Abstract
Goblet cells are the major mucus-producing cells of the intestine and are presumed to play an important role in mucosal protection. However, their functional role has not been directly assessed in vivo. In initial studies, a 5′ flanking sequence of the murine intestinal trefoil factor (ITF) gene was found to confer goblet cell–specific expression of a transgene. To assess the role of goblet cells in the intestine, we generated transgenic mice in which ∼60% of goblet cells were ablated by the expression of an attenuated diphtheria toxin (DT) gene driven by the ITF promoter; other cell lineages were unaffected. We administered 2 exogenous agents, dextran sodium sulfate (DSS) and acetic acid, to assess the susceptibility of mITF/DT-A transgenic mice to colonic injury. After oral administration of DSS, 55% of control mice died, whereas DT transgenic mice retained their body weight and less than 5% died. Similarly, 30% of the wild-type mice died after mucosal administration of acetic acid, compared with 3.2% of the transgenic mice. Despite the reduction in goblet-cell number, the total amount of ITF was increased in the mITF/DT-A transgenic mice, indicating inducible compensatory mechanisms. These results suggest that goblet cells contribute to mucosal protection and repair predominantly through production of trefoil peptides.
J. Clin. Invest. 104:1539–1547 (1999).
Introduction
The epithelium of the gastrointestinal mucosa provides a barrier against potentially injurious luminal agents, including acid, enzymes, bacteria, and toxins. Disruption of this barrier is the salient feature of a variety of common and important gastrointestinal disorders, including inflammatory bowel disease (IBD) and peptic ulcers (1, 2). Several general and specific protective factors are thought to contribute to maintenance of barrier function. Goblet cells are abundant constituents of the surface epithelium within the small and large intestine that have long been thought to play an important role in mucosal protection (3–5). However, direct assessment of their function has been generally lacking to date.
Past studies have demonstrated that targeted disruption of a specific cell lineage can be a useful method for evaluating its function. In these approaches, a toxin gene driven by a tissue-specific promoter is used to achieve the genetic ablation of a particular cell lineage. Targeted expression of γ2-crystallin promoter fused to diphtheria toxin (DT) A-chain gene (DT-A) caused microphthalmia in experimental transgenic mice (6). Similar targeted expression of the elastase I promoter fused to DT-A caused aplasia of the pancreas (7) in transgenic mice.
Within the gastrointestinal tract, targeted ablation of the parietal cell lineage of the stomach (8) and the Paneth’s cell lineage of the small intestine (9) have been achieved in transgenic murine models in which the expression of the DT-A gene is driven by the H+/K+-ATPase and cryptdin-2 promoters, respectively. In addition to these gastrointestinal tract–specific promoters, fatty acid binding protein promoter is known to lead to expression of the DT-A gene within the small intestine (10, 11). However, none of these promoters causes expression in goblet cells.
We previously reported the selective expression of intestinal trefoil factor (ITF) in goblet cells throughout the small and large intestine, and the ability of such expression to both protect intestinal mucosa from injury and to facilitate repair after injury (12–15). Subsequently, the goblet cell–specific transcriptional mechanism was partially clarified, and 6.35 kb of the 5′ flanking region of the ITF gene was found to be sufficient to promote goblet cell–specific expression (16, 17).
To assess the functional role of goblet cells in the intestine, we generated transgenic mice expressing attenuated DT gene driven by the ITF promoter. The highly selective targeted expression driven by the ITF promoter enabled targeted ablation of goblet cells, making possible functional assessment of diminished goblet cell populations.
Methods
Preparation of mITF promoter.
To obtain a sufficiently large segment of 5′ flanking promoter region to confer goblet cell–specific expression of the transgene, a 10-kb BamHI DNA fragment including 6,353 bp of 5′ flanking region of mouse ITF (mITF) gene was prepared from a mouse (129/SvJ) genomic bacterial artificial chromosome library (Genome Systems Inc., St. Louis, Missouri, USA) and subcloned into a pBluescript II KS+ phagemid vector (Stratagene, La Jolla, California, USA) (17). Because no appropriate restriction sites were present near the start codon of the mITF gene, a DNA fragment spanning nucleotides –6,353 to +24 of the mITF 5′ flanking region was made by the long and accurate PCR (LA-PCR) technology using LA Taq DNA polymerase (Takara Shuzo Co., Otsu, Japan) and a subcloned 10–kb fragment as a template. Primers used were as follows: T3(+)X: 5′-AGCTTGATAACGCGTTCCTGCAGCCCGGGGA-ATCC-3′ and 5′(–)X: 5′-AGCTTCAGGATCCCTGCACAGGATGTGCAAGGTCA-3′. Both primers contain the XhoI restriction site at the 5′ end.
Construction of transgene and generation of β-galactosidase ITF transgenic mice.
The β-galactosidase gene was obtained from pSV-β-galactosidase vector (Promega Corp., Madison, Wisconsin, USA) by digestion with BamHI and HindIII. The resulting 3.7 kb of β-galactosidase gene was inserted into the BamHI and HindIII sites of pBluescript II KS(+). The 6.3 kb of XhoI-digested 5′ end of the mITF promoter was then inserted into XhoI sites of pBluescript II KS(+) containing the β-galactosidase gene. The resulting plasmid was digested with BamHI, yielding a 10-kb DNA fragment consisting of the 6.35-kb mITF promoter followed by the β-galactosidase gene (3.7 kb). This fragment was gel purified, dialyzed, and injected into the mouse oocytes. The resulting offspring were screened both by Southern blotting (using the above β-galactosidase fragment as a probe) and by PCR using the following primers directed at the inserted β-galactosidase gene: sense: 5′-CCTGAGGCCGATACTGTCGTC-3′; antisense: 5′-CCAGATAACTGCCGTCACTCC-3′. PCR was performed according to the manufacturer’s guidelines (Promega Corp.) using 0.5 μL of Taq DNA polymerase (Promega Corp.) in a 50-μL reaction containing 1.5 mM MgCl2, 0.2 mM dNTP, 0.8 μM of each primer, and approximately 1 μg of extracted tail DNA. PCR conditions included an initial 10 minutes at 95°C followed by 36 cycles each of 95°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute, and a final 8-minute extension period.
Construction of transgene and generation of mITF/DT-A transgenic mice.
The 6.35-kb mITF 5′ flanking sequence digested with XhoI (as described above) was subcloned into the XhoI site of a pBluescript II KS+ phagemid containing 700 bp of attenuated mutant-type DT-A fragment followed by a polyadenylation signal between NotI and HindIII sites (6–9, 18, 19). This 700-bp DT-A fragment was excised with NotI and HindIII from pGlyCAM/DT-A (G128A) plasmid (6, 7, 20; our unpublished data). The subcloned DNA construct comprising nucleotides –6,353 to +24 of the mITF gene promoter linked to the DT-A fragment was transformed into competent Escherichia coli DH5α cells (CLONTECH Laboratories Inc., Palo Alto, California, USA). The subcloned DNA construct was subjected to restriction mapping and sequencing of the insertion junctions to confirm the correct orientation and preservation of the start codon.
To remove the vector sequence, a 7.1-kb DNA fragment comprising mITF promoter linked to DT-A cDNA was released by digestion with BamHI (mITF promoter 5′ end) and NotI (DT-A cDNA 3′ end) using standard techniques. The linearized DNA fragment was purified by agarose gel electrophoresis followed by extraction using QIAquick Gel Extraction Kits (QIAGEN Inc., Santa Clarita, California, USA). After dialysis against injection buffer (5 mM Tris [pH 7.4], 5 mM NaCl, and 0.1 mM EDTA [pH 8.0]), the DNA was used for pronuclear injection of 129/SvJ mouse oocytes that were transferred to pseudopregnant mice using standard techniques (21). Four-week-old live-born mice were screened for the presence of the DT-A transgene by Southern blot analysis using tail DNA. Briefly, after digestion of approximately 1 cm of cut tail with 0.5 mg/mL of proteinase K in digestion buffer (10 mM Tris [pH 7.6], 100 mM NaCl, 10 mM EDTA, and 0.5% SDS) overnight at 55°C, the DNA was purified by conventional phenol/chloroform extraction and ethanol precipitation techniques (22). Subsequently, the DNA was subjected to 1% agarose gel electrophoresis followed by blotting onto nylon membranes (Micron Seperations Inc., Westboro, Massachusetts, USA). The membrane was probed with a 32P-labeled probe — either a 700-bp fragment of DT-A, an 800-bp fragment of 5′-end mITF, or human GAPDH cDNA (CLONTECH Laboratories Inc.) — by random priming, and then exposed at –80°C overnight. Copy number of the transgene was determined by the density ratio of mITF 5′ end to GAPDH in DNA from transgenic mice compared to the ratio of mITF 5′ to GADPH in DT-A–negative littermates.
Ten mice expressing the DT-A transgene were successfully obtained from 24 live-born mice and then bred. All pedigrees were maintained by crosses to nontransgenic 129/SvJ littermates. Mice were kept on a standard chow diet ad libitum in a specific pathogen–free environment.
Histologic examination.
Various tissues from β-galactosidase transgene and DT-A transgene–positive and –negative mice were used for histologic examination, immediately after death or sacrifice by cervical dislocation. Samples from stomach, duodenum, jejunum, ileum, and proximal and distal portions of colon were fixed in 10% buffered formalin overnight followed by dehydration in 70% ethanol and embedding in paraffin wax. Serial sections 4 μm thick were deparaffinized, rehydrated, and stained with hematoxylin and eosin for routine histology, or with Alcian blue and periodic acid–Schiff (AB-PAS) to locate goblet cells as described previously (23, 24). The ratio of goblet cells to total epithelial cells was obtained by counting AB-PAS–positive cells and total epithelial cells in 10 high-power fields of well-oriented longitudinal sections under light microscopy. The results are shown as the mean ± SD from 3 mice.
Lac-Z staining.
After sacrifice of mice, tissues from β-galactosidase–ITF and control mice were removed and rinsed in cold 1× PBS (pH 7.4) with 2 mM MgCl2. Tissues were fixed for 20 minutes at 23°C in a solution containing 1% formaldehyde, 0.2% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA, and 0.02% NP-40 (Roche Molecular Biochemicals Inc., Indianapolis, Indiana, USA), followed by overnight staining at 37°C in a solution containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 2 mM MgCl2, 0.1% sodium deoxycholate, 0.02% NP-40, and 1 mg/mL X-galactosidase (5-bromo-4-chloro-3-indolyl-—D-galactoside; Promega Corp.) made up in 1× PBS (free of Mg2 and Ca2). Tissue samples were rinsed in PBS and postfixed in 2% paraformaldehyde with 0.1% glutaraldehyde for 1 hour, then dehydrated in xylene and embedded in paraffin. Sections of 5–10 μm were stained with hematoxylin and eosin Y or eosin Y alone, per standard protocols. (Unless otherwise noted, all reagents were purchased from Sigma-Aldrich, St. Louis, Missouri, USA.)
RNA blot analysis.
Eight-week-old mice (both DT-A transgene–positive and –negative) were sacrificed by cervical dislocation. Total cellular RNA from various tissues was extracted with Trizol (Life Technologies Inc., Gaithersburg, Maryland, USA) according to the manufacturer’s protocol. Thirty micrograms each of total RNA from various tissues was denatured with formamide, fractionated by electrophoresis on a 1% formaldehyde agarose gel, and transferred onto nylon membranes (Micron Seperations Inc.). Hybridization was performed under high-stringency conditions using 32P-labeled probes made by the random-primed DNA labeling method. The probes used were DT-A fragment, rITF cDNA (12), PCR products of rpS2 and rSP cDNA (24), and GAPDH (CLONTECH Laboratories Inc.). Rat MUC2 and mouse MUC3 probes corresponding to nucleotide positions 440–657 and 1,062–1,399 respectively were also prepared by PCR (25, 26). The membranes were exposed at –80°C overnight (ITF, pS2, and GAPDH), for 2 days (rSP), or for 3 days (DT-A, MUC2, and MUC3), and signal intensities of trefoil peptides and MUCs were compared with that of GAPDH by scanning densitometry.
Western blotting for ITF and mucin.
The entire colon and the distal 6 cm of the small bowel were removed, rinsed in ice-cold 1× PBS, and suspended ice-cold lysis buffer (600 μL/100 mg tissue) containing 1× PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 0.7 mM EDTA, and Roche Molecular Biochemicals complete Mini protease inhibitor cocktail tablets. Samples were homogenized for 30 seconds using a Polytron tissue homogenizer (Brinkmann Instruments Inc., Westbury, New York, USA), and then subjected to centrifugation at 16,000 g for 20 minutes at 4°C. Protein concentration was determined using the DC protein assay kit (Bio-Rad Laboratories Inc., Hercules, California, USA).
Immunoprecipitation was performed on approximately 200 μg of protein using a rabbit polyclonal antibody to ITF peptide (HM 88) (27). Thirty-five microliters of a solution of 20% protein A–Sepharose (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA) was added and the incubation continued for 8 hours at 4°C. After centrifugation at 16,000 g for 2 minutes, the samples were heated in loading buffer for 2 minutes at 85°C, and then loaded onto a 10–20% tricine gel (Novex, San Diego, California, USA) and transferred onto PVDF membrane (Millipore Corp., Bedford, Massachusetts, USA). Blots were blocked for 1 hour at 23°C in a blocking solution containing 5% dry milk, 0.1% BSA, and 0.05% Tween-20 in 1× PBS, and then incubated with the rabbit polyclonal antibody to ITF at 1:500 in the above blocking solution. The blots were then washed in 1× PBS and 0.05% Tween-20 (3 washes of 20 minutes each) and incubated with horseradish peroxidase–linked donkey anti-rabbit antibody (Amersham Life Sciences, Arlington Heights, Illinois, USA) at 1:10,000 in the same blocking solution for 60 minutes at 23°C. Antibody detection was carried out using Renaissance chemiluminescent reagents (NEN Life Science Products Inc., Boston, Massachusetts), according to the manufacturer’s instructions. Western blotting of mouse colonic or small bowel mucin was performed on 60 μg of protein from the above tissue homogenate supernatant. Protein was then separated on a 10–20% tricine gel and transferred and blocked as above. Blots were incubated overnight at 4°C with monoclonal anti-human colonic mucin antibody (R35.2.3) from mice immunized with unfractionated pure colonic mucin (27), followed by incubation with horseradish peroxidase–linked rabbit anti-mouse antibody (Amersham Life Sciences Inc.) at 1:10,000, and antibody detected using the Renaissance chemiluminescent reagents (NEN Life Science Products Inc.) as described above.
DSS and acetic acid–induced experimental colitis.
Experimental colitis was induced by two methods using 2 separate standard agents: oral administration of 2.5% (wt/vol) DSS (molecular weight 40 kDa; ICN, Costa Mesa, California, USA) or rectal instillation of 3% acetic acid. For the former treatment, 18 DT-A transgenic mice and 20 normal control mice were treated with 2.5% DSS in their drinking water ad libitum for 12 days (28, 29). Body weight was monitored daily and the survival rate was calculated. Immediately after death or sacrifice at the end of the experiment, tissues were prepared for histologic examination. For the latter treatment, after saline lavage, 3% acetic acid (10 μL/g of body weight) was instilled through a tube inserted 3 cm into the rectum of 28 DT-A transgenic and 28 normal mice under ether anesthesia (23, 24, 30). Survival rate was calculated and mice were examined histologically on days 1, 3, and 5 after administration.
Statistical analysis.
Goblet cell number was determined by counting under a microscope. Body weight changes caused by experimental colitis were analyzed using the Student’s t test. A P value of less than 0.05 was considered significant. Survival distribution of mice in experimental colitis was estimated by the Kaplan-Meier method. Difference in survival rate was tested by use of the log-rank test.
Results
Goblet cell–specific reporter gene expression by mITF promoter in transgenic mice.
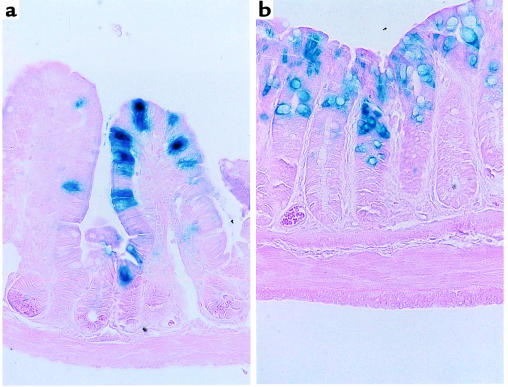
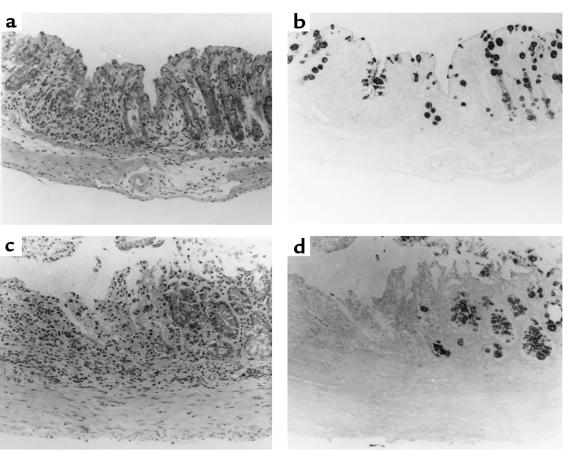
A construct comprising 6.35 kb of 5′ end of the mouse ITF gene ligated to the reporter β-galactosidase was used to establish transgenic murine lines. As demonstrated in Figure 1, the reporter gene β-galactosidase driven by the ITF promoter was specifically expressed in small and large intestinal goblet cells. The same pattern of expression was observed in each of 4 lines derived from individual founder mice. Of note, β-galactosidase was not detected in other tissues, confirming both the cell and tissue specificity of ITF promoter–driven gene expression.
Figure 1.
Goblet cell–specific expression of β-galactosidase by ITF promoter in transgenic mice. Transgenic mice were produced by injection of a construct composed of 6.35 kb of 5′ end of mITF ligated to β-galactosidase. Tissues were fixed and stained using Lac-Z to locate expression of β-galactosidase, as detailed in Methods. Counterstaining was done with eosin Y. (a) Small intestine. (b) Colon.
Expression of DT-A in transgenic mice.
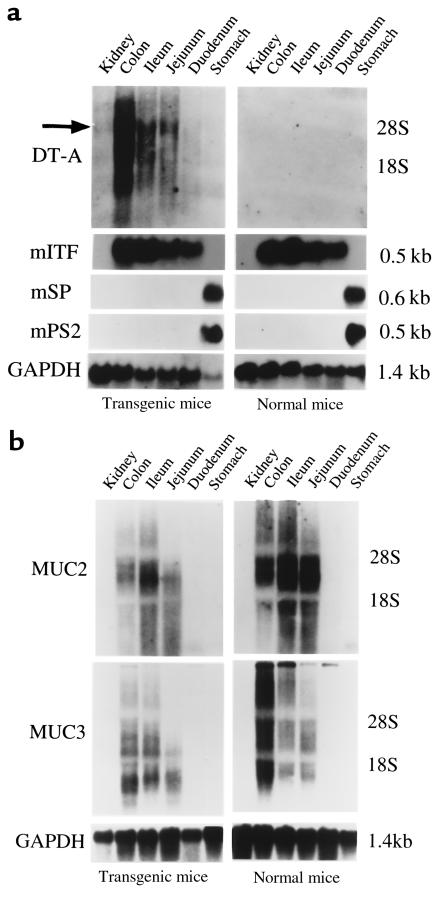
To study the functional importance of goblet cells, transgenic mice expressing attenuated DT under control of the goblet cell–specific mITF promoter were produced. Efforts to establish viable fetuses using a construct including wild-type DT gene were unsuccessful despite numerous attempts. The presence of the transgene was confirmed in 10 separate founder mice after initial oocyte injection of the mITF/DT-A construct. To assess the distribution of expression of the DT-A transgene and the mucosal expression of native ITF and GAPDH, mRNA from both positive and negative transgenic mice were analyzed by Northern blotting. As shown in Figure 2, DT mRNA was detected strongly in the colon, weakly in the jejunum and ileum, and faintly in the duodenum, but not at all in stomach, kidney, or other organs (data not shown) of DT-A–positive transgenic mice. This pattern of expression generally parallels that of the native ITF gene. As expected, DT-A mRNA was not detected in any tissues from normal control mice. These results indicate that the DT-A transgene was successfully and selectively expressed in the intestinal tissues but not in other tissues of positive transgenic mice.
Figure 2.
Expression of (a) DT-A, native endogenous trefoil peptides, and (b) the MUC2 and MUC3 genes in gastrointestinal tissues of mITF/DT-A transgenic mice and normal control mice. Northern blot analysis using 30 μg of total RNA from various tissues of both DT-A transgene–positive and –negative mice was performed as detailed in Methods; blots were hybridized under high-stringency conditions with 32P-labeled probes made by the random-primed DNA labeling method. The membranes were exposed at –80°C overnight (ITF and GAPDH) or for 3 days (DT-A, MUC2, and MUC3). DT mRNA was detected strongly in the colon, weakly in the jejunum and ileum, and faintly in the duodenum, but not at all in stomach or kidney of mITF/DT-A transgenic mice, in a manner parallel to that of the ITF gene. Arrow indicates predominant DT transcript. No DT-A mRNA was detected in tissues of normal control mice.
Goblet cells in transgenic mice.
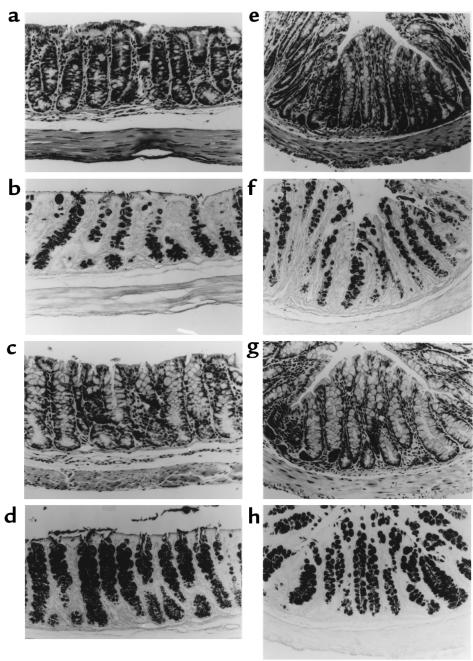
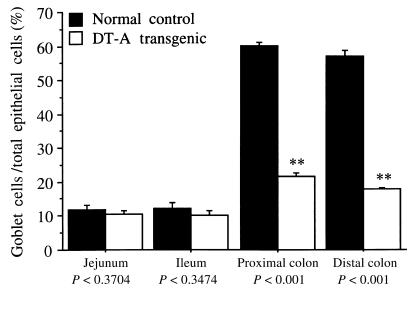
Histologic examination revealed that goblet cells were depleted by approximately half in the proximal (Figure 3a) and distal (Figure 3e) colon in the mITF/DT-A transgenic mice compared with normal littermates (Figure 3, c and g). Depletion of goblet cells was confirmed by AB-PAS staining (Figure 3, b, d, f, and h). Histologically, other cell lineages were unchanged in the transgenic mITF/DT-A mice. The ratio of goblet cells to total epithelial cells was determined by counting AB-PAS–positive and total epithelial cells in 10 high-power fields of well-oriented longitudinal sections under light microscopy. This quantitative analysis confirmed the histologic impression. Goblet cell numbers were decreased by 60% in the proximal and distal portions of the transgenic mice compared with control littermates. However, there was no apparent depletion of goblet cells in the jejunum or ileum (Figure 4).
Figure 3.
Histology of proximal (a–d) and distal (e–h) portions of the colon in mITF/DT-A transgenic mice and normal control mice. Tissues from DT-A transgene–positive and –negative mice were fixed in 10% buffered formalin and stained with hematoxylin and eosin or AB-PAS, as described in Methods. Goblet cells were depleted by approximately 60% in the proximal portion (a) and distal portion (e) of the colon in the transgenic mice, compared with normal littermates (c and g). Depletion of goblet cells was confirmed by AB-PAS staining (b, d, f, and h). Histologically, other cell lineages including Paneth’s cells were unaffected by expression of attenuated DT-A in the transgenic mice.
Figure 4.
Ratio of goblet cells to total epithelial cells in the small and large intestine of mITF/DT-A transgenic and normal control mice. The ratio of goblet cells to total epithelial cells was obtained by counting AB-PAS–positive cells and total epithelial cells in 10 high-power fields of well-oriented longitudinal sections under light microscopy. The results shown represent the mean ± SD of 3 mice.
Expression of ITF and mucin-associated genes in transgenic mice.
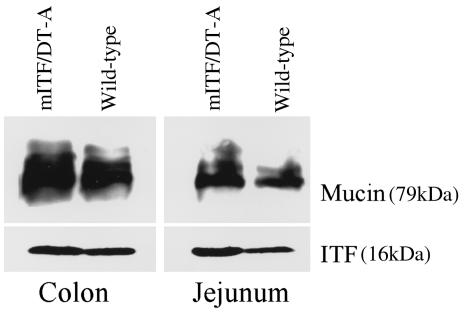
As shown in Figure 2, total native endogenous ITF and mucin-associated MUC2 and MUC3 mRNA expression in mITF/DT-A transgenic mice was essentially comparable to that of control normal mice, indicating that expression of these genes is preserved in mITF/DT-A transgenic mice despite the reduction in goblet cell number. Similarly, Western blot analysis confirmed preservation of both ITF peptide and mucin apoprotein (Figure 5). Because goblet cells of mITF/DT-A transgenic mice were depleted by 60%, these results suggest that ITF and MUC gene expression in goblet cells is increased in DT-A transgenic mice. Other trefoil peptides (mpS2 and mSP) are expressed only in the stomach of the transgenic mice as described previously for normal control mice (24); no ectopic expression of these peptides was found in the transgenic mice (data not shown), indicating that DT did not affect the expression of other trefoil peptides.
Figure 5.
Expression of mITF and MUC apoprotein in gastrointestinal tissues of mITF/DT-A transgenic and control mice. Presence of ITF and MUC apoprotein were evaluated by Western blot analysis as detailed in Methods using rabbit anti-ITF and murine monoclonal anti–colonic mucin apoprotein antibodies as first antibodies, and horseradish peroxidase–linked donkey anti-rabbit and horseradish peroxidase–linked rabbit anti-mouse as second antibodies, respectively. Equal loading was confirmed by Coomassie blue staining.
Susceptibility of transgenic mice to colonic injury.
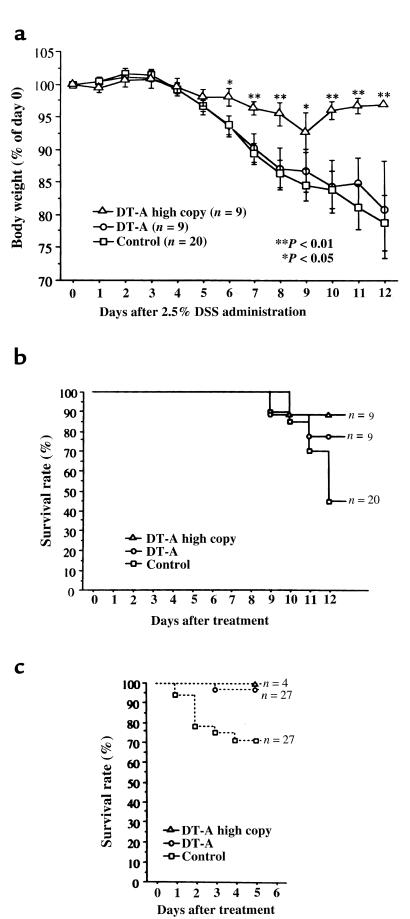
To assess the susceptibility to colonic injury of mITF/DT-A transgenic mice, the survival rate, body weight change, and histology after administration of 2 standard agents — oral 2.5% DSS or rectal 3% acetic acid — in separate experiments were assessed. Oral administration of 2.5% DSS resulted in histologically more severe colitis in normal mice than in DT-A transgenic mice (Figure 6, a and b). Epithelial restitution was more frequently seen in DT-A transgenic mice (Figure 6, c and d) than in controls. After administration of 2.5% DSS, normal mice also experienced progressive weight loss, whereas the mITF/DT-A transgenic mice with high gene copy numbers (> 3) maintained their body weight (P < 0.01 at days 7, 8, and 10–12; P < 0.05 at days 6 and 9; Figure 7a). Therefore, despite lower numbers of goblet cells, mITF/DT-A mice paradoxically appeared to be relatively resistant to DSS. This resistance was reflected in improved survival in the DT-A transgenic mice compared with control normal mice after exposure to DSS (P < 0.001). Only 1 mITF/DT-A transgenic mouse with a high number of gene copies and 2 mice with a single copy (P = 0.33) died during the 12 experimental days, whereas 11 of 20 normal control mice died by day 12, in addition to experiencing significant weight loss (P < 0.01) (Figure 7b). This resistance to injury was not unique to DSS-treated mice. The mITF/DT-A transgenic mice appeared to be generally resistant to injury by exogenous agents. Thus, 5 days after administration of 3% acetic acid, only 1 of 27 transgenic mice had died, whereas 8 of 27 normal control mice had died (P = 0.005; Figure 7c).
Figure 6.
Histology of the colon in mITF/DT-A transgenic mice (a and b) and in normal control mice (c and d) after oral 2.5% DSS administration. Both groups of mice were treated with 2.5% DSS in their drinking water for 12 days. Experimental colitis in normal mice caused by DSS was histologically more severe than in mITF/DT-A transgenic mice. (a and b) Hematoxylin and eosin staining. (c and d) AB-PAS staining. Epithelial restitution is more frequently seen in DT-A transgenic mice.
Figure 7.
Body weight (a) and survival rate after 2.5% DSS (b) and 3% acetic acid (c) treatment in mITF/DT-A transgenic and normal control mice. Eighteen DT-A transgenic mice and 20 normal control mice were treated with 2.5% DSS in their drinking water ad libitum for 12 days. Body weight was monitored daily and the survival rate was calculated. (a) After administration of 2.5% DSS, normal mice experienced a progressive loss of body weight, whereas the mITF/DT-A transgenic mice with high copy numbers (> 3) of the gene maintained their body weight (P < 0.01 at days 6 and 9). (b) Only 1 transgenic mouse with a high gene copy number (P < 0.005) and 2 mice with a single copy (P < 0.01) died during the 12 experimental days, whereas 11 of 20 normal control mice died by day 12, in addition to experiencing weight loss. (c) Five days after administration of 3% acetic acid, only 1 of 27 transgenic mice had died, compared with 8 of 27 normal control mice (P = 0.005).
Discussion
Previous studies using transient transfection approaches have suggested that 6.35 kb of 5′ flanking DNA of mITF was necessary and sufficient to drive goblet cell–specific expression of a reporter gene. In initial studies, we assessed the ability of this same flanking DNA of ITF to confer cell- and tissue-specific gene expression in vivo. As demonstrated in Figure 1, this 5′ flanking DNA was indeed sufficient to drive gene expression that appeared to be highly cell- and tissue-specific, with reporter β-galactosidase being detected only in goblet cells. This pattern complements that of other previously studied promoters derived from intestinal epithelium–associated transcripts that are primarily or exclusively expressed in colonic absorptive cells, e.g., fatty acid binding protein, sucrase-isomaltase, and lactase. Further comparative analysis may provide a more precise understanding of the regulatory elements conferring tissue-specific vs. cell-specific gene expression. Of interest, expression of the transgene was patchy, with some crypts and villi showing consistent expression of β-galactosidase in all goblet cells and other crypts and villi not showing expression. These observations indicate that goblet-cell expression may also be influenced by epigenetic events.
After confirmation of the ability of the ITF promoter to drive goblet cell–specific gene expression, we generated transgenic mice expressing DT-A gene driven by the ITF promoter in an attempt to achieve targeted ablation of goblet cells. Although the cells were not completely disrupted by this method, approximately 60% depletion was achieved in the proximal and distal portions of the colon of the transgenic mice, compared with normal littermates. The reason for incomplete ablation is unknown, but we speculate that attenuated DT may be too weak to disrupt all goblet cells, either because cell turnover is extremely fast in the intestinal epithelium (31) or because there is an alternative pathway for the development and differentiation of multipotent stem cells to goblet cells with minimal ITF gene expression. It may be that both of these mechanisms contribute to incomplete ablation.
The transgene appeared to be more highly expressed within the colon than in the small intestine, resulting in greater goblet cell ablation in the colon. However, transgene expression was not observed in other cell types, again demonstrating the highly cell-selective pattern of expression of the native ITF. Further, overall tissue specificity was preserved — aberrant expression was not present in tissues that do not normally express ITF or contain intestinal goblet cells. These findings suggest that the 6.35 kb of 5′ flanking sequence used to drive DT-A expression contains sufficient regulatory information to confer cell and tissue specificity, but may not contain other enhancer elements necessary for expression throughout the small intestine.
Despite a 60% decrease in goblet cell number in the large intestine, total native endogenous ITF and mucin-associated MUC2 and MUC3 expression (both mRNA and protein) in mITF/DT-A transgenic mice was equivalent to that of control mice. Therefore, ITF gene expression per goblet cell must be increased in the DT-A transgenic mice. In previous studies, an increase of as much as 3-fold has been observed in ITF expression during the recovery phase of experimental colitis, despite a constant number of goblet cells (23, 24). Similarly, the MUC2 and MUC3 genes are expressed in ulcerative colitis and Crohn’s disease at levels similar to those in normal mucosa, despite goblet cell depletion (32–34). Of note, in these settings, MUC genes are expressed in the goblet cells and the columnar cells of the surface epithelium (34), and trefoil peptides, including ITF, are expressed ectopically in a so-called ulcer-associated cell lineage (35, 36). Therefore, endogenous ITF and MUC genes may be expressed in mature and immature goblet cells of mITF/DT-A transgenic mice.
In contrast to the large intestine, the number of goblet cells in the small intestine was unchanged in mITF/DT-A transgenic mice. This suggests that either another enhancer element specific for small intestine located far upstream of the mITF promoter may be required, or that goblet cell number was unaffected by DT-A because of extremely high cell turnover and a lower number of goblet cells in the small intestine. Changes in ITF expression in the jejunum were not associated with an alteration in the number of goblet cells in a model of intestinal inflammation (23). Indeed, the production of ITF by jejunal goblet cells and its subsequent secretion into the lumen could have contributed to protection of the colon.
A variety of animal models of inflammatory bowel disease (IBD) induced by exogenous agents such as DSS (28, 29), acetic acid (23, 24, 30), and 2,4,6-trinitrobenzenesulfonic acid (TNBS) (37, 38) have been described. Although the pathogenesis of colitis induced by such agents is still unclear, it has been proposed that a direct toxic effect on mucosal epithelial cells is a key feature in DSS- and acetic acid–induced colitis (28, 30), whereas the persisting colitis induced by TNBS appears to be produced by T cell–mediated immunological response to haptenated proteins (37, 39). Therefore, the mechanism of colitis induction seems to differ among these agents. In this study, mITF/DT-A transgenic mice showed strong protection against exogenous agents such as DSS and acetic acid. Because the transgenic mice had 60% fewer goblet cells than the normal control mice had, our results suggest that goblet cells might be selectively affected by DSS and acetic acid. Of interest, the severity of TNBS-induced colitis in mITF/DT-A transgenic mice was not statistically different from that in normal control mice (unpublished data). Previous studies have shown that an absolute deficiency of ITF makes mice exquisitely sensitive to injury by DSS and acetic acid. Nevertheless, in this instance the colonic epithelium containing fewer goblet cells appears to produce sufficient ITF and mucin products to confer adequate or even enhanced healing in mITF/DT-A transgenic mice. Thus, we suggest that the goblet cell depletion usually seen in these specific forms of experimental colitis (and possibly also in nonspecific colitis) may reflect targeted damage of the goblet cells by various toxic agents.
Spontaneous development of IBD has been reported in knockout mice lacking IL-2 (40), IL-10 (41), or T-cell receptor a (42), and in dominant-negative N-cadherin–expressing mice (10). Colitis in these models is presumed to result from alteration of either the mucosal immune system or the epithelial cell-cell adhesion system, or both. An inappropriate immune response is likely to perturb intestinal homeostasis and induce IBD, but the role of epithelial cells — especially goblet cells — and mucosal protection, and the way in which these cells are damaged in IBD is still unknown. In this context, the mITF/DT-A transgenic mice provide a useful model with which to explore the role of the goblet cell in IBD through cross-breeding with mutant lines that spontaneously develop colitis.
In summary, transgenic mice showing partial ablation of the goblet cells were generated by using ITF gene promoter and attenuated DT gene. Paradoxically, these mice showed marked protection against exogenous toxic agents such as DSS and acetic acid. Because ITF expression and goblet cell numbers are increased in mITF/DT-A mice, mucosal protection may be determined more by adequate ITF expression than by goblet cells themselves.
Acknowledgments
These studies were supported by National Institutes of Health grants DK-43351 and DK-46906. The authors thank Ian Rosenberg for helpful comments.
Footnotes
Hiroshi Itoh and Paul L. Beck contributed equally to this work.
References
- 1.Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol. 1986;21:71–77. doi: 10.3109/00365528609093820. [DOI] [PubMed] [Google Scholar]
- 2.Owen DA, Kelly JK. Inflammatory diseases of the gastrointestinal tract. Mod Pathol. 1995;8:97–108. [PubMed] [Google Scholar]
- 3.Filipe MI. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979;2:195–216. [PubMed] [Google Scholar]
- 4.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260:C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 5.Verma M, Davidson EA. Mucin genes: structure, expression and regulation. Glycoconj J. 1994;11:172–179. doi: 10.1007/BF00731215. [DOI] [PubMed] [Google Scholar]
- 6.Breitman ML, et al. Genetic ablation: targeted expression of a toxin gene causes microphthalmia in transgenic mice. Science. 1987;238:1563–1565. doi: 10.1126/science.3685993. [DOI] [PubMed] [Google Scholar]
- 7.Palmiter RD, et al. Cell lineage ablation in transgenic mice by cell-specific expression of a toxin gene. Cell. 1987;50:435–443. doi: 10.1016/0092-8674(87)90497-1. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–3676. [PubMed] [Google Scholar]
- 9.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 10.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 11.Playford RJ, et al. Transgenic mice that overexpress the human trefoil peptide pS2 have an increased resistance to intestinal damage. Proc Natl Acad Sci USA. 1996;93:2137–2142. doi: 10.1073/pnas.93.5.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suemori S, Lynch-Devaney K, Podolsky DK. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci USA. 1991;88:11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Podolsky DK, et al. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993;268:6694–6702. [PubMed] [Google Scholar]
- 14.Babyatsky MW, deBeaumont M, Thim L, Podolsky DK. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996;110:489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- 15.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–265. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 16.Ogata H, Inoue N, Podolsky DK. Identification of a goblet cell-specific enhancer element in the rat intestinal trefoil factor gene promoter bound by a goblet cell nuclear protein. J Biol Chem. 1998;273:3060–3067. doi: 10.1074/jbc.273.5.3060. [DOI] [PubMed] [Google Scholar]
- 17.Itoh H, Inoue N, Podolsky DK. Goblet cell-specific transcription of mouse intestinal trefoil factor gene results from collaboration of distinctive positive and negative regulatory elements. Biochem J. 1999;341:461–472. [PMC free article] [PubMed] [Google Scholar]
- 18.Maxwell F, Maxwell IH, Glode LM. Cloning, sequence determination, and expression of transfected cells of the coding sequence for the tox 176 attenuated diphtheria toxin A chain. Mol Cell Biol. 1987;7:1576–1579. doi: 10.1128/mcb.7.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breitman ML, Rombola H, Maxwell IH, Klintworth GK, Bernstein A. Genetic ablation in transgenic mice with an attenuated diphtheria toxin A gene. Mol Cell Biol. 1990;10:474–479. doi: 10.1128/mcb.10.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evans GA. Dissecting mouse development with toxigenics. Genes Dev. 1989;3:259–263. doi: 10.1101/gad.3.3.259. [DOI] [PubMed] [Google Scholar]
- 21.Gordon JW. Production of transgenic mice. Methods Enzymol. 1993;225:747–771. doi: 10.1016/0076-6879(93)25048-7. [DOI] [PubMed] [Google Scholar]
- 22.Maniatis, T., Fritsch, E.F., and Sambrook, J. 1989. Molecular cloning: a laboratory manual. 2nd edition. Cold Spring Harbor Laboratory Press. Cold Spring Harbor, NY. 9.16–9.19.
- 23.Tomita M, et al. Molecular cloning of mouse intestinal trefoil factor and its expression during goblet cell changes. Biochem J. 1995;311:293–297. doi: 10.1042/bj3110293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itoh H, et al. cDNA cloning of rat pS2 peptide and expression of trefoil peptides in acetic acid-induced colitis. Biochem J. 1996;318:939–944. doi: 10.1042/bj3180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu G, et al. cDNA for the carboxyl-terminal region of a rat intestinal mucin-like peptide. J Biol Chem. 1992;267:5401–5407. [PubMed] [Google Scholar]
- 26.Shekels LL, et al. Cloning and characterization of mouse intestinal MUC3 mucin: 3′ sequence contains epidermal-growth-factor-like domains. Biochem J. 1998;330:1301–1308. doi: 10.1042/bj3301301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogata H, Podolsky DK. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am J Physiol. 1997;273:G348–G354. doi: 10.1152/ajpgi.1997.273.2.G348. [DOI] [PubMed] [Google Scholar]
- 28.Okayasu I, et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 29.Egger B, et al. Mice lacking transforming growth factor alpha have an increased susceptibility to dextran sulfate-induced colitis. Gastroenterology. 1997;113:825–832. doi: 10.1016/s0016-5085(97)70177-x. [DOI] [PubMed] [Google Scholar]
- 30.MacPherson BR, Pfeiffer CJ. Experimental production of diffuse colitis in rats. Digestion. 1978;17:135–150. doi: 10.1159/000198104. [DOI] [PubMed] [Google Scholar]
- 31.Gordon JI. Intestinal epithelial differentiation: new insights from chimeric and transgenic mice. J Cell Biol. 1989;108:1187–1194. doi: 10.1083/jcb.108.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang SK, et al. Localization of mucin (MUC2 and MUC3) messenger RNA and peptide expression in human normal intestine and colon cancer. Gastroenterology. 1994;107:28–36. doi: 10.1016/0016-5085(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 33.Tytgat KM, Opdam FJ, Einerhand AW, Büller HA, Dekker J. MUC2 is the prominent colonic mucin expressed in ulcerative colitis. Gut. 1996;38:554–563. doi: 10.1136/gut.38.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss AA, Babyatsky MW, Ogata S, Chen A, Itzkowitz SH. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996;44:1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- 35.Wright NA, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 36.Alison MR, et al. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995;175:405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- 37.Morris GP, et al. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 38.McHugh KJ, Weingarten HP, Keenan C, Wallace JL, Collins SM. On the suppression of food intake in experimental models of colitis in the rat. Am J Physiol. 1993;264:R871–R876. doi: 10.1152/ajpregu.1993.264.5.R871. [DOI] [PubMed] [Google Scholar]
- 39.Elson CO, et al. Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol. 1996;157:2174–2185. [PubMed] [Google Scholar]
- 40.Sadlack B, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 42.Mombaerts P, et al. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 1993;75:275–282. doi: 10.1016/0092-8674(93)80069-q. [DOI] [PubMed] [Google Scholar]