounier Y, Holy X, Stevens L. Compared properties of the contractile system of skinned slow and fast rat muscle fibres. Pflugers Arch. 1989;415:136–141. doi: 10.1007/BF00370583. [DOI] [PubMed] [Google Scholar]
- 51.Stephenson DG, Forrest QG. Different isometric force - [Ca2+] relationships in slow and fast twitch skinned muscle fibres of the rat. Biochim Biophys Acta. 1980;589:358–362. doi: 10.1016/0005-2728(80)90052-3. [DOI] [PubMed] [Google Scholar]
- 52.Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sieck GC, Prakash YS. Cross-bridge kinetics in respiratory muscles. Eur Respir J. 1997;10:2147–2158. doi: 10.1183/09031936.97.10092147. [DOI] [PubMed] [Google Scholar]
- 54.Sieck GC, Han YS, Prakash YS, Jones KA. Cross-bridge cycling kinetics, actomyosin ATPase activity and myosin heavy chain isoforms in skeletal and smooth respiratory muscles. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:435–450. doi: 10.1016/s0305-0491(98)00005-4. [DOI] [PubMed] [Google Scholar]
- 55.Jones EJ, Bishop PA, Woods AK, Green JM. Cross-sectional area and muscular strength: a brief review. Sports Med. 2008;38:987–994. doi: 10.2165/00007256-200838120-00003. [DOI] [PubMed] [Google Scholar]
- 56.Fukunaga T, Miyatani M, Tachi M, Kouzaki M, Kawakami Y, Kanehisa H. Muscle volume is a major determinant of joint torque in humans. Acta Physiol Scand. 2001;172:249–255. doi: 10.1046/j.1365-201x.2001.00867.x. [DOI] [PubMed] [Google Scholar]
- 57.Ikai M, Fukunaga T. Calculation of muscle strength per unit cross-sectional area of human muscle by means of ultrasonic measurement. Int Z Angew Physiol. 1968;26:26–32. doi: 10.1007/BF00696087. [DOI] [PubMed] [Google Scholar]
- 58.Moss BM, Refsnes PE, Abildgaard A, Nicolaysen K, Jensen J. Effects of maximal effort strength training with different loads on dynamic strength, cross-sectional area, load-power and load-velocity relationships. Eur J Appl Physiol Occup Physiol. 1997;75:193–199. doi: 10.1007/s004210050147. [DOI] [PubMed] [Google Scholar]
- 59.Fitts RH, McDonald KS, Schluter JM. The determinants of skeletal muscle force and power: their adaptability with changes in activity pattern. J Biomech. 1991;24:111–122. doi: 10.1016/0021-9290(91)90382-w. [DOI] [PubMed] [Google Scholar]
- 60.Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Widrick JJ, Trappe SW, Blaser CA, Costill DL, Fitts RH. Isometric force and maximal shortening velocity of single muscle fibers from elite master runners. Am J Physiol. 1996;271:C666–C675. doi: 10.1152/ajpcell.1996.271.2.C666. [DOI] [PubMed] [Google Scholar]
- 62.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 64.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–456. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 65.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 66.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS American College of Sports Medicine. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- 67.Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 68.Aagaard P, Magnusson PS, Larsson B, Kjaer M, Krustrup P. Mechanical muscle function, morphology, and fiber type in lifelong trained elderly. Med Sci Sports Exerc. 2007;39:1989–1996. doi: 10.1249/mss.0b013e31814fb402. [DOI] [PubMed] [Google Scholar]
- 69.Caspersen CJ, Kriska AM, Dearwater SR. Physical activity epidemiology as applied to elderly populations. Baillieres Clin Rheumatol. 1994;8:7–27. doi: 10.1016/s0950-3579(05)80222-5. [DOI] [PubMed] [Google Scholar]
- 70.Bassey EJ, Bendall MJ, Pearson M. Muscle strength in the triceps surae and objectively measured customary walking activity in men and women over 65 years of age. Clin Sci (Lond) 1988;74:85–89. doi: 10.1042/cs0740085. [DOI] [PubMed] [Google Scholar]
- 71.Martin HJ, Syddall HE, Dennison EM, Cooper C, Sayer AA. Relationship between customary physical activity, muscle strength and physical performance in older men and women: findings from the Hertfordshire Cohort Study. Age Ageing. 2008;37:589–593. doi: 10.1093/ageing/afn148. [DOI] [PubMed] [Google Scholar]
- 72.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 73.Murgia M, Giorgi C, Pinton P, Rizzuto R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J Mol Cell Cardiol. 2009;46:781–788. doi: 10.1016/j.yjmcc.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 75.Wallace DC, Fan W. Energetics, epigenetics, mitochondrial genetics. Mitochondrion. 2010;10:12–31. doi: 10.1016/j.mito.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Poderoso JJ, Boveris A, Cadenas E. Mitochondrial oxidative stress: a self-propagating process with implications for signaling cascades. Biofactors. 2000;11:43–45. doi: 10.1002/biof.5520110112. [DOI] [PubMed] [Google Scholar]
- 78.Crow MT, Mani K, Nam YJ, Kitsis RN. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- 79.Mattson MP, Kroemer G. Mitochondria in cell death: novel targets for neuroprotection and cardioprotection. Trends Mol Med. 2003;9:196–205. doi: 10.1016/s1471-4914(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 80.Zoratti M, Szabò I. The mitochondrial permeability transition. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 81.Saltin B, Rowell LB. Functional adaptations to physical activity and inactivity. Fed Proc. 1980;39:1506–1513. [PubMed] [Google Scholar]
- 82.Reichmann H, Hoppeler H, Mathieu-Costello O, von Bergen F, Pette D. Biochemical and ultrastructural changes of skeletal muscle mitochondria after chronic electrical stimulation in rabbits. Pflugers Arch. 1985;404:1–9. doi: 10.1007/BF00581484. [DOI] [PubMed] [Google Scholar]
- 83.Dimauro S. A history of mitochondrial diseases. J Inherit Metab Dis. 2011;34:261–276. doi: 10.1007/s10545-010-9082-x. [DOI] [PubMed] [Google Scholar]
- 84.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neder JA, Jones PW, Nery LE, Whipp BJ. Determinants of the exercise endurance capacity in patients with chronic obstructive pulmonary disease. The power-duration relationship. Am J Respir Crit Care Med. 2000;162:497–504. doi: 10.1164/ajrccm.162.2.9907122. [DOI] [PubMed] [Google Scholar]
- 86.Poole DC, Ward SA, Gardner GW, Whipp BJ. Metabolic and respiratory profile of the upper limit for prolonged exercise in man. Ergonomics. 1988;31:1265–1279. doi: 10.1080/00140138808966766. [DOI] [PubMed] [Google Scholar]
- 87.Poole DC, Ward SA, Whipp BJ. The effects of training on the metabolic and respiratory profile of high-intensity cycle ergometer exercise. Eur J Appl Physiol Occup Physiol. 1990;59:421–429. doi: 10.1007/BF02388623. [DOI] [PubMed] [Google Scholar]
- 88.Luo YM, Hart N, Mustfa N, Lyall RA, Polkey MI, Moxham J. Effect of diaphragm fatigue on neural respiratory drive. J Appl Physiol (1985) 2001;90:1691–1699. doi: 10.1152/jappl.2001.90.5.1691. [DOI] [PubMed] [Google Scholar]
- 89.Asmussen E. Muscle fatigue. Med Sci Sports. 1979;11:313–321. [PubMed] [Google Scholar]
- 90.Fujimoto T, Nishizono H. Involvement of membrane excitation failure in fatigue induced by intermittent submaximal voluntary contraction of the first dorsal interosseous muscle. J Sports Med Phys Fitness. 1993;33:107–117. [PubMed] [Google Scholar]
- 91.Westerblad H, Allen DG. Recent advances in the understanding of skeletal muscle fatigue. Curr Opin Rheumatol. 2002;14:648–652. doi: 10.1097/00002281-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 92.Westerblad H, Allen DG.Cellular mechanisms of skeletal muscle fatigue Adv Exp Med Biol 2003538563–570.discussion 571 [DOI] [PubMed] [Google Scholar]
- 93.Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moxham J, Edwards RH, Aubier M, De Troyer A, Farkas G, Macklem PT, Roussos C. Changes in EMG power spectrum (high-to-low ratio) with force fatigue in humans. J Appl Physiol. 1982;53:1094–1099. doi: 10.1152/jappl.1982.53.5.1094. [DOI] [PubMed] [Google Scholar]
- 95.Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586:161–173. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Hultman E, Kaijser L, Chwalbinska-Moneta J, Constantin-Teodosiu D, Macdonald IA, Greenhaff PL. Substrate availability limits human skeletal muscle oxidative ATP regeneration at the onset of ischemic exercise. J Clin Invest. 1998;101:79–85. doi: 10.1172/JCI1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Greenhaff PL, Timmons JA. Interaction between aerobic and anaerobic metabolism during intense muscle contraction. Exerc Sport Sci Rev. 1998;26:1–30. [PubMed] [Google Scholar]
- 98.Putman CT, Spriet LL, Hultman E, Dyck DJ, Heigenhauser GJ. Skeletal muscle pyruvate dehydrogenase activity during acetate infusion in humans. Am J Physiol. 1995;268:E1007–E1017. doi: 10.1152/ajpendo.1995.268.5.E1007. [DOI] [PubMed] [Google Scholar]
- 99.Ludvik B, Mayer G, Stifter S, Putz D, Barnas U, Graf H. Effects of dichloroacetate on exercise performance in healthy volunteers. Pflugers Arch. 1993;423:251–254. doi: 10.1007/BF00374403. [DOI] [PubMed] [Google Scholar]
- 100.Constantin-Teodosiu D, Greenhaff PL. The tricarboxylic acid cycle in human skeletal muscle: is there a role for nutritional intervention? Curr Opin Clin Nutr Metab Care. 1999;2:527–531. doi: 10.1097/00075197-199911000-00017. [DOI] [PubMed] [Google Scholar]
- 101.Eliason G, Abdel-Halim S, Arvidsson B, Kadi F, Piehl-Aulin K. Physical performance and muscular characteristics in different stages of COPD. Scand J Med Sci Sports. 2009;19:865–870. doi: 10.1111/j.1600-0838.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 102.Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, Correa J, Pearce D, Binnie M, Batt J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2010;42:461–471. doi: 10.1165/rcmb.2008-0382OC. [DOI] [PubMed] [Google Scholar]
- 103.Thériault ME, Paré ME, Maltais F, Debigaré R. Satellite cells senescence in limb muscle of severe patients with COPD. PLoS ONE. 2012;7:e39124. doi: 10.1371/journal.pone.0039124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitch WE. Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335:1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 105.Bailey JL, Wang X, England BK, Price SR, Ding X, Mitch WE. The acidosis of chronic renal failure activates muscle proteolysis in rats by augmenting transcription of genes encoding proteins of the ATP-dependent ubiquitin-proteasome pathway. J Clin Invest. 1996;97:1447–1453. doi: 10.1172/JCI118566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hasselgren PO, Fischer JE. Muscle cachexia: current concepts of intracellular mechanisms and molecular regulation. Ann Surg. 2001;233:9–17. doi: 10.1097/00000658-200101000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mitch WE, Bailey JL, Wang X, Jurkovitz C, Newby D, Price SR. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am J Physiol. 1999;276:C1132–C1138. doi: 10.1152/ajpcell.1999.276.5.C1132. [DOI] [PubMed] [Google Scholar]
- 108.Li YP, Reid MB. NF-kappaB mediates the protein loss induced by TNF-alpha in differentiated skeletal muscle myotubes. Am J Physiol. 2000;279:R1165–R1170. doi: 10.1152/ajpregu.2000.279.4.R1165. [DOI] [PubMed] [Google Scholar]
- 109.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294:1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 110.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem. 1996;271:26690–26697. doi: 10.1074/jbc.271.43.26690. [DOI] [PubMed] [Google Scholar]
- 112.Smith IJ, Lecker SH, Hasselgren PO. Calpain activity and muscle wasting in sepsis. Am J Physiol Endocrinol Metab. 2008;295:E762–E771. doi: 10.1152/ajpendo.90226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 2007;6:472–483. doi: 10.1016/j.cmet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 114.Du J, Wang X, Miereles C, Bailey JL, Debigaré R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115–123. doi: 10.1172/JCI200418330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McClung JM, Judge AR, Powers SK, Yan Z. p38 MAPK links oxidative stress to autophagy-related gene expression in cachectic muscle wasting. Am J Physiol Cell Physiol. 2010;298:C542–C549. doi: 10.1152/ajpcell.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 117.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 2007;6:458–471. doi: 10.1016/j.cmet.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 118.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, et al. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 120.Li YP, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hilder TL, Tou JC, Grindeland RE, Wade CE, Graves LM. Phosphorylation of insulin receptor substrate-1 serine 307 correlates with JNK activity in atrophic skeletal muscle. FEBS Lett. 2003;553:63–67. doi: 10.1016/s0014-5793(03)00972-4. [DOI] [PubMed] [Google Scholar]
- 122.Supinski GS, Ji X, Callahan LA. The JNK MAP kinase pathway contributes to the development of endotoxin-induced diaphragm caspase activation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R825–R834. doi: 10.1152/ajpregu.90849.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–C1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- 124.Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol. 2009;297:C1124–C1132. doi: 10.1152/ajpcell.00043.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lokireddy S, Wijesoma IW, Sze SK, McFarlane C, Kambadur R, Sharma M. Identification of atrogin-1-targeted proteins during the myostatin-induced skeletal muscle wasting. Am J Physiol Cell Physiol. 2012;303:C512–C529. doi: 10.1152/ajpcell.00402.2011. [DOI] [PubMed] [Google Scholar]
- 126.Lokireddy S, McFarlane C, Ge X, Zhang H, Sze SK, Sharma M, Kambadur R. Myostatin induces degradation of sarcomeric proteins through a Smad3 signaling mechanism during skeletal muscle wasting. Mol Endocrinol. 2011;25:1936–1949. doi: 10.1210/me.2011-1124. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 127.Vandenburgh HH, Karlisch P, Shansky J, Feldstein R. Insulin and IGF-I induce pronounced hypertrophy of skeletal myofibers in tissue culture. Am J Physiol. 1991;260:C475–C484. doi: 10.1152/ajpcell.1991.260.3.C475. [DOI] [PubMed] [Google Scholar]
- 128.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 129.1983 metropolitan height and weight tables. Stat Bull Metrop Life Found. 1983;64:3–9. [PubMed] [Google Scholar]
- 130.Harrison GG. Height-weight tables. Ann Intern Med. 1985;103:989–994. doi: 10.7326/0003-4819-103-6-989. [DOI] [PubMed] [Google Scholar]
- 131.Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, Sørensen TI, Lange P. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173:79–83. doi: 10.1164/rccm.200506-969OC. [DOI] [PubMed] [Google Scholar]
- 132.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 133.Schutz Y, Kyle UU, Pichard C. Fat-free mass index and fat mass index percentiles in Caucasians aged 18-98 y. Int J Obes Relat Metab Disord. 2002;26:953–960. doi: 10.1038/sj.ijo.0802037. [DOI] [PubMed] [Google Scholar]
- 134.Schols AM, Soeters PB, Dingemans AM, Mostert R, Frantzen PJ, Wouters EF. Prevalence and characteristics of nutritional depletion in patients with stable COPD eligible for pulmonary rehabilitation. Am Rev Respir Dis. 1993;147:1151–1156. doi: 10.1164/ajrccm/147.5.1151. [DOI] [PubMed] [Google Scholar]
- 135.Coronell C, Orozco-Levi M, Gea J.COPD and body weight in a Mediterranean population Clin Nutr 200221437–438.author reply 437–438 [DOI] [PubMed] [Google Scholar]
- 136.Engelen MP, Schols AM, Does JD, Wouters EF. Skeletal muscle weakness is associated with wasting of extremity fat-free mass but not with airflow obstruction in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2000;71:733–738. doi: 10.1093/ajcn/71.3.733. [DOI] [PubMed] [Google Scholar]
- 137.Caron MA, Debigaré R, Dekhuijzen PN, Maltais F. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J Appl Physiol (1985) 2009;107:952–961. doi: 10.1152/japplphysiol.00194.2009. [DOI] [PubMed] [Google Scholar]
- 138.Bernard S, LeBlanc P, Whittom F, Carrier G, Jobin J, Belleau R, Maltais F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158:629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 139.Eisner MD, Blanc PD, Sidney S, Yelin EH, Lathon PV, Katz PP, Tolstykh I, Ackerson L, Iribarren C. Body composition and functional limitation in COPD. Respir Res. 2007;8:7. doi: 10.1186/1465-9921-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Steuten LM, Creutzberg EC, Vrijhoef HJ, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15:84–91. doi: 10.1016/j.pcrj.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guerra S, Sherrill DL, Bobadilla A, Martinez FD, Barbee RA. The relation of body mass index to asthma, chronic bronchitis, and emphysema. Chest. 2002;122:1256–1263. doi: 10.1378/chest.122.4.1256. [DOI] [PubMed] [Google Scholar]
- 142.Franssen FM, O’Donnell DE, Goossens GH, Blaak EE, Schols AM. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63:1110–1117. doi: 10.1136/thx.2007.086827. [DOI] [PubMed] [Google Scholar]
- 143.Mancuso P. Obesity and lung inflammation. J Appl Physiol (1985) 2010;108:722–728. doi: 10.1152/japplphysiol.00781.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rutten EP, Breyer MK, Spruit MA, Hofstra T, van Melick PP, Schols AM, Wouters EF. Abdominal fat mass contributes to the systemic inflammation in chronic obstructive pulmonary disease. Clin Nutr. 2010;29:756–760. doi: 10.1016/j.clnu.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 145.Skyba P, Ukropec J, Pobeha P, Ukropcova B, Joppa P, Kurdiova T, Stroffekova K, Brusik M, Klimes I, Tkac I, et al. Metabolic phenotype and adipose tissue inflammation in patients with chronic obstructive pulmonary disease. Mediators Inflamm. 2010;2010:173498. doi: 10.1155/2010/173498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van den Borst B, Gosker HR, Wesseling G, de Jager W, Hellwig VA, Snepvangers FJ, Schols AM. Low-grade adipose tissue inflammation in patients with mild-to-moderate chronic obstructive pulmonary disease. Am J Clin Nutr. 2011;94:1504–1512. doi: 10.3945/ajcn.111.023911. [DOI] [PubMed] [Google Scholar]
- 147.Marquis K, Maltais F, Duguay V, Bezeau AM, LeBlanc P, Jobin J, Poirier P.The metabolic syndrome in patients with chronic obstructive pulmonary disease J Cardiopulm Rehabil 200525226–232.discussion 233–234 [DOI] [PubMed] [Google Scholar]
- 148.Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, Lam TH, Cheng KK, Adab P. Airflow obstruction and metabolic syndrome: the Guangzhou Biobank Cohort Study. Eur Respir J. 2010;35:317–323. doi: 10.1183/09031936.00024709. [DOI] [PubMed] [Google Scholar]
- 149.Schols AM, Slangen J, Volovics L, Wouters EF. Weight loss is a reversible factor in the prognosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1791–1797. doi: 10.1164/ajrccm.157.6.9705017. [DOI] [PubMed] [Google Scholar]
- 150.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–1861. doi: 10.1164/ajrccm.160.6.9902115. [DOI] [PubMed] [Google Scholar]
- 151.Poulain M, Doucet M, Drapeau V, Fournier G, Tremblay A, Poirier P, Maltais F. Metabolic and inflammatory profile in obese patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2008;5:35–41. doi: 10.1177/1479972307087205. [DOI] [PubMed] [Google Scholar]
- 152.van den Borst B, Gosker HR, Koster A, Yu B, Kritchevsky SB, Liu Y, Meibohm B, Rice TB, Shlipak M, Yende S, et al. Health, Aging, and Body Composition (Health ABC) Study. The influence of abdominal visceral fat on inflammatory pathways and mortality risk in obstructive lung disease. Am J Clin Nutr. 2012;96:516–526. doi: 10.3945/ajcn.112.040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ferguson GT, Calverley PM, Anderson JA, Jenkins CR, Jones PW, Willits LR, Yates JC, Vestbo J, Celli B. Prevalence and progression of osteoporosis in patients with COPD: results from the TOwards a Revolution in COPD Health study. Chest. 2009;136:1456–1465. doi: 10.1378/chest.08-3016. [DOI] [PubMed] [Google Scholar]
- 154.Graat-Verboom L, Wouters EF, Smeenk FW, van den Borne BE, Lunde R, Spruit MA. Current status of research on osteoporosis in COPD: a systematic review. Eur Respir J. 2009;34:209–218. doi: 10.1183/09031936.50130408. [DOI] [PubMed] [Google Scholar]
- 155.Silva DR, Coelho AC, Dumke A, Valentini JD, de Nunes JN, Stefani CL, da Silva Mendes LF, Knorst MM. Osteoporosis prevalence and associated factors in patients with COPD: a cross-sectional study. Respir Care. 2011;56:961–968. doi: 10.4187/respcare.01056. [DOI] [PubMed] [Google Scholar]
- 156.Coin A, Sergi G, Marin S, Vianello A, Perissinotto E, Sarti S, Rinaldi G, Mosele M, Inelmen EM, Enzi G, et al. Predictors of low bone mineral density in elderly males with chronic obstructive pulmonary disease: the role of body mass index. Aging Male. 2010;13:142–147. doi: 10.3109/13685531003657784. [DOI] [PubMed] [Google Scholar]
- 157.Bolton CE, Ionescu AA, Shiels KM, Pettit RJ, Edwards PH, Stone MD, Nixon LS, Evans WD, Griffiths TL, Shale DJ. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:1286–1293. doi: 10.1164/rccm.200406-754OC. [DOI] [PubMed] [Google Scholar]
- 158.Engelen MP, Schols AM, Lamers RJ, Wouters EF. Different patterns of chronic tissue wasting among patients with chronic obstructive pulmonary disease. Clin Nutr. 1999;18:275–280. doi: 10.1016/s0261-5614(98)80024-1. [DOI] [PubMed] [Google Scholar]
- 159.Kurosaki H, Ishii T, Motohashi N, Motegi T, Yamada K, Kudoh S, Jones RC, Kida K. Extent of emphysema on HRCT affects loss of fat-free mass and fat mass in COPD. Intern Med. 2009;48:41–48. doi: 10.2169/internalmedicine.48.1102. [DOI] [PubMed] [Google Scholar]
- 160.Rutten EP, Grydeland TB, Pillai SG, Wagers S, Dirksen A, Coxson HO, Gulsvik A, Wouters EF, Bakke PS. Quantitative CT: associations between emphysema, airway wall thickness and body composition in COPD. Pulm Med. 2011;2011:419328. doi: 10.1155/2011/419328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax. 2007;62:944–949. doi: 10.1136/thx.2007.078980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim HC, Mofarrahi M, Hussain SN. Skeletal muscle dysfunction in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3:637–658. doi: 10.2147/copd.s4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 1998;30:1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 164.Jobin J, Maltais F, Doyon JF, LeBlanc P, Simard PM, Simard AA, Simard C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulm Rehabil. 1998;18:432–437. doi: 10.1097/00008483-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 165.Vogiatzis I, Terzis G, Stratakos G, Cherouveim E, Athanasopoulos D, Spetsioti S, Nasis I, Manta P, Roussos C, Zakynthinos S. Effect of pulmonary rehabilitation on peripheral muscle fiber remodeling in patients with COPD in GOLD stages II to IV. Chest. 2011;140:744–752. doi: 10.1378/chest.10-3058. [DOI] [PubMed] [Google Scholar]
- 166.Larsson L. Histochemical characteristics of human skeletal muscle during aging. Acta Physiol Scand. 1983;117:469–471. doi: 10.1111/j.1748-1716.1983.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 167.Doucet M, Debigaré R, Joanisse DR, Côté C, Leblanc P, Grégoire J, Deslauriers J, Vaillancourt R, Maltais F. Adaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPD. Eur Respir J. 2004;24:971–979. doi: 10.1183/09031936.04.00020204. [DOI] [PubMed] [Google Scholar]
- 168.Gea JG, Pasto M, Carmona MA, Orozco-Levi M, Palomeque J, Broquetas J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J. 2001;17:939–945. doi: 10.1183/09031936.01.17509390. [DOI] [PubMed] [Google Scholar]
- 169.Gosker HR, Engelen MP, van Mameren H, van Dijk PJ, van der Vusse GJ, Wouters EF, Schols AM. Muscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr. 2002;76:113–119. doi: 10.1093/ajcn/76.1.113. [DOI] [PubMed] [Google Scholar]
- 170.Natanek SA, Riddoch-Contreras J, Marsh GS, Hopkinson NS, Man WD, Moxham J, Polkey MI, Kemp PR. Yin Yang 1 expression and localisation in quadriceps muscle in COPD. Arch Bronconeumol. 2011;47:296–302. doi: 10.1016/j.arbres.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 171.Fermoselle C, Rabinovich R, Ausín P, Puig-Vilanova E, Coronell C, Sanchez F, Roca J, Gea J, Barreiro E. Does oxidative stress modulate limb muscle atrophy in severe COPD patients? Eur Respir J. 2012;40:851–862. doi: 10.1183/09031936.00137211. [DOI] [PubMed] [Google Scholar]
- 172.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med. 2004;169:89–96. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 173.Rabinovich RA, Vilaró J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr Opin Pulm Med. 2010;16:123–133. doi: 10.1097/MCP.0b013e328336438d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Eliason G, Abdel-Halim SM, Piehl-Aulin K, Kadi F. Alterations in the muscle-to-capillary interface in patients with different degrees of chronic obstructive pulmonary disease. Respir Res. 2010;11:97. doi: 10.1186/1465-9921-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Gosker HR, Hesselink MK, Duimel H, Ward KA, Schols AM. Reduced mitochondrial density in the vastus lateralis muscle of patients with COPD. Eur Respir J. 2007;30:73–79. doi: 10.1183/09031936.00146906. [DOI] [PubMed] [Google Scholar]
- 176.Puente-Maestu L, Pérez-Parra J, Godoy R, Moreno N, Tejedor A, González-Aragoneses F, Bravo JL, Alvarez FV, Camaño S, Agustí A. Abnormal mitochondrial function in locomotor and respiratory muscles of COPD patients. Eur Respir J. 2009;33:1045–1052. doi: 10.1183/09031936.00112408. [DOI] [PubMed] [Google Scholar]
- 177.Maltais F, LeBlanc P, Whittom F, Simard C, Marquis K, Bélanger M, Breton MJ, Jobin J. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax. 2000;55:848–853. doi: 10.1136/thorax.55.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Green HJ, Bombardier E, Burnett M, Iqbal S, D’Arsigny CL, O’Donnell DE, Ouyang J, Webb KA. Organization of metabolic pathways in vastus lateralis of patients with chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol. 2008;295:R935–R941. doi: 10.1152/ajpregu.00167.2008. [DOI] [PubMed] [Google Scholar]
- 179.Picard M, Godin R, Sinnreich M, Baril J, Bourbeau J, Perrault H, Taivassalo T, Burelle Y. The mitochondrial phenotype of peripheral muscle in chronic obstructive pulmonary disease: disuse or dysfunction? Am J Respir Crit Care Med. 2008;178:1040–1047. doi: 10.1164/rccm.200807-1005OC. [DOI] [PubMed] [Google Scholar]
- 180.Remels AH, Schrauwen P, Broekhuizen R, Willems J, Kersten S, Gosker HR, Schols AM. Peroxisome proliferator-activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J. 2007;30:245–252. doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- 181.Rabinovich RA, Bastos R, Ardite E, Llinàs L, Orozco-Levi M, Gea J, Vilaró J, Barberà JA, Rodríguez-Roisin R, Fernández-Checa JC, et al. Mitochondrial dysfunction in COPD patients with low body mass index. Eur Respir J. 2007;29:643–650. doi: 10.1183/09031936.00086306. [DOI] [PubMed] [Google Scholar]
- 182.Puente-Maestu L, Pérez-Parra J, Godoy R, Moreno N, Tejedor A, Torres A, Lázaro A, Ferreira A, Agustí A. Abnormal transition pore kinetics and cytochrome C release in muscle mitochondria of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2009;40:746–750. doi: 10.1165/rcmb.2008-0289OC. [DOI] [PubMed] [Google Scholar]
- 183.Naimi AI, Bourbeau J, Perrault H, Baril J, Wright-Paradis C, Rossi A, Taivassalo T, Sheel AW, Rabøl R, Dela F, et al. Altered mitochondrial regulation in quadriceps muscles of patients with COPD. Clin Physiol Funct Imaging. 2011;31:124–131. doi: 10.1111/j.1475-097X.2010.00988.x. [DOI] [PubMed] [Google Scholar]
- 184.Gosker HR, Schrauwen P, Hesselink MK, Schaart G, van der Vusse GJ, Wouters EF, Schols AM. Uncoupling protein-3 content is decreased in peripheral skeletal muscle of patients with COPD. Eur Respir J. 2003;22:88–93. doi: 10.1183/09031936.03.00089802. [DOI] [PubMed] [Google Scholar]
- 185.Russell AP, Somm E, Debigaré R, Hartley O, Richard D, Gastaldi G, Melotti A, Michaud A, Giacobino JP, Muzzin P, et al. COPD results in a reduction in UCP3 long mRNA and UCP3 protein content in types I and IIa skeletal muscle fibers. J Cardiopulm Rehabil. 2004;24:332–339. doi: 10.1097/00008483-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 186.Sauleda J, García-Palmer F, Wiesner RJ, Tarraga S, Harting I, Tomás P, Gómez C, Saus C, Palou A, Agustí AG. Cytochrome oxidase activity and mitochondrial gene expression in skeletal muscle of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1413–1417. doi: 10.1164/ajrccm.157.5.9710039. [DOI] [PubMed] [Google Scholar]
- 187.Picard M, Csukly K, Robillard ME, Godin R, Ascah A, Bourcier-Lucas C, Burelle Y. Resistance to Ca2+-induced opening of the permeability transition pore differs in mitochondria from glycolytic and oxidative muscles. Am J Physiol Regul Integr Comp Physiol. 2008;295:R659–R668. doi: 10.1152/ajpregu.90357.2008. [DOI] [PubMed] [Google Scholar]
- 188.Anderson EJ, Neufer PD. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am J Physiol Cell Physiol. 2006;290:C844–C851. doi: 10.1152/ajpcell.00402.2005. [DOI] [PubMed] [Google Scholar]
- 189.Agustí AG, Sauleda J, Miralles C, Gomez C, Togores B, Sala E, Batle S, Busquets X. Skeletal muscle apoptosis and weight loss in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:485–489. doi: 10.1164/rccm.2108013. [DOI] [PubMed] [Google Scholar]
- 190.Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153:288–293. doi: 10.1164/ajrccm.153.1.8542131. [DOI] [PubMed] [Google Scholar]
- 191.Maltais F, Saey D, Debigaré R. Enhancing the benefits of pulmonary rehabilitation: doing more for a few or doing a little less for many? Am J Respir Crit Care Med. 2008;178:215–216. doi: 10.1164/rccm.200804-528ED. [DOI] [PubMed] [Google Scholar]
- 192.Sala E, Roca J, Marrades RM, Alonso J, Gonzalez De Suso JM, Moreno A, Barberá JA, Nadal J, de Jover L, Rodriguez-Roisin R, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:1726–1734. doi: 10.1164/ajrccm.159.6.9804136. [DOI] [PubMed] [Google Scholar]
- 193.Vilaro J, Rabinovich R, Gonzalez-deSuso JM, Troosters T, Rodríguez D, Barberà JA, Roca J. Clinical assessment of peripheral muscle function in patients with chronic obstructive pulmonary disease. Am J Phys Med Rehabil. 2009;88:39–46. doi: 10.1097/PHM.0b013e31818dff86. [DOI] [PubMed] [Google Scholar]
- 194.Debigaré R, Côté CH, Maltais F. Peripheral muscle wasting in chronic obstructive pulmonary disease. Clinical relevance and mechanisms. Am J Respir Crit Care Med. 2001;164:1712–1717. doi: 10.1164/ajrccm.164.9.2104035. [DOI] [PubMed] [Google Scholar]
- 195.Green HJ, Burnett ME, D’Arsigny CL, O’Donnell DE, Ouyang J, Webb KA. Altered metabolic and transporter characteristics of vastus lateralis in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2008;105:879–886. doi: 10.1152/japplphysiol.90458.2008. [DOI] [PubMed] [Google Scholar]
- 196.Saey D, Lemire BB, Gagnon P, Bombardier E, Tupling AR, Debigaré R, Côté CH, Maltais F. Quadriceps metabolism during constant workrate cycling exercise in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2011;110:116–124. doi: 10.1152/japplphysiol.00153.2010. [DOI] [PubMed] [Google Scholar]
- 197.Fiaccadori E, Del Canale S, Vitali P, Coffrini E, Ronda N, Guariglia A. Skeletal muscle energetics, acid-base equilibrium and lactate metabolism in patients with severe hypercapnia and hypoxemia. Chest. 1987;92:883–887. doi: 10.1378/chest.92.5.883. [DOI] [PubMed] [Google Scholar]
- 198.Green HJ, Bombardier E, Duhamel TA, Stewart RD, Tupling AR, Ouyang J. Metabolic, enzymatic, and transporter responses in human muscle during three consecutive days of exercise and recovery. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1238–R1250. doi: 10.1152/ajpregu.00171.2008. [DOI] [PubMed] [Google Scholar]
- 199.Jakobsson P, Jorfeldt L, Henriksson J. Metabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151:374–377. doi: 10.1164/ajrccm.151.2.7842194. [DOI] [PubMed] [Google Scholar]
- 200.Turan N, Kalko S, Stincone A, Clarke K, Sabah A, Howlett K, Curnow SJ, Rodriguez DA, Cascante M, O’Neill L, et al. A systems biology approach identifies molecular networks defining skeletal muscle abnormalities in chronic obstructive pulmonary disease. PLOS Comput Biol. 2011;7:e1002129. doi: 10.1371/journal.pcbi.1002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Barreiro E, Rabinovich R, Marin-Corral J, Barberà JA, Gea J, Roca J. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax. 2009;64:13–19. doi: 10.1136/thx.2008.105163. [DOI] [PubMed] [Google Scholar]
- 202.Rodriguez DA, Alcarraz-Vizán G, Diaz-Moralli S, Reed M, Gomez FP, Falciani F, Gunther U, Roca J, Cascante M. Plasma metabolic profile in COPD patients: effects of exercise and endurance training. Metabolomics. 2012;8:508–516. [Google Scholar]
- 203.Richardson RS, Sheldon J, Poole DC, Hopkins SR, Ries AL, Wagner PD. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:881–885. doi: 10.1164/ajrccm.159.3.9803049. [DOI] [PubMed] [Google Scholar]
- 204.Layec G, Haseler LJ, Hoff J, Richardson RS. Evidence that a higher ATP cost of muscular contraction contributes to the lower mechanical efficiency associated with COPD: preliminary findings. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1142–R1147. doi: 10.1152/ajpregu.00835.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Schols AM, Fredrix EW, Soeters PB, Westerterp KR, Wouters EF. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;54:983–987. doi: 10.1093/ajcn/54.6.983. [DOI] [PubMed] [Google Scholar]
- 206.Baarends EM, Schols AM, Westerterp KR, Wouters EF. Total daily energy expenditure relative to resting energy expenditure in clinically stable patients with COPD. Thorax. 1997;52:780–785. doi: 10.1136/thx.52.9.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kao CC, Hsu JW, Bandi V, Hanania NA, Kheradmand F, Jahoor F. Resting energy expenditure and protein turnover are increased in patients with severe chronic obstructive pulmonary disease. Metabolism. 2011;60:1449–1455. doi: 10.1016/j.metabol.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cavalheri V, Donária L, Ferreira T, Finatti M, Camillo CA, Cipulo Ramos EM, Pitta F. Energy expenditure during daily activities as measured by two motion sensors in patients with COPD. Respir Med. 2011;105:922–929. doi: 10.1016/j.rmed.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 209.Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don’t. J Appl Physiol (1985) 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 210.Javeshghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med. 2002;165:412–418. doi: 10.1164/ajrccm.165.3.2103028. [DOI] [PubMed] [Google Scholar]
- 211.Jackson MJ, Pye D, Palomero J. The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol (1985) 2007;102:1664–1670. doi: 10.1152/japplphysiol.01102.2006. [DOI] [PubMed] [Google Scholar]
- 212.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–R344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 213.Supinski G. Free radical induced respiratory muscle dysfunction. Mol Cell Biochem. 1998;179:99–110. doi: 10.1023/a:1006859920875. [DOI] [PubMed] [Google Scholar]
- 214.Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 215.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 216.de Batlle J, Barreiro E, Romieu I, Mendez M, Gómez FP, Balcells E, Ferrer J, Orozco-Levi M, Gea J, Antó JM, et al. Dietary modulation of oxidative stress in chronic obstructive pulmonary disease patients. Free Radic Res. 2010;44:1296–1303. doi: 10.3109/10715762.2010.500667. [DOI] [PubMed] [Google Scholar]
- 217.Rodriguez DA, Kalko S, Puig-Vilanova E, Perez-Olabarría M, Falciani F, Gea J, Cascante M, Barreiro E, Roca J. Muscle and blood redox status after exercise training in severe COPD patients. Free Radic Biol Med. 2012;52:88–94. doi: 10.1016/j.freeradbiomed.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 218.Ribera F, N’Guessan B, Zoll J, Fortin D, Serrurier B, Mettauer B, Bigard X, Ventura-Clapier R, Lampert E. Mitochondrial electron transport chain function is enhanced in inspiratory muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:873–879. doi: 10.1164/rccm.200206-519OC. [DOI] [PubMed] [Google Scholar]
- 219.Allaire J, Maltais F, LeBlanc P, Simard PM, Whittom F, Doyon JF, Simard C, Jobin J. Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Muscle Nerve. 2002;25:383–389. doi: 10.1002/mus.10039. [DOI] [PubMed] [Google Scholar]
- 220.Barreiro E, Gea J, Corominas JM, Hussain SN. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2003;29:771–778. doi: 10.1165/rcmb.2003-0138OC. [DOI] [PubMed] [Google Scholar]
- 221.Barreiro E, de la Puente B, Minguella J, Corominas JM, Serrano S, Hussain SN, Gea J. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1116–1124. doi: 10.1164/rccm.200407-887OC. [DOI] [PubMed] [Google Scholar]
- 222.Barreiro E, Gea J, Matar G, Hussain SN. Expression and carbonylation of creatine kinase in the quadriceps femoris muscles of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2005;33:636–642. doi: 10.1165/rcmb.2005-0114OC. [DOI] [PubMed] [Google Scholar]
- 223.Barreiro E, Schols AM, Polkey MI, Galdiz JB, Gosker HR, Swallow EB, Coronell C, Gea J ENIGMA in COPD project. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax. 2008;63:100–107. doi: 10.1136/thx.2007.078030. [DOI] [PubMed] [Google Scholar]
- 224.Couillard A, Maltais F, Saey D, Debigaré R, Michaud A, Koechlin C, LeBlanc P, Préfaut C. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1664–1669. doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- 225.Engelen MP, Schols AM, Does JD, Deutz NE, Wouters EF. Altered glutamate metabolism is associated with reduced muscle glutathione levels in patients with emphysema. Am J Respir Crit Care Med. 2000;161:98–103. doi: 10.1164/ajrccm.161.1.9901031. [DOI] [PubMed] [Google Scholar]
- 226.Heunks LM, Viña J, van Herwaarden CL, Folgering HT, Gimeno A, Dekhuijzen PN. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol. 1999;277:R1697–R1704. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- 227.Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Préfaut C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2004;169:1022–1027. doi: 10.1164/rccm.200310-1465OC. [DOI] [PubMed] [Google Scholar]
- 228.Koechlin C, Couillard A, Cristol JP, Chanez P, Hayot M, Le Gallais D, Préfaut C. Does systemic inflammation trigger local exercise-induced oxidative stress in COPD? Eur Respir J. 2004;23:538–544. doi: 10.1183/09031936.04.00069004. [DOI] [PubMed] [Google Scholar]
- 229.Koechlin C, Maltais F, Saey D, Michaud A, LeBlanc P, Hayot M, Préfaut C. Hypoxaemia enhances peripheral muscle oxidative stress in chronic obstructive pulmonary disease. Thorax. 2005;60:834–841. doi: 10.1136/thx.2004.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Marin-Corral J, Minguella J, Ramírez-Sarmiento AL, Hussain SN, Gea J, Barreiro E. Oxidised proteins and superoxide anion production in the diaphragm of severe COPD patients. Eur Respir J. 2009;33:1309–1319. doi: 10.1183/09031936.00072008. [DOI] [PubMed] [Google Scholar]
- 231.Rabinovich RA, Ardite E, Troosters T, Carbó N, Alonso J, Gonzalez de Suso JM, Vilaró J, Barberà JA, Polo MF, Argilés JM, et al. Reduced muscle redox capacity after endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:1114–1118. doi: 10.1164/ajrccm.164.7.2103065. [DOI] [PubMed] [Google Scholar]
- 232.Van Helvoort HA, Heijdra YF, Thijs HM, Viña J, Wanten GJ, Dekhuijzen PN. Exercise-induced systemic effects in muscle-wasted patients with COPD. Med Sci Sports Exerc. 2006;38:1543–1552. doi: 10.1249/01.mss.0000228331.13123.53. [DOI] [PubMed] [Google Scholar]
- 233.Barreiro E, Peinado VI, Galdiz JB, Ferrer E, Marin-Corral J, Sánchez F, Gea J, Barberà JA ENIGMA in COPD Project. Cigarette smoke-induced oxidative stress: A role in chronic obstructive pulmonary disease skeletal muscle dysfunction. Am J Respir Crit Care Med. 2010;182:477–488. doi: 10.1164/rccm.200908-1220OC. [DOI] [PubMed] [Google Scholar]
- 234.Barreiro E, del Puerto-Nevado L, Puig-Vilanova E, Pérez-Rial S, Sánchez F, Martínez-Galán L, Rivera S, Gea J, González-Mangado N, Peces-Barba G. Cigarette smoke-induced oxidative stress in skeletal muscles of mice. Respir Physiol Neurobiol. 2012;182:9–17. doi: 10.1016/j.resp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 235.Puente-Maestu L, Tena T, Trascasa C, Pérez-Parra J, Godoy R, García MJ, Stringer WW. Training improves muscle oxidative capacity and oxygenation recovery kinetics in patients with chronic obstructive pulmonary disease. Eur J Appl Physiol. 2003;88:580–587. doi: 10.1007/s00421-002-0743-9. [DOI] [PubMed] [Google Scholar]
- 236.Barreiro E, Ferrer D, Sanchez F, Minguella J, Marin-Corral J, Martinez-Llorens J, Lloreta J, Gea J. Inflammatory cells and apoptosis in respiratory and limb muscles of patients with COPD. J Appl Physiol (1985) 2011;111:808–817. doi: 10.1152/japplphysiol.01017.2010. [DOI] [PubMed] [Google Scholar]
- 237.Clark CJ, Cochrane LM, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J. 2000;15:92–97. doi: 10.1183/09031936.00.15109200. [DOI] [PubMed] [Google Scholar]
- 238.Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2000;20:353–360. doi: 10.1097/00008483-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 239.Debigaré R, Côte CH, Hould FS, LeBlanc P, Maltais F. In vitro and in vivo contractile properties of the vastus lateralis muscle in males with COPD. Eur Respir J. 2003;21:273–278. doi: 10.1183/09031936.03.00036503. [DOI] [PubMed] [Google Scholar]
- 240.Mador MJ, Bozkanat E, Kufel TJ. Quadriceps fatigue after cycle exercise in patients with COPD compared with healthy control subjects. Chest. 2003;123:1104–1111. doi: 10.1378/chest.123.4.1104. [DOI] [PubMed] [Google Scholar]
- 241.Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, Bouillon R, Decramer M. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58:752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Allaire J, Maltais F, Doyon JF, Noël M, LeBlanc P, Carrier G, Simard C, Jobin J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax. 2004;59:673–678. doi: 10.1136/thx.2003.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Coronell C, Orozco-Levi M, Méndez R, Ramírez-Sarmiento A, Gáldiz JB, Gea J. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J. 2004;24:129–136. doi: 10.1183/09031936.04.00079603. [DOI] [PubMed] [Google Scholar]
- 244.Franssen FM, Broekhuizen R, Janssen PP, Wouters EF, Schols AM. Limb muscle dysfunction in COPD: effects of muscle wasting and exercise training. Med Sci Sports Exerc. 2005;37:2–9. doi: 10.1249/01.mss.0000150082.59155.4f. [DOI] [PubMed] [Google Scholar]
- 245.Man WD, Hopkinson NS, Harraf F, Nikoletou D, Polkey MI, Moxham J. Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax. 2005;60:718–722. doi: 10.1136/thx.2005.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Vivodtzev I, Flore P, Lévy P, Wuyam B. Voluntary activation during knee extensions in severely deconditioned patients with chronic obstructive pulmonary disease: benefit of endurance training. Muscle Nerve. 2008;37:27–35. doi: 10.1002/mus.20867. [DOI] [PubMed] [Google Scholar]
- 247.Seymour JM, Ward K, Sidhu PS, Puthucheary Z, Steier J, Jolley CJ, Rafferty G, Polkey MI, Moxham J. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64:418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 248.Seymour JM, Spruit MA, Hopkinson NS, Natanek SA, Man WD, Jackson A, Gosker HR, Schols AM, Moxham J, Polkey MI, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36:81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Man WD, Soliman MG, Nikoletou D, Harris ML, Rafferty GF, Mustfa N, Polkey MI, Moxham J. Non-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax. 2003;58:665–669. doi: 10.1136/thorax.58.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Man WD, Soliman MG, Gearing J, Radford SG, Rafferty GF, Gray BJ, Polkey MI, Moxham J. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:562–567. doi: 10.1164/rccm.200302-162OC. [DOI] [PubMed] [Google Scholar]
- 251.Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11–16. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 252.Hopkinson NS, Man WD, Dayer MJ, Ross ET, Nickol AH, Hart N, Moxham J, Polkey MI. Acute effect of oral steroids on muscle function in chronic obstructive pulmonary disease. Eur Respir J. 2004;24:137–142. doi: 10.1183/09031936.04.00139003. [DOI] [PubMed] [Google Scholar]
- 253.Heijdra YF, Pinto-Plata V, Frants R, Rassulo J, Kenney L, Celli BR. Muscle strength and exercise kinetics in COPD patients with a normal fat-free mass index are comparable to control subjects. Chest. 2003;124:75–82. doi: 10.1378/chest.124.1.75. [DOI] [PubMed] [Google Scholar]
- 254.Couillard A, Koechlin C, Cristol JP, Varray A, Préfaut C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20:1123–1129. doi: 10.1183/09031936.02.00014302. [DOI] [PubMed] [Google Scholar]
- 255.Janaudis-Ferreira T, Wadell K, Sundelin G, Lindström B. Thigh muscle strength and endurance in patients with COPD compared with healthy controls. Respir Med. 2006;100:1451–1457. doi: 10.1016/j.rmed.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 256.Serres I, Gautier V, Varray A, Préfaut C. Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest. 1998;113:900–905. doi: 10.1378/chest.113.4.900. [DOI] [PubMed] [Google Scholar]
- 257.Van’t Hul A, Harlaar J, Gosselink R, Hollander P, Postmus P, Kwakkel G. Quadriceps muscle endurance in patients with chronic obstructive pulmonary disease. Muscle Nerve. 2004;29:267–274. doi: 10.1002/mus.10552. [DOI] [PubMed] [Google Scholar]
- 258.Swallow EB, Gosker HR, Ward KA, Moore AJ, Dayer MJ, Hopkinson NS, Schols AM, Moxham J, Polkey MI. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol (1985) 2007;103:739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- 259.Gouzi F, Préfaut C, Abdellaoui A, Vuillemin A, Molinari N, Ninot G, Caris G, Hayot M.Evidence of an early physical activity reduction in chronic obstructive pulmonary disease patients Arch Phys Med Rehabil 2011921611–1617.e2 [DOI] [PubMed] [Google Scholar]
- 260.van den Borst B, Slot IG, Hellwig VA, Vosse BA, Kelders MC, Barreiro E, Schols AM, Gosker HR. Loss of quadriceps muscle oxidative phenotype and decreased endurance in patients with mild-to-moderate COPD. J Appl Physiol. 2013;114:1319–1328. doi: 10.1152/japplphysiol.00508.2012. [DOI] [PubMed] [Google Scholar]
- 261.Newell SZ, McKenzie DK, Gandevia SC. Inspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitation. Thorax. 1989;44:903–912. doi: 10.1136/thx.44.11.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Zattara-Hartmann MC, Badier M, Guillot C, Tomei C, Jammes Y. Maximal force and endurance to fatigue of respiratory and skeletal muscles in chronic hypoxemic patients: the effects of oxygen breathing. Muscle Nerve. 1995;18:495–502. doi: 10.1002/mus.880180504. [DOI] [PubMed] [Google Scholar]
- 263.Killian KJ, Leblanc P, Martin DH, Summers E, Jones NL, Campbell EJ. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis. 1992;146:935–940. doi: 10.1164/ajrccm/146.4.935. [DOI] [PubMed] [Google Scholar]
- 264.Butcher SJ, Lagerquist O, Marciniuk DD, Petersen SR, Collins DF, Jones RL. Relationship between ventilatory constraint and muscle fatigue during exercise in COPD. Eur Respir J. 2009;33:763–770. doi: 10.1183/09031936.00014708. [DOI] [PubMed] [Google Scholar]
- 265.Mador MJ, Kufel TJ, Pineda LA, Steinwald A, Aggarwal A, Upadhyay AM, Khan MA. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am J Respir Crit Care Med. 2001;163:930–935. doi: 10.1164/ajrccm.163.4.2006125. [DOI] [PubMed] [Google Scholar]
- 266.Saey D, Debigaré R, LeBlanc P, Mador MJ, Côté CH, Jobin J, Maltais F. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:425–430. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 267.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol. 2010;299:R314–R324. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve. 1996;19:549–555. doi: 10.1002/(SICI)1097-4598(199605)19:5<549::AID-MUS1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 269.Saey D, Côté CH, Mador MJ, Laviolette L, LeBlanc P, Jobin J, Maltais F. Assessment of muscle fatigue during exercise in chronic obstructive pulmonary disease. Muscle Nerve. 2006;34:62–71. doi: 10.1002/mus.20541. [DOI] [PubMed] [Google Scholar]
- 270.Pepin V, Saey D, Whittom F, LeBlanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1517–1522. doi: 10.1164/rccm.200507-1037OC. [DOI] [PubMed] [Google Scholar]
- 271.Marquis N, Debigaré R, Bouyer L, Saey D, Laviolette L, Brouillard C, Maltais F. Physiology of walking in patients with moderate to severe chronic obstructive pulmonary disease. Med Sci Sports Exerc. 2009;41:1540–1548. doi: 10.1249/MSS.0b013e31819c717f. [DOI] [PubMed] [Google Scholar]
- 272.Gagnon P, Maltais F, Bouyer L, Ribeiro F, Coats V, Brouillard C, Noël M, Rousseau-Gagnon M, Saey D. Distal leg muscle function in patients with COPD. COPD. 2013;10:235–242. doi: 10.3109/15412555.2012.719047. [DOI] [PubMed] [Google Scholar]
- 273.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 274.Crul T, Spruit MA, Gayan-Ramirez G, Quarck R, Gosselink R, Troosters T, Pitta F, Decramer M. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest. 2007;37:897–904. doi: 10.1111/j.1365-2362.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 275.Vilaró J, Ramirez-Sarmiento A, Martínez-Llorens JM, Mendoza T, Alvarez M, Sánchez-Cayado N, Vega A, Gimeno E, Coronell C, Gea J, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med. 2010;104:1896–1902. doi: 10.1016/j.rmed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 276.Crul T, Testelmans D, Spruit MA, Troosters T, Gosselink R, Geeraerts I, Decramer M, Gayan-Ramirez G. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell Physiol Biochem. 2010;25:491–500. doi: 10.1159/000303054. [DOI] [PubMed] [Google Scholar]
- 277.Vermeeren MA, Schols AM, Wouters EF. Effects of an acute exacerbation on nutritional and metabolic profile of patients with COPD. Eur Respir J. 1997;10:2264–2269. doi: 10.1183/09031936.97.10102264. [DOI] [PubMed] [Google Scholar]
- 278.Vermeeren MA, Wouters EF, Geraerts-Keeris AJ, Schols AM. Nutritional support in patients with chronic obstructive pulmonary disease during hospitalization for an acute exacerbation; a randomized controlled feasibility trial. Clin Nutr. 2004;23:1184–1192. doi: 10.1016/j.clnu.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 279.Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:446–452. doi: 10.1164/rccm.200408-1054OC. [DOI] [PubMed] [Google Scholar]
- 280.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, Maltais F. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 281.Schols AM, Wouters EF, Soeters PB, Westerterp KR. Body composition by bioelectrical-impedance analysis compared with deuterium dilution and skinfold anthropometry in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1991;53:421–424. doi: 10.1093/ajcn/53.2.421. [DOI] [PubMed] [Google Scholar]
- 282.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, Moore AJ, Moxham J, Polkey MI. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Jones NL, Killian KJ. Cherniack NS. Philadelphia: W.B. Saunders; 1991. Limitation of exercise in chronic airway obstruction; Chronic obstructive pulmonary disease; pp. 196–206. [Google Scholar]
- 284.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153:976–980. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 285.Hamilton AL, Killian KJ, Summers E, Jones NL. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med. 1995;152:2021–2031. doi: 10.1164/ajrccm.152.6.8520771. [DOI] [PubMed] [Google Scholar]
- 286.Williams TJ, Patterson GA, McClean PA, Zamel N, Maurer JR. Maximal exercise testing in single and double lung transplant recipients. Am Rev Respir Dis. 1992;145:101–105. doi: 10.1164/ajrccm/145.1.101. [DOI] [PubMed] [Google Scholar]
- 287.Deschênes D, Pepin V, Saey D, LeBlanc P, Maltais F. Locus of symptom limitation and exercise response to bronchodilation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2008;28:208–214. doi: 10.1097/01.HCR.0000320074.73846.3b. [DOI] [PubMed] [Google Scholar]
- 288.Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 289.Maltais F, Bernard S, Jobin J, Belleau R, LeBlanc P. Lactate kinetics during exercise in chronic obstructive pulmonary disease. Can Respir J. 1997;4:251–257. [Google Scholar]
- 290.Maltais F, Jobin J, Sullivan MJ, Bernard S, Whittom F, Killian KJ, Desmeules M, Bélanger M, LeBlanc P. Metabolic and hemodynamic responses of lower limb during exercise in patients with COPD. J Appl Physiol (1985) 1998;84:1573–1580. doi: 10.1152/jappl.1998.84.5.1573. [DOI] [PubMed] [Google Scholar]
- 291.Steiner MC, Evans R, Deacon SJ, Singh SJ, Patel P, Fox J, Greenhaff PL, Morgan MD. Adenine nucleotide loss in the skeletal muscles during exercise in chronic obstructive pulmonary disease. Thorax. 2005;60:932–936. doi: 10.1136/thx.2004.038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Calvert LD, Singh SJ, Greenhaff PL, Morgan MD, Steiner MC. The plasma ammonia response to cycle exercise in COPD. Eur Respir J. 2008;31:751–758. doi: 10.1183/09031936.00164106. [DOI] [PubMed] [Google Scholar]
- 293.Calvert LD, Shelley R, Singh SJ, Greenhaff PL, Bankart J, Morgan MD, Steiner MC. Dichloroacetate enhances performance and reduces blood lactate during maximal cycle exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177:1090–1094. doi: 10.1164/rccm.200707-1032OC. [DOI] [PubMed] [Google Scholar]
- 294.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Gagnon P, Bussières JS, Ribeiro F, Gagnon SL, Saey D, Gagné N, Provencher S, Maltais F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:606–615. doi: 10.1164/rccm.201203-0404OC. [DOI] [PubMed] [Google Scholar]
- 296.Amann M, Proctor LT, Sebranek JJ, Eldridge MW, Pegelow DF, Dempsey JA. Somatosensory feedback from the limbs exerts inhibitory influences on central neural drive during whole body endurance exercise. J Appl Physiol (1985) 2008;105:1714–1724. doi: 10.1152/japplphysiol.90456.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Noakes TD, St Clair Gibson A, Lambert EV. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sports Med. 2005;39:120–124. doi: 10.1136/bjsm.2003.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S, Maltais F. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol (1985) 2009;107:832–840. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]
- 299.Burtin C, Saey D, Saglam M, Langer D, Gosselink R, Janssens W, Decramer M, Maltais F, Troosters T. Effectiveness of exercise training in patients with COPD: the role of muscle fatigue. Eur Respir J. 2012;40:338–344. doi: 10.1183/09031936.00111811. [DOI] [PubMed] [Google Scholar]
- 300.Mostert R, Goris A, Weling-Scheepers C, Wouters EF, Schols AM. Tissue depletion and health related quality of life in patients with chronic obstructive pulmonary disease. Respir Med. 2000;94:859–867. doi: 10.1053/rmed.2000.0829. [DOI] [PubMed] [Google Scholar]
- 301.Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J. 1997;10:417–423. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 302.Bossenbroek L, de Greef MH, Wempe JB, Krijnen WP, Ten Hacken NH. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8:306–319. doi: 10.3109/15412555.2011.578601. [DOI] [PubMed] [Google Scholar]
- 303.Booth FW, Gollnick PD. Effects of disuse on the structure and function of skeletal muscle. Med Sci Sports Exerc. 1983;15:415–420. [PubMed] [Google Scholar]
- 304.Larsson L, Ansved T. Effects of long-term physical training and detraining on enzyme histochemical and functional skeletal muscle characteristic in man. Muscle Nerve. 1985;8:714–722. doi: 10.1002/mus.880080815. [DOI] [PubMed] [Google Scholar]
- 305.Coyle EF, Martin WH, III, Bloomfield SA, Lowry OH, Holloszy JO. Effects of detraining on responses to submaximal exercise. J Appl Physiol (1985) 1985;59:853–859. doi: 10.1152/jappl.1985.59.3.853. [DOI] [PubMed] [Google Scholar]
- 306.Casaburi R. Deconditioning. Pulmonary rehabilitation. In: Fishman AP, editor. New York: Marcel Dekker; 1996. pp. 213–230. [Google Scholar]
- 307.Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease. Clin Med. 2006;6:190–196. doi: 10.7861/clinmedicine.6-2-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Couillard A, Préfaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719. doi: 10.1183/09031936.05.00139904. [DOI] [PubMed] [Google Scholar]
- 309.Polkey MI, Rabe KF. Chicken or egg: physical activity in COPD revisited. Eur Respir J. 2009;33:227–229. doi: 10.1183/09031936.00176808. [DOI] [PubMed] [Google Scholar]
- 310.Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease: an update. Clin Med. 2011;11:461–464. doi: 10.7861/clinmedicine.11-5-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11:681–686. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 312.Brønstad E, Rognmo O, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Håberg AK, Bjørk Ingul C, Wisløff U, Steinshamn S. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J. 2012;40:1130–1136. doi: 10.1183/09031936.00193411. [DOI] [PubMed] [Google Scholar]
- 313.Man WD, Kemp P, Moxham J, Polkey MI. Exercise and muscle dysfunction in COPD: implications for pulmonary rehabilitation. Clin Sci (Lond) 2009;117:281–291. doi: 10.1042/CS20080660. [DOI] [PubMed] [Google Scholar]
- 314.Troosters T, Gosselink R, Decramer M. Short- and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med. 2000;109:207–212. doi: 10.1016/s0002-9343(00)00472-1. [DOI] [PubMed] [Google Scholar]
- 315.Gosker HR, Schrauwen P, Broekhuizen R, Hesselink MK, Moonen-Kornips E, Ward KA, Franssen FM, Wouters EF, Schols AM. Exercise training restores uncoupling protein-3 content in limb muscles of patients with chronic obstructive pulmonary disease. Am J Physiol Endocrinol Metab. 2006;290:E976–E981. doi: 10.1152/ajpendo.00336.2005. [DOI] [PubMed] [Google Scholar]
- 316.Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, Da X, Casaburi R. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol (1985) 2007;103:1299–1310. doi: 10.1152/japplphysiol.00150.2007. [DOI] [PubMed] [Google Scholar]
- 317.Howald H, Hoppeler H, Claassen H, Mathieu O, Straub R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflugers Arch. 1985;403:369–376. doi: 10.1007/BF00589248. [DOI] [PubMed] [Google Scholar]
- 318.Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, Meier CA, Bell DR, Kralli A, Giacobino JP, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 319.Simoneau JA, Lortie G, Boulay MR, Marcotte M, Thibault MC, Bouchard C. Human skeletal muscle fiber type alteration with high-intensity intermittent training. Eur J Appl Physiol Occup Physiol. 1985;54:250–253. doi: 10.1007/BF00426141. [DOI] [PubMed] [Google Scholar]
- 320.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–546. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rehn TA, Munkvik M, Lunde PK, Sjaastad I, Sejersted OM. Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease-specific myopathy or a result of deconditioning? Heart Fail Rev. 2012;17:421–436. doi: 10.1007/s10741-011-9289-4. [DOI] [PubMed] [Google Scholar]
- 322.Späte U, Schulze PC. Proinflammatory cytokines and skeletal muscle. Curr Opin Clin Nutr Metab Care. 2004;7:265–269. doi: 10.1097/00075197-200405000-00005. [DOI] [PubMed] [Google Scholar]
- 323.Siu PM. Muscle apoptotic response to denervation, disuse, and aging. Med Sci Sports Exerc. 2009;41:1876–1886. doi: 10.1249/MSS.0b013e3181a6470b. [DOI] [PubMed] [Google Scholar]
- 324.Harris J. Autophagy and cytokines. Cytokine. 2011;56:140–144. doi: 10.1016/j.cyto.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 325.Doucet M, Russell AP, Léger B, Debigaré R, Joanisse DR, Caron MA, LeBlanc P, Maltais F. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 326.Koncarevic A, Jackman RW, Kandarian SC. The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J. 2007;21:427–437. doi: 10.1096/fj.06-6665com. [DOI] [PubMed] [Google Scholar]
- 327.Vasyutina E, Lenhard DC, Birchmeier C. Notch function in myogenesis. Cell Cycle. 2007;6:1451–1454. [PubMed] [Google Scholar]
- 328.Hurst JR, Donaldson GC, Perera WR, Wilkinson TM, Bilello JA, Hagan GW, Vessey RS, Wedzicha JA. Use of plasma biomarkers at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;174:867–874. doi: 10.1164/rccm.200604-506OC. [DOI] [PubMed] [Google Scholar]
- 329.Karadag F, Karul AB, Cildag O, Yilmaz M, Ozcan H. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung. 2008;186:403–409. doi: 10.1007/s00408-008-9106-6. [DOI] [PubMed] [Google Scholar]
- 330.Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-alpha system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 331.Montes de Oca M, Torres SH, De Sanctis J, Mata A, Hernández N, Tálamo C. Skeletal muscle inflammation and nitric oxide in patients with COPD. Eur Respir J. 2005;26:390–397. doi: 10.1183/09031936.05.00107404. [DOI] [PubMed] [Google Scholar]
- 332.Gosker HR, Kubat B, Schaart G, van der Vusse GJ, Wouters EF, Schols AM. Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. Eur Respir J. 2003;22:280–285. doi: 10.1183/09031936.03.00012803. [DOI] [PubMed] [Google Scholar]
- 333.Debigaré R, Maltais F, Côté CH, Michaud A, Caron MA, Mofarrahi M, Leblanc P, Hussain SN. Profiling of mRNA expression in quadriceps of patients with COPD and muscle wasting. COPD. 2008;5:75–84. doi: 10.1080/15412550801940457. [DOI] [PubMed] [Google Scholar]
- 334.Rabinovich RA, Figueras M, Ardite E, Carbó N, Troosters T, Filella X, Barberà JA, Fernandez-Checa JC, Argilés JM, Roca J. Increased tumour necrosis factor-alpha plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J. 2003;21:789–794. doi: 10.1183/09031936.03.00042702. [DOI] [PubMed] [Google Scholar]
- 335.Agustí A, Morlá M, Sauleda J, Saus C, Busquets X. NF-kappaB activation and iNOS upregulation in skeletal muscle of patients with COPD and low body weight. Thorax. 2004;59:483–487. doi: 10.1136/thx.2003.017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Moylan JS, Reid MB. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve. 2007;35:411–429. doi: 10.1002/mus.20743. [DOI] [PubMed] [Google Scholar]
- 337.Stanojkovic I, Kotur-Stevuljevic J, Milenkovic B, Spasic S, Vujic T, Stefanovic A, Llic A, Ivanisevic J. Pulmonary function, oxidative stress and inflammatory markers in severe COPD exacerbation. Respir Med. 2011;105:S31–S37. doi: 10.1016/S0954-6111(11)70008-7. [DOI] [PubMed] [Google Scholar]
- 338.Li YP, Chen Y, Li AS, Reid MB. Hydrogen peroxide stimulates ubiquitin-conjugating activity and expression of genes for specific E2 and E3 proteins in skeletal muscle myotubes. Am J Physiol Cell Physiol. 2003;285:C806–C812. doi: 10.1152/ajpcell.00129.2003. [DOI] [PubMed] [Google Scholar]
- 339.Li YP, Schwartz RJ, Waddell ID, Holloway BR, Reid MB. Skeletal muscle myocytes undergo protein loss and reactive oxygen-mediated NF-kappaB activation in response to tumor necrosis factor alpha. FASEB J. 1998;12:871–880. doi: 10.1096/fasebj.12.10.971. [DOI] [PubMed] [Google Scholar]
- 340.Buck M, Chojkier M. Muscle wasting and dedifferentiation induced by oxidative stress in a murine model of cachexia is prevented by inhibitors of nitric oxide synthesis and antioxidants. EMBO J. 1996;15:1753–1765. [PMC free article] [PubMed] [Google Scholar]
- 341.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 342.Lemire BB, Debigaré R, Dubé A, Thériault ME, Côté CH, Maltais F. MAPK signaling in the quadriceps of patients with chronic obstructive pulmonary disease. J Appl Physiol (1985) 2012;113:159–166. doi: 10.1152/japplphysiol.01518.2011. [DOI] [PubMed] [Google Scholar]
- 343.Magalhães J, Ascensão A, Soares JM, Ferreira R, Neuparth MJ, Oliveira J, Amado F, Marques F, Duarte JA. Acute and chronic exposition of mice to severe hypoxia: the role of acclimatization against skeletal muscle oxidative stress. Int J Sports Med. 2005;26:102–109. doi: 10.1055/s-2004-817858. [DOI] [PubMed] [Google Scholar]
- 344.Hoppeler H, Kleinert E, Schlegel C, Claassen H, Howald H, Kayar SR, Cerretelli P. Morphological adaptations of human skeletal muscle to chronic hypoxia. Int J Sports Med. 1990;11:S3–S9. doi: 10.1055/s-2007-1024846. [DOI] [PubMed] [Google Scholar]
- 345.Yun Z, Lin Q, Giaccia AJ. Adaptive myogenesis under hypoxia. Mol Cell Biol. 2005;25:3040–3055. doi: 10.1128/MCB.25.8.3040-3055.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Pitsiou G, Kyriazis G, Hatzizisi O, Argyropoulou P, Mavrofridis E, Patakas D. Tumor necrosis factor-alpha serum levels, weight loss and tissue oxygenation in chronic obstructive pulmonary disease. Respir Med. 2002;96:594–598. doi: 10.1053/rmed.2002.1322. [DOI] [PubMed] [Google Scholar]
- 347.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 348.Jatta K, Eliason G, Portela-Gomes GM, Grimelius L, Caro O, Nilholm L, Sirjsö A, Piehl-Aulin K, Abdel-Halim SM. Overexpression of von Hippel-Lindau protein in skeletal muscles of patients with chronic obstructive pulmonary disease. J Clin Pathol. 2009;62:70–76. doi: 10.1136/jcp.2008.057190. [DOI] [PubMed] [Google Scholar]
- 349.Favier FB, Costes F, Defour A, Bonnefoy R, Lefai E, Baugé S, Peinnequin A, Benoit H, Freyssenet D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1659–R1666. doi: 10.1152/ajpregu.00550.2009. [DOI] [PubMed] [Google Scholar]
- 350.Caron MA, Thériault ME, Paré ME, Maltais F, Debigaré R. Hypoxia alters contractile protein homeostasis in L6 myotubes. FEBS Lett. 2009;583:1528–1534. doi: 10.1016/j.febslet.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 351.Wagner PD. The biology of oxygen. Eur Respir J. 2008;31:887–890. doi: 10.1183/09031936.00155407. [DOI] [PubMed] [Google Scholar]
- 352.Vogiatzis I, Athanasopoulos D, Habazettl H, Aliverti A, Louvaris Z, Cherouveim E, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Intercostal muscle blood flow limitation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:1105–1113. doi: 10.1164/rccm.201002-0172OC. [DOI] [PubMed] [Google Scholar]
- 353.Puente-Maestu L, Lázaro A, Tejedor A, Camaño S, Fuentes M, Cuervo M, Navarro BO, Agustí A. Effects of exercise on mitochondrial DNA content in skeletal muscle of patients with COPD. Thorax. 2011;66:121–127. doi: 10.1136/thx.2010.153031. [DOI] [PubMed] [Google Scholar]
- 354.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 355.Hare JM. Nitric oxide and excitation-contraction coupling. J Mol Cell Cardiol. 2003;35:719–729. doi: 10.1016/s0022-2828(03)00143-3. [DOI] [PubMed] [Google Scholar]
- 356.Singel DJ, Stamler JS. Blood traffic control. Nature. 2004;430:297. doi: 10.1038/430297a. [DOI] [PubMed] [Google Scholar]
- 357.Hare JM. Nitroso-redox balance in the cardiovascular system. N Engl J Med. 2004;351:2112–2114. doi: 10.1056/NEJMe048269. [DOI] [PubMed] [Google Scholar]
- 358.Gonzalez NC, Wood JG. Alveolar hypoxia-induced systemic inflammation: what low PO(2) does and does not do. Adv Exp Med Biol. 2010;662:27–32. doi: 10.1007/978-1-4419-1241-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.de Theije C, Costes F, Langen RC, Pison C, Gosker HR. Hypoxia and muscle maintenance regulation: implications for chronic respiratory disease. Curr Opin Clin Nutr Metab Care. 2011;14:548–553. doi: 10.1097/MCO.0b013e32834b6e79. [DOI] [PubMed] [Google Scholar]
- 360.Payen JF, Wuyam B, Levy P, Reutenauer H, Stieglitz P, Paramelle B, Le Bas JF. Muscular metabolism during oxygen supplementation in patients with chronic hypoxemia. Am Rev Respir Dis. 1993;147:592–598. doi: 10.1164/ajrccm/147.3.592. [DOI] [PubMed] [Google Scholar]
- 361.Wuyam B, Payen JF, Levy P, Bensaïdane H, Reutenauer H, Le Bas JF, Benabid AL. Metabolism and aerobic capacity of skeletal muscle in chronic respiratory failure related to chronic obstructive pulmonary disease. Eur Respir J. 1992;5:157–162. [PubMed] [Google Scholar]
- 362.England BK, Chastain JL, Mitch WE. Abnormalities in protein synthesis and degradation induced by extracellular pH in BC3H1 myocytes. Am J Physiol. 1991;260:C277–C282. doi: 10.1152/ajpcell.1991.260.2.C277. [DOI] [PubMed] [Google Scholar]
- 363.Franch HA, Raissi S, Wang X, Zheng B, Bailey JL, Price SR. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am J Physiol Renal Physiol. 2004;287:F700–F706. doi: 10.1152/ajprenal.00440.2003. [DOI] [PubMed] [Google Scholar]
- 364.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 365.Van Vliet M, Spruit MA, Verleden G, Kasran A, Van Herck E, Pitta F, Bouillon R, Decramer M. Hypogonadism, quadriceps weakness, and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1105–1111. doi: 10.1164/rccm.200501-114OC. [DOI] [PubMed] [Google Scholar]
- 366.Kamischke A, Kemper DE, Castel MA, Lüthke M, Rolf C, Behre HM, Magnussen H, Nieschlag E. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998;11:41–45. doi: 10.1183/09031936.98.11010041. [DOI] [PubMed] [Google Scholar]
- 367.Karadag F, Ozcan H, Karul AB, Yilmaz M, Cildag O. Sex hormone alterations and systemic inflammation in chronic obstructive pulmonary disease. Int J Clin Pract. 2009;63:275–281. doi: 10.1111/j.1742-1241.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 368.Laghi F, Langbein WE, Antonescu-Turcu A, Jubran A, Bammert C, Tobin MJ. Respiratory and skeletal muscles in hypogonadal men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:598–605. doi: 10.1164/rccm.200412-1643OC. [DOI] [PubMed] [Google Scholar]
- 369.Debigaré R, Marquis K, Côté CH, Tremblay RR, Michaud A, LeBlanc P, Maltais F. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003;124:83–89. doi: 10.1378/chest.124.1.83. [DOI] [PubMed] [Google Scholar]
- 370.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 371.Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol. 2008;154:557–568. doi: 10.1038/bjp.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi R. The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N Engl J Med. 1996;335:1–7. doi: 10.1056/NEJM199607043350101. [DOI] [PubMed] [Google Scholar]
- 373.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 374.Elliott B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol (Oxf) 2012;205:324–340. doi: 10.1111/j.1748-1716.2012.02423.x. [DOI] [PubMed] [Google Scholar]
- 375.Roth SM, Walsh S. Myostatin: a therapeutic target for skeletal muscle wasting. Curr Opin Clin Nutr Metab Care. 2004;7:259–263. doi: 10.1097/00075197-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 376.Man WD, Natanek SA, Riddoch-Contreras J, Lewis A, Marsh GS, Kemp PR, Polkey MI. Quadriceps myostatin expression in COPD. Eur Respir J. 2010;36:686–688. doi: 10.1183/09031936.00032510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Hayot M, Rodriguez J, Vernus B, Carnac G, Jean E, Allen D, Goret L, Obert P, Candau R, Bonnieu A. Myostatin up-regulation is associated with the skeletal muscle response to hypoxic stimuli. Mol Cell Endocrinol. 2011;332:38–47. doi: 10.1016/j.mce.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 378.Ju CR, Chen RC. Serum myostatin levels and skeletal muscle wasting in chronic obstructive pulmonary disease. Respir Med. 2012;106:102–108. doi: 10.1016/j.rmed.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 379.Troosters T, Probst VS, Crul T, Pitta F, Gayan-Ramirez G, Decramer M, Gosselink R. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1072–1077. doi: 10.1164/rccm.200908-1203OC. [DOI] [PubMed] [Google Scholar]
- 380.Schols AM, Soeters PB, Mostert R, Saris WH, Wouters EF. Energy balance in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1991;143:1248–1252. doi: 10.1164/ajrccm/143.6.1248. [DOI] [PubMed] [Google Scholar]
- 381.Goris AH, Vermeeren MA, Wouters EF, Schols AM, Westerterp KR. Energy balance in depleted ambulatory patients with chronic obstructive pulmonary disease: the effect of physical activity and oral nutritional supplementation. Br J Nutr. 2003;89:725–731. doi: 10.1079/BJN2003838. [DOI] [PubMed] [Google Scholar]
- 382.King DA, Cordova F, Scharf SM. Nutritional aspects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:519–523. doi: 10.1513/pats.200707-092ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol. 2013;114:1253–1262. doi: 10.1152/japplphysiol.00790.2012. [DOI] [PubMed] [Google Scholar]
- 384.Decramer M, de Bock V, Dom R. Functional and histologic picture of steroid-induced myopathy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153:1958–1964. doi: 10.1164/ajrccm.153.6.8665061. [DOI] [PubMed] [Google Scholar]
- 385.Schakman O, Gilson H, Kalista S, Thissen JP. Mechanisms of muscle atrophy induced by glucocorticoids. Horm Res. 2009;72:36–41. doi: 10.1159/000229762. [DOI] [PubMed] [Google Scholar]
- 386.Hamilton B. Vitamin D and human skeletal muscle. Scand J Med Sci Sports. 2010;20:182–190. doi: 10.1111/j.1600-0838.2009.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Dirks-Naylor AJ, Lennon-Edwards S. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol. 2011;125:159–168. doi: 10.1016/j.jsbmb.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 388.Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, Dawson-Hughes B. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80:752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 389.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–633. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Menant JC, Close JC, Delbaere K, Sturnieks DL, Trollor J, Sachdev PS, Brodaty H, Lord SR. Relationships between serum vitamin D levels, neuromuscular and neuropsychological function and falls in older men and women. Osteoporos Int. 2012;23:981–989. doi: 10.1007/s00198-011-1637-7. [DOI] [PubMed] [Google Scholar]
- 391.Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, Knol DL, Lips P. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 392.Boland R. Role of vitamin D in skeletal muscle function. Endocr Rev. 1986;7:434–448. doi: 10.1210/edrv-7-4-434. [DOI] [PubMed] [Google Scholar]
- 393.Yoshikawa S, Nakamura T, Tanabe H, Imamura T. Osteomalacic myopathy. Endocrinol Jpn. 1979;26:65–72. doi: 10.1507/endocrj1954.26.supplement_65. [DOI] [PubMed] [Google Scholar]
- 394.Sørensen OH, Lund B, Saltin B, Lund B, Andersen RB, Hjorth L, Melsen F, Mosekilde L. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56:157–161. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 395.Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: a randomized controlled trial. Cerebrovasc Dis. 2005;20:187–192. doi: 10.1159/000087203. [DOI] [PubMed] [Google Scholar]
- 396.Boland R, de Boland AR, Ritz E, Hasselbach W. Effect of 1,25-dihydroxycholecalciferol on sarcoplasmic reticulum calcium transport in strontium-fed chicks. Calcif Tissue Int. 1983;35:190–194. doi: 10.1007/BF02405030. [DOI] [PubMed] [Google Scholar]
- 397.Boland R, de Boland AR, Marinissen MJ, Santillan G, Vazquez G, Zanello S. Avian muscle cells as targets for the secosteroid hormone 1,25-dihydroxy-vitamin D3. Mol Cell Endocrinol. 1995;114:1–8. doi: 10.1016/0303-7207(95)03650-v. [DOI] [PubMed] [Google Scholar]
- 398.Drittanti L, de Boland AR, Boland R. Stimulation of calmodulin synthesis in proliferating myoblasts by 1,25-dihydroxy-vitamin D3. Mol Cell Endocrinol. 1990;74:143–153. doi: 10.1016/0303-7207(90)90116-p. [DOI] [PubMed] [Google Scholar]
- 399.Pointon JJ, Francis MJ, Smith R. Effect of vitamin D deficiency on sarcoplasmic reticulum function and troponin C concentration of rabbit skeletal muscle. Clin Sci (Lond) 1979;57:257–263. doi: 10.1042/cs0570257. [DOI] [PubMed] [Google Scholar]
- 400.Janssens W, Lehouck A, Carremans C, Bouillon R, Mathieu C, Decramer M. Vitamin D beyond bones in chronic obstructive pulmonary disease: time to act. Am J Respir Crit Care Med. 2009;179:630–636. doi: 10.1164/rccm.200810-1576PP. [DOI] [PubMed] [Google Scholar]
- 401.Vogelmeier CF, Wouters EF. Treating the systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2011;8:376–379. doi: 10.1513/pats.201102-020RM. [DOI] [PubMed] [Google Scholar]
- 402.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, Coolen J, Mathieu C, Decramer M, Lambrechts D. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–220. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 403.Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Effect of cholecalciferol plus calcium on falling in ambulatory older men and women: a 3-year randomized controlled trial. Arch Intern Med. 2006;166:424–430. doi: 10.1001/archinte.166.4.424. [DOI] [PubMed] [Google Scholar]
- 404.Jackson AS, Shrikrishna D, Kelly JL, Hart N, Moxham J, Polkey MI, Kemp P, Hopkinson NS.Vitamin D and skeletal muscle strength and endurance in COPD Eur Respir J 2013. 41:309–316 [DOI] [PubMed] [Google Scholar]
- 405.Hopkinson NS, Li KW, Kehoe A, Humphries SE, Roughton M, Moxham J, Montgomery H, Polkey MI. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87:385–390. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]
- 406.Shrikrishna D, Astin R, Kemp PR, Hopkinson NS. Renin-angiotensin system blockade: a novel therapeutic approach in chronic obstructive pulmonary disease. Clin Sci (Lond) 2012;123:487–498. doi: 10.1042/CS20120081. [DOI] [PubMed] [Google Scholar]
- 407.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115:451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Russell ST, Wyke SM, Tisdale MJ. Mechanism of induction of muscle protein degradation by angiotensin II. Cell Signal. 2006;18:1087–1096. doi: 10.1016/j.cellsig.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 409.Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Danser AH, Schalekamp MA, Bax WA, van den Brink AM, Saxena PR, Riegger GA, Schunkert H. Angiotensin-converting enzyme in the human heart. Effect of the deletion/insertion polymorphism. Circulation. 1995;92:1387–1388. doi: 10.1161/01.cir.92.6.1387. [DOI] [PubMed] [Google Scholar]
- 411.Williams AG, Rayson MP, Jubb M, World M, Woods DR, Hayward M, Martin J, Humphries SE, Montgomery HE. The ACE gene and muscle performance. Nature. 2000;403:614. doi: 10.1038/35001141. [DOI] [PubMed] [Google Scholar]
- 412.Myerson S, Hemingway H, Budget R, Martin J, Humphries S, Montgomery H. Human angiotensin I-converting enzyme gene and endurance performance. J Appl Physiol (1985) 1999;87:1313–1316. doi: 10.1152/jappl.1999.87.4.1313. [DOI] [PubMed] [Google Scholar]
- 413.Zhang B, Tanaka H, Shono N, Miura S, Kiyonaga A, Shindo M, Saku K. The I allele of the angiotensin-converting enzyme gene is associated with an increased percentage of slow-twitch type I fibers in human skeletal muscle. Clin Genet. 2003;63:139–144. doi: 10.1034/j.1399-0004.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- 414.Hopkinson NS, Nickol AH, Payne J, Hawe E, Man WD, Moxham J, Montgomery H, Polkey MI. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:395–399. doi: 10.1164/rccm.200304-578OC. [DOI] [PubMed] [Google Scholar]
- 415.Folland J, Leach B, Little T, Hawker K, Myerson S, Montgomery H, Jones D. Angiotensin-converting enzyme genotype affects the response of human skeletal muscle to functional overload. Exp Physiol. 2000;85:575–579. [PubMed] [Google Scholar]
- 416.De Paepe B, Brusselle GG, Maes T, Creus KK, D’hose S, D’Haese N, Bracke KR, D’hulst AI, Joos GF, De Bleecker JL. TNF alpha receptor genotype influences smoking-induced muscle-fibre-type shift and atrophy in mice. Acta Neuropathol. 2008;115:675–681. doi: 10.1007/s00401-008-0348-4. [DOI] [PubMed] [Google Scholar]
- 417.Gosker HR, Langen RC, Bracke KR, Joos GF, Brusselle GG, Steele C, Ward KA, Wouters EF, Schols AM. Extrapulmonary manifestations of chronic obstructive pulmonary disease in a mouse model of chronic cigarette smoke exposure. Am J Respir Cell Mol Biol. 2009;40:710–716. doi: 10.1165/rcmb.2008-0312OC. [DOI] [PubMed] [Google Scholar]
- 418.Tang K, Wagner PD, Breen EC. TNF-alpha-mediated reduction in PGC-1alpha may impair skeletal muscle function after cigarette smoke exposure. J Cell Physiol. 2010;222:320–327. doi: 10.1002/jcp.21955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Rinaldi M, Maes K, De Vleeschauwer S, Thomas D, Verbeken EK, Decramer M, Janssens W, Gayan-Ramirez GN. Long-term nose-only cigarette smoke exposure induces emphysema and mild skeletal muscle dysfunction in mice. Dis Model Mech. 2012;5:333–341. doi: 10.1242/dmm.008508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Montes de Oca M, Loeb E, Torres SH, De Sanctis J, Hernández N, Tálamo C. Peripheral muscle alterations in non-COPD smokers. Chest. 2008;133:13–18. doi: 10.1378/chest.07-1592. [DOI] [PubMed] [Google Scholar]
- 421.Orlander J, Kiessling KH, Larsson L. Skeletal muscle metabolism, morphology and function in sedentary smokers and nonsmokers. Acta Physiol Scand. 1979;107:39–46. doi: 10.1111/j.1748-1716.1979.tb06440.x. [DOI] [PubMed] [Google Scholar]
- 422.Larsson L, Orlander J. Skeletal muscle morphology, metabolism and function in smokers and non-smokers. A study on smoking-discordant monozygous twins. Acta Physiol Scand. 1984;120:343–352. doi: 10.1111/j.1748-1716.1984.tb07394.x. [DOI] [PubMed] [Google Scholar]
- 423.Saey D, Michaud A, Couillard A, Côté CH, Mador MJ, LeBlanc P, Jobin J, Maltais F. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1109–1115. doi: 10.1164/rccm.200408-1005OC. [DOI] [PubMed] [Google Scholar]
- 424.Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J Physiol. 2011;589:5299–5309. doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/s0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 426.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 427.Romer LM, Lovering AT, Haverkamp HC, Pegelow DF, Dempsey JA. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol. 2006;571:425–439. doi: 10.1113/jphysiol.2005.099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Simon M, LeBlanc P, Jobin J, Desmeules M, Sullivan MJ, Maltais F. Limitation of lower limb VO(2) during cycling exercise in COPD patients. J Appl Physiol (1985) 2001;90:1013–1019. doi: 10.1152/jappl.2001.90.3.1013. [DOI] [PubMed] [Google Scholar]
- 429.Vogiatzis I, Habazettl H, Aliverti A, Athanasopoulos D, Louvaris Z, LoMauro A, Wagner H, Roussos C, Wagner PD, Zakynthinos S. Effect of helium breathing on intercostal and quadriceps muscle blood flow during exercise in COPD patients. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1549–R1559. doi: 10.1152/ajpregu.00671.2010. [DOI] [PubMed] [Google Scholar]
- 430.Hopkinson NS, Dayer MJ, Antoine-Jonville S, Swallow EB, Porcher R, Vazir A, Poole-Wilson P, Polkey MI. Central and peripheral quadriceps fatigue in congestive heart failure. Int J Cardiol. 2013;167:2594–2599. doi: 10.1016/j.ijcard.2012.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Holverda S, Rietema H, Westerhof N, Marcus JT, Gan CT, Postmus PE, Vonk-Noordegraaf A. Stroke volume increase to exercise in chronic obstructive pulmonary disease is limited by increased pulmonary artery pressure. Heart. 2009;95:137–141. doi: 10.1136/hrt.2007.138172. [DOI] [PubMed] [Google Scholar]
- 433.Hilde JM, Skjørten I, Hansteen V, Melsom MN, Hisdal J, Humerfelt S, Steine K. Haemodynamic responses to exercise in patients with COPD. Eur Respir J. 2013;41:1031–1041. doi: 10.1183/09031936.00085612. [DOI] [PubMed] [Google Scholar]
- 434.Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, Athanasopoulos D, Roussos C, Wagner PD, Zakynthinos S. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–310. doi: 10.1183/09031936.00112909. [DOI] [PubMed] [Google Scholar]
- 435.Sahlin K. Muscle fatigue and lactic acid accumulation. Acta Physiol Scand Suppl. 1986;556:83–91. [PubMed] [Google Scholar]
- 436.Sahlin K. Metabolic factors in fatigue. Sports Med. 1992;13:99–107. doi: 10.2165/00007256-199213020-00005. [DOI] [PubMed] [Google Scholar]
- 437.Sahlin K, Tonkonogi M, Söderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand. 1998;162:261–266. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- 438.Sahlin K, Söderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Physiol. 1997;273:C172–C178. doi: 10.1152/ajpcell.1997.273.1.C172. [DOI] [PubMed] [Google Scholar]
- 439.Mainwood GW, Renaud JM. The effect of acid-base balance on fatigue of skeletal muscle. Can J Physiol Pharmacol. 1985;63:403–416. doi: 10.1139/y85-072. [DOI] [PubMed] [Google Scholar]
- 440.Fitts RH. Cellular mechanisms of muscle fatigue. Physiol Rev. 1994;74:49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 441.Wilcox PG, Hards JM, Bockhold K, Bressler B, Pardy RL. Pathologic changes and contractile properties of the diaphragm in corticosteroid myopathy in hamsters: comparison to peripheral muscle. Am J Respir Cell Mol Biol. 1989;1:191–199. doi: 10.1165/ajrcmb/1.3.191. [DOI] [PubMed] [Google Scholar]
- 442.Tracey KJ, Lowry SF, Beutler B, Cerami A, Albert JD, Shires GT. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential. J Exp Med. 1986;164:1368–1373. doi: 10.1084/jem.164.4.1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Kutsuzawa T, Shioya S, Kurita D, Haida M, Ohta Y, Yamabayashi H. 31P-NMR study of skeletal muscle metabolism in patients with chronic respiratory impairment. Am Rev Respir Dis. 1992;146:1019–1024. doi: 10.1164/ajrccm/146.4.1019. [DOI] [PubMed] [Google Scholar]
- 444.Jakobsson P, Jorfeldt L, Brundin A. Skeletal muscle metabolites and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J. 1990;3:192–196. [PubMed] [Google Scholar]
- 445.Hildebrand IL, Sylvén C, Esbjörnsson M, Hellström K, Jansson E. Does chronic hypoxaemia induce transformations of fibre types? Acta Physiol Scand. 1991;141:435–439. doi: 10.1111/j.1748-1716.1991.tb09102.x. [DOI] [PubMed] [Google Scholar]
- 446.Dekhuijzen PN, Aben KK, Dekker I, Aarts LP, Wielders PL, van Herwaarden CL, Bast A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:813–816. doi: 10.1164/ajrccm.154.3.8810624. [DOI] [PubMed] [Google Scholar]
- 447.Steiner MC, Barton RL, Singh SJ, Morgan MD. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002;19:626–631. doi: 10.1183/09031936.02.00279602. [DOI] [PubMed] [Google Scholar]
- 448.Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41:810–817. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- 449.Rutten EP, Spruit MA, Wouters EF. Critical view on diagnosing muscle wasting by single-frequency bio-electrical impedance in COPD. Respir Med. 2010;104:91–98. doi: 10.1016/j.rmed.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 450.Engelen MP, Schols AM, Heidendal GA, Wouters EF. Dual-energy X-ray absorptiometry in the clinical evaluation of body composition and bone mineral density in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 1998;68:1298–1303. doi: 10.1093/ajcn/68.6.1298. [DOI] [PubMed] [Google Scholar]
- 451.Lerario MC, Sachs A, Lazaretti-Castro M, Saraiva LG, Jardim JR. Body composition in patients with chronic obstructive pulmonary disease: which method to use in clinical practice? Br J Nutr. 2006;96:86–92. doi: 10.1079/bjn20061798. [DOI] [PubMed] [Google Scholar]
- 452.Mathur S, Takai KP, Macintyre DL, Reid D. Estimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary disease. Phys Ther. 2008;88:219–230. doi: 10.2522/ptj.20070052. [DOI] [PubMed] [Google Scholar]
- 453.HajGhanbari B. Hamarneh G, Changizi N, Ward AD, Reid WD. MRI-based 3D shape analysis of thigh muscles patients with chronic obstructive pulmonary disease versus healthy adults. Acad Radiol. 2011;18:155–166. doi: 10.1016/j.acra.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 454.Coggan AR. Muscle biopsy as a tool in the study of aging. J Gerontol A Biol Sci Med Sci. 1995;50:30–34. doi: 10.1093/gerona/50a.special_issue.30. [DOI] [PubMed] [Google Scholar]
- 455.Bergström J. Muscle electrolytes in man. Determination by neutron activation analysis on needle biopsy specimens. A study on normal subjects, kidney patients and patients with chronic diarrhoea. Scand J Clin Lab Invest. 1962;14 [Google Scholar]
- 456.Bergström J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 457.Tarnopolsky MA, Pearce E, Smith K, Lach B. Suction-modified Bergström muscle biopsy technique: experience with 13,500 procedures. Muscle Nerve. 2011;43:717–725. doi: 10.1002/mus.21945. [DOI] [PubMed] [Google Scholar]
- 458.Hayot M, Michaud A, Koechlin C, Caron MA, LeBlanc P, Préfaut C, Maltais F. Skeletal muscle microbiopsy: a validation study of a minimally invasive technique. Eur Respir J. 2005;25:431–440. doi: 10.1183/09031936.05.00053404. [DOI] [PubMed] [Google Scholar]
- 459.van Wetering CR, van Nooten FE, Mol SJ, Hoogendoorn M, Rutten-Van Mölken MP, Schols AM. Systemic impairment in relation to disease burden in patients with moderate COPD eligible for a lifestyle program. Findings from the INTERCOM trial. Int J Chron Obstruct Pulmon Dis. 2008;3:443–451. doi: 10.2147/copd.s2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Kelly JL, Elkin SL, Fluxman J, Polkey MI, Soljak MA, Hopkinson NS. Breathlessness and skeletal muscle weakness in patients undergoing lung health screening in primary care. COPD. 2013;10:40–54. doi: 10.3109/15412555.2012.727923. [DOI] [PubMed] [Google Scholar]
- 461.Robles PG, Mathur S, Janaudis-Fereira T, Dolmage TE, Goldstein RS, Brooks D. Measurement of peripheral muscle strength in individuals with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2011;31:11–24. doi: 10.1097/HCR.0b013e3181ebf302. [DOI] [PubMed] [Google Scholar]
- 462.Zanotti E, Felicetti G, Maini M, Fracchia C. Peripheral muscle strength training in bed-bound patients with COPD receiving mechanical ventilation: effect of electrical stimulation. Chest. 2003;124:292–296. doi: 10.1378/chest.124.1.292. [DOI] [PubMed] [Google Scholar]
- 463.Hermans G, Clerckx B, Vanhullebusch T, Segers J, Vanpee G, Robbeets C, Casaer MP, Wouters P, Gosselink R, Van Den Berghe G. Interobserver agreement of Medical Research Council sum-score and handgrip strength in the intensive care unit. Muscle Nerve. 2012;45:18–25. doi: 10.1002/mus.22219. [DOI] [PubMed] [Google Scholar]
- 464.Stone CA, Nolan B, Lawlor PG, Kenny RA. Hand-held dynamometry: tester strength is paramount, even in frail populations. J Rehabil Med. 2011;43:808–811. doi: 10.2340/16501977-0860. [DOI] [PubMed] [Google Scholar]
- 465.Kaelin ME, Swank AM, Adams KJ, Barnard KL, Berning JM, Green A. Cardiopulmonary responses, muscle soreness, and injury during the one repetition maximum assessment in pulmonary rehabilitation patients. J Cardiopulm Rehabil. 1999;19:366–372. doi: 10.1097/00008483-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 466.Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, Fournier M, Storer TW. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 467.Mador MJ, Bozkanat E, Aggarwal A, Shaffer M, Kufel TJ. Endurance and strength training in patients with COPD. Chest. 2004;125:2036–2045. doi: 10.1378/chest.125.6.2036. [DOI] [PubMed] [Google Scholar]
- 468.Mathur S, Makrides L, Hernandez P. Test-retest reliability of isometric and isokinetic torque in patients with chronic obstructive pulmonary disease. Physiother Can. 2004;56:94–101. [Google Scholar]
- 469.Maffiuletti NA, Bizzini M, Desbrosses K, Babault N, Munzinger U. Reliability of knee extension and flexion measurements using the Con-Trex isokinetic dynamometer. Clin Physiol Funct Imaging. 2007;27:346–353. doi: 10.1111/j.1475-097X.2007.00758.x. [DOI] [PubMed] [Google Scholar]
- 470.Malaguti C, Napolis LM, Villaça D, Neder JA, Nery LE, Dal Corso S. Relationship between peripheral muscle structure and function in patients with chronic obstructive pulmonary disease with different nutritional status. J Strength Cond Res. 2011;25:1795–1803. doi: 10.1519/JSC.0b013e3181e501c1. [DOI] [PubMed] [Google Scholar]
- 471.Beauchamp MK, Sibley KM, Lakhani B, Romano J, Mathur S. Goldstein RS, Brooks D. Impairments in systems underlying control of balance in COPD. Chest. 2012;141:1496–1503. doi: 10.1378/chest.11-1708. [DOI] [PubMed] [Google Scholar]
- 472.Vieira L, Bottaro M, Celes R, Viegas CA, e Silva CA. Isokinetic muscle evaluation of quadriceps in patients with chronic obstructive pulmonary disease. Rev Port Pneumol. 2010;16:717–736. [PubMed] [Google Scholar]
- 473.Degens H, Sanchez Horneros JM, Heijdra YF, Dekhuijzen PN, Hopman MT. Skeletal muscle contractility is preserved in COPD patients with normal fat-free mass. Acta Physiol Scand. 2005;184:235–242. doi: 10.1111/j.1365-201X.2005.01447.x. [DOI] [PubMed] [Google Scholar]
- 474.Ju CR, Chen RC. Quadriceps strength assessed by magnetic stimulation of femoral nerve in patients with chronic obstructive pulmonary disease. Chin Med J (Engl) 2011;124:2309–2315. [PubMed] [Google Scholar]
- 475.Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–444. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- 476.Tomazin K, Verges S, Decorte N, Oulerich A, Millet GY. Effects of coil characteristics for femoral nerve magnetic stimulation. Muscle Nerve. 2010;41:406–409. doi: 10.1002/mus.21566. [DOI] [PubMed] [Google Scholar]
- 477.Hamnegård CH, Sedler M, Polkey MI, Bake B. Quadriceps strength assessed by magnetic stimulation of the femoral nerve in normal subjects. Clin Physiol Funct Imaging. 2004;24:276–280. doi: 10.1111/j.1475-097X.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 478.Hopkinson NS, Sharshar T, Ross ET, Nickol AH, Dayer MJ, Porcher R, Jonville S, Moxham J, Polkey MI. Corticospinal control of respiratory muscles in chronic obstructive pulmonary disease. Respir Physiol Neurobiol. 2004;141:1–12. doi: 10.1016/j.resp.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 479.Mohamed-Hussein AA, Hamed SA, Abdel-Hakim N. Cerebral cortical dysfunction in chronic obstructive pulmonary disease: role of transcranial magnetic stimulation. Int J Tuberc Lung Dis. 2007;11:515–521. [PubMed] [Google Scholar]
- 480.Ottenheijm CA, Heunks LM, Sieck GC, Zhan WZ, Jansen SM, Degens H, de Boo T, Dekhuijzen PN. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:200–205. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. Eur Respir Rev. 2010;19:24–29. doi: 10.1183/09059180.00007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Vivodtzev I, Gagnon P, Pepin V, Saey D, Laviolette L, Brouillard C, Maltais F. Physiological correlates of endurance time variability during constant-workrate cycling exercise in patients with COPD. PLoS ONE. 2011;6:e17007. doi: 10.1371/journal.pone.0017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:102–108. doi: 10.1164/rccm.200202-080OC. [DOI] [PubMed] [Google Scholar]
- 484.Saey D, Troosters T. Measuring skeletal muscle strength and endurance, from bench to bedside. Clin Invest Med. 2008;31:E307–E311. doi: 10.25011/cim.v31i5.4881. [DOI] [PubMed] [Google Scholar]
- 485.Cifrek M, Medved V, Tonković S, Ostojić S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin Biomech (Bristol, Avon) 2009;24:327–340. doi: 10.1016/j.clinbiomech.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 486.Rondelli RR, Dal Corso S, Simões A, Malaguti C. Methods for the assessment of peripheral muscle fatigue and its energy and metabolic determinants in COPD. J Bras Pneumol. 2009;35:1125–1135. doi: 10.1590/s1806-37132009001100011. [DOI] [PubMed] [Google Scholar]
- 487.Malaguti C, Nery LE, Dal Corso S, Nápolis L, De Fuccio MB, Castro M, Neder JA. Scaling skeletal muscle function to mass in patients with moderate-to-severe COPD. Eur J Appl Physiol. 2006;98:482–488. doi: 10.1007/s00421-006-0292-8. [DOI] [PubMed] [Google Scholar]
- 488.Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, Sangenis M, Broquetas JM, Casan P, Gea J. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166:1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 489.Boushel R, Langberg H, Olesen J, Gonzales-Alonzo J, Bülow J, Kjaer M. Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports. 2001;11:213–222. doi: 10.1034/j.1600-0838.2001.110404.x. [DOI] [PubMed] [Google Scholar]
- 490.Berton DC, Barbosa PB, Takara LS, Chiappa GR, Siqueira AC, Bravo DM, Ferreira LF, Neder JA. Bronchodilators accelerate the dynamics of muscle O2 delivery and utilisation during exercise in COPD. Thorax. 2010;65:588–593. doi: 10.1136/thx.2009.120857. [DOI] [PubMed] [Google Scholar]
- 491.Borghi-Silva A, Oliveira CC, Carrascosa C, Maia J, Berton DC, Queiroga F, Jr, Ferreira EM, Almeida DR, Nery LE, Neder JA. Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax. 2008;63:910–915. doi: 10.1136/thx.2007.090167. [DOI] [PubMed] [Google Scholar]
- 492.Vogiatzis I, Athanasopoulos D, Stratakos G, Garagouni C, Koutsoukou A, Boushel R, Roussos C, Zakynthinos S. Exercise-induced skeletal muscle deoxygenation in O-supplemented COPD patients. Scand J Med Sci Sports. 2009;19:364–372. doi: 10.1111/j.1600-0838.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 493.Siqueira AC, Borghi-Silva A, Bravo DM, Ferreira EM, Chiappa GR, Neder JA. Effects of hyperoxia on the dynamics of skeletal muscle oxygenation at the onset of heavy-intensity exercise in patients with COPD. Respir Physiol Neurobiol. 2010;172:8–14. doi: 10.1016/j.resp.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 494.Louvaris Z, Zakynthinos S, Aliverti A, Habazettl H, Vasilopoulou M, Andrianopoulos V, Wagner H, Wagner P, Vogiatzis I. Heliox increases quadriceps muscle oxygen delivery during exercise in COPD patients with and without dynamic hyperinflation. J Appl Physiol (1985) 2012;113:1012–1023. doi: 10.1152/japplphysiol.00481.2012. [DOI] [PubMed] [Google Scholar]
- 495.Lévy P, Wuyam B, Pépin JL, Reutenauer H, Payen JF. [Skeletal muscle abnormalities in chronic obstructive lung disease with respiratory insufficiency. Value of P31 magnetic resonance spectroscopy] Rev Mal Respir. 1997;14:183–191. [PubMed] [Google Scholar]
- 496.Spruit MA, Gosselink R, Troosters T, De Paepe K, Decramer M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J. 2002;19:1072–1078. doi: 10.1183/09031936.02.00287102. [DOI] [PubMed] [Google Scholar]
- 497.Bernard S, Whittom F, Leblanc P, Jobin J, Belleau R, Bérubé C, Carrier G, Maltais F. Aerobic and strength training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:896–901. doi: 10.1164/ajrccm.159.3.9807034. [DOI] [PubMed] [Google Scholar]
- 498.O’Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157:1489–1497. doi: 10.1164/ajrccm.157.5.9708010. [DOI] [PubMed] [Google Scholar]
- 499.Ortega F, Toral J, Cejudo P, Villagomez R, Sánchez H, Castillo J, Montemayor T. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:669–674. doi: 10.1164/rccm.2107081. [DOI] [PubMed] [Google Scholar]
- 500.Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19–38. doi: 10.1164/rccm.200408-1109SO. [DOI] [PubMed] [Google Scholar]
- 501.Beauchamp MK, Janaudis-Ferreira T, Goldstein RS, Brooks D. Optimal duration of pulmonary rehabilitation for individuals with chronic obstructive pulmonary disease - a systematic review. Chron Respir Dis. 2011;8:129–140. doi: 10.1177/1479972311404256. [DOI] [PubMed] [Google Scholar]
- 502.Beauchamp MK, Nonoyama M. Goldstein RS, Hill K, Dolmage TE, Mathur S, Brooks D. Interval versus continuous training in individuals with chronic obstructive pulmonary disease–a systematic review. Thorax. 2010;65:157–164. doi: 10.1136/thx.2009.123000. [DOI] [PubMed] [Google Scholar]
- 503.Dolmage TE, Goldstein RS. Response to one-legged cycling in patients with COPD. Chest. 2006;129:325–332. doi: 10.1378/chest.129.2.325. [DOI] [PubMed] [Google Scholar]
- 504.Dolmage TE, Goldstein RS. Effects of one-legged exercise training of patients with COPD. Chest. 2008;133:370–376. doi: 10.1378/chest.07-1423. [DOI] [PubMed] [Google Scholar]
- 505.Vogiatzis I, Terzis G, Nanas S, Stratakos G, Simoes DC, Georgiadou O, Zakynthinos S, Roussos C. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128:3838–3845. doi: 10.1378/chest.128.6.3838. [DOI] [PubMed] [Google Scholar]
- 506.Vogiatzis I, Stratakos G, Simoes DC, Terzis G, Georgiadou O, Roussos C, Zakynthinos S. Effects of rehabilitative exercise on peripheral muscle TNFalpha, IL-6, IGF-I and MyoD expression in patients with COPD. Thorax. 2007;62:950–956. doi: 10.1136/thx.2006.069310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 507.Maltais F, LeBlanc P, Simard C, Jobin J, Bérubé C, Bruneau J, Carrier L, Belleau R. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:442–447. doi: 10.1164/ajrccm.154.2.8756820. [DOI] [PubMed] [Google Scholar]
- 508.Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21:10–17. doi: 10.1097/00008483-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 509.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, Rao DC, Rankinen T. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol (1985) 2011;110:1160–1170. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Pitta F, Troosters T, Probst VS, Langer D, Decramer M, Gosselink R. Are patients with COPD more active after pulmonary rehabilitation? Chest. 2008;134:273–280. doi: 10.1378/chest.07-2655. [DOI] [PubMed] [Google Scholar]
- 511.Radom-Aizik S, Kaminski N, Hayek S, Halkin H, Cooper DM, Ben-Dov I. Effects of exercise training on quadriceps muscle gene expression in chronic obstructive pulmonary disease. J Appl Physiol (1985) 2007;102:1976–1984. doi: 10.1152/japplphysiol.00577.2006. [DOI] [PubMed] [Google Scholar]
- 512.Gouzi F, Préfaut C, Abdellaoui A, Roudier E, de Rigal P, Molinari N, Laoudj-Chenivesse D, Mercier J, Birot O, Hayot M. Blunted muscle angiogenic training-response in COPD patients versus sedentary controls. Eur Respir J. 2013;41:806–814. doi: 10.1183/09031936.00053512. [DOI] [PubMed] [Google Scholar]
- 513.Constantin D, Menon MK, Houchen-Wolloff L, Morgan MD, Singh SJ, Greenhaff P, Steiner MC. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax. 2013;68:625–633. doi: 10.1136/thoraxjnl-2012-202764. [DOI] [PubMed] [Google Scholar]
- 514.Rabinovich RA, Ardite E, Mayer AM, Polo MF, Vilaró J, Argilés JM, Roca J. Training depletes muscle glutathione in patients with chronic obstructive pulmonary disease and low body mass index. Respiration. 2006;73:757–761. doi: 10.1159/000094395. [DOI] [PubMed] [Google Scholar]
- 515.Mercken EM, Hageman GJ, Schols AM, Akkermans MA, Bast A, Wouters EF. Rehabilitation decreases exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:994–1001. doi: 10.1164/rccm.200411-1580OC. [DOI] [PubMed] [Google Scholar]
- 516.Mercken EM, Hageman GJ, Langen RC, Wouters EF, Schols AM. Decreased exercise-induced expression of nuclear factor-κB-regulated genes in muscle of patients with COPD. Chest. 2011;139:337–346. doi: 10.1378/chest.10-0275. [DOI] [PubMed] [Google Scholar]
- 517.Pascual-Guardia S, Wodja E, Gorostiza A, Lopez de Santamaria E, Gea J, Gáldiz JB, Sliwinski P, Barreiro E. Improvement in quality of life and exercise capacity without muscular biology changes after general training in patients with severe chronic obstructive pulmonary disease [in Spanish] Med Clin (Barc) 2013;140:200–206. doi: 10.1016/j.medcli.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 518.Laviolette L, Lands LC, Dauletbaev N, Saey D, Milot J, Provencher S, LeBlanc P, Maltais F. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food. 2010;13:589–598. doi: 10.1089/jmf.2009.0142. [DOI] [PubMed] [Google Scholar]
- 519.Williams MA, Haskell WL, Ades PA, Amsterdam EA, Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ American Heart Association Council on Clinical Cardiology; American Heart Association Council on Nutrition, Physical Activity, and Metabolism. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on Clinical Cardiology and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 520.O’Shea SD, Taylor NF, Paratz JD. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: a systematic review. Chest. 2009;136:1269–1283. doi: 10.1378/chest.09-0029. [DOI] [PubMed] [Google Scholar]
- 521.Houchen L, Steiner MC, Singh SJ. How sustainable is strength training in chronic obstructive pulmonary disease? Physiotherapy. 2009;95:1–7. doi: 10.1016/j.physio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 522.Burtin C, Decramer M, Gosselink R, Janssens W, Troosters T. Rehabilitation and acute exacerbations. Eur Respir J. 2011;38:702–712. doi: 10.1183/09031936.00079111. [DOI] [PubMed] [Google Scholar]
- 523.Puhan MA, Spaar A, Frey M, Turk A, Brändli O, Ritscher D, Achermann E, Kaelin R, Karrer W. Early versus late pulmonary rehabilitation in chronic obstructive pulmonary disease patients with acute exacerbations: a randomized trial. Respiration. 2012;83:499–506. doi: 10.1159/000329884. [DOI] [PubMed] [Google Scholar]
- 524.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 525.Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med. 2005;99:1297–1302. doi: 10.1016/j.rmed.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 526.Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, Yung B, Man WD, Hart N, Polkey MI, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65:423–428. doi: 10.1136/thx.2009.124164. [DOI] [PubMed] [Google Scholar]
- 527.Vivodtzev I, Lacasse Y, Maltais F. Neuromuscular electrical stimulation of the lower limbs in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2008;28:79–91. doi: 10.1097/01.HCR.0000314201.02053.a3. [DOI] [PubMed] [Google Scholar]
- 528.Sillen MJ, Speksnijder CM, Eterman RM, Janssen PP, Wagers SS, Wouters EF, Uszko-Lencer NH, Spruit MA. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest. 2009;136:44–61. doi: 10.1378/chest.08-2481. [DOI] [PubMed] [Google Scholar]
- 529.Neder JA, Sword D, Ward SA, Mackay E, Cochrane LM, Clark CJ. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD) Thorax. 2002;57:333–337. doi: 10.1136/thorax.57.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 530.Abdellaoui A, Préfaut C, Gouzi F, Couillard A, Coisy-Quivy M, Hugon G, Molinari N, Lafontaine T, Jonquet O, Laoudj-Chenivesse D, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J. 2011;38:781–788. doi: 10.1183/09031936.00167110. [DOI] [PubMed] [Google Scholar]
- 531.Dal Corso S, Nápolis L, Malaguti C, Gimenes AC, Albuquerque A, Nogueira CR, De Fuccio MB, Pereira RD, Bulle A, McFarlane N, et al. Skeletal muscle structure and function in response to electrical stimulation in moderately impaired COPD patients. Respir Med. 2007;101:1236–1243. doi: 10.1016/j.rmed.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 532.Vivodtzev I, Debigaré R, Gagnon P, Mainguy V, Saey D, Dubé A, Paré ME, Bélanger M, Maltais F. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest. 2012;141:716–725. doi: 10.1378/chest.11-0839. [DOI] [PubMed] [Google Scholar]
- 533.Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:513–518. doi: 10.1513/pats.200708-124ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 535.Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- 536.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 537.Gertz I, Hedenstierna G, Hellers G, Wahren J. Muscle metabolism in patients with chronic obstructive lung disease and acute respiratory failure. Clin Sci Mol Med. 1977;52:396–403. [PubMed] [Google Scholar]
- 538.Jakobsson P, Jorfeldt L. Long-term oxygen therapy may improve skeletal muscle metabolism in advanced chronic obstructive pulmonary disease patients with chronic hypoxaemia. Respir Med. 1995;89:471–476. doi: 10.1016/0954-6111(95)90122-1. [DOI] [PubMed] [Google Scholar]
- 539.O’Donnell DE, D’Arsigny C, Webb KA. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:892–898. doi: 10.1164/ajrccm.163.4.2007026. [DOI] [PubMed] [Google Scholar]
- 540.Maltais F, Simon M, Jobin J, Desmeules M, Sullivan MJ, Bélanger M, Leblanc P. Effects of oxygen on lower limb blood flow and O2 uptake during exercise in COPD. Med Sci Sports Exerc. 2001;33:916–922. doi: 10.1097/00005768-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 541.Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J. 2001;18:77–84. doi: 10.1183/09031936.01.00082201. [DOI] [PubMed] [Google Scholar]
- 542.Porszasz J, Emtner M, Goto S, Somfay A, Whipp BJ, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest. 2005;128:2025–2034. doi: 10.1378/chest.128.4.2025. [DOI] [PubMed] [Google Scholar]
- 543.Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168:1034–1042. doi: 10.1164/rccm.200212-1525OC. [DOI] [PubMed] [Google Scholar]
- 544.Nonoyama ML, Brooks D, Lacasse Y, Guyatt GH, Goldstein RS. Oxygen therapy during exercise training in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2007;(2):CD005372. doi: 10.1002/14651858.CD005372.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 545.Vermeeren MA, Creutzberg EC, Schols AM, Postma DS, Pieters WR, Roldaan AC, Wouters EF COSMIC Study Group. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med. 2006;100:1349–1355. doi: 10.1016/j.rmed.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 546.Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J. 1994;7:1793–1797. doi: 10.1183/09031936.94.07101793. [DOI] [PubMed] [Google Scholar]
- 547.Yang L, Zhou M, Smith M, Yang G, Peto R, Wang J, Boreham J, Hu Y, Chen Z. Body mass index and chronic obstructive pulmonary disease-related mortality: a nationally representative prospective study of 220,000 men in China. Int J Epidemiol. 2010;39:1027–1036. doi: 10.1093/ije/dyq051. [DOI] [PubMed] [Google Scholar]
- 548.Sundh J, Ställberg B, Lisspers K, Montgomery SM, Janson C. Co-morbidity, body mass index and quality of life in COPD using the Clinical COPD Questionnaire. COPD. 2011;8:173–181. doi: 10.3109/15412555.2011.560130. [DOI] [PubMed] [Google Scholar]
- 549.Katsura H, Yamada K, Kida K. Both generic and disease specific health-related quality of life are deteriorated in patients with underweight COPD. Respir Med. 2005;99:624–630. doi: 10.1016/j.rmed.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 550.de Marco R, Accordini S, Marcon A, Cerveri I, Antó JM, Gislason T, Heinrich J, Janson C, Jarvis D, Kuenzli N, et al. European Community Respiratory Health Survey (ECRHS) Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183:891–897. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 551.Weekes CE, Emery PW, Elia M. Dietary counselling and food fortification in stable COPD: a randomised trial. Thorax. 2009;64:326–331. doi: 10.1136/thx.2008.097352. [DOI] [PubMed] [Google Scholar]
- 552.Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2003;58:745–751. doi: 10.1136/thorax.58.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Efthimiou J, Fleming J, Gomes C, Spiro SG. The effect of supplementary oral nutrition in poorly nourished patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1988;137:1075–1082. doi: 10.1164/ajrccm/137.5.1075. [DOI] [PubMed] [Google Scholar]
- 554.Creutzberg EC, Wouters EF, Mostert R, Weling-Scheepers CA, Schols AM. Efficacy of nutritional supplementation therapy in depleted patients with chronic obstructive pulmonary disease. Nutrition. 2003;19:120–127. doi: 10.1016/s0899-9007(02)00841-9. [DOI] [PubMed] [Google Scholar]
- 555.Op den Kamp CM, Langen RC, Haegens A, Schols AM. Muscle atrophy in cachexia: can dietary protein tip the balance? Curr Opin Clin Nutr Metab Care. 2009;12:611–616. doi: 10.1097/MCO.0b013e3283319399. [DOI] [PubMed] [Google Scholar]
- 556.Baldi S, Aquilani R, Pinna GD, Poggi P, De Martini A, Bruschi C. Fat-free mass change after nutritional rehabilitation in weight losing COPD: role of insulin, C-reactive protein and tissue hypoxia. Int J Chron Obstruct Pulmon Dis. 2010;5:29–39. doi: 10.2147/copd.s7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85:431–439. doi: 10.1093/ajcn/85.2.431. [DOI] [PubMed] [Google Scholar]
- 558.Pison CM, Cano NJ, Chérion C, Caron F, Court-Fortune I, Antonini MT, Gonzalez-Bermejo J, Meziane L, Molano LC, Janssens JP, et al. IRAD Investigators. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax. 2011;66:953–960. doi: 10.1136/thx.2010.154922. [DOI] [PubMed] [Google Scholar]
- 559.Creutzberg EC, Schols AM, Weling-Scheepers CA, Buurman WA, Wouters EF. Characterization of nonresponse to high caloric oral nutritional therapy in depleted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:745–752. doi: 10.1164/ajrccm.161.3.9808075. [DOI] [PubMed] [Google Scholar]
- 560.Sugawara K, Takahashi H, Kasai C, Kiyokawa N, Watanabe T, Fujii S, Kashiwagura T, Honma M, Satake M, Shioya T. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir Med. 2010;104:1883–1889. doi: 10.1016/j.rmed.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 561.Sugawara K, Takahashi H, Kashiwagura T, Yamada K, Yanagida S, Homma M, Dairiki K, Sasaki H, Kawagoshi A, Satake M, et al. Effect of anti-inflammatory supplementation with whey peptide and exercise therapy in patients with COPD. Respir Med. 2012;106:1526–1534. doi: 10.1016/j.rmed.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 562.Broekhuizen R, Wouters EF, Creutzberg EC, Weling-Scheepers CA, Schols AM. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60:376–382. doi: 10.1136/thx.2004.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 563.van de Bool C, Steiner MC, Schols AM. Nutritional targets to enhance exercise performance in chronic obstructive pulmonary disease. Curr Opin Clin Nutr Metab Care. 2012;15:553–560. doi: 10.1097/MCO.0b013e328358bdeb. [DOI] [PubMed] [Google Scholar]
- 564.Rutten EP, Engelen MP, Gosker H, Bast A, Cosemans K, Vissers YL, Wouters EF, Deutz NE, Schols AM. Metabolic and functional effects of glutamate intake in patients with chronic obstructive pulmonary disease (COPD) Clin Nutr. 2008;27:408–415. doi: 10.1016/j.clnu.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 565.Marwood S, Jack S, Patel M, Walker P, Bowtell J, Calverley P. No effect of glutamine ingestion on indices of oxidative metabolism in stable COPD. Respir Physiol Neurobiol. 2011;177:41–46. doi: 10.1016/j.resp.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 566.Saudny-Unterberger H, Martin JG, Gray-Donald K. Impact of nutritional support on functional status during an acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;156:794–799. doi: 10.1164/ajrccm.156.3.9612102. [DOI] [PubMed] [Google Scholar]
- 567.van Wetering CR, Hoogendoorn M, Broekhuizen R, Geraerts-Keeris GJ, De Munck DR, Rutten-van Mölken MP, Schols AM. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc. 2010;11:179–187. doi: 10.1016/j.jamda.2009.12.083. [DOI] [PubMed] [Google Scholar]
- 568.Bhasin S, Jasuja R. Selective androgen receptor modulators as function promoting therapies. Curr Opin Clin Nutr Metab Care. 2009;12:232–240. doi: 10.1097/MCO.0b013e32832a3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 569.Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, Morton RA, Steiner MS. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 570.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 571.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, Yarasheski KE, Sinha-Hikim I, Dzekov C, Dzekov J, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 572.Dobs AS, Nguyen T, Pace C, Roberts CP. Differential effects of oral estrogen versus oral estrogen-androgen replacement therapy on body composition in postmenopausal women. J Clin Endocrinol Metab. 2002;87:1509–1516. doi: 10.1210/jcem.87.4.8362. [DOI] [PubMed] [Google Scholar]
- 573.Svartberg J, Aasebø U, Hjalmarsen A, Sundsfjord J, Jorde R. Testosterone treatment improves body composition and sexual function in men with COPD, in a 6-month randomized controlled trial. Respir Med. 2004;98:906–913. doi: 10.1016/j.rmed.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 574.Schols AM, Soeters PB, Mostert R, Pluymers RJ, Wouters EF. Physiologic effects of nutritional support and anabolic steroids in patients with chronic obstructive pulmonary disease. A placebo-controlled randomized trial. Am J Respir Crit Care Med. 1995;152:1268–1274. doi: 10.1164/ajrccm.152.4.7551381. [DOI] [PubMed] [Google Scholar]
- 575.Ferreira IM, Verreschi IT, Nery LE, Goldstein RS, Zamel N, Brooks D, Jardim JR. The influence of 6 months of oral anabolic steroids on body mass and respiratory muscles in undernourished COPD patients. Chest. 1998;114:19–28. doi: 10.1378/chest.114.1.19. [DOI] [PubMed] [Google Scholar]
- 576.Yeh SS, DeGuzman B, Kramer T M012 Study Group. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest. 2002;122:421–428. doi: 10.1378/chest.122.2.421. [DOI] [PubMed] [Google Scholar]
- 577.Casaburi R, Make B, Piquette C, Kramer T, Ries A. Oxandrolone increases weight and muscle mass in underweight COPD patients [abstract] Chest. 2002;122:73S. [Google Scholar]
- 578.Creutzberg EC, Wouters EF, Mostert R, Pluymers RJ, Schols AM. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124:1733–1742. doi: 10.1378/chest.124.5.1733. [DOI] [PubMed] [Google Scholar]
- 579.Sharma S, Arneja A, McLean L, Duerksen D, Leslie W, Sciberras D, Lertzman M. Anabolic steroids in COPD: a review and preliminary results of a randomized trial. Chron Respir Dis. 2008;5:169–176. doi: 10.1177/1479972308092350. [DOI] [PubMed] [Google Scholar]
- 580.Chavoshan B, Fournier M, Lewis MI, Porszasz J, Storer TW, Da X, Rambod M, Casaburi R. Testosterone and resistance training effects on muscle nitric oxide synthase isoforms in COPD men. Respir Med. 2012;106:269–275. doi: 10.1016/j.rmed.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 581.Hartman ML, Faria AC, Vance ML, Johnson ML, Thorner MO, Veldhuis JD. Temporal structure of in vivo growth hormone secretory events in humans. Am J Physiol. 1991;260:E101–E110. doi: 10.1152/ajpendo.1991.260.1.E101. [DOI] [PubMed] [Google Scholar]
- 582.Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717–797. doi: 10.1210/edrv.19.6.0353. [DOI] [PubMed] [Google Scholar]
- 583.Estívariz CF, Ziegler TR. Nutrition and the insulin-like growth factor system. Endocrine. 1997;7:65–71. doi: 10.1007/BF02778066. [DOI] [PubMed] [Google Scholar]
- 584.Liu H, Bravata DM, Olkin I, Friedlander A, Liu V, Roberts B, Bendavid E, Saynina O, Salpeter SR, Garber AM, et al. Systematic review: the effects of growth hormone on athletic performance. Ann Intern Med. 2008;148:747–758. doi: 10.7326/0003-4819-148-10-200805200-00215. [DOI] [PubMed] [Google Scholar]
- 585.Mador MJ, Bozkanat E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Res. 2001;2:216–224. doi: 10.1186/rr60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 586.Anand IS, Chandrashekhar Y, Ferrari R, Sarma R, Guleria R, Jindal SK, Wahi PL, Poole-Wilson PA, Harris P. Pathogenesis of congestive state in chronic obstructive pulmonary disease. Studies of body water and sodium, renal function, hemodynamics, and plasma hormones during edema and after recovery. Circulation. 1992;86:12–21. doi: 10.1161/01.cir.86.1.12. [DOI] [PubMed] [Google Scholar]
- 587.Scalvini S, Volterrani M, Vitacca M, Clark AL, Solfrini R, Panzali AM, Ferrari R, Levi GF. Plasma hormone levels and haemodynamics in patients with chronic obstructive lung disease. Monaldi Arch Chest Dis. 1996;51:380–386. [PubMed] [Google Scholar]
- 588.Creutzberg EC, Casaburi R. Endocrinological disturbances in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:76s–80s. doi: 10.1183/09031936.03.00004610. [DOI] [PubMed] [Google Scholar]
- 589.Zadik Z, Chalew SA, McCarter RJ, Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–516. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]
- 590.Wehrenberg WB, Janowski BA, Piering AW, Culler F, Jones KL. Glucocorticoids: potent inhibitors and stimulators of growth hormone secretion. Endocrinology. 1990;126:3200–3203. doi: 10.1210/endo-126-6-3200. [DOI] [PubMed] [Google Scholar]
- 591.Veldhuis JD, Iranmanesh A. Physiological regulation of the human growth hormone (GH)-insulin-like growth factor type I (IGF-I) axis: predominant impact of age, obesity, gonadal function, and sleep. Sleep. 1996;19:S221–S224. doi: 10.1093/sleep/19.suppl_10.s221. [DOI] [PubMed] [Google Scholar]
- 592.Graham MR, Baker JS, Evans P, Kicman A, Cowan D, Hullin D, Davies B. Short-term recombinant human growth hormone administration improves respiratory function in abstinent anabolic-androgenic steroid users. Growth Horm IGF Res. 2007;17:328–335. doi: 10.1016/j.ghir.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 593.Meinhardt U, Nelson AE, Hansen JL, Birzniece V, Clifford D, Leung KC, Graham K, Ho KK. The effects of growth hormone on body composition and physical performance in recreational athletes: a randomized trial. Ann Intern Med. 2010;152:568–577. doi: 10.7326/0003-4819-152-9-201005040-00007. [DOI] [PubMed] [Google Scholar]
- 594.Pape GS, Friedman M, Underwood LE, Clemmons DR. The effect of growth hormone on weight gain and pulmonary function in patients with chronic obstructive lung disease. Chest. 1991;99:1495–1500. doi: 10.1378/chest.99.6.1495. [DOI] [PubMed] [Google Scholar]
- 595.Gullett NP, Hebbar G, Ziegler TR. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. Am J Clin Nutr. 2010;91:1143S–1147S. doi: 10.3945/ajcn.2010.28608E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 596.Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156:1800–1806. doi: 10.1164/ajrccm.156.6.9704142. [DOI] [PubMed] [Google Scholar]
- 597.Suchner U, Rothkopf MM, Stanislaus G, Elwyn DH, Kvetan V, Askanazi J. Growth hormone and pulmonary disease. Metabolic effects in patients receiving parenteral nutrition. Arch Intern Med. 1990;150:1225–1230. [PubMed] [Google Scholar]
- 598.Khorram O. Use of growth hormone and growth hormone secretagogues in aging: help or harm. Clin Obstet Gynecol. 2001;44:893–901. doi: 10.1097/00003081-200112000-00026. [DOI] [PubMed] [Google Scholar]
- 599.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 600.Root AW, Root MJ. Clinical pharmacology of human growth hormone and its secretagogues. Curr Drug Targets Immune Endocr Metabol Disord. 2002;2:27–52. [PubMed] [Google Scholar]
- 601.Chappel S. Can GHRH or GH secretagogues re-initiate pituitary GH pulsatility? Clin Endocrinol (Oxf) 1999;50:547–556. doi: 10.1046/j.1365-2265.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- 602.Wang Y, Tomlinson B. Tesamorelin, a human growth hormone releasing factor analogue. Expert Opin Investig Drugs. 2009;18:303–310. doi: 10.1517/13543780802707658. [DOI] [PubMed] [Google Scholar]
- 603.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 604.Smith RG, Van der Ploeg LH, Howard AD, Feighner SD, Cheng K, Hickey GJ, Wyvratt MJ, Jr, Fisher MH, Nargund RP, Patchett AA. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18:621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- 605.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87:2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 606.Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG, Dhillo WS, Ghatei MA, Bloom SR. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992–5995. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 607.Granata R, Isgaard J, Alloatti G, Ghigo E. Cardiovascular actions of the ghrelin gene-derived peptides and growth hormone-releasing hormone. Exp Biol Med (Maywood) 2011;236:505–514. doi: 10.1258/ebm.2011.010365. [DOI] [PubMed] [Google Scholar]
- 608.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 609.Neary NM, Small CJ, Wren AM, Lee JL, Druce MR, Palmieri C, Frost GS, Ghatei MA, Coombes RC, Bloom SR. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:2832–2836. doi: 10.1210/jc.2003-031768. [DOI] [PubMed] [Google Scholar]
- 610.Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, Kangawa K. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- 611.Chopin L, Walpole C, Seim I, Cunningham P, Murray R, Whiteside E, Josh P, Herington A. Ghrelin and cancer. Mol Cell Endocrinol. 2011;340:65–69. doi: 10.1016/j.mce.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 612.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 613.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 614.Ying BW, Song XB, Fan H, Wang LL, Li YS, Cheng Z, Cheng H, Wen FQ. Plasma ghrelin levels and weight loss in Chinese Uygur patients with chronic obstructive pulmonary disease. J Int Med Res. 2008;36:1371–1377. doi: 10.1177/147323000803600626. [DOI] [PubMed] [Google Scholar]
- 615.Itoh T, Nagaya N, Yoshikawa M, Fukuoka A, Takenaka H, Shimizu Y, Haruta Y, Oya H, Yamagishi M, Hosoda H, et al. Elevated plasma ghrelin level in underweight patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:879–882. doi: 10.1164/rccm.200310-1404OC. [DOI] [PubMed] [Google Scholar]
- 616.Lainscak M, Andreas S, Scanlon PD, Somers VK, Anker SD. Ghrelin and neurohumoral antagonists in the treatment of cachexia associated with cardiopulmonary disease. Intern Med. 2006;45:837–844. doi: 10.2169/internalmedicine.45.1867. [DOI] [PubMed] [Google Scholar]
- 617.Kodama T, Ashitani J, Matsumoto N, Kangawa K, Nakazato M. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulm Pharmacol Ther. 2008;21:774–779. doi: 10.1016/j.pupt.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 618.Ferdinandi ES, Brazeau P, High K, Procter B, Fennell S, Dubreuil P. Non-clinical pharmacology and safety evaluation of TH9507, a human growth hormone-releasing factor analogue. Basic Clin Pharmacol Toxicol. 2007;100:49–58. doi: 10.1111/j.1742-7843.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 619.US Food and Drug Administration. FDA labeling information. Egrifta (tesamorelin for injection). FDA website. 2012. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022505s004lbl.pdf
- 620.Grunfeld C, Dritselis A, Kirkpatrick P. Tesamorelin. Nat Rev Drug Discov. 2011;10:95–96. doi: 10.1038/nrd3362. [DOI] [PubMed] [Google Scholar]
- 621.Abribat T, Chapdelaine A, Gravel D. Th 9507, a new growth hormone-releasing factor (GFR) analogue, is a powerful insulin-like growth factor -1 (IGF-1) inducer in 50–60 year old healthy subjects [abstract] Endocrin Soc. 2001;83:292. [Google Scholar]
- 622.Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011;96:150–158. doi: 10.1210/jc.2010-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 623.Schols AM, Maltais F, O’Donnell DE, Hernandez P, Tellier J, Lussier B, Allas S, Chapdelaine A, Abribat T, Vachon L. Anabolic effects and safety assessments of TH9507, a growth hormone releasing factor (GRF) analog, in patients with COPD-associated muscle wasting [abstract] Eur Respir J. 2004;24:292s. [Google Scholar]
- 624.Maltais F, O’Donnell DE, Schols AM, Hernandez P, Tellier J, Lussier B, Allas S, Abribat T, Vachon L. Effect of TH9507, a growth hormone releasing factor (GFR) analog, on functional performance in patients with COPD. Eur Respir J. 2004;24:245s. [Google Scholar]
- 625.Maltais F, O’Donnell DE, Schols AM, Hernandez P, Lussier B, Allas S, Deslauriers N, Abribat T, Vachon L. Effect of TH9507, a growth hormone-releasing factor (GFR) analogue, on health-related quality of life and respiratory muscle function in patients with COPD-associated wasting [abstract] Proc Am Thorac Soc. 2005;2:A127. [Google Scholar]
- 626.US National Institutes of Health. Efficacy and safety study of tesamorelin in chronic obstructive pulmonary disease (COPD) subjects with muscle wasting. 2012. Available from: http://clinicaltrialsfeeds.org/clinical-trials/show/NCT01388920
- 627.Yu H, Kim K. Direct nose-to-brain transfer of a growth hormone releasing neuropeptide, hexarelin after intranasal administration to rabbits. Int J Pharm. 2009;378:73–79. doi: 10.1016/j.ijpharm.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 628.Mantovani G, Macciò A, Lai P, Massa E, Ghiani M, Santona MC. Cytokine involvement in cancer anorexia/cachexia: role of megestrol acetate and medroxyprogesterone acetate on cytokine downregulation and improvement of clinical symptoms. Crit Rev Oncog. 1998;9:99–106. doi: 10.1615/critrevoncog.v9.i2.10. [DOI] [PubMed] [Google Scholar]
- 629.Yeh SS, Wu SY, Levine DM, Parker TS, Olson JS, Stevens MR, Schuster MW. The correlation of cytokine levels with body weight after megestrol acetate treatment in geriatric patients. J Gerontol A Biol Sci Med Sci. 2001;56:M48–M54. doi: 10.1093/gerona/56.1.m48. [DOI] [PubMed] [Google Scholar]
- 630.Weisberg J, Wanger J, Olson J, Streit B, Fogarty C, Martin T, Casaburi R. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest. 2002;121:1070–1078. doi: 10.1378/chest.121.4.1070. [DOI] [PubMed] [Google Scholar]
- 631.Branch JD. Effect of creatine supplementation on body composition and performance: a meta-analysis. Int J Sport Nutr Exerc Metab. 2003;13:198–226. doi: 10.1123/ijsnem.13.2.198. [DOI] [PubMed] [Google Scholar]
- 632.Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and strength gains with resistance exercise: a meta-analysis. J Appl Physiol (1985) 2003;94:651–659. doi: 10.1152/japplphysiol.00755.2002. [DOI] [PubMed] [Google Scholar]
- 633.Fuld JP, Kilduff LP, Neder JA, Pitsiladis Y, Lean ME, Ward SA, Cotton MM. Creatine supplementation during pulmonary rehabilitation in chronic obstructive pulmonary disease. Thorax. 2005;60:531–537. doi: 10.1136/thx.2004.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Faager G, Söderlund K, Sköld CM, Rundgren S, Tollbäck A, Jakobsson P. Creatine supplementation and physical training in patients with COPD: a double blind, placebo-controlled study. Int J Chron Obstruct Pulmon Dis. 2006;1:445–453. doi: 10.2147/copd.2006.1.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 635.Deacon SJ, Vincent EE, Greenhaff PL, Fox J, Steiner MC, Singh SJ, Morgan MD. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:233–239. doi: 10.1164/rccm.200710-1508OC. [DOI] [PubMed] [Google Scholar]
- 636.Al-Ghimlas F, Todd DC. Creatine supplementation for patients with COPD receiving pulmonary rehabilitation: a systematic review and meta-analysis. Respirology. 2010;15:785–795. doi: 10.1111/j.1440-1843.2010.01770.x. [DOI] [PubMed] [Google Scholar]
- 637.Brass EP. Supplemental carnitine and exercise. Am J Clin Nutr. 2000;72:618S–623S. doi: 10.1093/ajcn/72.2.618S. [DOI] [PubMed] [Google Scholar]
- 638.Villaça DS, Lerario MC, Dal Corso S, Neder JA. New treatments for chronic obstructive pulmonary disease using ergogenic aids. J Bras Pneumol. 2006;32:66–74. doi: 10.1590/s1806-37132006000100013. [DOI] [PubMed] [Google Scholar]
- 639.Borghi-Silva A, Baldissera V, Sampaio LM, Pires-DiLorenzo VA, Jamami M, Demonte A, Marchini JS, Costa D. L-carnitine as an ergogenic aid for patients with chronic obstructive pulmonary disease submitted to whole-body and respiratory muscle training programs. Braz J Med Biol Res. 2006;39:465–474. doi: 10.1590/s0100-879x2006000400006. [DOI] [PubMed] [Google Scholar]
- 640.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 641.Ferreira LF, Reid MB. Muscle-derived ROS and thiol regulation in muscle fatigue. J Appl Physiol (1985) 2008;104:853–860. doi: 10.1152/japplphysiol.00953.2007. [DOI] [PubMed] [Google Scholar]
- 642.Gosker HR, Bast A, Haenen GR, Fischer MA, van der Vusse GJ, Wouters EF, Schols AM. Altered antioxidant status in peripheral skeletal muscle of patients with COPD. Respir Med. 2005;99:118–125. doi: 10.1016/j.rmed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 643.Goldfarb AH. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999;24:249–266. doi: 10.1139/h99-021. [DOI] [PubMed] [Google Scholar]
- 644.Powers SK, DeRuisseau KC, Quindry J, Hamilton KL. Dietary antioxidants and exercise. J Sports Sci. 2004;22:81–94. doi: 10.1080/0264041031000140563. [DOI] [PubMed] [Google Scholar]
- 645.Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol (1985) 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- 646.Supinski G, Nethery D, Stofan D, DiMarco A. Effect of free radical scavengers on diaphragmatic fatigue. Am J Respir Crit Care Med. 1997;155:622–629. doi: 10.1164/ajrccm.155.2.9032204. [DOI] [PubMed] [Google Scholar]
- 647.Mantovani G, Macciò A, Madeddu C, Gramignano G, Lusso MR, Serpe R, Massa E, Astara G, Deiana L. A phase II study with antioxidants, both in the diet and supplemented, pharmaconutritional support, progestagen, and anti-cyclooxygenase-2 showing efficacy and safety in patients with cancer-related anorexia/cachexia and oxidative stress. Cancer Epidemiol Biomarkers Prev. 2006;15:1030–1034. doi: 10.1158/1055-9965.EPI-05-0538. [DOI] [PubMed] [Google Scholar]
- 648.Barreiro E, Gáldiz JB, Mariñán M, Alvarez FJ, Hussain SN, Gea J. Respiratory loading intensity and diaphragm oxidative stress: N-acetyl-cysteine effects. J Appl Physiol (1985) 2006;100:555–563. doi: 10.1152/japplphysiol.00780.2005. [DOI] [PubMed] [Google Scholar]
- 649.Rahman I, MacNee W. Antioxidant pharmacological therapies for COPD. Curr Opin Pharmacol. 2012;12:256–265. doi: 10.1016/j.coph.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 650.McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 651.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol (1985) 2004;97:1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- 652.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int. 2011;22:859–871. doi: 10.1007/s00198-010-1407-y. [DOI] [PubMed] [Google Scholar]
- 654.Dawson-Hughes B. Serum 25-hydroxyvitamin D and muscle atrophy in the elderly. Proc Nutr Soc. 2012;71:46–49. doi: 10.1017/S0029665111003260. [DOI] [PubMed] [Google Scholar]
- 655.Daly RM. Independent and combined effects of exercise and vitamin D on muscle morphology, function and falls in the elderly. Nutrients. 2010;2:1005–1017. doi: 10.3390/nu2091005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Bunout D, Barrera G, Leiva L, Gattas V, de la Maza MP, Avendaño M, Hirsch S. Effects of vitamin D supplementation and exercise training on physical performance in Chilean vitamin D deficient elderly subjects. Exp Gerontol. 2006;41:746–752. doi: 10.1016/j.exger.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 657.Kukuljan S, Nowson CA, Sanders K, Daly RM. Effects of resistance exercise and fortified milk on skeletal muscle mass, muscle size, and functional performance in middle-aged and older men: an 18-mo randomized controlled trial. J Appl Physiol (1985) 2009;107:1864–1873. doi: 10.1152/japplphysiol.00392.2009. [DOI] [PubMed] [Google Scholar]
- 658.Ringbaek T, Martinez G, Durakovic A, Thøgersen J, Midjord AK, Jensen JE, Lange P. Vitamin d status in patients with chronic obstructive pulmonary disease who participate in pulmonary rehabilitation. J Cardiopulm Rehabil Prev. 2011;31:261–267. doi: 10.1097/HCR.0b013e31821c13aa. [DOI] [PubMed] [Google Scholar]
- 659.Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, Decallonne B, Bouillon R, Decramer M, Janssens W. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156:105–114. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 660.Hornikx M, Van Remoortel H, Lehouck A, Mathieu C, Maes K, Gayan-Ramirez G, Decramer M, Troosters T, Janssens W. Vitamin D supplementation during rehabilitation in COPD: a secondary analysis of a randomized trial. Respir Res. 2012;13:84. doi: 10.1186/1465-9921-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Coin A, Sergi G, Minicuci N, Giannini S, Barbiero E, Manzato E, Pedrazzoni M, Minisola S, Rossini M, Del Puente A, et al. Fat-free mass and fat mass reference values by dual-energy X-ray absorptiometry (DEXA) in a 20-80 year-old Italian population. Clin Nutr. 2008;27:87–94. doi: 10.1016/j.clnu.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 662.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(4):CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 663.Ferreira IM, Brooks D, Lacasse Y, Goldstein RS, White J. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005;(2):CD000998. doi: 10.1002/14651858.CD000998.pub2. [DOI] [PubMed] [Google Scholar]