Abstract
Application of mechanical strain to neonatal rat ventricular myocytes in culture evokes changes in gene expression reminiscent of those that occur with hypertrophy in vivo, such as stimulation of brain natriuretic peptide (BNP) gene expression. Here, we show that a major component of strain-dependent BNP promoter activation results from stimulation of p38 mitogen-activated protein kinase (MAPK) in the cardiac myocyte. Strain increased p38 activity in a time-dependent fashion. The p38 inhibitor SB203580 led to a reduction of approximately 60% in strain-activated human BNP (hBNP) promoter activity. Cotransfection of wild-type p38 increased both basal and strain-dependent promoter activity, while cotransfection with MKK6AL, a dominant-negative inhibitor of p38 MAPK kinase, resulted in partial inhibition of either p38- or strain-activated hBNP promoter activity. p38 MAPK increased hBNP promoter activity through activation of the transcription factor NF-κB. Activation of the hBNP promoter by either p38 or strain was mediated by DNA elements present in the 5′ flanking sequence of the gene. Mechanical strain promoted assembly of NF-κB components on these DNA elements in vitro. Thus, induction of the hBNP promoter by mechanical strain depends, at least in part, on stimulation of p38 and subsequent activation of NF-κB. This activation may play an important role in signaling the increased BNP gene expression that accompanies hemodynamic overload and cardiac hypertrophy in vivo.
J. Clin. Invest. 104:1603–1612 (1999).
Introduction
Sustained hemodynamic overload elicits a series of functional and structural changes in the ventricular myocyte that culminate in cardiac hypertrophy. This is viewed as a compensatory response that, over the short term, results in improved cardiac performance; however, protracted exposure to the hypertrophic stimulus often results in an alteration in phenotype that leads to progressive heart failure. Clinical hypertrophy has, in fact, been linked to increased mortality independent of associated cardiovascular risk factors (1).
At the cellular level, hypertrophy is thought to develop in response to a combination of mechanical (i.e., load-dependent) and neurohumoral stimuli, such as angiotensin II (AII), endothelin (ET), and adrenergic agonists. In cultured neonatal rat cardiac myocytes, both mechanical (2–4) and biochemical (5–8) stimuli effect a series of changes in gene expression that closely parallel those seen in the hypertrophied heart in vivo (9). This includes the sequential activation of immediate early genes (e.g., protooncogenes like c-jun, c-fos, and c-myc), a fetal gene program (e.g., atrial natriuretic peptide [ANP], brain natriuretic peptide [BNP], skeletal α-actin, and β-myosin heavy chain) that is typically quiescent in the nonhypertrophied adult ventricular myocardium, and the structural sarcomeric genes that contribute the protein infrastructure associated with hypertrophy (e.g., cardiac α-actin and myosin light chain-2) (9).
The signaling cascades underlying the hypertrophic phenotype remain only partially understood. Protein kinase C (10), calcium/calmodulin (11), calcineurin (12–13), nonreceptor protein tyrosine kinases (14, 15), the Janus kinase/STAT system (16, 17), small G proteins (e.g., Ras, Rac, Rho, Cdc42) (18–21), and the various mitogen-activated protein kinases (i.e., ERKs, JNKs, and p38 MAPKs) (22–38) have each been implicated as playing a role in signaling hypertrophy. The MAPKs in particular have been the focus of considerable attention. Hypertrophic stimuli have been shown to activate ERK (22–24), JNK (30, 32), and p38 (34, 37, 38) in either cultured ventricular myocytes or intact myocardial tissue. Whereas interference with the ERK pathway has been shown to block some aspects of hypertrophy-dependent gene expression, it does not affect the morphological changes accompanying hypertrophy (27); in some instances (29) it has been dissociated from the hypertrophic program completely. JNKs have also been linked to hypertrophy (28–32), although recent studies have raised questions about the importance of these kinases in signaling various aspects of the phenotype (37). Activation of p38 by upstream kinases (e.g., MKK6) (35) or hypertrophic agonists (38) has been associated with effects on myocyte growth. Of note, the nature of the effect appears to be isoform specific. Overexpression of activated MKK3 (MKK3bE) elicited both characteristic hypertrophic changes and increased apoptosis in adenoviral vector–infected neonatal myocytes (33). The hypertrophic response was amplified by coinfection with wild-type p38β, whereas apoptosis was increased with wild-type p38α. It should be noted that, as with the ERKs and JNKs, some published studies argue against a major role for p38 in hypertrophy (32). Thus, whereas several signaling pathways appear to be involved, at present there is no consensus regarding the identity of the pathway or pathways that dominate initiation and maintenance of the hypertrophic phenotype in the cardiac myocyte.
We have recently shown that application of mechanical strain to neonatal rat ventricular myocytes in vitro results in increased expression of the BNP gene product and secretion of the encoded protein (4). This increase in gene expression results, in large part, from increased BNP gene promoter activity and seems to depend upon intact ERK and JNK signaling cascades. It is noteworthy that approximately half of the increase in promoter activity represents a primary response to the strain stimulus (39). The remainder results from activation of a local autocrine/paracrine system (or systems). This system links increased angiotensinogen- and angiotensin-converting enzyme gene expression to local generation of angiotensin II and subsequently of endothelin. Endothelin receptors are present on cardiac myocytes (40), and presumably signal the increase in BNP promoter activity (39).
In this study, we have examined the ability of mechanical strain to stimulate p38 activity in the neonatal rat ventricular myocyte model, and have explored the relationship of this activation to the subsequent increase in BNP gene promoter activity. Our findings suggest that p38 plays a major role in signaling strain-dependent activation of this promoter, and that it does so at least in part through specific cis-acting sites — shear stress response elements (SSREs) — present in the 5′ flanking sequence of the BNP gene.
Methods
Materials.
SB203580 was a gift from J. Lee of SmithKline Beecham Pharmaceuticals Inc. (King of Prussia, Pennsylvania, USA). PD98059 was obtained from Research Biochemicals International (Natick, Massachusetts, USA). Bovine myelin basic protein (MBP)was purchased from Upstate Biotechnology Inc. (Lake Placid, New York, USA). Polyclonal antibodies against p38, p50, and p65 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, California, USA). Anti-ACTIVE p38 polyclonal antibody and the luciferase assay system were purchased from Promega Corp. (Madison, Wisconsin, USA). NEN Life Science Products Inc. (Boston, Massachusetts, USA) provided [γ-32P]ATP. The enhanced chemiluminescence (ECL) Western blotting detection system was purchased from Amersham Life Sciences Inc. (Arlington Heights, Illinois, USA). Other reagents were obtained through standard commercial suppliers.
Cell culture and application of mechanical strain.
Ventricular myocytes were prepared from neonatal rat hearts (1–2 days old) by alternate cycles of 0.05% trypsin digestion and mechanical disruption as described previously (41). Cells (106) were cultured on collagen-coated Flex plates (Flexcell International Corp., McKeesport, Pennsylvania, USA) in DME-H21 medium containing 10% BCS (HyClone Laboratories Inc., Logan, Utah, USA), 2 mM glutamine, 10 U/mL penicillin, and 100 mg/mL streptomycin. A glass cloning cylinder (1 cm in diameter) was placed in the middle of each well to preclude cell attachment, thereby placing the majority of adherent cells on the outer 75% of the culture surface where distension is maximal (42). The medium was changed 24 hours before initiation of the experiment. Cells were subjected to cyclical strain (60 cycles/minute) on the Flexcell Strain apparatus (Flexcell International Corp.) at a level of distension sufficient to promote an increment of approximately 20% in surface area at the point of maximal distension on the culture surface (42).
Plasmid construction and site-directed mutagenesis.
The construction of –904 and –1,595 hBNP luciferase (hBNPLuc) has been described previously (43). The wild-type p38α expression vector pCMV-FLAG-p38MAPK (44) was provided by R. Davis (University of Massachusetts, USA). MKK6AL pcDNA3, a dominant-negative MKK6 mutant (45), was provided by J.R. Woodgett (Universtiy of Toronto, Toronto, Canada). The latter replaces Ser 207 and Thr 211 in MKK6 with alanine and leucine, respectively. These changes preclude phosphorylation and activation of the mutant kinase and permit it to function in a dominant-negative mode. Wild-type IκBα and IκBα mutants 2N, 3C, and 2N+3C (46) were provided by J. Hiscott (McGill University, Montreal, Quebec, Canada). NF-κB TKLuc, harboring a single copy of an NF-κB binding site [5′-AGGGACTTTCCGCTGGG-ACTTTCC-3′] upstream from a core (position –45 to +53), thymidine kinase promoter/luciferase reporter, came from D. Leitman (University of California–San Francisco, San Francisco, California, USA). TKLuc contains 109 bp of promoter sequence from the thymidine kinase gene (position –109 to +53) linked to the luciferase reporter.
Site-directed mutagenesis was carried out with the QuikChange kit (Stratagene, La Jolla, California, USA) using conditions recommended by the manufacturer. In brief, mixtures containing 10–50 ng of –904 hBNP luciferase, 2 complementary mutagenic primers, dNTPs, and Pfu DNA polymerase were added to the PCR buffer. PCR was carried out for 16–18 cycles using 30 seconds of denaturation at 95°C, 1 minute of annealing at 55°C, and 2 minutes/kb of extension at 68°C. After PCR, 1 μL of Dpn I was added to the reaction to cut parental DNA template, and 5 μL of this digest was used for transformation. Several candidate clones were identified by restriction mapping and were sequenced using a DNA sequence kit and [α-35S]dATP obtained from Amersham Life Sciences Inc. The oligonucleotides for site-directed mutagenesis (mutagenized primers) were as follows (mutagenized bases are identified by lowercase letters): mSSRE-1: 5′-GGTCGGCTCTGCCCtacagtCACCTCCCACGTCG-3′; mSSRE-2: 5′-GAAAGGTCTCGGAtacagtTTGTCCTTCGTCC-3′; mSSRE-3: 5′-GAGCATAGGGAAAtacagtGGAGGTCTCTTGTC-3′; mSSRE-2,3: 5′-GTGAGAGCATAGGGAAAtacagtGGAtacagtTTGTCCTTGCTCCACG-3′.
Transfection and luciferase assay.
Freshly prepared ventricular myocytes were transiently transfected with the indicated reporters and expression vectors by electroporation (Gene Pulser; Bio-Rad Laboratories, Richmond, California, USA) at 280 mV and 250 μF. DNA content in individual cultures was normalized with pUC 18 plasmid. After transfection, cells were plated and cultured as described above. Cells were harvested and lysed in 100 μL of cell culture lysis reagent (Promega Corp.). The protein concentration of each cell extract was measured using Coomassie protein reagent (Pierce Chemical Co., Rockford, Illinois, USA). Cell lysates were processed (20 μg protein per sample) and assayed for luciferase as described using a commercially available kit (Promega Corp.). To ensure reproducibility, experiments were repeated 3–5 times.
Immunoprecipitation and kinase assay.
Cells were harvested in 1 mL of lysis buffer (20 mM Tris-HCl at pH 7.9, 137 mM NaCl, 1% Triton X-100, 5 mM EDTA, 1 mM EGTA, 10% glycerol, 10 mM NaF, 1 mM β-glycerophosphate, 1 mM PMSF, 1.5 μg/mL aprotinin, and 1 μg/mL pepstatin) and centrifuged at 12,800 g for 30 minutes. Two hundred micrograms of supernatant protein was incubated with 1 μg of anti-p38 antibody and 10 μL protein G–Sepharose (Amersham Pharmacia Biotech Inc., Piscataway, New Jersey, USA) for 2 hours at 4°C. The immunoprecipitates were recovered by centrifugation, and then washed 3 times with cell lysis buffer and once with a kinase reaction buffer (25 mM HEPES at pH 7.4, 10 mM MgCl2, 10 mM MnCl2, and 1 mM DTT) without ATP. Twenty micrograms of myelin basic protein was then added to the immunoprecipitates in 30 μL of kinase reaction buffer containing 2 μCi [γ-32P]ATP. Reactions were incubated for 15 minutes at 30°C. Total reaction contents were electrophoresed on 15% SDS-polyacrylamide gels that were then dried and subjected to autoradiography. Autoradiographic signals were quantified using the NIH Image program.
Western blot analysis.
Immunoprecipitation using the anti-ACTIVE® p38 polyclonal antibody was carried out as described above. Immunoprecipitates were boiled for 5 minutes, electrophoresed on 10% SDS-polyacrylamide gels, and electrophoretically transferred onto Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). The membranes were incubated with rabbit anti-ACTIVE® p38 polyclonal antibody (1:2,000 dilution) in TBST buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween-20, and 5% nonfat dried milk) for 2 hours at room temperature with agitation, and then washed 3 times with TBST buffer. Membranes were later incubated with horseradish peroxidase–conjugated goat anti-rabbit antibody for 1 hour at room temperature and washed 3 times in TBST buffer. The blots were soaked for 1 minute in ECL Detection Reagent and exposed to ECL Hyperfilm (Amersham Pharmacia Biotech) for varying lengths of time. Signals were identified and quantified using NIH Image.
Preparation of nuclear extracts.
Ventricular myocytes were subjected to mechanical strain for varying lengths of time. Nuclear extracts were prepared using a modification of the method of Han and Brasier (47). Cells were harvested and lysed with buffer A (10 mM HEPES at pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5% NP-40, 1 mM DTT, 1 mM PMSF, 5 μg/mL leupeptin, and 5 μg/mL aprotinin) on ice for 10 minutes. Lysates were centrifuged for 5 minutes at 4°C. Particulates were resuspended in buffer B (1 M sucrose, 10 mM HEPES at pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 1 mM DTT, and the above protease inhibitors) and centrifuged at 12,800 g for 3 minutes at 4°C. Pelleted nuclei were resuspended in buffer C (20 mM HEPES at pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, and the above protease inhibitors) and kept on ice for 30 minutes. Nuclear suspensions were centrifuged at 12,800 g for 15 minutes and the supernatant was saved. Nuclear extracts were stored at –80°C until use.
Electrophoretic mobility shift assay.
Oligonucleotides used for electrophoretic mobility shift assay were as follows (SSRE sequences are underlined, and mutagenized bases are identified by lowercase letters): wild type: 5′- GTGAGAGCATAGGGAAAGGTCTCGGAGGTCTCTTGTCCTTGCTCCACG-3′, and mutant: 5′-GTGAGAGCATAGGGAAAtacagtGGAtacagtTTGTCCTTGCTCCACG-3′. Nuclear extracts (10 μg) were incubated in binding reaction buffer (10 mM HEPES at pH 7.9, 50 mM KCl, 0.2 mM EDTA, 2.5 mM DTT, 10% glycerol, and 0.05% NP-40) containing 0.5 μg poly(dI-dC) and 32P-endlabeled, double-stranded, wild-type oligonucleotide at room temperature for 30 minutes. For competition experiments, a 10- or 100-fold molar excess of unlabeled, double-stranded oligonucleotide was added to the binding reaction. For immunoperturbation experiments, nuclear extracts were preincubated on ice for 1 hour with 1 μg polyclonal antibody directed against NF-κB1 (p50) or RelA (p65). All samples were resolved on 5% nondenaturing polyacrylamide gels. Gels were dried and exposed to x-ray film.
Statistics.
Data were analyzed by ANOVA using the Newman-Keuls test to assess significance.
Results
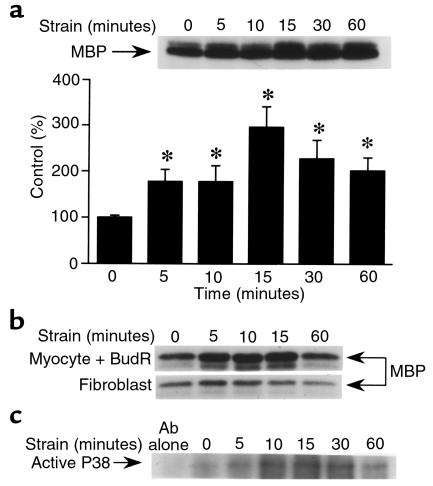
We used a conventional immune complex kinase assay to quantitate p38 activity after application of mechanical strain to neonatal rat ventricular myocytes in vitro. The strain stimulus effected a time-dependent increment in p38 activity (Figure 1a). The increase was first noticeable after 5 minutes, peaked at 15 minutes, and remained elevated after 60 minutes of mechanical strain. Activation of p38 was seen in myocyte-enriched cultures treated with bromodeoxyuridine, and was seen to a much lesser extent in fibroblasts cultured from the same neonatal hearts (Figure 1b). This indicates that the observed p38 induction occurs predominantly in the myocytes (as opposed to the nonmyocytes) in these cultures. Activation of p38 was confirmed by Western blot analysis using an antibody that selectively recognizes the activated form of the kinase. Again, the induction was apparent at 5 minutes, peaked at 10–15 minutes, and was still apparent after 1 hour of exposure to the strain stimulus (Figure 1c).
Figure 1.
Mechanical strain activates p38 in cultured neonatal rat ventricular myocytes. After 48 hours of culture, cells were subjected to cyclical strain for the times indicated. Cells were then collected, lysed, and assayed for p38 kinase activity using either an immune complex kinase assay (a and b) or Western blot analysis for the activated form of the enzyme (c). (a) Time course of p38 induction in conventional myocyte cultures; pooled data from 3 independent experiments. (b) p38 induction in cultures further enriched for myocytes after 48 hours of treatment with bromodeoxyuridine (BudR; 0.1 mM) or nonmyocytes (primarily fibroblasts) collected from the same neonatal hearts and expanded in culture over a 2-week period. Representative of duplicate experiments. (c) Cells were subjected to mechanical strain for varying periods of time, then assayed for active p38 using Western blot analysis. Representative of duplicate experiments. Data are presented as mean ± SD. *P < 0.01 vs. control.
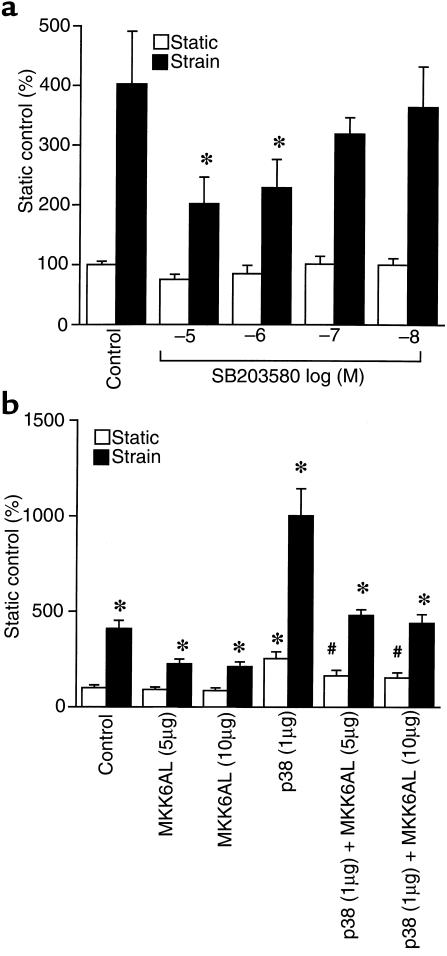
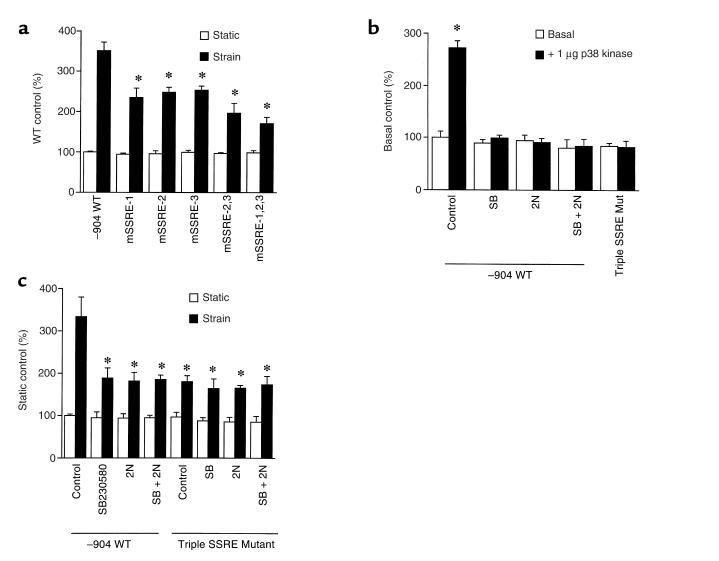
Next, we asked whether this increment in p38 activity could be linked to the strain-dependent increment in hBNP promoter activity that we have documented previously in this system (4, 39). As shown in Figure 2a, SB203580 effected a partial (∼60%), but dose-dependent reduction in strain-activated hBNP promoter activity. The p38 inhibitor had no effect on basal expression of this promoter. Cotransfection of MKK6AL, a dominant-negative mutant of MKK6 (an activating kinase positioned upstream from p38) also resulted in partial reduction in strain-activated hBNP promoter activity (Figure 2b). Again, the mutant had no effect on basal activity. Forced expression of wild-type p38 increased both basal and strain-dependent hBNP promoter activity, whereas MKK6AL effected a partial reversal of each. Independent experiments confirmed that the effects of SB203580 and MKK6AL were largely confined to the p38 (as opposed to the JNK) signaling pathway (data not shown). Collectively, these data indicate that the strain-dependent increase in p38 activity noted above participates directly in the activation of hBNP gene transcription.
Figure 2.
Effects of SB203580, MKK6AL, and wild-type p38 on strain-dependent hBNP promoter activity in neonatal rat ventricular myocytes. (a) Cells were transfected with 1 μg of –1,595 hBNP luciferase. After 24 hours of culture, cells were subjected to cyclical strain for 48 hours in the presence of different concentrations of SB203580. (b) Cells were cotransfected with 1 μg of –1,595 hBNP luciferase and 2 concentrations of MKK6AL with or without 1 μg of pCMV-FLAG-p38MAPK. After 24 hours of culture, cells were subjected to cyclical strain for 48 hours. Data are presented as mean ± SD of 4 separate experiments. *P < 0.01 vs. strain control.
It has been shown that p38 signals transcriptional activity through activation of NF-κB in some (48, 49) but not all (50) systems. Activation of NF-κB is determined in part by the phosphorylation status of the inhibitory protein IκB. IκB associates with NF-κB in the cytoplasmic compartment, precluding activation and entry of the latter into the nucleus. Activation of NF-κB occurs after phosphorylation of IκB on key serine residues (Ser 32 and Ser 36) at the amino terminus of the molecule. Phosphorylation marks IκB for ubiquination and subsequent degradation through the proteasome pathway (51). This frees NF-κB to enter the nuclear compartment, associate with its cognate recognition element, and stimulate transcription.
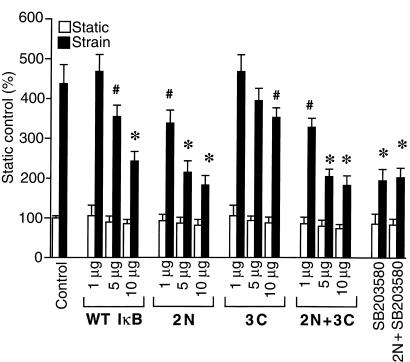
To investigate the role of NF-κB in signaling the p38 effect, we introduced an hBNPLuc reporter into ventricular myocytes together with either wild-type IκB or an IκB phosphorylation-defective mutant (i.e., a constitutive suppressor of NF-κB activity) before applying the strain stimulus. Wild-type IκB led to a concentration-dependent reduction (∼50%) in strain-associated, but not basal, reporter activity (Figure 3). 2N, an IκB mutant that is modified at 2 amino terminal serine residues (Ser 32 and Ser 36) that are thought to be the targets for the IκB kinase(s), effected a concentration-dependent reduction in stress-dependent hBNP promoter activity that was even more pronounced than that seen with wild-type IκB. 3C, an IκB mutant that targets Ser 283, Thr 291, and Thr 299 at the carboxyl terminus of IκB — residues that are not thought to be involved in the ubiquination/proteasome degradation pathway (46) — had essentially no effect, possibly reflecting the limited stability of this protein in intact cells. The combination mutant (2N+3C) was no more effective than 2N alone. Of interest, the maximal inhibition seen with the 2N mutant was roughly equivalent to that seen with SB203580, and the 2 were not additive, implying that p38 and NF-κB operate over shared pathways in contributing to the hBNP transcriptional response.
Figure 3.
Effects of IκBα mutants on strain-dependent hBNP promoter activity in neonatal rat ventricular myocytes. Cells were cotransfected with 1 μg of –1,595 hBNP luciferase and different concentrations of either wild-type (WT) IκBα or the mutant indicated (2N, 3C, or 2N + 3C). After 24 hours of culture, cells were subjected to cyclical strain for 48 hours. A separate subgroup was treated with 10 μM SB203580 before strain. The data are expressed as mean ± SD of 4 separate experiments. *P < 0.01 vs. strain control. #P < 0.05 vs. strain control.
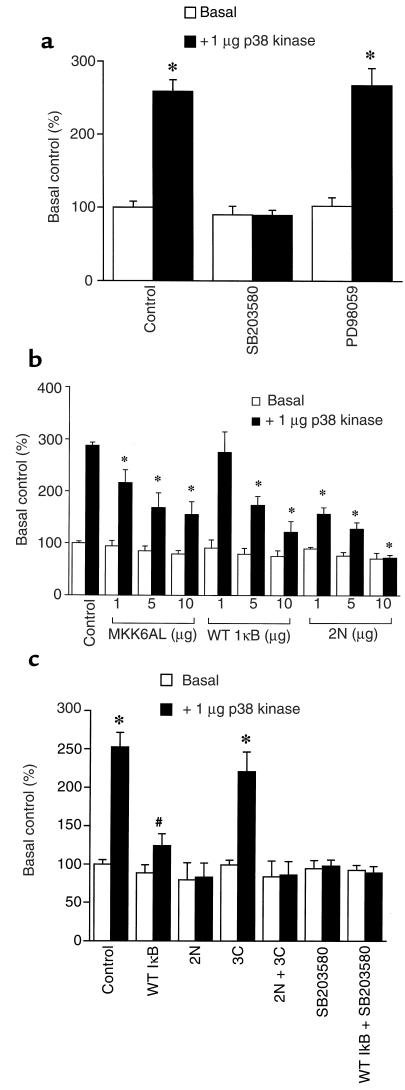
The p38-dependent increment (2.5- to 3-fold) in hBNP promoter activity (Figure 4a) was blocked completely by SB203580, but was unaffected by PD98059. The response was also partially inhibited by MKK6AL, by wild-type IκB, and even more effectively by the 2N mutant of IκB, which completely blocked the response to p38 (Figure 4b). A similar result was obtained when p38 was cotransfected with NF-κB TKLuc, a reporter plasmid harboring an NF-κB recognition sequence placed immediately upstream from the core thymidine kinase promoter (Figure 4c). A 2.5-fold induction of this reporter was effected by p38. This was inhibited partially by wild-type IκB and was completely blocked by the 2N mutant and SB203580.
Figure 4.
Effects of IκBα mutants and MKK6AL on p38-activated hBNPLuc or NF-κB TKLuc activity in neonatal rat ventricular myocytes. (a) Cells were cotransfected with 1 μg of –1,595 hBNP luciferase and 1 μg of pCMV-FLAG-p38MAPK. After 24 hours of culture, cells were treated with 10 μM SB203580 or 10 μM PD98059. (b) Cells were cotransfected with 1 μg of –1,595 hBNP luciferase, 1 μg of pCMV-FLAG-p38MAPK, and different concentrations of MKK6AL, WT IκBα, or the 2N mutant of IκBα. (c) Cells were cotransfected with 10 μg of NF-κB TKLuc, 1 μg of pCMV-FLAG-p38MAPK, and 10 μg of WT IκBα or one of the relevant mutants (2N, 3C, or 2N + 3C). A separate subgroup was treated with 10 μM SB203580. Data are presented as mean ± SD of 4 separate experiments. *P < 0.01 vs. basal control. #P < 0.05 vs. basal control.
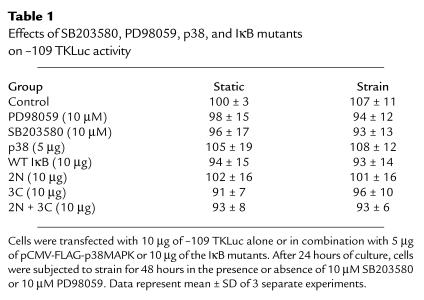
Nonspecific effects on transcriptional activity in these cultured myocytes were not seen after treatment with PD98059 or SB203580, or after cotransfection with p38 or the different IκΒ mutants. None of these perturbations had a significant effect on the activity of the transfected thymidine kinase promoter/luciferase reporter –109 TKLuc (see Table 1).
Table 1.
Effects of SB203580, PD98059, p38, and IκB mutants on –109 TKLuc activity
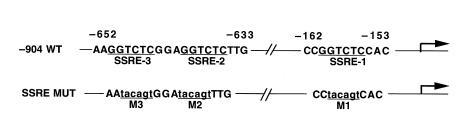
NF-κB has been implicated as playing a role in mediating shear stress activation of the PDGF receptor (52, 53). This activation involves the interaction of NF-κB with the so-called SSRE(s) in the proximal promoter of that gene. We scanned the hBNP 5′ flanking sequence for elements with potential homology to the SSRE, and identified 3 sites within 700 bp of the transcription start site. Two of these, positioned between –652 and –633, are arrayed as a direct repeat (Figure 5). To investigate the role of each of these candidate sites in signaling either p38- or strain-dependent hBNP promoter activity, we introduced site-directed mutations (Figure 5) into each candidate sequence, alone or in combination, and examined the effect of these mutations on both basal and strain-activated promoter activity. As shown in Figure 6a, mutation of each of the SSREs individually led to a near-equivalent reduction in the response to strain. Mutation of the upstream SSREs (sites 2 and 3) effected a further reduction, whereas mutation of all 3 sites led to the maximal level of inhibition observed (∼60% of the wild-type response). Wild-type p38 stimulated the –904 hBNPLuc reporter almost 3-fold (Figure 6b). As with the –1,595 reporter (see Figure 4), this stimulation was blocked by treatment with SB203580 or cotransfection with the IκB 2N mutant. Introduction of mutations at each of the SSREs identified in Figure 5 resulted in complete abrogation of the p38 effect. Similarly, the induction by mechanical strain (∼3.5-fold) was inhibited to an equivalent degree (∼60%) by treatment with SB230580, cotransfection with the IκB mutant (2N), and introduction of the triple mutation (sites 1–3) into the hBNP promoter (Figure 6c). Of note, none of the 3 perturbations proved to be additive with the others, implying use of a shared signaling pathway.
Figure 5.
Identification of SSRE-like structures in the 5′ flanking sequence of the hBNP gene (–904 WT). Positioning of the 3 elements relative to the transcription start site are indicated. Putative SSRE sequences are underlined. Mutated bases in each of the 3 SSRE mutant reporters (SSRE MUT) are indicated in lowercase letters.
Figure 6.
Effects of mutations in putative SSRE sequences on hBNP promoter activity. (a) Wild-type –904 hBNPLuc or site-directed mutants targeted at 1 or more of the SSREs described in Figure 5 were introduced into ventricular myocytes. After 24 hours, cells were subjected to mechanical strain for 48 hours; lysates were generated and analyzed for luciferase activity. (b) One microgram of wild-type –904 hBNPLuc or the triple SSRE mutant was cotransfected into ventricular cells with 1 μg of pCMV-FLAG-p38MAPK alone or in combination with 10 μg of the 2N mutant. Where indicated, cells were treated with 10 μM SB203580 for 48 hours. (c) Cells were cotransfected with 1 μg of wild-type –904 hBNPLuc or a –904 hBNPLuc harboring a triple SSRE mutation, and 10 μg of the 2N mutant of IκBα. After 24 hours of culture, cells were subjected to mechanical strain for 48 hours in the presence or absence of 10 μM SB203580, as indicated. Seventy-two hours after transfection, cells were harvested and luciferase activity was measured. Data are presented as mean ± SD of 3 separate experiments.
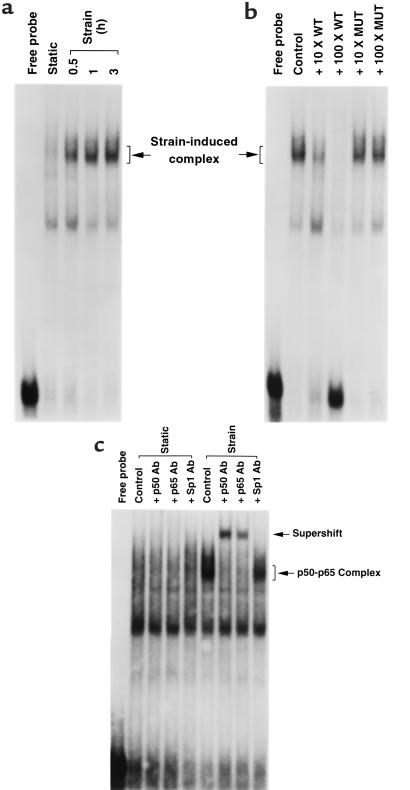
As noted above, NF-κB is typically concentrated in the cytoplasmic compartment in the inactivated state. Activation is followed by translocation to the nucleus and association with cognate DNA recognition elements (50). If our model is correct, we would predict that application of mechanical strain should promote interaction of NF-κB with the SSREs present in the proximal hBNP gene promoter. As shown in Figure 7a, application of strain led to a time-dependent increase in the association of myocyte nuclear protein with the SSRE direct repeat (sites 2 and 3) in the electrophoretic mobility shift assay. This interaction was blocked by increasing concentrations of unlabeled wild-type SSRE sequence, but not by mutant SSRE sequence (Figure 7b). In addition, inclusion of antibody directed against the p50 component or against the RelA component p65 resulted in a supershift of the strain-induced complex. Collectively, these data indicate that mechanical strain promotes a time-dependent association of NF-κB with specific SSRE-like sequences in the hBNP promoter, and that this association results in increased promoter activity.
Figure 7.
Nuclear extracts from myocytes subjected to mechanical strain interact with SSRE-like sites in hBNP promoter. (a) Cells were subjected to strain stimulus for different periods of time. Cells were harvested and nuclear extracts were prepared. Ten micrograms of nuclear extract was incubated with 32P-labeled oligonucleotide encoding SSRE-like sites (see Methods) and was subjected to electrophoretic mobility shift assay. (b) Ten micrograms of extract from cells subjected to 1 hour of mechanical strain was incubated with labeled oligonucleotide in the absence or presence of increasing concentrations of unlabeled oligonucleotide (10- to 100-fold excess) encoding wild-type or mutant SSRE sequence. (c) Ten micrograms of extract from myocytes subjected to strain for 1 hour was preincubated on ice for 1 hour with 1 μg of polyclonal antibody directed against p50, p65, or Sp1 before addition of labeled probe. Position of p50-p65 complex and supershift complex are indicated.
Discussion
We have documented previously that mechanical strain is a potent activator of hBNP gene transcription in neonatal rat ventricular myocytes (4, 39). The present work extends those findings in demonstrating that (a) mechanical strain, like other hypertrophic agonists (34, 38), increases p38 activity in these cells; (b) activation of p38 plays an important role in mediating strain-dependent hBNP promoter activity; and (c) p38 induction of hBNP promoter activity operates through an NF-κB–dependent pathway that targets 3 SSRE-like structures in the 5′ flanking sequence of the hBNP gene.
Considerable recent effort has been devoted to the identification of signaling pathways linking various mechanical and biochemical stimuli (e.g., phenylephrine, ET, AII, or mechanical strain) to the phenotypic changes (e.g., increased cell size and sarcomeric assembly) and changes in gene expression (e.g., increased ANP, BNP, skeletal α-actin, and β-myosin heavy chain expression) that accompany hypertrophy. Intracellular calcium (11), protein kinase C (10), nonreceptor protein tyrosine kinases (14, 15), calcineurin (12, 13), the small G proteins (18–21), and various members of the extended MAPK family (22–38) have been suggested as candidate mediators of various aspects of the hypertrophic phenotype. In the case of the MAPKs, the data have to some degree been inconclusive.
Evidence has been advanced supporting (25–27) or refuting (29, 32) a role for the Ras/Raf/ERK pathway in signaling hypertrophy-dependent phenotypic changes and gene expression in neonatal rat myocytes. Gillespie-Brown et al. (25) showed that dominant-negative MEK1, an activating kinase positioned immediately upstream from ERK, blocked phenylephrine-induced activation of a cotransfected rat ANP promoter. The inhibition was amplified further by cotransfection with dominant-negative ERK, whereas transfection of constitutively active MEK1 or wild-type ERK resulted in increased ANP promoter activity. Thorburn et al. confirmed the dependence of the phenylephrine induction of ANP promoter activity on ERK (27) and Raf (54), but noted that the cytoskeletal organization that typifies hypertrophy in this model did not appear to traffic through either. A subsequent study from Post et al. (29) seemingly dissociated activation of the ERK pathway from stimulation of ANP promoter activity, a finding supported by the later studies of Thorburn et al. (55), who found that activation of the ANP promoter by transfected MEKK1, a MAPK kinase kinase capable of activating either ERK or JNK signaled through the latter and not the former. Activation of ERK in this study resulted in, if anything, a reduction in ANP promoter activity.
The evidence in favor of JNK involvement in the hypertrophic process is somewhat stronger. In addition to the study of Thorburn et al. (55) alluded to above, the work of Ramirez et al. (30), Wang et al. (31), and Choukroun et al. (32) has established a close link between hypertrophic stimuli (i.e., either phenylephrine or endothelin), JNK activation, and different parameters associated with the hypertrophic phenotype. However, at least 1 study (37) has provided evidence that assigns a relatively minor role to the JNK pathway in signaling hypertrophy. Thus, as with the ERKs, the role of JNKs in signaling the hypertrophic process remains controversial.
Several researchers have identified p38 as an important signaling pathway in hypertrophy (33–38). Zechner et al. showed that overexpression of MKK6, a proximally positioned kinase that specifically activates p38 in ventricular myocytes, led to activation of ANP, BNP, and skeletal α-actin promoter activity, augmented cell size, and increased sarcomeric organization in a fashion similar to that seen with phenylephrine (34). It is noteworthy that activation of JNK with MEKK1COOH or of ERK with Raf-1 BXB each increased cell size and effected modest increases in natriuretic peptide and α-SkA promoter activity, but did not increase sarcomeric organization. The observed increase in ANP promoter activity appears to signal through a proximal serum response element, possibly through activation of an associated transcription factor, ATF6 (35). Clerk et al. (38) found that both phenylephrine and endothelin-1 increased p38 activity through a protein kinase C–dependent mechanism in neonatal myocytes; however, SB203580, the p38 antagonist, failed to prevent the morphological changes associated with myocyte hypertrophy after 4–24 hours of agonist stimulation. It did, however, diminish cell profile and myofibrillar organization after 48 hours, leading Clerk and colleagues to conclude that p38 does not play a role in the immediate induction of the morphological changes associated with hypertrophy, but may play a role in maintaining this phenotype over a longer period of time. The effects of p38 appear to be isoform specific. Infection of cardiac myocytes with adenoviral vectors encoding constitutively active MKK6 (MKK6bE) or MKK3 (MKK3bE) led to characteristic hypertrophic changes; however, forced expression of MKK3 also led to apoptosis in the myocyte population (33). Interestingly, constitutively active MKK6 has been shown to have antiapoptotic activity in these cells (36). The hypertrophic response was enhanced by coinfection with wild-type p38β, and was blocked by a dominant-negative p38β mutant. In contrast, the proapoptotic effects of MKK3bE were amplified by wild-type p38α and were blocked by dominant-negative p38α (33).
It has been shown that p38 signals through the transcription factor NF-κB in some (48, 49), though not all (50), systems. Our data indicate that p38 signals through NF-κB in activating the hBNP promoter. Both strain- and p38-activated promoter activity are blocked to varying degrees by a constitutive inhibitor of NF-κB activity, the 2N mutant of IκB; this inhibition is not additive with that produced by SB203580. Interestingly, Zechner et al. (35) found that NF-κB is activated by p38 in cardiac myocytes, but that this activation is not related to the antiapoptotic activity of p38 (see above). Thus, NF-κB may be linked to some but not all trophic activity associated with this signaling cascade.
The p38/NF-κB contribution to the strain response operates, at least in part, through a series of NF-κB binding sites present in the hBNP promoter. It is interesting that shear stress, another mechanical stimulus of transcription in vascular endothelial cells, has been shown to signal through a specific element — SSRE — harboring an NF-κB recognition site (52, 53). Thus, activation of NF-κB appears to be a consistent theme linking mechanical stimuli to activation of gene expression in the cardiovascular system.
A number of transcription factors have been identified as effectors of hypertrophic stimuli in driving gene expression, including transcription enhancing factor-1 (56, 57), serum response factor (58, 59), Sp1 (59), activator protein 1 (60, 61), and nuclear factor of activated T cells (NF-AT) (12, 13). NF-AT, in fact, has been linked specifically to activation of the hBNP gene in vitro (12). It is probable that these factors act in an overlapping (and possibly, at times, a redundant) fashion to link the upstream effector cascades to the genomic activity that culminates in hypertrophy. The potential interaction of these transcription factors with NF-κB in promoting these changes remains unexplored.
In summary, the induction of hBNP promoter activity by mechanical strain is, at least in part, dependent on stimulation of p38 in the cardiac myocyte and the subsequent activation of NF-κB. This activation may play an important role in signaling the increased BNP gene expression that accompanies hemodynamic overload and cardiac hypertrophy in the intact animal.
Acknowledgments
This work was supported by grant HL-35753 from the National Institutes of Health and by a postdoctoral fellowship (to F. Liang) from the Western Affiliate of the American Heart Association. The authors are grateful for the assistance of K. Nakamura, F. Roediger, and X.-C. Yuen with the cardiocyte preparations.
References
- 1.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 2.Komuro I, et al. Stretching cardiac myocytes stimulates protooncogene expression. J Biol Chem. 1990;265:3595–3598. [PubMed] [Google Scholar]
- 3.Sadoshima J, Jahn L, Takahashi T, Kulik TJ, Izumo S. Molecular characterization of the stretch-induced adaptation of cultured cardiac cells. An in vitro model of load-induced cardiac hypertrophy. J Biol Chem. 1992;267:10551–10560. [PubMed] [Google Scholar]
- 4.Liang F, Wu J, Garami M, Gardner DG. Mechanical strain increases expression of the brain natriuretic peptide gene in rat cardiac myocytes. J Biol Chem. 1997;272:28050–28056. doi: 10.1074/jbc.272.44.28050. [DOI] [PubMed] [Google Scholar]
- 5.Iwaki K, Sukhatme VP, Shubeita HE, Chien KR. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. J Biol Chem. 1990;265:13809–13817. [PubMed] [Google Scholar]
- 6.Nakagawa O, et al. Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. J Clin Invest. 1995;96:1280–1287. doi: 10.1172/JCI118162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanford DS, Thuerauf DJ, Murray SF, Glembotski CC. Brain natriuretic peptide is induced by alpha 1-adrenergic agonists as a primary response gene in cultured rat cardiac myocytes. J Biol Chem. 1994;269:26227–26233. [PubMed] [Google Scholar]
- 8.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Circ Res. 1993;73:413–422. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 9.Chien KR, Knowlton KU, Zhu H, Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991;5:3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- 10.Dunnmon PM, Iwaki K, Henderson S, Sen A, Chien KR. Phorbol esters induce immediate-early genes and activate cardiac gene transcription in neonatal rat myocardial cells. J Mol Cell Cardiol. 1990;22:901–910. doi: 10.1016/0022-2828(90)90121-h. [DOI] [PubMed] [Google Scholar]
- 11.McDonough PM, Stella SL, Glembotski CC. Involvement of cytoplasmic calcium and protein kinases in the regulation of atrial natriuretic factor secretion by contraction rate and endothelin. J Biol Chem. 1994;269:9466–9472. [PubMed] [Google Scholar]
- 12.Molkentin JD, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sussman MA, et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 14.Kovacic B, Ilic D, Damsky CH, Gardner DG. c-Src activation plays a role in endothelin-dependent hypertrophy of the cardiac myocyte. J Biol Chem. 1998;273:35185–35193. doi: 10.1074/jbc.273.52.35185. [DOI] [PubMed] [Google Scholar]
- 15.Fuller SJ, Gillespie-Brown J, Sugden PH. Oncogenic src, raf, and ras stimulate a hypertrophic pattern of gene expression and increase cell size in neonatal rat ventricular myocytes. J Biol Chem. 1998;273:18146–18152. doi: 10.1074/jbc.273.29.18146. [DOI] [PubMed] [Google Scholar]
- 16.Sheng Z, et al. Cardiotrophin 1 (CT-1) inhibition of cardiac myocyte apoptosis via a mitogen-activated protein kinase-dependent pathway. J Biol Chem. 1997;72:5783–5791. doi: 10.1074/jbc.272.9.5783. [DOI] [PubMed] [Google Scholar]
- 17.Mascareno E, Dhar M, Siddiqui MAQ. Signal transduction and activator of transcription (STAT) protein-dependent activation of angiotensinogen promoter: a cellular signal for hypertrophy in cardiac muscle. Proc Natl Acad Sci USA. 1998;95:5590–5594. doi: 10.1073/pnas.95.10.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorburn A, et al. Hras-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993;268:2244–2249. [PubMed] [Google Scholar]
- 19.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sah VP, Hoshijima M, Chien KR, Brown JH. Rho is required for Galphaq and alpha 1-adrenergic receptor signaling in cardiomyocytes. Dissociation of Ras and Rho pathways. J Biol Chem. 1996;271:31185–31190. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 21.Pracyk JB, et al. A requirement for the rac1 GTPase in the signal transduction pathway leading to cardiac myocyte hypertrophy. J Clin Invest. 1998;102:929–937. doi: 10.1172/JCI2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogoyevitch MA, et al. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 23.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamazaki T, et al. Mechanical stress activates protein kinase cascade of phosphorylation in neonatal rat cardiac myocytes. J Clin Invest. 1995;96:438–446. doi: 10.1172/JCI118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillespie-Brown J, Fuller SJ, Bogoyevitch MA, Cowley S, Sugden PH. The mitogen-activated protein kinase kinase MEK1 stimulates a pattern of gene expression typical of the hypertrophic phenotype in rat ventricular cardiomyocytes. J Biol Chem. 1995;270:26303–26310. doi: 10.1074/jbc.270.47.28092. [DOI] [PubMed] [Google Scholar]
- 26.Glennon PE, et al. Depletion of mitogen-activated protein kinase using an antisense oligodeoxynucleotide approach downregulates the phenylephrine-induced hypertrophic response in rat cardiac myocytes. Circ Res. 1996;78:954–961. doi: 10.1161/01.res.78.6.954. [DOI] [PubMed] [Google Scholar]
- 27.Thorburn J, Frost JA, Thorburn A. Mitogen-activated protein kinases mediate changes in gene expression, but not cytoskeletal organization associated with cardiac muscle cell hypertrophy. J Cell Biol. 1994;126:1565–1572. doi: 10.1083/jcb.126.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogoyevitch MA, Ketterman AJ, Sugden PH. Cellular stresses differentially activate c-Jun N-terminal protein kinases and extracellular signal-regulated protein kinases in cultured ventricular myocytes. J Biol Chem. 1995;270:29710–29717. doi: 10.1074/jbc.270.50.29710. [DOI] [PubMed] [Google Scholar]
- 29.Post GR, Goldstein D, Thuerauf DJ, Glembotski CC, Brown JH. Dissociation of p44 and p42 mitogen-activated protein kinase activation from receptor-induced hypertrophy in neonatal rat ventricular myocytes. J Biol Chem. 1996;271:8452–8457. doi: 10.1074/jbc.271.14.8452. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez MT, et al. The MEKK-JNK pathway is stimulated by α1-adrenergic receptor and ras activation and is associated with in vitro and in vivo cardiac hypertrophy. J Biol Chem. 1997;272:14057–14061. doi: 10.1074/jbc.272.22.14057. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, et al. Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells. J Biol Chem. 1998;273:5423–5426. doi: 10.1074/jbc.273.10.5423. [DOI] [PubMed] [Google Scholar]
- 32.Choukroun G, et al. Role of the stress-activated protein kinases in endothelin-induced cardiomyocyte hypertrophy. J Clin Invest. 1998;102:1311–1320. doi: 10.1172/JCI3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 34.Zechner D, Thuerauf DJ, Hanford DS, McDonough PM, Glembotski CC. A role for the p38 mitogen-activated protein kinase pathway in myocardial cell growth, sarcomeric organization, and cardiac-specific gene expression. J Cell Biol. 1997;139:115–127. doi: 10.1083/jcb.139.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zechner D, et al. MKK6 activates myocardial cell NF-κB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J Biol Chem. 1998;273:8232–8239. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]
- 36.Thuerauf DJ, et al. p38 mitogen-activated protein kinase mediates the transcriptional induction of the atrial natriuretic factor gene through a serum response element. J Biol Chem. 1998;273:20636–20643. doi: 10.1074/jbc.273.32.20636. [DOI] [PubMed] [Google Scholar]
- 37.Nemoto S, Sheng Z, Lin A. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol Cell Biol. 1998;18:3518–3526. doi: 10.1128/mcb.18.6.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clerk A, Michael A, Sugden PH. Stimulation of the p38 mitogen-activated protein kinase pathway in neonatal rat ventricular myocytes by the G protein-coupled receptor agonists, endothelin-1 and phenylephrine: a role in cardiac myocyte hypertrophy. J Cell Biol. 1998;142:523–535. doi: 10.1083/jcb.142.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang F, Gardner DG. Autocrine/paracrine determinants of strain-activated brain natriuretic peptide gene expression in cultured cardiac myocytes. J Biol Chem. 1998;273:14612–14619. doi: 10.1074/jbc.273.23.14612. [DOI] [PubMed] [Google Scholar]
- 40.Thibault G, Doubell AF, Garcia R, Lariviere R, Schiffrin EL. Endothelin-stimulated secretion of natriuretic peptides by rat atrial myocytes is mediated by endothelin A receptors. Circ Res. 1994;74:460–470. doi: 10.1161/01.res.74.3.460. [DOI] [PubMed] [Google Scholar]
- 41.Wu JP, LaPointe MC, West BL, Gardner DG. Tissue-specific determinants of human atrial natriuretic factor gene expression in cardiac tissue. J Biol Chem. 1989;264:6472–6479. [PubMed] [Google Scholar]
- 42.Banes AJ, Gilbert J, Taylor D, Monbureau O. A new vacuum-operated stress-providing instrument that applies static or variable duration cyclic tension or compression to cells in vitro. J Cell Sci. 1985;75:35–42. doi: 10.1242/jcs.75.1.35. [DOI] [PubMed] [Google Scholar]
- 43.LaPointe MC, Wu G, Garami M, Yang X-P, Gardner DG. Tissue-specific expression of the human brain natriuretic peptide gene in cardiac myocytes. Hypertension. 1996;27:715–722. doi: 10.1161/01.hyp.27.3.715. [DOI] [PubMed] [Google Scholar]
- 44.Enslen H, Raingeaud J, Davis RJ. Selective activation of p38 mitogen-activated protein (MAP) kinase isoforms by the MAP kinase kinases MKK3 and MKK6. J Biol Chem. 1998;273:1741–1748. doi: 10.1074/jbc.273.3.1741. [DOI] [PubMed] [Google Scholar]
- 45.Zanke BW, et al. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]
- 46.Beauparlant P, et al. Transdominant mutants of I kappa B alpha block Tat-tumor necrosis factor synergistic activation of human immunodeficiency virus type 1 gene expression and virus multiplication. J Virol. 1996;70:5777–5785. doi: 10.1128/jvi.70.9.5777-5785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han Y, Brasier AR. Mechanism for biphasic Rel A-NF-κB1 nuclear translocation in tumor necrosis factor α-stimulated hepatocytes. J Biol Chem. 1997;272:9825–9832. doi: 10.1074/jbc.272.15.9825. [DOI] [PubMed] [Google Scholar]
- 48.Vanden Berghe W, et al. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–3290. doi: 10.1074/jbc.273.6.3285. [DOI] [PubMed] [Google Scholar]
- 49.Maulik N, Sato M, Price BD, Das DK. An essential role of NF kappa B in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischemia. FEBS Lett. 1998;429:365–369. doi: 10.1016/s0014-5793(98)00632-2. [DOI] [PubMed] [Google Scholar]
- 50.Wesselborg S, Bauer MKA, Vogt M, Schmitz ML, Schulze-Osthoff K. Activation of transcription factor NF-κB and p38 mitogen-activated protein kinase is mediated by distinct and separate stress effector pathways. J Biol Chem. 1997;272:12422–12429. doi: 10.1074/jbc.272.19.12422. [DOI] [PubMed] [Google Scholar]
- 51.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 52.Resnick N, et al. Platelet-derived growth factor B chain promoter contains a cis-acting fluid shear-stress-responsive element. Proc Natl Acad Sci USA. 1993;90:4591–4595. doi: 10.1073/pnas.90.10.4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khachigian LM, Resnick N, Gimbrone MA, Jr, Collins T. Nuclear factor-kappa B interacts functionally with the platelet-derived growth factor B-chain shear-stress response element in vascular endothelial cells exposed to fluid shear stress. J Clin Invest. 1995;96:1169–1175. doi: 10.1172/JCI118106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thorburn J, McMahon M, Thorburn A. Raf-1 kinase activity is necessary and sufficient for gene expression changes but not sufficient for cellular morphology changes associated with cardiac myocyte hypertrophy. J Biol Chem. 1994;269:30580–30586. [PubMed] [Google Scholar]
- 55.Thorburn J, Xu S, Thorburn A. MAP kinase- and Rho-dependent signals interact to regulate gene expression but not actin morphology in cardiac muscle cells. EMBO J. 1997;16:1888–1900. doi: 10.1093/emboj/16.8.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kariya K, Karns LR, Simpson PC. An enhancer core element mediates stimulation of the rat beta-myosin heavy chain promoter by an alpha 1-adrenergic agonist and activated beta-protein kinase C in hypertrophy of cardiac myocytes. J Biol Chem. 1994;269:3775–3782. [PubMed] [Google Scholar]
- 57.Karns LR, Kariya K, Simpson PC. M-CAT, CArG and Sp1 elements are required for alpha 1-adrenergic induction of the skeletal alpha-actin promoter during cardiac myocyte hypertrophy. J Biol Chem. 1995;270:410–417. doi: 10.1074/jbc.270.1.410. [DOI] [PubMed] [Google Scholar]
- 58.MacLellan WR, Lee TC, Schwartz RJ, Schneider MD. Transforming growth factor-beta response elements of the skeletal alpha-actin gene. J Biol Chem. 1994;69:16754–16760. [PubMed] [Google Scholar]
- 59.Sprenkle AB, Murray SF, Glembotski CC. Involvement of multiple cis elements in basal- and alpha-adrenergic agonist-inducible atrial natriuretic factor transcription. Circ Res. 1995;77:1060–1069. doi: 10.1161/01.res.77.6.1060. [DOI] [PubMed] [Google Scholar]
- 60.Kovacic-Milivojevic B, Gardner DG. Divergent regulation of the human atrial natriuretic peptide gene by c-jun and c-fos. Mol Cell Biol. 1992;12:292–301. doi: 10.1128/mcb.12.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.von Harsdorf R, et al. Identification of a cis-acting regulatory element conferring inducibility of the atrial natriuretic factor gene in acute pressure overload. J Clin Invest. 1997;100:1294–1304. doi: 10.1172/JCI119643. [DOI] [PMC free article] [PubMed] [Google Scholar]