Abstract
Previous investigations revealed low activities of lactate dehydrogenase (LDH) and plasma membrane monocarboxylate transporters (MCT) in the pancreatic β cell. In this study the significance of these characteristics was explored by overexpressing type A LDH (LDH-A) and/or type 1 MCT (MCT-1) in the clonal INS-1 β cells and isolated rat islets. Inducible overexpression of LDH-A resulted in an 87-fold increase in LDH activity in INS-1 cells. Adenovirus-mediated overexpression of MCT-1 increased lactate transport activity 3.7-fold in INS-1 cells. Although overexpression of LDH-A, and/or MCT-1 did not affect glucose-stimulated insulin secretion, LDH-A overexpression resulted in stimulation of insulin secretion even at a low lactate concentration with a concomitant increase in its oxidation in INS-1 cells regardless of MCT-1 co-overexpression. Adenovirus-mediated overexpression of MCT-1 caused an increase in pyruvate oxidation and conferred pyruvate-stimulated insulin release to isolated rat islets. Although lactate did not stimulate insulin secretion from control or MCT-1–overexpressing islets, co-overexpression of LDH-A and MCT-1 evoked lactate-stimulated insulin secretion with a concomitant increase in lactate oxidation in rat islets. These results suggest that low expression of MCT and LDH is requisite to the specificity of glucose in insulin secretion, protecting the organism from undesired hypoglycemic actions of pyruvate and lactate during exercise and other catabolic states.
J. Clin. Invest. 104:1621–1629 (1999).
Introduction
Glucose homeostasis is precisely controlled in mammals. Its deterioration leads to diabetes mellitus, one of the most common diseases in the industrialized countries. Three major mechanisms contribute to maintain the glucose homeostasis: fine-tuning of insulin secretion by glucose from the pancreatic β cell, insulin-mediated glucose disposal into muscle and adipose tissue, and insulin-mediated suppression of glucose production from the liver (1). Insulin acts on its target cells by binding to specific receptors, which initiates an intracellular signaling cascade (2). On the other hand, the recognition of nutrients as stimuli by β cells is accomplished through metabolism-secretion coupling (3). This unusual mode of recognition of stimuli is dependent on the unique expression profiles of membrane transporters and metabolic enzymes. For example, the predominant isoform of hexokinase in β cells is glucokinase (hexokinase IV), whereas other cell types in general, except for hepatocytes, express hexokinase I or hexokinase II. In the β cell, where glucokinase is the flux-determining enzyme for glycolysis, glucose metabolism and insulin secretion are proportional to changes in the blood-glucose concentration (4). High expression levels of the mitochondrial enzymes, pyruvate carboxylase (5, 6), and FAD-linked glycerol phosphate dehydrogenase (mGPDH) (7, 8), are also prominent features of β-cell gene expression.
Mitochondrial ATP generation plays a key role in the coupling of glucose metabolism to insulin secretion (3, 4). Glucose is metabolized to pyruvate by the glycolytic pathway. Pyruvate supplies carbons to the tricarboxylic acid (TCA) cycle, generating reducing equivalents. This leads to stimulation of the respiratory chain and ATP synthesis. Glucose also promotes transfer of electrons from cytosolic NADH to the respiratory chain through the glycerol phosphate and the malate-aspartate shuttles. The importance of these shuttles has recently been substantiated in islets from mGPDH-deficient mice treated with aminooxyacetate (AOA), an inhibitor of the malate-aspartate shuttle (9). Islets are comprised of 65 to 70% β cells (10). In islet non–β cells (mainly glucagon-producing α cells), the activity of lactate dehydrogenase (LDH) is several fold higher than in β cells (6, 8). This may determine the fate of glucose-derived pyruvate and would explain why only as little as 15% of glucose carbons are oxidized in the mitochondria in islet non–β cells compared with about 90% in β cells (6).
In studies using whole rat or mouse islets, it has been observed that pyruvate cannot mimic the effect of glucose on insulin secretion, despite active metabolism (11–13). This has been referred to as the “pyruvate paradox.” Similar observations have been made for lactate (14, 15). Both pyruvate and lactate enter cells through the plasma membrane monocarboxylate transporter (MCT) (16). Recently, several MCTs have been cloned and characterized, and a family of at least 8 related cDNA sequences has been identified (17). They are abundantly expressed in several organs, such as skeletal muscle and brain, in which large quantities of lactate and pyruvate are released or taken up. The reported oxidation of pyruvate and lactate in whole islets may occur mainly in non–β cells, because these cells exhibit higher monocarboxylate transport activity than β cells (8). We postulate that pyruvate and lactate are not able to stimulate insulin secretion because of low expression of MCT and LDH in β cells.
The present study was designed to elucidate the pyruvate paradox, i.e., the failure of pyruvate and lactate to stimulate insulin secretion. To this end, we have explored the significance of low LDH and MCT activities in the β cell by overexpressing MCT-1 and LDH-A in rat insulinoma INS-1 cells and primary rat islets using both an inducible expression system and an adenoviral vector technique. Such gene manipulations affected neither glucose metabolism nor glucose-stimulated insulin secretion. Remarkably, increased MCT and LDH activities resulted in pyruvate- and lactate-stimulated insulin secretion in isolated rat islets.
Methods
Generation of INS-1 cells overexpressing rabbit LDH-A under the control of reverse tetracycline transactivator.
The INS-r7 cell line (18), which carries the reverse tetracycline/doxycycline-dependent transactivator, was secondarily transfected with a plasmid harboring rabbit type A LDH (LDH-A) cDNA (a generous gift from Y. Briand; ref. 19). Six out of 40 hygromycin-resistant clones showed more than 30-fold overexpression of the LDH activity with maximal induction. Clone number 31, which exhibited highest LDH activity after the induction, was used for the present study and is termed as INS(LDH) cells. Another clone, number 29, has also shown similar results. INS(LDH) cells were cultured in RPMI-1640, as seen previously (20). Doxycycline (500 ng/mL) induction was started 36 hours before the experiments.
Isolation of rat islets.
Islets were prepared from male Wister rats (250–350 g) by collagenase digestion and handpicking. After 3–5-hour culture in RPMI, islets were infected with recombinant adenoviruses and further cultured for 24 hours.
Generation of recombinant adenoviruses bearing hamster MCT-1 cDNA (AdCAMCT-1) and rabbit LDH-A cDNA (AdCALDH-A).
Hamster MCT-1 cDNA (21) was obtained from American Type Culture Collection (No. 77499; Rockville, Maryland, USA). Construction of recombinant adenoviruses was as described previously (22). AdCAlacZ, which express bacterial β-galactosidase, was used as a control adenovirus. INS(LDH) cells or islets were incubated with recombinant adenoviruses for 1 hour, washed once, and cultured in RPMI medium. INS(LDH) cells were infected 48 hours before experiments with recombinant adenoviruses at approximately 50 virus particles/cell, and islets were infected 24 hours before experiments at 2 × 105 virus particles/islet (50–70 virus particles/cell, assuming 3,000–4,000 cells/islet; ref. 10).
Immunoblot analysis.
INS(LDH) cells were homogenized in hypotonic buffer (20 mM Tris [pH 7.4] 40 mM NaCl, 1 mM DTT, 1 mM PMSF). Cell homogenates were first centrifuged at 1,500 g for 15 minutes at 4°C. The supernatants were used for detection of LDH-A. Crude membrane preparations were then obtained by centrifugation of this supernatant at 200,000 g for 30 minutes at 4°C. For detection of MCT-1 or LDH-A expression in islets, islets were collected and directly dissolved in SDS sample buffer after insulin secretion studies. Proteins were subjected to SDS-PAGE and were transferred to nitrocellulose membranes. Membranes were probed with chicken anti-rat MCT-1 antibody raised against the carboxy-terminal peptide (1:10000; ref. 23) or with rabbit anti-rat liver LDH (1:200, kindly supplied by A. Volkl; ref. 24) for 1 hour at room temperature, and then incubated for 1 hour with rabbit anti-chicken IgY (1:10,000) or with goat anti-rabbit IgG (1:3000) conjugated with horseradish peroxidase, respectively. Detection was accomplished with chemiluminescence (ECL; Amersham Switzerland, Zurich, Switzerland).
Measurement of LDH activity.
Cells or islets were homogenized in 20 mM Tris (pH 7.4), 5 mM EDTA, 120 mM KCl, and 2 mM DTT. After centrifugation at 12,000 g for 10 minutes, supernatants (approximately 10 μg protein) were incubated in a solution containing 80 mM Tris (pH 7.4), 5 mM EDTA, 2 mM pyruvate, and 20 μM NADH. The reduction of NADH fluorescence (excitation: 340 nm; emission: 460 nm) was monitored using an LS-50B fluorometer (Perkin-Elmer, Beaconsfield, United Kingdom).
Measurement of lactate transport.
INS(LDH) cells were harvested and cultured in a spinner culture flask for 2 hours. Cells (2 × 106) were suspended in a buffer (140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 10 mM Hepes [pH 7.4]) and loaded for 30 minutes with 5 μM of the acetoxy methyl ester of the pH-sensitive fluorescent dye 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF). Cells were placed in a cuvette in an LS-50B fluorometer, and BCECF fluorescence was measured at 530 nm (excitation at 500 nm) at room temperature (24–27°C). When needed, α-cyano-4-hydroxycinnamate (CHC) was added before starting the traces. Sodium salts of lactate, pyruvate, and acetate were used throughout the experiments. The fluorescence was calibrated to the pH value for each determination by adding HCl after permeabilization with 0.1% Triton X-100.
Measurement of insulin secretion.
INS(LDH) cells (0.2 × 106 cells/well of 24-well plates) or islets (10 islets/tube) were incubated over a period of 60 minutes in Krebs-Ringer-bicarbonate-HEPES buffer (KRBH; 140 mM NaCl, 3.6 mM KCl, 0.5 mM NaH2PO4, 0.5 mM MgSO4, 1.5 mM CaCl2, 2 mM NaHCO3, 10 mM HEPES [pH 7.4], 0.1% BSA) containing stimulators indicated. Insulin was detected by radioimmunoassay.
Measurement of mitochondrial oxidation.
INS(LDH) cells (0.5 × 106 cells/35-mm dish) or islets (30 islets/tube) were transferred into a glass chamber and preincubated for 30 minutes in KRBH. Oxidation was initiated by replacing the buffer with 1 mL (INS cells) or 0.1 mL (islets) containing 0.2 μCi/chamber of [U-14C]glucose, [1-14C]pyruvate, or [U-14C]lactate (DuPont-NEN, Boston, Massachusetts, USA). After 2-hour incubation at 37°C in the sealed chamber, 0.1 M HCl was added to cells and benzethonium hydroxide (Fluka Chemie AG, Buchs, Switzerland) was injected into the bottom of the chamber to bind the liberated CO2. Following an overnight incubation at room temperature, 14CO2 production was counted in a LS6500 liquid scintillation counter (Beckman Instruments Inc., Palo Alto, California, USA).
Statistical analyses.
Data are presented as mean ± SEM. Differences between groups are assessed by Student’s t test for unpaired data.
Results
Overexpression of LDH-A and/or MCT-1 in INS-1 cells.
INS-1 cells were stably transfected with an expression vector harboring LDH-A cDNA under the tetracycline-inducible promoter. One of the clones, designated INS(LDH), with the highest LDH-A expression after doxycycline induction, has been chosen for subsequent analyses. Doxycycline strongly induced the expression of a 35-kDa protein as detected by Western blot analysis probed with anti-liver LDH antibody (Figure 1a). Noninduced control cells do not express LDH-A, confirming the previous data that the endogenous LDH isoform in INS-1 cells is LDH-B4 (8). In good agreement with the results of immunoblot analysis, nearly a 100-fold increase in LDH activity was found in doxycycline-treated cells (Figure 1b). LDH-A overexpression was not affected by MCT-1 co-overexpression (Figures 1a and 1b). Immunocytochemical and cell fractionation analyses have shown that overexpressed LDH-A was almost exclusively localized in the cytosol (data not shown).
Figure 1.
Doxycycline-inducible overexpression of LDH-A in INS-1 cells. (a) INS(LDH) cells were infected with either AdCAlacZ or AdCAMCT-1 and cultured in the absence or presence of doxycycline (DOX; 500 ng/mL). Cellular homogenates (100 μg) were dissolved in 11% SDS-PAGE, transferred to nitrocellulose, and immunoblotted with antibody against purified rat liver LDH. (b) LDH activity was determined in cell homogenates using pyruvate as a substrate. Data show the mean ± SEM of 3 independent experiments. **Difference from control cells (noninduced and infected with AdCAlacZ) at P < 0.01.
To overexpress MCT-1 simultaneously with LDH-A, a recombinant adenovirus, AdCAMCT-1, was constructed. Immunoblot analysis probed with anti–MCT-1 antibody demonstrated that 42-kDa protein was abundantly expressed only in INS(LDH) cells infected with AdCAMCT-1 (Figure 2a). Induction of LDH-A overexpression did not affect MCT-1 overexpression in INS(LDH) cells. Immunocytochemical analysis showed that overexpressed MCT-1 was correctly targeted to the plasma membrane (Figure 2b). Although the staining intensity was heterogeneous, essentially 100% of cells expressed the exogenous MCT-1. The MCT-1 expression was low in the control cells. So far, 8 MCT-related cDNA sequences have been identified (17). It remains to be established which of the isoforms are expressed in primary β cells or INS-1 cells.
Figure 2.
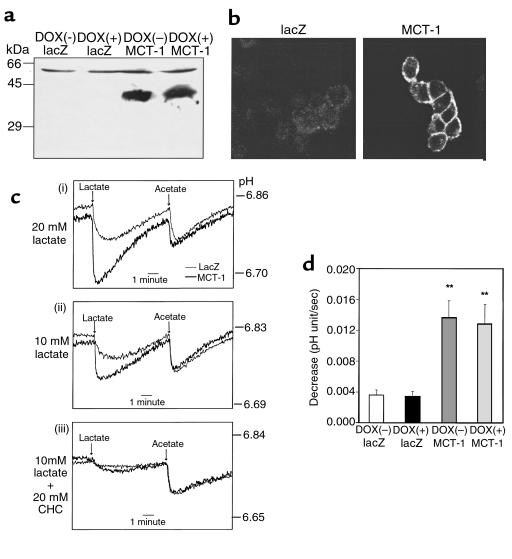
Overexpression of MCT-1 through a recombinant adenovirus in INS-1 cells. (a) INS(LDH) cells were treated as indicated in Figure 1a. Crude membrane preparations (50 μg) were dissolved in 9% SDS-PAGE and immunoblotted with antibody against COOH-terminal peptide of rat MCT-1. (b) Infected INS(LDH) cells were fixed and immunostained with antibody against MCT-1. (c) Infected INS(LDH) cells were loaded with the pH sensitive dye BCECF and challenged with 20 mM lactate (i) or 10 mM lactate in the absence (ii) or presence (iii) of CHC (20 mM). Traces are representative of more than 4 traces from 2 to 5 experiments. (d) Initial rates of pH decrease were calculated from 4 traces each from 4 different cell preparations. **Difference from control cells at P < 0.01.
To confirm functions of the expressed MCT-1, lactate transport activities were estimated by monitoring the transient acidification upon addition of lactate in control and MCT-1–overexpressing cells loaded with the pH-sensitive fluorescent dye, BCECF. This well-established method avoids the use of radiolabeled compounds (25). Typical traces of cells loaded with BCECF are shown in Figure 2c. Basal intracellular pH values were not different between control and MCT-1–overexpressing cells. Reduction of pH in response to 20 mM lactate was significantly greater in MCT-1–overexpressing cells compared with control cells. In contrast, the response to 10 mM acetate, which enters cells mainly by simple diffusion (16), was not altered (Figure 2c [i]). To verify that lactate enters the cells by MCTs, 10 mM lactate was tested in the absence (Figure 2c [ii]) and presence (Figure 2c [iii]) of 20 mM CHC, a competitive inhibitor of MCTs (16). CHC inhibited lactate-evoked acidification by 84% in control cells (from 2.2 ± 0.4 to 0.4 ± 0.2 pH unit × 10–3/sec; n = 4, P < 0.01). CHC also reduced the effect of 10 mM lactate by 82% in MCT-1–overexpressing INS(LDH) cells (from 7.7 ± 0.4 to 1.2 ± 0.3; n = 4, P < 0.01). This demonstrates that increased rate of pH reduction in MCT-1–overexpressing cells is indeed due to overexpression of functional MCT-1. Initial pH decrease rates in response to 20 mM lactate were calculated to estimate the lactate transport activity (Figure 2d). It was found that cells infected with AdCAMCT-1 have a 3.7-fold greater lactate transport activity than cells infected with the control virus. The increase in the lactate transport activity was independent of LDH co-overexpression and was also evident at 2 mM lactate (data not shown). Pyruvate (10 mM) transport activity was also increased after MCT-1 overexpression (2.1 ± 0.4 vs. 4.4 ± 0.9 pH unit × 10–3/sec in control and MCT-1–overexpressing INS(LDH) cells; n = 4, P < 0.05).
Effects of LDH-A and/or MCT-1 overexpression on insulin secretion from INS(LDH) cells.
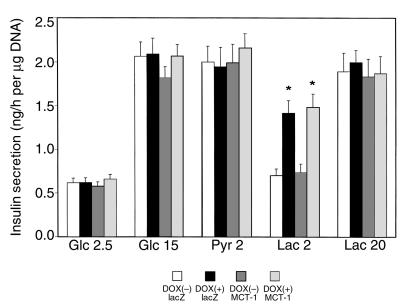
Insulin secretion in response to several secretagogues was assayed in cells treated with doxycycline and/or AdCAMCT-1 (Figure 3). In all 4 types of cell preparations, the stimulatory glucose concentration (15 mM) induced more than 3-fold increases in insulin secretion, and there were no differences among the 4 cell preparations. Pyruvate is a potent stimulus for insulin secretion in INS-1 cells (8). Overexpression of MCT-1 and/or LDH-A, however, did not affect insulin secretion evoked by pyruvate (2 mM). In noninduced INS(LDH) cells, 2 mM lactate did not elicit insulin secretion. Remarkably, this concentration of lactate was able to stimulate insulin secretion (2-fold) after the overexpression of LDH-A in INS-1 cells. In contrast, 20 mM lactate stimulated insulin secretion both in control and LDH-A overexpressing INS-1 cells. These effects were not altered by co-overexpression of MCT-1 (Figure 3). At 10 mM lactate, the incremental insulin secretion over the basal level was 0.32 ± 0.07 ng/h per micrograms DNA in control cells (n = 3, NS), which rose to 1.11 ± 0.13 after overexpression of LDH-A (n = 3, P < 0.05).
Figure 3.
Insulin secretion from INS-1 cells overexpressing LDH-A and/or MCT-1. INS(LDH) cells cultured in the presence or absence of doxycycline and infected with either AdCAlacZ or AdCAMCT-1 were incubated in KRBH with the indicated stimuli for 60 minutes. Insulin secreted in KRBH was quantified by radioimmunoassay and normalized by cellular DNA content. Data show the mean ± SEM of 4 or 5 independent experiments. *Difference from control cells (noninduced and infected with AdCAlacZ) at P < 0.05.
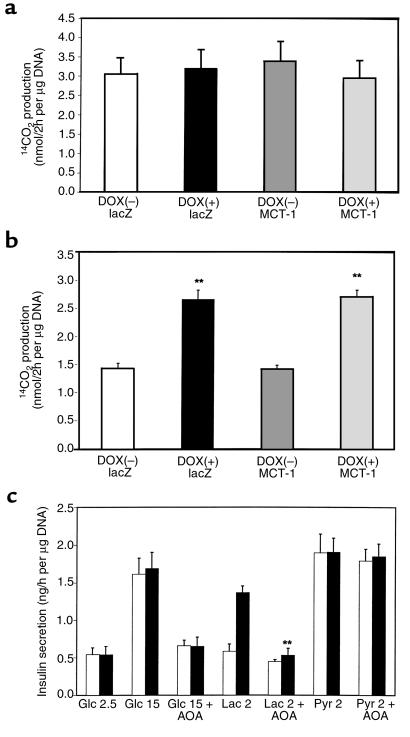
To gain insight into the mechanism underlying lactate-evoked insulin secretion in INS-1 cells, the metabolic consequences of LDH-A and/or MCT-1 overexpression in INS(LDH) cells were studied. The rate of [U-14C]glucose (15 mM) oxidation was not altered by overexpression of LDH-A and/or MCT-1 (Figure 4a). Importantly, the [U-14C]lactate (2 mM) oxidation rate was doubled in LDH-A overexpressing cells, regardless of MCT-1 co-overexpression, compared with that in control cells (Figure 4b). This emphasizes the good correlation between mitochondrial substrate oxidation and insulin secretion. The results obtained with AOA, an inhibitor of the malate-aspartate shuttle, suggest the importance of this shuttle for the reoxidation of cytosolic NADH (Figure 4c). Thus, lactate-stimulated insulin secretion, which is associated with NADH production in the cytosol, was abolished by 0.25 mM AOA in INS(LDH) cells. Insulin release by pyruvate, not generating cytosolic NADH, was unaffected by AOA. Glucose-induced insulin secretion, on the other hand, was blocked by AOA (Figure 4c).
Figure 4.
Mitochondrial oxidation in INS(LDH) cells overexpressing LDH-A and/or MCT-1. 14CO2 formation during 2-hour incubation with 15 mM [U-14C]glucose (a) or 2 mM [U-14C]lactate (b) were measured. Data show the mean ± SEM of 3 independent experiments. **Difference from control cells (noninduced and infected with AdCAlacZ) at P < 0.01. (c) INS(LDH) cells cultured in the presence (filled bars) or absence (open bars) of doxycycline were incubated in KRBH with the indicated stimuli. AOA (0.25 mM), an inhibitor of the malate-aspartate shuttle, was included where indicated. Statistical significance was obtained (P < 0.01) in lactate-stimulated insulin secretion between AOA-treated and nontreated cells overexpressing LDH-A.
Engineering of pyruvate-stimulated insulin secretion in rat pancreatic islets.
INS-1 cells deviate from native β cells in that pyruvate stimulates insulin secretion in the former but not latter cells. We hypothesize that this difference is due to low transport capacity for pyruvate in native β cells. The fact that a membrane-permeable analogue of pyruvate, methylpyruvate, is a potent secretagogue in isolated islets supports this idea (12, 13, 26). To test this hypothesis, we infected isolated rat islets with AdCAMCT-1 to increase the monocarboxylate transport activity in the β cell.
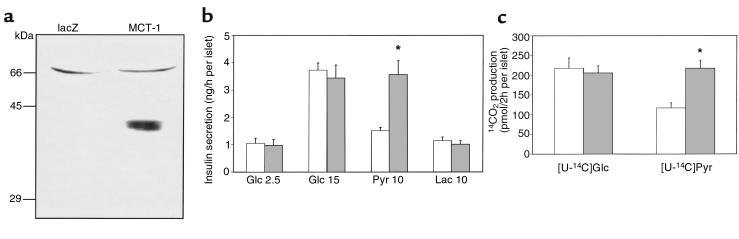
MCT-1 protein was not detected in islets infected with AdCAlacZ, whereas islets infected with AdCAMCT-1 exhibited significant levels of expression of MCT-1 after 24-hour culture (Figure 5a). The insulin secretory response was then examined in these islets (Figure 5b). Insulin secretion in response to 15 mM glucose was not different between control islets and islets overexpressing MCT-1. Pyruvate (10 mM) failed to stimulate insulin secretion significantly in islets infected with AdCAlacZ. After infection with AdCAMCT-1, pyruvate caused 3-fold greater insulin secretory response compared to that at the basal glucose (Figure 5b). To examine whether the enhanced pyruvate-stimulated insulin secretion was due to an increase in the pyruvate metabolism in islets, the rate of [1-14C]pyruvate oxidation was measured. Pyruvate oxidation was increased by 2-fold in islets overexpressing MCT-1 compared with control islets (Figure 5c). On the other hand, overexpression of MCT-1 did not affect the rate of [U-14C]glucose oxidation.
Figure 5.
Overexpression of MCT-1 through a recombinant adenovirus and its effects on isolated islets. (a) One hundred twenty islets infected with either AdCAlacZ or AdCAMCT-1 were used for immunoblot analyses. The result is representative of 3 independent experiments. (b) Ten islets infected with recombinant adenoviruses in each tube were incubated in KRBH with indicated stimuli for 60 minutes. Data show the mean ± SEM of 5 independent experiments. Statistical significance between MCT-1–overexpressing islets and control islets was obtained at 10 mM pyruvate (P < 0.05). (c) 14CO2 formation during 2-hour incubation with 15 mM [U-14C]glucose or 10 mM [1-14C]pyruvate was measured. Data show the mean ± SEM of 5 independent experiments. *Difference from control islets at P < 0.05. Open bars, islets infected with AdCAlacZ; shaded bars, islets infected with AdCAMCT-1.
Engineering of lactate-stimulated insulin secretion in rat pancreatic islets.
Although rat islets became responsive to pyruvate after overexpression of MCT-1, lactate did not elicit insulin secretion from islets overexpressing MCT-1 (Figure 5b). The enhanced lactate-stimulated insulin secretion after overexpression of LDH-A in INS-1 cells suggests that an efficient conversion of lactate to pyruvate is necessary for the stimulation by lactate. This was examined by overexpressing LDH-A simultaneously with MCT-1 in isolated rat islets.
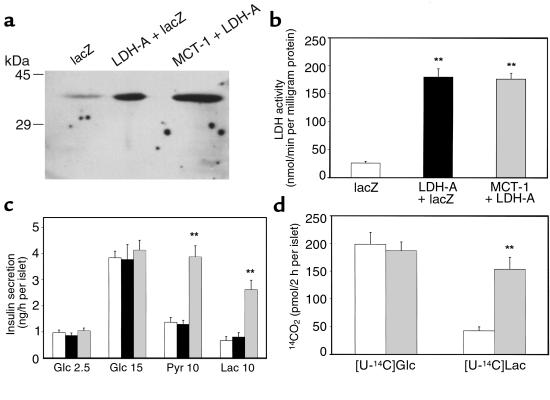
Adenovirus-mediated introduction of LDH-A cDNA increased the amount of LDH-A protein by approximately 5-fold in rat islets (Figure 6a). The LDH activity was 7-fold greater in islets infected with AdCALDH-A alone or with both AdCALDH-A and AdCAMCT-1 (Figure 6b). Because the endogenous LDH activity is lower in β cells than in non–β cells (6, 8), the degree of LDH overexpression in β cells would be higher than that observed in whole islets. As was the case with INS-1 cells, insulin secretion evoked by glucose was not affected by overexpression of MCT-1 and LDH-A (Figure 6c). Although islets overexpressing LDH-A alone did not respond to pyruvate challenge, overexpression of both LDH-A and MCT-1 again produced pyruvate-stimulated insulin secretion. Interestingly, the same manipulation brought about marked (3.5-fold) stimulation by 10 mM lactate compared with control islets (Figure 6c). Lactate-induced insulin secretion was accompanied by a 3.7-fold enhancement of the [U-14C]lactate oxidation rate in rat islets overexpressing both LDH-A and MCT-1 (Figure 6d).
Figure 6.
Co-overexpression of MCT-1 and LDH-A through recombinant adenoviruses and its effects on isolated islets. (a) One hundred twenty islets infected with either AdCAlacZ, AdCALDH-A plus AdCAlacZ (AdCAlacZ was combined to adjust total virus amounts), or AdCAMCT-1 plus AdCALDH-A were used for immunoblot analyses. The result is representative of 2 independent experiments. (b) Lysates of 100 islets infected with recombinant adenoviruses were assayed for LDH activity. Data show the mean ± SEM of 3 independent experiments. **Difference from control islets at P < 0.01. (c) Ten islets infected with recombinant adenoviruses in each tube were incubated in KRBH with the indicated stimuli for 60 minutes. Data show the mean ± SEM of 4 independent experiments. Statistical significance between islets overexpressing both MCT-1 and LDH-A and control islets were obtained at 10 mM pyruvate and 10 mM lactate (P < 0.01). (d) 14CO2 formation during 2-hour incubation with 15 mM [U-14C]glucose or 10 mM [U-14C]lactate were measured. Data show the mean ± SEM of 3 independent experiments. **Difference from control islets at P < 0.01. Open bars, islets infected with AdCAlacZ; filled bars, islets infected with AdCALDH-A plus AdCAlacZ; shaded bars, islets infected with AdCAMCT-1 plus AdCALDH-A.
Discussion
Recent advances in the adenoviral vector technology have facilitated the transfer of multiple foreign genes into well-differentiated cell lines or primary cells, such as islet cells. To explore the significance of the documented low activities of LDH and MCT in the β cell relative to islet non–β cells (6, 8) and other cell types (8, 16), we have investigated the consequences of overexpression of LDH-A and MCT-1 on glucose-, pyruvate-, or lactate-stimulated insulin secretion in INS-1 cells and isolated rat islets. Because the chicken actin promoter used in our recombinant adenovirus constructs (27) is active in both β cells and non–β cells (28), this approach diminishes differences in expression levels of LDH and MCT between these cells.
Pyruvate and lactate have been reported to be metabolized, but do not cause insulin secretion in pancreatic islets (11–15). Hence, the oxidation rate of 30 mM [1-14C]pyruvate was found to be as high as that of 16.7 mM [U-14C]glucose without any increase in insulin secretion (11). This dissociation between mitochondrial substrate oxidation and insulin secretion is known as the pyruvate paradox. It is most probable that the paradox could be explained by higher rates of pyruvate oxidation in the 30–35% non–β cells present in rat islets (10), which transport monocarboxylates 3 times more efficiently than β cells (8). This view is supported by results showing that islets from ob/ob mice, comprised of 90% β cells, oxidize 17 mM [U-14C]pyruvate 6 times less efficiently than 20 mM [U-14C]glucose (29). Also note that 5 mM pyruvate plus 3.3 mM glucose stimulates glucagon secretion but not insulin secretion in the perfused rat pancreas (30). The differences between pyruvate and glucose oxidation in ob/ob mouse islets may reside mainly in the much lower transport capacity for pyruvate compared with glucose across the β-cell plasma membrane. In contrast, pyruvate was reported to be avidly metabolized by mitochondria isolated from ob/ob mouse islets (31), indicating that the transport capacity of pyruvate across the inner mitochondrial membrane is not limiting. Our data demonstrating an increased pyruvate oxidation after overexpression of MCT-1 indicate that pyruvate transport by the plasma membrane MCT indeed is flux determining for pyruvate metabolism in isolated islets. Nonetheless, it cannot be excluded that the difference in efficiency between glucose and pyruvate metabolism could be determined not only by low expression of MCTs, but also by stimulation of mitochondrial shuttles (see below) by the former but not by the latter.
In INS-1 cells MCT activity is not rate limiting for the metabolism of pyruvate and lactate, because, first, pyruvate stimulates insulin secretion in these cells (Figure 3 and ref. 8), and second, lactate oxidation was not affected following overexpression of MCT-1. Nonetheless, MCT activity is markedly lower in INS-1 cells compared with that of RINm5F cells derived from the same rat insulinoma (8). The different potencies of pyruvate and lactate in INS-1 cells appear to be due to distinct metabolic fates of the 2 substrates. Because LDH-A but not MCT-1 overexpression led to insulin secretion at low lactate concentrations in INS-1 cells, initiation of secretion by lactate depends on pyruvate generation in the cell. This conclusion is supported by the results with AOA, which blocks reoxidation of NADH by the malate-aspartate shuttle (Figure 4c). The compound did not reduce pyruvate-stimulated insulin secretion while abolishing the action of lactate. The treatment with AOA also inhibited glucose-stimulated insulin secretion in INS-1 cells. This suggests that the glycerol phosphate shuttle is less active than the malate-aspartate shuttle in the regeneration of cytosolic NAD+ consumed by the glycolysis in INS-1 cells. In rat islets a 20-fold higher concentration of AOA inhibits glucose-stimulated insulin secretion by about 50% (32, 33), whereas this compound is less efficient in mouse islets (9). It appears, thus, that in mouse β cells the activity of the glycerol phosphate shuttle can substitute for the malate-aspartate shuttle, whereas this is not the case in INS-1 cells, despite high activity of mGPDH (8, 34). The reason for this discrepancy may lie in substrate availability or in other regulatory factors.
In contrast to pyruvate, lactate did not elicit insulin secretion in islets infected with AdCAMCT-1 alone. The simultaneous overexpression of MCT-1 and LDH-A caused marked insulin secretion in response to lactate, which was associated with increased lactate oxidation by the islets. The combined results from INS-1 cells and islets demonstrate that endogenous LDH activity does not allow generation of sufficient amounts of pyruvate, the essential intermediate, to accomplish insulin secretion by lactate. This can be brought about by LDH-overexpression. In INS-1 cells, high concentrations of extracellular lactate elicit insulin secretion by forcing the LDH reaction to provide sufficient quantities of pyruvate even without LDH-A overexpression.
It has been reported that more than 90% of glucose-derived pyruvate enters into the mitochondria in the β cell (6). Low activities of LDH and MCT might contribute to the efficient channeling of glucose-derived pyruvate into the mitochondria by preventing pyruvate from escaping from the cell. However, neither glucose metabolism nor glucose-stimulated insulin secretion was affected in both INS-1 cells and rat islets after overexpression of LDH-A and/or MCT-1 (Figures 3 and 6c). Moreover, lactate output was not changed significantly in INS(LDH) cells exposed to 15 mM glucose after overexpression of LDH-A and MCT-1 (data not shown). These observations suggest that efficient metabolic channeling of glucose-derived pyruvate to the mitochondria is not dependent on low LDH and MCT activities. An attenuated glucose-stimulated insulin secretion was reported in LDH-A overexpressing stable clones of MIN6 cells relative to wild-type MIN6 cells, another well-differentiated tumoral β-cell line (35). A possible explanation for the differences between our results and the data obtained in MIN6 cells is that the stable overexpression of LDH-A might exert untoward effects rendering the cells less responsive to glucose. Note that glucose-stimulated insulin secretion was also unaffected in MIN6 cells transiently overexpressing LDH-A through a recombinant adenovirus (36). In any case, our results suggest that unknown mechanisms of efficient coupling of glycolysis to mitochondrial metabolism exist. Further studies are needed to reveal the mechanism of the efficient channeling of glycolytic intermediates to the mitochondria.
What would be the consequence if lactate would also act as a nutrient in β cells? Lactate is now considered to be an important energy source in several cells such as neurons and heart muscle cells, especially when circulating glucose is insufficient (37, 38). Plasma lactate levels in humans can increase up to nearly 10 mM during exercise (39). Such a high concentration of lactate could stimulate insulin secretion if the β cell would express high levels of MCT and LDH. The results here, therefore, suggest that low LDH and MCT activities protect the β cell from stimulatory effects of lactate, which could otherwise cause undesired insulin secretion during a catabolic state such as exercise. In other terms, low LDH and MCT activities confer specific selectivity to glucose of insulin release and avoid stimulation by pyruvate and lactate.
Acknowledgments
We are grateful to O. Dupont, A. Gjinovci, and J. Bassy for their expert technical assistance. We thank A. Volkl (University of Heidelberg, Germany) and Y. Briand (University Blaise Pascal-Clermont-Ferrand, France) for kindly supplying anti-liver LDH antibody and rabbit LDH-A cDNA, respectively. We also thank P. Antinozzi for helpful discussions. H. Ishihara is a recipient of a fellowship from Manpei Suzuki Foundation for Diabetes Research (Tokyo, Japan), a fellowship from the Cell Science Research Foundation (Osaka, Japan), and a fellowship from the Juvenile Diabetes Foundation International. This work was supported by a grant from the Swiss National Science Foundation (32-49755.96 to C.B. Wollheim) and grants from the National Institutes of Health (NS-37764 to L.R. Drewes) and the American Heart Association (9708013A to L.R. Drewes).
References
- 1.DeFronzo RA. Lilly Lecture 1987. The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 2.Virkarmaki A, Ueki K, Kahn CR. Protein-protein interaction in insulin signaling and the molecular mechanism of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prentki M. New insights into pancreatic β-cell metabolic signaling in insulin secretion. Eur J Endocrinol. 1996;134:272–286. doi: 10.1530/eje.0.1340272. [DOI] [PubMed] [Google Scholar]
- 4.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45:223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 5.MacDonald MJ. Influence of glucose on pyruvate carboxylase expression in pancreatic islets. Arch Biochem Biophys. 1995;319:128–132. doi: 10.1006/abbi.1995.1274. [DOI] [PubMed] [Google Scholar]
- 6.Schuit F, et al. Metabolic fate of glucose in purified islet cells. J Biol Chem. 1997;272:18572–18579. doi: 10.1074/jbc.272.30.18572. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald MJ. High content of mitochondrial glycerol 3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J Biol Chem. 1981;256:8287–8290. [PubMed] [Google Scholar]
- 8.Sekine N, et al. Low lactate dehydrogenase and high mitochondrial glycerol phosphate dehydrogenase in pancreatic β-cells. J Biol Chem. 1994;269:4895–4902. [PubMed] [Google Scholar]
- 9.Eto K, et al. Role of NADH shuttling system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science. 1999;283:981–985. doi: 10.1126/science.283.5404.981. [DOI] [PubMed] [Google Scholar]
- 10.Baetens D, Malaisse-Lagae F, Perrelet A, Orci L. Endocrine pancreas: three-dimensional reconstitution shows two type of islets of Langerhans. Science. 1979;206:1323–1325. doi: 10.1126/science.390711. [DOI] [PubMed] [Google Scholar]
- 11.Sener A, et al. The stimulus-secretion coupling of glucose-induced insulin release: effect of exogenous pyruvate on islet function. Biochem J. 1978;176:217–232. doi: 10.1042/bj1760217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mertz RJ, Worley JF, III, Spencer B, Johnson JH, Dukes ID. Activation of stimulus-secretion coupling in pancreatic β-cells by specific products of glucose metabolism. J Biol Chem. 1996;271:4838–4845. doi: 10.1074/jbc.271.9.4838. [DOI] [PubMed] [Google Scholar]
- 13.Zawalich WS, Zawalich KC. Influence of pyruvic acid methyl ester on rat pancreatic islets. J Biol Chem. 1997;272:3527–3531. doi: 10.1074/jbc.272.6.3527. [DOI] [PubMed] [Google Scholar]
- 14.Malaisse WJ, et al. The stimulus-secretion coupling of glucose-induced insulin release: effect of exogenous lactate upon islet function. Arch Biochem Biophys. 1979;194:49–62. doi: 10.1016/0003-9861(79)90594-0. [DOI] [PubMed] [Google Scholar]
- 15.Lenzen S, Panten U. Effects of pyruvate, L-lactate and 3-phenylpyruvate on function of ob/ob mouse pancreatic islets. Biochem Med. 1981;25:366–372. doi: 10.1016/0006-2944(81)90095-8. [DOI] [PubMed] [Google Scholar]
- 16.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am J Physiol. 1993;264:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 17.Price NT, Jackson VN, Halestrap AP. Cloning and sequencing of four new mammalian monocarboxylate transporter (MCT) homologues confirms the existence of a transporter family with an ancient past. Biochem J. 1998;329:321–328. doi: 10.1042/bj3290321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H, Iynedjan PB. Modulation of glucose responsiveness of insulinoma β-cells by graded overexpression of glucokinase. Proc Natl Acad Sci USA. 1997;94:4372–4377. doi: 10.1073/pnas.94.9.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sass C, Briand M, Benslimane S, Renaud M, Briand Y. Characterization of rabbit lactate dehydrogenase-M and lactate dehydrogenase-H cDNAs. J Biol Chem. 1989;264:4076–4081. [PubMed] [Google Scholar]
- 20.Asfari M, et al. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130:167–178. doi: 10.1210/endo.130.1.1370150. [DOI] [PubMed] [Google Scholar]
- 21.Garcia CK, Goldstein JL, Pathak RK, Anderson RGW, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implication for cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 22.Miyake S, et al. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerhart D, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- 24.Baumgart E, Fahimi HD, Stich A, Volkl A. L-lactate dehydrogenase A4- and A3B isoforms are bona fide peroxisomal enzymes in rat liver. J Biol Chem. 1996;271:3846–3855. doi: 10.1074/jbc.271.7.3846. [DOI] [PubMed] [Google Scholar]
- 25.Jackson VN, Halestrap HP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of the rat liver cells determined using the fluorescent intracellular pH indicator, 2′,7′-bis(2-carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem. 1996;271:861–868. doi: 10.1074/jbc.271.2.861. [DOI] [PubMed] [Google Scholar]
- 26.Malaisse WJ, et al. Insulinotropic action of methyl pyruvate: enzymatic and metabolic aspects. Arch Biochem Biophys. 1996;335:229–244. doi: 10.1006/abbi.1996.0505. [DOI] [PubMed] [Google Scholar]
- 27.Niwa H, Yamamura K, Miyazaki J-I. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–200. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 28.Ueda K, et al. Overexpression of mitochondrial FAD-linked glycerol-3-phosphate dehydrogenase does not correct glucose-stimulated insulin secretion from diabetic GK rat pancreatic islets. Diabetologia. 1998;41:649–653. doi: 10.1007/s001250050963. [DOI] [PubMed] [Google Scholar]
- 29.Hellman B, Sehlin J, Taljedal I-B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971;123:513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leclercq V, Marchand J, Malaisse WJ. An arginine-like effect of the “fumarate + glutamate + pyruvate” mixture on glucagon release. Life Sci. 1977;20:1193–1198. doi: 10.1016/0024-3205(77)90492-1. [DOI] [PubMed] [Google Scholar]
- 31.Lembert N, Idahl LA. Alpha-ketoisocaproate is not a true substrate for ATP production by pancreatic β-cell mitochondria. Diabetes. 1998;47:339–344. doi: 10.2337/diabetes.47.3.339. [DOI] [PubMed] [Google Scholar]
- 32.MacDonald MJ. Evidence for the malate aspartate shuttle in pancreatic islets. Arch Biochem Biophys. 1982;213:643–649. doi: 10.1016/0003-9861(82)90594-x. [DOI] [PubMed] [Google Scholar]
- 33.Malaisse WJ, Malaisse-Lagae F, Sener A. The stimulus-secretion coupling of glucose-induced insulin release: effect of aminooxyacetate upon nutrient-stimulated insulin secretion. Endocrinology. 1982;111:392–397. doi: 10.1210/endo-111-2-392. [DOI] [PubMed] [Google Scholar]
- 34.Rutter GA, Pralong WF, Wollheim CB. Regulation of mitochondrial glycerol-phosphate dehydrogenase by Ca2+ within electropermeabilized insulin-secreting cells (INS-1) Biochem Biophys Acta. 1992;1175:107–113. doi: 10.1016/0167-4889(92)90016-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Rutter GA. Overexpression of lactate dehydrogenase A attenuates glucose-induced insulin secretion in stable MIN-6 β-cell lines. FEBS Lett. 1998;430:213–216. doi: 10.1016/s0014-5793(98)00600-0. [DOI] [PubMed] [Google Scholar]
- 36.Ishihara H, Kizuki N, Oka Y. Control of glucose metabolism underlying normal glucose sensing in β-cell line MIN6. Diabetes. 1996;45(Suppl. 2):243–(Abstr.). [Google Scholar]
- 37.Izumi Y, Benz AM, Zurumaski CF, Olney JW. Effects of lactate and pyruvate on glucose deprivation in rat hippocampal slices. Neuroreport. 1994;5:617–620. doi: 10.1097/00001756-199401000-00021. [DOI] [PubMed] [Google Scholar]
- 38.Pellerin L, Pellegri G, Martin J-L, Magistretti P. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci USA. 1998;95:3990–3995. doi: 10.1073/pnas.95.7.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindinger M, McKelvie RS, Heigenhauser GJF. K+ and lac- distribution in humans during and after high-intensity exercise: role in muscle fatigue attenuation? J Appl Physiol. 1995;78:765–777. doi: 10.1152/jappl.1995.78.3.765. [DOI] [PubMed] [Google Scholar]