Abstract
Indirect fluorescence analysis (IFA), the gold standard for determining herpesvirus antibody titers, is labor-intensive and poorly suited for large population-based studies. The enzyme-linked immunosorbent assay (ELISA) is used widely for measuring antiviral antibodies but also suffers drawbacks such as reduced specificity and the qualitative nature of the results due to limited interpretation of the optical density (OD) units. This paper describes a method to titer herpesvirus antibodies using microplates coated with virally-infected cells in which a standard curve, derived from IFA-scored samples, allowed OD units to be converted into titers. A LOOKUP function was created in order to report the data as traditional IFA-based (i.e., 2-fold) titers. The modified ELISA correlated significantly with IFA and was subsequently used to compute endpoint antibody titers to Epstein-Barr virus (EBV)-virus capsid antigen (VCA) and cytomegalovirus (CMV) in blood samples taken from 398 pregnant Hispanic women. Four women were EBV negative (1%), while 58 women were CMV negative (14.6%). EBV VCA antibody titers were significantly higher than CMV antibody titers (p <0.001). This method allows titering of herpesvirus antibodies by ELISA suitable for large population-based studies. In addition, the LOOKUP table enables conversion from OD-derived titers into 2-fold titers for comparison of results with other studies.
Keywords: herpesvirus, EBV, CMV, ELISA, antibody titer, IFA
1. Introduction
Herpesviruses commonly establish latent infections in the majority of adults worldwide. The best known members of this family include cytomegalovirus (CMV) and Epstein-Barr virus (EBV). Herpesviruses are medically important viruses; EBV infects over 90% of the adult population and is the causative agent of infectious mononucleosis, Burkitt's lymphoma, nasopharyngeal carcinoma, and diffuse polyclonal B-cell lymphoma. As with EBV, infection with CMV usually occurs asymptomatically during childhood but may result in a mononucleosis-like syndrome, central nervous system infections, and febrile illnesses. Notably, EBV and CMV infections can be severe in immunocompromised individuals such as AIDS and post transplant patients.
While the medical importance and effects of symptomatic herpesvirus infections is well documented, recent attention has focused on the effects of life-long infection and subclinical reactivation of these viruses. Studies have found an association between herpesvirus seropositivity and inflammation, kidney disease, and frailty (Trzonkowski et al., 2003; Schmaltz et al., 2005; Wall et al., 2013). CMV in particular has been associated with an immune risk phenotype, cardiovascular disease, and higher mortality (Wikby et al., 2006; Simanek et al., 2011).
Maladaptive alterations in cellular immune function can enhance latent herpesvirus reactivation and replication, resulting in elevated antibody titers. Elevated herpesvirus antibodies have been linked to a number of diseases. As an extreme case, organ transplant patients provide a dramatic illustration of the association between dysregulated cellular immune function and high antiviral antibody titers (Gray et al., 1995). High levels of herpesvirus antibodies have also been linked to premature mortality (Aiello et al., 2006; Aiello et al., 2008), development of coronary artery disease (Kendall et al., 1992), fatigue before cancer treatment (Fagundes et al., 2012), and cognitive impairment in elderly adults with cardiovascular disease (Strandberg et al., 2003). In addition, elevated CMV antibodies were associated with cognitive impairment even after controlling for numerous covariates including age, education, and health conditions (Aiello et al., 2006).
The gold standard for measuring antiviral antibodies is the indirect fluorescence analysis (IFA) method. In general, a blood serum or plasma sample containing antibody is serially diluted (e.g., 1:10, 1:20, 1:40, 1:80). The dilutions are then applied to slide wells containing both virally-infected cells and uninfected cells (built-in control to distinguish non-specific reactions). Following a short incubation time, an appropriate fluorescent antibody (IgG or IgM) conjugate with counterstain is added to each of the slide wells. The slides are then read manually on a fluorescent microscope with appropriate filters. The assigned titer is indicative of the last dilution in which the antibody was detected. For example, if antibody was detected in each of the tubes listed above except for the 1:80 dilution, the titer is said to be 40. Therefore the titer is the degree to which the antibody-serum solution can be diluted and still contain detectable amounts of antibody.
Although highly specific, IFA is labor-intensive and not suitable for large numbers of samples. For instance, measurement of antibody titers to three herpesviruses by IFA in samples from 1,457 subjects (Stowe et al., 2010) required 4–5 months. In addition, results can vary between laboratories due to differences in type or condition of fluorescence microscope employed as well as the experience level of personnel performing the assay. Accordingly, the enzyme-linked immunosorbent assay (ELISA) has become a widespread method of screening for antiviral antibodies in population-based studies. If the viral antibody is present in the patient sample, the antibody will bind to the antigen on the plates. Upon addition of an enzyme-linked secondary antibody, a color-generating reaction occurs that increases the optical density (OD). The results are reported as positive if the observed OD for a patient sample is above the specified threshold for the assay or negative if the OD is below the threshold. However, this method also has several drawbacks including: 1) synthetic peptides as antigens are less sensitive and less specific; 2) the number and quantity of antigens has not been standardized resulting in manufacturer-to-manufacturer test variability, and 3) the results are qualitative (i.e., negative/positive) or semi-quantitative due to the limited interpretation of the OD units (Hess, 2004).
This paper describes a new method to titer EBV and CMV antibodies for use in population-based studies. Microplates coated with the same virally-infected cells used for IFA substrate slides were used as the source of antigen. IFA-scored plasma samples were then used to create a standard curve allowing OD units to be converted into titers. To report the results as 2-fold titers, a LOOKUP function was created to transform the OD-derived values. The results show that this approach constitutes a rapid, quantitative method to titer EBV and CMV antibodies in large scale studies.
2. Materials and Methods
2.1 Samples
Plasma samples from 398 pregnant Hispanic women (ages 14–45; mean age 25 ± 6) were used for this study (Ruiz et al., 2012). In addition, plasma was obtained from peripheral blood samples taken from 32 healthy adults (20 males and 12 non-pregnant females, mean age = 29) as a comparison group. The Institutional Review Board at the University of Texas Austin approved the study, and all participants gave informed consent.
2.2 Measurement of anti-viral antibodies by IFA
Anti-viral antibody titers were determined by indirect immunofluorescence as previously described (Stowe et al., 2001; Stowe et al., 2007; Stowe et al., 2010). Commercially prepared substrate slides and control sera (Bion Enterprises, Park Ridge, IL) were used for determining IgG antibody titers to EBV-viral capsid antigen (VCA) and CMV. Briefly, 30 ul of serially diluted plasma were pipetted onto spot slides and incubated for 30 minutes at room temperature. For VCA, the antigen substrate slides contained a mixture of EBV (P3HR1 strain) infected and uninfected lymphocytic cells fixed onto each well. For CMV, the antigen substrate slides contained a mixture of CMV (clinical specimen) infected and uninfected human diploid fibroblast cells fixed onto each well. Each well contained an average of 10–20% infected cells per 200X field. After incubation, the spot slides were rinsed for 5 minutes in PBS. The secondary antibody used was anti-human IgG conjugated with fluorescein isothiocynate (FITC). Evans blue was used as a counterstain. After a second incubation (30 minutes at room temperature), slides were washed, lightly blotted, and mounted with mounting medium. All specimens were batch-analyzed and read blind-coded. Slides were read using an Axioskop microscope (Zeiss, Oberkochen, Germany) with appropriate barrier and excitation filters for FITC visualization. The endpoint titer was determined as the highest dilution of serum demonstrating immunofluorescent-positive cells.
2.3 ELISA-based method for titer determination
Ninety-six well microtiter plates, coated with virally infected cells, were obtained from EuroImmun (Morris Plains, NJ). Antigen source for VCA plates (EI 2791-9601-G) were inactivated cell lysates of lymphocytes infected with the P3HR1 strain of EBV. The antigen sources for the CMV plates (EI 2570-9601-G) were inactivated cell lysates of MRC-5 (diploid fibroblast) cells infected with the CMV AD169 strain. These kits include cut-off calibrators to differentiate samples from individuals with prior exposure (i.e., seropositive).
Plasma samples with high IFA-scored antibody titers (i.e., 2560), obtained from prior studies, were used as the top standards for both VCA and CMV. Seven two-fold serial dilutions of the top standards were made with PBS in separate tubes. After diluting, the VCA and CMV standards were 2560, 1280, 640, 320, 160, 80, 40, and 20. One hundred microliters of positive and negative controls, standards, and diluted patient samples (all dilutions were at 1:101 with PBS) were pipetted in duplicate into individual microplate wells followed by a 30 min incubation (all steps were carried out at room temperature). The plates were then washed 3 times with 350ul wash buffer (provided) using an Embla microplate washer (Molecular Devices, Menlo Park, CA). Next, 100ul of enzyme conjugate (peroxidase labeled anti-human IgG) was pipetted into the wells followed by another 30 min incubation period. The plates were then washed 3 times, and 100ul of chromogen substrate (TMB/H2O2) was pipetted into the wells. The plates were then covered to protect from direct light and incubated for 15 min. One hundred microliters of 0.5 M sulphuric acid) was added to each well to stop the reaction. Absorbance was then read at 450nm (reference wavelength 620nm) using a SpectraMax Plus 384 (Molecular Devices). The values of the unknown samples were assigned in relation to the standard curve.
2.4 Excel look up function
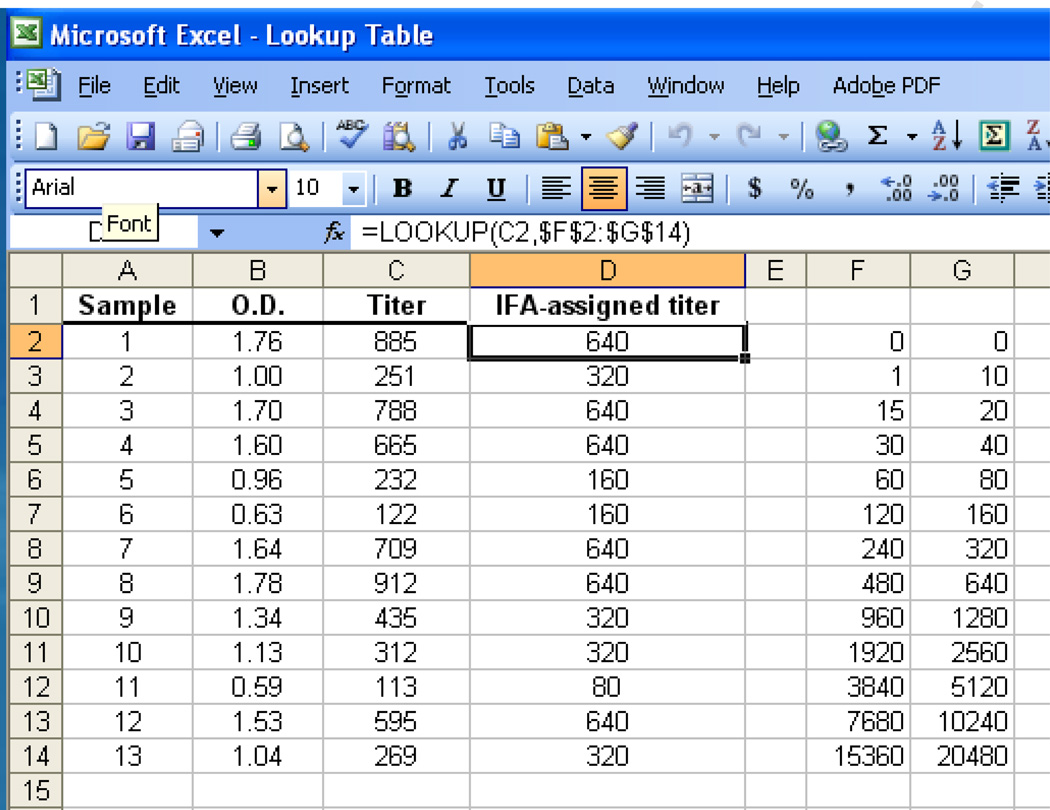
A LOOKUP function was created in order to transform OD-derived titers into 2-fold titers (as would be obtained with IFA). The LOOKUP function returns a value either from a one-row or one-column range or from an array. A data table was written that returns a specific titer depending on the titer range (Fig. 1). For instance, a titer of 80 was assigned for OD-derived titers that ranged from 60 to 119; a titer of 160 was assigned for OD-derived titers that ranged from 120 to 239. The syntax was as follows: LOOKUP (lookup_value, lookup_vector, result_vector). The function was then copied onto cells adjacent to the data column containing the OD-derived titer values for subsequent transformation.
Fig. 1.
LOOKUP table for assigning titers (2-fold) from ELISA-derived titer data. The sample numbers are indicated in column A. Columns B and C show the (raw) OD and titer (calculated from the standard curve) data, respectively. The titer values are then compared against the table data (contained in columns F2:G14) for conversion into an IFA-assigned titer (column D).
2.5 Statistical Analysis
Statistical analysis was performed using SigmaStat software v2.03 (SPSS, Chicago, IL). Normality was assessed using the Kolmogorov-Smirnov normality test. Data not normally distributed were subjected to natural log transformations to normalize the distributions prior to analyses. Since the method of doubling dilutions was used to obtained antibody titer results, a log base-2 transformation was used to reduce variance for statistical comparisons. Where expressed, results are mean ± SE and p values less than 0.05 were considered significant.
3. Results
3.1 Validation of the ELISA method for antiviral antibody titers
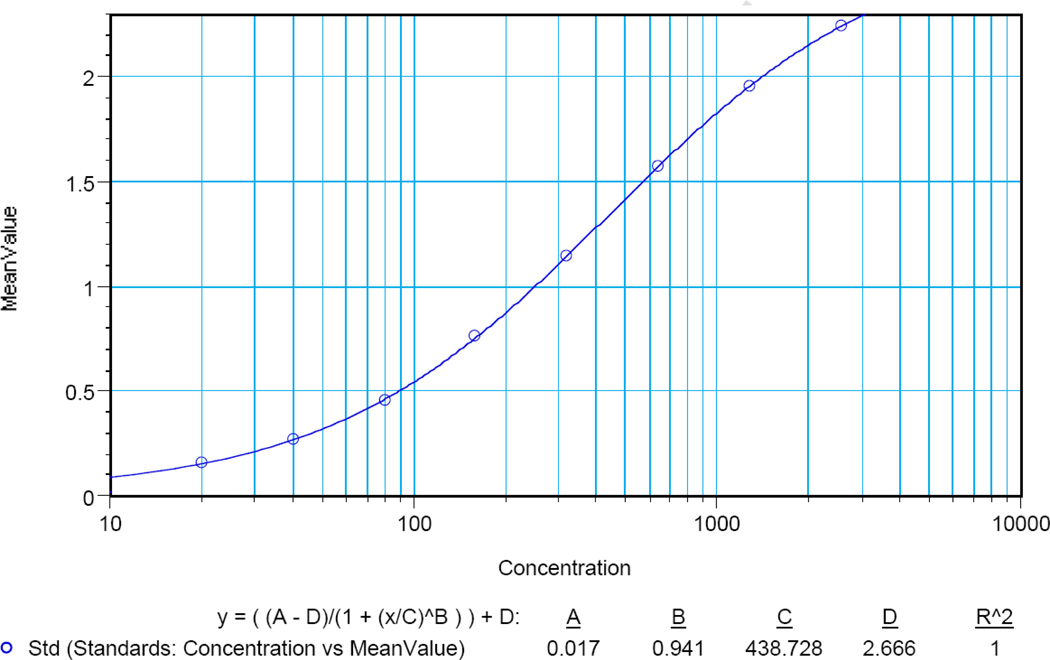
Typical analyses of VCA standards are shown in Table 1. The highest standard (St01) yielded a mean OD value of 2.245 and a standard deviation of 0.023 (CV = 1.0%). Serial dilutions yielded results with excellent standard deviations (range 0.002 – 0.021) and CVs (range 0.1 – 3.0). Similar results were found for CMV (Table 2). The highest standard (St01) yielded a mean OD value of 2.248 and a standard deviation of 0.071 (CV = 3.2%). Serial dilutions also yielded results with excellent standard deviations (range 0.000 – 0.071) and CVs (range 0.0 – 3.2). Initial analyses showed that the data points for the VCA and CMV standards were not linear (data not shown). However, using a non-linear regression curve (a 4–parameter logistic curve fit commonly used for immunoassays) gave excellent results (Fig. 2; VCA). Data for CMV also showed an excellent correlation (r2=0.999; data not shown).
Table 1.
Analysis of EBV VCA standards
| Sample | Concentration | Value Range | Mean Value | Std.Dev. | CV% |
|---|---|---|---|---|---|
| St01 | 2560.00 | 2.229 – 2.262 | 2.245 | 0.023 | 1.0 |
| St02 | 1280.00 | 1.953 – 1.956 | 1.954 | 0.002 | 0.1 |
| St03 | 640.00 | 1.569 – 1.582 | 1.576 | 0.009 | 0.6 |
| St04 | 320.00 | 1.145 – 1.146 | 1.145 | 0.001 | 0.1 |
| St05 | 160.00 | 0.748 – 0.778 | 0.763 | 0.021 | 2.8 |
| St06 | 80.00 | 0.451 – 0.457 | 0.454 | 0.005 | 1.0 |
| St07 | 40.00 | 0.261 – 0.272 | 0.267 | 0.008 | 3.0 |
| St08 | 20.00 | 0.156 – 0.159 | 0.158 | 0.002 | 1.6 |
Table 2.
Analysis of CMV standards
| Sample | Concentration | Value Range | Mean Value | Std.Dev. | CV% |
|---|---|---|---|---|---|
| St01 | 2560.00 | 2.197 – 2.298 | 2.248 | 0.071 | 3.2 |
| St02 | 1280.00 | 2.087 – 2.087 | 1.954 | 0.000 | 0.0 |
| St03 | 640.00 | 1.873 – 1.895 | 1.884 | 0.015 | 0.8 |
| St04 | 320.00 | 1.513 – 1.517 | 1.515 | 0.002 | 0.2 |
| St05 | 160.00 | 1.162 – 1.205 | 1.184 | 0.030 | 2.5 |
| St06 | 80.00 | 0.780 – 0.783 | 0.782 | 0.002 | 0.3 |
| St07 | 40.00 | 0.536 – 0.538 | 0.537 | 0.002 | 0.3 |
| St08 | 20.00 | 0.349 – 0.359 | 0.354 | 0.007 | 1.9 |
Fig. 2.
A typical standard curve for VCA antibodies. Seven dilutions of the highest standard, yielding a range from 2560 – 20, were analyzed in duplicate. The OD (mean value) is indicated on the Y-axis, whereas the X-axis (concentration) indicates the titer value.
The assay precision, defined using three different samples, has been reported by the manufacturer: CVs within and between assays were 4.2–7.4% (n = 20) and 3.2–8.2% (n= 20), respectively. The intra-assay variation of the VCA and CMV plates was tested by running 16 replicates on the same plate. The mean OD and standard error was 2.0 ± 0.03 for VCA (CV =5.6%) and 1.6 ± 0.03 for CMV (CV =8.3%); these results are in line with data from the manufacturers test runs. The VCA and CMV assays are reported by the kit manufacturer not to be cross-reactive with other herpesviruses, measles, mumps, and rubella. In the current study cross reactivity between herpesviruses, including herpes simplex virus type 1 (HSV-1), was not found after testing these samples which is in agreement with the manufacturer’s results (data not shown). Regarding sensitivity, the 20 standard (St08) for both VCA and CMV was used as the lower cutoff (i.e., to determine whether the subject was seropositive or seronegative) as this dilution yielded similar ODs as the #2 calibrator supplied with the kit used to determine negative/positive results.
Thirty-one plasma samples, previously titered by IFA for VCA and CMV, were analyzed using the ELISA-based method in order to compare methods. There was a significant correlation (r2 = 0.98; p <0.001) between the IFA-derived titers and the OD-derived titers. After transformation by the LOOKUP function, the correlation remained significant (r2 = 0.97; p <0.001). A similar relationship was found for CMV (r2 = 0.92; p <0.001) after transformation by the LOOKUP function. Of note, the ELISA-based method yielded results slightly more sensitive that the IFA method in that one sample for VCA and two samples for CMV were scored one dilution higher by the ELISA-based method after transformation by the LOOKUP function. Samples seronegative by IFA for EBV VCA (n=4) and CMV (n=10) were also analyzed by the ELISA-based method; the results also showed that the samples were seronegative and not borderline or unequivocal.
3.2 EBV and CMV antibody titers in Hispanic pregnant women and control subjects
After validation, this method was used to titer EBV and CMV antibodies in plasma from a group of 398 pregnant women and a group of 32 healthy adults. The results are shown in Table 3. Of the 398 pregnant females, 394 were EBV positive (99%) while 340 were CMV positive (85%). The 4 EBV negative subjects were CMV positive; the 58 subjects who were CMV negative were EBV positive. Thus, 62 subjects were infected with only one herpesvirus while 336 subjects were co-infected with both viruses.
Table 3.
Herpesvirus IgG antibody titers1 in a group of pregnant Hispanic females and a group of healthy adults
| Pregnant females2 Number (%) or Mean titer ± SE (range) |
Adult subjects3 Number (%) or Mean titer ± SE (range) |
P value | |
|---|---|---|---|
| EBV positive | 394 (99%) | 32 (100%) | |
| CMV positive | 340 (85%) | 28 (88%) | |
| EBV VCA | 8.0 ± 0.07 (5.5 – 12.3) | 7.6 ± 0.07 (5.6 – 10.4) | 0.33 |
| EBV VCA ≥640 | 94 (23.9%) | 11 (34.4%) | |
| EBV VCA ≥320 | 210 (53.3%) | 18 (56.3%) | |
| CMV | 7.3 ± 0.05 (0 – 10.4) | 5.6 ± 0.4 (0 – 8.5) | 0.002 |
| CMV ≥640 | 21 (6.2%) | 0 (0%) | |
| CMV ≥320 | 84 (24.7%) | 4 (14.3%) |
Log 2;
N = 398;
N =32
The mean VCA antibody titer was significantly higher than the mean CMV antibody titer (8.0 vs. 7.3; p <0.001). In comparison, none of the samples from the 32 healthy adults were EBV negative while four were CMV negative. The mean VCA antibody titer was significantly higher than the mean CMV antibody titer (7.6 vs. 5.6; p <0.001). When comparing between groups, there were no significant differences in VCA IgG antibody titers. However, the mean CMV antibody titer for the group of pregnant females was significantly higher than that of the adult subject group (p<0.002).
The OD-derived titers were then transformed using the LOOKUP table. EBV VCA titers ≥320 are considered elevated and are correlated with reactivation in immunosuppressed individuals (Horwitz et al., 1985; Jenson, 2011). Other investigators, using EBV VCA titers ≥640 as a cutoff, found an increased risk of disease progression (Schetter et al., 2008). Therefore, the EBV and CMV data was further analyzed by using both cutoffs (i.e., ≥320 and ≥640).
When comparing EBV and CMV titers within groups, both groups had a significantly greater number of subjects with elevated VCA titers (≥320 and ≥640) than with elevated CMV titers (p<0.01; chi-square test). There were no significant differences in VCA or CMV antibody titers between the two groups. Further analysis of the pregnant female group showed that 8 of the 336 co-infected subjects (2%) had IgG antibody titers ≥640 for both VCA and CMV, while 23 subjects (7%) had VCA antibody titers ≥640 and CMV antibody titers ≥320 (data not shown). There were no significant differences in EBV antibody titers between the CMV-negative and –positive pregnant Hispanic women. However, VCA antibody titers were significantly greater in pregnant Hispanic women with high CMV IgG titers (≥ 320) as compared to those with less than 320 (p<0.05).
4. Discussion
In the current study the utility of a new ELISA-based method to rapidly titer (< 2 hr) CMV and EBV antibodies was demonstrated with a large number of samples. Using IFA-scored plasma samples to create a standard curve similar to cytokine ELISA assays, an OD reading was able to be converted into a meaningful unit of measure (i.e., a titer). For instance, a titer is required to determine if viral reactivation has occurred by observing a 4-fold or greater increase between paired blood samples; elevated antibodies may also indicate reactivation (Stowe et al., 2000; Stowe et al., 2010). A high positive correlation was found between the IFA and ELISA methods, partly due to the use of the same substrate (i.e., virally-infected cells), which validated the assay for use in population-based studies. In addition, this approach (i.e., IFA-scored samples for use as standards) has been used to titer antibodies to EBV early antigen (EA), HSV-1, and varicella zoster virus (VZV) (unpublished data), and IFA pre-titered plasma may be obtained commercially (e.g., Plasma Services Group) to aid with large multi-site studies. Lastly, the LOOKUP table allows conversion from OD-derived titers into 2-fold titers for comparison of results with other studies.
However, this approach is not without limitations. For instance, selection of the appropriate IFA-scored samples for use as standards is important. As shown by the LOOKUP table, samples with an assigned IFA titer of 1280 actually encompass a range of titers determined by the ELISA method. Multiple plasma samples pre-titered at 2560 by IFA were screened in order to find a sample that gave an OD reading near the mean of these samples. This helped reduce potential error that might have resulted from using samples with values near either end of the range. In addition, this assay also has the typical drawback of non-specific reactions (e.g., autoantibodies), although this problem would not be expected to be prevalent in samples from healthy adults. Lastly, serological assays alone are not encouraged in immunosuppressed individuals due to dysfunction in the production and maintenance of antibodies (Hess, 2004).
This assay was used to measure EBV and CMV antibody titers in pregnant Hispanic women. In agreement with prior studies of Hispanics (Stowe et al., 2010), the majority of these individuals were found to be infected with multiple herpesviruses. In addition, EBV antibodies were significantly higher than CMV antibodies. Notably, subjects with elevated CMV antibodies had significantly higher EBV antibodies than those with lower CMV antibodies. Notably, this type of relationship has been associated with an inflammatory state in older adults (Bennett et al., 2011) and is consistent with previous literature linking total pathogen burden and inflammation (Zhu et al., 2000; Nazmi et al., 2010; Roberts et al., 2010).
CMV antibodies were significantly greater in pregnant Hispanic females as compared to the healthy adult group. Notably, impaired cellular immunity results in higher antiviral IgG antibodies reflecting viral reactivation (Glaser et al., 1991; Glaser et al., 1994; Stowe et al., 2000; Glaser and Kiecolt-Glaser, 2005). It is possible that the results from the current study may reflect downregulation of cell-mediated immunity, perhaps due in part to the Th2-type immunity associated with pregnancy (Wegmann et al., 1993; Piccinni et al., 1998). Future studies are needed to examine the role of herpesviruses in pregnancy and preterm birth.
Overall these results demonstrate a new approach to measuring antiviral antibodies. Previous studies (e.g., NHANES) used standard ELISA approaches for determining EBV and CMV serostatus which limited interpretation of the viral data. For instance, viral data in NHANES III for subjects over age 49 were top-coded making them unusable as a predictor of mortality (Dowd and Aiello, 2009; Simanek et al., 2011). In addition, OD values alone make it impossible to correlate antibody levels as an indicator of reactivation. Although some of these studies used upper quartiles to define high antibody levels, using an arbitrary cutoff can skew the data interpretation depending on the size of the sample. For instance, further analysis of the current data showed that the upper quartile was not a significant predictor of infant physical characteristics. However, when using a defined titer cutoff similar to prior work (Stowe et al., 2010), it was found that elevated titers predicted specific infant physical characteristics. Future studies should include measurement of antibody titers along with serostatus to examine the role of herpesvirus reactivation in poor health outcomes.
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases (R03AI081021, R03AI083062, and R01AI084898), the National Institute of Nursing Research (R01NR007891), and the National Space and Aeronautics Administration (NNJ06HB73A).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Haan MN, Pierce CM, Simanek AM, Liang J. Persistent infection, inflammation, and functional impairment in older Latinos. J Gerontol A Biol Sci Med Sci. 2008;63:610–618. doi: 10.1093/gerona/63.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett JM, Glaser R, Malarkey WB, Beversdorf DQ, Peng J, Kiecolt-Glaser JK. Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav Immun. 2011;26:739–746. doi: 10.1016/j.bbi.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE. Socioeconomic differentials in immune response. Epidemiology. 2009;20:902–908. doi: 10.1097/EDE.0b013e3181bb5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Alfano CM, Bennett JM, Povoski SP, Lipari AM, Agnese DM, Yee LD, Carson WE, 3rd, Farrar WB, Malarkey WB, Kiecolt-Glaser JK. Fatigue and herpesvirus latency in women newly diagnosed with breast cancer. Brain Behav Immun. 2012;26:394–400. doi: 10.1016/j.bbi.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearl DK, Kiecolt-Glaser JK, Malarkey WB. Plasma cortisol levels and reactivation of latent Epstein-Barr virus in response to examination stress. Psychoneuroendocrinology. 1994;19:765–772. doi: 10.1016/0306-4530(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Glaser R, Pearson GR, Jones JF, Hillhouse J, Kennedy S, Mao HY, Kiecolt-Glaser JK. Stress-related activation of Epstein-Barr virus. Brain Behav Immun. 1991;5:219–232. doi: 10.1016/0889-1591(91)90018-6. [DOI] [PubMed] [Google Scholar]
- Gray J, Wreghitt TG, Pavel P, Smyth RL, Parameshwar J, Stewart S, Cary N, Large S, Wallwork J. Epstein-Barr virus infection in heart and heart-lung transplant recipients: incidence and clinical impact. J Heart Lung Transplant. 1995;14:640–646. [PubMed] [Google Scholar]
- Hess RD. Routine Epstein-Barr virus diagnostics from the laboratory perspective: still challenging after 35 years. J Clin Microbiol. 2004;42:3381–3387. doi: 10.1128/JCM.42.8.3381-3387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz CA, Henle W, Henle G, Rudnick H, Latts E. Long-term serological follow-up of patients for Epstein-Barr virus after recovery from infectious mononucleosis. J Infect Dis. 1985;151:1150–1153. doi: 10.1093/infdis/151.6.1150. [DOI] [PubMed] [Google Scholar]
- Jenson HB. Epstein-Barr virus. Pediatr Rev. 2011;32:375–383. doi: 10.1542/pir.32-9-375. quiz 384. [DOI] [PubMed] [Google Scholar]
- Kendall TJ, Wilson JE, Radio SJ, Kandolf R, Gulizia JM, Winters GL, Costanzo-Nordin MR, Malcom GT, Thieszen SL, Miller LW, McManus BM. Cytomegalovirus and other herpesviruses: do they have a role in the development of accelerated coronary arterial disease in human heart allografts? J Heart Lung Transplant. 1992;11:S14–S20. [PubMed] [Google Scholar]
- Nazmi A, Diez-Roux AV, Jenny NS, Tsai MY, Szklo M, Aiello AE. The influence of persistent pathogens on circulating levels of inflammatory markers: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. BMC Public Health. 2010;10:706. doi: 10.1186/1471-2458-10-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccinni MP, Beloni L, Livi C, Maggi E, Scarselli G, Romagnani S. Defective production of both leukemia inhibitory factor and type 2 T-helper cytokines by decidual T cells in unexplained recurrent abortions. Nat Med. 1998;4:1020–1024. doi: 10.1038/2006. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz RJ, Stowe RP, Brown A, Wommack J. Acculturation and biobehavioral profiles in pregnant women of Hispanic origin: generational differences. ANS Adv Nurs Sci. 2012;35:E1–E10. doi: 10.1097/ANS.0b013e3182626199. [DOI] [PubMed] [Google Scholar]
- Schetter AJ, You WC, Lennette ET, Gail MT, Rabkin CS. Association of Epstein-Barr virus antibody levels with precancerous gastric lesions in a high-risk cohort. Cancer Sci. 2008;99:350–354. doi: 10.1111/j.1349-7006.2007.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Peek MK, Perez NA, Yetman DL, Cutchin MP, Goodwin JS. Herpesvirus reactivation and socioeconomic position: a community-based study. J Epidemiol Community Health. 2010;64:666–671. doi: 10.1136/jech.2008.078808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe RP, Pierson DL, Barrett AD. Elevated stress hormone levels relate to Epstein-Barr virus reactivation in astronauts. Psychosom Med. 2001;63:891–895. doi: 10.1097/00006842-200111000-00007. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Pierson DL, Feeback DL, Barrett AD. Stress-induced reactivation of Epstein-Barr virus in astronauts. Neuroimmunomodulation. 2000;8:51–58. doi: 10.1159/000026453. [DOI] [PubMed] [Google Scholar]
- Strandberg TE, Pitkala KH, Linnavuori KH, Tilvis RS. Impact of viral and bacterial burden on cognitive impairment in elderly persons with cardiovascular diseases. Stroke. 2003;34:2126–2131. doi: 10.1161/01.STR.0000086754.32238.DA. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machala M, Mysliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- Wall NA, Chue CD, Edwards NC, Pankhurst T, Harper L, Steeds RP, Lauder S, Townend JN, Moss P, Ferro CJ. Cytomegalovirus seropositivity is associated with increased arterial stiffness in patients with chronic kidney disease. PLoS One. 2013;8:e55686. doi: 10.1371/journal.pone.0055686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14:353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- Wikby A, Nilsson BO, Forsey R, Thompson J, Strindhall J, Lofgren S, Ernerudh J, Pawelec G, Ferguson F, Johansson B. The immune risk phenotype is associated with IL-6 in the terminal decline stage: findings from the Swedish NONA immune longitudinal study of very late life functioning. Mech Ageing Dev. 2006;127:695–704. doi: 10.1016/j.mad.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]