Gastric acid secretion plays an essential role in the pathogenesis of peptic ulcer disease and gastroesophageal reflux disease. These chronic recurring diseases affect approximately 20–40% of the adult population (1) and result in significant morbidity, lost work, and billions of health care dollars. Although peptic ulcer disease has decreased dramatically, gastroesophogeal reflux disease is increasing in prevalence.
Although hydrochloric acid in gastric juice was discovered in 1823 (2), it took three-quarters of a century before most of the major mediators — vagal acetylcholine (ACh), gastrin, and histamine — were identified. However, it would take another 75 years of advances in protein chemistry, immunoassay techniques, isolation of nearly pure cell populations by counterflow elutriation (3), and other cellular and molecular biological methods before the markedly complex regulation of acid secretion would be unveiled in the form summarized below and in ref. 4 and depicted in Figure 1.
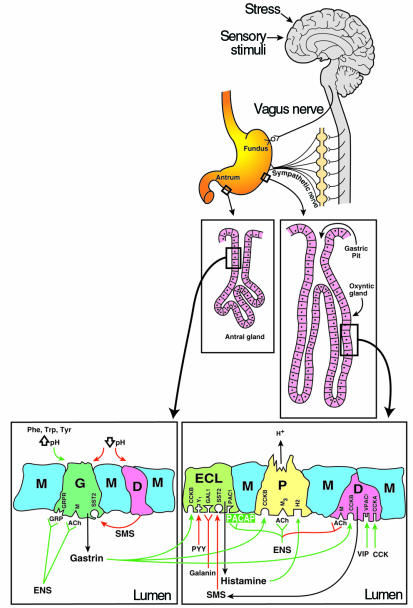
Figure 1.
Cephalic (neural) and peripheral (gastric and intestinal) endocrine and paracrine regulation of gastric acid secretion. G, G cell; P, parietal cell; D, D cell; M cell, mucus cell; ENS, enteric nervous system; GRP, gastrin-releasing peptide; GRPR, GRP receptor; SMS, somatostatin; VIP, vasoactive intestinal polypeptide; M, muscarinic receptor; M3, muscarinic receptor type 3; SST2, somatostatin type 2 receptor; CCKA and CCKB, cholecystokinin type A and B receptors; Y1, PYY type 1 receptor; PYY, peptide YY; H2, histamine type 2 receptor; Gal1, galanin type 1 receptor. Green lines indicate stimulation; red lines indicate inhibition.
Visual and chemical stimuli, hypoglycemia, and stress signals that activate secretion originate in the central nervous system, initiating the cephalic phase of gastric control. Hypoglycemic stimuli are also received in the nucleus tractus solitarius along with taste and modulatory stimuli from the peripheral visceral organs. All of these neuronal signals integrate in the dorsal motor nucleus of the vagus and are transmitted to the stomach via vagal efferents that synapse with the enteric nervous system. The enteric nervous system consists of secondary muscarinic neurons in the gastric wall. These neurons loosely innervate mucosal endocrine cells through the release of ACh and possibly one or more neuropeptide transmitters. ACh directly stimulates acid secretion by gastric parietal (P) cells within fundic oxyntic glands; it also acts indirectly on these cells by stimulating the release of gastrin from antral G cells.
Regulation of histamine production by enterochromaffin-like cells.
Integrated with the cephalic phase of digestion, the peripheral gastric phase of digestion originates in response to the chemical composition and mechanical distension of a meal. Gastrin, released into the circulation from antral G cells in response to luminal aromatic amino acids and pH elevation, acts on enterochromaffin-like (ECL) cells to promote the release of histamine. Histamine, which interacts with histamine type 2 receptors on P cells, serves as the most important direct stimulus for P cells, as evidenced by the effectiveness of histamine type 2 receptor antagonists in the control of acid secretion. The direct action of gastrin on P cells is minimal in the absence of the permissive effect of costimulation by histamine. Similarly, the direct action of ACh at the M3 muscarinic receptor on the P cell is weak and transient compared with its ability to stimulate G cells and (indirectly) ECL cells. In light of the central role of histamine in this pathway, the ECL cell has emerged as a pivotal regulator of acid secretion.
A variety of hormones and neurotransmitters negatively regulate acid secretion, serving to check excess acid secretion and to terminate the process after digestion is complete. Somatostatin released from D cells in the antrum and fundus acts in both a paracrine and an endocrine fashion, feeding back to G cells and ECL cells to tonically inhibit the release of gastrin and histamine, respectively. Antral D cells are stimulated to secrete somatostatin by reduced gastric luminal pH (hence the hypergastrinemia associated with proton pump inhibitors and atrophic gastritis), and are inhibited by ACh. Fundic D cells are stimulated by the release of gastrin, duodenal cholecystokinin, and vasoactive intestinal polypeptide (VIP), a neuropeptide. Enterogastrones (hormones secreted from the intestinal mucosa that inhibit gastric secretion and motility) such as peptide YY, and neuropeptides such as galanin, also inhibit ECL cell function.
The signal from the central nervous system that provokes ECL cells to secrete histamine has been a long-standing puzzle. In the rat, this aspect of gastric acid regulation is mediated primarily by M1 receptors (5). However, cultured ECL cells have responded poorly to the M1 agonist carbachol, raising the possibility that the effect of ACh is indirect, and precipitating a 10-year search for a suspected neural mediator. Pituitary adenylate cyclase-activating polypeptide (PACAP) has become an increasingly likely candidate since its recent identification in gastric enteric neurons (6) and the discovery of its ability to stimulate histamine secretion (7, 8) and proliferation by ECL cells in culture (9). However, peripheral injection of PACAP has been shown to inhibit gastrin-stimulated acid secretion in vivo (10). This finding may be understood as an artifact of peripheral injection: other cell types within the gastric gland might give rise to an opposing (but perhaps nonphysiologic) response to PACAP. Nevertheless, direct evidence that PACAP mediates the effects of central M1 receptor stimulation has been lacking.
Dissecting out cell type–specific responses.
The dispersed nature of gastric epithelial endocrine cells, along with their complex neural interface, makes the task of unraveling the physiologic role of a new mediator of acid secretion especially daunting. It is in this context that one must appreciate the significance of the contribution of the article by Zeng et al. in this issue of the JCI (11). These authors use a variety of methods necessary to dissect out the direct role of PACAP in regulating ECL cell function within the cephalic phase of acid regulation. The strength of their findings lies in their multilevel experimental approach, which builds from the isolated cell to the isolated gland, and finally to the whole animal.
Using a nearly pure population of rat ECL cells, Zeng et al. demonstrate the presence of mRNA for PACAP-specific receptors (PAC1) by RT-PCR. The absence of expression of the vasoactive intestinal peptide receptor VPAC in parallel RT-PCR experiments argues that PAC1 is indeed expressed by ECL cells, rather than by contaminating D cells. The 100-fold greater potency of PACAP relative to vasoactive intestinal peptide in inducing a rise in intracellular calcium concentration and release of histamine from these cultures strongly confirms that PAC1 is biologically active in ECL cells. The authors show that isolated D cells, on the other hand, respond equally to these 2 stimuli, as would be expected if their response is mediated by VPAC1 receptors. Crucially, in an integrated glandular model consisting of superfused PACAP in intact rabbit gastric glands, the effects of PACAP are shown to be specific for ECL cells. Together with in vivo data from the rat, in which PACAP stimulates acid secretion in the presence of somatostatin-neutralizing antibodies, these findings confirm that PACAP directly stimulates ECL cell secretion of histamine and that this effect is opposed by the nonselective activation of D cells expressing VPAC1.
Although stimulation of the central nervous system would have reproduced the location and concentration of PACAP release in the stomach more faithfully than does peripheral injection of PACAP, this approach to assessing the contribution of PACAP to ECL cell– or D cell–mediated acid secretion would have required presently unavailable potent and specific antagonists to PAC1 and VPAC1. The available data strongly support the role of PACAP in the neural regulation of ECL cells, but it is by no means clear that PACAP accounts for the entire effect of M1-dependent cholinergic stimulation of acid secretion mediated by histamine (5); other centrally regulated neurotransmitters may also activate the M1-dependent response in ECL cells. With the recent creation of a PAC1-deficient mouse (12), it may be possible to measure the contribution of this pathway, but it will be difficult to determine whether proliferative or signaling effects of PAC1 stimulation are responsible for an ECL cell phenotype in these animals (9).
The findings of Zeng et al. (11) are unlikely to result in new therapies for gastric acid–related illnesses — PACAP accounts for only a portion of central stimulation and none of the peripheral stimulation, and effective histamine type 2 receptor antagonists and inhibitors of acid secretion are already available to clinicians. Nevertheless, these impressive studies of PACAP and its recently discovered receptor (13) fill a long-standing hole in our understanding of the regulation of gastric acid secretion.
References
- 1.Malfertheiner P. Current concepts in dyspepsia: a world perspective. Eur J Gastroenterol Hepatol. 1999;11(Suppl. 1):25–29. [PubMed] [Google Scholar]
- 2.Baron JH. The discovery of gastric acid. Gastroenterology. 1979;76:1056–1064. [PubMed] [Google Scholar]
- 3.Soll AH. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978;61:370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh, J.H. 1994. Gastrointestinal hormones. In: Physiology of the gastrointestinal tract. L.R. Johnson, editor. Raven Press. New York, NY. 1–129.
- 5.Yanagisawa K, Yang H, Walsh JH, Tache Y. Role of acetylcholine, histamine and gastrin in the acid response to intracisternal injection of TRH analog, RX 77368, in the rat. Regul Pept. 1990;27:161–170. doi: 10.1016/0167-0115(90)90036-v. [DOI] [PubMed] [Google Scholar]
- 6.Sundler F, et al. Pituitary adenylate cyclase activating peptide: a novel vasoactive intestinal peptide-like neuropeptide in the gut. Neuroscience. 1992;46:439–454. doi: 10.1016/0306-4522(92)90064-9. [DOI] [PubMed] [Google Scholar]
- 7.Zeng N, et al. Selective ligand-induced intracellular calcium changes in a population of rat isolated gastric endocrine cells. Gastroenterology. 1996;110:1835–1846. doi: 10.1053/gast.1996.v110.pm8964409. [DOI] [PubMed] [Google Scholar]
- 8.Lindstrom E, et al. Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept. 1997;71:73–86. doi: 10.1016/s0167-0115(97)01018-5. [DOI] [PubMed] [Google Scholar]
- 9.Lauffer JM, et al. Pituitary adenylate cyclase activating polypeptide (PACAP) modulates gastric enterochromaffin-like cell proliferation in rats. Gastroenterology. 1999;116:623–635. doi: 10.1016/s0016-5085(99)70184-8. [DOI] [PubMed] [Google Scholar]
- 10.Mungan Z, et al. Effect of PACAP on gastric acid secretion in rats. Peptides. 1995;16:1051–1056. doi: 10.1016/0196-9781(95)00083-v. [DOI] [PubMed] [Google Scholar]
- 11.Zeng N, et al. PACAP type I receptor activation regulates ECL cells and gastric acid secretion. J Clin Invest. 1999;104:1383–1391. doi: 10.1172/JCI7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekblad E, Jongsma H, Brabet P, Bockaert J, Sundler F. Characterization of intestinal receptors for VIP and PACAP in rat and in PAC1 receptor knock-out mouse. Regul Pept. 1999;83:A45. doi: 10.1111/j.1749-6632.2000.tb06960.x. [DOI] [PubMed] [Google Scholar]
- 13.Pisegna JR, Wank SA. Molecular cloning and functional expression of the pituitary adenylate cyclase-activating polypeptide type I receptor. Proc Natl Acad Sci USA. 1993;90:6345–6349. doi: 10.1073/pnas.90.13.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]