The unprecedented and growing number of older adults in the US and other developed countries has crystallized scientific interest in the health consequences of aging. Perhaps the most feared of these consequences is Alzheimer’s disease (AD). As the leading cause of dementia, AD not only extracts a terrible toll from patients and their families but is an enormous public health burden, with annual costs in the US alone approaching 100 billion dollars. The major risk factor for AD is living longer; AD prevalence doubles every 5 years after age 65 and approaches 50% by age 85 (1). Some epidemiologists report that the prevalence of AD continues to increase in the very old and conclude that “AD … may become universal when age is sufficiently advanced” (2). As we enter the 21st century with increasing life expectancies, the obvious question becomes, “If we live long enough, will we all be demented?” (3). Although this question cannot be definitely resolved at present, accumulating evidence suggests that not all of us will.
Before reviewing the data that led me to consider aging and AD as discrete rather than continuous processes, I should acknowledge 3 biases. First, I believe that for studies of cognitive aging, longitudinal study designs are preferable to cross-sectional designs, which may be confounded by cohort effects that overestimate age-associated decline. A lower cognitive test score for an 85-year-old in comparison with that of a 65-year-old, for example, may reflect generational differences in education rather than an age effect. Although longitudinal studies also have biases (e.g., nonrepresentative samples and selective attrition tend to overestimate cognitive ability), they permit repeated observations of the same individual and allow optimal performance to be observed. Using this approach, recent studies reveal little or no cognitive decline for the large majority of elderly individuals (4–6), in contrast to previous cross-sectional studies that found decreasing performance with age. My second bias is that ageism colors our expectations for what is normal at a given age. Too often, ageism may mask disease by accepting cognitive and functional decline as a natural consequence of aging, as when someone exclaims, “He’s doing fine … for age 90!” It is important to recognize that diseases that accompany aging may be responsible for much of the impairment attributed to age alone (7, 8). Finally, I believe that sensitive clinical assessment methods are necessary to identify truly nondemented elderly individuals. Cognitive tests commonly are used to screen elderly persons for dementia but may fail to detect early impairment, particularly in high-functioning individuals and those who are well educated. Reliance on cognitive tests alone thus may result in control samples that are contaminated with individuals who have unrecognized very mild dementia. We and others use the observations of an informant to obtain the critical diagnostic information as to whether or not an individual has declined in cognitive ability relative to their past performance. We find that informants are sensitive to the earliest symptoms of AD, even when cognitive test performance is “normal” (9, 10), and also may help distinguish those individuals currently labeled with “mild cognitive impairment” (11) who have incipient AD from those with nondementing conditions.
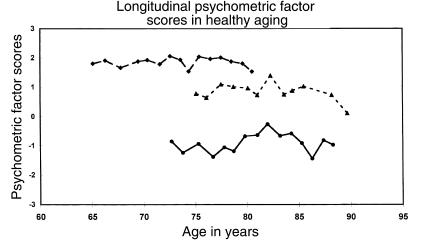
We have been studying individuals without detectable cognitive impairment at Washington University since 1979, using both clinical and psychometric assessments. The clinical protocol incorporates interviews with both the subject and an informant to obtain information sufficient to derive a Clinical Dementia Rating (CDR) for the subject, where CDR = 0 indicates no dementia and CDR = 0.5, 1, 2, or 3 indicates very mild, mild, moderate, or severe dementia, respectively. The psychometric battery includes measures of verbal and nonverbal episodic memory, semantic memory, speeded psychomotor performance, executive function, and visuospatial abilities. For those subjects who remain nondemented (CDR = 0), cognitive performance on these tests has shown no appreciable decline when measured annually for 15 years up to age 90 (Figure 1) (4). Similar cognitive stability has been demonstrated in single-site (7) and multicenter (5) studies of nondemented older adults who were identified with similar assessment methods, including the CDR. A caveat for these data is that they reflect a subset of older adults who are aging “successfully,” and thus the findings cannot easily be extrapolated to the general elderly population. The appearance of cognitive stability, moreover, does not exclude the possibility that minor age-related cognitive declines occurred but were overcome by learning or practice effects. Nonetheless, the prevailing notion of inevitable cognitive deterioration with advancing age needs re-examination in light of the finding that when even very mild dementia is carefully excluded, the cognitive abilities of healthy older adults generally remain intact well into the ninth decade of life.
Figure 1.
Principal-components analysis of psychometric test scores yielded a general factor of cognitive ability in nondemented elderly subjects, where the mean performance level is represented by a factor score of 0. Factor scores from 3 of these subjects with different baseline levels of cognitive ability are shown by age at each annual assessment and show relative stability of performance. Reproduced with permission from Lippincott Williams & Wilkins (21).
Quantitative postmortem studies also support the concept that healthy brain aging is possible. For example, brains from our control subjects (carefully determined to be truly nondemented during life) up to age 89 years show little, if any, neocortical pathology (9, 12). In contrast, subjects just at the earliest symptomatic stages of AD (corresponding to a CDR score of 0.5) already display abundant β-amyloid–containing senile plaques (SPs) throughout the neocortex. Similar distinctions between normal aging and AD have been reported by others (13, 14). Both our healthy control (CDR = 0) and very mildly demented (CDR = 0.5) subjects had neurofibrillary tangles (NFTs) in limbic regions, particularly the CA1 region of the hippocampus and the entorhinal cortex, but virtually none outside of the temporal lobe (15). Neurons of the entorhinal cortex are in a critical path for neural systems that subserve memory and are highly vulnerable in AD. It is notable, therefore, that using unbiased stereological techniques in brains from our nondemented subjects, no decrement with age was observed in entorhinal neuronal number between the sixth and ninth decades of life, whereas a 32% reduction from control values already had occurred in very mildly demented (CDR = 0.5) subjects (16). The absence of neocortical NFTs and SPs and the preservation of entorhinal neurons in nondemented controls contrast with the increase in neocortical SP density and the dramatic entorhinal neuronal loss in subjects just at the earliest detectable stage of AD. These findings provide pathoanatomical evidence that aging and AD are not part of a continuum.
Further investigations with larger numbers of nondemented control subjects revealed that AD may be present neuropathologically but was not associated with clinically detectable cognitive change during life (17, 18). J. Price from our center recently reported neuromorphometric data in 39 control (CDR = 0) subjects who were age 51–93 years at death, in comparison with similar data from AD subjects who died at different stages of dementia severity (19). At least a few NFTs were present in all nondemented subjects in the entorhinal and perirhinal cortical areas and in hippocampal field CA1 and appeared to increase in density as a function of age; a very few NFTs also were present in inferior temporal and orbital cortex in the older controls. Very mildly demented (CDR = 0.5) AD subjects had greater NFT densities than did nondemented (CDR = 0) subjects in the same vulnerable areas and had slightly more NFTs in neocortex. A similar distribution for NFTs was observed in severely demented AD subjects, but neocortical densities were much greater. Tangle density thus followed a predictable pattern of burden and distribution from aging to AD. Three different patterns of SP densities, however, marked the controls. One pattern included all subjects younger than 75 years of age and about one third of the older subjects (up to 88 years) and consisted of the absence of SPs from the entire neocortex. The second pattern, in approximately 40% of subjects older than 75 years, showed a few SPs (all diffuse) limited to patches in the neocortex. The remaining pattern was found in about 25–30% of control subjects older than 75 years and featured extensive diffuse and neuritic SPs distributed throughout the neocortex.
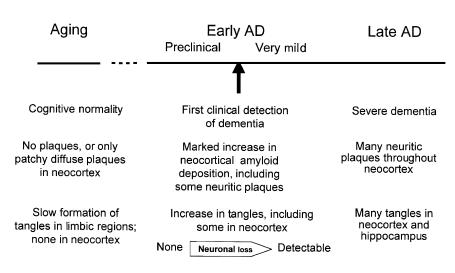
Our synthesis of these findings is shown in Figure 2. NFTs appear in vulnerable brain regions as a function of age and are not associated with neuronal loss (16, 20). The presence of extensive SPs appears to accelerate the base rate of age-dependent NFT formation, which is associated with neuronal degeneration and death and correlates with the first clinical recognition of AD (CDR = 0.5). We propose that the beginning of AD occurs with the deposition of β-amyloid in the form of large numbers of SPs. The presence of SPs exacerbates neurofibrillary degeneration and culminates in neuronal dysfunction and death, which, in turn, leads to the appearance of clinical symptoms. Preclinical AD, which affects perhaps 25% of cognitively normal individuals older than the age of 75 years, is present when death comes after SP formation but before sufficient neuronal loss has occurred to produce even very mild dementia.
Figure 2.
Hypothetical sequence of clinicopathologic findings in aging and AD.
Many questions remain. Does the frequency of preclinical and clinical AD increase in nonagenarians and centenarians? Does the presence of a few patchy neocortical SPs represent a still earlier stage of preclinical AD? Would all preclinical AD cases eventually become demented if they lived long enough, or might some of these individuals enjoy protection from cognitive decline? Is it possible to develop more sensitive clinical detection methods so that AD can be diagnosed at an earlier stage than is now accepted? (The ability to assay or image cerebral β-amyloid deposits in cognitively normal individuals who are followed-up longitudinally for development of dementia would be extremely useful in addressing these questions.) Finally, we view amyloid deposition as the critical pathogenetic event for AD, but an alternative view is that the first AD lesion is neurofibrillary change in the form of NFTs in entorhinal cortex. This would imply that the appearance of even a single NFT marks the initiation of a cascade that culminates in recognizable AD. In this view, essentially all individuals older than 50 years of age might be considered to have preclinical AD, with the age at onset of symptoms being modulated by factors such as the presence of the ApoE-ε4 allele.
Despite these unresolved issues, I interpret the data in hand to indicate that older adults can remain nondemented and free of neuropathological AD, at least into the ninth decade of life. The brain changes of normal aging are distinguished from those of the earliest symptomatic stages of AD and from preclinical stages of AD (which lack sufficient neuronal degeneration to produce symptoms) by the absence of extensive β-amyloid deposition, which likely represents the initiating event for AD. In conclusion, although aging and AD are unquestionably related, the evidence suggests that they are distinct conditions and that AD is not inevitable with age.
Acknowledgments
Much of the work presented here is attributable, directly or indirectly, to my colleagues in the Memory and Aging Project and Alzheimer’s Disease Research Center at Washington University. Among these colleagues, Leonard Berg, Daniel McKeel, Philip Miller, Joseph Price, Eugene Rubin, and Martha Storandt have been particularly helpful in the conceptualization of ideas discussed in this article. Credit for the scheme depicted in Figure 2 goes to J. Price. My research is supported in part by National Institute on Aging grants AG03991 and AG05681.
References
- 1.Evans DA, et al. Prevalence of Alzheimer’s disease in a community population of older persons: higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 2.Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old: results from the Canadian Study of Health and Aging. Neurology. 1994;44:1593–1600. doi: 10.1212/wnl.44.9.1593. [DOI] [PubMed] [Google Scholar]
- 3.Drachman DA. If we live long enough, will we all be demented? Neurology. 1994;44:1563–1565. doi: 10.1212/wnl.44.9.1563. [DOI] [PubMed] [Google Scholar]
- 4.Rubin EH, et al. A prospective study of cognitive function and onset of dementia in cognitively healthy elders. Arch Neurol. 1998;55:395–401. doi: 10.1001/archneur.55.3.395. [DOI] [PubMed] [Google Scholar]
- 5.Unger J, van Belle G, Heyman A. Cross-sectional vs. longitudinal estimates of cognitive change in the non-demented elderly: a CERAD study. J Am Geriatr Soc. 1999;47:559–563. doi: 10.1111/j.1532-5415.1999.tb02570.x. [DOI] [PubMed] [Google Scholar]
- 6.Haan MN, Shemanki L, Jagust WJ, Manolio TA, Kuller L. The role of APOE ε4 in modulating effects of other risk factors for cognitive decline in elderly persons. JAMA. 1999;282:40–46. doi: 10.1001/jama.282.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Howieson DB, Holm LA, Kaye JA, Oken BS, Howieson J. Neurologic function in the optimally healthy oldest old: neuropsychological evaluation. Neurology. 1993;43:1882–1886. doi: 10.1212/wnl.43.10.1882. [DOI] [PubMed] [Google Scholar]
- 8.Sliwinski M, Lipton R, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol. 1996;51:P217–P225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- 9.Morris JC, et al. Very mild Alzheimer’s disease: informant-based clinical, psychometric, and pathologic distinction from normal aging. Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- 10.Jorm AF. Methods of screening for dementia: a meta-analysis of studies comparing an informant questionnaire with a brief cognitive test. Alzheimer Dis Assoc Disord. 1997;11:158–162. [PubMed] [Google Scholar]
- 11.Petersen RC, et al. Mild cognitive impairment. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 12.Morris JC, et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 13.Troncoso JC, Martin LJ, Dal Forno G, Kawas CH. Neuropathology in controls and demented subjects from the Baltimore Longitudinal Study of Aging. Neurobiol Aging. 1996;17:365–371. doi: 10.1016/0197-4580(96)00028-0. [DOI] [PubMed] [Google Scholar]
- 14.Haroutunian V, et al. Regional distribution of neuritic plaques in the nondemented elderly and subjects with very mild Alzheimer disease. Arch Neurol. 1998;55:1185–1191. doi: 10.1001/archneur.55.9.1185. [DOI] [PubMed] [Google Scholar]
- 15.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Isla T, et al. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hulette CM, et al. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol. 1998;57:1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 20.Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. 1999;58:188–197. doi: 10.1097/00005072-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Morris, J. C. 1999. Clinical presentation and course of Alzheimer disease. In Alzheimer disease. 2nd edition. R.D. Terry, R. Katzman, K.L. Bick, and S.S. Sisodia, editors. Lippincott Williams & Wilkins. Philadelphia, PA. 11–24.