Abstract
Homeobox transcription factors specify body plan by regulating differentiation, proliferation, and migration at a cellular level. The homeobox transcription factor Gax is expressed in quiescent vascular smooth muscle cells (VSMCs), and its expression is downregulated by vascular injury or other conditions that lead to VSMC proliferation. Previous investigations demonstrate that Gax may regulate VSMC proliferation by upregulating the cyclin-dependent kinase (cdk) inhibitor p21. Here we examined whether Gax influences VSMC migration, a key feature in the development of stenotic lesions after balloon injury. Transduction of a Gax cDNA inhibited the migratory response of VSMCs toward PDGF-BB, basic fibroblast growth factor, or hepatocyte growth factor/scatter factor. Gax expression also inhibited migration of NIH·3T3 fibroblasts and embryonic fibroblasts lacking p53. Gax was unable to inhibit the migration of fibroblasts lacking p21, but this effect could be restored in these cells by providing exogenous p21 or by overexpressing another cdk inhibitor, p16. Flow cytometric analysis implicated a Gax-mediated downregulation of αvβ3 and αvβ5 integrin expression in VSMCs as a potential cause for reduced cell motility. Gax specifically downregulated β3 and β5 in VSMCs in culture and after acute vascular injury in vivo. Repression of integrin expression was also found in NIH 3T3 cells and p53 knockout fibroblasts, but not in p21-knockout fibroblasts, unless these cells express exogenous p21 or p16. These data suggest that cycle progression, integrin expression, and cell migration can be regulated in VSMCs by the homeobox gene product Gax.
J. Clin. Invest. 104:1469–1480 (1999).
Introduction
Remodeling in the cardiovascular system occurs during normal development and in various pathological states. Homeobox genes encode transcription factors that have been shown to be regulators of body-plan formation, cell growth, differentiation, and region-specific cell migration in developing embryos (1–5). Homeobox transcription factors contain a characteristic homeodomain, a 60–amino acid DNA-binding domain that is conserved through evolution. One of these factors, Gax (growth-arrest specific homeobox) is unusual among the homeobox class of transcription factors, as its expression is rapidly downregulated in cultured vascular smooth muscle cells (VSMCs) upon stimulation by serum PDGF (6, 7) or angiotensin II (8), and in the rat carotid artery during the proliferative response to balloon injury (9). Conversely, gax expression is upregulated by conditions that favor differentiation and cell-cycle arrest (7, 8), indicating that Gax may be required for the expression of proteins in VSMCs that are associated with the quiescent or contractile phenotype in smooth muscle cells.
In adult organisms, Gax is expressed in cardiovascular tissues including heart, lung, and the medial smooth muscle cells of arteries (7), and embryonic expression was detected in all 3 muscle lineages (cardiac, smooth, and skeletal muscle) (10). Gax overlaps in its expression pattern with myocyte-specific enhancer factor 2 (MEF2), which is a known regulator of gene transcription in all muscle types (11, 12). MEF2 transactivates the gax promoter (13), suggesting that MEF2 may participate in the regulation of Gax expression during embryogenesis. Taken together, these observations suggest that Gax might play a role in regulating muscle differentiation and the responses of these myocytes to proliferative signals (6, 7). Recently, the antiproliferative effects of Gax overexpression have been documented in VSMCs and fibroblasts (14). The upregulation of the general cyclin-dependent kinase (cdk) inhibitor p21 is essential for growth arrest by Gax.
Proliferation of VSMCs is generally regarded as a key event in the development of atherosclerosis and restenosis after balloon angioplasty (15, 16), and the migration of proliferating VSMCs from the medial to the luminal side of the vessel is an important mechanism in intimal thickening (17). The chemotactic growth factors PDGF (18, 19) and basic fibroblast growth factor (bFGF) (20, 21) are likely to direct VSMC migration, and the local extracellular matrix (ECM) environment may provide an additional level of motility regulation (22–25). Integrin cell-surface receptors serve as a link between ECM and the cytoskeleton (26), and both adhesion and migration can be regulated by altering integrin expression patterns (27–31). Whereas these exogenous regulators of cell migration are relatively well described, little is known about the nuclear proteins that function to coordinate cell growth and motility.
In this study, transduction of the gax gene with a replication-defective adenoviral vector in VSMCs led to a marked decrease in cell motility on extracellular matrix proteins in vitro. This decrease was dose dependent and was independent of either chemotactic growth factor or receptor expression. Cell-cycle arrest appeared important for the antimigratory activity of Gax, as Gax overexpression had no effect on migration of cells lacking the cdk inhibitor p21 (p21–/– cells). Furthermore, the antimigratory effect of Gax could be restored in p21–/– cells when cell-cycle arrest was induced by coexpression with exogenous p21 or the cdk inhibitor, p16. However, overexpression of p21 or p16 alone did not influence cell migration. These data suggest that cell-cycle arrest is required in Gax-induced inhibition of cell migration, but inhibition of cell cycle by itself is not sufficient to suppress migration. Overexpression of Gax led to a downregulation of the integrins αvβ3 and αvβ5 through the specific suppression of β3 and β5 subunits in vitro and in vivo. In parallel with its effects on migration, Gax-induced integrin downregulation did not occur in p21–/– cells unless cell-cycle arrest was induced by coexpression with p21 or p16. These data suggest that Gax can function to coordinate cell growth and motility through its ability to regulate integrin expression in a cell cycle–dependent manner.
Methods
Cell culture and reagents.
Rat primary VSMCs were obtained by enzymatic digestion of the media from the thoracic aorta of male Sprague-Dawley rats as described previously (32), maintained in high-glucose DMEM (GIBCO BRL, Gaithersburg, Maryland, USA) containing 100 U/mL penicillin G, 100 U/mL of streptomycin sulfate (GIBCO BRL), and 15% FBS (GIBCO BRL) and used before passage 10. Before use, primary smooth muscle cell cultures were stained with an mAb to smooth muscle α-actin (Sigma Chemical Co., St. Louis, Missouri, USA) to verify homogeneity. Human VSMCs were derived from unused saphenous vein segments excised at the time of coronary bypass surgery at St. Elizabeth’s Medical Center (33). Mouse embryonic fibroblasts (MEFs) with either wild-type p21 allele (+/+) or a homozygous disruption (–/–) (34) were maintained in DMEM with penicillin/streptomycin and 10% FBS. MEFs with a homozygous deletion of the p53 allele (35) and BALB/c NIH 3T3 cells obtained from the American Type Culture Collection (Rockville, Maryland, USA) were maintained in DMEM with penicillin/streptomycin and 10% FBS. Synchronous populations of cells were obtained by maintaining cultures in low-serum media (0.5% FBS) for 48 hours. Viral transduction was conducted in VSMCs using mitogen-deprived quiescent cultures in fresh low-mitogen medium containing 0.5% FBS. Fibroblast cells were not mitogen-deprived before viral transduction. Polymerized and monomer collagen was prepared as described previously (36). PDGF-BB and bFGF were purchased from Genzyme Corp. (Cambridge, Massachusetts, USA); hepatocyte growth factor/scatter factor (HGF/SF) was purchased from Genentech (South Francisco, California, USA).
Adenoviral infection in vitro.
Rat VSMCs were plated at approximately 10% confluence in 6-well tissue culture dishes in DMEM containing 15% FBS overnight. Cultures were made quiescent by incubation for 2 days in DMEM containing 0.5% FBS. Cells from 1 well were harvested by trypsinization, and the cell number per well was determined by counting with a hemacytometer. Viral dilutions were prepared from purified viral stocks in DMEM containing 0.5% FBS and infections were conducted in 0.5 mL/well for 24 hours. At the end of the infection period, the virus containing medium was removed, the cultures were washed with PBS, and fresh DMEM containing 0.5% FBS was added. Preparation of replication-defective adenoviral constructs encoding either the rat gax gene (Ad-gax) (14), the human p16 (Ad-p16) or p21 gene (Ad-p21) (37, 38), or the Escherichia coli β-galactosidase gene (Ad-βgal) (39) were described previously.
Cell-viability assay.
For evaluation of cell viability, the colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)2H-tetrazolium (MTS) assay with the electron coupling reagent phenazine methosulfate (PMS) was performed (CellTiter 96 AQ; Promega Corp., Madison, Wisconsin, USA) (40). Cells were seeded in a 96-well plate (5 × 104 cells per well) in 0.1 mL serum-supplemented medium and allowed to attach overnight. Cells were made quiescent for 48 hours in low-serum medium and infected with adenovirus (300 moi) as already described here. At 12 hours after virus removal, 20 μL of a MTS/PMS mixture was added per well and allowed to incubate for 1 hour at 37°C before measuring the absorbance at 490 nm in an ELISA plate reader. Background absorbance was subtracted, and 7 wells were performed in parallel for each condition.
Cell-migration assay.
Cell-migration assays were performed using a 48-well microchemotaxis chamber (Neuroprobe Inc., Cabin John, Maryland, USA) (41). For some experiments, 2 chemotaxis chambers were used in parallel. Polyvinylpyrrolidone-free polycarbonate filters with a pore size of 8 μm (Nuclepore Corp., Cambridge, Massachusetts, USA) were coated with a mixture of 100 μg/mL type I collagen and 10 μg/mL vitronectin (19, 31, 42–44) for at least 6 hours at room temperature and dried under sterile air (similar results for VSMC migration were obtained when coating the filters with 0.1% gelatin). Test substances were diluted to appropriate concentrations in DMEM supplemented with 1% FBS, and 25 μL of the final dilution was placed in the lower chamber of the modified Boyden apparatus. Subconfluent rat VSMC or fibroblast cultures were washed with calcium- and magnesium-free PBS and detached by incubation with 0.5% EDTA for 30 minutes at 37°C and careful pipetting. Removal of cells in clumps was excluded by microscopy. After collecting the cells by centrifugation at 1,000 g for 3 minutes and placement of the filter, 2.5 × 105 cells suspended in 50 μL DMEM containing 1% FBS were seeded in the upper compartment and the apparatus was incubated for 5 hours at 37°C in a humidified chamber with 5% CO2 to allow cell migration. After the incubation period, the filter was removed and the upper side of the filter was scraped with a rubber policeman to remove nonmigrating cells. The filters were fixed with methanol and stained with a Giemsa solution (Diff-Quick; Baxter Healthcare Corp., Deerfield, Illinois, USA). Migration was quantified by counting cells of 3 random high-power fields (×100) in each well, and all conditions were evaluated in quadruplicate.
Cell-adhesion assay.
To simulate the chemotaxis conditions, a mixture of adhesive substrates, as already described here, was used to coat the wells of a 96-well plate (Costar, Cambridge, Massachusetts, USA) for 6 hours at room temperature. Excess liquid was removed, and the wells were allowed to dry for 4 hours. Wells were then rinsed with PBS, and nonspecific binding sites were blocked by the addition of 10 mg/mL BSA in PBS for 1 hour at 37°C. VSMCs prepared as described for the cell-migration assay were washed with 10% FBS-DMEM. After centrifugation and resuspension in DMEM supplemented with 1 mg/mL BSA, cell number was determined by counting with a hemacytometer, and 3.0 × 105 cells were plated per well. The plates were incubated at 37°C to allow cells to adhere. After 60 minutes, each well was rinsed with PBS and the cells were fixed with methanol and stained with Giemsa solution (Diff-Quick) and quantified in a microtiter plate reader at 595 nm. Each test group was performed in quadruplicate.
Radioligand binding assay.
Radioiodinated PDGF-B was purchased from DuPont NEN (NEN Life Science Products, Inc., Boston, Massachusetts, USA). For binding assays, VSMCs were plated in 24-well plates (2 × 104 cells/cm2) and allowed to attach overnight. After 48 hours of serum starvation, cells were transduced with Ad-gax or Ad-βgal for 24 hours as already described here. Twelve hours after adding fresh medium containing 0.5% FBS, VSMCs were chilled on ice, washed twice with ice-cold PBS/1% FBS, and incubated in ice-cold binding buffer (DMEM, 1% FBS, 25 mM HEPES). For Scatchard analysis, 25–800 pM [125I]PDGF was added to the binding buffer for 2 hours at 4°C on an orbital shaker. The binding medium was removed, and VSMCs were washed and lysed in PBS containing 1% Triton-X 100. Lysates were counted using a Beckman Gamma 5500B counter (Beckman Coulter Inc., Fullerton, California, USA). Triplicate samples of each condition were obtained. Nonspecific binding was determined by competition with a 200-fold molar excess of nonradiolabeled PDGF-BB.
Flow cytometric analysis of integrin expression.
VSMCs were harvested with Dulbecco’s phosphate buffered saline (D-PBS) containing 0.5% EDTA for 30 minutes. Detached cells were gently collected, washed with PBS containing 10% FBS, and aliquoted in 50-μL volumes (5 × 105 cells), and the specific primary murine antibody or mouse control IgG was added to the cell suspension for 45 minutes on ice. After washing, secondary polyclonal anti-mouse antibody was added for 30 minutes on ice in the dark. Cells were fixed in 250 μL 1% paraformaldehyde in PBS, and fluorescence intensity was measured using a FaxScan flow cytometer (FACS; Becton Dickinson and Co., Franklin Lakes, New Jersey, USA) with Lysis II software (Becton Dickinson and Co., Franklin Lakes, New Jersey, USA). The IgG1 isotypic control α1 and β1 mAb’s were purchased from Immunotech (Marseille, France); αvβ3 (clone LM609) and αvβ5 (clone P1F6), from Chemicon Inc. (Temecula, California, USA).
Immunohistochemical analysis of integrin expression in vivo.
Male Sprague-Dawley rats (400–500 g) were anesthetized with an intraperitoneal injection of sodium pentobarbital (45 mg/kg; Abbott Laboratories, Abbott Park, Illinois, USA), and both carotid arteries were exposed through midline incision and injured with a 2F embolectomy catheter (Baxter Edwards Healthcare, Deerfield, Illinois, USA). Injured vessels were treated for 15 minutes with saline (n = 9) or adenoviral solution (Ad-gax 1 × 109 pfu, n = 9; or Ad-βgal 1 × 109 pfu, n = 9). Other vessels were not injured or treated with adenovirus to serve as a control. Some rats were sacrificed at 3 days with an intraperitoneal injection of pentobarbital (100 mg/kg). Vessels were harvested and snap frozen in liquid nitrogen. Frozen arteries were cut into 5-μm cross-sections. After fixation in 4% paraformaldehyde solution, sections were immunostained with anti-β3 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, California, USA), anti-β5 antibody (Chemicon) or IgG (Santa Cruz). Antibody distribution over each cryosection was determined with a biotin streptavidin amplified detection system (Super Sensitive Immunodetection System; BioGenex Laboratories, San Ramon, California, USA). All immunostained sections were subjected to counterstain with hematoxylin and eosin to view nuclei. Some rats were also sacrificed 2 weeks after injury/gene delivery to assess neointima formation (n = 6 for each experimental condition). These vessels were fixed in 100% methanol and embedded in paraffin. Several 5-μm sections from each specimen were stained with hematoxylin and eosin and projected onto a digitizing board to quantify intimal, medial, and luminal areas using a computerized sketching program. This experimental protocol was approved by St. Elizabeth’s Medical Center Institutional Animal Care and Use Committee and complied with National Institutesof Health standards for care of laboratory animals.
Western blot analysis of integrin expression.
Cultured VSMCs or frozen carotid arteries as already described here were homogenized in RIPA buffer (50 mM Tris-HCl [pH 7.5]; 150 mM NaCl; 1% NP-40; 0.5% Na-deoxycholic acid; 0.1% SDS) containing 10 μg/mL leupeptin, 5 μg/mL aprotinin, and 2 mM PMSF. Protein concentration of each whole-cell extract was determined using a BCA assay (Pierce Chemical Co., Rockford, Illinois, USA). A total of 30 μg of each extract was separated by SDS-PAGE with a 7.5% polyacrylamide gel and transferred to 0.2-μm PVDF membrane (Bio-Rad Laboratories, Hercules, California, USA). Membranes were blocked in 10% nonfat dry milk, 0.2% Tween-20 in PBS (TPBS). Immunocomplexes were viewed by chemiluminescence using ECL reagent (Amersham Life Sciences, Arlington Heights, Illinois). Immunoblots were performed with mouse monoclonal anti-β3 antibody (clone F-11; Santa Cruz), rabbit polyclonal anti-β5 antibody (Chemicon), rabbit polyclonal anti-α1 antibody (Chemicon), mouse monoclonal anti-β1 antibody (clone HUTS-4; Chemicon), or anti-tubulin antibody (Sigma Chemical Co.). All antibodies were used at a 1:1,000 dilution at 4°C overnight. Blots were washed with TPBS and incubated with horseradish peroxidase–linked goat anti-rabbit antibody for 45 minutes.
Statistical analysis.
Results were expressed as mean ± SEM. Statistical significance was evaluated using unpaired Student’s t test for comparisons between 2 means, or ANOVA analysis followed by Scheffe’s procedure for more than 2 means. A value of P < 0.05 was regarded as statistically significant.
Results
Adenoviral transduction of the gax homeobox gene inhibits directed VSMC migration.
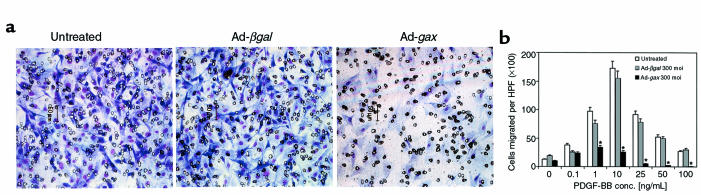
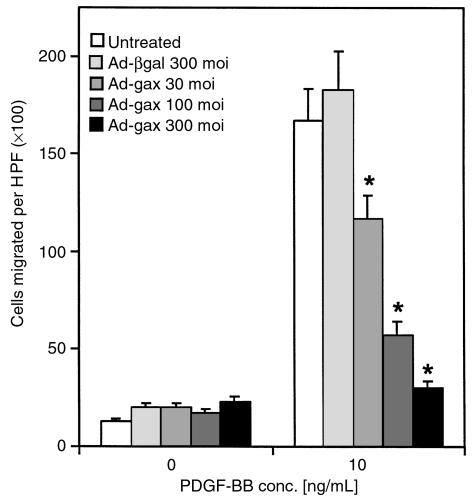
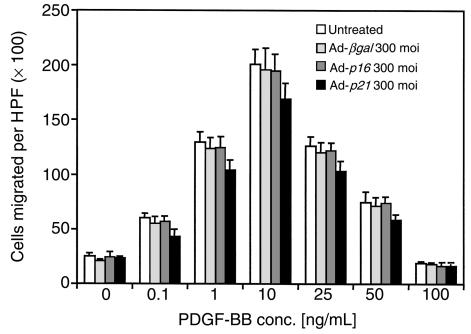
To investigate the role of Gax in migration, a replication-defective adenovirus encoding the full-length rat gax cDNA downstream from the cytomegalovirus promoter was used to transduce Gax into rat VSMCs (14). Migration experiments were initially conducted with PDGF-BB as chemotactic agent, because PDGF-BB is known to be a potent chemotactic growth factor for VSMCs (45). Migration assays were performed using a modified Boyden chamber with rat VSMCs (Figure 1a). Characteristic of most chemotactic factors (18, 46), a bell-shaped dose-response curve was observed over a wide range of concentrations with a maximal response at 10 ng/mL PDGF-BB (Figure 1b). Rat VSMCs transduced with a control virus expressing β-galactosidase (Ad-βgal) displayed a similar chemotactic response toward PDGF-BB as untreated cells, whereas adenoviral overexpression of gax led to a marked decrease in cell motility (Figure 1) at an moi of 300 (at PDGF-BB 10 ng/mL: 170 ± 12 untreated cells migrated per ×100 high-power field versus 152 ± 13 migrated Ad-βgal–infected cells, NS; and 24 ± 2 migrated Ad-gax–infected cells; P < 0.001 versus Ad-βgal–infected cells). The extent of inhibition correlated with the amount of virus applied, as shown in Figure 2. There was a dose-dependent increase in gax inhibitory activity, which seemed to be saturating at 300 moi (93.9% inhibition versus Ad-βgal). Parallel experiments examining transgene expression from the Ad-βgal vector by X-gal staining revealed that 30 moi produced approximately 40% transduction efficiency, whereas 300 moi led to a quantitative transduction of VSMCs. The dose of Ad-gax required to inhibit migration is well within the range used by others to transduce rat or human VSMCs efficiently (14, 47, 48).
Figure 1.
Effect of gax transduction on VSMC migration. Quiescent VSMCs were transduced with Ad-βgal or Ad-gax at an moi of 300 for 24 hours. The migration assay was performed 12 hours later in a chemotaxis chamber for 5 hours with a polycarbonate membrane coated with type I collagen and vitronectin and designated concentrations of PDGF-BB in the lower well. (a) Representative membranes using 10 ng/mL PDGF-BB as chemoattractant and stained with a Giemsa-solution. Untreated (left), Ad-βgal–transduced (middle), and Ad-gax–transduced (right) cells. (b) Quantitative analysis of the chemotactic response of VSMCs toward PDGF-BB at different concentrations. Results represent the average of 3 experiments. Each condition was performed in quadruplicate, and 3 high-power fields were counted per well. The bars show the mean ± SEM. *P < 0.01 versus Ad-βgal–transduced cells. There was no statistically significant difference between untreated and Ad-βgal–transduced cells. HPF = high power field.
Figure 2.
Effect of gax gene dosage on VSMC migration using increasing moi’s. Migratory response of untreated (no virus applied), Ad-βgal–transduced (300 moi), or Ad-gax–transduced (30, 100, and 300 moi) rat VSMCs toward PDGF-BB (10 ng/mL). The experiment was done as described in Figure 1. *P < 0.01 versus Ad-βgal–transduced cells. There was no statistically significant difference between untreated and Ad-βgal–transduced cells. Bars show mean ± SEM.
Because constitutive Gax expression can induce apoptosis in cells at 24–48 hours after mitogen stimulation (49), we also performed an MTS assay (40) to ensure that the antimigratory effect was not due to cell loss at these earlier time points. At the time point when the migration experiments were performed (5 hours) and also at 12 hours, MTS assays showed no detectable decrease in cell viability at 300 moi (not shown). These data indicate a specific antimigratory effect of gax, rather than an apoptotic effect resulting from the sustained expression of this transgene or viral toxicity.
Adhesion to coated polycarbonate membranes is a prerequisite for migration in this assay. Thus, the extent of rat VSMCs adhesion to 96-well plates coated with the same mixture of adhesive ligands used for the migration assay was determined for transduced and nontransduced cells. The concentrations of ECM proteins were chosen as described previously (19, 31, 42–44). Under these conditions, the number of Ad-βgal–treated (95.3 ± 3.1%) and Ad-gax–treated cells (97.1 ± 4.4%) adhering to the wells did not differ significantly from uninfected control cells (data not shown). Therefore, an alteration in adhesion to ECM was not responsible for the attenuated cell migration caused by gax overexpression.
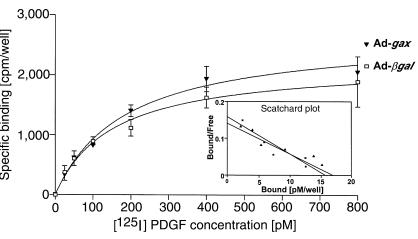
Radioligand binding experiments with 125I-labeled PDGF were performed to test whether the reduced migratory response of Ad-gax–transduced VSMCs toward PDGF-BB is due to a downregulation of PDGF-receptor expression (43). Figure 3 shows the binding curves of Ad-βgal– and Ad-gax–transduced VSMCs. Scatchard plot of both binding curves indicates a single high-affinity PDGF-BB binding site in both cases (Figure 3, inset). Comparison of Ad-βgal– to Ad-gax–transduced cells by Scatchard analysis revealed no statistically significant difference of either PDGF-receptor affinity (KD 108 ± 10 pM for Ad-βgal versus 115 ± 11 pM for Ad-gax; NS) or total receptor number per well (Bmax 15.7 pM for Ad-βgal pM versus 16.5 pM for Ad-gax).
Figure 3.
Transduction of gax does not alter PDGF receptor expression or affinity for ligand. Specific binding curve of [125I]PDGF-BB to rat VSMCs. Subconfluent rat VSMCs were transduced with Ad-βgal or Ad-gax at an moi of 300 for 24 hours. Twelve hours after removal of virus, cell were incubated in binding buffer containing 25–800 pM [125I]PDGF-BB for 2 hours on ice. Triplicate cell lysates were counted using a gamma counter. Nonspecific binding, determined by competition with a 200-fold molar excess of non-radiolabeled PDGF-BB, was subtracted. The graph insert shows the Scatchard plot of both binding curves, indicating a single high-affinity PDGF-BB binding site. No statistically significant differences were obtained for either KD or total receptor number/well (Bmax) between Ad-gax– and Ad-βgal–transduced VSMCs.
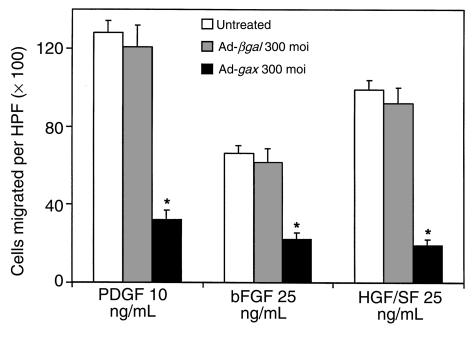
Migration to other known VSMC chemotactic growth factors was evaluated to determine if the antimigratory effect of Ad-gax overexpression is restricted to PDGF-BB–mediated chemotaxis. Concentrations of bFGF (bFGF = FGF-2) and HGF/SF were chosen to provide an optimal migratory response; the maximal chemotactic effect for both growth factors was lower than that obtained for PDGF-BB (Figure 4), in agreement with the results of others (20). Transduction with Ad-gax at an moi of 300 resulted in nearly complete inhibition of directed VSMC migration independent of the chemotactic agent used, whereas transduction with Ad-βgal had no effect (Figure 4).
Figure 4.
Transduction of gax inhibits VSMC migration to multiple growth factors. Effect of adenoviral overexpression of Gax on chemotaxis toward different VSMC growth factors. The migration experiment was performed using PDGF-BB, bFGF, and HGF/SF. The concentration used for each growth factor was previously determined to provide the optimal migratory response. *P < 0.01 versus Ad-βgal–transduced cells. Bars show mean ± SEM.
Overexpression of p21 or p16 is not sufficient to reduce VSMC motility, but Gax-induced inhibition of migration requires cell-cycle block.
p21 serves as a negative regulator of cell-cycle progression by inhibiting cyclin-dependent kinase activity through binding to cyclin-cdk complexes (50). Previous experiments have shown that Ad-gax induced cell-cycle arrest in wild-type fibroblasts, but no growth inhibition was found in a p21–/– fibroblast cell line, suggesting that inhibition of cell growth by gax depends on its ability to upregulate p21 expression (14). To explore the relationship between the antiproliferative and antimigratory activities of Gax, the effect of adenoviral overexpression of p21 (Ad-p21) on the migration of rat VSMCs was analyzed. Moreover, to investigate the effect of the second cdk-inhibitor family (INK4s), which bind to cyclin D-cdk4/6 (51, 52), on cell migration, we overexpressed a member of this family, p16, as an alternative way to induce cell-cycle arrest in VSMCs. Transduction of VSMCs with Ad-p21 or Ad-p16 at 300 moi did not result in statistically significant attenuation of directed cell migration toward different concentrations of PDGF-BB (Figure 5). These data show that overexpression of negative regulators of cell cycle activity is not sufficient to influence VSMC motility.
Figure 5.
Transduction of cell-cycle regulatory genes p21 and p16 does not inhibit VSMC migration. Effect of adenoviral overexpression of p16 (Ad-p16) or p21CIP1 (Ad-p21) on chemotaxis of rat VSMCs toward PDGF-BB. Subconfluent rat VSMCs were transduced with Ad-βgal, Ad-p16, or Ad-p21 at an moi of 300 for 24 hours. The migration experiment was performed 12 hours after removal of virus, using PDGF-BB as chemoattractant. Bars show mean ± SEM. The differences between uninfected and Ad-βgal–, Ad-p16–, and Ad-p21–transduced cells are not statistically significant.
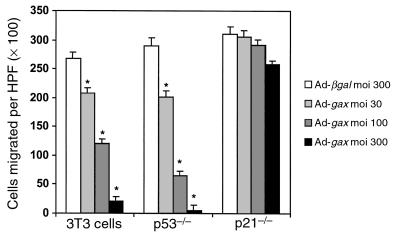
The ability of Gax overexpression to inhibit cell migration was also examined in NIH·3T3 fibroblasts and in MEFs defective for either p53 (p53–/– MEFs) or p21 (p21–/– MEFs). It is well established that p53 can induce growth arrest by transactivating p21 expression (53). In 3T3 fibroblasts and p53–/– MEFs, adenoviral overexpression of Gax led to a dose-dependent inhibition of directed migration toward PDGF-BB compared with βgal control virus, in a manner similar to that observed with VSMCs (at moi 100: in 3T3 cells 55 ± 6.7%, in p53–/– cells 78 ± 12% inhibition of migration versus Ad-βgal; P < 0.01) (Figure 6). Surprisingly, gax transduction in p21–/– cells had no significant effect on cellular motility, even at high moi’s (moi 300: 10.1 ± 3.4% inhibition versus Ad-βgal; P = NS). Collectively, these data suggest that p21 is essential for the antimigratory effects of Gax but that overexpression of p21 is not sufficient to alter motility.
Figure 6.
The cdk inhibitor p21 is essential for Gax-mediated inhibition of migration. Effect of adenoviral overexpression of Gax at different moi’s (30, 100, 300) compared with Ad-βgal (moi 300) on the migratory response toward PDGF-BB (10 ng/mL) of wild-type 3T3 cells, p53–/–MEFs, and p21–/– MEFs, derived from homozygous disruption of the gene encoding p53 and p21, respectively. The experiment was done as described in Figure 1. *P < 0.01 versus Ad-βgal–transduced cells. Bars show mean ± SEM. Migration of untreated cells did not differ from that of Ad-βgal–infected cells (not shown).
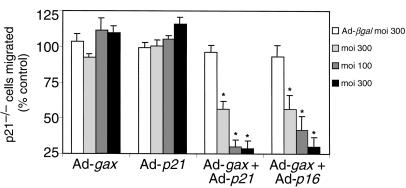
To demonstrate that the inability of gax to inhibit migration in p21–/– cells was due to a specific lack of p21, leading to an inability to induce cell-cycle arrest, we tested whether the gax-mediated antimigratory effect could be rescued by reconstitution of p21–/– cells with p21, or, alternatively, with p16. As shown in Figure 7, sole replacement of p21 by Adeno-p21 transduction, or forced expression of p16 by Adeno-p16 (data not shown), did not result in any significant inhibition of directed migration, which was comparable to the lack of any effect by Ad-gax in these p21–/– cells at any moi examined. However, concomitant transduction with Ad-p21 and Ad-gax resulted in an inhibition of directed cell migration that depended on the dose of Ad-gax (at moi 100: 29 ± 5.0% migration for Ad-gax + Ad-p21 versus 96 ± 4.3% Ad-βgal; P < 0.01; Figure 7). Alternatively, concomitant transduction with Ad-p16 and Ad-gax significantly reduced migration of these p21–/– cells in a comparable manner (41 ± 10% for Ad-gax+Ad-p16 versus 93 ± 7.7% Ad-βgal; P < 0.01; Figure 7, right bars). Thus, the ability of Ad-gax to inhibit p21–/– cell migration after reconstitution with either exogenous p21 or p16, to induce cell-cycle block, was similar to that seen in quiescent VSMCs and NIH·3T3 fibroblasts.
Figure 7.
The antimigratory effect of Gax in p21–/– cells can be rescued by reconstitution with p21 or by overexpression of p16. Effect of adenoviral overexpression of Gax, p21, or a combination of Gax and p21 or Gax and p16 at different moi’s (30, 100, or 300 each) is compared with the effect of Ad-βgal (moi 300) on the migratory response of p21–/– MEFs toward 10 ng/mL PDGF-BB. The bars indicate the percentage of cells migrated compared with their untreated controls. The experiment was done as described in Figure 1. *P < 0.01 versus Ad-βgal–transduced cells. Bars show mean ± SEM.
It was reported previously that plating of smooth muscle cells on polymerized in contrast to monomer collagen induces cell-cycle arrest mainly through induction of p27, a member of the cip/kip family of cdk-inhibitors (36). We therefore plated p21–/– cells on polymerized collagen for 48 hours to inhibit cell-cycle progression or on monomer collagen, before transduction with Ad-gax or Ad-βGal at 100 moi for further 24 hours and analysis of cell migration toward 10 ng/mL PDGF-BB. In p21–/– cells plated on monomerized collagen (“proliferative state” according to Koyama et al. [36]), there was no statistically significant antimigratory effect of Ad-gax versus Ad-βgal (211 ± 24 versus 249 ± 29 migrated cells; NS; data not shown). In contrast, in p21–/– cells plated on polymerized collagen (G1 arrest by upregulation of p27 [ref. 36]), Ad-gax exhibited a significant antimigratory activity compared with Ad-βgal (188 ± 18 versus 89 ± 22 migrated cells; P < 0.05; data not shown). This experiment provides additional evidence that gax-induced inhibition of cell migration depends on cell-cycle arrest.
Overexpression of gax gene alters integrin expression.
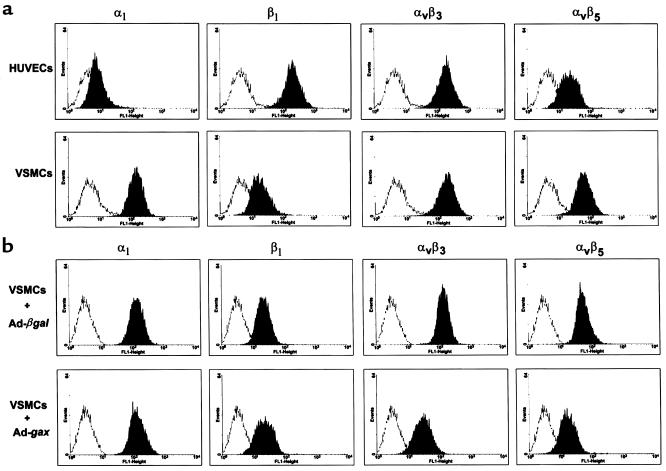
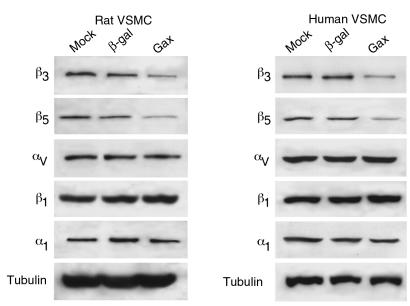
In VSMCs, integrins of the β1 (31, 54, 55) as well as the β3 family (55, 56) have been noted to be involved in promoting migration. Recently, it has also been shown that the vitronectin receptor αvβ3 is required for VSMC motility (57). We therefore analyzed the cell-surface expression of the integrins α1, β1, αvβ3, and αvβ5 by FACS after transduction with Ad-βgal or Ad-gax at 300 moi in VSMCs. Although mAb’s LM609 and P1F6 are not suitable for immunohistochemical detection of αvβ3 and αvβ5 in rodent tissues, FACS analyses with these reagents produced well-defined peaks with high mean fluorescence intensities in rat VSMCs as well as human umbilical vein endothelial cells (HUVECs) (Figure 8a). As shown in Figure 8b, α1 and β1 integrin content on VSMCs was unaffected by gax overexpression, whereas expression of the vitronectin receptors αvβ3 and αvβ5 were significantly reduced compared with Ad-βgal (mean fluorescence 190 ± 18 versus 94.7 ± 12 and 145 ± 21 versus 76.2 ± 14 respectively; P < 0.01). Similarly, Ad-gax decreased αvβ3 and αvβ5 expression on HUVECs (data not shown). Western blot analysis of whole cell extracts of rat or human VSMCs revealed that the β3 and β5 integrin subunit content was decreased in Ad-gax–infected cells, whereas αv subunit expression was unaffected (Figure 9). Also consistent with the results of the FACS analyses (Figure 8), infection with Ad-gax had no effect on β1 or α1 integrin subunit expression (Figure 9), indicating the specificity of Gax action.
Figure 8.
Gax downregulates αvβ3 and αvβ5 expression on VSMCs. (a) Comparison of integrin signal intensities on rat VSMCs and HUVECs by flow cytometric analysis. (b) Flow cytometric analysis of integrin expression in rat VSMCs after transduction with Ad-βgal (upper row) or Ad-gax (lower row). Quiescent cells were transduced at an moi of 300 for 24 hours, and fresh medium was added for an additional 12 hours. VSMCs were harvested with EDTA, incubated with anti-integrin antibodies for 45 minutes followed by incubation with FITC-conjugated anti-mouse IgG, and analyzed by flow cytometry. The histograms show fluorescence intensity (x axis) plotted against number of events (y axis). The fluorescence of the corresponding isotype-matched control IgG appears on the left of each histogram.
Figure 9.
Gax specifically downregulates expression of β3 and β5 integrin subunits in rat and human VSMCs. Cultured VSMCs (rat: left panels; human: right panels) were infected with adenovirus at an moi of 300 (Ad-gax: Gax; Ad-βgal: β-gal) or uninfected (mock) in low-serum media (0.5% FBS) for 12 hours. After infection, cell were incubated with 10% FBS–containing media for 24 hours as described in Methods. Whole-cell extracts (30 μg protein) were prepared from the cultures and subjected to SDS-PAGE on 7.5% polyacrylamide gels. Immunoblot analysis was performed for each indicated integrin subtype (β3, β5, β1, α1) or tubulin as a control, and immunoreactive bands were viewed by chemiluminescence.
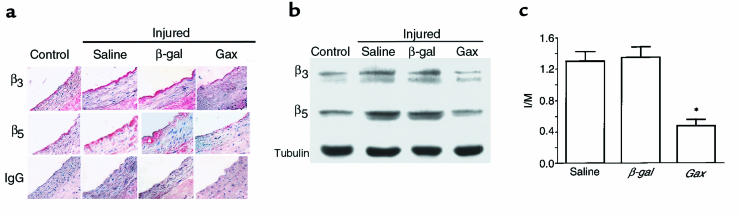
Treatment with Ad-gax decreases injury-induced intimal hyperplasia (14, 58, 59). Therefore, we evaluated the effects of Ad-gax infection on β3 and β5 integrin expression in balloon-injured rat carotid arteries. Acute balloon injury led to an upregulation of both β3 and β5 subunits in medial VSMCs at 3 days after injury (Figure 10), consistent with previous reports (60, 61). Similar to its expression pattern in injured baboon brachial arches (60), β3 expression was notable on the luminal surface of the injured vessel, whereas β5 expression was more uniform throughout the media at 3 days after injury (Figure 10a). Infection with Ad-gax at the time of injury markedly decreased β3 and β5 expression at 3 days as determined by immunohistochemistry (Figure 10a) and by quantitative immunoblot analysis of these tissues (Figure 10b). At 2 weeks after injury, local delivery of Ad-gax inhibited neointima formation under these experimental conditions (Figure 10c).
Figure 10.
Transduction of the gax gene inhibits injury-induced integrin expression and intimal hyperplasia in rat carotid arteries. (a) Upregulation of β3 integrin and β5 integrin is inhibited by adenovirus-mediated gax gene transfer. Arteries were harvested 3 days after balloon injury and incubation with the indicated adenoviral construct (βgal or Gax) or saline. Uninjured, contralateral arteries served as a control. β3 and β5 integrin antibody immunoreactivity was detected in frozen sections using SuperSensitive Immuno Detection System (red). Each specimen was counterstained with hematoxylin (blue). (b) Immunoblot analysis was performed using extracts (30 μg) prepared from rat carotid arteries treated as described in the text. Immunoreactivity to integrin β3, β5, and tubulin was detected by chemiluminescence. (c) Transduction of the gax gene inhabits neointima formation at 2 weeks after injury and gene transfer. The ratio of intimal (I) to medial (M) area is shown.
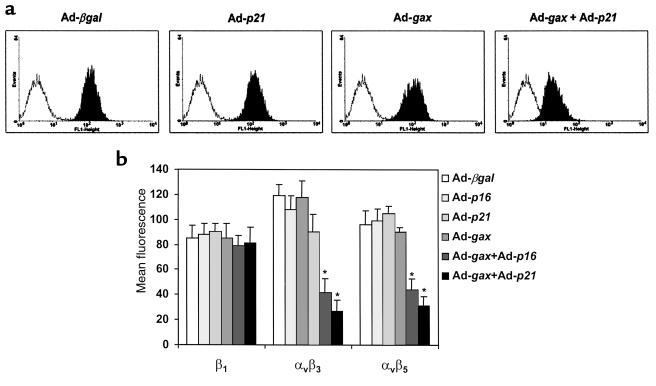
Taken together, these results suggest that the downregulation of the vitronectin receptors αvβ3 and αvβ5 by Gax overexpression might be the cause for reduced cell motility on ECM containing vitronectin. To determine whether Gax-regulated integrin expression is linked to cell-cycle activity in the same manner as Gax-regulated migration, we tested integrin surface expression on p21–/– MEFs depending on the presence of different cell-cycle regulators. Although sole overexpression of p16, p21, or Gax had no effect on the expression of αvβ3 or αvβ5 on p21–/– MEFs, the combination of Ad-gax with adenoviral cell-cycle inhibitor p16 or, alternatively, by phenotype rescue with Ad-p21 significantly downregulated surface expression of these 2 integrin heterodimers (αvβ3: mean fluorescence 42 ± 11 for Ad-gax + Ad-p16 and 27 ± 9 for Ad-gax + Ad-p21 versus 119 ± 9 for Ad-βgal; P < 0.01 each; αvβ5: 44 ± 9 and 31 ± 8 versus 96 ± 11, respectively; P < 0.01; Figure 11, a and b). Expression of α1 (data not shown) and β1 integrin (Figure 11b, left bars) did not change for all conditions tested. Taken together, Gax-mediated downregulation of αvβ3 and αvβ5 was observed only under conditions of cell-cycle arrest, a pattern of regulation that was identical to the observed effects of Gax on cell migration.
Figure 11.
The cdk inhibitor p21 is essential for Gax-mediated downregulation of integrin expression, and reconstitution of p21–/– cells with p21, or overexpression of p16, will restore the ability of Gax to suppress integrin expression. (a) Flow cytometric analysis of αvβ3 integrin expression in p21–/– MEFs after transduction with Ad-βgal, Ad-p21, Ad-gax or a combination of Ad-gax and Ad-p21 at an moi of 300. The experiment was done as described in Figure 8. (b) Integrin β1, αvβ3, and αvβ5 expression in p21–/– cells after transduction with either Ad-βgal, Ad-p21, Ad-p16 or Ad-gax alone, or Ad-gax in combination with Ad-p21 or Ad-p16. Cells were transduced at an moi of 300 for 24 hours followed by addition of fresh medium for further 12 hours. Bars show mean ± SEM from 3 experiments. *P < 0.01 versus Ad-βgal–transduced cells.
Discussion
Gax is a member of the growth-arrest specific (gas) (62) and growth arrest and DNA damage-inducible (gadd) (63) families of genes. Like gax, these genes are expressed at their highest levels in quiescent cells and are downregulated after mitogen activation. Recently, it was demonstrated that recombinant Gax homeodomain protein inhibits cell growth by microinjection of recombinant Gax protein or by gax expression from plasmid or replication-defective adenovirus vectors (14). In addition to their involvement in proliferation and differentiation, homeobox transcription factors are also known to affect cell migration. It has been shown that the homeobox genes goosecoid in Xenopus (1) and the HOM-C gene mab-5 in Caenorhabditis elegans (2) not only direct region-specific patterns of cell division and differentiation, but can also act upon migrating cells to induce region-specific migratory behavior during development through unknown mechanisms. The study presented here provides direct evidence that the Gax homeobox transcription factor can inhibit migration through a cell cycle–dependent mechanism that is associated with the downregulation of the integrins αvβ3 and αvβ5. The observed reduction of migratory behavior depended on the dose of the adenoviral construct expressing Gax, but it was not due to nonspecific viral toxicity nor did it depend on a particular growth factor–receptor interaction.
Previous work has shown that the cdk inhibitor p21CIP1 mediates the growth inhibitory actions of Gax (14). Gax overexpression does not have a growth inhibitory effect in cells derived from p21-knockout mice, suggesting that the upregulation of this cdk inhibitor can largely account for the antiproliferative actions of Gax. In this study, overexpression of p21 alone did not alter directed migration of VSMCs. This result suggests that signaling pathways leading to cell migration are distinct from those of proliferation. Consistent with this hypothesis, it has been shown that although both PDGF-BB and IGF-1 are chemotactic for VSMCs, IGF-1 has a low mitogenic potency in human arterial smooth muscle cells (64). Moreover, studies with labeled VSMCs in an injured rat carotid artery indicate that a fraction of the cells migrate into the intima without proliferating (65), further supporting the contention that SMC proliferation and migration are separable phenomena. However, previous studies have demonstrated an outside-in link between integrin engagement and the cdk inhibitor p21 (66), providing evidence for cross-talk between regulatory pathways that control cell migration and growth.
Here, evidence is provided for overlapping regulatory mechanisms in the transcriptional control of VSMC proliferation and migration. It is shown that Gax was unable to suppress the chemotactic response of cells toward PDGF when these cells contained a homozygous disruption of the gene encoding p21 (p21–/– cells), whereas lack of p53, an upstream regulator or p21, did not alter migratory behavior of cells with an isogenic background. Adenovirus-mediated reconstitution of p21 rescued the defect in p21–/– cells, and Gax overexpression was able to block PDGF-induced chemotaxis. The rescuing effect of p21 could be replaced by overexpression of p16, a member of the INK4 cdk-inhibitor family, or, alternatively, by plating cells on polymerized collagen, which induces cell-cycle arrest by the upregulation of p27 (36). Although neither p21 nor p16 is sufficient to inhibit migration by itself, cell-cycle arrest by one of these negative cell-cycle regulators is essential for Gax-induced inhibition of migration. Collectively, these data suggest that overlapping regulatory mechanisms can coordinate VSMC proliferation and migration at the transcriptional level.
It has been proposed that homeobox transcription factors control pattern formation during embryogenesis partly through their ability to regulate the expression of extracellular membrane proteins that mediate cell-matrix interactions and cell movement (67). Potential targets of gax action that might influence migration include integrins, key regulators of adhesion and cell motility that are believed to be of importance in intimal lesion formation upon vascular injury (68–71). Adhesion to and migration on ECM proteins are mediated by different integrin heterodimers. To date, several β1 (27–31, 54) and β3 (55, 56, 60, 61) integrins have been identified on smooth muscle cells. Although integrins of the β1 family appear to be the dominant integrins responsible for VSMC adhesion to the ECM (27, 56), and also appear to be involved in VSMC migration (20, 31, 55), migration of VSMCs has been noted to depend on β3 integrins (55, 56). In particular, the vitronectin receptor (VNR) αvβ3 appears to be required for SMC motility (57). During wound healing response, migrating cells exhibit enhanced expression of VNR integrins, including αvβ3 (71–73). Our finding that αvβ3 is downregulated after gax transduction could account for the observed reduction in cell motility on an ECM substrate containing vitronectin. In addition, we show that the other known VNR, αvβ5, is expressed on rat VSMCs and downregulated by gax transduction, although its role in VSMC motility has not been clearly defined yet. Western immunoblot analyses revealed that VNR downregulation by Gax was mediated by the specific repression of β3 and β5 subunits, whereas αv, α1, and β1 expression were not affected by Gax. The observed attenuation of SMC migration by gax was not due to an inhibition of cellular adhesion. This could be explained by the fact that expression of β1 integrin, which is able to bind multiple adhesive ligands including type I collagen (26), was unchanged. Consistent with our observations, it has been demonstrated that blocking αvβ3 function with the monoclonal anti-αvβ3 antibody LM609 does not alter VSMC attachment, but it does significantly reduce cellular migration on vitronectin- (57) or fibronectin-coated (74) membranes.
Our findings also show that cdk inhibitors are essential for Gax-mediated alterations in integrin expression. Gax was found to have no effect on αvβ3 or αvβ5 expression in p21–/– cells unless the defect was rescued by p21 or p16 gene transfer. Therefore, the observation that cdk inhibitors are also essential for the inhibition of migration resulting from Gax overexpression provides compelling evidence that Gax-induced inhibition of migration is due to a cell cycle–dependent alteration in integrin expression. As integrins are also recognized as key regulators of cell viability and proliferation (75), these data also suggest that the pleiotropic activities of Gax overexpression on growth and survival (14, 49, 59) may be mediated, at least in part, through its ability to interfere with integrin-regulated signaling pathways within vascular cells.
We have previously shown that gax is downregulated in the rat carotid artery in response to balloon injury (6, 9), which provides a proliferative signal to VSMCs (76), and that adenoviral overexpression of gax reduces neointima formation in rat carotid (14, 59) and rabbit iliac (58) models of balloon angioplasty. Because migration of VSMCs from the medial to the luminal side of the vessel is regarded as a key event in restenosis after balloon angioplasty (16), the antimigratory properties of Gax on VSMCs may contribute to the observed reduction in neointima formation. Consistent with this hypothesis, reductions in β3 and β5 integrin expression were observed after gax gene transfer to injured arterial vessels under conditions that inhibit neointima formation. Furthermore, αvβ3 appears to have a role in VSMC migration and neointima formation after endothelial denudation (61, 68, 69, 77, 78), and our finding that Gax downregulates αvβ3 integrin expression in VSMCs fits well into this paradigm. Therefore, the integrin-regulatory activities of gax suggest that transduction of this gene represents a promising molecular tool for dissecting the regulatory pathways that control the behavior of VSMCs in proliferative lesions of the vessel wall.
Acknowledgments
The authors thank R.C. Smith for helpful discussions and careful reading of the manuscript. This study was supported by grants from the National Institutes of Health (RO1HL50692, RO1AR40197, and R01AG15052).
References
- 1.Niehrs C, Keller R, Cho KWY, De Robertis EM. The homeobox gene goosecoid controls cell migration in Xenopus embryos. Cell. 1993;72:491–503. doi: 10.1016/0092-8674(93)90069-3. [DOI] [PubMed] [Google Scholar]
- 2.Salser SJ, Kenyon C. Activation of a C. elegans Antennapedia homologue in migrating cells controls their direction of migration. Nature. 1992;355:255–258. doi: 10.1038/355255a0. [DOI] [PubMed] [Google Scholar]
- 3.Gorski DH, Patel CV, Walsh K. Homeobox transcription factor regulation in the cardiovascular system. Trends Cardiovasc Med. 1993;3:184–190. doi: 10.1016/1050-1738(93)90004-P. [DOI] [PubMed] [Google Scholar]
- 4.Olson EN, Rosenthal N. Homeobox genes and muscle patterning. Cell. 1994;79:9–12. doi: 10.1016/0092-8674(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 5.Scott MP, Tamkun JW, Hartzell GW. The structure and function of the homeodomain. Biochim Biophys Acta. 1989;989:25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- 6.Gorski DH, Walsh K. Mitogen-responsive nuclear factors that mediate growth control signals in vascular myocytes. Cardiovasc Res. 1995;30:585–592. [PubMed] [Google Scholar]
- 7.Gorski DH, LePage DF, Copeland NG, Jenkins NA, Walsh K. Molecular cloning of a diverged homeobox gene that is rapidly down-regulated during the G0/G1 transition in vascular smooth muscle cells. Mol Cell Biol. 1993;13:3722–3733. doi: 10.1128/mcb.13.6.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamashita J, et al. Opposite regulation of gax homeobox expression by angiotensin II and C-type natriuretic peptide. Hypertension. 1997;29:381–387. doi: 10.1161/01.hyp.29.1.381. [DOI] [PubMed] [Google Scholar]
- 9.Weir L, Chen D, Pastore C, Isner JM, Walsh K. Expression of gax, a growth arrest homeobox gene, is rapidly down-regulated in the rat carotid artery during the proliferative response to balloon injury. J Biol Chem. 1995;270:5457–5461. doi: 10.1074/jbc.270.10.5457. [DOI] [PubMed] [Google Scholar]
- 10.Skopicki HA, et al. Embryonic expression of the gax homeodomain protein in cardiac, smooth, and skeletal muscle. Circ Res. 1997;80:452–462. doi: 10.1161/01.res.80.4.452. [DOI] [PubMed] [Google Scholar]
- 11.Edmondson DG, Lyons GE, Martin JF, Olson EN. Mef2 gene expression marks the cardiac and skeletal muscle lineages during mouse embryogenesis. Development. 1994;120:1251–1263. doi: 10.1242/dev.120.5.1251. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki E, Guo K, Kolman M, Yu Y-T, Walsh K. Serum induction of MEF2/RSRF expression in vascular myocytes is mediated at the level of transcription. Mol Cell Biol. 1995;15:3415–3423. doi: 10.1128/mcb.15.6.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andres V, Fisher S, Wearsch P, Walsh K. Regulation of gax homeobox gene transcription by a combination of positive factors including myocyte-specific enhancer factor 2. Mol Cell Biol. 1995;15:4272–4281. doi: 10.1128/mcb.15.8.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith RC, et al. p21CIP1-mediated inhibition of cell proliferation by overexpression of the gax homeodomain gene. Genes Dev. 1997;11:1674–1689. doi: 10.1101/gad.11.13.1674. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz SM, Campbell GR, Campbell JH. Replication of smooth muscle cells in vascular disease. Circ Res. 1986;58:427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- 16.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 17.Casscells W. Endothelial and smooth muscle cell migration: critical factors in restenosis. Circulation. 1992;86:723–729. doi: 10.1161/01.cir.86.3.723. [DOI] [PubMed] [Google Scholar]
- 18.Grotendorst CR, Chang T, Seppa HEJ, Kleinman HK, Martin GR. Platelet-derived growth factor is a chemoattractant for vascular smooth muscle cells. J Cell Physiol. 1982;113:261–266. doi: 10.1002/jcp.1041130213. [DOI] [PubMed] [Google Scholar]
- 19.Bilato C, et al. Intracellular signaling pathways required for rat vascular smooth muscle cell migration. J Clin Invest. 1995;96:1905–1915. doi: 10.1172/JCI118236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickering JG, et al. Fibroblast growth factor-2 potentiates vascular smooth muscle cell migration to platelet-derived growth factor. Upregulation of α2β1 integrin and disassembly of actin filaments. Circ Res. 1997;80:627–637. doi: 10.1161/01.res.80.5.627. [DOI] [PubMed] [Google Scholar]
- 21.Jackson CL, Reidy MA. Basic fibroblast growth factor: its role in the control on smooth muscle cell migration. Am J Pathol. 1993;143:1024–1031. [PMC free article] [PubMed] [Google Scholar]
- 22.Boudreau N, Turley E, Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991;143:235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- 23.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molossi S, et al. Blockade of very late antigen-4 integrin binding to fibronectin with connecting segment-1 peptide reduces accelerated coronary arteriopathy. J Clin Invest. 1995;95:2601–2610. doi: 10.1172/JCI117962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savani RC, et al. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J Clin Invest. 1995;95:1158–1168. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hynes RO. Integrins: versatility, modulation, and signalling in cell adhesion. Cell. 1992;69:11–24. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 27.Clyman RI, McDonald KA, Kramer RH. Integrin receptors on aortic smooth muscle cells mediate adhesion to fibronectin, laminin, and collagen. Circ Res. 1990;67:175–186. doi: 10.1161/01.res.67.1.175. [DOI] [PubMed] [Google Scholar]
- 28.Clyman RI, Turner DC, Kramer RH. An α1/β1-like integrin receptor on rat aortic smooth muscle cells mediates adhesion to laminin and collagen types I and IV. Arteriosclerosis. 1990;10:402–409. doi: 10.1161/01.atv.10.3.402. [DOI] [PubMed] [Google Scholar]
- 29.Bottger BA, Hedin U, Johansson S, Thyberg J. Integrin-type fibronectin receptors of rat arterial smooth muscle cells: isolation, partial characterization and role in cytoskeletal organization and control of differentiated properties. Differentiation. 1989;41:158–167. doi: 10.1111/j.1432-0436.1989.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 30.Lee RT, Berditchevski F, Cheng GC, Hemler ME. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res. 1995;76:209–214. doi: 10.1161/01.res.76.2.209. [DOI] [PubMed] [Google Scholar]
- 31.Skinner MP, Raines EW, Ross R. Dynamic expression of α1β1 and α2β2 integrin receptors by human vascular smooth muscle cells: α2β1 integrin is required for chemotaxis across type I collagen coated membranes. Am J Pathol. 1994;145:1070–1081. [PMC free article] [PubMed] [Google Scholar]
- 32.Mader SL. Influence of animal age on the β-adrenergic system in cultured rat aortic and mesenteric artery smooth muscle cells. J Gerontol. 1992;47:B32–B36. doi: 10.1093/geronj/47.2.b32. [DOI] [PubMed] [Google Scholar]
- 33.Pickering JG, et al. Smooth muscle cell outgrowth from human atherosclerotic plaque: implications for the assessment of lesion biology. J Am Coll Cardiol. 1992;20:1430–1439. doi: 10.1016/0735-1097(92)90259-p. [DOI] [PubMed] [Google Scholar]
- 34.Deng C, Zhang P, Harper J, Elledge S, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–684. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 35.Harvey D, Levine A. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 1991;5:2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 36.Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of cdk2 inhibitors. Cell. 1996;87:1069–1078. doi: 10.1016/s0092-8674(00)81801-2. [DOI] [PubMed] [Google Scholar]
- 37.Clayman GL, et al. Gene therapy for head and neck cancer. Comparing the tumor suppressor gene p53 and a cell cycle regulator WAF1/CIP1 (p21) Arch Otolaryngol Head Neck Surg. 1996;122:489–493. doi: 10.1001/archotol.1996.01890170025006. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Guo K, Wills KN, Walsh K. Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 1997;57:351–354. [PubMed] [Google Scholar]
- 39.Stratford-Perricaudes LD, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1993;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buttke TM, McCubrey JA, Owen TC. Use of an aqueous soluble tetrazolium/formazan assay to measure viability and proliferation of lymphokine-dependent cell lines. J Immunol Methods. 1993;157:233–240. doi: 10.1016/0022-1759(93)90092-l. [DOI] [PubMed] [Google Scholar]
- 41.Falk W, Goodwin RHJ, Leonard EJ. A 48 well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 42.Pauly RR, et al. Role of calcium/calmodulin-dependent protein kinase II in the regulation of vascular smooth muscle cell migration. Circulation. 1995;91:1107–1115. doi: 10.1161/01.cir.91.4.1107. [DOI] [PubMed] [Google Scholar]
- 43.Kundra V, et al. Regulation of chemotaxis by the platelet-derived growth factor receptor-β. Nature. 1994;367:474–476. doi: 10.1038/367474a0. [DOI] [PubMed] [Google Scholar]
- 44.Grzesiak JJ, Davies GE, Kirchhofer D, Pierschbacher MD. Regulation of α2β1-mediated fibroblast migration on type I collagen by shifts in the concentrations of extracellular Mg2+ and Ca2+ J Cell Biol. 1992;117:1109–1117. doi: 10.1083/jcb.117.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koyama N, Hart CE, Clowes AW. Different functions of the Platelet-derived growth factor-α and -β receptors for the migration and proliferation of cultured baboon smooth muscle cells. Circ Res. 1994;75:682–691. doi: 10.1161/01.res.75.4.682. [DOI] [PubMed] [Google Scholar]
- 46.Gruber B, Marchese M, Kew R. Angiogenic factors stimulate mast-cell migration. Blood. 1995;86:2488–2493. [PubMed] [Google Scholar]
- 47.Clesham GJ, Browne H, Efstathiou S, Weissberg PL. Enhancer stimulation unmasks latent gene transfer after adenovirus-mediated gene delivery into human vascular smooth muscle cells. Circ Res. 1996;79:1188–1195. doi: 10.1161/01.res.79.6.1188. [DOI] [PubMed] [Google Scholar]
- 48.Lee SW, Trapnell BC, Rade JJ, Virmani R, Dichek DA. In vivo vector-mediated gene transfer into balloon-injured rat carotid arteries. Circ Res. 1993;73:797–807. doi: 10.1161/01.res.73.5.797. [DOI] [PubMed] [Google Scholar]
- 49.Perlman H, et al. Bax-mediated cell death by the Gax homeoprotein requires mitogen-activation but is independent of cell cycle activity. EMBO J. 1998;17:3576–3586. doi: 10.1093/emboj/17.13.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 51.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 52.Peter M, Herskowitz I. Joining the complex: cyclin-dependent kinase inhibitory proteins and the cell cycle. Cell. 1994;79:181–184. doi: 10.1016/0092-8674(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 53.El-Deiry WS, et al. WAF-1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 54.Seki J, et al. Regulation of β1-integrin function in cultured human vascular smooth muscle cells. Circ Res. 1996;78:596–605. doi: 10.1161/01.res.78.4.596. [DOI] [PubMed] [Google Scholar]
- 55.Yue T-L, et al. Osteopontin-stimulated vascular smooth muscle cell migration is mediated by β3 integrin. Exp Cell Res. 1994;214:459–464. doi: 10.1006/excr.1994.1282. [DOI] [PubMed] [Google Scholar]
- 56.Clyman RI, Mauray F, Kramer RH. β1 and β3 integrins have different roles in the adhesion and migration of vascular smooth muscle cells on extracellular matrix. Exp Cell Res. 1992;200:272–284. doi: 10.1016/0014-4827(92)90173-6. [DOI] [PubMed] [Google Scholar]
- 57.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin α3β3 binding to vitronectin. Nature. 1996;383:441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 58.Maillard L, et al. Percutaneous delivery of the gax gene inhibits vessel stenosis in a rabbit model of balloon angioplasty. Cardiovasc Res. 1997;35:536–546. doi: 10.1016/s0008-6363(97)00147-8. [DOI] [PubMed] [Google Scholar]
- 59.Perlman H, et al. Adenoviral-mediated delivery of the Gax transcription factor to rat carotid arteries inhibits smooth muscle proliferation and induces apoptosis. Gene Ther. 1999;6:758–763. doi: 10.1038/sj.gt.3300893. [DOI] [PubMed] [Google Scholar]
- 60.Stouffer G, et al. Beta3 integrins are upregulated after vascular injury and modulate thrombospondin- and thrombin-induced proliferation of cultured smooth muscle cells. Circulation. 1998;97:907–915. doi: 10.1161/01.cir.97.9.907. [DOI] [PubMed] [Google Scholar]
- 61.Srivatsa S, et al. Selective alpha v beta 3 integrin blockade potently limits neointimal hyperplasia and lumen stenosis following deep coronary arterial stent injury: evidence for the functional importance of integrin alpha v beta 3 and osteopontin expression during neointimal formation. Cardiovasc Res. 1997;36:408–428. doi: 10.1016/s0008-6363(97)00184-3. [DOI] [PubMed] [Google Scholar]
- 62.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–793. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 63.Fornace AJ, Jackman J, Hollander MC, Hoffman-Liebermann B, Liebermann DA. Genotoxic-stress-response genes and growth arrest genes: gadd, MyD, and other genes induced by treatments eliciting growth arrest. Ann NY Acad Sci. 1992;663:139–153. doi: 10.1111/j.1749-6632.1992.tb38657.x. [DOI] [PubMed] [Google Scholar]
- 64.Bornfeldt KE, et al. Insulin-like growth factor-I and platelet-derived growth factor-BB induce directed migration of human arterial smooth muscle cells via signaling pathways that are distinct from those of proliferation. J Clin Invest. 1994;93:1266–1274. doi: 10.1172/JCI117081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res. 1985;56:139–145. doi: 10.1161/01.res.56.1.139. [DOI] [PubMed] [Google Scholar]
- 66.Stroemblad S, Becker JC, Yebra M, Brooks PC, Cheresh DA. Suppression of p53 activity and p21WAF1/CIP1 expression by vascular cell integrin αvβ3 during angiogenesis. J Clin Invest. 1996;98:426–433. doi: 10.1172/JCI118808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edelman GM, Jones FS. Outside and downstream of the homeobox. J Biol Chem. 1993;268:20683–20686. [PubMed] [Google Scholar]
- 68.Choi ET, et al. Inhibition of neointimal hyperplasia by blocking αvβ3 integrin with a small peptide antagonist GpenGRGDSPCA. J Vasc Surg. 1994;19:125–134. doi: 10.1016/s0741-5214(94)70127-x. [DOI] [PubMed] [Google Scholar]
- 69.Matsuno H, Stassen JM, Vermylen J, Deckmyn H. Inhibition of integrin function by a cyclic RGD-containing peptide prevents neointimal formation. Circulation. 1994;90:2203–2206. doi: 10.1161/01.cir.90.5.2203. [DOI] [PubMed] [Google Scholar]
- 70.Topol E, et al. Randomized trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. Lancet. 1994;343:881–886. doi: 10.1016/s0140-6736(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 71.Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 72.Clark RAF, Tonnesen MG, Gailit J, Cheresh DA. Transient functional expression of alphavbeta 3 on vascular cells during wound repair. Am J Pathol. 1996;148:1407–1421. [PMC free article] [PubMed] [Google Scholar]
- 73.Brooks PC, et al. Integrin αvβ3 antagonists promote further tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 74.Bilato C, et al. The inhibition of vascular smooth muscle cell migration by peptide and antibody antagonists of the αvβ3 integrin complex is reversed by activated calcium/calmodulin-dependent protein kinase II. J Clin Invest. 1997;100:693–704. doi: 10.1172/JCI119582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shattil SJ, Ginsberg MH. Perspectives series: cell adhesion in vascular biology. Integrin signaling in vascular biology. J Clin Invest. 1997;100:1–5. doi: 10.1172/JCI119500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wei GL, et al. Temporally and spatially coordinated expression of cell cycle regulatory factors after angioplasty. Circ Res. 1997;80:418–426. [PubMed] [Google Scholar]
- 77.Deitch J, et al. Effects of beta3-integrin blockade (c7E3) on the response to angioplasty and intra-arterial stenting in atherosclerotic nonhuman primates. Arterioscler Thromb Vasc Biol. 1998;18:1730–1737. doi: 10.1161/01.atv.18.11.1730. [DOI] [PubMed] [Google Scholar]
- 78.van der Zee R, et al. Reduced intimal thickening following αvβ3 blockade is associated with smooth muscle cell apoptosis. Cell Adhes Commun. 1998;6:371–379. doi: 10.3109/15419069809109146. [DOI] [PubMed] [Google Scholar]