Abstract
The pathogenesis of atherosclerosis and abdominal aortic aneurysm involves breakdown of the elastic laminae. Elastolytic cysteine proteases, including cathepsins S and K, are overexpressed at sites of arterial elastin damage, but whether endogenous local inhibitors counterbalance these proteases is unknown. We show here that, whereas cystatin C is normally expressed in vascular wall smooth muscle cells (SMCs), this cysteine protease inhibitor is severely reduced in both atherosclerotic and aneurysmal aortic lesions. Furthermore, increased abdominal aortic diameter among 122 patients screened by ultrasonography correlated inversely with serum cystatin C levels. In vitro, cytokine-stimulated vascular SMCs secrete cathepsins, whose elastolytic activity could be blocked when cystatin C secretion was induced by treatment with TGF-β1. The findings highlight a potentially important role for imbalance between cysteine proteases and cystatin C in arterial wall remodeling and establish that cystatin C deficiency occurs in vascular disease.
Introduction
Atherosclerosis and abdominal aortic aneurysm (AAA) are inflammatory diseases that involve extensive extracellular matrix degradation and vascular wall remodeling. Cardiovascular events correlated with the presence of inflammation and atheroma tend to rupture at sites of matrix remodeling (1, 2). The exact proteases involved in these pathological events are currently unknown. Previous studies have implicated both matrix metalloproteases (MMPs) (3, 4) and serine proteases (5, 6). Recently, our laboratory isolated 2 potent elastolytic cysteine proteases, cathepsins S and K (7, 8), and have demonstrated overexpression of these proteases in atherosclerotic lesions compared with normal arteries. Macrophages and smooth muscle cells (SMCs) in atherosclerotic plaques express cathepsins S and K (9). In the presence of proinflammatory cytokines found in atheroma, cultured vascular SMCs secrete active cathepsin S capable of degrading extracellular elastin (9). Monocyte-derived macrophages also degrade elastin through release of active cathepsins (10). Thus, elastolytic and collagenolytic cysteine proteases likely participate in vascular wall remodeling.
By regulating protease activities, protease inhibitors also play a pivotal role in tissue remodeling (11). The most abundant extracellular inhibitor of cysteine proteases is cystatin C, a 13-kDa protein constitutively secreted shortly after its synthesis (12, 13). Cystatin C belongs to the type 2 cystatin gene family, and clusters together with other members including cystatins D, S, SA, and SN on chromosome 20 (14). Cystatins D, S, and SA are primarily expressed in salivary glands, cystatin C is expressed in virtually all organs of the body. Owing to its high concentration in biologic fluids, cystatin C is probably one of the most important extracellular inhibitors of cysteine proteases (15–19). Aside from a rare mutation in cystatin C that leads to its precipitation as amyloid in cerebral blood vessels and causes cerebral hemorrhage (20), no evidence thus far has implicated cystatin C in disease. In vitro, alveolar macro-phages from cigarette smokers or monocytes stimulated by IFN-γ secrete less cystatin C than unstimulated macrophages or monocytes, raising the possibility of reduced cystatin C levels at sites of inflammation (21, 22). Whether cystatin C expression actually changes in situ in diseases in which inflammation is prominent, and how changes in cystatin C levels might be affected, are currently unknown.
This study explored the possibility that cystatin C is deficient in diseased arteries. Normal SMCs highly express cystatin C, raising the possibility that this inhibitor could counterbalance augmented cysteine proteases. Surprisingly, initial immunohistochemical analyses of samples from atherosclerotic plaques revealed virtually no cystatin C antigen within plaques. This result prompted further study of the amounts of cystatin C in both atherosclerotic plaques and aneurysmal tissues, as well as measurement of circulating levels of cystatin C in patients with normal or dilated aortas. We also examined the regulation of cystatin C expression by vascular SMCs in vitro, as well as the capacity of pericellular cystatin C levels to influence the elastolytic activity of these cells. The results reveal a previously unsuspected protective role for cystatin C in vascular remodeling and indicate that a cytokine whose circulating levels are reported to be depressed in atherosclerosis — TGF-β1 (23) — is a major inducer of SMC cystatin C secretion.
Methods
Immunohistochemistry.
Serial cryostat sections (6 μm) of human atherosclerotic plaques from coronary (n = 10) and carotid (n = 6) arteries, aortic aneurysms (n = 6), and nonatherosclerotic arteries (carotids from autopsies [n = 4] and aortas from cardiac transplantation donors [n = 4]) were fixed in acetone (–20°C, 5 minutes), air-dried, and stained by an avidin-biotin-peroxidase method as described previously (9). Tissue sections were treated with 0.3% hydrogen peroxide to inhibit endogenous peroxidase activity, followed by primary antibodies diluted in PBS supplemented with 4% species-appropriate normal serum. The subsequent processing was performed according to the manufacturer’s recommendations (Universal DAKO LSAB kit, peroxidase; DAKO Corp., Carpinteria, California, USA). The reaction was viewed with 3-amino-9-ethylcarbazole (Sigma Chemical Co., St. Louis, Missouri, USA). Sections were counterstained with Gill’s hematoxylin solution (Sigma Chemical Co.). Cell types were identified with monoclonal anti-muscle actin HHF-35 (Enzo Diagnostics Inc., Syosset, New York, USA) or monoclonal anti-human CD68 (macrophages).
Western blot analysis.
Frozen atherosclerotic plaques (n = 7), AAA aortas (n = 4), and normal arteries (n = 11) were pulverized and lysed into buffer containing 1% Triton X-100, 40 mM sodium acetate, and 1 mM EDTA (pH 5.0). After incubation at 37°C for 1 hour, lysate protein concentrations were determined using Bio-Rad Dc Protein Assay kit according to the manufacturer’s directions (Bio-Rad Laboratories Inc., Hercules, California, USA). Thirty micrograms of protein from each sample was separated on SDS-PAGE, blotted onto nitrocellulose filter, and probed with rabbit anti-human cystatin C (1:1,000; DAKO Corp.) or human cathepsin S (1:1,000).
Human saphenous vein SMCs were cultured in a 6-well plate in DMEM containing 10% FBS until confluent. Cells were then cultured in serum-free DMEM for 24 hours, followed by stimulation with IFN-γ (500 U/mL) for an additional 24 hours in the presence or absence of 10 ng/mL TGF-β1. Culture medium was then collected and centrifuged at 16,000 g for 10 minutes. Proteins were precipitated with 20% trichloroacetic acid on ice for 30 minutes followed by repeat centrifugation. The protein pellet was briefly washed with 100% ethanol and resuspended in electrophoresis sample buffer. SMCs were lysed directly into sample buffer after 2 washes with 1× PBS. Both cell lysates and proteins from culture medium were separated on SDS-PAGE for immunoblot analysis with antibodies against human cathepsin S and cystatin C as already described here. All Western blots were developed with Western Blot Chemiluminescence Reagent (NEN Life Science Products Inc., Boston, Massachusetts, USA).
Northern blot analysis.
After IFN-γ and TGF-β1 treatments, 1.5 × 107 SMCs were lysed in 5 mL of guanidium isothiocyanate lysis buffer for RNA preparation as described elsewhere (9). Twenty micrograms of total RNA from SMCs was separated on 1.2% agarose gel and blotted on Zeta-Probe GT blotting membrane (Bio-Rad Laboratories Inc.). A human cystatin C probe was generated by PCR using cystatin C cDNA (a kind gift from M. Abrahamson, University of Lund, Lund, Sweden). The 500-bp cystatin C cDNA fragment and cathepsin S cDNA probe (9) were labeled with [32P]dCTP and purified with NENSORB 20 column (NEN Life Science Products Inc.) before hybridizing as described elsewhere (9).
Elastase assay.
SMCs were cultured on 24-well plates in 10% FBS DMEM until 100% confluence (5 × 105 cells per well). Cells were then cultured in serum-free DMEM for 24 hours before stimulation with IFN-γ (500 U/mL) and/or TGF-β1 (1–25 ng/mL). Bovine neck ligament elastins (Elastin Products Inc., Pacific, Missouri, USA) were tritiated with [3H]sodium borohydride (NEN Life Science Products Inc.) as described previously (24). Both stimulated and nonstimulated SMCs were incubated with 300 μg [3H]elastin (1,300 cpm/μg) for 3 days at 37°C. The inhibitory effect of cystatin C was examined by incubating SMCs with recombinant cystatin C (0–500 ng/mL) or rabbit anti-human cystatin C IgG (100 μg/mL; Cortex Biochem, San Leandro, California, USA). Culture media were then collected by centrifugation at 16,000 g for 15 minutes. The digested elastin radioactivity was counted using 200 μL of medium, and the data were presented as micrograms of elastin degraded per 106 cells in 24 hours.
Patient population and ELISA.
Outpatients older than 50 years of age, referred to the Noninvasive Cardiac Laboratory of the Brigham and Women’s Hospital for a transthoracic echocardiogram, were invited to participate in a study examining markers of vascular disease (25, 26). Patients with a clinical history consistent with active infection, systemic inflammatory disease, or heart transplant and those taking corticosteroids were excluded. Overall, 122 eligible patients agreed to participate and constituted the study population. Ultrasound evaluations were performed by 2 experienced ultrasonographers, using commercially available equipment (Hewlett Packard Sonos 2500; Hewlett Packard Medical Products, Andover, Massachusetts, USA) and a 2.7/3.5 MHz phase-array transducer (for the aorta) or a 5.5/7.0 MHz linear transducer (for the carotid imaging) as described elsewhere (26). Before the imaging procedures, individual data concerning atherosclerosis risk factors, prior cardiovascular history, and other comorbidities were obtained. The protocol was reviewed and approved by the Human Research Committee of the Brigham and Women’s Hospital, and informed consent was obtained for all patients. Among these subjects, 8 patients were identified with abdominal aortic dilatation as defined with periumbilical aorta diameter larger than 2.5 cm. Serum samples from all patients were collected and used for cystatin C ELISA as described elsewhere (17–19). Briefly, 96-well immunosorb plates (NUNC Co., Naperville, Illinois, USA) were coated with cystatin C polyclonal IgG (10 μg/mL; Cortex Biochem) in NaHCO3 (pH 8.2) overnight at 4°C. After wash and 3% BSA blocking, plates were incubated with diluted human serum samples and were again incubated overnight at 4°C. Cystatin C mAb’s (1 μg/mL) were used as detecting antibody followed with peroxidase-conjugated goat anti-mouse IgG/IgM (1:1,000; Kirkegaard & Perry Laboratories, Gaithersburg, Maryland, USA). ELISA plates were developed with OPD (Sigma Chemical Co.). Recombinant cystatin C was used as standard of each plate.
Results
Immunostaining of normal vessels and atherosclerotic plaques.
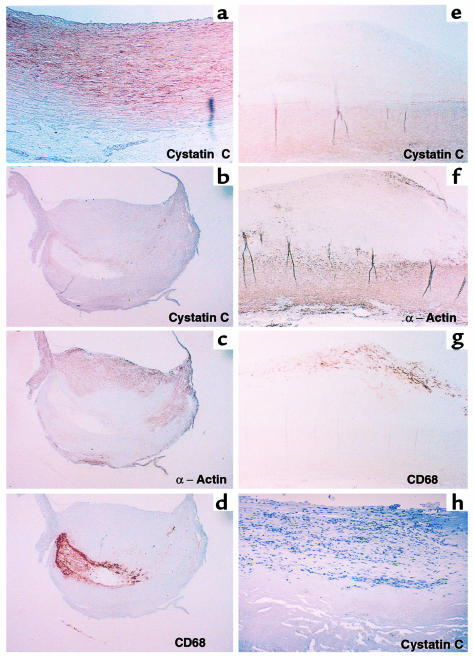
Atherosclerotic plaques overexpress elastolytic cathepsins S and K (9). To examine whether the lesions also exhibit altered levels of their endogenous inhibitor cystatin C, both plaques and normal vessels were immunostained with rabbit anti-human cystatin C polyclonal antibodies. Normal arteries had abundant cystatin C staining (n = 6) (Figure 1a). However, atherosclerotic plaques (n = 10) showed little immunoreactive cystatin C (Figure 1b). Macrophages and SMCs in the developing plaques were identified by immunostaining plaques with mouse anti-human CD68 (macrophage-specific) and mouse anti-human α-actin (SMC-specific) antibodies. As expected, vivid CD68 staining was found at the lipid core and shoulder areas of the plaque (Figure 1d), whereas α-actin staining was found mostly in the fibrous cap (Figure 1c). The specificity of immunohistochemistry was confirmed using nonimmune rabbit serum (data not shown). Interestingly, the level of cystatin C in atherosclerotic plaques appeared to correlate inversely with disease progression. Early stages of plaque development (fatty streaks; n = 4) showed very little immunodetectable cystatin C, whereas SMC of underlying media (α-actin positive; Figure 1f) demonstrated stronger staining for cystatin C (Figure 1e). AAAs (n = 6) displayed a similar lack of cystatin C staining (Figure 1h) as well as increased cathepsin S immunostaining (data not shown). Thus, AAAs and atherosclerotic plaques contain scant cystatin C.
Figure 1.
Decreased expression of cystatin C in atherosclerotic plaques and AAAs. Frozen sections of normal arteries (×100) (a), atherosclerotic plaques (×20) (b–d), fatty streaks (n = 4; ×40) (e–g), and AAAs (×100) (h) were used for immunohistochemistry with rabbit anti–cystatin C polyclonal antibodies (a, b, e, and h). The distribution of SMCs and macrophages within the lesions was determined by cellular staining with α-actin (c and f) and CD68 mAb’s (d and g), respectively.
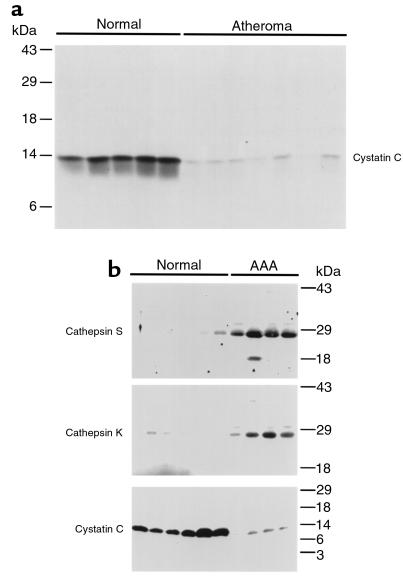
To explore biochemically the apparent cystatin C deficiency in these lesions, protein extracts of atherosclerotic artery (n = 7), aneurysmal tissue (n = 4), and normal control vessel (n = 11) were examined by immunoblotting with cathepsin and cystatin C antibodies. Similar to the findings with immunostaining (Figure 1), the immunoblot analysis with cystatin C antibodies showed a dramatic decrease of 13-kDa cystatin C antigen in atherosclerotic plaque extracts (Figure 2a) and AAA extracts (Figure 2b) compared with normal arteries. As expected, cathepsin S and K expression (Figure 2b) in AAA extracts was increased, consistent with previously reported data for atherosclerotic plaques (9). These findings provide further evidence for a disturbed balance between proteases and antiproteases involving elastolytic and collagenolytic cysteine proteases in these diseased vessel walls.
Figure 2.
Immunoblotting of normal arteries, atherosclerotic plaques, and aortic aneurysms. Equivalent total protein from detergent extracts of normal arteries (n = 11), atherosclerotic plaques (n = 7), and AAAs (n = 4) was separated on SDS-PAGE and immunoblotted with rabbit polyclonal antibodies against cystatin C or cathepsins S and K as indicated in the figure. Both atherosclerotic plaques (a) and aortic aneurysms (b) demonstrated markedly decreased cystatin C antigen, whereas both cathepsins S and K were increased in aneurysmal aortas (b).
Correlation between AAA aorta diameter and serum cystatin C levels.
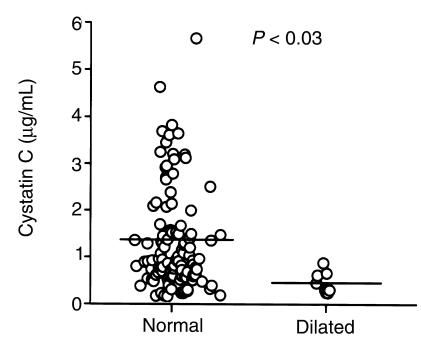
On the basis of evidence detailed here indicating a deficiency of cystatin C in aneurysmal aortic tissue, we explored the possibility that circulating levels of cystatin C may relate to the development of AAAs. A cohort of patients (n = 122) referred to an outpatient cardiology clinic for echocardiographic testing underwent measurements of carotid artery intimal to medial thickness (IMT) ratios and aortic diameter. Correlations between these measurements and circulating indices of inflammation in this cohort have been published previously (25, 26). We measured the serum cystatin C levels in these patients using a cystatin C ELISA. This method has been previously used to quantify cystatin C levels in human gingiva (17), cerebrospinal fluid (18), and normal serum (19). The serum cystatin C levels were quantitated against a recombinant cystatin C standard. No significant correlation between IMT and cystatin C levels was apparent. However, a significant negative correlation of abdominal aortic diameters with serum cystatin C levels was observed (P < 0.03; r = 0.203). The correlation remained significant when aortic diameter was corrected for body surface area and when cystatin C was normalized to serum creatinine, glomerular filtration being a known determinant of cystatin C levels (27) (not shown). Although the number of patients with frankly dilated aortas (> 2.5 cm) is small (n = 8), all patients with dilated aortas had relatively low circulating cystatin C (0.516 ± 0.233 μg/mL; mean ± SD, Mann-Whitney) (Figure 3). As a group, these subjects had significantly lower serum cystatin C levels than that of subjects with aortic diameters less than 2.5 cm (1.303 ± 1.064 μg/mL; mean ± SD; P < 0.03) (Figure 3). There was no significant association of cystatin C with age, race, sex, history of smoking, diabetes, myocardial infarction, revascularization, or lipoprotein levels. Thus, these findings concur with biochemical evidence indicating that a disruption in the balance between cysteine protease expression and cystatin C levels may play a role in vascular remodeling.
Figure 3.
Comparison of serum cystatin C levels between patients with dilated (diameter > 2.5 cm) and normal aorta (diameter < 2.5 cm). A cohort of outpatients (n = 122) underwent ultrasound measurements of their abdominal aortas. Patients with dilated aorta (n = 8) had lower serum cystatin C (0.516 ± 0.233 μg/mL; mean ± SD) than patients with normal aorta (n = 114) (1.303 ± 1.064 μg/mL; mean ± SD; P < 0.03, Mann-Whitney). The aortic diameters of all subjects were inversely correlated with serum cystatin C levels (P < 0.03; r = 0.203; simple regression analysis) in blood drawn at the time of the outpatient visit. A similar correlation (P < 0.04) was observed with indexed aortic diameter (diameter/body surface area) or with aortic diameter as a function of cystatin C corrected for serum creatinine, a known determinant of cystatin C levels (data not shown).
Cystatin C expression and regulation of vascular SMC elastolytic activity.
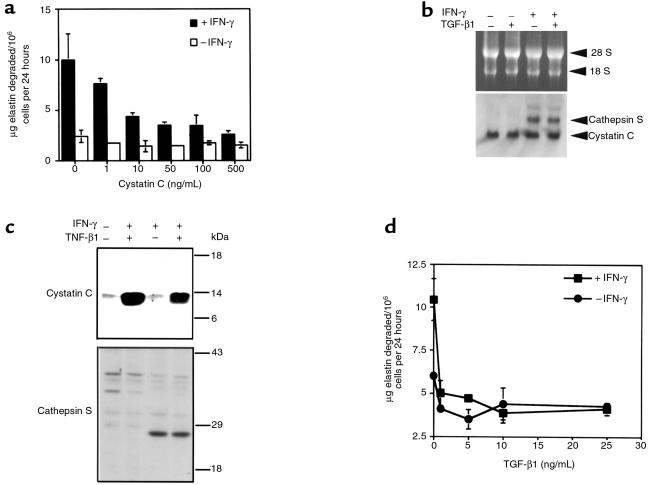
Prior studies documenting the capacity of macrophages to use cysteine proteases to degrade elastin found macrophage elastolytic activity to be insensitive to the presence of pericellular serum protease inhibitors (10, 28). These findings raise questions as to whether, in spite of the marked reduction of cystatin C in atherosclerotic and aneurysmal lesions and the clinical correlation (Figures 1, 2, 3), cystatin C can actually regulate vessel wall matrix remodeling by vascular cells. To address this uncertainty, the influence of cystatin C on the elastolytic activity of cultured vascular SMCs was examined. Vascular SMCs were stimulated with IFN-γ (500 U/mL) for 24 hours to augment cathepsin S–dependent elastase activity (9). In subsequent cocultures with insoluble elastin, the addition of as little as 10 ng/mL of recombinant cystatin C inhibited elastin degradation (Figure 4a). Higher concentrations of cystatin C blocked more than 75% of the IFN-γ–induced activity, confirming that SMC elastase activity depends largely on elastolytic cysteine proteases.
Figure 4.
Regulation of SMCs elastase activity by cystatin C. (a) SMC elastase activity is inhibited by recombinant cystatin C. Interferon-γ stimulated SMCs were cocultured in serum-free medium with insoluble elastin in the presence of increasing concentrations of cystatin C as indicated in the panel. After 72 hours, media were collected and soluble radioactivity measured as an index of elastin degradation. (b) TGF-β1 does not alter cystatin C and cathepsin S mRNA levels. Northern blot analysis of SMC stimulated with IFN-γ (500 U/mL) or TGF-β1 (10 ng/mL) for 24 hours. (c) TGF-β1 stimulates SMC cystatin C secretion. Cells were stimulated with TGF-β1 as described in the text and media collected for immunoblots after 24 hours. As previously reported (9), IFN-γ induced cathepsin S mRNA (b) and secretion of active cathepsin S (c) but had no effect on cystatin C secretion. (d) TGF-β1 blocks SMC elastase activity. IFN-stimulated SMCs were cocultured with elastin as described in the text in the presence of increasing amounts of TGF-β1 and elastin degradation quantified after 72 hours.
Given that normal medial SMCs express cystatin C (Figure 1), we next examined the regulation of cystatin C secretion by cultured SMCs. Total RNAs were isolated from control and IFN-γ−stimulated SMCs for Northern blot analysis. As expected, cathepsin S mRNA was markedly increased in stimulated cells compared with controls. However, the level of cystatin C mRNA was unchanged (Figure 4b), consistent with the constitutive nature of the cystatin C promoter (29). To explore further the regulation of cystatin C expression in SMC, we also tested a cytokine previously reported to induce other classes of protease inhibitors, TGF-β1 (30–32). TGF-β1 (5 ng/mL) did not change the level of cathepsin S mRNA or protein or of cystatin C mRNA (Figure 4b). However, TGF-β1 markedly increased the level of secreted cystatin C (Figure 4c, top panel). Additional experiments indicate that cystatin C biosynthesis increases as little as 1 hour after exposure of SMCs to TGF-β1 (not shown). Consistent with this observation, as little as 1 ng/mL of recombinant TGF-β1 almost completely blocked the elastase activity of IFN-γ−stimulated SMCs (Figure 4d). To test further the link between the elastase “protective” effect of TGF-β1 and increased cystatin C secretion, INF-γ–induced SMCs from 2 individuals were cultured with 10 ng/mL of TGF-β1 and 100 μg/mL of rabbit anti-human cystatin C IgG (Cortex Biochem) or normal rabbit IgG (Sigma Chemical Co.). Addition of cystatin C antibodies completely restored the elastase activity induced by IFN-γ in the presence of TGF-β1 (9.803 ± 2.79 μg elastin degraded per 106 cells per 24 hours; mean ± SD) to that of SMCs stimulated by IFN-γ alone (10.00 ± 3.4). In contrast, the control IgG had no effect on elastin degradation by either cytokine-stimulated or unstimulated SMCs. Thus, cytokine-induced SMC elastase activity depends on pericellular cystatin C levels, and TGF-β1 can inhibit elastin degradation by induction of cystatin C secretion.
Discussion
Imbalance of proteolytic and antiproteolytic levels may result in lung destruction (33, 34), tumor progression (35, 36), and possibly vascular wall remodeling (37–41). Several lines of evidence reported in this study indicate that the severely depressed levels of cystatin C found in lesions of patients with atherosclerosis and aortic aneurysms contribute to progression of vascular injury. First, cysteine proteases with potent elastase and collagenase activities are markedly elevated at sites of elastin and collagen degradation, both in advanced plaques in vascular arteries susceptible to rupture and in aneurysms of the aorta, a large elastic vessel where there is massive elastin breakdown (ref. 9; Figure 2). Second, IFN-γ−stimulated SMCs secrete an elastolytic cathepsin, cathepsin S, and IFN-γ is present within diseased arteries (42, 43), providing a basis for cysteine proteases to overwhelm residual pericellular cystatin C. Third, we observed a significant inverse correlation between blood cystatin C levels and aortic dilatation, consistent with a protective effect of cystatin C on matrix breakdown (Figure 3). And finally, a cytokine identified in this study as a major inducer of SMC cystatin C secretion, TGF-β1 (Figure 4), is reportedly severely depressed in plasma of patients with atherosclerosis (23). Taken together, these findings indicate that deficiency of cystatin C likely contributes to progression of vascular remodeling and, by inference, that elastolytic cysteine proteases are also important contributors to the pathophysiology of these diseases.
The severe depression of cystatin C antigen levels within SMCs of atherosclerotic plaques implies a marked change in the functional properties of plaque SMC. Normal medial SMCs express abundant cystatin C (Figure 1). Such changes in the phenotypic properties of these SMCs are consistent with other evidence that migrating vascular SMCs have protease and protease inhibitor profiles distinctly different from resident cells of the vessel wall. SMCs in atheromatous arteries have augmented MMP levels (44, 45). Macrophages within atherosclerotic and aneurysmal tissues also exhibit increased expression of MMPs (46, 47). However, in contrast to results reported here for cystatin C, tissue inhibitors of matrix metalloproteinases (TIMPs) have been found to be either unchanged or overexpressed in both atherosclerotic lesions (38, 39) and AAAs (48, 49). Inflammatory cytokines and growth factors increase the expression of TIMP-1 and TIMP-3 in atherosclerotic lesion–related macrophages and vascular SMCs (38).
To our knowledge, the marked suppression of cystatin C concurrent with augmented expression of cysteine proteases observed in our studies of atherosclerosis and abdominal aneurysms represents the first acquired cysteine protease inhibitor deficiency in human disease.
Our findings of a significant inverse correlation between cystatin C levels and distal aortic diameters (Figure 3) must be viewed as preliminary. Only a small fraction (n = 8) of the screened subjects had distinctly dilated aortas. Most subjects also had normal carotid intimal thickness. None of these outpatients had severe wall thickening of the common carotid (> 2 mm IMT), and only 6 patients had larger than 2 mm of carotid bifurcation IMT. This may explain the relatively low correlation coefficient (r = 0.203) relating aortic diameter to circulating cystatin C levels. Cystatin C levels likely have little influence on variation in aortic dimensions among normal subjects. Rather, our data suggest that cystatin C levels contribute to progression of vascular remodeling, consistent with significantly lower cystatin C levels among subjects with dilated aortas (Figure 3). Additional studies of more severely affected populations will be needed to test the relationship revealed in our studies.
We also think it is unlikely that alterations in the circulating levels of cystatin C contribute to the severe depression of lesional cystatin C observed in injured vessel walls. Rather, the downward skewing of circulating cystatin C levels among subjects with dilated aortas, and the severe depletion of cystatin C within atherosclerotic and aneurysmal lesions, likely indicate a common response to similar local and systemic changes in the cytokine milieu in these settings. Numerous studies have documented evidence of increased local and systemic markers of inflammation, e.g., the cytokine IL-6 and the acute-phase reactant, C-reactive protein, in atherosclerosis and aneurysmal disease (43, 50). In addition, both decreased systemic active TGF-β1 and local resistance to TGF-β1 activity have been reported in atherosclerosis (23, 51). Low TGF-β1 activity could explain the low levels of lesional cystatin C and, potentially, the inverse correlation with aortic dilatation observed in this study, because we found TGF-β1 to be a potent inducer of cystatin C secretion by vascular SMCs (Figure 4c). Reversal of TGF-β1–mediated elastase inhibition by cystatin C antibodies confirms that induction of cystatin C secretion underlies the protective effect of TGF-β1 on elastin degradation. Thus, our results indicate an additional mechanism by which TGF-β1 deficiency could contribute to the pathophysiology of vascular wall remodeling and atherogenesis. The restoration of a physiological balance between the elastolytic cathepsins and inhibitors of these proteases merits study as a novel therapeutic strategy, especially in aneurysmal disease in which matrix breakdown is dramatic.
Acknowledgments
We thank E. Shvartz, M. Muszynski, I. Chulsky, and Y. Sun for technical assistance. This work was supported by grants from the National Heart, Lung, and Blood Institute to G-P. Shi (HL 60942), P. Libby (HL 34636), and H. Chapman (HL 48261).
Footnotes
Guo-Ping Shi and Galina K. Sukhova contributed equally to this work.
References
- 1.Davies MJ. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation. 1996;94:2913–2020. doi: 10.1161/01.cir.94.8.2013. [DOI] [PubMed] [Google Scholar]
- 2.Ghorpade A, Baxter BT. Biochemistry and molecular regulation of matrix macromolecules in abdominal aortic aneurysms. Ann NY Acad Sci. 1996;800:138–150. doi: 10.1111/j.1749-6632.1996.tb33305.x. [DOI] [PubMed] [Google Scholar]
- 3.Newby AC, Southgate KM, Davies M. Extracellular matrix degrading metalloproteinases in the pathogenesis of arteriosclerosis. Basic Res Cardiol. 1994;89(Suppl. 1):59–70. doi: 10.1007/978-3-642-85660-0_6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysm. Ann NY Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17:439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 6.Rao SK, Reddy KV, Cohen JR. Role of serine proteases in aneurysm development. Ann NY Acad Sci. 1996;800:131–137. doi: 10.1111/j.1749-6632.1996.tb33304.x. [DOI] [PubMed] [Google Scholar]
- 7.Shi G-P, Munger JS, Meara JP, Rich DH, Chapman HA. Molecular cloning and expression of human alveolar macrophage cathepsin S, an elastolytic cysteine protease. J Biol Chem. 1992;267:7258–7262. [PubMed] [Google Scholar]
- 8.Shi G-P, et al. Molecular cloning of human cathepsin O, a novel endopeptidase and homologue of rabbit OC2. FEBS Lett. 1995;357:129–134. doi: 10.1016/0014-5793(94)01349-6. [DOI] [PubMed] [Google Scholar]
- 9.Sukhova GK, Shi G-P, Simon DI, Chapman HA, Libby P. Expression of elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy VY, Zhang QY, Weiss SJ. Pericellular mobilization of the tissue-destructive cysteine proteinases, cathepsins B, L, and S, by human monocyte-derived macrophages. Proc Natl Acad Sci USA. 1995;92:3849–3853. doi: 10.1073/pnas.92.9.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werb Z, Chin JR. Extracellular matrix remodeling during morphogenesis. Ann NY Acad Sci. 1998;857:110–118. doi: 10.1111/j.1749-6632.1998.tb10111.x. [DOI] [PubMed] [Google Scholar]
- 12.Barrett AJ, Davies ME, Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984;120:631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- 13.Merz GS, et al. Human cystatin C forms an inactive dimer during intracellular trafficking in transfected CHO cells. J Cell Physiol. 1997;173:423–432. doi: 10.1002/(SICI)1097-4652(199712)173:3<423::AID-JCP15>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamson M, Islam MQ, Szpirer J, Szpirer C, Levan G. The human cystatin C gene (CST3), mutated in hereditary cystatin C amyloid angiopathy, is located on chromosome 20. Hum Genet. 1989;82:223–226. doi: 10.1007/BF00291159. [DOI] [PubMed] [Google Scholar]
- 15.Abrahamson M, Barrett AJ, Salvesen G, Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. J Biol Chem. 1986;261:11282–11289. [PubMed] [Google Scholar]
- 16.Takeyabu K, et al. Cysteine proteinases and cystatin C in bronchoalveolar lavage fluid from subjects with subclinical emphysema. Eur Respir J. 1998;12:1033–1039. doi: 10.1183/09031936.98.12051033. [DOI] [PubMed] [Google Scholar]
- 17.Skaleric U, Babnik J, Curin V, Lah T, Turk V. Immunochemical quantitation of cysteine proteinase inhibitor cystatin C in inflamed human gingiva. Arch Oral Biol. 1998;34:301–305. doi: 10.1016/0003-9969(89)90072-1. [DOI] [PubMed] [Google Scholar]
- 18.Shimode K, Fujihara S, Nakamura M, Kobayashi S, Tsunematsu T. Diagnosis of cerebral amyloid angiopathy by enzyme-linked immunosorbent assay of cystatin C in cerebral fluid. Stroke. 1991;22:860–866. doi: 10.1161/01.str.22.7.860. [DOI] [PubMed] [Google Scholar]
- 19.Ishiguro H, Ohkubo I, Mizokami M, Titani K, Sasaki M. The use of monoclonal antibodies to define levels of cystatin C in normal human serum. Hybridoma. 1989;8:303–313. doi: 10.1089/hyb.1989.8.303. [DOI] [PubMed] [Google Scholar]
- 20.Jensson O, et al. Cystatin C mutation causing amyloid angiopathy and brain hemorrhage. Biol Chem Hoppe Seyler. 1990;371(Suppl.):229–232. [PubMed] [Google Scholar]
- 21.Chapman HA, Reilly JJ, Yee R, Grubb A. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. Am Rev Respir Dis. 1990;141:698–705. doi: 10.1164/ajrccm/141.3.698. [DOI] [PubMed] [Google Scholar]
- 22.Warfel AH, Zucker-Franklin D, Frangione B, Ghiso J. Constitutive secretion of cystatin C (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J Exp Med. 1987;166:1912–1917. doi: 10.1084/jem.166.6.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grainger DJ, et al. The serum concentration of active transforming growth factor-beta is severely depressed in advanced atherosclerosis. Nat Med. 1995;1:74–79. doi: 10.1038/nm0195-74. [DOI] [PubMed] [Google Scholar]
- 24.Banda MJ, Werb Z, McKerrow JH. Elastin degradation. Methods Enzymol. 1987;144:288–305. doi: 10.1016/0076-6879(87)44184-0. [DOI] [PubMed] [Google Scholar]
- 25.Rohde LE, et al. Circulating cell adhesion molecules are correlated with ultrasound-based assessment of carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 1998;18:1765–1770. doi: 10.1161/01.atv.18.11.1765. [DOI] [PubMed] [Google Scholar]
- 26.Rohde LE, et al. Plasma concentrations of interleukin-6 and abdominal aortic diameter among subjects without aortic dilatation. Arterioscler Thromb Vasc Biol. 1999;19:1695–1699. doi: 10.1161/01.atv.19.7.1695. [DOI] [PubMed] [Google Scholar]
- 27.Kyhse-Andersen J, et al. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40:1921–1926. [PubMed] [Google Scholar]
- 28.Chapman HA, Jr, Stone OL. Comparison of live human neutrophil and alveolar macrophage elastolytic activity in vitro. Relative resistance of macrophage elastolytic activity to serum and alveolar proteinase inhibitors. J Clin Invest. 1984;74:1693–1700. doi: 10.1172/JCI111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olafsson I. The human cystatin C gene promoter: functional analysis and identification of heterogeneous mRNA. Scand J Clin Lab Invest. 1995;55:597–607. doi: 10.3109/00365519509110259. [DOI] [PubMed] [Google Scholar]
- 30.Buisson A, et al. Up-regulation of a serine protease inhibitor in astrocytes mediates the neuroprotective activity of transforming growth factor beta1. FASEB J. 1998;12:1683–1691. [PubMed] [Google Scholar]
- 31.Cao HJ, Hogg MG, Martino LJ, Smith TJ. Transforming growth factor-beta induces plasminogen activator inhibitor type-1 in cultured human orbital fibroblasts. Invest Ophthalmol Vis Sci. 1995;36:1411–1419. [PubMed] [Google Scholar]
- 32.Su S, DiBattista JA, Sun Y, Li WQ, Zafarullah M. Up-regulation of tissue inhibitor of metalloproteinases-3 gene expression by TGF-beta in articular chondrocytes is mediated by serine/threonine and tyrosine kinases. J Cell Biochem. 1998;70:517–527. [PubMed] [Google Scholar]
- 33.Snider GL. Emphysema: the first two centuries — and beyond. A historical overview, with suggestions for future research: Part 2. Am Rev Respir Dis. 1992;146:1615–1622. doi: 10.1164/ajrccm/146.6.1615. [DOI] [PubMed] [Google Scholar]
- 34.Tetley TD. New perspectives on basic mechanisms in lung disease. VI. Proteinase imbalance: its role in lung disease. Thorax. 1993;48:560–565. doi: 10.1136/thx.48.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeClerck YA, et al. Proteases and protease inhibitors in tumor progression. Adv Exp Med Biol. 1997;425:89–97. doi: 10.1007/978-1-4615-5391-5_9. [DOI] [PubMed] [Google Scholar]
- 36.Calkins CC, Sloane BF. Mammalian cysteine protease inhibitors: biochemical properties and possible roles in tumor progression. Biol Chem Hoppe Seyler. 1995;376:71–80. [PubMed] [Google Scholar]
- 37.Allaire E, Forough R, Clowes M, Starcher B, Clowes AW. Local overexpression of TIMP-1 prevents aortic aneurysm degeneration and rupture in a rat model. J Clin Invest. 1998;102:1413–1420. doi: 10.1172/JCI2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fabunmi RP, Sukhova GK, Sugiyama S, Libby P. Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells, a potential protective mechanism in plaque stability. Circ Res. 1998;83:270–278. doi: 10.1161/01.res.83.3.270. [DOI] [PubMed] [Google Scholar]
- 39.Knox JB, Sukhova GK, Whittemore AD, Libby P. Evidence for altered balance between matrix metalloproteinases and their inhibitors in human aortic diseases. Circulation. 1997;95:205–212. doi: 10.1161/01.cir.95.1.205. [DOI] [PubMed] [Google Scholar]
- 40.Thompson RW, et al. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms: an elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMillan WD, et al. In situ localization and quantification of mRNA for 92-kD type IV collagenase and its inhibitor in aneurysmal, occlusive, and normal aorta. Arterioscler Thromb Vasc Biol. 15:1139–1144. doi: 10.1161/01.atv.15.8.1139. [DOI] [PubMed] [Google Scholar]
- 42.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 43.Szekanecz Z, Shah MR, Pearce WH, Koch AE. Human atherosclerotic abdominal aortic aneurysms produce interleukin (IL)-6 and interferon-gamma but not IL-2 and IL-4: the possible role for IL-6 and interferon-gamma in vascular inflammation. Agents Actions. 1994;42:159–162. doi: 10.1007/BF01983484. [DOI] [PubMed] [Google Scholar]
- 44.Schonbeck U, et al. Regulation of matrix metalloproteinase expression in human vascular smooth muscle cells by T lymphocytes: a role for CD40 signaling in plaque rupture? Circ Res. 1997;81:448–454. doi: 10.1161/01.res.81.3.448. [DOI] [PubMed] [Google Scholar]
- 45.Galis ZS, Muszynski M, Sukhova GK, Simon-Morrissey E, Libby P. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann NY Acad Sci. 1995;748:501–507. doi: 10.1111/j.1749-6632.1994.tb17348.x. [DOI] [PubMed] [Google Scholar]
- 46.Libby P, et al. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Pearce WH, Koch AE. Cellular components and features of immune response in abdominal aortic aneurysms. Ann NY Acad Sci. 1996;800:175–185. doi: 10.1111/j.1749-6632.1996.tb33308.x. [DOI] [PubMed] [Google Scholar]
- 48.Elmore JR, Keister BF, Franklin DP, Youkey JR, Carey DJ. Expression of matrix metalloproteinases and TIMPs in human abdominal aortic aneurysms. Ann Vasc Surg. 1998;12:221–228. doi: 10.1007/s100169900144. [DOI] [PubMed] [Google Scholar]
- 49.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997;122:264–271, discussion 271–272. doi: 10.1016/s0039-6060(97)90017-9. [DOI] [PubMed] [Google Scholar]
- 50.Ridker PM, Haughie P. Prospective studies of C-reactive protein as a risk factor for cardiovascular disease. J Investig Med. 1998;46:391–395. [PubMed] [Google Scholar]
- 51.McCaffrey TA, et al. Genomic instability in the type II TGF-beta 1 receptor gene in atherosclerotic and restenotic vascular cells. J Clin Invest. 1997;100:2182–2188. doi: 10.1172/JCI119754. [DOI] [PMC free article] [PubMed] [Google Scholar]