Abstract
The female genital tract is a portal of entry for sexual HIV transmission and a possible viral reservoir. In this study, the ectocervical CD8+ T cell distribution was explored in situ and was related to expression of CD3 and HLA-DR and presence of HIV RNA. For this purpose, ectocervical tissue samples and genital secretions were collected from HIV-seropositive (HIV+) Kenyan female sex workers (FSWs) (n = 20), HIV-seronegative (HIV−) FSWs (n = 17), and HIV− lower-risk women (n = 21). Cell markers were assessed by in situ staining and by quantitative PCR. HIV RNA expression in tissue was analyzed by in situ hybridization, and viral shedding was assessed by quantitative PCR. The HIV+FSW group had a higher amount of total cells and CD8+, CD3+, and HLA-DR+ cells compared with the HIV−FSW group and HIV− lower-risk women. The majority of CD8+ cells were CD3+ T cells, and the numbers of CD8+ cells correlated significantly with plasma and cervical viral load. HIV RNA expression in situ was found in 4 of the 20 HIV+FSW women but did not correlate with cervical or plasma viral load. Thus, the HIV+ women displayed high numbers of CD8+, CD3+, and HLA-DR+ cells, as well as a limited number of HIV RNA+ cells, in their ectocervical mucosa; hence, this localization cannot be neglected as a potential viral reservoir. The elevated levels of CD8+ T cells may play a role in the immunopathogenesis of HIV in the female genital tract.
A broad range of immune cells in the epithelial and sub-mucosal layer of the female genital tract mucosa, as well as the epithelial cells themselves, have the capacity to respond rapidly to pathogen exposure. Because the distribution and function of various immune cells differ at the systemic and mucosal level, it is important to study the immune response in both of these compartments. The T cell responses, as well as influx of APCs and release of inflammatory cytokines at genital mucosal sites, have the capacity to affect viral replication and systemic spread of the infection. Cervical T cells are predominantly Ag experienced and highly differentiated, with effector memory T cells being the most predominant subset (1). However, although HIV-specific T cells are present in the cervix (2), they are suggested to be largely monofunctional and, thereby, may have limited functional antiviral capacity (3–5). Unfortunately, exposure to seminal fluid and to various pathogens, including HIV, can cause mucosal inflammation and, thereby, also may increase the number of target cells for HIV and promote local viral replication (6–8). We recently documented a higher expression of immune activation markers, in situ, by intact ectocervical tissue samples from HIV-infected women. In the same study, it also was observed that, although the blood CD4+ T cell numbers were lower, the ectocervical CD4+ T cell numbers were comparable to those of healthy uninfected control women (9).
Sexual HIV transmission is correlated with plasma viral RNA levels and likely occurs through mucosal contact with the virus in genital secretions (10). Furthermore, genital HIV RNA levels often correspond to plasma HIV RNA levels and can predict transmission risk in some cases (11–13). However, some studies (14–17) reported only a modest correlation between systemic and local virus levels, and this discrepancy may be explained, in part, by mucosal immune activation resulting in increased local viral replication without affecting other anatomical sites. Several conditions can increase the risk for genital viral shedding, including genital infections, general mucosal inflammation, vaginal douching, hormonal contraceptive use, and pregnancy (18–28). Genital viral shedding also may be intermittent and can be detected even though plasma viral load is low or undetectable (29, 30).
The local T cell response against HIV and factors involved in genital viral shedding at the female genital tract have been investigated extensively, primarily by assessing cervicovaginal secretions (CVSs) and cytobrush-derived cervical cells (1, 4, 31, 32). Studies (5, 9, 33–36) at the single-cell level discriminating the epithelial and submucosal distribution of immune markers in intact cervical tissue of HIV-infected women have been limited in sample size and have been lacking information about corresponding plasma or cervical viral shedding. Using in situ techniques assessing snap-frozen human tissue samples, it is possible to visualize the exact distribution of local T cells and HIV RNA expression. Therefore, in the current study we investigated the in situ distribution and quantity of CD8-, CD3-, or HLA-DR–expressing cells, as well as the presence of HIV RNA+ cells. These results were correlated with plasma and cervical viral load.
Materials and Methods
Ethical approval
This study was reviewed and approved by the research ethics boards at Kenyatta National Hospital (Nairobi, Kenya), The Regional Ethical Review Board (Stockholm, Sweden), and the University of Manitoba. All study participants provided written informed consent.
Study population and procedures
HIV-seropositive (HIV+) and HIV-seronegative (HIV−) female sex workers (FSWs), recruited through the Majengo Sex Worker Clinic, and HIV− lower-risk nonsex working women (LRs), recruited through a Maternal Health Clinic at the Pumwani Maternity Hospital (37–39), were included in this study. General inclusion criteria included age ≥ 18 y, uterus and cervix present, not actively menstruating, no symptomatic or clinically apparent cervical inflammation, and willingness to undergo ectocervical biopsy collection and to abstain from vaginal sex during a healing period of 2 wk. All participants were provided with HIV/sexually transmitted infections prevention counseling, male and female condoms, family-planning services, treatment of sexually transmitted infections, medical care for acute and chronic illnesses, access to adequate diagnostic testing, and referral for specialist consultant and/or hospitalization at Kenyatta National Hospital, as needed.
Specimen collection
CVS samples were collected from all women by rotating one cotton-tipped swab 360° in the cervical os and one swab to collect secretions from the posterior vaginal fornix. Both swabs were transferred into a vial containing 5 ml PBS. Furthermore, two ectocervical biopsies (3 mm2) were collected from the superior portion of the ectocervix with Schubert biopsy forceps (B. Braun Aesculap, Tuttlingen, Germany); one biopsy was placed in a vial containing RNAlater solution (QIAGEN, Valencia, CA), and the other was snap-frozen in liquid nitrogen and cryopreserved at −80°C.
HIV RNA quantification
Detection of HIV-1 RNA in plasma and CVS samples was assessed using real-time RT-PCR-assay—the COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0. (Roche, Basel, Switzerland)—with a threshold sensitivity of 20 HIV RNA copies/ml. The samples were centrifuged prior to performing the test.
Detection of HIV RNA+ cells by in situ hybridization
HIV RNA+ cells within the ectocervical tissue sections were detected as previously described (40, 41). Briefly, three consecutive 8-μm sections of the cryopreserved cervical biopsies were cut and adhered to a Menzel SuperFrost Plus GOLD slides (Histolab Products, Göteborg, Sweden), fixed in freshly prepared 4% paraformaldehyde, and air-dried. The sections were then rehydrated, permeabilized, and acetylated prior to hybridization to 35S-labeled HIV riboprobes. After washing and digestion with RNase, sections were coated with nuclear track emulsion, exposed for 14 d, developed, and counterstained with H&E stain. HIV RNA+ cells were enumerated in sections using the Spectrum Plus analysis program (Version 9.1; Aperio ePathology Solutions, San Diego, CA). Negative control consisted of ectocervical tissue from an HIV− individual; endocervical tissues from humanized mice experimentally infected with HIV were used as a positive control.
Detection and quantification of mRNA
Quantification of mRNA expression was performed as previously described (9, 42). In brief, the biopsies stored in RNAlater solution were thawed and disrupted in lysis buffer using a mechanical rotor. RNA extraction was performed according to the manufacturer’s protocol (RNeasy; QIAGEN, Hilden, Germany). Total mRNA was converted to cDNA using superscript reverse transcriptase (Invitrogen, Carlsbad, CA) and random hexanucleotide primers (Roche). The ABI PRISM 7700 sequence detection system and FAM dye–labeled TaqMan minor groove binder probes and primers (Applied Biosystems, Foster City, CA) were used to detect, amplify, and quantify the following targets: ubiquitin C (UBC), CD3, CD8, and HLA-DR. Each sample was run in duplicate, and cycle threshold (Ct) values for each target gene were normalized to UBC. Fold change of the target genes was calculated as 2−dCt.
Detection of immune markers by in situ staining
Immunohistochemistry was performed on 8-μm-thick sections of the cryopreserved cervical biopsies. The tissue sections were fixed in 2% formaldehyde, and the peroxidase-labeled streptavidin-biotin amplification method was used, as previously described (43, 44). In brief, endogenous biotin was blocked using an Avidin/Biotin Blocking Kit (Vector Laboratories, Burlingame, CA), followed by the addition of selected mouse IgG mAbs detecting human CD3 (clone SK7), CD8 (clone SK1), or HLA-DR (clone L243; all from BD Biosciences, Stockholm, Sweden). Thereafter, secondary biotinylated polyclonal rabbit anti-mouse IgG Ab (Dako Sweden) was added prior to the use of peroxidase-based VECTASTAIN (VECTASTAIN Elite Standard; Vector Laboratories). Staining reactions were developed with diaminobenzidine tetrahydrochloride (Vector Laboratories), and the sections were counterstained with hematoxylin. Negative controls consisted of incubations in the presence of secondary Ab alone.
For immunofluorescent double staining of CD3+CD8+ cells and CD3+ CD4+ cells, samples were stained sequentially. CD3+ cells were first detected by addition of a rabbit monoclonal anti-human CD3 Ab (clone SP7; Abcam, Cambridge, U.K.) in combination with an Alexa Fluor 488– or an Alexa Fluor 594–conjugated donkey anti-rabbit IgG Ab (Molecular Probes, Life Technologies Europe, Stockholm, Sweden). Thereafter, CD8+ cells were detected with an Alexa Fluor 647–conjugated monoclonal mouse anti-human CD8 Ab (RPA-T8; BD Pharmingen), and CD4+ cells were detected with a monoclonal mouse anti-human CD4 Ab (clone SK3; BD Biosciences), followed by the addition of an Alexa Fluor 488–conjugated donkey anti-mouse IgG Ab (Molecular Probes). The tissues were stained with DAPI to visualize all cell nuclei prior to being mounted in SlowFade Gold Antifade Reagents (Molecular Probes).
Quantitative analysis of in situ staining
The immunohistochemically stained sections were examined using a DMR-X microscope (Leica, Wetzlar, Germany). Digital images were transferred from the microscope (Leica) to the Quantimet, Q 550 IW computerized image analysis system (Leica Imaging Systems, Cambridge, U.K.) (45). The epithelium and the submucosa were analyzed separately, including the total area of the epithelium and a submucosal area measured from the basal membrane to ~400 μm (355–415 μm) depth into the mucosa. For each tissue section, about eight fields of the epithelium and seven fields of the submucosa were scanned at ×20 magnification. Each field represents an average area of 1.4 ×105 μm2 epithelium and 1.8 ×105 μm2 submucosa; thus, a total area of 1.1 × 106 μm2 epithelium and 1.2 × 106 μm2 sub-mucosal tissue was analyzed. The percentage of cells within the total tissue area was calculated by measuring the hematoxylin-stained cellular area in the total tissue area. The frequency of positively stained cells (CD3+, CD8+, and HLA-DR+ cells) was expressed as the percentage of stained area of the total tissue area. All imaging analyses were performed by a blinded investigator.
Statistical analysis
Nonparametric comparisons were performed between the HIV+FSW group and the HIV−FSW and HIV−LR groups (e.g., the statistical method of choice was for comparison of two groups: HIV+FSW versus HIV−FSW or HIV+ FSW versus HIV−LR). Statistical significance was assessed using the Mann–Whitney U test for comparisons of continuous variables and the Fisher exact test for categorical variables between the study groups. The Spearman rank correlation coefficient test was used to assess correlations. All tests were two-sided, and a p value < 0.05 was considered significant. Prism 5.00 for Windows (GraphPad Software, CA) was used for statistical analysis.
Results
Study population
Demographic data, sexual risk-taking profile, and laboratory results of the participants are outlined in Table I. The main focus of this study was to investigate the expression of the immune markers of interest in HIV+ women compared with HIV− women. Because the HIV+ women were recruited from a sex-worker cohort (HIV+FSW group), an additional group of HIV− women was recruited from the same cohort (HIV−FSW group) to control for factors such as sexually transmitted infections and high-risk behavior. Thus, the study contains two HIV− control groups, one including sex workers and one including lower-risk women (HIV−LR group). In summary, all FSWs enrolled were active in sex work and had a minimum of five clients per day, as reported at the last formal resurvey visit 6 mo earlier. The two FSW study groups had comparable data for “median years in sex work,” “condom use,” and “median number of clients per week.” The HIV+FSWs had been infected with HIV for a median of 3 y (1–21 y), and none had received antiretroviral treatment or had a history of AIDS-defining illnesses or acute health issues. All study participants were within the same age range, were similarly distributed in the different stages of the menstrual cycle, reported similar use of hormonal contraception, and had a similar number of pregnancies. Furthermore, sexual activity during menses, detectable levels of prostate-specific Ag (PSA), and the prevalence of bacterial vaginosis, Candida, Chlamydia trachomatis, and Neisseria gonorrhea were also similar across the groups. Although there were no differences in syphilis, HSV-2 serostatus, or the performance of vaginal douching between the two FSW groups, these three parameters were more common when comparing the HIV+FSW and HIV−LR groups (p = 0.04, p = 0.0001, p = 0.001, respectively).
Table I.
Characteristics of the study population at date of biopsy
| HIV+FSW (n = 20) | HIV−FSW (n = 17) | HIV−LR (n = 21) | p Valuea | |
|---|---|---|---|---|
| Age (y; median [range]) | 42 (24–58) | 42 (27–51) | 38 (24–47) | NS |
| Pregnancies (median [range])b | 3 (0–6) | 3 (1–13) | 3 (0–9) | NS |
| Hormonal contraception (n [%])c | 5 (25) | 9 (53) | 6 (29) | NS |
| Menstrual cycle stage (n [%]) | ||||
| Follicular | 3 (15) | 2 (12) | 2 (10) | NS |
| Periovulatory | 3 (15) | 0 (0) | 3 (14) | NS |
| Luteal | 8 (40) | 5 (29) | 7 (33) | NS |
| Not applicabled | 6 (30) | 10 (59) | 9 (43) | n/a |
| Bacterial vaginosis (n [%]) | 3 (15) | 3 (18) | 3 (14) | NS |
| Candida (n [%]) | 1 (5) | 2 (12) | 2 (10) | NS |
| Chlamydia trachomatis (n [%]) | 0 (0) | 0 (0) | 1 (5) | n/a |
| Neisseria gonorrhea (n [%]) | 0 (0) | 0 (0) | 0 (0) | n/a |
| Syphilis seropositive (n [%]) | 6 (30) | 3 (18) | 1 (5) | 0.05e |
| HSV-2+ (n [%]) | 20 (100) | 16 (94) | 9 (43) | 0.001e |
| Condom use < 50% (n [%])f | 7 (35) | 9 (53) | 14 (67) | NS |
| Sex during menses (n [%]) | 3 (15) | 2 (12) | 0 (0) | NS |
| PSA+ (n [%])g | 1 (5) | 1 (6) | 1 (5) | NS |
| Vaginal douching (n [%])h | 20 (100) | 17 (100) | 11 (52) | 0.01e |
The statistical analyses were performed by comparing the HIV+FSW group versus the HIV−FSW group and the HIV+FSW group versus the HIV−LR group.
Fisher exact test.
Includes abortions.
Includes oral contraception, Depo-Provera, and Norplant.
Subjects with amenorrhea due to long-term hormonal treatment or menopause.
HIV+FSW versus HIV−LR.
Self-reported use of condoms with regular partners defined as husband, boyfriend, or lover.
PSA detected in CVS > 1 ng/ml.
Any douching performed by inserting water or water and soap in the vagina. n/a, not applicable.
In situ quantification reveals higher numbers of total cells in ectocervical tissue of HIV+ women
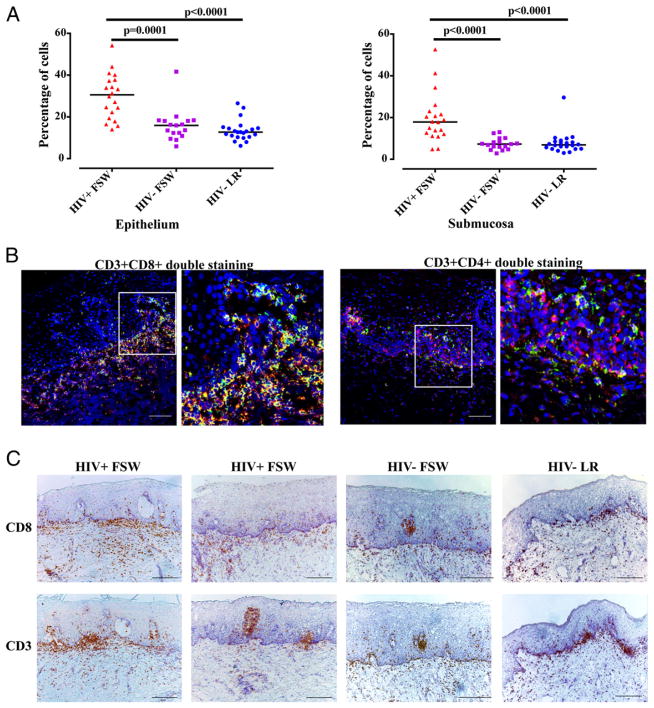
In the initial experiments, the total number of cells was quantified in situ in ectocervical tissue samples from the HIV+FSW (n = 20), HIV−FSW (n = 17), and HIV−LR (n = 21) groups. The percentage of total cells in the tissue sections (including the whole epithelium and a specified area of the submucosa: 400 μm from the basal membrane into the submucosal compartment) was analyzed by in situ staining. The HIV+FSW group had a significantly higher percentage of cells in the epithelium (range: 16–54%; median, 31%) compared with the HIV−FSW group (range: 6–42%; median, 16%; p = 0.0001) and the HIV−LR group (range: 6–26%; median, 13%; p < 0.0001; Fig. 1A). The HIV+FSW group also had a significantly higher percentage of cells in the submucosa (range: 5–53%; median, 18%) compared with the HIV−FSW group (range: 3–13%; median 7%; p < 0.0001) and the HIV−LR group (range: 3–30%; median, 7%; p < 0.0001; Fig. 1A).
FIGURE 1.
Enumeration and in situ staining of immune cells in ectocervical tissue. (A) Distribution and median of the percentage of total cells in the ectocervical epithelium and submucosa of tissue samples from the three study groups. A nonparametric, two-tailed Mann–Whitney U test was used to compare the HIV+FSW group versus the HIV−FSW group and the HIV+FSW group versus the HIV−LR group. (B) Immunofluorescent images of ectocervical tissue sections from an HIV+FSW subject. The image on the right for each pair is a magnified view of the region indicated in the box in the image to the left. The majority of CD8+ cells (red) were also CD3+ (green) and vice versa (left panels); the majority of CD3+ cells (red) were not CD4+ cells (green) (right panels). Scale bar, 100 μm. (C) Bright-field images of ectocervical tissue stained with hematoxylin (blue) for visualization of cell nuclei and stained for CD8+ cells (brown; upper panels) and CD3+ cells (brown; lower panels). Scale bar, 200 μm.
Elevated levels of CD8+ and CD3+ cells in ectocervical tissue of HIV+ women
Next, we specifically aimed at characterizing the ectocervical CD8+ cell population; therefore, their phenotype was determined by double staining with the T cell marker CD3. The majority of CD8+ cells in the samples from the HIV+FSW group were CD3+ cells (Fig. 1B). Further analyses showed that the majority of CD3+ cells were CD8+ cells (Fig. 1B) and that CD3+CD4+ cells, as well as CD3−CD4+ cells (e.g., macrophages and dendritic cells), were also present (Fig. 1B). The distribution of CD8+ and CD3+ cells was examined further, which revealed that the majority of these cells were localized near both sides of the basal membrane. Clusters of both CD8+ and CD3+ cells also were seen in the epithelium, particularly in those samples that had a higher percentage of CD8+ and CD3+ cells (Fig. 1C).
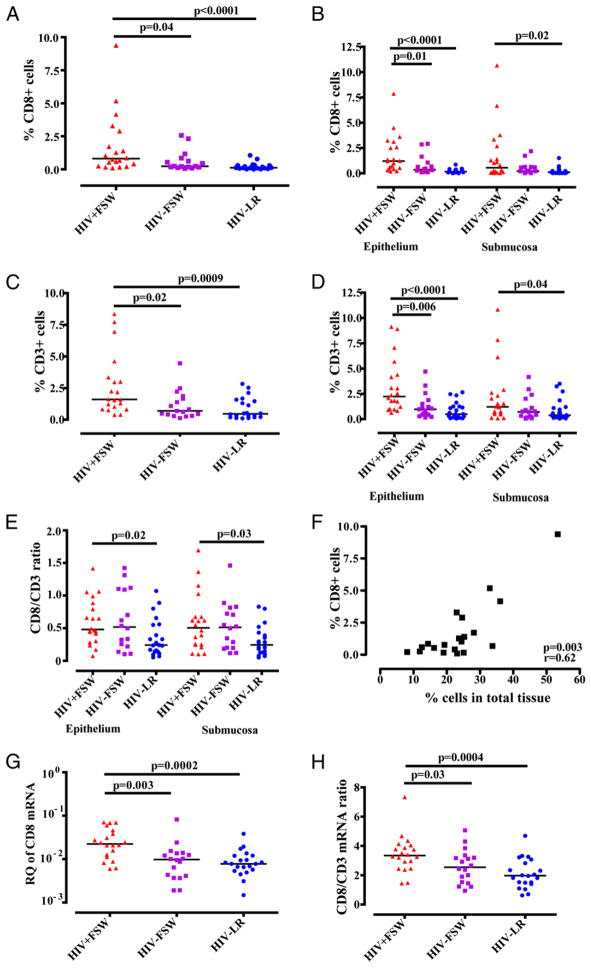
The HIV+FSW group was subsequently shown to have significantly more CD8+ cells (range: 0.1–9.4%; median, 0.8%) and CD3+ cells (range: 0.4–8.3%; median, 1.6%) compared with the HIV−FSW group (CD8+: range: 0.1–2.6%; median, 0.2%; p = 0.04; CD3+: range: 0.1–4.5%; median, 0.69%; p = 0.02) and the HIV−LR group (CD8+: range: 0.01–1.1%; median, 0.12%; p < 0.0001; CD3+: range: 0.1–2.8%; median, 0.5%; p = 0.0009) (Fig. 2A, 2C). When specified for the respective mucosal compartment, the differences were most pronounced in the epithelium: the HIV+FSW group had significantly more cells expressing both markers in the epithelium compared with the HIV−FSW group (CD8: p = 0.01; CD3: p = 0.006) and the HIV−LR group (CD8: p < 0.0001; CD3: p < 0.0001) (Fig. 2B, 2D). For the submucosal compartment, no significant differences were found in the expression of CD8+ and CD3+ cells between the HIV+FSW group and the HIV−FSW group, whereas the HIV+FSW group displayed significantly more CD8+ and CD3+ cells compared with the HIV− LR group (p = 0.02 and p = 0.04, respectively) (Fig. 2B, 2D). When calculating the CD8/CD3 ratio, no difference between the HIV+FSW group and the HIV−FSW group was seen, whereas the HIV+FSW group displayed a significantly higher CD8/CD3 ratio in both the epithelium and submucosa compared with the HIV− LR group (p = 0.02 and p = 0.03, respectively) (Fig. 2E), confirming our in situ double-staining results. Furthermore, the HIV+ FSW subjects displayed a significant correlation between the percentage of CD8+ cells and the percentage of total cells within the ectocervical tissue area (p = 0.003: r = +0.62) (Fig. 2F).
FIGURE 2.
Enumeration of CD8+ and CD3+ cells in ectocervical tissue. Distribution and median of the percentage of positively stained (A) CD8+ cells in the total tissue analyzed (B) and in the ectocervical epithelial and submucosal tissue area analyzed separately. Percentage of positively stained (C) CD3+ cells in the total tissue analyzed and (D) in the ectocervical epithelial and sub-mucosal tissue area analyzed separately, as assessed by imaging analysis. (E) CD8+/CD3+ cell ratio in the total ectocervical epithelial area and submucosal area. (F) Correlation between the percentage of CD8+ cells and the percentage of the total cells within the ectocervical tissue area analyzed by imaging analysis. (G) Distribution and median relative quantification (RQ; UBC = 1) of CD8 mRNA expression, which was assessed by qPCR. (H) CD8/CD3 mRNA ratio. The Ct values for each target gene were normalized to UBC. Fold change of the target genes was calculated as 2−dCt. A nonparametric, two-tailed Mann–Whitney U test was used to analyze the statistical significance between the study groups.
Because the parameters “syphilis seropositive,” “HSV-2 sero-positive,” and “vaginal douching” were overrepresented in the HIV+FSW study group compared with the HIV−LR group, we investigated whether these parameters had an impact on the mucosal expression of CD8. Because all women in the HIV+FSW study group were HSV-2 seropositive and performed vaginal douching, this evaluation was restricted to the HIV−LR group. Thus, the percentage of CD8+ cells was compared between HIV−LR subjects who did or did not perform vaginal douching (11 versus 10) and who were or were not HSV-2 seropositive (9 versus 12), but no statistical differences were found (data not shown). Furthermore, the syphilis-seropositive women did not have higher CD8+ cell numbers than the syphilis seronegative women (data not shown). Together with the fact that the two FSW study groups had comparable risk factors at enrollment (Table I), the increased percentage of CD8+ cells seen in the HIV+FSW group does not seem to be associated with sex work per se or with these confounders.
In summary, the HIV+FSW group had significantly more CD8+ and CD3+ cells, as well as a higher CD8+/CD3+ cell ratio, within the defined ectocervical tissue areas compared with the HIV−LR group, whereas the differences were less pronounced compared with the HIV−FSW group. The increased number of CD8+ cells was most pronounced in the epithelium, and the majority of these cells were CD3+.
Elevated expression of CD8 and CD3 mRNA in ectocervical tissue of HIV+ women
To complement the in situ staining, the mRNA expression of CD8 and CD3 was measured by quantitative PCR (qPCR). This method does not allow discrimination between the epithelial and submucosal compartment, because mRNA is extracted from the whole ectocervical tissue block. Nevertheless, using this method, the HIV+FSW group had a significantly higher expression of total CD8 mRNA compared with the HIV−FSW group (p = 0.003) and the HIV−LR group (p = 0.0002) (Fig. 2G). The CD8/CD3 ratio also was significantly higher in the HIV+FSW group compared with the HIV−FSW group (p = 0.03) and the HIV−LR group (p = 0.0004) (Fig. 2H). Hence, these mRNA data confirm our imaging analysis data.
Plasma viral load correlates significantly with cervical viral load but not with presence of HIV RNA–expressing cells in ectocervical tissue of HIV+ women
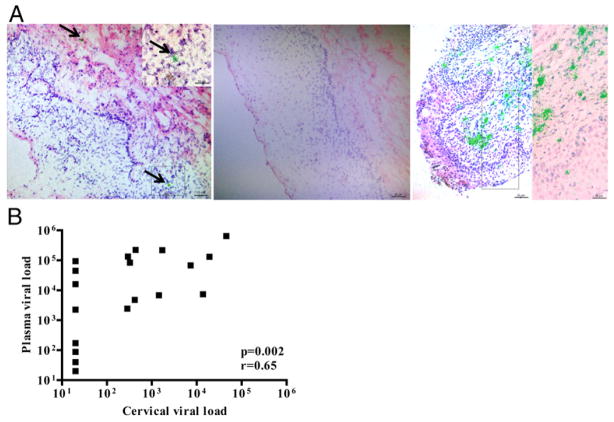
The HIV+FSW group was evaluated for the presence of HIV RNA by in situ hybridization of three ectocervical tissue sections per individual. Thus, a limited number of HIV RNA–expressing cells was found in 4 of the 20 subjects. The HIV RNA+ cells were localized to both the epithelium and the submucosa (Fig. 3A).
FIGURE 3.
Detection and quantification of HIV RNA in ectocervical tissue and in plasma and cervical secretions. (A) Bright-field images of ectocervical tissue sections stained with H&E (in blue and red) to visualize cell nuclei and showing the presence of HIV RNA+ cells (a cluster of green silver grains illuminated under epipolarized light after radioautography), detected by in situ hybridization. Arrows are pointing at HIV RNA+ cells. Ectocervical tissue from an HIV+FSW (left panel), an HIV−FSW (middle panel), and a humanized mouse experimentally infected with HIV, which was used as a positive control (right panel). Scale bars, 25 μm (lower-magnification images); 50 μm (higher-magnification images). (B) Correlation between plasma and cervical viral load in the HIV+ FSW group, as determined by Spearman rank correlation coefficient test.
The HIV+FSW group also was assessed for plasma and cervical viral load. A total of 19 of 20 women had detectable plasma viral load in the range of 40 to 648,000 HIV RNA copies/ml (median = 16,100), and 11 of 20 patients had detectable cervical viral load in the range of 284 to 45,800 HIV RNA copies/ml (median 1,440). The plasma and cervical viral load levels correlated significantly with each other (p = 0.002; r = +0.65) (Fig. 3B). However, the four women with detectable HIV RNA, as assessed by in situ hybridization, did not have higher plasma or cervical viral load than the 16 women with negative staining (plasma viral load: 44,800; 16,100; 40; 20 HIV RNA copies/ml, respectively; cervical viral load: <20 HIV RNA copies/ml in all four women).
CD8+ cell and CD8 mRNA expression in ectocervix correlates with plasma and cervical viral load but not with presence of HIV RNA+ cells
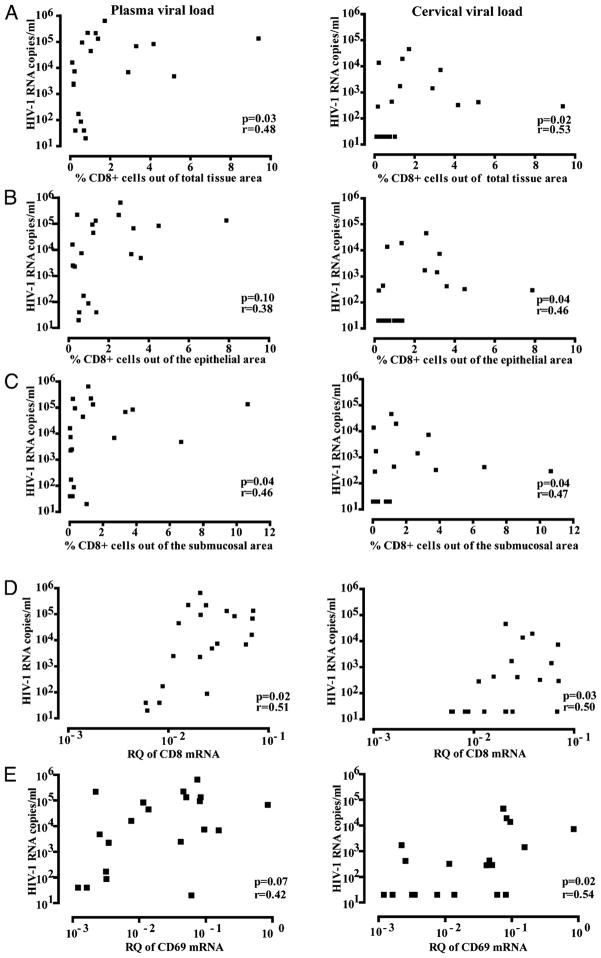
Both plasma and cervical viral load correlated significantly with the percentage of CD8+ cells within the ectocervical tissue area, as assessed by imaging analysis (p = 0.03, r = +0.48 and p = 0.02, r = +0.53, respectively) (Fig 4A), whereas no correlation was found with the total percentage of cells or the percentage of CD3+ cells within the ectocervical tissue area (data not shown). This was scrutinized further by assessing the epithelial and submucosal compartments separately; although the percentage of epithelial CD8+ cells did not correlate with plasma viral load, it correlated significantly with cervical viral load (p = 0.04, r = +0.46) (Fig. 4B). Furthermore, the percentage of submucosal CD8+ cells correlated significantly with both plasma and cervical viral load (p = 0.04, r = +0.46 and p = 0.04, r = +0.47) (Fig. 4C). The four women who had detectable HIV RNA, as assessed by in situ hybridization, did not differ with respect to the percentage of CD8+ cells or CD8 mRNA expression levels from the other 16 HIV+FSWs (data not shown).
FIGURE 4.
Comparisons between cellular markers versus plasma and cervical viral load. Correlation between plasma viral load (left panels) and cervical viral load (right panels) and the percentage of CD8+ cells (A) within the total ectocervical tissue area (B), the epithelial tissue area, and (C) the submucosal tissue area, as assessed by imaging analysis. Relative quantification (RQ; UBC = 1) of (D) CD8 mRNA and (E) CD69 mRNA levels, as assessed by qPCR. Spearman rank correlation coefficient test was used to assess correlations.
To complement these data, the mRNA expression of CD8 and CD3 was compared with plasma and cervical viral load levels. Similar results were achieved: the CD8 mRNA levels correlated significantly with both plasma (p = 0.02, r = +0.51) and cervical viral load (p = 0.03, r = +0.50) (Fig. 4D), whereas no correlation was found for the mRNA expression of CD3 with either plasma or cervical viral load (data not shown). CD8 and CD3 mRNA expression did not correlate with in situ expression of HIV RNA (data not shown).
In summary, although the total percentage of cells, percentage of CD3+ cells, and CD3 mRNA expression within the ectocervical tissue samples from the HIV+FSW group did not correlate with plasma or cervical viral load, the percentage of CD8+ cells and CD8 mRNA expression correlated significantly with both plasma and cervical viral load. However, the percentage of CD8+ cells and CD8 mRNA expression did not correlate with the presence of HIV RNA+ cells in the ectocervical tissue.
Elevated expression of HLA-DR in ectocervical tissue but no correlation with plasma or cervical viral load
We (9) previously showed that the HIV+FSW group had upregulated mRNA expression of a number of proinflammatory cytokines and cellular markers of immune activation in ectocervical tissue compared with the HIV−FSW and HIV−LR groups. Because we now had access to the cervical viral loads of these individuals, a retrospective analysis could be performed. Among all cellular phenotypes (CD3, CD68, CD1a, CD11c), immune activation markers (CD69, CCR5, Langerin, DC-SIGN, mannose receptor), and inflammatory cytokines (IFN-α, IFN-γ, TNF-α, IL-6, IL-17, IL-22) analyzed, mRNA expression of CD69 correlated significantly with cervical viral load (p = 0.02; r = +0.54) but not with plasma viral load (Fig. 4E). The mRNA levels of CD69 also correlated significantly with CD8 mRNA levels in the HIV+FSW group (p = 0.03; r = +0.47) (data not shown).
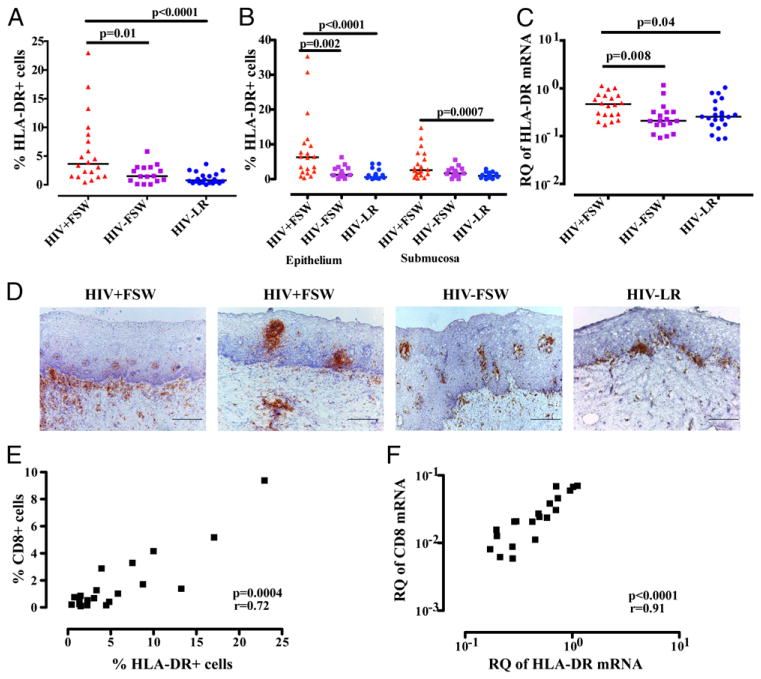
These results were extended with analyses of HLA-DR expression, both at the protein and mRNA level, as a surrogate marker of immune activation and APCs. Thus, the HIV+FSW group was shown to have significantly more cells expressing HLA-DR (range: 0.4–23%; median, 3.6%) compared with the HIV−FSW group (range: 0.1–5.8%; median, 1.5%, p = 0.01) and the HIV−LR group (range: 0.0002–3.6%; median, 0.8%, p < 0.0001) (Fig. 5A). A detailed analyses of the two mucosal layers revealed that the HIV+FSW group had significantly more cells expressing HLA-DR in the epithelium compared with the HIV−FSW group (p = 0.002) and the HIV-LR group (p < 0.0001) (Fig. 5B). Within the submucosal compartment, a similar number of HLA-DR+ cells was found between the HIV+FSW group and the HIV−FSW group, whereas the HIV+FSW group had significantly more cells expressing HLA-DR compared with the HIV−LR group (p = 0.0007) (Fig. 5B). Comparable results were seen when assessing the mRNA expression of HLA-DR, which was significantly higher in the HIV+ FSW group compared with the HIV−FSW group (p = 0.008) and the HIV−LR group (p = 0.04) (Fig. 5C).
FIGURE 5.
Enumeration of HLA-DR+ cells and HLA-DR mRNA expression in ectocervical tissue. Distribution and median of the percentage of positively stained HLA-DR+ cells in (A) the total tissue analyzed (B) and in the ectocervical epithelial and submucosal tissue area analyzed separately, as assessed by imaging analysis. (C) Relative quantification (RQ; UBC = 1) of HLA-DR mRNA expression, which was assessed by qPCR. A nonparametric, two-tailed Mann–Whitney U test was used to analyze statistical significance between the HIV+FSW group versus the HIV−FSW group and the HIV+FSW group versus the HIV−LR group. (D) Bright-field images of human ectocervical tissue stained with hematoxylin (blue) for visualization of cell nuclei and stained for HLA-DR (brown). Scale bars, 200 μm. (E) Correlation between the percentage of CD8+ cells and percentage of HLA-DR+ cells within the total ectocervical tissue area, as assessed by imaging analysis in the HIV+FSW group. (F) Correlation between the RQ of CD8 and HLA-DR mRNA levels, as assessed by qPCR, in the HIV+FSW group. Spearman rank correlation coefficient test was used to assess the correlation.
HLA-DR staining showed a similar expression pattern as seen for CD3+ and CD8+ cells; hence, the HLA-DR+ cells also were expressed close to the basal membrane, and clusters of HLA-DR+ cells could be found within both the epithelial and submucosal sites. The submucosal clusters of HLA-DR+ cells were more frequent than seen for CD3+ and CD8+ cells (Fig. 5D).
There was no correlation between the percentage of HLA-DR+ cells and plasma or cervical viral load in the HIV+FSW group (data not shown), but CD8 and HLA-DR expression correlated with each other at the cellular and mRNA levels (p = 0.0004, r = +0.72 and p < 0.0001, r = +0.91, respectively) (Fig. 5E, 5F). Among the four HIV-infected individuals with detectable HIV RNA within the tissue (as analyzed by in situ hybridization), none had higher HLA-DR+ cell numbers or HLA-DR mRNA expression than any of the other individuals (data not shown).
Discussion
We found that HIV+FSWs had significantly more total cells in their ectocervical tissue compartment; among these cells, CD8+ and CD3+ cells were significantly more frequent compared with either of the two HIV− control groups. The control groups consisted of FSWs (to control for the influence of high-risk sexual activity) and non-FSWs from the same geographical area. We also demonstrated, for the first time, to our knowledge, that HIV RNA+ cells were present in the ectocervical tissue samples, as detected by in situ hybridization. The expression of HLA-DR, used as a general marker for immune activation and presence of APCs, was also higher in the HIV+ women than in the control groups. Among these immune cell populations, only CD8+ cells correlated significantly with levels of plasma and cervical viral load, thus indicating that CD8+ cells in the female genital tract may play a role in the immunopathogenesis of HIV. A more in-depth analysis of the different subpopulations of CD8+ cells is needed to elucidate whether they display functional phenotypes that are beneficial and/or disadvantageous for the local immune control of HIV. Furthermore, costaining of the CD8/CD4 markers, together with the in situ expression of HIV RNA, also would contribute to understanding of the mucosal interplay between the cells and their impact on local viral replication.
In this unique set of ectocervical tissue samples, the localization and enumeration of mucosal CD8+ cells could be assessed in detail. The majority of CD8+ cells in the ectocervical tissue of HIV+ women were shown to be CD3+ T cells, and, likewise, the majority of CD3+ T cells expressed CD8. Single-stained CD8+ and CD3+ cells were also present. Thus, the percentage of total cells, CD8+ cells, and CD3+ cells within the tissue sections were significantly higher in the HIV+ women than in either of the two control groups, and they also had a significantly higher CD8/CD3 cell ratio than did the HIV− low-risk group. Furthermore, the HIV+ women displayed a significant correlation between percentages of CD8+ cells and total cells in their ectocervical tissue. The CD8+ cell dominance of the HIV+ women was more pronounced in the epithelium compared with the submucosa. In all study groups, the CD8+ and CD3+ cells mainly were localized in proximity to both sides of the basal membrane. Clusters of CD8+ cells also were seen in the epithelial compartment, especially in the tissue samples of the HIV+ group. It was suggested that it is primarily effector memory T cells that migrate between blood, spleen, and the female genital tract (e.g., peripheral tissue), whereas tissue-resident T cells are primarily found in the epithelial compartment of the female genital tract (46, 47). Thus, one might speculate that the increased number of CD8+ cells observed in the epithelial layer of HIV+ women might be tissue-resident cells, whereas the CD8+ cells in the submucosa are not. However, to prove this, a careful characterization, using several cell markers, would be needed.
To complement these in situ quantifications, assessment of the mRNA expression of CD8 and CD3, as well as the CD8/CD3 ratio, confirmed the significantly elevated expression of CD8+ and CD3+ cells in the HIV+ group. We (9) showed previously that these HIV+ women had similar levels of ectocervical CD4+ cells (e.g., CD4+ T cells, macrophages, and dendritic cells) as did HIV− women engaged in comparable sex-work activities. Therefore, it was interesting to analyze whether those levels of CD4+ cells correlated with the present levels of CD8+ cells; however, no association was seen (data not shown). Thus, our data indicate a selective infiltration or local expansion of CD8+ T cells in the ectocervix of HIV+ women. The elevated CD8+ cell numbers were not merely an effect of sex work per se, or of HSV-2 or syphilis serostatus, because the two FSW study groups were comparable with respect to these parameters. The lack of association between HSV-2 seropositivity and CD8+ cell numbers can be expected because the women were asymptomatic at the time of sample collection, and previous lesions may have been localized at other tissue sites. These observations are in line with other studies (5, 31, 32, 36, 48) demonstrating higher numbers of CD8+ T cells in female genital tract samples (e.g., cervical biopsy specimens and cytobrush-derived cervical cells) obtained from HIV+ individuals.
In our previous study (9), it was shown that ectocervical immune cells of HIV+ women displayed a higher level of general immune activation than those in the HIV− control groups. When retrospectively assessing the mRNA expression of inflammatory markers available from that study, the CD8+ cell numbers did not correlate significantly with those values, with the exception of a positive correlation with CD69. However, no formal double staining of CD69 and CD8 was performed to determine whether the two markers were present on the same cell population. In agreement with these results, Jaspan et al. (48) showed that cytobrush-derived T cells from HIV-infected women expressed higher frequencies of T cell–activation markers, including HLA-DR, compared with uninfected women and that these cell numbers predicted viral shedding. We also showed in this study that the HIV+ women had higher levels of HLA-DR expression, both at the mRNA and cellular levels, and that this expression correlated with CD8+ cell numbers. However, the relative numbers of HLA-DR+ cells were higher than those of CD8+ cells, which is expected because HLA-DR is a marker for general immune activation and is expressed on both T cells and APCs. Chronic HIV infection is associated with immune activation in the systemic and lymphoid compartments (49). Thus, this increased expression of HLA-DR may indicate an influx of APCs and even may include plasmacytoid dendritic cells, which were shown to accumulate in the female genital tract of SIV-infected monkeys shortly postinfection (50).
Because we had access to cervical viral loads, we could perform a retrospective analysis showing that, among a set of cellular phenotypes, immune activation markers and inflammatory cytokines analyzed previously by qPCR in the ectocervical tissue (9), only CD69 mRNA correlated significantly with cervical viral load. Thus, although the vast majority of immune-activation markers, as assessed in CVSs, show a clear correlation with genital viral load (16, 17), this was not the case for the tissue-bound immune-activation markers. This discrepancy may reflect that the proteins within the secretions are also derived from other parts of the female genital tract, as well as the systemic circulation (plasma exudates). The correlation between CD69 mRNA and cervical viral load is interesting because CD69 is not only an activation marker, it also has a role in tissue trafficking and tissue residence (46, 47). When assessing the correlation of viral load with CD8, CD3, or HLA-DR, only the number of CD8+ cells and expression of CD8 mRNA correlated significantly with plasma and cervical viral load. Future studies using in situ enumeration of CD69+ cells, HIV-specific CD8+ cells, and functional assays will be very informative to elucidate the specificity and antiviral capacity of these CD8+ cells, because it was suggested that they may have limited antiviral capacity (3–5).
HIV RNA+ cells were detected within the ectocervical tissue site of 4 of the 20 HIV+ women. The HIV RNA–expressing cells were located both in the epithelium and in the submucosa. For ethical reasons, it was not possible to obtain larger tissue samples that would have allowed costaining of HIV RNA and the cell markers in the mucosa. However, by reassessing the data from our previous study (9), we could determine that the CD4+ cell expression in these four individuals was above the median value for the whole group, but this difference was not statistically significant (data not shown). In the current study, none of the immune markers—CD8, CD3, or HLA-DR—was overrepresented in the HIV RNA+ samples. Our finding of local HIV RNA–expressing cells supports previous findings of infectious HIV particles, HIV nucleic acids, and HIVp24 Ags in cervical tissue samples of HIV+ subjects (33, 34, 36) and indicates that HIV can actively replicate in the ectocervix of chronically HIV-infected women. The in situ hybridization technique used in the current study has been evaluated extensively in studies (51) of SIV-infected cells in experimentally infected nonhuman primates showing SIV replication at the endocervical site. There was no clear correlation between cervical viral load and presence of HIV RNA in the ectocervical tissue samples. The cervical viral load most likely represents a mixture of HIV RNA shed from both plasma and other parts of the genital tract mucosa. It would be interesting to compare HIV sequence data in samples representing these different localities, including the ectocervix, to better understand their relative importance for sexual viral transmission.
From these limited numbers of study subjects, and by assessing only three sections/individual, it obviously cannot be excluded that larger foci of viral replication can be present in the ectocervix, or elsewhere in the female genital tract, as shown in a study (52) of vaginal tissue samples. It can furthermore not excluded that more than four of the samples were positive if the whole biopsies had been scanned. However, the low number of HIV RNA+ cells detected is not believed to be due to assay sensitivity, because our in situ hybridization technique is well established with a detection limit of ~20 copies of HIV RNA/cell (53). Collectively, these data suggest that the female genital tract should not be neglected as a potential viral reservoir if the immune cell balance were disrupted.
It must be noted that this was a cross-sectional study of women in the chronic stage of HIV infection, and genital samples were only collected from a single subcompartment of the female genital tract. Thus, one cannot exclude altered cellular ratios during acute infection or at other sites of the female genital tract. Further limitations of the study include the fact that only HIV RNA, and not proviral DNA, was analyzed. These women also represent a highly selected risk group and may not be representative of nonsex working HIV+ women with a lower prevalence of concomitant genital infections. We also may have missed low levels of HIV RNA in the plasma, as well as in the secretions and tissue samples, because of assay sensitivity. Some of the HIV− women may further represent the well-described group of relatively HIV-resistant women (women with >7 y of high-risk behavior without sero-converting to HIV) who may have a lower immune-activation profile (54–56). Although a formal comparison between the two HIV− control groups was not the focus of the current study, the HIV−FSWs displayed comparable levels of total cells, as well as levels of CD3+ and HLA-DR+ cells. However, the HIV−FSWs had significantly higher CD8+ cell numbers compared with the HIV− nonsex working women. Thus, some of these HIV−FSWs might display an altered immune phenotype, which may contribute to their relative resistance against infection.
In the current study, we assessed genital tissue samples and showed that the HIV+ group had elevated numbers of total cells and CD8+, CD3+, and HLA-DR+ cells, as well as a higher CD8/ CD3 ratio, in their ectocervical compartment. Both CD8+ cell number and CD8 mRNA expression correlated significantly with plasma and cervical viral load but not with the presence of the few HIV-replicating cells found in the ectocervix. Furthermore, the HIV+ women displayed a significant correlation between percentages of CD8+ cells and total cells, HLA-DR+ cells, and CD69 mRNA expression in their ectocervical tissue. An improved understanding of how immune effector cells and inflammatory cytokines in the genital tissue contribute to HIV pathogenesis and viral transmission could aid in the design of interventions aimed at effectively disrupting sexual HIV transmissions.
Acknowledgments
This work was supported by the Swedish Physicians against AIDS Research Foundation (to A.G. and A.T.), the Karolinska Institutet’s faculty funds for a graduate program in international ranking (to A.T. and A.G.), the Swedish Society for Medical Research (to A.T.), the Swedish Society of Medicine (to A.T.), the Swedish Research Council (to K. Broliden and T.H.), the Swedish International Development Cooperation Agency (to K. Broliden and T.H.), the Erik and Edith Fernström Foundation (to T.H.), the Bill and Melinda Gates Foundation through the Grand Challenges in Global Health Initiative (to F.A.P.), and the Canadian Institutes of Health Research. F.A.P. is a Tier I Canada Research Chair.
We thank the study participants and the staff at the Kenyan AIDS Control Project at the University of Nairobi, especially the staff of the Majengo/ Pumwani clinics. We also acknowledge Dr. Angela Muliro and Juliana Cheruiyot for clinical assistance and Pernilla Petersson and Anette Hofmann for technical assistance.
Abbreviations used in this article
- Ct
cycle threshold
- CVS
cervicovaginal secretion
- FSW
female sex worker
- LR
lower-risk nonsex working woman
- PSA
prostate-specific Ag
- qPCR
quantitative PCR
- UBC
ubiquitin C
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Nkwanyana NN, Gumbi PP, Roberts L, Denny L, Hanekom W, Soares A, Allan B, Williamson AL, Coetzee D, Olivier AJ, et al. Impact of human immunodeficiency virus 1 infection and inflammation on the composition and yield of cervical mononuclear cells in the female genital tract. Immunology. 2009;128(1 Suppl):e746–e757. doi: 10.1111/j.1365-2567.2009.03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bere A, Denny L, Naicker P, Burgers WA, Passmore JA. HIV-specific T-cell responses detected in the genital tract of chronically HIV-infected women are largely monofunctional. Immunology. 2013;139:342–351. doi: 10.1111/imm.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gumbi PP, Jaumdally SZ, Salkinder AL, Burgers WA, Mkhize NN, Hanekom W, Coetzee D, Williamson A, Passmore JS. CD4 T cell depletion at the cervix during HIV infection is associated with accumulation of terminally differentiated T cells. J Virol. 2011;85:13333–13341. doi: 10.1128/JVI.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olaitan A, Johnson MA, MacLean A, Poulter LW. The distribution of immunocompetent cells in the genital tract of HIV-positive women. AIDS. 1996;10:759–764. doi: 10.1097/00002030-199606001-00010. [DOI] [PubMed] [Google Scholar]
- 6.Bebell LM, Passmore JA, Williamson C, Mlisana K, Iriogbe I, van Loggerenberg F, Karim QA, Karim SA. Relationship between levels of inflammatory cytokines in the genital tract and CD4+ cell counts in women with acute HIV-1 infection. J Infect Dis. 2008;198:710–714. doi: 10.1086/590503. [DOI] [PubMed] [Google Scholar]
- 7.Roberts L, Passmore JA, Mlisana K, Williamson C, Little F, Bebell LM, Walzl G, Abrahams MR, Woodman Z, Abdool Karim Q, Abdool Karim SS. Genital tract inflammation during early HIV-1 infection predicts higher plasma viral load set point in women. J Infect Dis. 2012;205:194–203. doi: 10.1093/infdis/jir715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Southern PJ. Missing out on the biology of heterosexual HIV-1 transmission. Trends Microbiol. 2013;21:245–252. doi: 10.1016/j.tim.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Hirbod T, Kimani J, Tjernlund A, Cheruiyot J, Petrova A, Ball TB, Mugo N, Jaoko W, Plummer FA, Kaul R, Broliden K. Stable CD4 expression and local immune activation in the ectocervical mucosa of HIV-infected women. J Immunol. 2013;191:3948–3954. doi: 10.4049/jimmunol.1301220. [DOI] [PubMed] [Google Scholar]
- 10.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, Meehan MO, Lutalo T, Gray RH Rakai Project Study Group. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 11.Cu-Uvin S, Snyder B, Harwell JI, Hogan J, Chibwesha C, Hanley D, Ingersoll J, Kurpewski J, Mayer KH, Caliendo AM. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr. 2006;42:584–587. doi: 10.1097/01.qai.0000229997.52246.95. [DOI] [PubMed] [Google Scholar]
- 12.Kovacs A, Wasserman SS, Burns D, Wright DJ, Cohn J, Landay A, Weber K, Cohen M, Levine A, Minkoff H, et al. DATRI Study Group; WIHS Study Group. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–1601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 13.Mostad SB. Prevalence and correlates of HIV type 1 shedding in the female genital tract. AIDS Res Hum Retroviruses. 1998;14(Suppl 1):S11–S15. [PubMed] [Google Scholar]
- 14.Baeten JM, Kahle E, Lingappa JR, Coombs RW, Delany-Moretlwe S, Nakku-Joloba E, Mugo NR, Wald A, Corey L, Donnell D, et al. Partners in Prevention HSV/HIV Transmission Study Team. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3:77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baeten JM, Overbaugh J. Measuring the infectiousness of persons with HIV-1: opportunities for preventing sexual HIV-1 transmission. Curr HIV Res. 2003;1:69–86. doi: 10.2174/1570162033352110. [DOI] [PubMed] [Google Scholar]
- 16.Blish CA, McClelland RS, Richardson BA, Jaoko W, Mandaliya K, Baeten JM, Overbaugh J. Genital Inflammation Predicts HIV-1 Shedding Independent of Plasma Viral Load and Systemic Inflammation. J Acquir Immune Defic Syndr. 2012;61:436–440. doi: 10.1097/QAI.0b013e31826c2edd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukura LR, Ghosh M, Fahey JV, Cu-Uvin S, Wira CR. Genital tract viral load in HIV Type 1-positive women correlates with specific cytokine levels in cervical-vaginal secretions but is not a determinant of infectious virus or anti-HIV activity. AIDS Res Hum Retroviruses. 2012;28:1533–1539. doi: 10.1089/aid.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson BL, Wang CC, Delong AK, Liu T, Kojic EM, Kurpewski J, Ingersoll J, Mayer K, Caliendo AM, Cu-Uvin S. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin Infect Dis. 2008;47:1216–1221. doi: 10.1086/592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Magaret A, Wald A, Corey L, Celum C. Herpes simplex virus (HSV)-suppressive therapy decreases plasma and genital HIV-1 levels in HSV-2/HIV-1 coinfected women: a randomized, placebo-controlled, cross-over trial. J Infect Dis. 2008;198:1804–1808. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RA, Theall KP, Amedee AM, Kissinger PJ. Frequent douching and clinical outcomes among HIV-infected women. Sex Transm Dis. 2007;34:985–990. doi: 10.1097/OLQ.0b013e31811ec7cb. [DOI] [PubMed] [Google Scholar]
- 21.Clemetson DB, Moss GB, Willerford DM, Hensel M, Emonyi W, Holmes KK, Plummer F, Ndinya-Achola J, Roberts PL, Hillier S, et al. Detection of HIV DNA in cervical and vaginal secretions. Prevalence and correlates among women in Nairobi, Kenya. JAMA. 1993;269:2860–2864. [PubMed] [Google Scholar]
- 22.Heffron R, Donnell D, Rees H, Celum C, Mugo N, Were E, de Bruyn G, Nakku-Joloba E, Ngure K, Kiarie J, et al. Partners in Prevention HSV/HIV Transmission Study Team. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012;12:19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–959. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 24.Mcclelland RS, Wang CC, Mandaliya K, Overbaugh J, Reiner MT, Panteleeff DD, Lavreys L, Ndinya-Achola J, Bwayo JJ, Kreiss JK. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS. 2001;15:105–110. doi: 10.1097/00002030-200101050-00015. [DOI] [PubMed] [Google Scholar]
- 25.McClelland RS, Wang CC, Overbaugh J, Richardson BA, Corey L, Ashley RL, Mandaliya K, Ndinya-Achola J, Bwayo JJ, Kreiss JK. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–2430. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 26.Nagot N, Ouédraogo A, Foulongne V, Konaté I, Weiss HA, Vergne L, Defer MC, Djagbaré D, Sanon A, Andonaba JB, et al. ANRS 1285 Study Group. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–799. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Garcia M, Patel MV, Wira CR. Innate and adaptive anti-HIV immune responses in the female reproductive tract. J Reprod Immunol. 2013;97:74–84. doi: 10.1016/j.jri.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CC, McClelland RS, Overbaugh J, Reilly M, Panteleeff DD, Mandaliya K, Chohan B, Lavreys L, Ndinya-Achola J, Kreiss JK. The effect of hormonal contraception on genital tract shedding of HIV-1. AIDS. 2004;18:205–209. doi: 10.1097/00002030-200401230-00009. [DOI] [PubMed] [Google Scholar]
- 29.Cu-Uvin S, DeLong AK, Venkatesh KK, Hogan JW, Ingersoll J, Kurpewski J, De Pasquale MP, D’Aquila R, Caliendo AM. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–2497. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 30.Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, Ingerman MJ, Witek J, Kedanis RJ, Natkin J, DeSimone J, Pomerantz RJ. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 31.Coombs RW, Reichelderfer PS, Landay AL. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 2003;17:455–480. doi: 10.1097/00002030-200303070-00001. [DOI] [PubMed] [Google Scholar]
- 32.Iqbal SM, Ball TB, Kimani J, Kiama P, Thottingal P, Embree JE, Fowke KR, Plummer FA. Elevated T cell counts and RANTES expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infect Dis. 2005;192:728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 33.Behbahani H, Walther-Jallow L, Klareskog E, Baum L, French AL, Patterson BK, Garcia P, Spetz AL, Landay A, Andersson J. Proinflammatory and type 1 cytokine expression in cervical mucosa during HIV-1 and human papillomavirus infection. J Acquir Immune Defic Syndr. 2007;45:9–19. doi: 10.1097/QAI.0b013e3180415da7. [DOI] [PubMed] [Google Scholar]
- 34.Nuovo GJ, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 35.Olaitan A, Johnson MA, Reid WM, Poulter LW. Changes to the cytokine microenvironment in the genital tract mucosa of HIV+ women. Clin Exp Immunol. 1998;112:100–104. doi: 10.1046/j.1365-2249.1998.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pomerantz RJ, de la Monte SM, Donegan SP, Rota TR, Vogt MW, Craven DE, Hirsch MS. Human immunodeficiency virus (HIV) infection of the uterine cervix. Ann Intern Med. 1988;108:321–327. doi: 10.7326/0003-4819-108-3-321. [DOI] [PubMed] [Google Scholar]
- 37.Embree JE, Njenga S, Datta P, Nagelkerke NJ, Ndinya-Achola JO, Mohammed Z, Ramdahin S, Bwayo JJ, Plummer FA. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–2541. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 38.Fowke KR, Nagelkerke NJ, Kimani J, Simonsen JN, Anzala AO, Bwayo JJ, MacDonald KS, Ngugi EN, Plummer FA. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 39.Hasselrot K, Cheruiyot J, Kimani J, Ball TB, Kaul R, Hirbod T. Feasibility and safety of cervical biopsy sampling for mucosal immune studies in female sex workers from Nairobi, Kenya. PLoS ONE. 2012;7:e47570. doi: 10.1371/journal.pone.0047570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV-1-infected lymphatic tissue to antiretroviral therapy. J Infect Dis. 2004;189:572–582. doi: 10.1086/381396. [DOI] [PubMed] [Google Scholar]
- 42.Brismar Wendel S, Kaldensjö T, Peterson P, Andersson S, Broliden K, Hirbod T. Slumbering mucosal immune response in the cervix of human papillomavirus DNA-positive and -negative women. Int J Oncol. 2010;37:1565–1573. doi: 10.3892/ijo_00000810. [DOI] [PubMed] [Google Scholar]
- 43.Andersson J, Fehniger TE, Patterson BK, Pottage J, Agnoli M, Jones P, Behbahani H, Landay A. Early reduction of immune activation in lymphoid tissue following highly active HIV therapy. AIDS. 1998;12:F123–F129. doi: 10.1097/00002030-199811000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Hirbod T, Bailey RC, Agot K, Moses S, Ndinya-Achola J, Murugu R, Andersson J, Nilsson J, Broliden K. Abundant expression of HIV target cells and C-type lectin receptors in the foreskin tissue of young Kenyan men. Am J Pathol. 2010;176:2798–2805. doi: 10.2353/ajpath.2010.090926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjork L, Fehniger TE, Andersson U, Andersson J. Computerized assessment of production of multiple human cytokines at the single-cell level using image analysis. J Leukoc Biol. 1996;59:287–295. doi: 10.1002/jlb.59.2.287. [DOI] [PubMed] [Google Scholar]
- 46.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 47.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 48.Jaspan HB, Liebenberg L, Hanekom W, Burgers W, Coetzee D, Williamson AL, Little F, Myer L, Coombs RW, Sodora D, Passmore JA. Immune activation in the female genital tract during HIV infection predicts mucosal CD4 depletion and HIV shedding. J Infect Dis. 2011;204:1550–1556. doi: 10.1093/infdis/jir591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miedema F, Hazenberg MD, Tesselaar K, van Baarle D, de Boer RJ, Borghans JA. Immune Activation and Collateral Damage in AIDS Pathogenesis. Front Immunol. 2013;4:298. doi: 10.3389/fimmu.2013.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdulhaqq SA, Martinez MI, Kang G, Foulkes AS, Rodriguez IV, Nichols SM, Hunter M, Sariol CA, Ruiz LA, Ross BN, et al. Serial cervicovaginal exposures with replication-deficient sivsm induce higher dendritic cell (pdc) and cd4+ t-cell infiltrates not associated with prevention but a more severe sivmac251 infection of rhesus macaques. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0000000000000047. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohn MA, Frankel SS, Rugpao S, Young MA, Willett G, Tovanabutra S, Khamboonruang C, VanCott T, Bhoopat L, Barrick S, et al. Chronic inflammation with increased human immunodeficiency virus (HIV) RNA expression in the vaginal epithelium of HIV-infected Thai women. J Infect Dis. 2001;184:410–417. doi: 10.1086/322780. [DOI] [PubMed] [Google Scholar]
- 53.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 54.Card CM, McLaren PJ, Wachihi C, Kimani J, Plummer FA, Fowke KR. Decreased immune activation in resistance to HIV-1 infection is associated with an elevated frequency of CD4(+)CD25(+)FOXP3(+) regulatory T cells. J Infect Dis. 2009;199:1318–1322. doi: 10.1086/597801. [DOI] [PubMed] [Google Scholar]
- 55.Chege D, Chai Y, Huibner S, Kain T, Wachihi C, Kimani M, Barasa S, McKinnon LR, Muriuki FK, Kariri A, et al. Blunted IL17/IL22 and pro-inflammatory cytokine responses in the genital tract and blood of HIV-exposed, seronegative female sex workers in Kenya. PLoS ONE. 2012;7:e43670. doi: 10.1371/journal.pone.0043670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLaren PJ, Ball TB, Wachihi C, Jaoko W, Kelvin DJ, Danesh A, Kimani J, Plummer FA, Fowke KR. HIV-exposed seronegative commercial sex workers show a quiescent phenotype in the CD4+ T cell compartment and reduced expression of HIV-dependent host factors. J Infect Dis. 2010;202(Suppl 3):S339–S344. doi: 10.1086/655968. [DOI] [PubMed] [Google Scholar]