Abstract
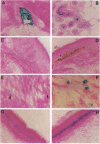
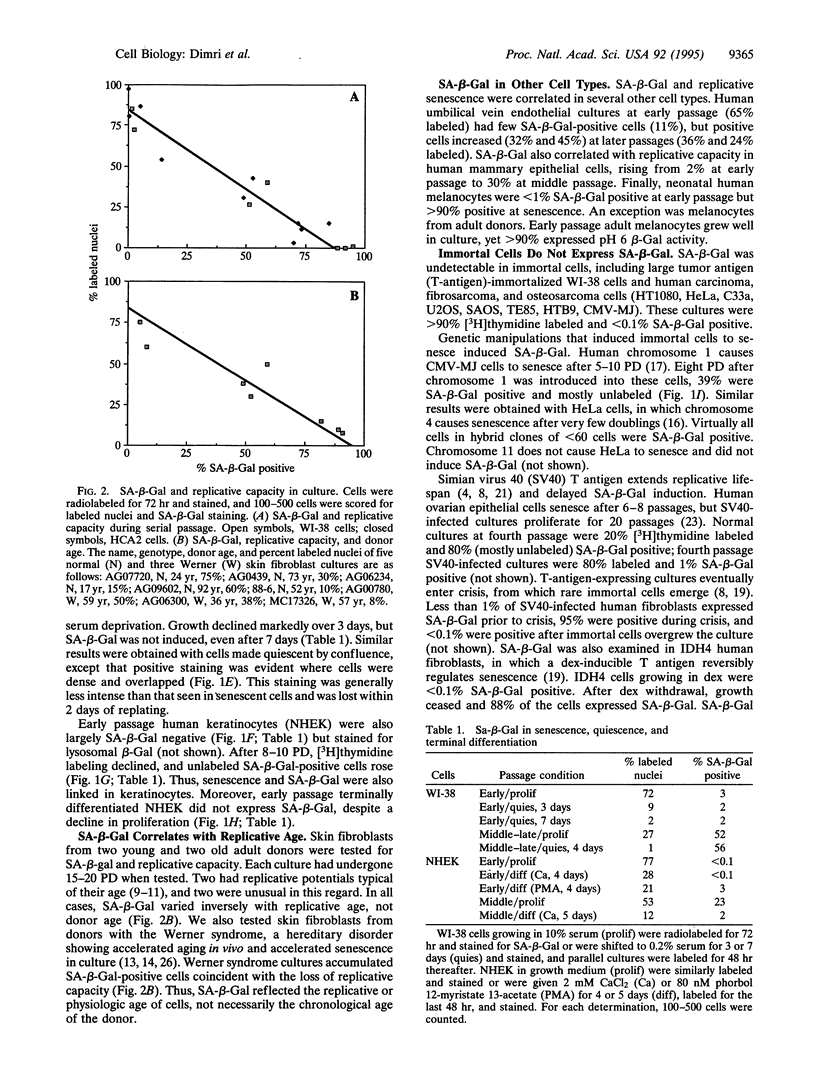
Normal somatic cells invariably enter a state of irreversibly arrested growth and altered function after a finite number of divisions. This process, termed replicative senescence, is thought to be a tumor-suppressive mechanism and an underlying cause of aging. There is ample evidence that escape from senescence, or immortality, is important for malignant transformation. By contrast, the role of replicative senescence in organismic aging is controversial. Studies on cells cultured from donors of different ages, genetic backgrounds, or species suggest that senescence occurs in vivo and that organismic lifespan and cell replicative lifespan are under common genetic control. However, senescent cells cannot be distinguished from quiescent or terminally differentiated cells in tissues. Thus, evidence that senescent cells exist and accumulate with age in vivo is lacking. We show that several human cells express a beta-galactosidase, histochemically detectable at pH 6, upon senescence in culture. This marker was expressed by senescent, but not presenescent, fibroblasts and keratinocytes but was absent from quiescent fibroblasts and terminally differentiated keratinocytes. It was also absent from immortal cells but was induced by genetic manipulations that reversed immortality. In skin samples from human donors of different age, there was an age-dependent increase in this marker in dermal fibroblasts and epidermal keratinocytes. This marker provides in situ evidence that senescent cells may exist and accumulate with age in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosmann H. B., Guthell R. L., Jr, Case K. R. Loss of a critical neutral protease in ageing WI-38 cells. Nature. 1976 Jun 10;261(5560):499–501. doi: 10.1038/261499a0. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Ham R. G. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983 Jul;81(1 Suppl):33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Cristofalo V. J., Kabakjian J. Lysosomal enzymes and aging in vitro: subcellular enzyme distribution and effect of hydrocortisone on cell life-span. Mech Ageing Dev. 1975 Jan-Feb;4(1):19–28. doi: 10.1016/0047-6374(75)90004-4. [DOI] [PubMed] [Google Scholar]
- Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990 Sep 7;249(4973):1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- Hensler P. J., Annab L. A., Barrett J. C., Pereira-Smith O. M. A gene involved in control of human cellular senescence on human chromosome 1q. Mol Cell Biol. 1994 Apr;14(4):2291–2297. doi: 10.1128/mcb.14.4.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994 Dec 23;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- Kruk P. A., Maines-Bandiera S. L., Auersperg N. A simplified method to culture human ovarian surface epithelium. Lab Invest. 1990 Jul;63(1):132–136. [PubMed] [Google Scholar]
- Kuo C. H., Wells W. W. beta-Galactosidase from rat mammary gland. Its purification, properties, and role in the biosynthesis of 6beta-O-D-galactopyranosyl myo-inositol. J Biol Chem. 1978 May 25;253(10):3550–3556. [PubMed] [Google Scholar]
- Maciag T., Hoover G. A., Stemerman M. B., Weinstein R. Serial propagation of human endothelial cells in vitro. J Cell Biol. 1981 Nov;91(2 Pt 1):420–426. doi: 10.1083/jcb.91.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J. A., Statuto M., Ragnotti G. Senescence stimulates U937-endothelial cell interactions. Exp Cell Res. 1993 Sep;208(1):270–274. doi: 10.1006/excr.1993.1246. [DOI] [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Epstein C. J. Replicative life-span of cultivated human cells. Effects of donor's age, tissue, and genotype. Lab Invest. 1970 Jul;23(1):86–92. [PubMed] [Google Scholar]
- Medrano E. E., Nordlund J. J. Successful culture of adult human melanocytes obtained from normal and vitiligo donors. J Invest Dermatol. 1990 Oct;95(4):441–445. [PubMed] [Google Scholar]
- Morreau H., Galjart N. J., Gillemans N., Willemsen R., van der Horst G. T., d'Azzo A. Alternative splicing of beta-galactosidase mRNA generates the classic lysosomal enzyme and a beta-galactosidase-related protein. J Biol Chem. 1989 Dec 5;264(34):20655–20663. [PubMed] [Google Scholar]
- Ning Y., Weber J. L., Killary A. M., Ledbetter D. H., Smith J. R., Pereira-Smith O. M. Genetic analysis of indefinite division in human cells: evidence for a cell senescence-related gene(s) on human chromosome 4. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5635–5639. doi: 10.1073/pnas.88.13.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontén J. The relationship between in vitro transformation and tumor formation in vivo. Biochim Biophys Acta. 1976 Dec 23;458(4):397–422. doi: 10.1016/0304-419x(76)90009-3. [DOI] [PubMed] [Google Scholar]
- Sager R. Senescence as a mode of tumor suppression. Environ Health Perspect. 1991 Jun;93:59–62. doi: 10.1289/ehp.919359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E. L., Mitsui Y. The relationship between in vitro cellular aging and in vivo human age. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3584–3588. doi: 10.1073/pnas.73.10.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Shay J. W., Wright W. E., Werbin H. Defining the molecular mechanisms of human cell immortalization. Biochim Biophys Acta. 1991 Apr 16;1072(1):1–7. doi: 10.1016/0304-419x(91)90003-4. [DOI] [PubMed] [Google Scholar]
- Stanulis-Praeger B. M. Cellular senescence revisited: a review. Mech Ageing Dev. 1987 Mar;38(1):1–48. doi: 10.1016/0047-6374(87)90109-6. [DOI] [PubMed] [Google Scholar]
- West M. D., Pereira-Smith O. M., Smith J. R. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989 Sep;184(1):138–147. doi: 10.1016/0014-4827(89)90372-8. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]