Abstract
Microtus californicus scirpensis is an endangered, isolated subspecies of California vole. It requires water pools and riparian bulrush (Schoenoplectus americanus) and occupies some of the rarest habitat of any North American mammal. The minimally vegetated, extremely arid desert surrounding the pools is essentially uninhabitable for Ixodes species ticks. We describe an enzootic cycle of Borrelia carolinensis in Ixodes minor ticks at a site 3500 km distant from the region in which I. minor is known to occur in Tecopa Host Springs, Inyo County, eastern Mojave Desert, California. Voles were live-trapped, and ticks and blood samples queried by PCR and DNA sequencing for identification and determination of the presence of Borrelia spp. Between 2011–2013, we found 21 Ixodes minor ticks (prevalence 4–8%) on Amargosa voles and Reithrodontomys megalotis. DNA sequencing of 16S rRNA from ticks yielded 99% identity to I. minor. There was 92% identity with I. minor in the calreticulin gene fragment. Three ticks (23.1%), 15 (24%) voles, three (27%) house mice, and one (7%) harvest mice were PCR positive for Borrelia spp. Sequencing of the 5S-23S intergenic spacer region and flagellin gene assigned Amargosa vole Borrelia strains to B. carolinensis. Ixodes minor, first described in 1902 from a single Guatemalan record, reportedly occurs only in the southeast American on small mammals and birds. The source of this tick in the Mojave Desert and time scale for introduction is not known but likely via migratory birds. Borrelia strains in the Amargosa ecosystem most closely resemble B. carolinensis. B. carolinensis occurs in a rodent-I. minor enzootic cycle in the southeast U.S. although its epidemiological significance for people or rodents is unknown. The presence of a tick and Borrelia spp. only known from southeast U.S. in this extremely isolated habitat on the other side of the continent is of serious concern because it suggests that the animals in the ecosystem could be vulnerable to further incursions of pathogens and parasites.
Keywords: 16S rRNA, Amargosa vole, Borrelia burgdorferi sensu lato, calreticulin, Microtus californicus scirpensis, Migration
Introduction
The Amargosa vole (Microtus californicus scirpensis, Kellogg, 1918) is a critically endangered and very isolated subspecies of California vole found only in the eastern Mojave Desert in Inyo County, California (U.S. Fish and Wildlife Service 1997; Cudworth and Koprowski 2010). This approximately 75 g microtine rodent requires riparian habitat dominated by bulrush (Schoenoplectus americanus, (Persoon) Volkart, 1905) and thus has one of the narrowest niche breadths and occupies some of the rarest habitat of any North American mammal. Suitable, occupied habitat occurs at present only within the Amargosa River basin near the town of Tecopa and is surrounded by harsh, alkali desert.
Federal protection under the U.S. Endangered Species Act for the Amargosa vole was motivated by habitat loss and degradation, with very low population size, habitat fragmentation, genetic impoverishment, and disease all cited as contributory factors (U.S. Fish and Wildlife Service 1997). Since 2011, a collaborative group of researchers has begun to catalog pathogens and ectoparasites of the Amargosa vole and sympatric small mammals, initially identifying trombiculid mite larvae as a cause of severe ear tissue destruction in many vole individuals (Foley et al. 2013). In the course of this survey, we also obtained ixodid ticks from some of the voles and detected Borrelia burgdorferi (Johnson et al., 1984) sensu lato DNA from ear tissue of individuals. In California, Borrelia burgdorferi sensu stricto appears to be the most significant agent of Lyme disease and its reservoirs are small mammals such as the western gray squirrel (Sciurus griseus, Ord, 1818) (Lane et al. 2005). A cluster of genospecies designated B. burgdorferi sensu lato occurs in various small mammals but most, with the exception of B. burgdorferi sensu stricto, do not cause human Lyme disease (Girard et al. 2011). The ticks on the Amargosa voles, including adult stage ticks, appeared morphologically consistent with Ixodes pacificus (Cooley and Kohls, 1943), a species that is the main vector for B. burgdorferi in the western U.S. (Burgdorfer et al. 1985; Clover and Lane 1995) but is extraordinarily rare as an adult on small mammals and never occurs at the low elevation, extremely arid, and unforested site occupied by the Amargosa vole (Furman and Loomis 1984). Because of this, we pursued further evaluation of the ticks and spirochetes including molecular characterization. In the present study, we describe an enzootic cycle of B. carolinensis (Rudenko et al. 2011) in I. minor (Neumann 1902) ticks at a site 3500 km distant from the region in which I. minor is known to occur.
Materials and Methods
Small mammals were trapped in Sherman (HB Sherman, Tallahassee, FL) live traps along the Amargosa River in the vicinity of Tecopa Hot Springs in southeastern Inyo County, California (UTM Zone 11 N 3969717-3971539 and W 568016-569168) between November 2011 and February 2013. In these sites, riparian vegetation extends for only a few meters beyond river or pool margins and predominantly consists of bulrush (Schoenoplectus americanus) interspersed with cattails (Typha domingensis, Persoon, 1807), desert salt grass (Distichlis spicata, (L.) Greene, 1887), and rushes (Juncus spp.). Appropriate biosecurity was implemented including sterilization of traps, footwear, and equipment among sites. Traps were set at dusk and baited with peanut butter and oats and then animals recovered at dawn. Captured individuals were identified to species, sex, and age class, then weighed, evaluated for reproductive condition, and sampled for parasites and pathogens. Based on physical examination, animals were assigned a body condition score from 1 to 5 (Ullman-Cullere and Foltz 1999), all ectoparasites were collected into 70% ethanol, and a piece of marginal pinna was collected with a sterile scissors. Animals were then given a uniquely numbered metal ear tag (1005-1 Monel, National Band and Tag Co., Newport, KY) and released at the capture site. Each successful capture event was localized with a handheld global positioning system device (Garmin 62S, Garmin International, Olathe, KS). All work with animals was performed in compliance with the UC Davis IACUC and overseen by the campus attending veterinarian, under valid scientific collecting permits from California Department of Fish and Wildlife, US Fish and Wildlife Service, and Bureau of Land Management.
Initially, we attempted to identify Ixodes spp. ticks to species using keys (Furman and Loomis 1984; Webb et al. 1990) with larvae viewed under a compound microscope in a depression slide as well as a dissecting microscope before identification was confirmed. For further evaluation of tick morphology, we utilized an expanded set of resources from east of the Rocky Mountains (Cooley and Kohls 1945; Clifford et al. 1961; Keirans and Clifford 1978). DNA was extracted from ticks using ammonium hydroxide, modified from Humair et al. (2007). Ticks were individually placed in microcentrifuge tubes, cooled in liquid nitrogen for 3 min, and crushed using a microcentrifuge pestle. 100 mL of 0.7 mol/L NH4OH was added, and samples were placed on a 100°C heat block for 15 min. Tubes were then cooled on ice for 30 sec followed by an additional 15 min of heating at 100°C with open lids in order to evaporate the ammonia. In order to confirm tick identification, the 16S rRNA gene of the tick mitochondrion was amplified as previously described with modifications (Black and Piesman 1994) from four randomly chosen ticks using primers 16S+1 and 16S−2 to produce a 287-base-pair product. The 25 μL PCR mix contained GoTaq Green Master Mix (Promega, Madison, WI), 0.5 μmol/L of the forward primer, 0.5 μmol/L of the reverse primer, and 1 μL of DNA, and run conditions were as described in the original publication. A new PCR for a 242-base-pair fragment of the calreticulin gene was designed by hand after obtaining all sequences of I. pacificus and I. minor calreticulin fragments available on GenBank. The assay used forward primer 5′-GACGGAGGTAAGCCCCATTTTC-3′ and reverse primer 5′-GAACTTGCCGGCGGACAGCTTG-3′, mastermix as described for the 16S PCR, and the following run conditions: after denaturation at 94°C for 5 min, 30 cycles of 94°C for 30 sec, 58°C for 15 sec, and 72°C for 30 sec, with a final extension of 72°C for 1 min. Following PCR and agarose gel electrophoresis, DNA was purified from gels using a kit (QiaQuick, Qiagen, Valencia, CA) and submitted for sequencing on an ABI 3730 sequencer (Davis Sequencing, Davis, CA) using the forward PCR primer. Sequenced amplicons were evaluated by BLAST search of GenBank (NCBI; http://blast.ncbi.nlm.nih.gov/Blast.cgi). For final confirmation and for submission to the GenBank database, PCR amplicons were gel-extracted and then cloned using the pGEM-T easy vector system (Promega) followed by DNA sequencing using plasmid primer T7.
Ear tissue was kept frozen at −20°C, and then, DNA extraction carried out using the Qiagen Purification kit, following manufacturer's guidelines. TaqMan real-time PCR for Borrelia spp. was performed on ear and tick DNA as described previously (Barbour et al. 2009), modified to use only the forward and reverse primers, and the probe identified as specific for B. burgdorferi (although in silico analysis, our internal unpublished data show that both primers are generic for borreliae, while the probe has 100% homology to most if not all B. burgdorferi sensu lato genospecies including B. bissettii, B. garinii, and others). Reactions were run in a combined thermocycler/fluorometer (ABI Prism 7700, Applied Biosystems, Foster City, CA). Water negative controls were included in each run, while a panel of positive controls consisted of DNA from cultured strains of B. burgdorferi sensu stricto and B. bissettii. Strongly, PCR-positive samples with a cycle threshold <35 were evaluated using conventional PCR for three additional targets. 5S-23S intergenic spacer sequence (IGS) and flagellin protocols were performed as described with modifications to use GreenGoTaq Master Mix (Rudenko et al. 2011). PCR for the Borrelia 16S gene was carried out as described (Rudenko et al. 2011) with the following modifications: Amplitaq Gold Mastermix 360 (Life Technologies, Carlsbad, CA) was used in a 50 μL reaction volume containing 1.0 μmol/L primers and 10 μL DNA. Initial denaturation was changed to 10 min at 96°C to initiate hotstart, and the rest of the cycle conditions remained the same as initially published. Amplicons were excised, cloned, and sequenced as for tick products. Tick 16S and calreticulin and Borrelia 16S, fla, and 5S-23 IGS sequences were deposited in GenBank, accession numbers (to be inserted).
Results
Between autumn 2011 and spring 2013, we assessed 62 individual Amargosa voles, 15 harvest mice (Reithrodontomys megalotis (Baird, 1857)), and 11 house mice (Mus musculus, L., 1758) in marshes near Tecopa, CA. All ticks (N = 21) were morphologically confirmed as I. minor, differentiated from I. pacificus primarily on the basis of overall size and shape of the auriculae. All of the ticks except two on harvest mice were from voles. There were 13 adults (nine female and four male), three larvae, and five nymphs. The harvest mouse ticks were collected in October 2012, while the other ticks were collected in February (larvae, nymphs, and adults), March (nymphs and adults), and April (larvae). DNA sequencing from 16S rRNA from an I. minor larva, nymph, and a male and female adult yielded complete coverage of the 287 nucleotides, each with 99% identity to I. minor accession AF549841.1 in the GenBank database and only 91% identity with the next closest match (I. muris, Bishopp and Smith, 1937). All I. minor were identical to each other in the 16S gene. There was 92% identity with I. minor accession A4395264.1 in the calreticulin fragment, compared with 89% for I. muris and I. jellisoni (Cooley and Kohls, 1938) and 88% for I. pacificus.
Of the ticks and rodents tested by PCR for Borrelia spp., three ticks (of 13, 23.1%) were positive, while 15 of 62 voles (24.2%), three of 11 house mice (27.3%) and one of 15 harvest mice (6.7%) were positive. Results of DNA sequencing showed that Amargosa vole Borrelia strains were closely related to several species of B. burgdorferi sensu lato. Sequencing of the 5S-23S IGS assigned Amargosa vole Borrelia strains to B. carolinensis. Three samples from Amargosa voles had identical sequences over the 253 base pairs and were 100% homologous with 15 different B. carolinensis strains in the database (e.g., strain SCSC-1, EU072440.1) and only 95% homology with the next closest match, B. garinii. In the fla gene, four amplicons had identical sequences and, over 494 base pairs, had 99% homology with multiple B. carolinensis accessions including strain SCCH-6, SCGT-18, and SCGT-21 (EU076485.1, EU076498.1, and EU076499.1), while the relatedness to B. americanum was only 97% and other genospecies were lower. For the 16S rRNA gene, PCR from two individuals yielded invariant sequences, but there were scattered differences across the fragment with sequences in GenBank and no consistently best match (Fig. 1). Rather, across the 18 single nucleotide polymorphisms (SNP) observed when vole sequences were aligned with well-defined sequences from multiple B. americana, B. californiensis, B. carolinensis, B. bissettii, and B. burgdorferi sensu stricto, two SNPs were unique to vole strains, two specifically matched only B. californiensis, while four ruled out B. californiensis (but did not distinguish among the other genospecies), four ruled out B. americana, four ruled out B. carolinensis, and two ruled out B. burgdorferi sensu stricto. Thus, overall Amargosa vole Borrelia strains appear to be more closely related to B. carolinensis.
Figure 1.
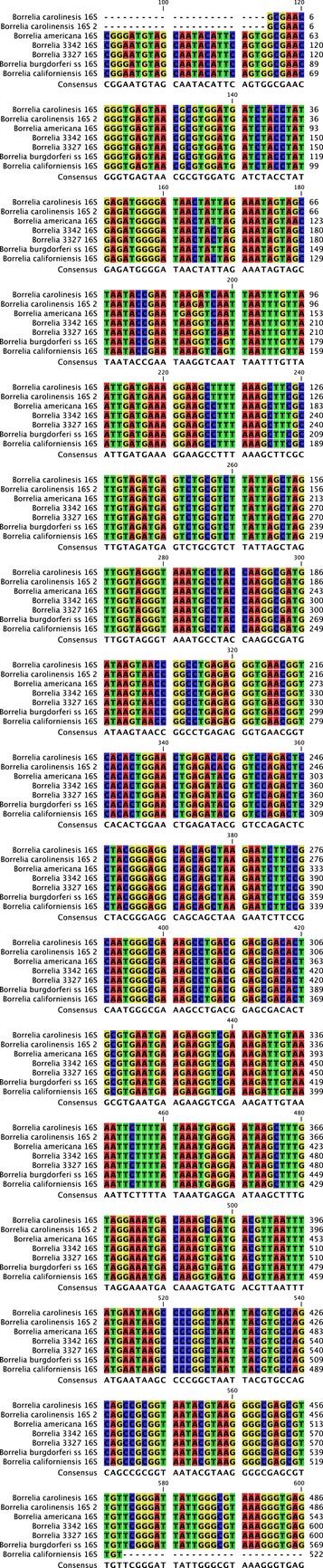
Multiple-sequence alignment for Borrelia genospecies from Amargosa vole tissue with other well-characterized Borrelia accessions from GenBank in the 16S rDNA. Amargosa vole sequences are designated 3327 and 3342, GenBank accession numbers in process. Other GenBank accessions are as follows: B. carolinensis strain SCCH-11, EU085416.1; B. carolinensis strain SCW-19, EU085409.1; B. americana strain SCW-41, EU081285.1; B. burgdorferi sensu stricto strain B31, AE000783.1; B. californiensis strain CA 443, DQ393303.1.
Discussion
South of Shoshone, California, the Amargosa River comprises stretches of southward-flowing water, subterranean flows, and occasional pools and springs until it finally makes a sharp turn northwest and terminates in Death Valley. While much of the riparian habitat along these waterways may have been occupied historically by Amargosa voles, all but the Tecopa population have been extirpated. Beyond the narrow strip of bulrush that fringes a few of the Amargosa pools near Tecopa, the eastern Mojave ecosystem is minimally vegetated, has a mean annual rainfall of only about 12 cm, and has a substrate of salt-encrusted alkaline clay flats (Williams et al. 1984). This landscape, by virtue of extreme aridity and lack of vegetated microhabitats, is essentially uninhabitable for Ixodes species ticks. Thus, an established colony of I. minor in a relict rodent population was a very unexpected finding.
Ixodes minor, first described in 1902 from a Guatemalan collection of a “Hesperomys” (possibly Calomys sp.)(Neumann 1902), was considered an invalid species in 1945 because of the lack of corroborating data (Cooley and Kohls 1945), rediscovered in Georgia as I. bishoppi (Smith and Gouck 1947), and then re-established with I. bishoppi as a junior synonym in 1961 (Clifford et al. 1961). The species is well documented from the 20th century in the American southeast from Florida to South Carolina, where it reportedly feeds in all stages primarily on small mammals, ground-feeding birds, and from a report on the eastern spotted skunk (Spilogale putorius, L, 1758) (Keirans and Clifford 1978; Tedders et al. 1992). This species rarely bites humans (Oliver et al. 2003). While I. minor and I. pacificus are both in the same subgenus (Ixodes), analysis of the 16S shows them to be in separate clades (Xu et al. 2003). Morphological characteristics such as size may allow for confirmation of species, but few resources directly compare I. pacificus with I. minor, and thus, we performed molecular analysis to confirm the species, to initialize a database from this western population, and to determine similarity with eastern specimens. We used the commonly reported 16S fragment but also chose the calreticulin gene, which at the time of the study was the only other gene reported in GenBank from both I. pacificus and I. minor. This analysis confirms the Amargosa vole tick as I. minor or a very close, not previously described relative.
We know little about the ecology of I. minor in the Amargosa ecosystem. In the American southeast, reports document adult activity in summer and October, nymphs during November and early summer, and larvae in November (Banks et al. 1998). In Tecopa however, trapping was restricted to months when ambient temperature does not endanger the voles in traps. House mice (Mus musculus) are infested in the eastern U.S. and have been introduced into some of the Tecopa marshes. It would be valuable to assess small mammals from all potential vole habitat near Tecopa for this tick and such studies are underway. The source of this tick into the Mojave Desert and time scale for its introduction are not known. As small mammals generally are not particularly vagile, a more plausible source is on migratory birds; indeed, movement on migratory birds of ticks including I. minor onto barrier islands in the southeast has been hypothesized (Wilson and Durden 2003). However, migration routes tend to be along north-south flyways, and birds would be unlikely to travel from Georgia or Florida directly to southern California. Rather, we posit that some individuals may have migrated from southeastern North America to Mexico or Central America, with occasional movement of birds not back to the eastern U.S. but rather up the west coast flyway. If our hypothesis is accurate, it suggests two important directions for further investigation. Of the two large tick studies from Mexico and Central America, neither the tick fauna from Panama nor Mexico list this species (Hoffmann 1962); however, Mexican and Central American regions where birds overwinter should be further evaluated for this tick, as it was first found in Guatemala and its migration may be an important piece of the puzzle of its ecology. Additionally, Tecopa marshes are a very small (albeit important) target for northward migrating birds and their ticks: if I. minor were introduced via this migration route, it would seem even more probable that other larger suitable habitat patches in the western U.S. might be targets as well, and perhaps, I. minor has been overlooked or mis-identified as I. pacificus elsewhere.
The B. burgdorferi sensu lato group of related strains and genospecies has experienced considerable revision, with discovery of new strains as well as phylogenetic and ecological studies that reveal significant distinctions among the strains. However, without full genome sequencing of multiple replicates, coupled with extensive ecological studies to understand host and vector extent and competence for each of the various genospecies, species boundaries seem slightly insecure at present, especially given that the GenBank database is full of older deposited material with nomenclature reflecting the designation at the time of submission rather than updated species names. Nevertheless, comprehensive comparison of fla, 16S, and 5S-23S IGS sequences from Borrelia strains in the Amargosa ecosystem to accessions that were recent and well supported in the literature revealed that Amargosa strains most closely resemble B. carolinensis. B. carolinensis was formally described in 2011 and has been cultured from the cotton mouse (Peromyscus gossypinus, (Le Conte, 1850) and eastern woodrat [Neotoma floridana, (Ord, 1818)] and from an I. minor on a woodrat in South Carolina (Rudenko et al. 2011). It has not previously been reported from outside the American southeast and has unknown epidemiological significance for humans or rodents. It is closely related to B. bissettii, which is present in California and has been associated with human Lyme disease (Rudenko et al. 2009). Oliver et al. (2003) reported enzootic maintenance of B. bissettii-like bacteria in I. minor and small mammals in the southeastern U.S. Very recent research has expanded our understanding of the pathogenic potential for B. burgdorferi and related genospecies such as B. miyamotoi and B. americanum (Clark et al. 2013; Krause et al. 2013), suggesting further consideration of disease association for B. carolinensis is warranted.
The Amargosa vole, with its dependence on very limited, patchily-distributed, and highly specialized habitat, is at considerable danger of extinction. Endemic diseases may fail to persist in such small patches, and the remoteness of the ecosystem would tend to limit the introduction of pathogens. However, we show that a tick and bacterium from the farthest possible side of the continent are relatively common in Amargosa voles. It is not known when these organisms were introduced, although further molecular study could reveal their phylogeographic history. Their presence is of serious concern because it suggests that the animals in the ecosystem could be vulnerable to further incursions of pathogens and parasites. In addition to the multiple infectious challenges to which these voles already are exposed, introductions of non-native disease must also be identified and managed in order to contribute to a realistic recovery plan for the species.
Acknowledgments
We thank Tammy Branston, Daniel Rejmanek, and Robert Klinger for assistance in the field and laboratory and Blaine Glover and Colin Foley for help with graphics. Robert Lane, Joyce Kleinjian, Yvette Girard, and Stan Wright contributed valuable data and interpretation of tick and Borrelia biology. Special thanks are due to Russell Scofield and Christopher Otahal at BLM, Susan Sorrels, and the Amargosa Conservancy for housing and logistical support as well as project leadership. This project was funded by the California Department of Fish and Wildlife and the Bureau of Land Management.
Conflict of Interest
None declared.
Funding Information
This project was funded by the California Department of Fish and Wildlife and the Bureau of Land Management.
References
- Banks CW, Phillips JH, Jr, Oliver JB, Clark KL. Life cycle of Ixodes minor (Acari: Ixodidae) in the laboratory. J. Med. Entomol. 1998;35:496–499. doi: 10.1093/jmedent/35.4.496. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black W, Piesman J. Phylogeny of hard-and soft-tick taxa (Acari:Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Nat. Acad. Sci. USA. 1994;91:10034–10038. doi: 10.1073/pnas.91.21.10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Lane RS, Barbour AG, Gresbrink RA, Anderson JR. The western black-legged tick, Ixodes pacificus: a vector of Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 1985;34:925–930. doi: 10.4269/ajtmh.1985.34.925. [DOI] [PubMed] [Google Scholar]
- Clark K, Leydet B, Hartman S. Lyme borreliosis in human patients in Florida and Georgia, USA. Int. J. Med. Sci. 2013;10:915–931. doi: 10.7150/ijms.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford C, Anastos G, Elbl A. The larval ixodid ticks of the eastern United States (Acarina: Ixodidae) 1961. Miscellaneous Publication 2, Entomological Society of America. [Google Scholar]
- Clover J, Lane R. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am. J. Trop. Med. Hyg. 1995;53:237–240. doi: 10.4269/ajtmh.1995.53.237. [DOI] [PubMed] [Google Scholar]
- Cooley R, Kohls G. 1945. The genus Ixodes in North America US National Institution Health Bulletin.
- Cudworth N, Koprowski J. Microtus californicus (Rodentia: Cricetidae) Mamm. Spec. 2010;42:230–243. [Google Scholar]
- Foley J, Branston T, Woods L, Clifford D. Severe ulceronecrotic dermatitis associated with mite infestation in the critically endangered Amargosa vole (Microtus californicus scirpensis. J. Parasitol. 2013;99:595–598. doi: 10.1645/12-4.1. [DOI] [PubMed] [Google Scholar]
- Furman DP, Loomis EC. The ticks of California (Acari: Ixodida) Berkeley, CA: Univ. of California Press; 1984. [Google Scholar]
- Girard YA, Fedorova N, Lane RS. Genetic diversity of Borrelia burgdorferi and detection of B. bissettii-like DNA in serum of north-coastal California residents. J. Clin. Microbiol. 2011;49:945–954. doi: 10.1128/JCM.01689-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A. Monografia de los Ixodoidea de Mexico. Revis. de la Soc. Mex. de His. Nat. 1962;23:191–307. [Google Scholar]
- Humair PF, Douet V, Moran Cadenas F, Schouls LM, Gern I, Van De Pol L. Molecular identification of bloodmeal source in Ixodes ricinus ticks using 12S rDNA as a genetic marker. J. Med. Entomol. 2007;44:869–880. doi: 10.1603/0022-2585(2007)44[869:miobsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Keirans J, Clifford C. The genus Ixodes in the United States: a scanning electron microscope study and key to the adults. J. Med. Entomol. 1978;20:1–149. doi: 10.1093/jmedent/15.suppl2.1. [DOI] [PubMed] [Google Scholar]
- Krause PJ, Narasimhan S, Wormser GP, Rollend L, Fikrig E, Lepore T. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS, Mun J, Eisen RJ, Eisen L. Western gray squirrel (Rodentia: Sciuridae): a primary reservoir host of Borrelia burgdorferi in Californian oak woodlands? J. Med. Entomol. 2005;42:388–396. doi: 10.1093/jmedent/42.3.388. [DOI] [PubMed] [Google Scholar]
- Neumann L. Notes sur les Ixodides. Archiv. de Parasit. 1902;6:109–128. [Google Scholar]
- Oliver JH, Jr, Lin T, Gao L, Clark KL, Banks CW, Durden LA. An enzootic transmission cycle of Lyme borreliosis spirochetes in the southeastern United States. Proc. Nat. Acad. Sci. USA. 2003;100:11642–11645. doi: 10.1073/pnas.1434553100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Ruzek D, Piskunova N, Mallatova N, Grubhoffer L. Molecular detection of Borrelia bissettii DNA in serum samples from patients in the Czech Republic with suspected borreliosis. FEMS Microbiol. Lett. 2009;292:274–281. doi: 10.1111/j.1574-6968.2009.01498.x. [DOI] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH., Jr Borrelia carolinensis sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex isolated from rodents and a tick from the south-eastern USA. Int. J. Syst. Evol. Microbiol. 2011;61:381–383. doi: 10.1099/ijs.0.021436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C, Gouck H. Ixodes bishoppi, a new species from Georgia (Acarina: Ixodidae) Ann. Entomol. Soc. Am. 1947;40:75–81. [Google Scholar]
- Tedders SH, Keirans JE, Williams DC, Gwinn TA. First report of Ixodes (Ixodes) minor Neumann (Acari: Ixodidae) from South Carolina. J. Med. Entomol. 1992;29:282–283. doi: 10.1093/jmedent/29.2.282. [DOI] [PubMed] [Google Scholar]
- Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab. Anim. Sci. 1999;49:319–323. [PubMed] [Google Scholar]
- U.S. Fish and Wildlife Service. 1997. p. 43. Amargosa voles (Microtus californicus scirpensis) recovery plan pp, Portland, Or.
- Webb J, Bennett SN, Challet G. The larval ticks of the genus Ixodes Latreille (Acari: Ixodidae) of California. Bull. Soc. Vector Ecol. 1990;5:73–124. [Google Scholar]
- Williams J, Kobetich G, Benz C. Management aspects of relict populations inhabiting the Amargosa Canyon ecosystem. In: Warner R, Hendrix K, editors. California Ripraian systems. Berkeley: Univ. of California Press; 1984. pp. 706–715. [Google Scholar]
- Wilson N, Durden LA. Ectoparasites of terrestrial vertebrates inhabiting the Georgia Barrier Islands, USA: an inventory and preliminary biogeographical analysis. J. Biogeogr. 2003;30:1207–1220. [Google Scholar]
- Xu G, Fang Q, Keirans JE, Durden L. Molecular phylogenetic analyses indicate that the Ixodes ricinus complex is a paraphyletic group. J. Parasitol. 2003;89:452–457. doi: 10.1645/0022-3395(2003)089[0452:MPAITT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]


