Abstract
During the last 15 years several laboratories have attempted to generate rabbit monoclonal antibodies, mainly because rabbits recognize antigens and epitopes that are not immunogenic in mice or rats, two species from which monoclonal antibodies are usually generated. Monoclonal antibodies from rabbits could not be generated, however, because a plasmacytoma fusion partner was not available. To obtain a rabbit plasmacytoma cell line that could be used as a fusion partner we generated transgenic rabbits carrying two transgenes, c-myc and v-abl. These rabbits developed plasmacytomas, and we obtained several plasmacytoma cell lines from which we isolated hypoxanthine/aminopterin/thymidine-sensitive clones. One of these clones, when fused with spleen cells of immunized rabbits, produced stable hybridomas that secreted antibodies specific for the immunogen. The hybridomas can be cloned and propagated in nude mice, and they can be frozen without change in their ability to secrete specific monoclonal antibodies. These rabbit-rabbit hybridomas will be useful not only for production of monoclonal antibodies but also for studies of immunoglobulin gene rearrangements and isotype switching.
Full text
PDF

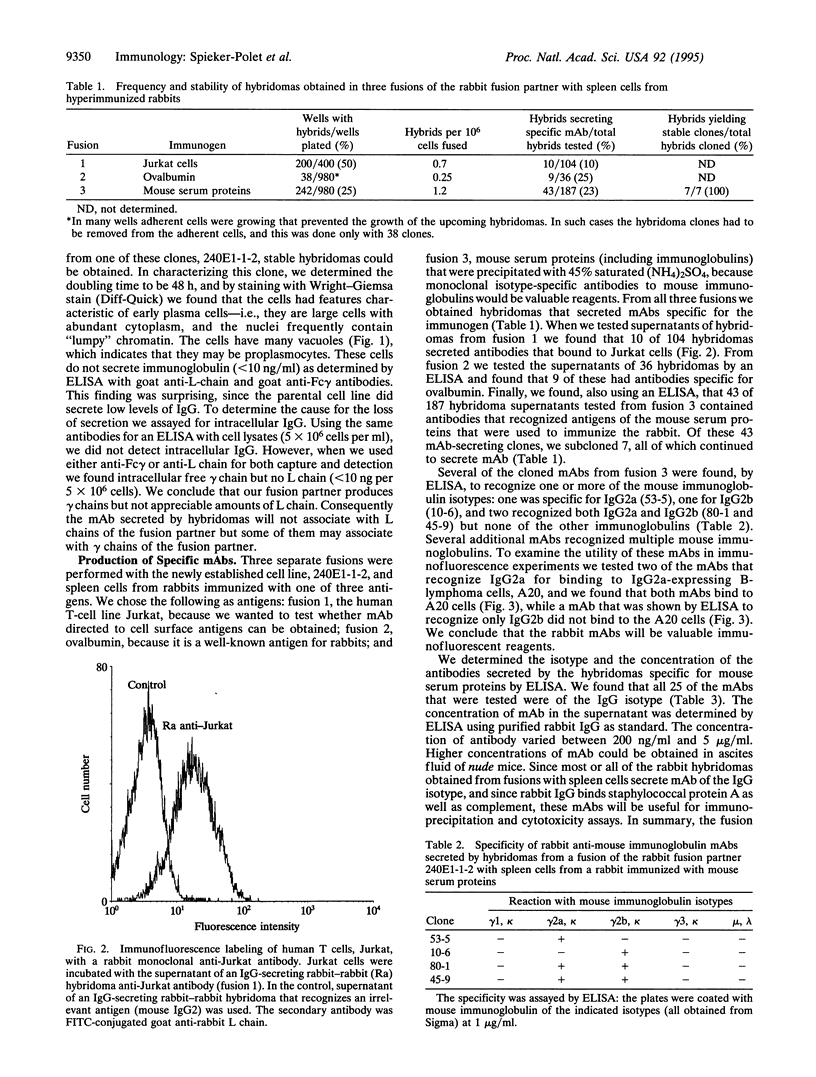
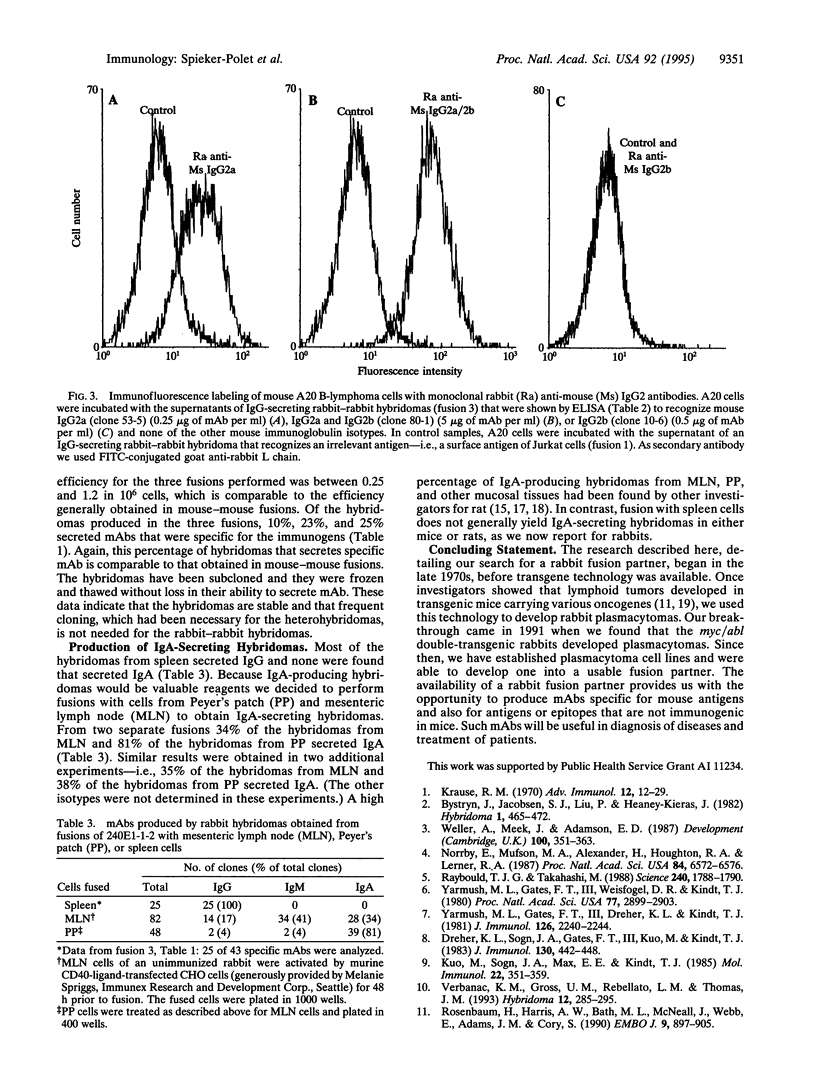

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bystryn J. C., Jacobsen J. S., Liu P., Heaney-Kieras J. Comparison of cell-surface human melanoma-associated antigens identified by rabbit and murine antibodies. Hybridoma. 1982;1(4):465–472. doi: 10.1089/hyb.1.1982.1.465. [DOI] [PubMed] [Google Scholar]
- Dreher K. L., Sogn J. A., Gates F. T., 3rd, Kuo M. C., Kindt T. J. Allotype-defined mRNA for rabbit immunoglobulin H and L chains isolated from rabbit-mouse hybridomas. J Immunol. 1983 Jan;130(1):442–448. [PubMed] [Google Scholar]
- Knight K. L., Spieker-Polet H., Kazdin D. S., Oi V. T. Transgenic rabbits with lymphocytic leukemia induced by the c-myc oncogene fused with the immunoglobulin heavy chain enhancer. Proc Natl Acad Sci U S A. 1988 May;85(9):3130–3134. doi: 10.1073/pnas.85.9.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisar J. L., Fuhrman J. A., Cebra J. J. IgA-producing hybridomas are readily derived from gut-associated lymphoid tissue. J Immunol. 1982 May;128(5):2376–2378. [PubMed] [Google Scholar]
- Kuo M. C., Sogn J. A., Max E. E., Kindt T. J. Rabbit-mouse hybridomas secreting intact rabbit immunoglobulin. Mol Immunol. 1985 Apr;22(4):351–359. doi: 10.1016/0161-5890(85)90119-1. [DOI] [PubMed] [Google Scholar]
- Norrby E., Mufson M. A., Alexander H., Houghten R. A., Lerner R. A. Site-directed serology with synthetic peptides representing the large glycoprotein G of respiratory syncytial virus. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6572–6576. doi: 10.1073/pnas.84.18.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudghiri M., Seguin J., Deslauriers N. IgA hybridomas derived from naturally primed salivary gland B cells. Adv Exp Med Biol. 1987;216B:1215–1222. [PubMed] [Google Scholar]
- Peppard J. V., Jackson L. E. Variability in the molecular sizes of IgA secreted by individual hybridoma cell lines. Adv Exp Med Biol. 1987;216B:1207–1213. [PubMed] [Google Scholar]
- Raybould T. J., Takahashi M. Production of stable rabbit-mouse hybridomas that secrete rabbit mAb of defined specificity. Science. 1988 Jun 24;240(4860):1788–1790. doi: 10.1126/science.3289119. [DOI] [PubMed] [Google Scholar]
- Rosenbaum H., Harris A. W., Bath M. L., McNeall J., Webb E., Adams J. M., Cory S. An E mu-v-abl transgene elicits plasmacytomas in concert with an activated myc gene. EMBO J. 1990 Mar;9(3):897–905. doi: 10.1002/j.1460-2075.1990.tb08187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethupathi P., Spieker-Polet H., Polet H., Yam P. C., Tunyaplin C., Knight K. L. Lymphoid and non-lymphoid tumors in E kappa-myc transgenic rabbits. Leukemia. 1994 Dec;8(12):2144–2155. [PubMed] [Google Scholar]
- Stewart T. A., Pattengale P. K., Leder P. Spontaneous mammary adenocarcinomas in transgenic mice that carry and express MTV/myc fusion genes. Cell. 1984 Oct;38(3):627–637. doi: 10.1016/0092-8674(84)90257-5. [DOI] [PubMed] [Google Scholar]
- Verbanac K. M., Gross U. M., Rebellato L. M., Thomas J. M. Production of stable rabbit-mouse heterohybridomas: characterization of a rabbit monoclonal antibody recognizing a 180 kDa human lymphocyte membrane antigen. Hybridoma. 1993 Jun;12(3):285–295. doi: 10.1089/hyb.1993.12.285. [DOI] [PubMed] [Google Scholar]
- Weller A., Meek J., Adamson E. D. Preparation and properties of monoclonal and polyclonal antibodies to mouse epidermal growth factor (EGF) receptors: evidence for cryptic EGF receptors in embryonal carcinoma cells. Development. 1987 Jun;100(2):351–363. doi: 10.1242/dev.100.2.351. [DOI] [PubMed] [Google Scholar]
- Yarmush M. L., Gates F. T., 3rd, Dreher K. L., Kindt T. J. Serologic and structural characterization of immunoglobulin chains secreted by rabbit-mouse hybridomas. J Immunol. 1981 Jun;126(6):2240–2244. [PubMed] [Google Scholar]
- Yarmush M. L., Gates F. T., 3rd, Weisfogel D. R., Kindt T. J. Identification and characterization of rabbit-mouse hybridomas secreting rabbit immunoglobulin chains. Proc Natl Acad Sci U S A. 1980 May;77(5):2899–2903. doi: 10.1073/pnas.77.5.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]




