Abstract
Hematopoietic stem cell (HSC) gene therapy using integrating vectors has a potential leukemogenic risk due to insertional mutagenesis. To reduce this risk, a limitation of ≤2 average vector copy number (VCN) per cell is generally accepted. We developed an assay for VCN among transduced CD34+ cells that reliably predicts in vivo VCN in 16 rhesus recipients of CD34+ cells transduced with a green fluorescent protein (GFP) (or yellow fluorescent protein (YFP))-encoding lentiviral vector. Using GFP (or YFP)-specific probe/primers by real-time PCR, VCN among transduced CD34+ cells had no correlation with VCN among granulocytes or lymphocytes in vivo assayed 6 months post-transplantation. This was a likely result of residual plasmids present in the vector preparation. We then designed self-inactivating long terminal repeat (SIN-LTR)-specific probe/primers, which detect only integrated provirus. Evaluation with SIN-LTR probe/primers resulted in a positive correlation of VCN among transduced CD34+ cells with granulocytes and lymphocytes in vivo. The transduced CD34+ cells had higher VCN (25.1 ± 5.6) as compared with granulocytes (2.8 ± 1) and lymphocytes (2.4 ± 0.7). In summary, an integrated provirus-specific real-time PCR system demonstrated nine- to tenfold higher VCN in transduced CD34+ cells in vitro, as compared with VCN in vivo. Therefore, the restriction of ≤2 VCN before infusion might unnecessarily limit gene transfer efficacy.
Keywords: CD34+ cells, hematopoietic stem cell transplantation, large animal model, lentiviral vector
Introduction
Hematopoietic stem cell (HSC)-targeted gene therapy is potentially curative for congenital and acquired diseases affecting the blood, and efficacy has been demonstrated in several clinical trials, mostly in hereditary immunodeficiency.1,2,3,4,5,6 However, in an X-linked severe combined immunodeficiency gene therapy trial, T cell type acute lymphoblastic leukemia was observed and determined to result from insertional mutagenesis involving the proto-oncogenes LMO2 and CCND2.7 In another gene therapy trial for X-linked chronic granulomatous disease, in vivo clonal expansion of transduced cells was associated with γ-retroviral vector insertions into common integration sites, such as MDS1-EVI1, PRDM16, and SETBP1.5 Finally, in a recent β-thalassemia gene therapy trial, a dominant clone was demonstrated in vivo which harbored an activation of the HMGA2 gene by insertion of a human immunodeficiency virus type 1 (HIV-1)-based lentiviral vector.3
To reduce the risk for insertional mutagenesis, an average vector copy number (VCN) per cell of ≤2 is generally thought to be acceptable. Therefore, evaluation of VCN among transduced CD34+ cells determined before infusion of the cells into patients should be accurate and reliable in predicting VCN in vivo. However, CD34+ cells contain not only HSCs which reconstitute peripheral blood for long term, but also progenitor cells which reconstitute only for short term. Therefore, we sought to evaluate whether VCN among transduced CD34+ cells could predict VCN in vivo 6 months after transplantation of the transduced CD34+ cells. In addition, as plasmid DNAs utilized for lentiviral vector preparation could be contained in vector stocks,8 we also sought to determine whether these residual vector plasmids could result in an overestimation of VCN.
Previously, we developed a large animal model for HSC transplantation with lentiviral transduction using rhesus macaques, and have demonstrated efficient long-term gene marking in vivo.9,10 In this study, we utilized this model to develop an assay for VCN among both transduced CD34+ cells and peripheral blood cells after transplantation of the transduced CD34+ cells in 16 rhesus macaques previously transplanted under identical transduction conditions.
Results
Positive correlation of transgene expression rates (%GFP or %YFP) between transduced CD34+ cells and peripheral blood cells in transplanted rhesus macaques
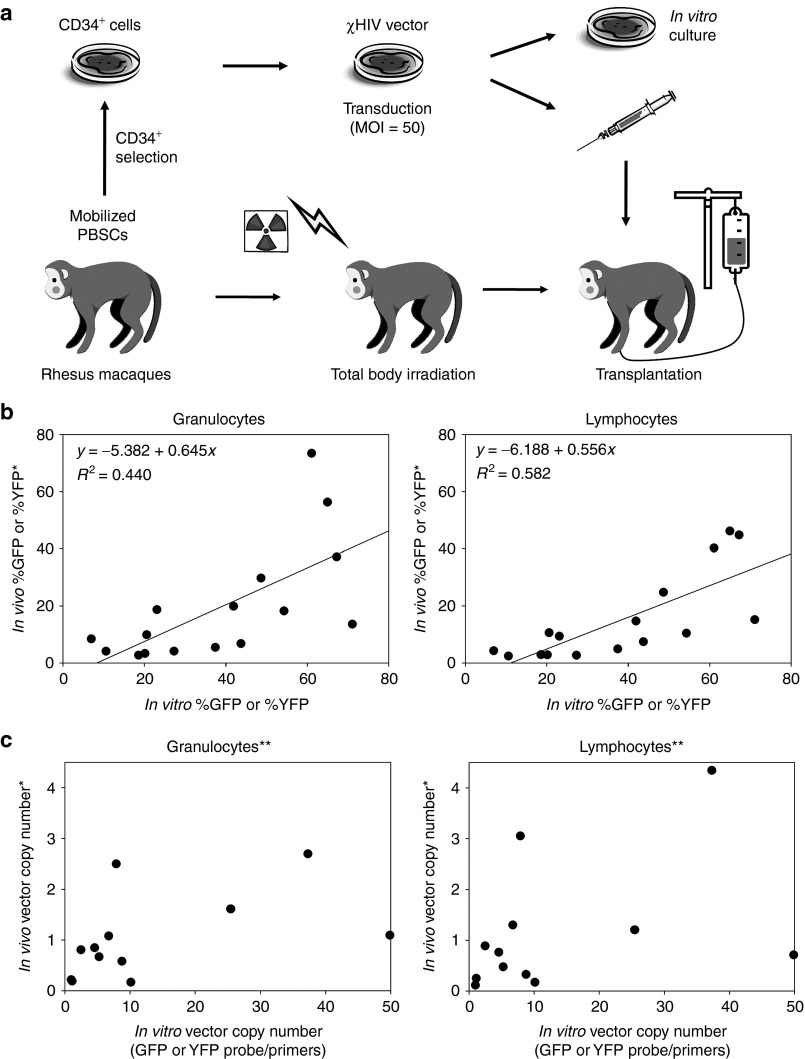
To investigate whether transduction rates in vitro could predict marking levels in vivo, we evaluated transduction rates in both transduced CD34+ cells and peripheral blood cells 6 months after transplantation of the transduced CD34+ cells using our rhesus model (Figure 1a). CD34+ cells were transduced with a green fluorescent protein (GFP)- or yellow fluorescent protein (YFP)-expressing chimeric HIV-1–based lentiviral vector (χHIV vector) at multiplicity of infection of 50 and these cells were transplanted into 16 rhesus macaques following a split dose of 10 Gy total body irradiation. VCN in transduced CD34+ cells (6 days after in vitro culture) and both granulocytes and lymphocytes (6 months after transplantation) was evaluated by real-time PCR and standardized by ribosomal RNA (rRNA) gene signals. In addition, transgene expression rates (%GFP or %YFP) were evaluated by flow cytometry.
Figure 1.
Positive correlation of %GFP or %YFP between transduced CD34+ cells and peripheral blood cells. (a) Mobilized rhesus CD34+ cells were transduced with GFP- or YFP-expressing chimeric HIV-1 vector (χHIV vector) at multiplicity of infection (MOI) of 50, and these cells were transplanted into a total of 16 rhesus macaques following 10 Gy total body irradiation. The transduction efficiency in transduced CD34+ cells (6 days after transduction) and granulocytes and lymphocytes (6 months after transplantation) were evaluated by flow cytometry (transgene expression rates: %GFP or %YFP) and real-time PCR (average vector copy number (VCN) per cell). (b) We observed a positive correlation of %GFP or %YFP in transduced CD34+ cells with granulocytes (P < 0.01) and lymphocytes (P < 0.01). (c) Real-time PCR with GFP- or YFP-specific probe/primers (which can detect both the integrated provirus and the plasmid) resulted in no correlation of VCN in transduced CD34+ cells with granulocytes (P = 0.10) and lymphocytes (P = 0.18). *Twofold scores were used in competitive assay; **Excluding in vivo VCN >6 and in vitro VCN >600. GFP, green fluorescent protein; HIV-1, human immunodeficiency virus type 1; PBSC, peripheral blood stem cells; YFP, yellow fluorescent protein.
All 16 animals demonstrated normal blood counts 1–5 years after transplantation and stable transgene expression rates in vivo (1–40%).10,11,12 We observed a positive correlation between %GFP or %YFP among transduced CD34+ cells and both granulocytes (R 2 = 0.44, P < 0.01) and lymphocytes (R 2 = 0.58, P < 0.01) in vivo evaluated by flow cytometry (Figure 1b). However, evaluation by real-time PCR with GFP- or YFP-specific probe/primers, which detected both integrated provirus and vector plasmids, resulted in no correlation between VCN among transduced CD34+ cells and both granulocytes (R 2 = 0.25, P = 0.10) and lymphocytes (R 2 = 0.17, P = 0.18) in vivo, even when excluding outliers (in vivo VCN >6 and in vitro VCN >600, Figure 1c).
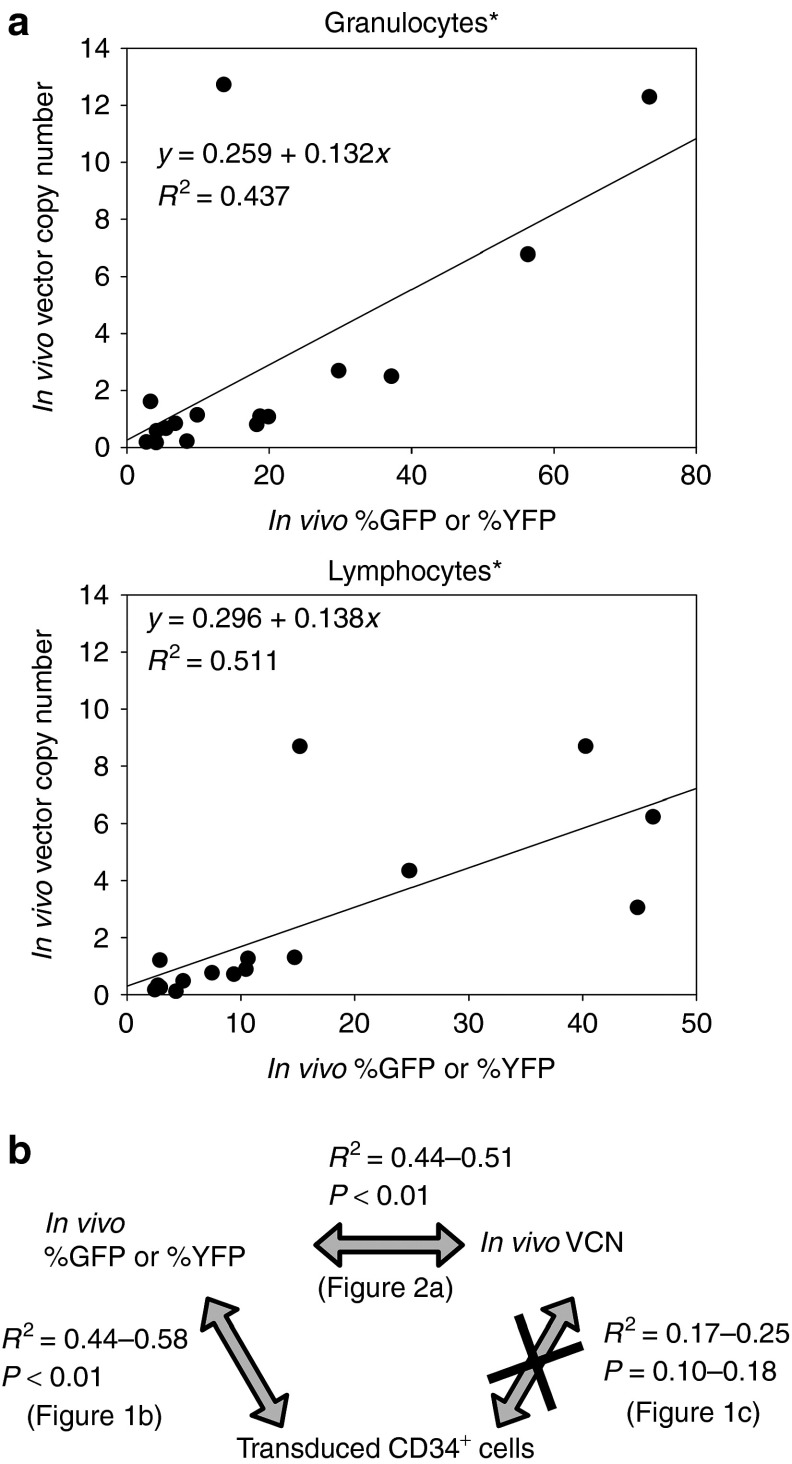
To further delineate the lack of correlation between in vitro VCN and in vivo VCN, we compared VCN to %GFP or %YFP among transduced CD34+ cells, granulocytes, and lymphocytes (Figure 2a,b). Among both granulocytes and lymphocytes, in vivo VCN was positively correlated with %GFP or %YFP (R 2 = 0.44, P < 0.01 and R 2 = 0.51, P < 0.01, respectively; Figure 2a), whereas no correlation between VCN and %GFP or %YFP (R 2 = 0.06, P = 0.37) was observed among transduced CD34+ cells.
Figure 2.
No correlation between average vector copy number (VCN) per cell and %GFP or %YFP in transduced CD34+ cells. (a) To evaluate why there was no correlation of VCN in transduced CD34+ cells with granulocytes and lymphocytes, we compared VCN to %GFP or %YFP in both granulocytes and lymphocytes. The VCN was positively correlated with %GFP or %YFP in both granulocytes (P < 0.01) and lymphocytes (P < 0.01). (b) We summarized comparison data between in vitro and in vivo. *Twofold scores were used in competitive assay. GFP, green fluorescent protein; YFP, yellow fluorescent protein.
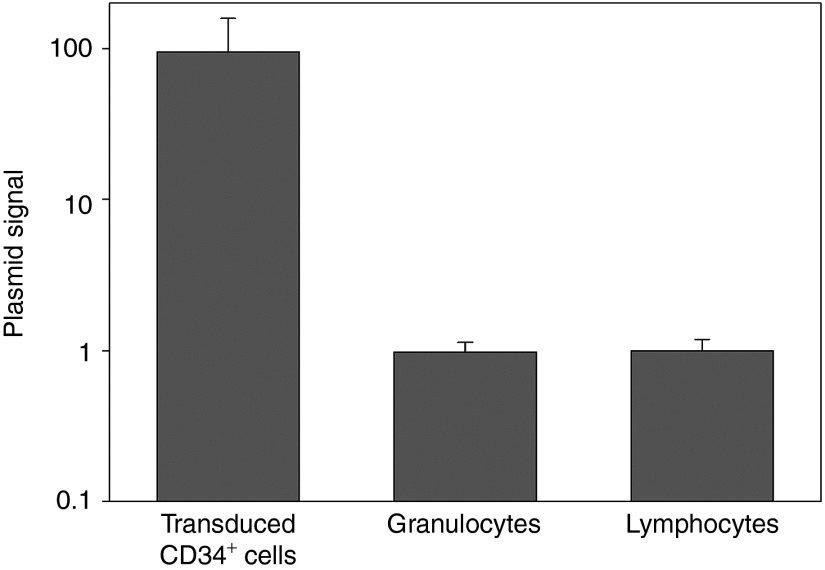
We hypothesized that VCN among transduced CD34+ cells might be overestimated by residual vector plasmids, which were utilized in viral preparation and contained in vector stocks. Therefore, we performed real-time PCR for the ampicillin resistance gene (AmpR) which was specific for plasmid DNA. The plasmid signals were 95-fold higher in transduced CD34+ cells, as compared with granulocytes and lymphocytes in vivo (Figure 3). These data reveal that the residual plasmids in transduced CD34+ cells overestimated VCN evaluated by transgene-specific probe/primers.
Figure 3.
Presence of residual plasmids in transduced CD34+ cells. We hypothesized that transduced CD34+ cells with lentiviral vectors contain vector plasmids which were utilized in vector preparation. Residual plasmids were evaluated in all the samples using ampicillin resistance gene-specific probe/primers which detect plasmids but not integrated provirus. The plasmid signals were 95-fold more detected in transduced CD34+ cells, as compared with granulocytes and lymphocytes 6 months after transplantation.
Development of integrated provirus-specific real-time PCR system
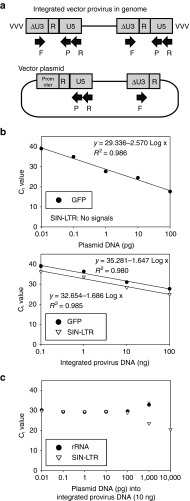
To reduce overestimation of VCN due to residual plasmids, we designed self-inactivating long terminal repeat (SIN-LTR)-specific probe/primers (Figure 4a). In a tenfold dose escalation of plasmid DNA or integrated provirus DNA (both DNA contains GFP-encoding HIV-1 vector sequence), the SIN-LTR probe/primers detected provirus signals, with logarithmic regression (R 2 = 0.98) between Ct (threshold cycle) value and provirus DNA concentration (0.1–100 ng; Figure 4b, lower panel), but did not detect plasmid signals (0.01–100 pg; Figure 4b, upper panel). On the other hand, the GFP probe/primers detected both the provirus (R 2 = 0.98) and the plasmids (R 2 = 0.99) with logarithmic regression. Only when adding >100 pg plasmids (comparable to 3.8 × 103 copy number per cell) into 10 ng provirus DNA did we observe an increase of SIN-LTR signals and a reduction of rRNA signals (Figure 4c). These data demonstrate that the SIN-LTR probe/primers differentiated between integrated provirus DNA and plasmid DNA, and allowed evaluation of VCN in transduced cells with <100 pg plasmids.
Figure 4.
Development of integrated provirus-specific real-time PCR system. (a) To reduce overestimation of vector copy number (VCN) per cell due to residual plasmids, we designed self-inactivating long terminal repeat (SIN-LTR)-specific probe/primers. (b) In tenfold dose escalation of GFP-encoding plasmid DNA (upper) or provirus-integrated DNA (VCN = 1, lower), the SIN-LTR–specific probe/primers detect only provirus signals (concentration 0.1–100 ng, lower), and not detect plasmid signals (0.01–100 pg, upper). The GFP-specific probe/primers detect both the integrated provirus and the plasmid signals. (c) Addition of >100 pg plasmids (comparable to 3.8 × 103 copy number per cell) into provirus DNA (10 ng) resulted in increase of SIN-LTR signals and reduction of ribosomal RNA (rRNA) signals. Ct, threshold cycle; F, forward primer; GFP, green fluorescent protein; No signals, signals are less than threshold for detection at 40 cycles; P, probe; R, reverse primer.
Positive correlation of VCN between transduced CD34+ cells and peripheral blood cells when using provirus-specific real-time PCR
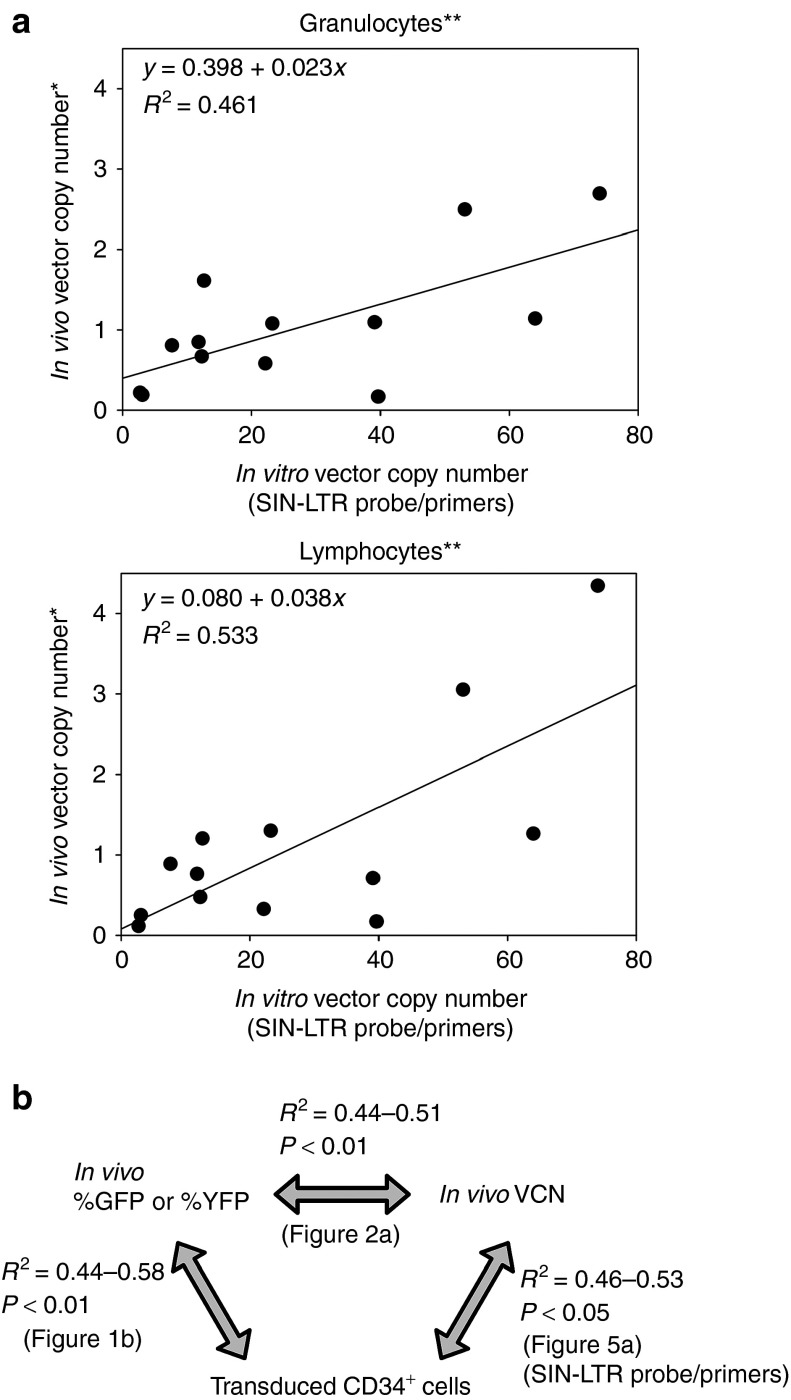
We next quantified VCN among transduced CD34+ cells using the SIN-LTR probe/primers. In all the 16 animals, transduced CD34+ cells had nine- to tenfold higher VCN (25.1 ± 5.6) as compared with granulocytes (2.8 ± 1) and lymphocytes (2.4 ± 0.7) 6 months after transplantation. There was also a positive correlation of VCN in transduced CD34+ cells with both granulocytes (R 2 = 0.46, P = 0.01) and lymphocytes (R 2 = 0.53, P < 0.01) in vivo after excluding outliers of three high-marking animals (VCN >6, Figure 5a,b). These data demonstrate that the SIN-LTR probe/primers allow the evaluation of VCN among transduced CD34+ cells containing residual plasmids, and that under our conditions, transduced CD34+ cells had nine- to tenfold higher VCN as compared with granulocytes or lymphocytes in vivo 6 months after reconstitution.
Figure 5.
Positive correlation of average vector copy number (VCN) per cell in transduced CD34+ cells with peripheral blood cells when using provirus-specific real-time PCR system. (a) We evaluated VCN in transduced CD34+ cells using the SIN-LTR–specific probe/primers, which resulted in positive correlation of VCN in transduced CD34+ cells with both granulocytes (P = 0.01) and lymphocytes (P < 0.01) when excluding outliers of three animals with high-marking levels (VCN >6). The transduced CD34+ cells had nine- to tenfold higher VCN, as compared with granulocytes and lymphocytes. (b) We summarized comparison data between in vitro and in vivo, when using SIN-LTR probe/primers. *Twofold scores were used in competitive assay; **Excluding in vivo VCN >6. GFP, green fluorescent protein; SIN-LTR, self-inactivating long terminal repeat; YFP, yellow fluorescent protein.
Discussion
Estimating VCN before infusion of integrating vector-modified cells should enable us to quantify the risk of insertional mutagenesis, yet conventional PCR methods led to an overestimation of VCN, simultaneously limiting potential benefit. Generally, vector sequence-specific probe/primers (such as GFP or YFP probe/primers) are used to evaluate VCN in transduced cells, but our data demonstrate that this method does not differentiate between vector plasmid and integrated provirus. These vector sequence-specific probe/primers have the advantage that vector plasmids can be utilized as templates to create standard curves. However, we found that genomic DNA extracted from transduced CD34+ cells contained plasmids, leading to an overestimation of VCN. These results are not unexpected, because these plasmids were utilized for lentiviral preparation and thus vector stocks should contain residual plasmids. Adding vector to CD34+ cells invariably adds some residual plasmids to the cells in culture.
To reduce overestimation of VCN due to residual plasmids, we developed integrated provirus-specific real-time PCR system in which the forward primer was designed to recognize U3 region and both reverse primer and probe to recognize U5 region. The integrated provirus have the full-length of both LTR including U3, R, and U5 regions, whereas vector plasmid contains only partial LTR; 5′LTR (R and U5 regions) and 3′LTR (U3 and R regions). This provirus-specific real-time PCR allowed evaluation of VCN among transduced CD34+ cells even with documented residual plasmids, to predict in vivo VCN.
Another strategy often employed is to allow cells to remain in culture for 14 days before evaluating VCN in CD34+ cells because nonintegrated vector DNA (preintegration complex) in transduced cells should decrease during the 14 days in in vitro culture and expansion. Theoretically, the SIN-LTR–specific probe/primers can recognize not only integrated provirus but also nonintegrated vector DNA including one-LTR circles, two-LTR circles, and linear forms. When we previously evaluated vector DNA amounts in lentivirally transduced human CD34+ cells at various timepoints, a peak of vector DNA was observed at 24 hours after transduction.13 Vector DNA amounts in transduced CD34+ cells fell sharply at 2–3 days reaching a plateau that persisted between 6 and 14 days after transduction (relative vector amounts 1.0 ± 0.2 at 6 days versus 1.2 ± 0.5 at 14 days, P = 0.36).13 In addition, when nonintegrated two-LTR circular DNA was quantified in transduced 293T cells using the same HIV-1 vector construct, a peak of the DNA amount was observed at 2 days after transduction, and it decreased more than tenfold until 6 days after transduction.14 These data suggest that nonintegrated vector DNA has minimal effects on quantification of integrated provirus amounts 6 days after transduction, and 6 days after transduction (as we performed in this analysis) it should be sufficient to evaluate integrated VCN in transduced CD34+ cells.
Interestingly, when we used Stemline II hematopoietic stem cell expansion media (Sigma-Aldrich, St Louis, MO) instead of X-VIVO10 media, in vitro VCN in human CD34+ cells increased sevenfold from 2 to 6 days after transduction.13 On the other hand, we observed similar VCN between 2 and 6 days after transduction in X-VIVO10 media.13 These data suggest that longer in vitro culture in cell expansion media may induce selected proliferation of efficiently transduced progenitor cells, which results in higher VCN in transduced CD34+ cells and demonstrate that VCN determined after longer culture periods is highly dependent upon culture conditions.
To evaluate whether a large amount of vector plasmids could affect the provirus-specific real-time PCR system, increasing doses of vector plasmids were added into a fixed dose of provirus DNA (10 ng). The PCR amplification with SIN-LTR probe/primers resulted in a nonspecific increase of SIN-LTR signals when adding >100 pg plasmids. The vector plasmids do not have a full-length of target sequence for PCR reaction with SIN-LTR probe/primers. Only SIN-LTR reverse primer and probe can bind to the 5′LTR in the plasmids to allow subsequent linear amplifications of SIN-LTR signal. This should be much less efficient, as compared with the PCR reaction of the integrated provirus. However, the PCR amplification of rRNA might be interfered with by excess DNA; 100 pg plasmids, a very large amount of contamination comparable to 3.8 × 103 VCN in provirus DNA, proved sufficient to increase the SIN-LTR signals from provirus-specific PCR, and reduced rRNA signals. Therefore, a very large amount of plasmid contamination could lead to overestimation of VCN by increasing SIN-LTR signals and decreasing rRNA signals.
VCN was evaluated among rhesus CD34+ cells that were transduced using identical culture conditions in 16 transplanted animals, from which the cells were cultured in X-VIVO10 media containing stem cell factor, FMS-related tyrosine kinase 3 ligand, and thrombopoietin (all 100 ng/ml). We previously demonstrated that %GFP in transduced human CD34+ cells was increased by addition of interleukin-3 (IL-3) in the culture media, but it did not increase %GFP in peripheral blood cells in xenograft mice after transplantation of the transduced CD34+ cells.15 These data suggest that IL-3 expanded only progenitor cells with high %GFP, which did not reconstitute peripheral blood cells in vivo for long term. Simply adding IL-3 changed the ability of transduced CD34+ cells in vitro to predict %GFP among reconstituted cells in vivo. Identical culture conditions for transduced CD34+ cells are therefore necessary to predict in vivo %GFP and VCN.
A limit of two VCN is generally accepted to reduce the risk for insertional mutagenesis in HSC-targeted gene therapy using integrating vectors. Using this guideline, when transduced CD34+ cells have >2 VCN, the cells would not pass lot-release criteria, and therefore would not be infused into patients. However, transduced CD34+ cells with ≤2 VCN might be insufficient to ameliorate the phenotype for a number of disorders, especially sickle cell disease where around two VCN in peripheral blood cells is thought to be required.16 In this study, we demonstrated that transduced CD34+ cells had nine- to tenfold higher VCN (25.1 ± 5.6), as compared with granulocytes (2.8 ± 1) or lymphocytes (2.4 ± 0.7) in vivo, even when using integrated provirus-specific real-time PCR system. These data suggest that a restriction of ≤2 VCN before infusion may unnecessarily limit efficacy. Indeed, we demonstrated that transduced CD34+ cells with ≤40 VCN resulted in ≤2 VCN in vivo, when excluding outliers of three high-marking monkeys with VCN >6.
In HSC gene therapy, VCN among peripheral blood cells in vivo is one of the useful factors to evaluate effects from integrated vector constructs, and VCN is one means generally utilized to quantify the risk of insertional mutagenesis. However, no clear evidence linking in vivo VCN and insertional mutagenesis has been reported. Insertional mutagenesis is caused by vector integration into or around oncogenes, which are activated by enhancers in vector LTRs or internal promoters. This activation can be reduced when using SIN-LTRs, tissue-specific promoters, and chromatin insulators.17,18,19,20 Integration patterns of viral vectors are also influenced by the target disease and vector types.7,21 Therefore, the risk of insertional mutagenesis should be evaluated by not only in vivo VCN but also vector construct, disease type, and integration patterns.
In this study, we demonstrated a positive correlation between in vitro VCN and in vivo VCN when using the SIN-LTR–specific probe/primers; however, it required exclusion of three high-marking animals. Though our study does not permit us to assign a “safe” in vitro VCN, it does demonstrate that current methods may unnecessarily limit efficacy without providing additional safety. In addition, this correlation was preserved in %GFP or %YFP between transduced CD34+ cells and peripheral blood cells in vivo, even when including these high-marking animals. However, flow cytometry for such marker genes may not be useful in gene therapy trials, as most of the therapeutic vectors do not contain them. When testing therapeutic vectors in our large animal model, we have begun to separately transduce a small aliquot of CD34+ cells with a GFP-expressing vector to predict transduction efficiency in vivo. This strategy may be useful for prediction of in vivo marking levels in gene therapy trials. Importantly, all animals including the three high-marking animals have stable blood counts and gene-marking levels among peripheral blood cells.10,11,12
CD34+ cells contain HSCs as well as hematopoietic progenitor cells. After transplantation of CD34+ cells, peripheral blood cells are temporally derived from hematopoietic progenitor cells (around 3 months after transplantation), and reconstitution by HSCs ensues over the long term.22 In the current study, we evaluated in vivo %GFP and in vivo VCN using granulocytes and lymphocytes 6 months after transplantation, since gene-marking levels plateaued in the peripheral blood cells in this timepoint, a timepoint at which peripheral blood is reconstituted from HSCs.10,11,12 We observed nine- to tenfold higher VCN in transduced CD34+ cells than granulocytes and lymphocytes 6 months after transplantation. In addition, we previously demonstrated that in vitro IL-3 exposure of CD34+ cells resulted in higher in vitro %GFP in transduced CD34+ cells and similar in vivo %GFP in peripheral blood cells after xenograft mouse transplantation.15 These data suggest that hematopoietic progenitor cells were more efficiently transduced with lentiviral vectors, as compared with HSCs.
Benzonase treatment during vector preparation was reported to reduce ~100-fold residual plasmids in vector stocks.23,24 In these reports, residual plasmids in vector stocks were not transfected into target cells during in vitro exposure to lentiviral vectors. Treatment with benzonase is not, however, routine in most preclinical laboratory studies. In addition, residual plasmids in vector stocks could be reduced by concentration of vector products with ultracentrifugation or ultrafiltration.23
In this study, we demonstrated positive correlation between in vivo %GFP or %YFP and in vivo VCN. In addition, we evaluated VCN in either GFP-positive or GFP-negative fractions in both granulocytes and lymphocytes in three rhesus macaques 3–3.8 years after transplantation (Supplementary Figure S1). We observed 18.5-fold higher VCN in GFP-positive fraction (10.8 ± 3.5 VCN) in both granulocytes and lymphocytes, as compared with GFP-negative fraction (0.6 ± 0.1 VCN). The vector signals in the GFP-negative fraction might be derived from transduced cells with no or little transgene expression. These data suggest that vector signals in real-time PCR were mainly derived from GFP-positive fraction, and most integrated vector could express GFP transgene without gene silencing for long term in vivo.
In summary, we developed a system using integrated provirus-specific real-time PCR to determine VCN in transduced CD34+ cells containing residual plasmids. Even when using this system, transduced CD34+ cells had nine- to tenfold higher VCN as compared with in vivo VCN. Our findings are important in developing safety restrictions for gene therapy trials, and suggest that a restriction of ≤2 VCN before infusion may be too stringent and unnecessarily limit efficacy.
Materials and methods
Lentiviral vector preparation. We previously developed a chimeric HIV-1–based lentiviral vector system (χHIV vector) in which the HIV-1 genome is packaged into simian immunodeficiency virus capsid with vesicular stomatitis virus glycoprotein envelope, to efficiently transduce rhesus hematopoietic cells.10,11 The χHIV vectors encoding GFP or YFP were prepared by cotransfection of four plasmids into 293T cells, which contain gag/pol, rev/tat, envelope, and vector plasmids.10,25 The HIV-1 vector plasmids were kindly provided by Dr Arthur Nienhuis (St Jude Children's Research Hospital, Memphis, TN).26,27 Two days after transfection, conditioned media from the transfected 293T cells were 100-fold concentrated by ultracentrifugation. The vector stocks were stored in a −80 °C freezer. Deoxyribonucleases were not used for our viral preparation.
Rhesus HSC transplantation with lentiviral transduction. We previously developed a large animal model for HSC transplantation with lentiviral transduction in rhesus macaques.10,12 Granulocyte colony-stimulating factor (Amgen, Thousand Oaks, CA) and stem cell factor (Amgen) or plerixafor (Genzyme, Cambridge, MA)-mobilized rhesus CD34+ cells were cultured in X-VIVO10 media (Lonza, Allendale, NJ) containing stem cell factor, FMS-related tyrosine kinase 3 ligand, and thrombopoietin (all 100 ng/ml; R&D Systems, Minneapolis, MN).15 After 1 day prestimulation, the CD34+ cells were transduced with χHIV vectors at a multiplicity of infection of 50, and next day, these cells were infused into rhesus macaques following a split dose (2 × 5 Gy) of 10 Gy total body irradiation. A small amount of the transduced CD34+ cells were cultured in vitro in fresh media with same cytokines.
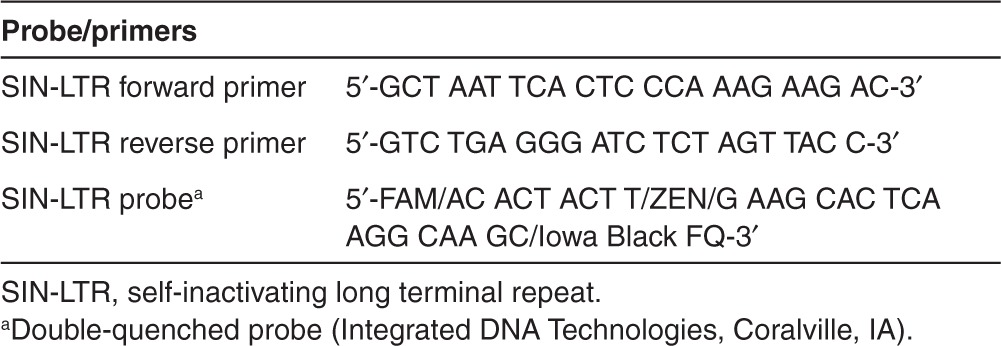
Real-time PCR. Genomic DNA was extracted from the transduced rhesus CD34+ cells 6 days after transduction and from both granulocytes and lymphocytes 6 months after transplantation, which were separated by Ficoll-Paque PLUS density gradient centrifugation (GE Healthcare, Uppsala, Sweden). The extracted DNA (10 ng) was used as templates, and specific sequences were amplified by real-time PCR (Mx3000P QPCR Systems; Agilent Technologies, Santa Clara, CA) using GFP or YFP probe/primers,10,11 SIN-LTR probe/primers (Table 1 and Figure 4a), and AmpR probe/primers28 for 40 cycles of 30 seconds at 95 °C, 30 seconds at 60 °C, and 15 seconds at 72 °C. TaqMan Ribosomal RNA control reagents (Applied Biosystems, Foster City, CA) were used to determine the amount of genomic DNA. Average VCN per cell was calculated by total VCN per total cell number, which was compared to a monoclonal cell line with single copy of integrated provirus (VCN = 1).10 The relative plasmid signals using AmpR probe/primers were calculated by compared to average plasmid signal in lymphocytes.
Table 1. Self-inactivating long terminal repeat-specific probe/primers.
In addition, transgene expression rates (%GFP or %YFP) were evaluated by flow cytometry (FACSCalibur; BD Biosciences, Franklin Lakes, NJ) 3–4 days after transduction among CD34+ cells and 6 months after transplantation among both granulocytes and lymphocytes from 16 transplanted rhesus macaques. In rhesus macaques undergoing competitive repopulation assays, twofold scores of VCN and %GFP or %YFP were used in both granulocytes and lymphocytes since each represented only half of the transduced product.
Statistical analyses. Statistical analyses were performed using the JMP 9 software (SAS Institute, Cary, NC). We evaluated correlation using two factors of (i) significance of relationship expressed by t-test for coefficient of correlation, and (ii) strength of relationship measured by R 2 in regression analysis. A P value of <0.01 or 0.05 was deemed significant. SEM are shown as error bars in figures. We excluded outliers (in vivo VCN >6 and in vitro VCN >600 in Figure 1c and in vivo VCN >6 in Figure 5a), which were defined by more than the upper limit of “(third quartile) + 1.5 × (interquartile range)” or less than the lower limit of “(first quartile) − 1.5 × (interquartile range).”
SUPPLEMENTARY MATERIAL Figure S1. Vector copy number per cell in GFP-positive and GFP-negative fractions in peripheral blood cells of transplanted rhesus macaques.
Acknowledgments
This work was supported by the intramural research program of the National Heart, Lung, and Blood Institute and the National Institute of Diabetes, Digestive, and Kidney Diseases at the National Institutes of Health. We thank the animal care staff and technicians at 5 Research Court for their excellent care and handling of the animals. The authors declared no conflict of interest.
Supplementary Material
Vector copy number per cell in GFP-positive and GFP-negative fractions in peripheral blood cells of transplanted rhesus macaques.
References
- Aiuti A, Cattaneo F, Galimberti S, Benninghoff U, Cassani B, Callegaro L, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360:447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. Transfusion independence and HMGA2 activation after gene therapy of human ß-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]
- Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat Med. 2006;12:401–409. doi: 10.1038/nm1393. [DOI] [PubMed] [Google Scholar]
- Schwarzwaelder K, Howe SJ, Schmidt M, Brugman MH, Deichmann A, Glimm H, et al. Gammaretrovirus-mediated correction of SCID-X1 is associated with skewed vector integration site distribution in vivo. J Clin Invest. 2007;117:2241–2249. doi: 10.1172/JCI31661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Garrigue A, Wang GP, Soulier J, Lim A, Morillon E, et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J Clin Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- Hanawa H, Hematti P, Keyvanfar K, Metzger ME, Krouse A, Donahue RE, et al. Efficient gene transfer into rhesus repopulating hematopoietic stem cells using a simian immunodeficiency virus-based lentiviral vector system. Blood. 2004;103:4062–4069. doi: 10.1182/blood-2004-01-0045. [DOI] [PubMed] [Google Scholar]
- Uchida N, Washington KN, Hayakawa J, Hsieh MM, Bonifacino AC, Krouse AE, et al. Development of a human immunodeficiency virus type 1-based lentiviral vector that allows efficient transduction of both human and rhesus blood cells. J Virol. 2009;83:9854–9862. doi: 10.1128/JVI.00357-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Hargrove PW, Lap CJ, Evans ME, Phang O, Bonifacino AC, et al. High-efficiency transduction of rhesus hematopoietic repopulating cells by a modified HIV1-based lentiviral vector. Mol Ther. 2012;20:1882–1892. doi: 10.1038/mt.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Bonifacino A, Krouse AE, Metzger ME, Csako G, Lee-Stroka A, et al. Accelerated lymphocyte reconstitution and long-term recovery after transplantation of lentiviral-transduced rhesus CD34(+) cells mobilized by G-CSF and plerixafor. Exp Hematol. 2011;39:795–805. doi: 10.1016/j.exphem.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, Tisdale JF, Uchida N. Determining limitations in human CD34+ cell transduction with an HIV1-based lentiviral vector [abstract]. Blood. 2011;118:Abstract 4171. [Google Scholar]
- Hanawa H, Yamamoto M, Zhao H, Shimada T, Persons DA. Optimized lentiviral vector design improves titer and transgene expression of vectors containing the chicken beta-globin locus HS4 insulator element. Mol Ther. 2009;17:667–674. doi: 10.1038/mt.2009.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Hsieh MM, Hayakawa J, Madison C, Washington KN, Tisdale JF. Optimal conditions for lentiviral transduction of engrafting human CD34+ cells. Gene Ther. 2011;18:1078–1086. doi: 10.1038/gt.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestina TI, Hargrove PW, Jay D, Gray JT, Boyd KM, Persons DA. Correction of murine sickle cell disease using gamma-globin lentiviral vectors to mediate high-level expression of fetal hemoglobin. Mol Ther. 2009;17:245–252. doi: 10.1038/mt.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam PI, Higashimoto T, Urbinati F, Modlich U, Nestheide S, Xia P, et al. Genotoxic potential of lineage-specific lentivirus vectors carrying the beta-globin locus control region. Mol Ther. 2009;17:1929–1937. doi: 10.1038/mt.2009.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruggi G, Porcellini S, Facchini G, Perna SK, Cattoglio C, Sartori D, et al. Transcriptional enhancers induce insertional gene deregulation independently from the vector type and design. Mol Ther. 2009;17:851–856. doi: 10.1038/mt.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlich U, Navarro S, Zychlinski D, Maetzig T, Knoess S, Brugman MH, et al. Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors. Mol Ther. 2009;17:1919–1928. doi: 10.1038/mt.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans-Galea MV, Wielgosz MM, Hanawa H, Srivastava DK, Nienhuis AW. Suppression of clonal dominance in cultured human lymphoid cells by addition of the cHS4 insulator to a lentiviral vector. Mol Ther. 2007;15:801–809. doi: 10.1038/sj.mt.6300103. [DOI] [PubMed] [Google Scholar]
- Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Zickler P, Hoffmann G, Haas S, Wissler M, Muessig A, et al. Polyclonal long-term repopulating stem cell clones in a primate model. Blood. 2002;100:2737–2743. doi: 10.1182/blood-2002-02-0407. [DOI] [PubMed] [Google Scholar]
- Sastry L, Xu Y, Cooper R, Pollok K, Cornetta K. Evaluation of plasmid DNA removal from lentiviral vectors by benzonase treatment. Hum Gene Ther. 2004;15:221–226. doi: 10.1089/104303404772680029. [DOI] [PubMed] [Google Scholar]
- Bandeira V, Peixoto C, Rodrigues AF, Cruz PE, Alves PM, Coroadinha AS, et al. Downstream processing of lentiviral vectors: releasing bottlenecks. Hum Gene Ther Methods. 2012;23:255–263. doi: 10.1089/hgtb.2012.059. [DOI] [PubMed] [Google Scholar]
- Uchida N, Washington KN, Lap CJ, Hsieh MM, Tisdale JF. Chicken HS4 insulators have minimal barrier function among progeny of human hematopoietic cells transduced with an HIV1-based lentiviral vector. Mol Ther. 2011;19:133–139. doi: 10.1038/mt.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Persons DA, Nienhuis AW. Mobilization and mechanism of transcription of integrated self-inactivating lentiviral vectors. J Virol. 2005;79:8410–8421. doi: 10.1128/JVI.79.13.8410-8421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanawa H, Kelly PF, Nathwani AC, Persons DA, Vandergriff JA, Hargrove P, et al. Comparison of various envelope proteins for their ability to pseudotype lentiviral vectors and transduce primitive hematopoietic cells from human blood. Mol Ther. 2002;5:242–251. doi: 10.1006/mthe.2002.0549. [DOI] [PubMed] [Google Scholar]
- Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K. Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 2002;9:1155–1162. doi: 10.1038/sj.gt.3301731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vector copy number per cell in GFP-positive and GFP-negative fractions in peripheral blood cells of transplanted rhesus macaques.