Abstract
Background
Cortical and subcortical hyperintensities in magnetic resonance imaging (MRI) scans are thought to represent areas of ischemic damage to brain tissue. Researchers have focused on the possible role these lesions may have in psychiatric disorders, including bipolar disorder. In 1997, the proposed ‘vascular mania’ diagnosis suggested utilizing not only the presence of strokes, but also confluent hyperintensities in its diagnostic criteria. This study was conducted to use meta-analytic techniques to investigate the association of hyperintensities and bipolar illness and to evaluate the current state of the literature.
Methods
Using the PubMed and MEDLINE databases, we conducted a systematic literature search of studies investigating hyperintensities in subjects with bipolar disorder and controls or other psychiatric illnesses. We identified 44 publications from which 35 studies were included for review and 27 were selected for meta-analysis. Summary statistics of the prevalence were estimated through odds-ratios and confidence interval. Heterogeneity of the results across studies was tested using Q-statistics.
Results
Meta-analysis identified an odds ratio of 2.5 (95% CI 1.9, 3.3) for hyperintensities in bipolar subjects compared to controls; however, there was significant heterogeneity among the studies (Q-statistics =32; p =0.04). This finding was most prominent for adolescents and children where the odds ratio was 5.7 (95% CI 2.3, 13.7). Deep white matter hyperintensities (odd ratio 3.2; 95% CI 2.2, 4.5) and subcortical grey matter hyperintensities (odds ratio 2.7; 95% CI 1.3, 2.9) were more strongly associated with bipolar subjects. There were no differences between bipolar subjects and controls for perivascular hyperintensities (odds ratio 1.3; 95% CI 0.8, 1.9). Though hyperintensities were numerically greater in bipolar subjects, meta-analysis did not demonstrate any significant differences between bipolar subjects and unipolar depression subjects (OR 1.6; 95% CI 0.9, 2.7) nor subjects with schizophrenia (OR 1.5; 95% CI 0.9, 2.7).
Conclusions
This meta-analysis continues to support the association of bipolar disorder and hyperintensities, especially in the deep white matter and subcortical grey matter. It also highlights the increased incidence in children and adolescence with bipolar disorder. However, hyperintensities are not specific to bipolar disorder, but appear at similar rates in unipolar depression and schizophrenia. Thus, the role of hyperintensities in the pathogenesis, pathophysiology, and treatment of bipolar disorder remains unclear. Further studies are required that are large enough to decrease the heterogeneity of the samples and MRI techniques, assess size and location of hyperintensities, and the impact on treatment response. Coordination with newer imaging techniques, such as diffusion tensor imaging (DTI) may be especially helpful in understanding the pathology of these lesions.
Introduction
Subcortical hyperintensities are bright areas viewed on T2-weighted brain magnetic resonance images (MRI) located in the deep white (DWM), periventricular white (PVWM), or subcortical grey (SCGM) matter. They could represent infracts areas of poor perfusion or lacunes. Small changes could be enlarged Virchow Robin spaces, which are enlarged perivascular spaces that surround blood vessels for a short distance as they enter the brain. Neuropathological examination has found that hyperintensities primarily reflect ischaemic damage and correspond to focal demyelination, loss of axons/nerve fibres, and sometimes lacunar infarctions or necrosis (Awad et al., 1986; Braffman et al., 1988; Chimowitz et al., 1992; Fazekas et al., 1993; George et al., 1986; Schmidt et al., 1999; van Swieten et al., 1991). Fujikawa et al. (1995) coined the term ‘silent cerebral ischaemia’ to refer to these hyperintensities.
Hyperintensities are a frequent neuroimaging finding in the general population, especially in the elderly (Sachdev et al., 1997). The prevalence of hyperintensities is strongly associated with age. Nearly all individuals over the age of 60 may exhibit hyperintense lesions on brain MRI (Sachdev et al., 2005; Wen & Sachdev, 2004). Although frequently reported as ‘non-specific’ or ‘incidental’ brain findings (Vernooij et al., 2007; Zanetti et al., 2007), numerous correlative studies have described subtle neuropsychological deficits in elderly individuals with these lesions (Boone et al., 1992; Breteler et al., 1994; Junqué et al., 1990; Mirsen et al., 1991; Schmidt et al., 1993; Ylikoski et al., 1993) suggesting that they may directly effect cortical functioning.
It is not surprising then that subcortical hyper-intensities have also been associated with various psychiatric disorders. Studies have especially focused on the potential association of subcortical hyperintensities and late life depression. It is theorized that subcortical hyperintensities may occur in a subgroup of patients with an underlying condition (e.g. cerebrovascular disease) that predisposes them to develop depression by disrupting the frontostriatal circuitry implicated in mood regulation (Alexopoulos et al., 1997; Brown et al., 1992; Krishnan et al., 1997). Krishnan et al. (1997) and Alexopoulos et al. (1997) have proposed criteria for a vascular-related subtype of depression based on research data and clinical experience with elderly depression. Further, the presence and increased severity of the hyperintense lesions have been related to a poor prognosis, treatment non-response, increased chronicity of symptoms, and long-term disability in depression (Heiden et al., 2005; Hickie et al., 1995, 1997; O’Brien et al., 1998; Simpson et al., 1997; Taylor et al., 2003).
Similarly, hyperintensities have also been hypothesized to be related to bipolar disorder. Steffens and Krishnan (1998) have proposed criteria for the diagnosis of a vascular mania (see Table I). Within the suggested diagnostic criteria, they have proposed that not only ischaemic strokes, but also larger, confluent hyperintensities may contribute to the development of mania. Many early studies have demonstrated an association between subcortical hyperintensities and bipolar illness (Aylward et al., 1994; Dupont et al., 1995b; Figiel et al., 1991; Swayze et al., 1990). Not all investigators have found a significantly greater prevalence of these lesions in bipolar patients (Brown et al., 1992; Lewine et al., 1995), but many of the negative studies have found trends toward a relationship (Altshuler et al., 1995; Botteron et al., 1995; Persaud et al., 1997; Strakowski et al., 1993), and most of these studies used a relatively small sample with a large range in ages. When meta-analytic tests were applied to these early studies, a strong odds ratio for hyperintensities in bipolar subjects was noted (Altshuler et al., 1995; Videbech, 1997).
Table I.
Studies of T2 hyperintensities in bipolar disorder.
| Study | Bipolar
|
Controls
|
Location | Presence of hyperintensities
|
Other psychiatric disorder comparators
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean age ± SD | N | Mean age ± SD | BP (%) | CON (%) | Odds ratio | N | Mean age ± SD | Disorder (%) | Odds ratio | ||
| Ahearn, 1998 | 9 | 41 | 10 | 37 | Total SC* | 9 (100%) | 6 (60%) | 12.18 | ||||
| DWM | 4 (44%) | 1 (10%) | 7.20 | |||||||||
| SCGM | 8 (89%) | 6 (60%) | 5.33 | |||||||||
| Ahn, 2004 | 43 | 36.9 ± 11.5 | 39 | 35.1 ± 9.7 | Total WM* | 12 (28%) | 4 (8%) | 3.39 | ||||
| DWM | 12 (28%) | 3 (8%) | 4.65 | |||||||||
| PVH | 4 (9%) | 1 (2.5%) | 3.90 | |||||||||
| Altshuler, 1995 | 29 | 41.6 ± 11.6 | 20 | 35.2 ± 9.9 | Total SC* | 19 (66%) | 8 (40%) | 2.85 | ||||
| DWM | 3 (10%) | 2 (10%) | 1.04 | |||||||||
| SCGM | 2 (7%) | 1 (5%) | 1.41 | |||||||||
| PVWM | 18 (62%) | 6 (30%) | 3.82 | |||||||||
| Aylward, 1994 | 32 | 39.3 ± 11.1 | 31 | 37.6 ± 9.0 | Total * | 11 (34%) | 1 (3%) | 15.71 | ||||
| DWM | 11 (34%) | 1 (3%) | 15.71 | |||||||||
| SCGM | 1 (3%) | 0 (0%) | 7.16 | |||||||||
| Botteron, 1995 | 8 | 11.3 ± 3.1 | 5 | 11.8 ± 2.9 | Total WM* | 2 (25%) | 0 (0%) | 1.33 | ||||
| Brown, 1992 | 22 | 37.7 ± 7.6 | 154 | 34 ± 9.5 | Total WM* | 1 (0.5%) | 14 (9.1%) | 0.48 | ||||
| Chang, 2005 | 20 | 14.6 (2.8) | 20 | 14.1 (2.8) | DWM* | 12 (63%) | 10 (50%) | 1.5 | ||||
| SCGM | 17 (85%) | 13 (65%) | 3.05 | |||||||||
| PVH | 4 (20%) | 5 (25%) | 0.75 | |||||||||
| Dupont, 1995 | 44 | 36.6 ± 10.7 | 32 | 39.2 ± 8.9 | DWM* | 20 (46%) | 7 (22%) | 2.22 | 33 UP | 38.9 ± 10.2 | 9 (27%) | 1.34 |
| Figiel, 1991 | 18 | 37.5 | 18 | 34.7 | DWM* | 8 (44%) | 1 (6%) | 13.61 | ||||
| SCGM | 0 (18%) | 0 (18%) | 0 | |||||||||
| Gulseren, 2006 | 12 | 30.9 ± 3.6 | 12 | 30.4 ± 3.6 | Total WM | 8/12 (67%) | 4/12 (33%) | |||||
| DWM | 2/12 | 1/12 | 2.2 | |||||||||
| (other)SCH | 6/12 | 3/12 | 3 | |||||||||
| PVH | 0/12 | 0/12 | NaN | |||||||||
| Krabbendam, 2000a | 22 | 47.7 ± 8.3 | 22 | 41.4 ± 11.3 | DWM* | 14 (64%) | 11 (50%) | 1.75 | 22 SZ | 39.6 ± 6.6 | 11 (50%) | 1.00 |
| PVH | 3 (14%) | 0 (0%) | 8.15 | 4 (18%) | 8.59 | |||||||
| Lewine, 1995 | 20 | 34.6 ± 6.6 | 150 | 33.7 ± 9.1 | DWM | 1 (5%) | 7 (5%) | 1.08 | 108 SZ | 32.8 ± 7.7 | 7 (6%) | 1.42 |
| 20 SZA | 39.5 ± 9.6 | 1 (5%) | 2.55 | |||||||||
| 27 UP | 40.9 ± 11.1 | 3 (11%) | ||||||||||
| Lyoo, 2002 | 56 | 13.6 ± 2.1 | 83 | 9.9 ± 3.3 | TotalWM* | 10 (18%) | 1 (1%) | 17.97 | 42 SZ | 12.8 ± 2.3 | 1 (2%) | 2.0 |
| DWM* | 10 (18%) | 1 (1%) | 17.97 | 94 UP | 12.9 ± 2.1 | 13 (15%) | 13.1 | |||||
| 103 CD/ADD | 12.9 ± 2.2 | 14 (14%) | ||||||||||
| McDonald, 1999b | 70 | 49.9 ± 19.7 | 70 | 53.2 ± 18.1 | DWM* | 33 (47%) | 18 (26%) | 2.58 | ||||
| SCGM | 5 | 0 | 7.84 | |||||||||
| PV | 22 | 20 | 1.15 | |||||||||
| Moore, 2004c | 29 | 44.7 | 15 | 41.9 ± 12.6 | DWM* | 8 (26%) | 0 (0%) | 5.34 | ||||
| PVH | 18 (62%) | 7 (47%) | 1.87 | |||||||||
| Persaud, 1997 | 21 | 35.6 | 34 | 31.6 | TotalWM* | 13 (62%) | 29 (85%) | 0.28 | 48 SZ | 31.1 | 41 (85%) | 1.01 |
| Pillai, 2002 | 15 | 15 ± 2.4 | 16 | 16 ± 1.8 | DWM* | 10 (66%) | 5 (31%) | 4.4 | 19 SZ | 15 ± 2.2 | 7 (37%) | 1.28 |
| Sassi, 2003 | 24 | 34.2 ± 9.9 | 38 | 36.8 ± 9.7 | Total SC* | 12 (50%) | 22 (58%) | 0.85 | 17 UP | 42.8 ± 9.2 | 7 (41%) | 0.51 |
| DWM | 2 (8%) | 4 (11%) | 0.71 | |||||||||
| SCGM | 1 (4%) | 3 (8%) | 0.51 | |||||||||
| PVWM | 10 (42%) | 21 (55%) | 0.58 | |||||||||
| Silverstone, 2003d | 13 | 40 | 19 | 36 | DWM* | 7 (54%) | 5 (26%) | 3.27 | 11 UP | 34.4 | 3 (27%) | 1.05 |
| PVH | 3 (23%) | 8 (42%) | 0.41 | |||||||||
| Swayze, 1990 | 48 | 33.9 | 47 | 34.4 | TotalWM* | 9 (19%) | 2 (4%) | 5.19 | ||||
| Strakowski, 1993 | 18 | 31 ± 11.8 | 15 | 32.4 ± 8.8 | TotalWM* | 4 (22%) | 2 (13%) | 1.86 | 54 SZ | 33.3 | 5 (9%) | 2.30 |
| Full sample | 573 | 35 | 850 | 33.2 | 223/350 | 153/697 | 2.90 (2.28–3.70) | |||||
Calculated from reported occurrence of ‘small’ DWM.
Calculated from the highest reported incidence of lesions.
Calculated from reported occurrence of DWM.
Calculated from highest reported incidence of lesions in either DWM or PVH.
Calculated from reported occurrence of DWM.
SC =subcortical hyperintensities; DWM =deep white matter hyperintensities; SCGM =subcortical gray matter hyperintensities; WM =white matter hyperintensities; PVWM =periventricular white matter hyperintensities; SCH =subcortical hyperintensities; PVH =periventricular hyperintensities; UP =unipolar depression; SZ =schizophrenia; SZA =schizoaffective disorder; CD =conduct disorder; ADD =attention deficit disorder
In the decade since the first proposal of the vascular mania diagnostic sub-type and the early meta-analyses, there have been more concerted studies on hyperintense lesions and bipolar disorder. Therefore, it seemed timely that an updated review and meta-analysis of studies of subcortical hyper-intensities in bipolar disorder be conducted. We hypothesized that the rate of subcortical hyperintensities would be greater in individuals with bipolar disorder (regardless of age) compared with healthy controls. Our secondary hypotheses were: (1) subcortical hyperintensities would be most consistently found in the deep white matter; (2) the rate of subcortical hyperintensities would increase with age but that children and adolescents with bipolar disorder would also demonstrate increased subcortical hyperintensities compared with controls; and (3) the rate of subcortical hyperintensities in bipolar subjects would be greater than that seen in unipolar depressed comparison subjects but less than the rate seen in comparison subjects with schizophrenia. Finally, we wished to summarize the current literature of other related research questions that are not yet available for meta-analysis, such as the association of hyperintensities in bipolar disorder subjects with specific brain regions, age of onset, treatment response, family history and neuropsychological deficits.
Method
Search strategy
We searched the Ovid MEDLINE database (1902 to 2008) and PubMed (1966–2008) using combinations of the Medical Subject Headings (MESH) ‘bipolar and magnetic resonance imaging (MRI)’ and keywords ‘hyperintensities’, ‘white matter’, ‘grey matter’, ‘subcortical’, and ‘periventricular’. In addition, the reference lists of all the studies were inspected for more published reports and citations of unpublished research.
Studies included
Studies were included if they reported on hyper-intensities in patients with bipolar disorder and met the following criteria: (1) the sample had a predominant diagnosis of bipolar disorder, (2) the sample was comprised of more than three subjects with bipolar disorder, (3) the sample included a comparison group, (4) MRI reviewers were blinded to diagnosis, and (5) an adequate description of hyperintensity measurement was provided. The studies could either be prospective or retrospective in design, as long as the MRI scanning was consistent or adequately controlled. In addition, other information potentially related to hyperintensities were recorded from the studies, including location of hyperintensities, demographic information of subjects, clinical features or data regarding treatment outcomes or course of illness data.
Data analysis
Means, standard deviations are used to describe the summary statistics. In the meta-analysis, summary statistics of the prevalence is estimated through odds-ratios and its confidence interval. Heterogeneity of the results across studies is tested using Q-statistics. Because not all studies identified various hyperintense lesions (i.e. DWM, SCGM, PVH), we subdivided the analysis to assess for each of these individually. Forest plots are used to graphically represent the summary of the studies used in the meta-analysis. All the analysis is conducted in the freeware software MIX (Bax et al., 2006, 2008).
Results
Description of studies
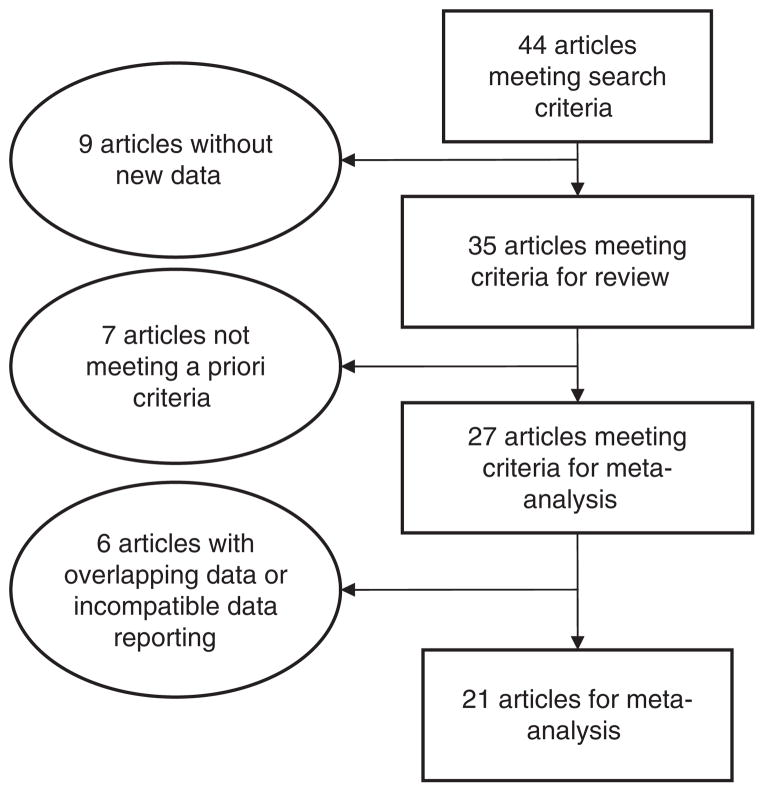
Forty-four studies were initially identified that matched our search criteria (see Figure 1). Thirty-five of these studies evaluated the presence of hyperintensities in bipolar patients. Applying the five a priori criteria to these studies, eight studies were excluded: four (Breeze et al., 2003; Deicken et al., 1991; Hickie et al., 1995; Pompili et al., 2007) were excluded because the hyperintensity data reported was not differentiated by the specific psychiatric diagnosis; three (Botteron et al., 1992; Böhm et al., 2006; Zanetti et al., 2007) were individual case reports; and one (Kato et al., 2000) did not include a control group. Finally, of the 27 studies meeting criteria for meta-analysis, six of these were excluded (see Table II). Five studies (Ahearn et al., 2002; Dupont et al., 1987, 1990, 1995a; McDonald et al., 1991) were reports of hyperintensities in bipolar subjects in which samples overlapped with later reports. In these cases, only the latest (and larger) study was included to avoid duplication of subjects. One study (de Asis et al., 2006) was excluded from the meta-analysis because it did not report categorical presence or absence of hyperintensities.
Figure 1.
Diagram of article selection.
Table II.
Comparison of commonly used hyperintensity measurement scales.
| Fazekas | Coffey | Boyko | |
|---|---|---|---|
| Periventricular hyperintensity | |||
| 0 | Absent | Absent | Absent |
| 1 | Caps/pencil-thin lining | Caps | Hypointense to grey matter and hyperintense to CSF on proton density images |
| 2 | Smooth halo | Smooth halo | Isointense to grey matter and hyperintense to CSF on proton density images |
| 3 | Irregular and extending into deep white matter | Irregular and extending into deep white matter | Hyperintense to CSF on T2-weighted images |
| 4 | NA | NA | Hyperintense to CSF on T2-weighted images and isointense to CSF on T1-weighted images |
| Deep white matter hyperintensity | |||
| 0 | Absent | Absent | Absent |
| 1 | Punctate foci | Punctuate foci | Punctate |
| 2 | Beginning of confluence of foci | Beginning confluence of foci | Rounded ( <5 mm) |
| 3 | Large confluent areas | Large confluent areas | Irregular ( >5 mm) |
| 4 | NA | NA | Confluent lesions |
| Subcortical grey matter hyperintensity | |||
| 0 | NA | Absent | Absent |
| 1 | NA | Punctuate | Punctuate |
| 2 | NA | Multi-punctate | Rounded (<5 mm) |
| 3 | NA | Diffuse | Irregular (>5 mm) |
| 4 | NA | NA | Confluent lesions |
| Frontal caps | |||
| 0 | NA | NA | Absent |
| 1 | NA | NA | Present |
| Subependymal hyperintensity | |||
| 0 | NA | NA | Absent |
| 1 | NA | NA | Thin |
| 2 | NA | NA | Thick (>2 mm) |
The demographic characteristics of the samples varied amongst the studies. While 17 studies focused on adults of any age, four studies focused on youth and adolescents (see Table I). Control subjects were primarily non-psychiatrically ill adults from the community, though one study also included a sibling comparison group (Gulseren et al., 2006), and one used a larger family as the comparison group (Ahearn et al., 1998).
While all studies included in this analysis evaluated bipolar subjects, most did not indicate diagnostic criteria, phase of illness, or bipolar subtype. One study in the meta-analysis (Altshuler et al., 1995) did indicate inclusion of some bipolar II subjects though the results were not reported separately. Eight studies in the meta-analysis also included subject cohorts with other psychiatric diagnoses, primarily schizophrenia and unipolar depression (see Table I).
Quality of studies
Sample size
The sample size of most studies was relatively small with a mean of 28.1 (SD =15.9) bipolar subjects. Thirteen of the studies included fewer than 25 bipolar subjects; the largest had 70. Studies focusing on genetics or treatment response typically had even fewer participants. The smaller sample size limited the power calculations in subgroup analyses, and may have contributed to negative findings (such as comparing older adults with younger adults or subdividing samples based on age of onset).
MRI technology and technique
The published literature spans 18 years, from 1990–2007. During this time there have been several refinements in both MRI machines and imaging technique. For example, magnet strength has been one such variable that has changed over this time span. The most common magnet strength used was 1 Tesla, although six studies used a 0.5-Tesla magnet and one used a 3-Tesla magnet. All of these magnet strengths are adequate for viewing hyperintensities and estimating size.
Adequate quantitation of hyperintensities depends on the image slice thickness and number of images obtained. Fewer image slices, thicker image slices, or larger gaps between slices may ‘hide’ hyperintensities and alter findings. The trend in later studies was toward both smaller image slices and smaller gaps. Studies reported slice thickness varying from 1 mm–10 mm, the most common being 5 mm (17 of 22 studies). Not all studies identified whether slices were contiguous or if a gap was present. Of the 16 studies that did include this information in their method section, 14 noted gap widths ranging from 0.5 mm to 2 mm, while two reported contiguous slices. Since most studies identified hyperintensities as small as 1 mm, it is possible that hyperintensities could be overlooked depending on the MRI technique employed. This risk, however, should be equivalent between subject and control groups.
Finally, not all studies evaluated all described hyperintensities. For example, 16 studies described defined DWM hyperintensities, eight studies defined PV hyperintensities, six studies described a value of ‘total white matter hyperintensities’ and only nine studies identified SCGM hyperintensities. Therefore, some studies likely under-represent the total presence of hyperintensities.
MRI interpretation
Various scales have been used to describe hyperintensities, the most common being the scales of Coffey (Krishnan et al., 1993), Boyko et al. (1994), and Fazekas et al. (1986). Each scale assigns a numerical value to hyperintensities based on location (white matter, grey matter, periventricular regions, and frontal poles) and size (see Table III). However, eight studies created their own descriptive scale, generally correlating with the three published primary scales.
Limitations
There were several limitations in comparing and contrasting the MRI studies. First, the use of different scales limited exact comparison. Second, various studies had different thresholds for reporting hyperintensities. For example, Strakoswki et al. (1993) and Altshuler et al. (1995) noted subcortical hyperintensities if they were 2 mm or larger. Persaud et al. (1997), Ahearn et al. (1998), and McDonald et al. (1999) identified hyperintensities larger than 1 mm. The latter criterion is consistent with the common use of ‘grade 1’ on the rating scales. However, while most studies used the Fazekas or Coffey scale cut-off of grade 1, Silverstone et al. (2003) and Botteron et al. (1995) grouped their categories into ‘mild’ (Coffey score of 0–1) or ‘more extensive’ (Coffey score 2–3). This meant that hyperintensities considered significant were those that had at least ‘early confluence’, a much higher standard than in other studies. Third, studies also reported their findings differently. For example, de Asis et al. (2006) reported average severity of hyperintensities. Therefore, although they found higher hyperintensity scores in bipolar subjects, comparison with other studies could not be made because the number of subjects with hyperintensities was not noted. Another limitation in comparing studies is that authors used different terms for similar regions of interest. For example, the use of the general term ‘subcortical hyperintensities’ could refer to either ‘deep white matter hyperintensities’ or ‘deep white matter and periventricular white matter hyperintensities’. Furthermore, while most studies reported a ‘total’ number of subjects with hyperintensities, some studies focused only on certain regions, such as subcortical hyperintensity results (Strakowski et al., 1993), deep white matter results (Pillai et al., 2002; Krabbendam et al., 2000; Chang et al., 2005), deep white matter and subcortical hyperintensities (Figiel et al., 1991), or deep white matter and periventricular hyperintensities (Silverstone et al., 2003; Moore et al., 2001). Therefore, calculations were based on the highest number of subjects reported, which may have underestimated the number of subjects with hyperintensities. Finally, most authors did not include information about comorbid diagnoses. This included information on substance abuse, a common comorbid condition in these patients and a potential confound in hyperintensity research.
Results
Demographics
Among the 21 studies used in the meta-analysis, there were a total of 573 bipolar subjects and 850 non-psychiatrically ill comparison subjects. The bipolar subjects had an average age of 35; the comparison group had an average of 33.2 years. Four of the studies (Botteron et al., 1995; Chang et al., 2005; Lyoo et al., 2002; Pillai et al., 2002) focused on children and adolescents.
Prevalence of hyperintensities
Table I presents the rate of hyperintense lesions found in patient and comparison groups. Overall, there is a wide range in percentage of subjects with hyperintensities in the studies: 18–100% in the bipolar subjects and 0–85% in the normal control groups. On average, 38.9% of bipolar subjects and 18% of control subjects had hyperintensities. Eleven of the 21 studies (Ahearn et al., 1998; Ahn et al., 2004; Aylward et al., 1994; Dupont et al., 1995b; Figiel et al., 1991; Gulseren et al., 2006; Lyoo et al., 2002; McDonald et al., 1999; Moore et al., 2001; Pillai et al., 2002; Swayze et al., 1990) reported statistically significant differences in hyperintensities between bipolar subjects and controls with hyperintensities. Of the remaining ten studies, all but three (Brown et al., 1992; Lewine et al., 1995; Sassi et al., 2003) reported a trend toward increased hyperintensities in bipolar subjects compared with controls. In the meta-analysis, the odds ratio was 2.5 (95% CI 1.9, 3.3) (see Figure 2). Mantel-Haenszel test suggested possible heterogeneity among the studies (Q-statistics =32; p =0.04), most likely due to the large variability in hyperintensity findings.
Figure 2.
Plot of hyperintensities in bipolar disorder odds ratios by study.
Prevalence of hyperintensities in brain regions
Of the 21 studies in the meta-analysis, 16 reported the percentage of subjects with hyperintensities specifically in the deep white matter regions. Meta-analysis found an odds ratio for DWM hyperintensities in bipolar subjects was 3.2 (95% CI 2.2, 4.5) compared with controls. In this analysis there was no heterogeneity (Q-statistics =15.7; p =0.4). Seven studies also specifically reported the percentage of subjects with hyperintensities in the subcortical grey matter regions. Meta-analysis found an odds ratio for SCGM hyperintensities of 2.7 (95% CI 1.3, 2.9) in bipolar subjects compared with controls. There was no heterogeneity in these studies (Q-statistics 3.6; p-value =0.7). Eight studies reported the percentage of subjects with hyperintensities in the periventricular regions (PVH). Meta-analysis found an odds ratio for PVH hyperintensities of 1.3 (95% CI 0.8, 1.9) in bipolar subjects and no heterogeneity.
Prevalence of hyperintensities and age
Seventeen of the 21 studies focused on adult subjects with bipolar disorder. Meta-analysis found that odds ratio of any hyperintensity was 2.2 (95% CI 1.6, 3.0) in bipolar subjects compared with controls. However, these studies also had possible heterogeneity (Q-statistics =27; p-value =0.04). In the four studies that examined children and adolescents with bipolar disorder, the meta-analysis found an odds ratio of 5.7 (95% CI 2.3, 13.7) for hyperintensities in adolescents with bipolar disorder compared with controls. There was no heterogeneity (Q-statistics =1.9; p-value =0.6).
Prevalence of hyperintensities in bipolar disorder compared with other psychiatric disorders
Five of the studies included a comparison group of subjects with unipolar depression. Meta-analysis found an odds ratio for any hyperintensity was 1.6 (95% CI 0.9, 2.7) for subjects with bipolar disorder compared with unipolar disorder. Six of the studies included a comparison group of subjects with schizophrenia. Meta-analysis found an odds ratio for any hyperintensity of 1.5 (95% CI 0.9, 2.7) for subjects with bipolar disorder compared with schizophrenia. Neither test found heterogeneity.
Discussion and review
Prevalence of hyperintensities in bipolar disorder compared with controls
Our findings supported our primary hypothesis that bipolar subjects had an increased rate of hyperintensities compared to controls. The common odds ratio of 2.5 is most likely an underestimation of the prevalence of hyperintensities, since many of the studies did not include an evaluation of the full brain, but focused only on specific regions, i.e. deep white matter. This odds ratio, though, is consistent with two previous meta-analyses conducted in the mid 1990s. Altshuler and colleagues (1995) and Videbech (1997) evaluated a smaller group of studies and calculated the common odds ratio of 3.3 in both studies.
The positive meta-analysis in the presence of multiple studies demonstrating a positive trend (18 of the 21 studies) would suggest that a major limitation of the individual studies has been the small sample size. However, this does not fully explain the marked variability of hyperintensities found in the studies. For example, the large range of percentages of hyperintensities in both subjects and controls is the probable reason for the possible heterogeneity found on the Mantel-Haenszel chi-square test (Q). This would suggest that either individual MRI procedures (such as assessment scales, acquisition techniques, locations of interest, etc.) or samples are significantly different among the studies. Neither Altshuler et al. (1995) nor Videbech (1997) found this heterogeneity in their meta-analyses.
If we were to test the possibility that different MRI assessment techniques were the cause, then we would expect that studies which used the most similar acquisition procedures, scales, and areas of interest (e.g. DWM), would show greater consistency. When we limited the data to only those studies that used standardized measures, MRI acquisition, and focused on DWM (Ahn et al., 2004; Altshuler et al., 1995; Chang et al., 2005; Figiel et al., 1991; Lyoo et al., 2002; McDonald et al., 1999; Moore et al., 2001; Sassi et al., 2003; Silverstone et al., 2003), the percentage of hyperintensities continued to show a fairly wide range though not as large (subjects 8%–67%, controls 9%–50%). This finding suggests that sample selection or subject characteristics may provide significant variability to the findings. One critique of negative study by Brown et al. (1992) was that selection bias may have occurred since they evaluated over 200 subjects with varying psychiatric disorders who were recruited by general newspaper advertising. Other factors that may contribute to differences in study findings include medication use, medical illnesses such as hypertension or hyperlipidaemia, smoking, age, duration of illness, age, or age at onset. The studies differed in whether they assessed for these factors.
Location of hyperintensities
Some studies have suggested that hyperintensity location may be important in the expression of bipolar symptoms. One of our secondary hypotheses was that, similar to findings in late life unipolar depression, lesions will more consistently found in the DWM. Our meta-analysis found that both DWM and SCGM areas had increased rates of hyperintensities in bipolar subjects compared with controls, though this finding was strongest for DWM (the DWM odds ratio was 3.2; SCGM was 2.3). We did not find that PVWM lesions were more common in bipolar disorder than in controls. As was true regarding prevalence, studies differed in their findings.
While Ahn et al. (2004), Aylward et al. (1994), Dupont et al. (1995), Figiel et al. (1991), Lyoo et al. (2002), Moore et al. (2001), Pillai et al. (2002), and Silverstone et al. (2003) all reported that hyperintensities were more likely to occur in the DWM in bipolar subjects compared with controls, Ahearn et al. (1998), Chang et al. (2005), and McDonald et al. (1999) noted higher rates of both DWM and SCGM hyperintensities. McDonald et al. (1999) suggested that the increased rate of SCGM hyperintensities in their bipolar group could be related to their use of smaller MRI slices or to the advanced group age (mean age 68). However, de Asis et al. (2006) evaluated MRI findings in older adults and did not find any increase in SCGM hyperintensities although they used contiguous slices, and Chang et al. (2005) reported fairly high numbers of SCGM hyperintensities in their adolescent sample.
In contrast, while Altshuler et al. (1995) found relatively few DWM and SCGM hyperintensities in their sample, they found a larger number of PVWM hyperintensities in bipolar subjects compared with controls. However, the PVWM hyperintensities were significant only in those above the age of 30. McDonald et al. (1999) also reported elevated PVWM hyperintensities in both younger (mean age 36 years) and older (mean age 68 years) samples; however, only the younger sample had significantly more PVWM hyperintensities than controls.
Despite their similar appearances and their frequent combined analysis, some authors (Spilt et al., 2006) have suggested that hyperintensities in differing areas of the brain have different causes and functional consequences. For example, research using magnetic transfer imaging have found that the magnetic transfer ratio (MTR) better reflects histological differences than standard MRIs. PVWM hyperintensities have been found to have a lower MTR than DWM hyperintensities (Spilt et al., 2006; Tanabe et al., 1999) suggesting that the lesions may involve different mechanisms that lead to ischaemia and different severity of the resulting ischaemia. The PVWM is an arterial border zone that is supplied by long perforating arteries and may be particularly vulnerable to decreases in cerebral blood flow (Pantoni & Garcia, 1997). Therefore, perfusion in this area may be jeopardized by more mechanisms (hypotension, diffuse small vessel disease, atherosclerosis, or emboli) in the PVWM than in the DWM (atherosclerosis or emboli), resulting in a higher accumulation of ischaemic damage in the periventricular location. Further, in studies in which WMH are sub-classified, different functional correlates have been found for PVWMH and DWMH. PVWMH are correlated with cognitive decline (Wahlund et al., 1994), and DWMH are correlated with late-onset depression (de Groot et al., 2000). In this case, our findings suggest a process more similar to that seen in late life depression.
In several studies, investigators commented on the location of hyperintensities within the DWM. Gulseren et al. (2006) found that in subjects with bipolar disorder, hyperintensities were found only in the frontal/parietal areas; and that for both bipolar subjects and their siblings compared with controls, the lesions were found exclusively in the right cerebral hemisphere. Aylward et al. (1994) also noted that the most common location for hyperintensities in bipolar subjects was in the DWM of the frontal lobes and the frontal/parietal junction, though evenly divided between the left and right. Dupont et al. (1995b), Pillai et al. (2002), Lyoo et al. (2002), and Figiel et al. (1991) also noted that the predominant location of the hyperintensities was in the frontal lobes, but they did not comment on laterality. In a further analysis, Dupont et al. (1995b) reported that in comparison with bipolar depressed patients, unipolar depressed patients had a slightly more posterior distribution. Lyoo et al. (2002), studying children and adolescents, noted that the frontal lobes were also the predominant location of hyperintensities for subjects with unipolar depression and conduct disorder/ADD. Therefore, while evidence supports potential disruption in bipolar disorder of frontal lobe circuits that modulate moods, this pathology is not necessarily specific to bipolar disorder.
Prevalence of hyperintensities compared with other psychiatric diagnoses
Our final secondary hypothesis concerned the prevalence of hyperintensities in bipolar subjects compared to subjects with other psychiatric diagnoses. Within the studies in our meta-analysis, five studies included a unipolar depression comparison group. Lewine et al. (1995), Silverstone et al. (2003) and Sassi et al. (2003) found no difference of hyperintensities among bipolar and unipolar depressed subjects compared with controls. Lyoo et al. (2002) evaluated a group of adolescents and found that both bipolar disorder and unipolar depressed subjects had significantly higher percentage of hyperintensities (17.9% and 13.8% respectively) than controls (1.2%), but there was no difference between these two groups. Dupont et al. (1995b) found that though bipolar subjects had more hyperintensities than controls, unipolar depressive subjects had an intermediate number of hyperintensities that was not statistically different from either the bipolar subjects or the control group.
Within this group of studies, bipolar subjects had a significantly higher odds ratio for hyperintensities compared to controls (OR 3.1; 95% CI 1.9358 to 4.9208), and unipolar depressed subjects had an odds ratio of 2.4 (95% CI 1.3842 to 4.1469). Though bipolar subjects had more hyperintensities than unipolar depressed subjects, the meta-analysis did not demonstrate this to be significant (OR 1.6; 95% CI 0.9, 2.7). There was no significant difference in results when the adolescent studies were removed from the calculations. In the studies not included in our meta-analysis, two reports included a comparison group of unipolar subjects, but did not find a significant difference in hyperintensities between the two groups (Breeze et al., 2003; Hickie et al., 1995).
Within our meta-analysis studies, six studies included a schizophrenia comparison group, and results are more mixed. Lyoo et al. (2002) and Pillai et al. (2002) found bipolar subjects had significantly more severe hyperintensities than both the schizophrenia and control groups. Strakowski et al. (1993) found an increase in the number of focal hyperintensities in bipolar subjects when compared with controls, but not with the schizophrenia group. Lewine et al. (1995) and Persaud et al. (1997) did not find any significant difference in the number of hyperintensities among the bipolar, schizophrenia, and control groups, though Persaud et al. (1997) did find that subjects with schizophrenia had larger areas of hyperintensities than either bipolar or control groups. Interestingly, Pillai et al. (2002), in their adolescent study, found that though a larger percentage of the bipolar group had hyperintensities compared with both schizophrenia and control groups, only the schizophrenia group had subjects with the larger, more severe hyperintensities. Finally, Krabbendam et al. (2000) found a greater number of hyperintensities in the bipolar group, but the three groups did not differ statistically.
Similar to the findings in the unipolar analysis, though bipolar subjects had more total reported hyperintensities than schizophrenia subjects, the meta-analysis did not demonstrate this to be significant (OR 1.5; 95% CI 0.9, 2.7). Among the studies not included in our meta-analysis, Breeze et al. (2003) evaluated 600 MRIs of patients with various psychiatric diagnoses, including bipolar disorder and schizophrenia, and did not find differences in hyperintensities among the different diagnoses.
Review of other factors with potential impact on hyperintensity studies in bipolar disorder
Age and medical illness
In general, increasing age is associated with the presence of hyperintensities (Campbell & Coffey, 2001; Longstreth et al., 1996). Several studies have reported that some type of hyperintensity finding is present in up to 100% of people over the age of 60 years (Sachdev et al., 2005; Wen & Sachdev, 2004). However, the relationship of bipolar disorder, hyperintensities, and age is less clear.
Silverstone noted that the main determinant for the presence of hyperintensities in their sample was age rather than diagnosis. When they attempted to control for age by excluding subjects over the age of 50, there was no difference in the number of hyperintensities between the two groups. McDonald et al. (1999), however, studied a larger number of bipolar subjects, dividing the groups into older (>50 years) and younger (<50 years) subjects. They found that both groups had more hyperintensities than the comparison groups, and that there was no significant interaction between age and diagnosis for DWM and SCGM hyperintensities. They did note that PVWM hyperintensities were associated with age rather than diagnoses. Interestingly, Altshuler et al. (1995) and Moore et al. (2001), looking at smaller samples, did not find any differences in DWM or SCGM hyperintensities between bipolar and control groups, but they both noted that older bipolar subjects had more PVH than controls. Consistent with McDonald et al. (1999), Figiel et al. (1991), Dupont et al. (1995a), Krabbendam et al. (2000), Persaud et al. (1997), and Strakowski et al. (1993) found that the increased number and size of SC hyperintensities in bipolar subjects was not associated with age. Complicating this, Aylward et al. (1994) noted that younger bipolar subjects did not differ from control groups, but older bipolar subjects had more hyperintensities than older controls.
Given the disparity in conclusions about age and hyperintensities in bipolar disorder, it is possible that age may serve as an inexact marker for other pathologic processes associated with hyperintensities. For example, Aylward et al. (1994) suggested that the differences in ages and hyperintensities may be due in part to cardiac risk factors prevalent in their bipolar group. Thus, hyperintensities may be related to medical illness such as hypertension that tends to occur with older age, and bipolar subjects may be at a higher likelihood of developing these illnesses or developing them earlier in life (see Beyer et al., 2005; McIntyre et al., 2007). This explanation may be especially pertinent to the increased number of PVWM hyperintensities noted in older adults by McDonald et al. (1999), Altshuler et al. (1995), and Aylward et al. (1994), since it is believed that PVWM is particularly vulnerable to vascular changes.
Other factors, including familial effects, may also be associated with hyperintensities, especially in DWM and SCGM. Ahearn et al. (1998) evaluated the presence of hyperintensities in a family with a strong history of bipolar disorder. They found that all of the members with bipolar disorder and 60% of the members without bipolar disorder had hyperintensities. No age or cardiovascular risk factor effect was noted, suggesting that at least in this family, there may be a genetic association between leukoencephalopathy and bipolar disorder. The studies in children and adolescents with bipolar disorder may provide an especially important perspective here since they are not believed to be old enough to have developed cardiac or lifestyle risk factors that would induce hyperintensities. Only two (Lyoo et al., 2002; Pillai et al., 2002) of the four (Botteron et al., 1995; Chang et al., 2005) published studies in bipolar adolescents found a significant difference in hypertensities between bipolar and control groups. However, the odds ratio for the presence of hyperintensities in bipolar subjects was 3.5 (95% CI 1.7739 to 6.7698). This again suggests that another process besides medical illness and the aging brain may be related to hyperintensities in bipolar disorder.
Size of lesions
Beyond counting the presence of hyperintensities, attention has also turned to whether the size of hyperintensities is associated with bipolar illness. In Ahearn’s family study (1998), no correlation was found between the size of hyperintensities and the presence of the illness. However, Ahn et al. (2004), Figiel et al. (1991), Lyoo et al. (2002), McDonald et al. (1999), and Moore et al. (2001) noted that bipolar subjects had not only increased numbers of hyperintensities, but also increased size of hyperintensities compared with controls. Further, although Botteron et al. (1995), Chang et al. (2005), and Silverstone et al. (2003) did not find a significant increase in the presence of hyperintensities, they did find that bipolar subjects had a significantly higher grade of hyperintensities than controls. In contrast, Gulseren et al. (2006), Krabbendam et al. (2000), Persaud et al. (1997), and Pillai et al. (2002) did not note any difference in hyperintensity size compared to controls. However, both Persaud et al. (1997) and Pillai et al. (2002) reported the schizophrenic subjects had larger hyperintensity areas than either bipolar or control subject groups. In other published studies, de Asis et al. (2006) noted that DWM (but not SCGM) hyperintensity scores were larger in their sample of older adults (>60 yrs of age) with bipolar disorder compared with aged controls.
Symptoms and treatment
Several studies have addressed clinical features that may be associated with the presence of hyperintensities in bipolar patients. These have primarily been medications, response to treatment, rehospitalization, and number of episodes.
Breeze et al. (2003), in a study of over 600 subjects with a variety of psychiatric diagnoses, found that though cardiovascular disease and advanced age were the strongest predictors for the presence of hyperintensities in all subjects, repeated psychiatric admissions (>3) and lithium use (primarily in men) were also associated with the presence of hyperintensities. The authors speculated that the use of lithium in vulnerable individuals may have some vascular effect that may lead to WMH. Figiel et al. (1991), Dupont et al. (1995a), and Persaud et al. (1997), who did find increased WMH in bipolar subjects compared to controls, did not observe an association between the use of lithium and WMH. Kato et al. (2000) commented that subjects with higher hyperintensity scores actually had a better response to lithium; however, Moore et al. (2001) noted that subjects with DWM hyperintensities were less likely to respond to lithium. As for other medications, Altshuler et al. (1995) did not find that medications were associated with the increase PVWM hyperintensities in older bipolar subjects. Persaud et al. (1997) did not find any associations between hyperintensities and the use of tricyclic antidepressants in bipolar patients.
In relation to course and outcome, Gulseren et al. (2006) noted an increased number of manic episodes in subjects with increased hyperintensities, but there was no relationship to depressive symptoms. Moore et al. (2001) found that subjects with DWM hyperintensities had an overall poorer response to treatment, as defined by chronic symptoms over a two-year period despite adequate treatment. Dupont et al. (1987, 1990, 1995b) consistently found that all types of hyperintensities were associated with an increase in the number of hospitalizations, and McDonald et al. (1999) noted that the higher the hyperintensity scores, the more likely a patient was to be rehospitalized. However, contrary to the results noted above, Altshuler et al. (1995) did not find that greater number of PVWM hyperintensities in bipolar patients was related to the number of previous hospitalizations; Silverstone et al. (2003) did not find an association between increased hyperintensities and treatment resistance; and Kato et al. (2000) did not find a relationship with length of illness.
As to the presence of symptoms in bipolar subjects with hyperintensities, the reports were also mixed. Although McDonald et al. (1999) found a relationship between hyperintensities and psychotic features, Altshuler et al. (1995), Dupont et al. (1990, 1987) and Figiel et al. (1991) did not find a relationship between hyperintensities and psychosis. Dupont et al. (1987, 1990) noted a trend toward increased depressive symptoms in subjects with greater hyperintensities, but Gulseren et al. (2006) did not. Pompili et al. (2007) reported that WMH were associated with history of suicide attempts.
Neuropsychological testing performance
Bipolar disorder has been associated with some cognitive changes, especially when a patient is in an episode; however, neuropsychologists have not found a specific cognitive profile in bipolar disorder (Bearden et al., 2001). There is some evidence that bipolar subjects may demonstrate deficits in tasks of conceptual ability and memory processes independent of current mood state. Verbal skills appear to be relatively less impaired in bipolar patients than are visuo-spatial, non-verbal memory, and abstraction skills; verbal memory may be impaired when subjects are in an affective episode, and in patients with longstanding illness.
Given the association of hyperintensities with dementia, one would anticipate that the presence of hyperintensities may be related to cognitive changes as well. However, there is very little information hyperintensities and cognitive performance in bipolar subjects. Four studies on hyperintensities included some findings on neuropsychological test performance. Dupont et al. (1990) noted that hyperintensities in bipolar patients were associated with lower verbal fluency and digit symbol scores. In a follow-up study, Dupont et al. (1995a) found that bipolar but not unipolar or control subjects with hyperintensities had impairment on neuropsychological testing, suggesting greater subcortical dysfunction. Hickie et al. (1995) also found that DWM, but not SCGM, hyperintensities were associated with poorer performance on testing, although age differences confounded interpretation. Krabbendam et al. (2000) performed the Auditory Verbal Learning Test, Stroop Colour Word Test, Concept Shifting Test, Letter Digit Substitution Test, and Fluency Test in bipolar and schizophrenia outpatients. Although they did find that bipolar subjects in remission have a rather diffuse pattern of cognitive deficits, similar to the schizophrenia group, they did not find an association between cognitive performance and hyperintensities.
Gender
Breeze et al. (2003) found that in their sample of subjects with various psychiatric disorders, women had more DWM and PVH than men. However, Ahn et al. (2004), Aylward et al. (1994), Hickie et al. (1995), Kato et al. (2000), Krabbendam et al. (2000), McDonald et al. (1999), and Persaud et al. (1997) did not find any gender differences in hyperintensities.
Age at onset
Zanetti et al. (2007) provided a case report of an elderly woman with treatment-resistant depression who had prominent white matter hyperintensities on her brain MRI scan that developed late-life mania. They postulated that vascular-related WMH may provoke late-onset bipolar disorder by damaging frontolimbic circuits. De Asis et al. (2006) conducted a study in elderly bipolar subjects and found that a later age of onset for mania was associated with an increased severity of right frontal hyperintense lesions. This was consistent with the observation by Fujikawa et al. (1995) who had noted more silent cerebral infarcts in late-onset manic patients compared with both early-onset bipolar and early- and late-onset unipolar depressed patients. Dupont et al. (1987, 1990) initially did not observe a relationship between age of onset and hyperintensities in their early studies, but in their larger sample (Dupont et al., 1995) they found that increased volumes of white matter lesions was associated with a later age of onset. This is similar to Hickie et al. (1995) who noted subjects that were 50 years or older when they experienced their first manic episode had more white matter hyperintensities. However, Figiel et al. (1991) and McDonald et al. (1999) did not find any association of hyperintensities with age at onset of illness.
Family history
Ahearn et al. (1998) conducted an MRI study on a large family with a high prevalence of bipolar disorder. They found that both bipolar and non-affected family members had a high prevalence of hyperintensities; however, the incidence of hyperintensities in bipolar family members was 100%, regardless of age, compared to 60% in non-affected family members. The authors suggested that hyperintensities may serve as a genetic or biological marker for bipolar disorder. Gulseren et al. (2006) conducted a study on 12 patients with bipolar disorder, their siblings who had no history of bipolar disorder, and a control group. They found that hyperintensities were more likely in bipolar subjects than siblings or controls. The sibling and control group did not differ in number of hyperintensities, though they did differ in location. Initially, Dupont et al. (1987) reported that there was no association between a family history of psychiatric disorder and the presence of hyperintensities in subjects. However, Dupont et al. (1995a) noted that if a subject had a high number of hyperintensities, more family members would have been reported to have various psychiatric disorders. However, Hickie et al. (1995), Figiel et al. (1991), Kato et al. (2000), and Moore et al. (2001) did not find any association between the presence of white matter hyperintensities and reports of family psychiatric history.
Conclusions and clinical implications
Bipolar disorder is a heterogenous condition in which biological, familial, social and psychological factors may play etiological roles. Our study systematically reviewed published MRI studies to evaluate whether the presence of hyperintensities is associated with bipolar disorder. The results indicate that hyperintensities are associated with bipolar disorder, particularly those located in the DWM and SCGM of bipolar subjects compared with controls. However, the presence of hyperintensities is not specific to bipolar subjects since both unipolar depressed and schizophrenic subjects also have excess hyperintensities. Further, the role of hyperintensities in the pathogenesis and pathophysiology bipolar disorder is unclear. Aging may play a part in the presence of hyperintensities; yet age, and its associated cardiovascular risk factors and medical illnesses, do not fully explain the presence of hyperintensities in the bipolar subject. The literature concerning hyperintensities in bipolar disorder is conflicting but includes heuristically useful themes. The increased rate of psychiatric histories in families of bipolar subjects with hyperintensities suggests a genetic mechanism. The location of hyperintensities (e.g. the frontal/parietal DWM) may be important in the expression of bipolar disorder. Finally, hyperintensities may influence illness course and treatment outcomes in bipolar disorder.
The findings of this analysis and review also highlight methodological directions for future research in the role of hyperintensities in bipolar illness. Longitudinal studies are especially needed, and neuropsychological assessment may be a valuable component. In addition to assessment of volumes of hyperintensities, newer techniques such as diffusion tensor imaging may be especially helpful. In this method, the integrity of the white matter tracts can be calculated by assessment of the diffusion rate and direction of water molecules. Thus it is anticipated that we will be able to assess the functional significance of white matter hyperintensities in the future. Research on hyperintensities and related aspects of brain morphology in bipolar disorder promises to contribute to the assessment of prognosis, management decisions, and perhaps preventative strategies.
Acknowledgments
This study was supported by NIMH Grant #MH57027.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahearn EP, Speer MC, Chen YT, Steffens DC, Cassidy F, Van Meter S, et al. Investigation of Notch3 as a candidate gene for bipolar disorder using brain hyperintensities as an endophenotype. American Journal of Medical Genetics. 2002;114:652–658. doi: 10.1002/ajmg.10512. [DOI] [PubMed] [Google Scholar]
- Ahearn EP, Steffens DC, Cassidy F, Van Meter SA, Provenzale JM, Seldin MF, et al. Familial leukoencephalopathy in bipolar disorder. American Journal of Psychiatry. 1998;155:1605–1607. doi: 10.1176/ajp.155.11.1605. [DOI] [PubMed] [Google Scholar]
- Ahn KH, Lyoo IK, Lee HK, Song IC, Oh JS, Hwang J, et al. White matter hyperintensities in subjects with bipolar disorder. Psychiatry and Clinical Neurosciences. 2004;58:516–521. doi: 10.1111/j.1440-1819.2004.01294.x. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Archives of General Psychiatry. 1997;54:915–922. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. American Journal of Psychiatry. 1995;152:1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- Awad IA, Johnson PC, Spetzler RF, Hodak JA. Incidental subcortical lesions identified on magnetic resonance imaging in the elderly. II. Postmortem pathological correlations. Stroke. 1986;17:1090–1097. doi: 10.1161/01.str.17.6.1090. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, et al. Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. American Journal of Psychiatry. 1994;151:687–693. doi: 10.1176/ajp.151.5.687. [DOI] [PubMed] [Google Scholar]
- Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. Development and validation of MIX: Comprehensive free software for meta-analysis of causal research data. BMC Medical Research Methodology. 2006;6:50. doi: 10.1186/1471-2288-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bax L, Yu LM, Ikeda N, Tsuruta H, Moons KGM. [Accessed on June 9, 2008];MIX: Comprehensive free software for meta-analysis of causal research data. Version 1.7. 2008 http://mix-for-meta-analysis.info.
- Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: A critical review. Bipolar Disorders. 2001;3:106–150. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151–153. [DOI] [PubMed] [Google Scholar]
- Beyer J, Kuchibhatla M, Gersing K, Krishnan KR. Medical comorbidity in a bipolar outpatient clinical population. Neuropsychopharmacology. 2005;30:401–404. doi: 10.1038/sj.npp.1300608. [DOI] [PubMed] [Google Scholar]
- Böhm D, Hoffmann K, Laccone F, Wilken B, Dechent P, Frahm J, et al. Association of Jacobsen syndrome and bipolar affective disorder in a patient with a de novo 11q terminal deletion. American Journal of Medical Genetics A. 2006;140:378–382. doi: 10.1002/ajmg.a.31088. [DOI] [PubMed] [Google Scholar]
- Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, et al. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Archives of Neurology. 1992;49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- Botteron KN, Figiel GS, Wetzel MW, Hudziak J, VanEerdewegh M. MRI abnormalities in adolescent bipolar affective disorder. Journal of American Academy of Child & Adolescent Psychiatry. 1992;31:258–261. doi: 10.1097/00004583-199203000-00012. [DOI] [PubMed] [Google Scholar]
- Botteron KN, Vannier MW, Geller B, Todd RD, Lee BC. Preliminary study of magnetic resonance imaging characteristics in 8- to 16-year-olds with mania. Journal of American Academy of Child & Adolescent Psychiatry. 1995;34:742–749. doi: 10.1097/00004583-199506000-00014. [DOI] [PubMed] [Google Scholar]
- Boyko OB, Alston SR, Fuller GN, Julette CM, Johnson GA, Burger PC. Utility of postmortem MR imaging in neuropathology. Archives of Pathology & Laboratory Medicine. 1994;118(3):218–222. [PubMed] [Google Scholar]
- Braffman BH, Zimmerman RA, Trojanowski JQ, Gonatas NK, Hickey WF, Schlaepfer WW. Brain MR: Pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. American Journal of Roentgenology. 1988;151:559–566. doi: 10.2214/ajr.151.3.559. [DOI] [PubMed] [Google Scholar]
- Breeze JL, Hesdorffer DC, Hong X, Frazier JA, Renshaw PF. Clinical significance of brain white matter hyperintensities in young adults with psychiatric illness. Harvard Review of Psychiatry. 2003;11:269–283. [PubMed] [Google Scholar]
- Breteler MM, van Swieten JC, Bots ML, Grobbee DE, Claus JJ, van den Hout JH, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: The Rotterdam study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- Brown FW, Lewine RJ, Hudgins PA, Risch SC. White matter hyperintensity signals in psychiatric and non-psychiatric subjects. American Journal of Psychiatry. 1992;149:620–625. doi: 10.1176/ajp.149.5.620. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, III, Coffey CE. Neuropsychiatric significance of subcortical hyperintensity. Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13:261–288. doi: 10.1176/jnp.13.2.261. [DOI] [PubMed] [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, Simeonova DI, Barnes P, Ketter T, et al. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biological Psychiatry. 2005;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Chimowitz MI, Estes ML, Furlan AJ, Awad IA. Further observations on the pathology of subcortical lesions identified on magnetic resonance imaging. Archives of Neurology. 1992;49:747–752. doi: 10.1001/archneur.1992.00530310095018. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, et al. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Archives of General Psychiatry. 1993;50:7–16. doi: 10.1001/archpsyc.1993.01820130009002. [DOI] [PubMed] [Google Scholar]
- de Asis JM, Greenwald BS, Alexopoulos GS, Kiosses DN, Ashtari M, Heo M, et al. Frontal signal hyperintensities in mania in old age. American Journal of Geriatric Psychiatry. 2006;14:598–604. doi: 10.1097/01.JGP.0000200603.70504.d5. [DOI] [PubMed] [Google Scholar]
- de Groot JC, de Leeuw FE, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and cognitive function: The Rotterdam Scan Study. Annals of Neurology. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Reus VI, Manfredi L, Wolkowitz OM. MRI deep white matter hyperintensity in a psychiatric population. Biological Psychiatry. 1991;29(9):918–922. doi: 10.1016/0006-3223(91)90058-t. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Butters N, Delis D, Hesselink JR, Heindel W, et al. Subcortical abnormalities detected in bipolar affective disorder using magnetic resonance imaging. Clinical and neuropsychological significance. Archives of General Psychiatry. 1990;47:55–59. doi: 10.1001/archpsyc.1990.01810130057008. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Gillin JC, Butters N, Delis DC, Hesselink JR. Subcortical signal hyperintensities in bipolar patients detected by MRI. Psychiatry Research. 1987;21:357–358. doi: 10.1016/0165-1781(87)90020-5. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Jernigan TL, Heindel W, Butters N, Shafer K, Wilson T, Hesselink J, Gillin JC. Magnetic resonance imaging and mood disorders. Localization of white matter and other subcortical abnormalities. Archives of General Psychiatry. 1995a;52:747–755. doi: 10.1001/archpsyc.1995.03950210041009. [DOI] [PubMed] [Google Scholar]
- Dupont RM, Butters N, Schafer K, Wilson T, Hesselink J, Gillin JC. Diagnostic specificity of focal white matter abnormalities in bipolar and unipolar mood disorder. Biological Psychiatry. 1995b;38:482–486. doi: 10.1016/0006-3223(95)00100-u. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, et al. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43:1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MRI signal abnormalities at 1.5T in Alzheimer. American Journal of Neuroradiology. 1987;8:421–426. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/wnl.43.9.1683. [DOI] [PubMed] [Google Scholar]
- Figiel GS, Krishnan KR, Rao VP, Doraiswamy M, Ellinwood CB, Jr, Evans D, et al. Subcortical hyperintensities on brain magnetic resonance imaging: a comparison of normal and bipolar subjects. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:18–22. doi: 10.1176/jnp.3.1.18. [DOI] [PubMed] [Google Scholar]
- Fujikawa T, Yamawaki S, Tahouda Y. Silent cerebral infarctions in patients with late-onset mania. Stroke. 1995;26:946–949. doi: 10.1161/01.str.26.6.946. [DOI] [PubMed] [Google Scholar]
- George AE, de Leon MJ, Kalnin A, Rosner L, Goodgold A, Chase N. Leukoencephalopathy in normal and pathologic aging: 2. MRI of brain lucencies. American Journal of Neuroradiology. 1986;7:567–570. [PMC free article] [PubMed] [Google Scholar]
- Gulseren S, Gurcan M, Gulseren L, Gelal F, Erol A. T2 hyperintensities in bipolar patients and their healthy siblings. Archives of Medical Research. 2006;37:79–85. doi: 10.1016/j.arcmed.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hajek T, Carrey N, Alda M. Neuroanatomical abnormalities as risk factors for bipolar disorder. Bipolar Disorder. 2005;7:393–403. doi: 10.1111/j.1399-5618.2005.00238.x. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, Licalzi EM, et al. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biological Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Heiden A, Kettenbach J, Fischer P, Schein B, Ba-Ssalamah A, Frey R, et al. White matter hyperintensities and chronicity of depression. Journal of Psychiatric Research. 2005;39:285–293. doi: 10.1016/j.jpsychires.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Hickie I, Scott E, Mitchell P, Wilhelm K, Austin MP, Bennett B. Subcortical hyperintensities on magnetic resonance imaging: Clinical correlates and prognostic significance in patients with severe depression. Biological Psychiatry. 1995;37:151–160. doi: 10.1016/0006-3223(94)00174-2. [DOI] [PubMed] [Google Scholar]
- Hickie I, Scott E, Wilhelm K, Brodaty H. Subcortical hyperintensities on magnetic resonance imaging in patients with severe depression — A longitudinal evaluation. Biological Psychiatry. 1997;42:367–374. doi: 10.1016/S0006-3223(96)00363-0. [DOI] [PubMed] [Google Scholar]
- Junqué C, Pujol J, Vendrell P, Bruna O, Jódar M, Ribas JC, et al. Leuko-araiosis on magnetic resonance imaging and speed of mental processing. Archives of Neurology. 1990;47:151–156. doi: 10.1001/archneur.1990.00530020047013. [DOI] [PubMed] [Google Scholar]
- Kato T, Fujii K, Kamiya A, Kato N. White matter hyperintensity detected by magnetic resonance imaging and litium response in bipolar disorder: A preliminary observation. Psychiatry and Clinical Neurosciences. 2000;54:117–120. doi: 10.1046/j.1440-1819.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbendam L, Honig A, Wiersma J, Vuurman EF, Hofman PA, Derix MM, Nolen WA, Jolles J. Cognitive dysfunctions and white matter lesions in patients with bipolar disorder in remission. Acta Psychiatrica Scandinavica. 2000;101(4):274–280. [PubMed] [Google Scholar]
- Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. American Journal of Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- Krishnan KRR, Boyko OB, McDonald WM, Charles HC, MacFall JR, Upchurch L. MR morphometry: Image analysis methodology development for affective disorder. Depression. 1993;1:159–171. [Google Scholar]
- Lewine RR, Hudgins P, Brown F, Caudle J, Risch SC. Differences in qualitative brain morphology findings in schizophrenia, major depression, bipolar disorder, and normal volunteers. Schizophrenia Research. 1995;15(3):253–259. doi: 10.1016/0920-9964(94)00055-d. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Hwang J, Sim M, Dunn BJ, Renshaw PF. Advances in magnetic resonance imaging methods for the evaluation of bipolar disorder. CNS Spectrums. 2006;11:269–280. doi: 10.1017/s1092852900020770. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Lee HK, Jung JH, Noam GG, Renshaw PF. White matter hyperintensities on magnetic resonance imaging of the brain in children with psychiatric disorders. Comprehensive Psychiatry. 2002;43:361–368. doi: 10.1053/comp.2002.34636. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Mino I, Renshaw PF, Lee HK. White matter hyperintensities. Journal of American Academy of Child & Adolescent Psychiatry. 1995;34:833–834. doi: 10.1097/00004583-199507000-00004. [DOI] [PubMed] [Google Scholar]
- McDonald WM, Krishnan KR, Doraiswamy PM, Blazer DG. Occurrence of subcortical hyperintensities in elderly subjects with mania. Psychiatry Research. 1991;40:211–220. doi: 10.1016/0925-4927(91)90013-g. [DOI] [PubMed] [Google Scholar]
- McDonald WM, Tupler LA, Marsteller FA, Figiel GS, DiSouza S, Nemeroff CB, Krishnan KR. Hyperintense lesions on magnetic resonance images in bipolar disorder. Biological Psychiatry. 1999 Apr 15;45(8):965–971. doi: 10.1016/s0006-3223(98)00341-2. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Soczynska JK, Beyer JL, Woldeyohannes HO, Law CW, Miranda A, et al. Medical comorbidity in bipolar disorder: Re-prioritizing unmet needs. Current Opinion in Psychiatry. 2007;20:406–416. doi: 10.1097/YCO.0b013e3281938102. [DOI] [PubMed] [Google Scholar]
- Mirsen TR, Lee DH, Wong CJ, Diaz JF, Fox AJ, Hachinski VC, et al. Clinical correlates of white-matter changes on magnetic resonance imaging scans of the brain. Archives of Neurology. 1991;48:1015–1021. doi: 10.1001/archneur.1991.00530220031015. [DOI] [PubMed] [Google Scholar]
- Monkul ES, Malhi GS, Soares JC. Anatomical MRI abnormalities in bipolar disorder: Do they exist and do they progress? Australian and New Zealand Journal of Psychiatry. 2005;39:222–226. doi: 10.1080/j.1440-1614.2005.01571.x. [DOI] [PubMed] [Google Scholar]
- Moore PB, Shepherd DJ, Eccleston D, Macmillan IC, Goswami U, McAllister VL, et al. Cerebral white matter lesions in bipolar affective disorder: Relationship to outcome. British Journal of Psychiatry. 2001;178:172–176. doi: 10.1192/bjp.178.2.172. [DOI] [PubMed] [Google Scholar]
- Norris SD, Krishnan KR, Ahearn E. Structural changes in the brain of patients with bipolar affective disorder by MRI: A review of the literature. Prog Neuropsychopharmacol Biological Psychiatry. 1997;21:1323–1337. doi: 10.1016/s0278-5846(97)00167-x. [DOI] [PubMed] [Google Scholar]
- O’Brien J, Ames D, Chiu E, Schweitzer I, Desmond P, Tress B. Severe deep white matter lesions and outcome in elderly patients with major depressive disorder: follow up study. British Medical Journal. 1998;317:982–984. doi: 10.1136/bmj.317.7164.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: A review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Persaud R, Russow H, Harvey I, Lewis SW, Ron M, Murray RM, et al. Focal signal hyperintensities in schizophrenia. Schizophrenia Research. 1997;27:55–64. doi: 10.1016/S0920-9964(97)00060-1. [DOI] [PubMed] [Google Scholar]
- Pillai JJ, Friedman L, Stuve TA, Trinidad S, Jesberger JA, Lewin JS, et al. Increased presence of white matter hyperintensities in adolescent patients with bipolar disorder. Psychiatry Research. 2002;114:51–56. doi: 10.1016/s0925-4927(01)00129-9. [DOI] [PubMed] [Google Scholar]
- Pompili M, Ehrlich S, De Pisa E, Mann JJ, Innamorati M, Cittadini A, et al. White matter hyperintensities and their associations with suicidality in patients with major affective disorders. European Archives of Psychiatry and Clinical Neurosciences. 2007;257:494–499. doi: 10.1007/s00406-007-0755-x. [DOI] [PubMed] [Google Scholar]
- Regenold WT, Hisley KC, Obuchowski A, Lefkowitz DM, Marano C, Hauser P. Relationship of white matter hyperintensities to cerebrospinal fluid glucose polyol pathway metabolites — A pilot study in treatment-resistant affective disorder patients. Journal of Affective Disorders. 2005;85:341–350. doi: 10.1016/j.jad.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Sachdev P, Wen W, Chen X, Brodaty H. Progression of white matter hyperintensities in elderly individuals over 3 years. Neurology. 2007;68(3):214–222. doi: 10.1212/01.wnl.0000251302.55202.73. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are related to physical disability and poor motor function. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76:362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassi RB, Brambilla P, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. White matter hyperintensities in bipolar and unipolar patients with relatively mild-to-moderate illness severity. Journal of Affective Disorders. 2003;77:237–245. doi: 10.1016/s0165-0327(02)00170-2. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Offenbacher H, Dusek T, Zach E, Reinhart B, et al. Neuropsychologic correlates of MRI white matter hyperintensities: A study of 150 normal volunteers. Neurology. 1993;43:2490–2494. doi: 10.1212/wnl.43.12.2490. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Kapeller P, Schmidt H, Hartung HP. MRI white matter hyperintensities: Three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53:132–139. doi: 10.1212/wnl.53.1.132. [DOI] [PubMed] [Google Scholar]
- Silverstone T, McPherson H, Li Q, Doyle T. Deep white matter hyperintensities in patients with bipolar depression, unipolar depression and age-matched control subjects. Bipolar Disorders. 2003;5:53–57. doi: 10.1034/j.1399-5618.2003.01208.x. [DOI] [PubMed] [Google Scholar]
- Simpson SW, Jackson A, Baldwin RC, Burns A. 1997 IPA/Bayer Research Awards in Psychogeriatrics. Subcortical hyperintensities in late-life depression: Acute response to treatment and neuropsychological impairment. International Psychogeriatric. 1997;9:257–275. doi: 10.1017/s1041610297004432. [DOI] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders–review of structural neuroimaging studies. Biological Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Spilt A, Goekoop R, Westendorp RG, Blauw GJ, de Craen AJ, van Buchem MA. Not all age-related white matter hyperintensities are the same: A magnetization transfer imaging study. American Journal of Neuroradiology. 2006;27:1964–1968. [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Krishnan DRR. Structural neuroimaging and mood disorders: Recent findings, implications for classification, and future directions. Biological Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- Stoll AL, Renshaw PF, Yurgelun-Todd DA, Cohen BM. Neuroimaging in bipolar disorder: What have we learned? Biological Psychiatry. 2000;48:505–517. doi: 10.1016/s0006-3223(00)00982-3. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Woods BT, Tohen M, Wilson DR, Douglass AW, Stoll AL. MRI subcortical signal hyperintensities in mania at first hospitalization. Biological Psychiatry. 1993;33:204–206. doi: 10.1016/0006-3223(93)90140-9. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello M, Adler CMKM, Sax KW. Neuroimaging in bipolar disorder. Bipolar Disorders. 2000;2:148–164. doi: 10.1034/j.1399-5618.2000.020302.x. [DOI] [PubMed] [Google Scholar]
- Swayze VW, II, Andreasen NC, Alliger RJ, Ehrhardt JC, Yuh WT. Structural brain abnormalities in bipolar affective disorder. Ventricular enlargement and focal signal hyperintensities. Archives of General Psychiatry. 1990;47:1054–1059. doi: 10.1001/archpsyc.1990.01810230070011. [DOI] [PubMed] [Google Scholar]
- Tanabe JL, Ezekiel F, Jagust WJ, Reed BR, Norman D, Schuff N, Weiner MW, Chui H, Fein G. Magnetization transfer ratio of white matter hyperintensities in subcortical ischemic vascular dementia. American Journal of Neuroradiology. 1999;20:839–844. [PMC free article] [PubMed] [Google Scholar]
- Taylor WD, Steffens DC, MacFall JR, McQuoid DR, Payne ME, Provenzale JM, Krishnan KR. White matter hyperintensity progression and late-life depression outcomes. Archives of General Psychiatry. 2003;60(11):1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114:761–774. doi: 10.1093/brain/114.2.761. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. The New England Journal of Medicine. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- Videbech P. MRI findings in patients with affective disorder: A meta-analysis. Acta Psychiatrica Scandinavica. 1997;96:157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- Wahlund LO, Basun H, Almkvist O, Andersson-Lundman G, Julin P, Sääf J. White matter hyperintensities in dementia: Does it matter? Journal of Magnetic Resonance Imaging. 1994;12:387–394. doi: 10.1016/0730-725x(94)92531-3. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev P. The topography of white matter hyperintensities on brain MRI in healthy 60- to 64-year-old individuals. Neuroimage. 2004;22:144–154. doi: 10.1016/j.neuroimage.2003.12.027. [DOI] [PubMed] [Google Scholar]
- Ylikoski R, Ylikoski A, Erkinjuntti T, Sulkava R, Raininko R, Tilvis R. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Archives of Neurology. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- Zanetti MV, Cordeiro Q, Busatto GF. Late onset bipolar disorder associated with white matter hyperintensities: A pathophysiological hypothesis. Prog Neuropsychopharmacol Biological Psychiatry. 2007;31:551–556. doi: 10.1016/j.pnpbp.2006.10.004. [DOI] [PubMed] [Google Scholar]