Abstract
The chemokine SDF-1 plays a central role in the repopulation of the bone marrow (BM) by circulating CD34+ progenitors, but the mechanisms of its action remain obscure. To extravasate to target tissue, a blood-borne cell must arrest firmly on vascular endothelium. Murine hematopoietic progenitors were recently shown in vivo to roll along BM microvessels that display selectins and integrins. We now show that SDF-1 is constitutively expressed by human BM endothelium. In vitro, human CD34+ cells establish efficient rolling on P-selectin, E-selectin, and the CD44 ligand hyaluronic acid under physiological shear flow. ICAM-1 alone did not tether CD34+ cells under flow, but, in the presence of surface-bound SDF-1, CD34+ progenitors rolling on endothelial selectin rapidly developed firm adhesion to the endothelial surface, mediated by an interaction between ICAM-1 and its integrin ligand, which coimmobilized with SDF-1. Human CD34+ cells accumulated efficiently on TNF-activated human umbilical cord endothelial cells in the absence of SDF-1, but they required immobilized SDF-1 to develop firm integrin-mediated adhesion and spreading. In the absence of selectins, SDF-1 also promoted VLA-4–mediated, Gi protein–dependent tethering and firm adhesion to VCAM-1 under shear flow. To our knowledge, this is the first demonstration that SDF-1 expressed on vascular endothelium is crucial for translating rolling adhesion of CD34+ progenitors into firm adhesion by increasing the adhesiveness of the integrins VLA-4 and LFA-1 to their respective endothelial ligands, VCAM-1 and ICAM-1.
Introduction
Transplanted human hematopoietic stem cells must retain specific adhesive capacity to interact with the vascular endothelium that lines the bone marrow (BM) (1–3). The mechanisms by which these cells home to and engraft the BM are still obscure. Murine hematopoietic progenitors (HPCs) have been shown to interact in vivo with both P-selectin and E-selectin on vascular endothelium of murine BM (4). In addition, VCAM-1 has been shown to support rolling of HPC on BM endothelium in the absence of endothelial selectins (4). Nevertheless, optimal recruitment of HPC to the BM requires the combined action of both selectins and VCAM-1 (5). Thus, the initiation of primary rolling adhesion of blood-borne cells to the lining of the BM microvessels under shear flow is critical for the recruitment of HPC to the BM. HPC rolling on BM endothelium is likely to be accompanied by a coordinated sequence of adhesive and activation events leading to cell arrest, a key step in the successful extravasation of blood-borne cells to extravascular beds (6, 7). In these prior studies, the mechanisms by which HPCs develop firm adhesion to BM endothelium in the presence of physiological shear flow have not been elucidated.
We recently established a key role for the chemokine SDF-1 and its receptor CXCR4 in murine BM engraftment by human severe combined immunodeficiency (SCID) repopulating stem cells (8). In light of the growing evidence that chemokines can regulate the arrest of leukocytes on blood vessels through integrin-dependent interactions with IgSF ligands, we asked whether SDF-1 regulates CD34+ progenitors interaction with BM endothelium, and if so, how. We now report that human BM endothelium constitutively expresses high levels of SDF-1. Using in vitro flow chamber assays, we have tested the specific effects of SDF-1 on the ability of human cord blood CD34+ progenitors to initiate rolling interactions and arrest on human vascular endothelium and on isolated endothelial receptors, including E-selectin, P-selectin, ICAM-1, and VCAM-1, which have all been shown to be constitutively expressed on both murine and human BM endothelium (9). Our results suggest that SDF-1 is a key player in the ability of CD34+ cells to develop firm adhesion to BM vascular endothelium under physiological shear flow conditions.
Methods
Reagents and mAbs.
Human ICAM-1 (affinity purified from spleen; a kind gift of L. Klickstein, Brigham and Women’s Hospital, Boston, Massachusetts, USA) and P-selectin (affinity purified from human platelets; a gift of R. McEver, University of Oklahoma, Oklahoma City, Oklahoma, USA) were suspended in PBS containing 1% octyl glucoside and stored at –30°C. Recombinant soluble 7-domain human VCAM-1 (sVCAM-1; ref. 10), a generous gift of R. Lobb (Biogen Inc., Cambridge, Massachusetts, USA), was stored in PBS. Recombinant IgG1 fusion proteins of human P-selectin and E-selectin (11) were gifts of T.S. Kupper (Brigham and Women’s Hospital). Human SDF-1α and MIP-1α were purchased from R&D Systems Inc. (Minneapolis, Minnesota, USA). BSA (Fraction V), HBSS (free of Ca2+ and Mg2+), EGTA, HEPES, hyaluronic acid (HA), heparin, pertussis toxin, platelet activating factor, and Ficoll-Hypaque 1077 were obtained from Sigma Chemical Co. (St. Louis, Missouri, USA). HSA was from Fraction V, and pertussis toxin was from Calbiochem-Novabiochem Corp. (La Jolla, California, USA). Human fibronectin was obtained from Chemicon International (Temecula, California, USA). The anti–VLA-4 integrin mAb HP1/2 (12), the anti–E-selectin mAb BB11 (13) (gifts of R. Lobb), the P-selectin mAb G1 (ref. 14; a gift of R. McEver, University of Oklahoma), the anti–LFA-1 (CD11a) mAb MCA1149 (Serotec Ltd., Kidlington, United Kingdom), and the anti-CD44 mAb 515 (a gift of G. Kansas, Northwestern University, Chicago, Illinois, USA) were all used as purified Ig. The anti-CXCR4 mAb 12g5 was purchased from PharMingen (San Diego, California, USA) and was used as purified Ig. The anti–P-selectin glycoprotein ligand-1 (anti–PSGL-1) mAb KPL-1 (15), a gift of G. Kansas, was used in blocking studies as ascites at a 1:100 dilution. Purified mouse IgG (Zymed Laboratories Inc., South San Francisco, California, USA) was used as a control antibody.
Immunohistology of BM sections.
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded tissue sections using the avidin-biotin-peroxidase complex method and the DAB chromogen kit from DAKO A/S (Glostrup, Denmark). Sections were stained with the anti–SDF-1 mAb K15C (1:50 dilution; ref. 16). The immunohistochemical staining was performed using the LSAB+ peroxidase kit from DAKO A/S. To enhance immunostaining, the antigen was preretrieved in citrate buffer using pressure cooking in a calibrated microwave for 15 minutes. The antigen was allowed to cool and was then reheated for 2.5 minutes. Slides were counterstained with Mayer’s hematoxylin. The specificity of the anti–SDF-1 mAb was confirmed by the complete absence of peroxidase staining on identical tissue sections using an isotype-matched control mAb.
Cells.
The human experimentation and ethics committees of The Weizmann Institute of Science approved the use of human cells according to the indicated procedures. Human cord blood cells were obtained from full-term deliveries. The blood samples were diluted 1:1 in PBS and supplemented with 1% FBS (Biological Industries, Bet Haemek, Israel). Low-density mononuclear cells were collected after standard separation on Ficoll-Paque (Pharmacia Biotech AB, Uppsala, Sweden), and then washed in RPMI with 1% FCS. Enrichment of human CD34+ cells was performed with a magnetic bead separation kit (miniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. The purity of the enriched CD34+ cells was higher than 95%, as confirmed by cytofluorometry. Human peripheral blood lymphocytes (PBL) obtained from healthy donors were isolated from citrate-anticoagulated whole blood by dextran sedimentation and density separation over Ficoll-Hypaque (17). The mononuclear cells thus obtained were washed and further purified on nylon wool. The resulting PBL consisted of more than 90% CD3+ T lymphocytes.
Human umbilical cord endothelial cells (HUVEC) were isolated from umbilical cord veins according to the method of Jaffe et al. (18). The cells were then pooled and established as primary cultures in M199 containing 10% FCS, 8% pooled human serum, 50 μg/mL endothelial cell (EC) growth factor (Sigma Chemical Co.), porcine intestinal heparin (10 U/mL; Sigma Chemical Co.), and antibiotics. Primary cultures were serially passaged (1:3 split ratio), and passages 2 and 3 were used for adhesion experiments.
Preparation of adhesive substrates and HUVEC monolayers.
Laminar flow adhesion assays were performed as described previously (19). The various adhesion proteins were diluted at the indicated concentrations in coating medium (PBS buffered with 20 mM bicarbonate at pH 8.5) and were adsorbed as 20-μL spots on polystyrene plates (60 × 15 mm; Becton Dickinson and Co., Lincoln Park, New Jersey, USA), either for 2 hours at 37°C (sVCAM-1) or overnight at 4°C (P-selectin, ICAM-1, HA, or chondroitin sulfate). sVCAM-1 was coated at 1–10 μg/mL in the presence of 2 μg/mL HSA carrier. The plates were then washed 3 times with PBS and blocked with HSA (20 mg/mL in PBS) for 2 hours at room temperature. To co-coat the adhesive spots with SDF-1, washed plates were coated with 2–10 μg/mL SDF-1 in PBS for 30 minutes at room temperature before being blocked with HSA. Substrates coated with either P-selectin– or E-selectin–IgG fusion proteins were prepared as described previously (20). Protein A (20 μg/mL in coating medium) was spotted onto a polystyrene plate and the substrate was blocked with 2% HSA in PBS. The protein A substrate was overlaid overnight at 4°C with supernatant containing 1–2 μg/mL of selectin fusion protein. All substrates were washed 5 times with PBS and blocked with 2% HSA in PBS before use. The plates were assembled as the lower stage of a parallel plate laminar flow chamber (260-μm gap) and mounted on the stage of an inverted phase contrast microscope (Diaphot 300; Nikon Inc., Tokyo, Japan) as described previously (19, 21). All flow experiments were performed at 37°C; temperature was maintained by warming the microscope stage with heating lamps in a humidified atmosphere. For adhesion experiments on resting or activated EC, primary HUVEC (passage 2 or 3) were plated at confluent density for 1 hour on FALCON tissue culture plates (Becton Dickinson UK Ltd., Plymouth, United Kingdom) spotted with human fibronectin (25 μg/mL in PBS). Nonadherent EC were gently rinsed out and adherent cells were grown on the fibronectin-coated spots for 24 hours before cytokine treatment. The EC monolayers were left intact or were stimulated for 18 hours with heparin-free culture media supplemented with TNF-α (2 ng/mL, 50 units/mL; R&D Systems Inc.). Before assays, the various EC-coated plates were washed 3 times with binding medium and assembled to form the lower wall of the flow chamber, where a portion of the monolayer (5 × 30 mm) was exposed to flow. The monolayer was perfused with 1 mL of binding medium to remove nonadherent material. CD34+ cells or PBL were perfused through the chamber at controlled flow rates, and cellular interactions on 2–4 different fields of view (each with an area of 0.17 mm2) were viewed through an objective lens (×10 or ×4). The perfusion periods were recorded in their entirety on videotape with a long-integration LIS-700 CCD video camera (Applitech, Holon, Israel) and an SVHS video recorder with time-lapse (AG-6730; Panasonic, Osaka, Japan).
Analysis of cell accumulation and resistance to detachment and rolling at elevated shear stress. Freshly isolated cells were stored at room temperature in H/H medium (cation-free HBSS containing 10 mM, pH 7.4 HEPES, and 2 mg/mL BSA) for up to 2 hours before use in the adhesion assays. CD34+ cells or PBL were resuspended at 5 × 105 to 5 × 106 cells/mL in binding medium (H/H supplemented with Ca2+ and Mg2+ at 1 mM each), and then perfused through the flow chamber at the desired shear stress. In all flow experiments using substrates coated with purified selectins, the binding medium included only 2 mM Ca2+. Shear stress was generated with an automated syringe pump (Harvard Apparatus Co., South Natick, Massachusetts, USA) attached to the outlet side of the flow chamber. For antibody inhibition studies, 2.5–5 × 106 cells/mL were preincubated for 5 minutes at 4°C in H/H medium with the mAb of interest (20 μg/mL), and then diluted 5-fold with warm binding medium (H/H medium containing 1 mM Ca2+ and 1 mM Mg2+) without washing out the antibodies. Intact or treated cells were perfused into the flow chamber at the desired flow rate. Adherent cells that had accumulated in the field of view during a 45-second perfusion period at a wall shear stress of 0.75 dyn/cm2 or 1 dyn/cm2 were subjected to detachment by incremented flow. The wall shear stress was increased stepwise every 5 seconds with a programmed set of flow rates delivered by the syringe pump. The first 3 increments were of 0.5 dyn/cm2 each; these were followed by 3 successive increments of 1 dyn/cm2, 3 increments of 2 dyn/cm2, and 3 increments of 3 dyn/cm2. All cells accumulating under flow on the adhesive substrates were manually tracked by analysis of images replayed directly from the monitor screen. During the detachment assay, the number of cells that remained bound at the end of each 5-second interval of incremented shear was expressed relative to the number of cells that accumulated on the adhesive ligand at the end of the first 45-second accumulation period. Detachment assays on HA were performed with CD34+ cells that bound at stasis to HA-coated plates for a 30-second period; bound cells were then subjected to wall shear stresses that were increased stepwise every 5 seconds as described above. The contribution of cells rolling into the observation field from upstream fields was minimized by locating the field at the upstream edge of the spot of adsorbed ligands. Fractions of rolling or stationary (arrested) cells within the cells adhered to the adhesive substrates were determined at different shear stresses during the detachment experiment. Rolling velocities were determined for cells during the incremented shear assay by analysis of cell displacements over 3–4 seconds.
Analysis of categories of tethered cells.
In some cases, the motion of cells that were stably tethered to the adhesive substrate at a shear stress of 1 dyn/cm2 was monitored from the initial tether point until the cell came to full arrest. Three categories of stable cell tethers to the adhesive substrates were defined. Tethers were defined as rolling motions if rolling persisted as the cell was subjected to increments of shear stress (each lasting 5 seconds) up to a shear stress of 2.5 dyn/cm2. Spontaneous arrests during rolling were defined as cells tethered at 1 dyn/cm2 that came to irreversible arrest during 5–40 seconds of rolling at 1 dyn/cm2 and that remained arrested when subjected to increments of shear stress up 2.5 dyn/cm2. The final category consisted of cells that, immediately upon tethering to the ligand-coated substrate under a shear stress of 1 dyn/cm2, came to full arrest and remained adherent up to a stress of 2.5 dyn/cm2. In each experiment, all events were normalized to a constant number of cells in the population that came into immediate proximity with the substrate.
Statistical analysis.
Data are expressed as mean ± SD or mean ± SEM. Statistical comparison of means was performed by a 2-tailed unpaired Student’s t test.
Results
SDF-1 is displayed constitutively by human BM endothelium.
The potential role of SDF-1 in human CD34+ progenitor adherence to BM endothelium has been studied in this work. We immunohistologically assayed the expression of SDF-1 by EC in biopsies of human BM. SDF-1 was expressed at high levels by BM venules. Anti–SDF-1 mAb reacted with EC lining the BM venules (Figure 1a, arrow), capillaries (Figure 1b, arrow), and sinusoids (data not shown), all of which have been recently reported to support stem cell rolling at sites of progenitor HPC emigration within the BM (22). These results suggest a key role for SDF-1 in the regulation of CD34+ progenitor recruitment to the human BM. Chemokines are implicated in the conversion of rolling interactions of circulating leukocytes into integrin-mediated arrest and emigration (6, 7). We therefore tested whether exposure of CD34+ cells to SDF-1 may drive rolling progenitors to develop firm adhesion to endothelial integrin ligands under physiological shear flow.
Figure 1.
BM venules and capillaries reactive to anti–SDF-1 in human BM sections. SDF-1 immunostaining (arrows) of venous (a) and capillary (b) BM endothelium. ×100 (original magnification). No peroxidase staining of control mAb was observed on identical tissue sections.
Human CD34+ cells tether and roll on endothelial selectins and HA at physiological shear stresses.
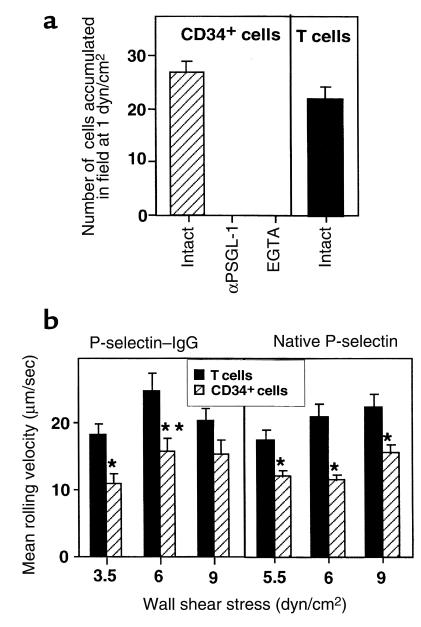
The potential for human CD34+ cells to roll on endothelial selectins expressed on BM vessel walls under physiological shear flow has not been studied before. We first characterized the efficiency by which CD34+ progenitors interact with purified selectins in vitro using parallel plate flow chamber assays designed to simulate the wall shear forces that exist in the BM microvasculature (4). Highly purified human CD34+ progenitors isolated from fresh cord blood were perfused over surfaces coated with physiological densities of endothelial selectins, either native or in the form of recombinant IgG fusion proteins. CD34+ cells express high and uniform levels of PSGL-1, the major P-selectin ligand, and highly heterogeneous levels of the cutaneous lymphocyte antigen (Figure 2a), a carbohydrate ligand whose expression on leukocytes closely correlates with their E-selectin binding activity (23–25). FACS® staining with an E-selectin–IgM chimera failed to detect high-affinity E-selectin ligands on human HPC, but revealed high-affinity ligands to a P-selectin–IgM chimera (26). Nevertheless, a similar fraction of CD34+ cells accumulated on both P-selectin and E-selectin substrates under different physiological shear stresses ranging between 0.5 dyn/cm2 and 2.5 dyn/cm2 (Figure 3a). All CD34+ cells tethered under flow to both selectins continued to roll on the selectins either immediately or when shear stresses were elevated (Figure 3b). Ca2+ chelation by EGTA abolished all adhesive interactions between CD34+ cells and P-selectin or E-selectin (Figure 4a and data not shown). P-selectin–mediated rolling of CD34+ cells was exclusively mediated by PSGL-1, the major P-selectin ligand on leukocytes (27, 28). This was confirmed by blocking CD34+ cell rolling on P-selectin with specific mAb to PSGL-1 (Figure 4a). When CD34+ cells accumulated on P-selectin or E-selectin were subjected to detachment by elevated shear forces, a higher fraction of CD34+ progenitors remained adherent to E-selectin than to P-selectin (Figure 3a). This was associated with a rolling velocity of CD34+ cells that was 2- to 2.5-fold lower on E-selectin than on P-selectin at every shear flow tested (Figure 3b). CD34+ cell rolling velocities on P-selectin increased by 50–80% when the site density of the selectin was reduced 2-fold (Figures 3b and 4b). However, neither P-selectin–mediated nor E-selectin–mediated rolling velocities varied with shear stress (Figures 3b and 4b). CD34+ cell rolling was comparable on both recombinant and native platelet-derived P-selectin (Figure 4b). The slower rolling and higher resistance to detachment of CD34+ cells adhered to E-selectin suggest that CD34+ cells express higher levels of adhesive ligands to E-selectin than adhesive ligands to P-selectin. CD34+ cells express moderate levels of L-selectin (29). Previously, L-selectin interactions with leukocyte ligands (including PSGL-1) were shown to prime homotypic interactions between myeloid cells and to augment secondary adhesion of circulating cells to adherent leukocytes and endothelium (30–32). In spite of the coexpression of functional PSGL-1 and L-selectin by CD34+ cells, we did not observe homotypic interactions between progenitors under any shear flow tested. These results and the previous findings by Mazo et al. (4) demonstrating the lack of endothelial ligands for L-selectin in the BM vasculature suggest that L-selectin plays little if any role in either primary or secondary adhesion of human CD34+ progenitors on the BM endothelium.
Figure 2.
Immunofluorescence flow cytometry of human CD34+ progenitors stained with antibodies to major vascular adhesion receptors and to the SDF-1 receptor CXCR4. (a) Cutaneous lymphocyte antigen (CLA) expression (detected by the HECA-452 mAb PSGL-1) and CD44 expression are shown. (b) Expression levels of the LFA-1 integrin CXCR4 and the integrin VLA-4. Negative control staining with nonbinding preimmune mouse IgG is shown in the filled histograms.
Figure 3.
CD34+ cells roll on endothelial selectins and CD44 ligand under physiological shear flow. (a) Accumulation of CD34+ cells continuously perfused on substrates coated with E-selectin–IgG or P-selectin–IgG at stepwise incremented shear stresses; resistance of accumulated cells to detachment by elevated shear stresses. Cells were perfused for 45 seconds at 1 dyn/cm2, and then the flow was increased by stepwise increments every 5 seconds. The number of cells bound at the end of each interval of incremented shear stress was determined as described in Methods. Data points are presented as mean ± SD of 4 fields of view. Note that cells continued to attach to the substrate to a maximum shear stress of 3 dyn/cm2. Selectin-IgG fusion proteins were each coated at 4 μg/mL on substrate-immobilized protein A, yielding 130 sites/μm2 of fusion protein, corresponding to 260 selectin sites/μm2. (b) Velocities of CD34+ cells rolling at representative shear stresses on the P-selectin–IgG or E-selectin–IgG substrate as described in a. Each mean value represents a minimum of 15 cells ± SEM, determined in 2 fields of view. (c) Effect of shear stress on the CD44-mediated rolling of CD34+ cells tethered to immobilized hyaluronan. CD34+ cells were allowed to settle for 30 seconds on HA (coated at 1 mg/mL) and then subjected to a shear stress of 0.5 dyn/cm2 for 5 seconds, followed by 3 increments each of 0.5 dyn/cm2, 1 dyn/cm2, 2 dyn/cm2, and 3 dyn/cm2, with each increment lasting 5 seconds. The number of cells remaining adherent at the end of the indicated shear interval was determined in 4 representative fields and was expressed relative to the number of cells adhering to the HA-coated substrate at a shear stress of 2.5 dyn/cm2. The percentage of rolling cells within the adherent cells at low and high shear stresses is shown above the data points. The mean velocity of 20 CD34+ cells rolling on HA at representative shear stresses is shown in black squares below the graph. SEM of mean velocities determined at 6.5 dyn/cm2 and 9.5 dyn/cm2 were 2.8 μm/s and 2.7 μm/s, respectively. The data shown in a–c are representative of 3 independent assays using CD34+ cells from different donors.
Figure 4.
CD34+ cells and adult PBL express comparable levels of functional P-selectin ligand. (a) Accumulation of CD34+ cells and PBL perfused in identical numbers over immobilized P-selectin–IgG coated at 2 μg/mL. In control experiments, cells were briefly pretreated with the PSGL-1–blocking mAb KPL-1(αPSGL-1), or were perfused in a cation-free binding medium in the presence of EGTA. (b) Velocities of CD34+ cells and PBL at representative shear stresses on P-selectin–IgG or native (platelet-purified) P-selectin coated on substrates at densities supporting a comparable strength of adhesion of each cell type. Each mean value represents a minimum of 15 cells ± SEM, determined in 2 fields of view. *P < 0.002. **P < 0.016.
Blocking of the vascular receptor CD44 on HPC has been shown to interfere with their engraftment in mouse BM (1). CD44 can support rolling interactions of tumor cells and subsets of activated lymphocytes on its carbohydrate ligand, hyaluronate, as well as on activated vascular endothelium (33–35). Although high and uniform CD44 levels were detected on human CD34+ cells (Figure 2a), only a fraction of cells adhered to purified HA; yet all adherent cells could roll on the CD44 ligand when subjected to elevated shear stresses (Figure 3c). CD34+ cell rolling on HA was entirely CD44 dependent, as verified by mAb blocking, and did not require divalent cations as did selectin-mediated rolling (data not shown). A fraction of cells arrested on HA without rolling even at elevated shear stresses, but more than 70% of the cells that remained adherent to HA in high physiological shear flow rolled on the CD44 ligand (Figure 3c). Adherent cells rolling on high-density HA were as resistant to detachment by physiological shear stresses as those with E-selectin–mediated rolling were (Figure 3, a and c); rolling velocities were comparable for E-selectin and CD44 (Figure 3, b and c). Neither CD34+ progenitors nor CD44-expressing lymphocytes adhered to other glycosaminoglycans such as heparin and chondroitin sulfate, which were identically immobilized on the substrate (data not shown). This is consistent with the notion that the bond between CD44 and HA is specialized in mediating rolling adhesions under shear flow. Nevertheless, CD44 failed to promote initial CD34+ progenitor tethering to HA at shear stresses higher than 1 dyn/cm2, in contrast to tethering to E-selectin and P-selectin (Figure 3a and data not shown). This suggests that CD44 interactions with HA are unlikely to take place in the absence of endothelial selectins, which are obligatory for initial cell tethering to BM endothelium.
CD34+ cells express moderate levels of the P-selectin ligand PSGL-1 (Figure 2a). PBL express about 10-fold higher levels of PSGL-1, as determined by FACS® staining (data not shown). However, only a portion of the protein is properly glycosylated and can bind P-selectin (36). It was therefore necessary to compare the level of adhesiveness to P-selectin of CD34+ cells and fresh PBL under physiological shear flow. Although similar fractions of CD34+ cells and PBL accumulated at low physiological flow on P-selectin (Figure 4a), CD34+ cells rolled on the selectin at significantly reduced velocities compared with PBL (Figure 4b). CD34+ cells also resisted detachment from P-selectin by elevated shear stresses more effectively than did PBL (data not shown). Because slower rolling and higher resistance to detachment correspond to a higher number of adhesive bonds (37), these data collectively suggest that CD34+ cells express more functional P-selectin ligands than do freshly isolated adult T lymphocytes.
SDF-1 triggers LFA-1–mediated firm adhesion to ICAM-1 of CD34+ cells and PBL rolling on P-selectin.
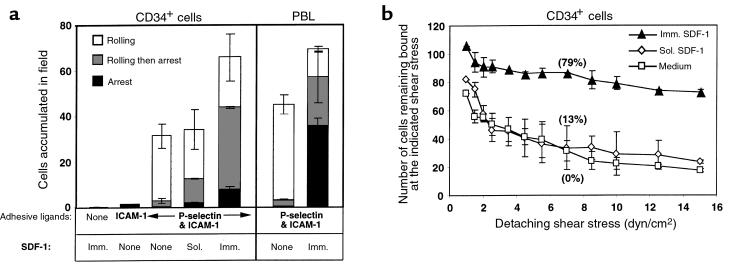
All CD34+ cells express moderate levels of the integrin LFA-1 (Figure 2b). In stasis, strong LFA-1–dependent adhesion of CD34+ cells to the major LFA-1 ligand ICAM-1 could be induced with short pretreatment with agonists such as phorbol esters and the LFA-1–activating mAb TS2.1 (data not shown). In contrast to the efficient attachment with which CD34+ cells tethered to endothelial selectins, CD34+ cells failed to adhere to substrates coated with ICAM-1 under physiological shear flow (Figure 5a) or at stasis (data not shown). However, when ICAM-1 was coimmobilized with P-selectin (P-selectin/ICAM-1), it supported efficient CD34+ cell tethering under physiological shear flow (Figure 5a). Nevertheless, the vast majority of tethered and rolling cells failed to spontaneously arrest on the substrate. The presence of ICAM-1 did not increase the percentage of CD34+ cells tethering to the substrate, which was comparable to that seen on P-selectin alone (data not shown). Short pretreatment of CD34+ cells with soluble SDF-1 for 1 minute did not increase the rate of cell tethering to the P-selectin/ICAM-1 substrate at 1 dyn/cm2, but caused about 30% of the cells accumulating on the adhesive substrate to arrest on the P-selectin/ICAM-1 substrate (Figure 5a). However, these arrested CD34+ cells did not develop firm adhesion to the substrate — at elevated shear stresses they exhibited poor resistance to detachment and resumed rolling, as did CD34+ cells accumulated and rolling on identical P-selectin/ICAM-1 substrates in the absence of SDF-1 (Figure 5b). Prolonged pretreatment of CD34+ cells with saturating levels of SDF-1 (1 μg/mL for up to 5 minutes) caused cell aggregation or deformation and reduced CD34+ progenitor accumulation on identical adhesive substrates (data not shown). In contrast to cells treated with soluble SDF-1, cells tethered to P-selectin/ICAM-1 substrate containing immobilized SDF-1 developed high adhesiveness to the substrate. About 60% of the cells accumulating on the P-selectin/ICAM-1/SDF-1 substrate came to full arrest either immediately or after a short period of rolling on the substrate (Figure 5a), and remained firmly adhered to the substrate even when the shear stress was elevated to the supraphysiological level of 15 dyn/cm2 (Figure 5b). The resistance to detachment by elevated shear stresses of cells tethered to P-selectin/ICAM-1 substrate containing immobilized SDF-1 was far greater than that of cells accumulated in flow on P-selectin/ICAM-1 alone or after treatment with soluble SDF-1 (Figure 5b). At all shear stresses tested, the vast majority of adherent cells remained arrested on the adhesive substrate that contained immobilized SDF-1 (Figure 5b and data not shown), but none of the cells adhering to the P-selectin/ICAM-1 substrate remained arrested in the absence of SDF-1 (Figure 5b). CD34+ cells accumulating on P-selectin/ICAM-1 substrate containing immobilized SDF-1 also readily spread on the substrate, whereas little or no spreading was detected of unstimulated CD34+ cells or of cells stimulated with soluble SDF-1 adhering to the P-selectin/ICAM-1 substrate (data not shown). Immobilized SDF-1 alone, on the other hand, failed to tether CD34+ cells under shear flow, although it slightly enhanced the attachment of CD34+ cells to ICAM-1 substrates (Figure 5a). In contrast to its dramatic effect on the conversion of rolling adhesions to firm arrests on P-selectin/ICAM-1, immobilized SDF-1 elevated only marginally the number of cells that initially accumulated on P-selectin/ICAM-1 (Figure 5b). Similar results were obtained with PBL: under identical experimental conditions, the vast majority of PBL rolling on P-selectin came to full arrest either immediately or after a short rolling period on the P-selectin/ICAM-1 substrate only when in the presence of coimmobilized SDF-1 (Figure 5a). Immobilized SDF-1 also stimulated firm, shear-resistant adhesion of PBL to the P-selectin/ICAM-1 substrate (Figure 5b); this adhesion was blocked by treatment with LFA-1–specific mAbs (data not shown). These experiments demonstrate a considerable synergy among P-selectin, ICAM-1, and immobilized SDF-1 with respect to the ability of CD34+ cells and of fresh lymphocytes to develop firm LFA-1–mediated arrest subsequent to rolling on P-selectin. Because soluble SDF-1 failed to stimulate LFA-1 adhesiveness with comparable efficiency to immobilized SDF-1, regardless of the time course of treatment, presentation of the chemokine on the adhesive surface appears to be crucial for its activity in stimulating firm integrin adhesion under physiological shear flow.
Figure 5.
Tethering and rolling on P-selectin are prerequisites for SDF-1–triggered firm arrest of CD34+ cells on ICAM-1–containing surfaces. (a) CD34+ cell and T lymphocyte tethering to various adhesive substrates containing P-selectin, SDF-1, and ICAM-1 at a shear stress of 1 dyn/cm2. Motions of individual cells tethered to the different substrates were monitored over a 45-second period, and were divided into 3 categories as described in Methods. The fraction of each category within each experimental group (i.e., rolling, rolling-associated arrests, and immediate arrests) is presented in the stacked bars. The categories were analyzed only for cells that remained bound to the substrate at a shear stress of 2.5 dyn/cm2. (b) Resistance to detachment by incremented shear stresses of CD34+ cells accumulated at 1 dyn/cm2 on P-selectin/ICAM-1. The absolute numbers of cells accumulated during 1 minute at 1 dyn/cm2 and the number of cells remaining bound at the end of a 5-second interval of each shear increment are depicted. The percentage of stationary (arrested) cells within the cells remaining bound at a median shear stress (7.5 dyn/cm2) is shown in parentheses near the data points for each experimental group. ICAM-1 and SDF-1 were coated at 0.4 μg/mL and 10 μg/mL, respectively. P-selectin/ICAM-1 spots were prepared by mixing P-selectin and ICAM-1 in PBS/1% octyl glucoside and diluting the mixture in coating medium to final concentrations of 1 μg/mL and 0.5 μg/mL, respectively. Substrates were washed and then coated with SDF-1 as described in Methods. To assess the effect of soluble SDF-1, cells were preincubated in binding medium containing 1 μg/mL SDF-1 for 1 minute and perfused unwashed over the P-selectin/ICAM-1 substrate. Results shown in a and b are presented as mean of 2 determinations ± range. Sol., soluble; Imm., immobilized.
VLA-4 supports stable tethering of CD34+ cells to VCAM-1 under flow that is augmented by SDF-1.
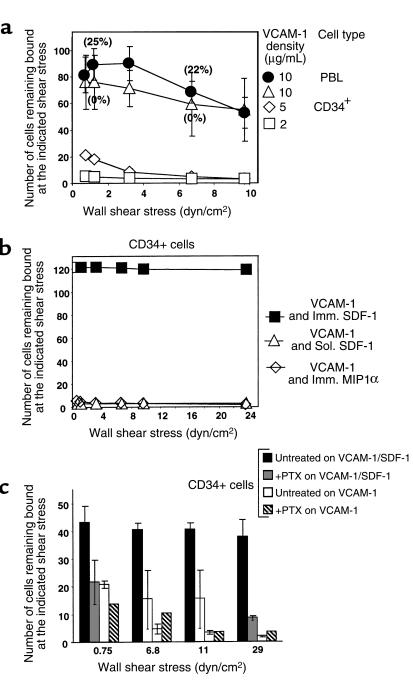
VLA-4 is a key CD34+ stem cell integrin (Figure 2b) that has recently been shown to work in parallel with selectins in promoting primary rolling adhesions of murine HPC on BM endothelium (4). We therefore asked whether VLA-4 on human CD34+ cells could support rolling adhesions on isolated VCAM-1, and whether SDF-1 could modulate VLA-4 adhesiveness to VCAM-1 under shear flow. Efficient tethering to VCAM-1 occurred at 0.75 dyn/cm2. This is a lower shear stress than that seen to allow CD34+ cell tethering to endothelial selectins, yet tethering efficiency of CD34+ cells was comparable to that of PBL under the same conditions (Figure 6a). Initial tethering on VCAM-1 was completely inhibited by treatment with mAb against the α4 integrin subunit as well as by EDTA chelation of divalent cations (data not shown). Tethering and accumulation of CD34+ cells varied strongly with the concentration of VCAM-1 coating on the substrate (Figure 6a and data not shown). Tethering was diminished on VCAM-1 coated at 0.5 μg/mL and lower concentrations, suggesting that VLA-4–mediated cell adhesion to VCAM-1 is supported by multivalent VLA-4:VCAM-1 bonds. After tethering to high-density VCAM-1, CD34+ cells spontaneously arrested on the VLA-4 ligand without rolling, even at elevated shear stresses. In contrast, PBL established both rolling adhesions and spontaneous arrests on various VCAM-1 substrates (Figure 6a; ref. 38).
Figure 6.
Immobilized SDF-1 stimulates CD34+ cell adhesion to VCAM-1. (a) Accumulation of CD34+ cells or PBL on sVCAM-1–coated substrates at 0.75 dyn/cm2, and resistance to detachment of accumulated cells by incremented shear stresses. (b) Effect of soluble (1 μg/mL) or immobilized SDF-1 or MIP-1α (co-coated at 2 μg/mL with sVCAM-1) on the accumulation of CD34+ cells at 0.75 dyn/cm2 and on the resistance of accumulated cells to detachment by elevated shear forces. (c) Inhibition by pertussis toxin (PTX) of SDF-1–triggered firm adhesion of CD34+ cells to sVCAM-1 alone or coated together with 2 μg/mL of SDF-1. In b and c, sVCAM-1 was coated at 2 μg/mL and 5 μg/mL, respectively.
To study the effect of SDF-1 on VLA-4–mediated accumulation and adhesion strengthening on VCAM-1 under shear flow, CD34+ cells were perfused at 0.75 dyn/cm2 on a substrate coated with low-density VCAM-1, which alone was incapable of supporting CD34+ cell accumulation under shear flow (Figure 6b). Brief preactivation of CD34+ cells with soluble SDF-1 failed to enhance the attachment of CD34+ cells or PBL to the VCAM-1 substrate. Reminiscent of the lack of effect of soluble SDF-1 on LFA-1 adhesiveness shown in Figure 6, prolonged pretreatment of CD34+ cells with soluble SDF-1 deformed the cells and reduced their VLA-4–dependent tethering to a level below that of intact cells (data not shown). In sharp contrast, immobilized SDF-1 induced a high fraction of the CD34+ cells tethered to VCAM-1 at 0.75 dyn/cm2 to immediately arrest on the VLA-4 ligand. All arrested cells developed high resistance to detachment by elevated shear forces (Figure 6b). A major portion of cells also readily spread on the VCAM-1/SDF-1 substrate (data not shown). The strong proadhesive effect of immobilized SDF-1 was G-protein sensitive, as shown by the fact that pertussis toxin treatment suppressed almost all SDF-1–stimulated adhesion to VCAM-1 (Figure 6c). In contrast, MIP-1α , a stimulant of β1 integrin–mediated CD34+ cell adhesion to fibronectin (39), failed to enhance adhesion of cells to VCAM-1 under shear flow (Figure 6b), although it stimulated VLA-4 adhesiveness of T cells to VCAM-1 under similar experimental conditions (data not shown). Platelet activating factor, a lipid stimulant of HPC differentiation (40), similarly failed to enhance CD34+ cell adhesion when coimmobilized with VCAM-1 (data not shown). This may reflect the lower expression levels of MIP-1α and platelet activating factor receptors on CD34+ cells relative to the levels of the SDF-1 receptor CXCR4. Indeed, chemokine triggering of integrin adhesiveness under shear flow requires a high level of functional chemoattractant receptors (41).
SDF-1 adsorbed onto TNF-activated HUVEC triggers firm adhesion of CD34+ cells under shear flow.
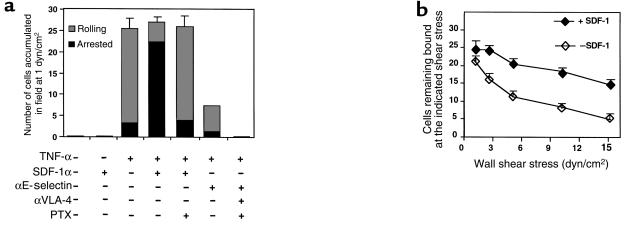
To elucidate the mechanism by which SDF-1 affects stem cell adhesiveness to endothelium under shear flow, we studied the interactions of cord blood–derived CD34+ progenitors with primary cytokine–activated HUVEC, a well-established vascular EC system. Upon cytokine activation, this endothelium expresses high levels of E-selectin, ICAM-1, and VCAM-1 (42, 43), and supports rolling and firm adhesion of leukocytes under physiological shear flow (44–46). Because the availability of human BM-derived EC is limited, we used this primary model as a surrogate for BM vascular endothelium. CD34+ cells accumulated efficiently on activated HUVEC under shear flow of 1 dyn/cm2, but failed to accumulate on resting HUVEC. The majority of CD34+ cells that accumulated on activated HUVEC at physiological shear flow started to roll on the endothelial monolayer when subjected to elevated shear stresses. CD34+ cell rolling on TNF-stimulated HUVEC was slow, with mean velocities similar to those observed in parallel experiments on purified E-selectin (data not shown). The majority of primary rolling adhesions to activated HUVEC were mediated by E-selectin (Figure 7a). The remaining rolling interactions of CD34+ cells with TNF-activated HUVEC pretreated with an E-selectin–blocking mAb were eliminated by blocking VLA-4 function on CD34+ cells (Figure 7a). Because VCAM-1 is the exclusive vascular ligand for VLA-4, these data suggest that endothelial VCAM-1 accounts for a small portion of CD34+ cell tethering and rolling in this vascular endothelium model, but together, VCAM-1 and E-selectin support all primary adhesions of CD34+ cells to TNF-activated HUVEC under physiological shear flow. Pretreatment of the activated endothelium with anti–P-selectin mAb did not interfere with CD34+ cell accumulation or rolling on the activated HUVEC, consistent with a lack of P-selectin, as verified by flow cytometry (data not shown). L-selectin ligands were also absent from the HUVEC cells tested in this study (data not shown), and appear to be absent from BM. Cytokine-activated endothelium expresses glycosaminoglycan ligands for CD44 that support rolling adhesions of lymphoid cells in a divalent cation–independent manner (35, 47). However, pretreatment of CD34+ cells with a CD44-blocking mAb did not affect tethering or rolling of CD34+ cells on TNF-stimulated HUVEC, indicating that this EC model does not express CD44 ligands that are functional under shear flow. Rolling of CD34+ cells on TNF-activated HUVEC was also eliminated by chelation of Ca2+ and Mg2+, consistent with exclusive roles for E-selectin and VLA-4, but not for CD44, in promoting rolling of CD34+ cells in this EC model. This is probably due to the absence of hyaluronan-like CD44 ligand on the lumenal surface of stimulated HUVEC under the experimental conditions.
Figure 7.
CD34+ cell accumulation and development of firm adhesion on TNF-activated HUVEC under physiological shear flow. (a) Accumulation of CD34+ cells on intact and TNF-activated HUVEC; effect of EC-associated SDF-1 and blocking mAb’s against E-selectin or VLA-4. Cells were perfused at 1 dyn/cm2 for 45 seconds and then subjected to incremented shear stresses as described in Methods. The number of cells accumulated on the different HUVEC monolayers under the indicated experimental conditions was determined in 4 representative fields of view. Accumulated cells were divided into 2 categories: firmly arrested cells were cells that came to full arrest during accumulation at 1 dyn/cm2 and remained bound and stationary throughout the detachment assay (i.e., at a shear stress of 15 dyn/cm2). Rolling cells were defined as cells that continued to roll on the HUVEC monolayer immediately after tethering at 1 dyn/cm2, or cells that began to roll on the EC at elevated shear stresses and either remained adherent or moved from the field of view. The fractions of arrested or rolling cells among the cells initially accumulated in the field of view are shown in the stacked bars. (b) Resistance to detachment by shear flow of CD34+ cells accumulated at 1 dyn/cm2 on TNF-activated HUVEC. The absolute number of cells accumulated during 1 minute at 1 dyn/cm2 and the number of cells remaining bound at the end of each shear increment (each lasting 5 seconds) are depicted. Values are given as mean ± range of determinations in 4 fields of view. One of 4 independent experiments.
We next asked whether SDF-1 made available to flowing CD34+ cells on the lumenal surface of activated HUVEC can induce firm adhesion of CD34+ cells under shear flow. Because TNF-activated HUVEC do not produce endogenous SDF-1, soluble SDF-1 was overlaid on the cytokine-activated HUVEC monolayer for several minutes, and free chemokine was removed by extensive washing with binding medium. The presence of SDF-1 did not enhance CD34+ cell accumulation on stimulated EC, but did increase the percentage of CD34+ cells that, upon tethering and rolling on HUVEC, became firmly adherent and remained arrested even at high shear stresses (Figure 7a). In the absence of SDF-1, 80% of the cells originally tethered to the TNF-activated EC continued to roll on it without coming to arrest (Figure 7a). SDF-1 also increased the resistance of cells to detachment or to clearance from the site of initial accumulation by accelerated rolling at elevated shear stresses (Figure 7b). The proadhesive effects of SDF-1 on CD34+ cell adhesion to activated HUVEC were totally inhibited by pretreating the cells with pertussis toxin (Figure 7a). TNF-stimulated HUVEC are known to produce various chemokines and lipid mediators, such as GRO and platelet activating factor (48–50). However, pertussis toxin treatment of CD34+ progenitors had no effect on the low levels of spontaneous progenitor arrest on TNF-activated HUVEC that were observed in the absence of SDF-1 (Figure 7a and data not shown). Taken together, these results suggest that neither chemokines nor other Gi-protein stimulants produced by TNF-activated HUVEC trigger firm integrin adhesion of CD34+ cells to the endothelium under shear flow. Notably, firm adhesion of CD34+ cells triggered by endothelium-associated SDF-1 was fully integrin dependent — it could be blocked by a mixture of anti–LFA-1 and anti–VLA-4 mAbs (data not shown). These results indicate that SDF-1 on activated HUVEC can stimulate CD34+ cell integrins under physiological shear flow. This stimulation is essential to translate selectin-mediated rolling into firm shear-resistant arrest of CD34+ cells on vascular endothelium expressing the integrin ligands ICAM-1 and VCAM-1.
Discussion
The BM microenvironment constitutes a homing compartment for transplanted human hematopoietic stem cells (1–3). The microvasculature in most organs expresses endothelial selectins and vascular integrin ligands only upon exposure to inflammatory stimuli (4, 7). However, BM venules and sinusoids constitutively express both endothelial selectins and integrin ligands such as VCAM-1 and ICAM-1 (51, 52), and have recently been shown to support rolling of HPC through these receptors (4, 5). Although establishment of rolling adhesions on the lining of BM microvessels could be a critical step in the homing of HPC to the BM, firm adhesion between these cells and the BM is probably mandatory for successful extravasation, as shown in numerous studies on leukocyte trafficking from the blood to sites of inflammation or to lymphoid organs (6, 7). Integrin-mediated firm arrest of human CD34+ progenitors could be crucial for these cells to initiate diapedesis through the vascular endothelium into the BM stroma. LFA-1 and VLA-4 are constitutively expressed by circulating cord blood CD34+ cells (53), but occur largely in inactive states and must be functionally upregulated during the initial adhesive contacts between the blood-borne cell and the BM endothelium, i.e., during the short period of cell rolling on the blood vessel wall. LFA-1 binding to endothelial ICAM-1 is the major adhesive interaction that mediates the firm arrest of leukocytes on vascular endothelium (54–56). ICAM-1 may therefore play a crucial role in the establishment of firm arrest of stem cells rolling on BM endothelium (51). Our recent studies have implicated both VLA-4 and LFA-1 in human CD34+ cell homing to murine BM of SCID/NOD mice (Peled et al., manuscript in preparation). We have also recently identified a crucial role for the BM-derived chemoattractant SDF-1 in the engraftment of human CD34+ cells in murine BM (8). Our present finding that SDF-1 is expressed at high levels on BM endothelium strongly suggests a potential role for this chemokine in stimulating firm integrin-dependent adhesion of circulating CD34+ cells to the BM microvasculature.
Chemokines are expressed by vascular endothelium at sites of inflammation and lymphoid organs through association with heparan sulfate proteoglycans (50, 57–60). The immunohistochemical staining of SDF-1 on BM sections suggests that the chemokine is produced by BM EC rather than being secreted by the extravascular BM tissue. It is likely that presentation of this chemokine by vascular proteoglycans helps to retain the chemokine on the walls of the BM vessels, preventing it from being washed away by blood flow (57). In fact, SDF-1 associates at high affinity with heparan sulfates (61). The chemokine clustering induced by these scaffolds may also potentiate the rapid signaling by the chemokine receptor that is required to trigger integrin activation on cells that are tethered to or rolling on the vascular endothelium under shear flow. The major role established in this study for SDF-1 displayed on stimulated EC, or in the context of surface-immobilized ICAM-1 and VCAM-1, is the induction of cellular arrest and firm adhesion of CD34+ cells to these ligands (Figures 5,6,7). Notably, although chemoattractants were reported to trigger integrin adhesiveness both in their soluble and surface-bound forms (19, 41, 62–65), we found that SDF-1 could upregulate integrin adhesiveness of CD34+ cells only when immobilized on the adhesive surface. SDF-1 lacked adhesive activity on its own; all its proadhesive effects involved coupling of G-protein signaling to integrin activation. Interestingly, pre-exposure of CD34+ cells to soluble SDF-1 did not stimulate VLA-4 or LFA-1 adhesiveness, regardless of the time course of cell exposure to the chemokine (0.5–5 minutes). It is unlikely that desensitization or internalization of SDF-1 receptor CXCR4, induced by the soluble chemokine, was responsible for this finding. CXCR4 desensitization by soluble SDF-1 occurs over minutes (data not shown), much more slowly than integrin activation by chemokines. Immobilized chemokines have been noted to activate integrin adhesiveness within subseconds of cellular contact (64 and Grabovsky et al., manuscript in preparation). Therefore, chemokines expressed at high localized density within the contact zone of the cell and the adhesive surface appear to trigger integrin-mediated adhesion more efficiently than do their soluble analogues. The ability of immobilized (but not soluble) SDF-1 to rapidly augment integrin adhesiveness of CD34+ cells within restricted contact zones also raises the intriguing possibility that SDF-1 must be juxtaposed to integrin ligands within these zones (48).
Our results predict that SDF-1 is a key, if not an exclusive, stimulant of human stem cell adherence to EC in the BM microvasculature. SDF-1 is the most potent proadhesive chemokine known to act on CD34+ cells (66, 67). Our study failed to demonstrate proadhesive effectiveness comparable to VLA-4 or LFA-1 activation on CD34+ cells by other weak HPC chemoattractants such as MIP-3β, MIP-1α, or the HPC stimulant platelet activating factor (39, 68, 69) under identical experimental conditions (Figure 6b and data not shown). Although stem cell factor — a potent HPC cytokine — can modulate integrin-dependent CD34+ cell migration and adhesion (67), it was incapable of rapidly triggering CD34+ cell adherence to ICAM-1 or VCAM-1 under shear flow when coimmobilized with these ligands. Also, we could not detect any proadhesive activity of chemokines produced in culture by TNF-activated HUVEC. Nevertheless, it is possible that unidentified BM-derived chemokines or cytokines synergize with SDF-1 in triggering integrin adhesion of CD34+ cells to the BM endothelium. Alternatively, these cytokines may contribute to postadhesion strengthening events of human CD34+ progenitors, such as cell spreading on the BM EC and diapedesis.
Our results suggest that the presence of either P-selectin or E-selectin is necessary for human CD34+ cells to initiate primary adhesion under flow, extending recent in vivo data on the central role of selectins in murine HPC rolling on BM vasculature (4). Although VCAM-1 has been implicated in primary murine HPC adhesion to BM, VCAM-1 alone or on stimulated HUVEC supported initial human CD34+ cell tethering with much lower efficiency than did E-selectin or P-selectin. In the presence of SDF-1, however, the ability of VCAM-1 to tether cells in physiological flow was significantly increased (Figure 6). In contrast, ICAM-1 alone or on stimulated HUVEC could not tether CD34+ progenitor cells under physiological shear flow, even when coimmobilized with SDF-1. These results implicate the endothelial selectins as the major adhesion receptors that control the initial tethering and rolling adhesions of human CD34+ cells on vascular endothelium. LFA-1 plays no role in primary adhesion, whereas VLA-4 plays a secondary role in promoting initial tethering or rolling, depending on the density of VCAM-1 or SDF-1 on the adhesive surface. Interactions between VLA-4 and VCAM-1 may work in parallel with the endothelial selectins in stabilizing rolling adhesions, particularly at sites where selectin expression is low. Our in vitro study also revealed that E-selectin supports considerably slower rolling of CD34+ cells than does P-selectin when both selectins are present at identical densities on an adhesive substrate. This observation is reminiscent of the slower E-selectin–mediated leukocyte rolling seen in various in vivo and in vitro settings (37, 70–72). E-selectin–mediated rolling has recently been shown to facilitate stable integrin-dependent leukocyte adhesion to inflamed microvascular endothelium (72). The slower E-selectin–mediated rolling of CD34+ cells may play a similar role, facilitating temporary arrest of CD34+ cells on the EC until complete stimulation of their integrins is successfully accomplished. This specialized role of E-selectin, and possibly of CD44, may be particularly important at sites that lack high levels of integrin ligands. Although we have demonstrated the ability of CD44 expressed on CD34+ cells to support slow rolling adhesion to its major ligand, hyaluronate, the presence and density of this ligand on BM EC must be further investigated. Both CD34+ cell subsets and the BM microvasculature vary in their expression of functional vascular counter-receptors (3, 4). Further studies will be required to elucidate the significance of the dynamic in vivo specialization between E-selectin, P-selectin, CD44, and VLA-4 revealed in the present study.
VLA-4 occurs in several activation states on resting leukocytes and contributes to tethering, rolling, and firm adhesion to VCAM-1 (38, 73, 74). We found the VLA-4 of resting CD34+ cells to require a high density of VCAM-1 to support initial tethering under physiological shear flow. VLA-4 also supported weak rolling of human CD34+ cells on HUVEC-expressing VCAM-1 (Figure 7) in the absence of functional endothelial selectins. Recent reports have consistently implicated VLA-4 as an important rolling receptor of murine HPC on vessel walls expressing VCAM-1, but its contribution to HPC rolling and homing to BM has been shown to be much more pronounced in the absence of endothelial selectins (4, 5). Our demonstration of the dramatic proadhesive effect of SDF-1 coimmobilized with VCAM-1 on a surface argues that endothelial SDF-1 may also contribute to in vivo VLA-4–mediated rolling of murine HPC in BM microvasculature (4). Further studies will be necessary to test this intriguing possibility — that SDF-1 may augment not only VLA-4–mediated arrest of HPC on BM endothelium, but may also regulate VLA-4 rolling on endothelial VCAM-1.
Several chemokines, including SDF-1, have recently been shown to convert L-selectin–mediated rolling of T lymphocytes on endothelial ligands into firm LFA-1–dependent arrests on ICAM-1 (60, 64). Our observations support the idea that SDF-1 can also promote firm LFA-1–dependent arrests of PBL tethered and rolling on P-selectin or E-selectin (Figure 5). Notably, PBL and CD34+ responded with comparable efficiency to SDF-1 stimulation of their integrin adhesiveness under identical experimental conditions. The repertoire of endothelial selectins and integrin ligands constitutively expressed by BM endothelium resembles that induced at sites of chronic inflammation, although the composition of chemokines produced at these sites is different than that of the BM (51). Although our results predict that subsets of CD34+ cells may roll on inflamed vessels, they may fail to arrest on inflamed endothelia due to insufficient chemokine stimulation. On the other hand, PBL subsets such as skin-homing lymphocytes, as well as monocytes, express high levels of ligands to E-selectin and P-selectin (24, 75). These mononuclear cells are responsive to SDF-1 (76) and express high levels of VLA-4 and LFA-1, and may therefore successfully arrest on BM endothelium. The failure to detect mononuclear cell recruitment to BM may reside in the inability of these cells to either extravasate through the BM endothelium or migrate through the BM microenvironment. However, our study raises the intriguing possibility that subsets of mononuclear cells that express high levels of SDF-1 receptors may successfully adhere to BM endothelium. The physiological implications of such adherence remain to be elucidated.
Acknowledgments
The authors wish to thank G. Kansas, I. Clark-Lewis, L. Klickstein, R. Lobb, R. McEver, and T. Kupper for providing reagents. We also thank Naomi Lanir for helping with HUVEC isolation, S. Schwarzbaum for editorial assistance, and D. Zipori and O. Lider for critical reading of the manuscript. Parts of this study were supported by the Israel Science Foundation (founded by the Israel Academy of Sciences and Humanities), and by the Minnerva Foundation (R. Alon). T. Lapidot is the incumbent Pauline Recanati Career Development Chair for Immunology. R. Alon is the incumbent Tauro Career Development Chair in Biomedical Research and is a recipient of the Yigal Allon Fellowship.
References
- 1.Vermeulen M, et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 2.Quesenberry PJ, Becker PS. Stem cell homing: rolling, crawling, and nesting. Proc Natl Acad Sci USA. 1998;95:15155–15157. doi: 10.1073/pnas.95.26.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verfaillie CM. Adhesion receptors as regulators of the hematopoietic process. Blood. 1998;92:2609–2612. [PubMed] [Google Scholar]
- 4.Mazo IB, et al. Hematopoietic progenitor cell rolling in bone marrow microvessels: parallel contributions by endothelial selectins and vascular cell adhesion molecule 1. J Exp Med. 1998;188:465–474. doi: 10.1084/jem.188.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci USA. 1998;95:14423–14428. doi: 10.1073/pnas.95.24.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 7.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;6:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 8.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 9.Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992;141:673–683. [PMC free article] [PubMed] [Google Scholar]
- 10.Lobb R, et al. Expression and functional characterization of a soluble form of vascular cell adhesion molecule 1. Biochem Biophys Res Commun. 1991;178:1498–1504. doi: 10.1016/0006-291x(91)91063-i. [DOI] [PubMed] [Google Scholar]
- 11.Rossiter H, et al. Skin disease-related T cells bind to endothelial selectins: expression of cutaneous lymphocyte antigen (CLA) predicts E-selectin but not P-selectin binding. Eur J Immunol. 1994;24:205–210. doi: 10.1002/eji.1830240132. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Madrid F, et al. VLA-3: a novel polypeptide associated with the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986;16:1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- 13.Lobb R, et al. Expression and functional characterization of a soluble form of endothelial-leukocyte adhesion molecule 1. J Immunol. 1991;147:124–129. [PubMed] [Google Scholar]
- 14.Geng JG, et al. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343:757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 15.Snapp KR, et al. A novel P-selectin glycoprotein ligand-1 (PSGL-1) monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood. 1997;91:154–164. [PubMed] [Google Scholar]
- 16.Coulomb-L’Hermin A, et al. Stromal cell-derived factor 1 (SDF-1) and antenatal human B cell lymphopoiesis: expression of SDF-1 by mesothelial cells and biliary ductal plate epithelial cells. Proc Natl Acad Sci USA. 1999;96:8585–8590. doi: 10.1073/pnas.96.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr MW, Alon R, Springer TA. The C-C chemokine MCP-1 differentially modulates the avidity of beta 1 and beta 2 integrins on T lymphocytes. Immunity. 1996;2:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 20.Fuhlbrigge RC, Alon R, Puri KD, Lowe JB, Springer TA. Sialylated, fucosylated ligands for L-selectin expressed on leukocytes mediate tethering and rolling adhesions in physiologic flow conditions. J Cell Biol. 1996;135:837–848. doi: 10.1083/jcb.135.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dwir O, et al. GlyCAM-1 supports leukocyte tethering and rolling: evidence for a greater dynamic stability of L-selectin rolling of lymphocytes than of neutrophils. Cell Adhes Commun. 1998;6:349–370. doi: 10.3109/15419069809010793. [DOI] [PubMed] [Google Scholar]
- 22.Mazo IB, von Andrian UH. Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol. 1999;66:25–32. doi: 10.1002/jlb.66.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Berg EL, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picker LJ, Kishimoto TK, Smith CW, Warnock RA, Butcher EC. ELAM-1 is an adhesion molecule for skin-homing T cells. Nature. 1991;349:796–799. doi: 10.1038/349796a0. [DOI] [PubMed] [Google Scholar]
- 25.Knibbs RN, et al. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zannettino AC, et al. Primitive human hematopoietic progenitors adhere to P-selectin (CD62P) Blood. 1995;85:3466–3477. [PubMed] [Google Scholar]
- 27.Moore KL, et al. P-selectin glycoprotein ligand-1 mediates rolling of human neutrophils on P-selectin. J Cell Biol. 1995;128:661–671. doi: 10.1083/jcb.128.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sako D, et al. A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell. 1995;83:323–331. doi: 10.1016/0092-8674(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 29.Dercksen MW, et al. Expression of adhesion molecules on CD34+ cells: CD34+ L-selectin+ cells predict a rapid platelet recovery after peripheral blood stem cell transplantation. Blood. 1995;85:3313–3319. [PubMed] [Google Scholar]
- 30.Bargatze RF, Kurk S, Butcher EC, Jutila MA. Neutrophils roll on adherent neutrophils bound to cytokine-induced endothelial cells via L-selectin on the rolling cells. J Exp Med. 1994;180:1785–1792. doi: 10.1084/jem.180.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alon R, Fuhlbrigge RC, Finger EB, Springer TA. Interactions through L-selectin between leukocytes and adherent leukocytes nucleate rolling adhesions on selectins and VCAM-1 in shear flow. J Cell Biol. 1996;135:849–865. doi: 10.1083/jcb.135.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walchek B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark RA, Alon R, Springer TA. CD44 and hyaluronan-dependent rolling interactions of lymphocytes on tonsillar stroma. J Cell Biol. 1996;134:1075–1087. doi: 10.1083/jcb.134.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeGrendele HC, Estess P, Picker LJ, Siegelman MH. CD44 and its ligand hyaluronate mediate rolling under physiologic flow: a novel lymphocyte-endothelial cell primary adhesion pathway. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Estess P, DeGrendele HC, Pascual V, Siegelman MH. Functional activation of lymphocyte CD44 in peripheral blood is a marker of autoimmune disease activity. J Clin Invest. 1998;102:1173–1182. doi: 10.1172/JCI4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEver RP, Cummings RD. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:485–492. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puri KD, Finger EB, Springer TA. The faster kinetics of L-selectin than of E-selectin and P-selectin rolling at comparable binding strength. J Immunol. 1996;158:405–413. [PubMed] [Google Scholar]
- 38.Alon R, et al. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Durig J, Testa NG, Heyworth CM. Distinct biological effects of macrophage inflammatory protein-1alpha and stroma-derived factor-1alpha on CD34+ hemopoietic cells. Stem Cells. 1999;17:62–71. doi: 10.1002/stem.170062. [DOI] [PubMed] [Google Scholar]
- 40.Dupuis F, et al. Effect of platelet-activating factor on the growth of human erythroid and myeloid CD34+ progenitors. Mediators Inflamm. 1998;7:99–103. doi: 10.1080/09629359891243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bevilacqua MP, Gimbrone MA., Jr Inducible endothelial functions in inflammation and coagulation. Semin Thromb Hemost. 1987;13:425–433. doi: 10.1055/s-2007-1003519. [DOI] [PubMed] [Google Scholar]
- 43.Rice GE, Munro JM, Bevilacqua MP. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. Science. 1990;246:1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luscinskas FW, et al. Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, beta 1-integrins, and beta 2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luscinskas FW, Ding H, Lichtman AH. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J Exp Med. 1995;181:1179–1186. doi: 10.1084/jem.181.3.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luscinskas FW, et al. L- and P-selectins, but not CD49d (VLA-4) integrins, mediate monocyte initial attachment to TNF-alpha-activated vascular endothelium under flow in vitro. J Immunol. 1996;157:326–335. [PubMed] [Google Scholar]
- 47.Catalina MD, Estess P, Siegelman MH. Selective requirements for leukocyte adhesion molecules in models of acute and chronic cutaneous inflammation: participation of E- and P- but not L-selectin. Blood. 1999;93:580–589. [PubMed] [Google Scholar]
- 48.Lorant DE, et al. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Utgaard JO, Jahnsen FL, Bakka A, Brandtzaeg P, Haraldsen G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J Exp Med. 1998;188:1751–1756. doi: 10.1084/jem.188.9.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber KS, von Hundelshausen P, Clark-Lewis I, Weber PC, Weber C. Differential immobilization and hierarchical involvement of chemokines in monocyte arrest and transmigration on inflamed endothelium in shear flow. Eur J Immunol. 1999;29:700–712. doi: 10.1002/(SICI)1521-4141(199902)29:02<700::AID-IMMU700>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 51.Schweitzer KM, et al. Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol. 1996;148:165–175. [PMC free article] [PubMed] [Google Scholar]
- 52.Frenette PS, Mayadas TN, Rayburn H, Hynes RO, Wagner DD. Susceptibility to infection and altered hematopoiesis in mice deficient in both P- and E-selectins. Cell. 1996;84:563–574. doi: 10.1016/s0092-8674(00)81032-6. [DOI] [PubMed] [Google Scholar]
- 53.Timeus F, et al. Cell adhesion molecule expression in cord blood CD34+ cells. Stem Cells. 1998;16:120–126. doi: 10.1002/stem.160120. [DOI] [PubMed] [Google Scholar]
- 54.Bargatze RF, Butcher EC. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer’s patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 56.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med. 1998;187:205–216. doi: 10.1084/jem.187.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanaka Y, et al. T-cell adhesion induced by proteoglycan-immobilized cytokine MIP-1β. Nature. 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 58.Middleton J, et al. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385–395. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 59.Gunn MD, et al. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Campbell JJ, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 61.Amara A, et al. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 62.Laudanna C, Campbell JJ, Butcher EC. Role of Rho in chemoattractant-activated leukocyte adhesion through integrins. Science. 1996;271:981–983. doi: 10.1126/science.271.5251.981. [DOI] [PubMed] [Google Scholar]
- 63.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 65.Gerszten RE, et al. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 66.Kim CH, Pelus LM, White JR, Broxmeyer HE. Macrophage-inflammatory protein-3 beta/EBI1-ligand chemokine/CK beta-11, a CC chemokine, is a chemoattractant with a specificity for macrophage progenitors among myeloid progenitor cells. J Immunol. 1998;161:2580–2585. [PubMed] [Google Scholar]
- 67.Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91:100–110. [PubMed] [Google Scholar]
- 68.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levesque JP, Haylock DN, Simmons PJ. Cytokine regulation of proliferation and cell adhesion are correlated events in human CD34+ hemopoietic progenitors. Blood. 1996;88:1168–1176. [PubMed] [Google Scholar]
- 70.Lawrence MB, Berg EL, Butcher EC, Springer TA. Rolling of lymphocytes and neutrophils on peripheral node addressin and subsequent arrest on ICAM-1 in shear flow. Eur J Immunol. 1995;25:1025–1031. doi: 10.1002/eji.1830250425. [DOI] [PubMed] [Google Scholar]
- 71.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- 72.Milstone DS, et al. Mice lacking E-selectin show normal numbers of rolling leukocytes but reduced leukocyte stable arrest on cytokine-activated microvascular endothelium. Microcirculation. 1998;5:153–171. [PubMed] [Google Scholar]
- 73.Berlin C, et al. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;74:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 74.Chen C, et al. High affinity VLA-4 subsets expressed on T cells are mandatory for spontaneous adhesion strengthening but not for rolling on VCAM-1 in shear flow. J Immunol. 1999;162:1084–1095. [PubMed] [Google Scholar]
- 75.Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on alpha1, 3-fucosyltransferase VII gene expression. J Exp Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor (SDF-1) J Exp Med. 1996;184:1–9>. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]