Abstract
SUMMARY
During Mycobacterium tuberculosis and other respiratory infections, optimal T cell activation requires pathogen transport from the lung to a local draining lymph node (LN). However, the infected inflammatory monocyte-derived dendritic cells (DCs) that transport M. tuberculosis to the local lymph node are relatively inefficient at activating CD4 T cells, possibly due to bacterial inhibition of antigen presentation. We found that infected migratory DCs release M. tuberculosis antigens as soluble, unprocessed proteins for uptake and presentation by uninfected resident lymph node DCs. This transfer of bacterial proteins from migratory to local DCs results in optimal priming of antigen-specific CD4 T cells, which are essential in controlling tuberculosis. Additionally, this mechanism does not involve transfer of the whole bacterium and is distinct from apoptosis or exosome shedding. These findings reveal a mechanism that bypasses pathogen inhibition of antigen presentation by infected cells and generates CD4 T cell responses that control the infection.
INTRODUCTION
Although activation of naive antigen-specific T cells takes place in secondary lymphoid tissues such as lymph nodes, most pathogens are initially localized in peripheral nonlymphoid tissues, such as the respiratory or gastrointestinal tracts or the skin. This spatial separation between the location of a pathogen and the site of T cell activation must be resolved to allow timely development of adaptive immune responses. While soluble antigens, certain viruses (Junt et al., 2007), and motile bacteria (Kastenmüller et al., 2012) can enter lymph nodes by lymphatic flow, other viral, bacterial, and fungal antigens require transport from the site of entry in a peripheral tissue to the local lymph node by migratory dendritic cells (DCs). Once they arrive in a lymph node, migratory DCs may directly present antigens to T cells, or they may cooperate with resident lymph node DCs, which then activate T cells.
Antigen presentation after acquisition from another cell was first described for an MHC II-restricted antigen acquired by DCs through phagocytosis of antigen-bearing B cells in vitro (Inaba et al., 1998). In vivo, antigen transfer is best characterized for MHC I-dependent cross-presentation of viral antigens. After cutaneous infection with herpes simplex virus (HSV), Langerhans cells and a dermal DC subset transport viral antigens to lymph nodes, where CD8α+ DCs acquire antigen from them to activate HSV antigen-specific CD8 T cells (Allan et al., 2003, 2006). Likewise, after subcutaneous inoculation with an attenuated vaccine strain of Blastomyces dermatiditis, migratory DCs transport the yeast to the local draining lymph node, where they deliver yeast to resident DCs, which in turn present antigen for CD4 T cell activation (Ersland et al., 2010). In the respiratory tract, migratory DCs can directly present influenza virus antigens to CD4 and CD8 T cells (Ballesteros-Tato et al., 2010; Belz et al., 2004; Kim and Braciale, 2009; Mount et al., 2008); however, antigen transfer to lymph node-resident DCs also contributes to CD8 T cell activation (Belz et al., 2004). After respiratory infection with fungi (Aspergillus or Blastomyces) or a bacterium (M. tuberculosis), Ly6Chi, CCR2+ inflammatory monocytes or DCs transport the pathogen from the site of entry in the respiratory tract to the local lymph node (Ersland et al., 2010; Hohl et al., 2009; Samstein et al., 2013; Wüthrich et al., 2012). In some, but not all of the cases examined so far, the migratory cells themselves were not able to prime naive CD4 T cells; they required the cooperation of resident lymph node DCs. This cooperation clearly includes transfer of pathogen antigens from migratory to resident DCs, yet the mechanism(s) underlying antigen transfer are not defined, and it is unclear whether antigen transfer occurs independently of pathogen transfer.
Mycobacterium tuberculosis is an exceptionally successful bacterial pathogen, due to its ease of aerosol transmission and its multiple mechanisms for evading and exploiting immune responses, including inhibition of MHC class II antigen presentation (Philips and Ernst, 2012). CD4 T cells are essential for control of tuberculosis in humans (Kwan and Ernst, 2011), mice (Mogues et al., 2001), cattle (Waters et al., 2011), and nonhuman primates (Diedrich et al., 2010). Despite their importance in immunity to tuberculosis, the mechanisms of initial activation of CD4 T cells remain incompletely understood. While the lung alveoli are the first sites of implantation of the bacteria, there is considerable evidence that M. tuberculosis antigen-specific CD4 T cells are initially activated in the mediastinal lymph node (MDLN), which drains the lungs. First, activation of naive antigen-specific CD4 T cells occurs in the MDLN, coincides with the appearance of live M. tuberculosis in the MDLN (Chackerian et al., 2002; Wolf et al., 2008), and is detectable in the MDLN earlier than in the lungs. The timing of T cell activation in the MDLN depends on the genetic background of the mice, and earlier T cell activation in the MDLN is associated with superior control of M. tuberculosis in the lungs (Chackerian et al., 2002). Second, CD4 T cell activation in the MDLN depends on transport of bacteria from the lungs by infected DCs (Khader et al., 2006) and production of bacterial antigen in the MDLN (Wolf et al., 2008). Third, a high fraction of the cells that contain bacteria in the lungs are CD11bhi DCs, and CD11bhi DCs account for nearly all of the infected cells in the MDLN (Wolf et al., 2007), consistent with their role in transporting live M. tuberculosis from the lungs. However, CD11bhi DCs isolated from the MDLN of M. tuberculosis-infected mice are less efficient at presenting peptide to antigen-specific CD4 T cells than are other lymph node DCs (Wolf et al., 2007). Thus, it is unclear whether the DCs that transport bacteria to the MDLN directly activate M. tuberculosis-specific naive CD4 T cells or transfer antigen and/or bacteria to other DC subsets that then prime naive CD4 T cells. This is especially relevant in tuberculosis, since there is considerable evidence that M. tuberculosis interferes with MHC class II antigen presentation in the cells that it infects (reviewed in Baena and Porcelli, 2009). Indeed, a recent study revealed that after aerosol infection with M. tuberculosis, CCR2+ inflammatory monocytes, which differentiate into DCs during or after transit from the lungs to the lymph node, are incapable of activating antigen-specific CD4 T cells, despite their expression of MHC II (Samstein et al., 2013).
In agreement with the findings of Samstein and colleagues (Samstein et al., 2013), we report here that infected migratory DCs activate naive CD4 T cells poorly in mice infected with M. tuberculosis and that optimal CD4 T cell priming requires antigen transfer to uninfected lymph node DCs. We found that transfer of antigen to lymph node DCs occurs without transfer of the bacteria, involves full-length unprocessed antigen, and is decreased, not increased, by promoting apoptosis of the infected cells. Cell-to-cell antigen transfer and cooperation between migratory and resident lymph node DCs optimize activation of naive CD4 T cells and may compensate for M. tuberculosis inhibition of antigen presentation in infected cells.
RESULTS
Migratory DCs Cooperate with Lymph Node Resident DCs to Optimize Activation of Naive M. tuberculosis Antigen-Specific CD4 T Cells
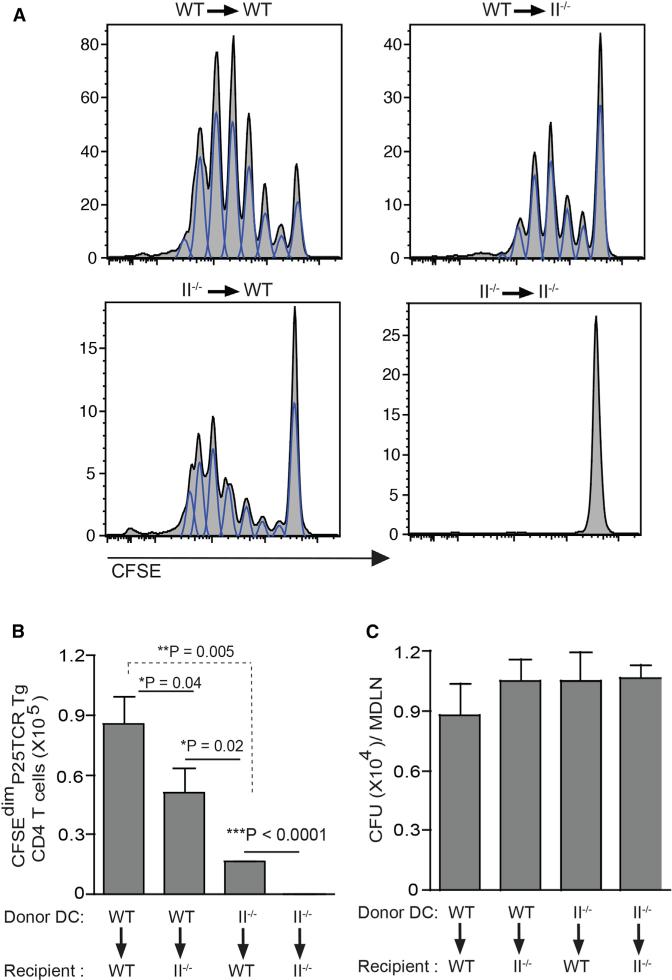
Since migratory CD11c+CD11bhi DCs or CD11c+CD103+ DCs (Samstein et al., 2013) from M. tuberculosis-infected mice are less efficient at stimulating CD4 T cells than are other DCs, we hypothesized that other DCs contribute to stimulating M. tuberculosis antigen-specific CD4 T cells. To test this hypothesis, we used intratracheal transfer of M. tuberculosis-infected bone marrow-derived dendritic cells (BMDCs) (Bhatt et al., 2004; Divangahi et al., 2010; Khader et al., 2006). We initially validated this method by transferring M. tuberculosis-infected BMDCs from wild-type mice into wild-type recipients and assaying proliferation of adoptively transferred TCR transgenic CD4 T cells specific for aa 240–254 (peptide 25) from M. tuberculosis Ag85B, bound to I-Ab (Blomgran and Ernst, 2011; Bold et al., 2011; Olmos et al., 2010; Wolf et al., 2008) (P25TCR-Tg cells). After transfer of infected BMDCs, a large fraction of the P25TCR-Tg CD4 T cells proliferated in the MDLN (Figures 1A and 1B), indicating that intratracheal transfer of M. tuberculosis-infected BMDCs activates naive P25TCR-Tg CD4 T cells in vivo. Consistent with our previous finding that proliferation of naive P25TCR-Tg CD4 cells requires antigen production by bacteria in the MDLN (Wolf et al., 2008), intratracheal transfer of infected BMDCs resulted in the appearance of M. tuberculosis in the MDLN (Figure 1C). Proliferation was Ag85B specific, as transfer of BMDCs infected with Ag85B null M. tuberculosis (H37Rv:ΔAg85B) (Bold et al., 2011; Wolf et al., 2008) did not stimulate proliferation (Figures S1A and S1B). Proliferation of P25TCR-Tg CD4 T cells required MHC class II, as transfer of infected MHC II−/− BMDCs into MHC II−/− recipients did not result in T cell proliferation (Figures 1A and 1B). To determine the contribution of direct presentation of Ag85B by migratory DCs, we transferred M. tuberculosis-infected wild-type BMDCs into MHC II−/− mice, so that only migratory DCs could present antigen. This resulted in proliferation of P25TCR-Tg CD4 T cells that was less extensive than when wild-type BMDCs were transferred into wild-type mice (Figures 1A and 1B). This implies that MHC II is required on both migratory and resident lymph node DCs for optimal stimulation of naive CD4 T cells in response to M. tuberculosis. Notably, transfer of infected MHC II−/− BMDC into wild-type mice, in whom only resident DCs can present antigen, also resulted in proliferation of P25TCRTg CD4 T cells (Figures 1A and 1B), confirming that resident lymph node DCs contribute to naive CD4 T cell activation in the context of tuberculosis.
Figure 1. Migratory and Lymph Node Resident DCs Collaborate for Optimal Activation of Naive Antigen-Specific CD4 T Cells in Tuberculosis.
(A–C) Wild-type C57BL/6 or MHC-II−/− mice received CFSE-labeled naive Ag85B-specific P25TCR-Tg CD4+ T cells followed by intratracheal transfer of M. tuberculosis-infected wild-type or MHC-II−/− DCs (moi 1:1; 1 × 106 bacteria/1 × 106 DCs/ mouse). (A) Proliferation of CFSE-labeled P25TCR-Tg CD4+ T cells in mediastinal lymph nodes (MDLNs) of wild-type or MHC-II−/− mice 60 hr after intratracheal transfer of M. tuberculosis-infected wild-type or MHC-II−/− DCs. Flow cytometry plots represent a pool of cells from MDLN of two mice (three pools from six mice per group). (B) Quantitation of P25TCR-Tg CD4+ T cells that have undergone at least one cycle of proliferation (CFSEdim) in MDLN of the groups of mice shown in (A). (C) Quantitation of bacteria in MDLN of the groups of mice shown in (A) and (B). Data are expressed as mean ± SEM of three pools of mice (n = 6) per experimental group and represent three independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. See also Figure S1.
These results indicate that cooperation between migratory and lymph node DCs is necessary for optimal activation of naive antigen-specific CD4 T cells in tuberculosis. The finding that transfer of MHC II−/− DCs into wild-type mice resulted in activation of naive CD4 T cells suggests that migratory DCs transfer antigen to lymph node resident DCs during M. tuberculosis infection.
DC Transport of M. tuberculosis from the Lungs to the MDLN Is Required for Activation of M. tuberculosis Antigen-Specific CD4+ T Cells
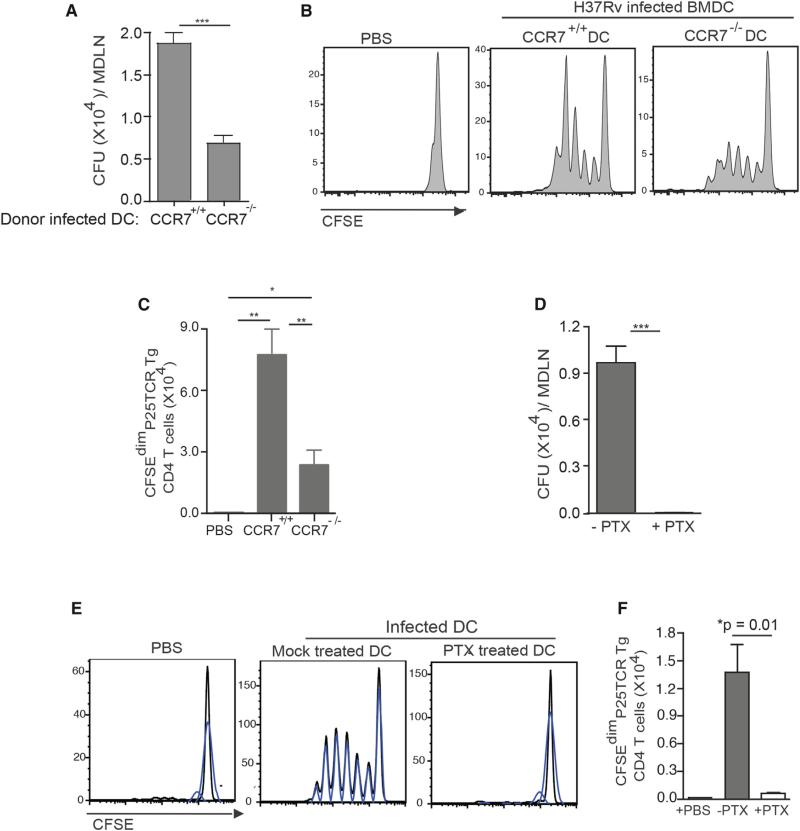
While the results of the DC transfer experiments are consistent with transfer of antigen from migratory DCs to other DCs in the MDLN, they are also consistent with an alternative mechanism: release of bacteria and/or antigen from cells in the lungs, followed by drainage or carriage to the lymph node and uptake by lymph node DCs. To determine whether chemokine receptor-dependent migration of transferred DCs from the lungs to the MDLN is essential for activation of naive CD4 T cells, we initially examined CD4 T cell activation after intratracheal transfer of M. tuberculosis-infected BMDCs from CCR7−/− mice into wild-type mice. Compared with wild-type DCs, CCR7−/− DCs transported fewer bacteria to the MDLN (Figure 2A) and stimulated less proliferation of P25TCR-Tg CD4 T cells (Figures 2B and 2C). The finding that the absence of CCR7 did not completely abrogate trafficking of bacteria or T cell proliferation is consistent with two possibilities. One is that mechanisms independent of DC migration contribute to trafficking of M. tuberculosis and CD4 T cell activation. The other is that chemokine receptors other than CCR7 can direct DC migration from the lungs to the MDLN, consistent with previously published findings (Olmos et al., 2010). To distinguish these two possibilities, we treated M. tuberculosis-infected wild-type BMDCs with pertussis toxin (PTX) prior to transfer. PTX treatment of infected DCs prior to transfer resulted in retention of bacteria in the lungs (Figure S2A) and completely blocked the appearance of bacteria in the MDLN (Figure 2D), indicating that transport of M. tuberculosis from the lungs to the MDLN depends on G protein-coupled receptor-dependent migration of infected DCs. Likewise, PTX treatment of infected BMDCs prior to intratracheal administration abrogated proliferation of P25TCR-Tg CD4 T cells in the MDLN (Figures 2E and 2F), indicating that migration of M. tuberculosis-infected DCs from the lungs to the MDLN is essential for activation of naive P25TCR-Tg CD4 T cells.
Figure 2. DC Migration Is Required for Transport of M. tuberculosis from the Lungs to the MDLN and Priming of Ag85B-Specific CD4 T Cells.
(A) Quantitation of bacteria in the MDLNs of wild-type mice 60 hr after intratracheal transfer of M. tuberculosis-infected wild-type or CCR7−/− DCs (moi 0.6:1; 6 × 105 bacteria/1 × 106 DC/mouse).
(B) Proliferation profile of CFSE-labeled P25TCR-Tg CD4+ T cells in MDLNs of the groups of mice shown in (A). Control mice received PBS.
(C) Quantitation of P25TCR-Tg CD4+ T cells that have undergone at least one cycle of proliferation (CFSEdim) in MDLNs of groups of mice shown in (A) and (B).
(D) Quantitation of bacteria in wild-type mice 60 hr after intratracheal transfer of infected wild-type DCs (moi 0.5:1; 5 × 105 bacteria/1 × 106 DC/mouse) that were either mock treated or treated with pertussis toxin (PTX).
(E) Proliferation profile of CFSE-labeled P25TCR-Tg CD4+ T cells in MDLN of the groups of mice shown in (C) and (D). Control mice received PBS. Each CFSE histogram represents a pool of cells from two mice (three pools from a total of six mice per group).
(F) Quantitation of P25TCR-Tg CD4+ T cells that have undergone at least one cycle of proliferation (CFSEdim) in MDLNs of groups of mice in (D) and (E). Data are expressed as mean ± SEM of either individual mice (n = 4–5; A–C) or three pools of mice (n = 6; D–F) per experimental group and represent two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. See also Figure S2.
Although the preceding experiment indicated that release of bacteria from donor DCs in the lungs was not responsible for activation of CD4 T cells in the MDLN, we further evaluated the potential contribution of free bacteria to naive CD4 T cell activation. When cell-free M. tuberculosis was administered intratracheally, up to 5 × 106 cell-free bacteria/mouse (5- to 10-fold more than that administered with infected BMDCs) failed to stimulate P25TCR-Tg CD4 T cells in the MDLN (Figures S2B and S2C). Proliferation of P25TCR-Tg CD4 cells occurred only after intratracheal inoculation of a large excess of bacteria (3 × 107) (Figures S2B and S2C), indicating that cell-free translocation of M. tuberculosis from the lungs to the MDLN does not account for activation of Ag85B-specific naive CD4 T cells after intratracheal administration of infected DCs. Likewise, bacteria appeared in the MDLN only after intratracheal administration of at least 5 × 106 cell-free M. tuberculosis; administration of 3 × 107 cell-free bacteria was required to achieve the number of bacteria in the lymph node (~1,200) (Wolf et al., 2008) required to stimulate naive CD4 T cells (Figure S2D).
Together, these results indicate that M. tuberculosis reaches the MDLN from the lungs via DC migration and not through passive lymphatic drainage of intact bacteria or soluble antigen secreted by cell-free bacteria. These findings are consistent with our previously reported findings that activation of M. tuberculosis antigen-specific naive CD4 T cells follows transport of live bacteria to the MDLN by DCs after aerosol infection (Wolf et al., 2008) and are consistent with results obtained after aerosol infection (Samstein et al., 2013). Additionally, our results suggest that the cell-to-cell antigen transfer that leads to naive CD4 T cell activation occurs locally in the MDLN after infected migratory DCs transport bacteria from the lungs. Antigen transfer is efficient, since transfer of as few as 4 × 104 M. tuberculosis-infected MHC II−/− BMDCs stimulated proliferation of P25TCR-Tg CD4 T cells in wild-type recipients (data not shown).
M. tuberculosis-Infected DC Release Bacterial Antigen for Uptake and MHC Class II Antigen Presentation to CD4 T Cells
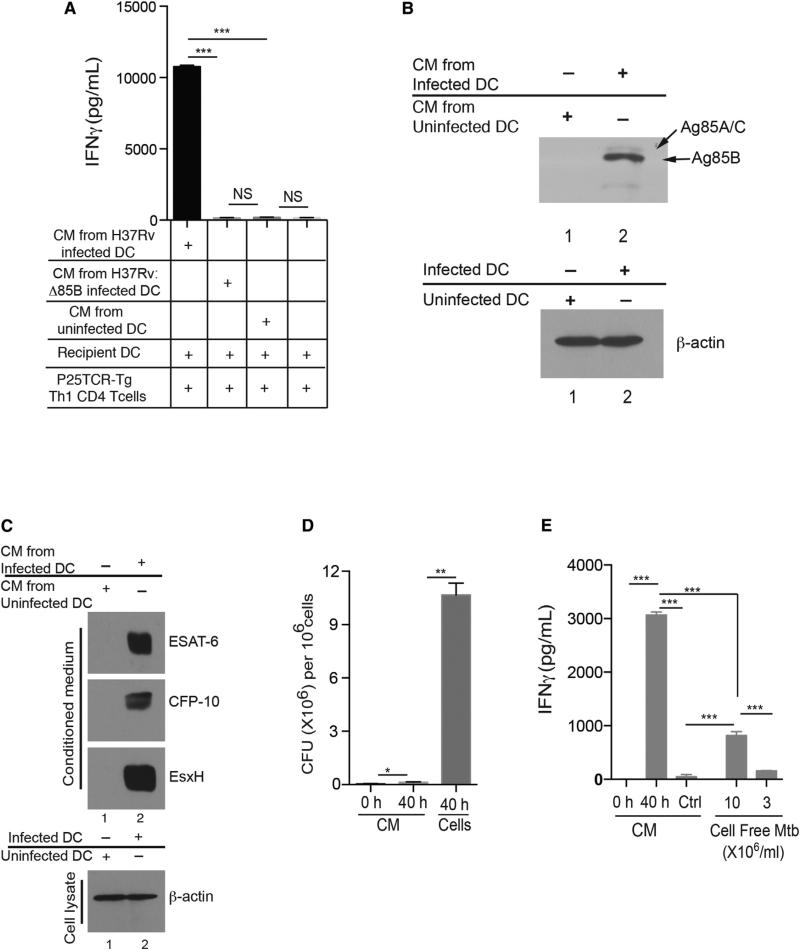
The finding that transfer of M. tuberculosis-infected, MHC II−/− BMDCs into wild-type mice results in activation of naive P25TCR-Tg cells, and that this requires migration of DCs from the lungs to the MDLN, suggests that migratory DCs transfer antigen to resident lymph node DCs. To determine whether M. tuberculosis-infected DCs release Ag85B for uptake by other cells, we examined conditioned medium (CM) from M. tuberculosis-infected BMDCs. CM that was filtered to remove bacteria and then transferred to uninfected BMDCs stimulated slight yet significant proliferation of naive P25TCR-Tg CD4 T cells (Figures S3A and S3B) and stimulated robust IFNg secretion from P25TCR-Tg Th1 effector cells (Figure 3A). Stimulation of P25TCR-Tg Th1 cells by CM-treated BMDC required infection of the donor DCs by M. tuberculosis that expressed Ag85B, as BMDCs treated with CM obtained from DCs infected with Ag85B null M. tuberculosis (H37Rv:Δ85B) (Figure 3A) failed to stimulate P25TCR-Tg Th1 cells. We confirmed the presence of Ag85B in CM obtained from infected BMDCs by immunoblot and found that Ag85B in CM is full-length, unprocessed protein (Figure 3B). We also detected other M. tuberculosis proteins in CM from infected cells: ESAT-6 and CFP-10 (secreted by the ESX-1 system) and EsxH (secreted by ESX-3) (Figure 3C). These data indicate that infected BMDCs release Ag85B and other antigenic bacterial proteins into the extracellular environment, where they can be taken up by uninfected DCs and presented to CD4 T cells.
Figure 3. M. tuberculosis-Infected DCs Release Ag85B for Uptake and Presentation by Uninfected DCs to CD4 T Cells.
(A–E) Conditioned medium (CM) was collected 40 hr after infection of DCs with M. tuberculosis (moi = 2), sterile filtered, and added to uninfected DCs along with Th1-polarized P25TCR-Tg cells. Sixty hours later, IFNg was quantitated in supernatants. (A) Antigen-dependent IFNg secretion by P25TCR-Tg CD4+ Th1 T cells cultured with conditioned medium collected from uninfected DCs or DCs infected with either wild-type M. tuberculosis (H37Rv) or Ag85B null M. tuberculosis (H37Rv:Δ85B). (B) Immunoblot of Ag85B in conditioned medium from M. tuberculosis-infected DCs (lane 2) or uninfected DCs (lane 1). (C) Immunoblot showing presence of ESAT-6, CFP-10, and EsxH in conditioned medium (CM) collected from infected DCs (moi 2) (lane 2) and uninfected DCs (lane 1) after 40 hr. The infected DCs and uninfected DCs used to generate CM were lysed and probed for actin expression. (D) Quantitation of bacteria in: CM from M. tuberculosis-infected DCs at time = 0 (CM = 0 hr); CM from infected DCs after 40 hr of culture (CM = 40 hr); or in lysates of DCs infected and cultured for 40 hr (cells = 40 hr). (E) IFNg secretion by P25 TCR-Tg Th1 CD4+ T cells when cocultured with uninfected DCs in the presence of: CM collected immediately after addition of fresh medium to M. tuberculosis-infected BMDCs (0 hr); CM collected after 40 hr (40 hr); media from bacteria cultured without cells at 3 × 106/ml or 10 × 106/ml in BMDC media. Data are expressed as mean ± SEM of three replicates and represent 3–4 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. See also Figure S3.
Antigenic Activity in CM Is Derived from Intracellular Bacteria
To evaluate the possibility that Ag85B produced by cell-free bacteria might contribute to the antigenic activity in CM, we compared the number of bacteria in BMDCs and bacteria in the medium after 40 hr of infection. More than 95% of the bacteria were associated with the DCs and not free in the medium (Figure 3D). We also compared antigenic activity in medium from M. tuberculosis-infected BMDCs and in medium containing an equivalent number of bacteria in the absence of BMDCs and found that the latter stimulated < 10% the amount of IFNg from P25TCR-Tg Th1 cells after addition to uninfected BMDCs (Figure 3E). Therefore, cell-free bacteria make an insignificant contribution to the Ag85B present in CM from M. tuberculosis-infected DCs, and the bulk of Ag85B in the extracellular medium is derived from bacteria residing in the DCs.
M. tuberculosis-Infected Migratory DCs Transfer Antigen to Lymph Node Antigen-Presenting Cells In Vivo
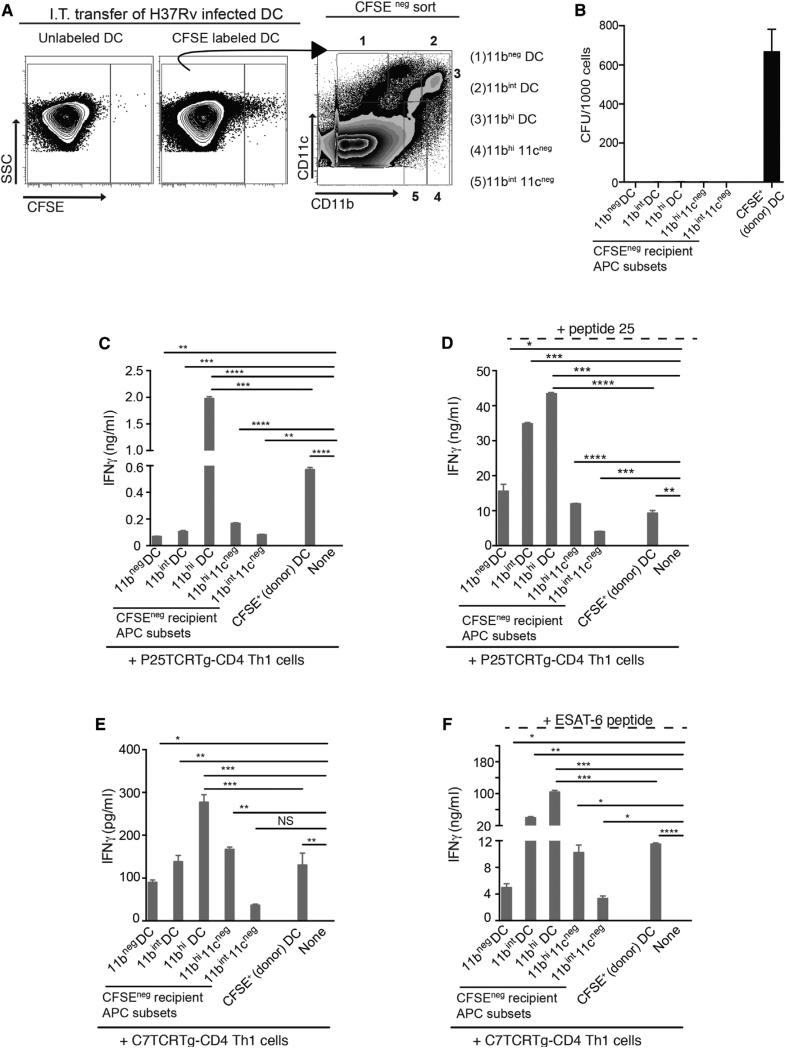
The evidence that administration of infected MHC II−/− BMDCs to wild-type mice resulted in activation of naive antigen-specific CD4 T cells in the MDLN, together with the finding that M. tuberculosis-infected BMDCs release antigen to extracellular culture medium in vitro, suggested that infected cells release antigen for uptake and MHC II antigen presentation by other cells. To determine whether M. tuberculosis-infected DCs transfer antigen to lymph node resident DCs in vivo, we labeled infected BMDCs with CFSE and transferred them intratracheally to wild-type mice. After 60 hr, we isolated MDLN cells from the recipient mice and used fluorescent cell sorting to separate the CFSE+ (donor, infected) BMDCs and the CFSE− recipient MDLN DCs and macrophage subsets (Wolf et al., 2007) (Figure 4A). Following isolation from the MDLN, the CFSE+ (donor) cells contained ~700 M. tuberculosis cfu/1,000 sorted cells, while the CFSE− (recipient) MDLN cells contained % 0.2 cfu/ 1,000 sorted cells (Figure 4B). The CFSE− recipient MDLN DCs and macrophage subsets all activated P25TCR-Tg CD4+ Th1 cells, although the individual subsets varied in their effectiveness over a 10-fold range (Figure 4C). Likewise, the same recipient cell subsets activated ESAT-63-15-specific C7 transgenic TCR CD4 Th1 cells with similar relative efficacy (Figure 4E). Since the only source of antigen for activation of CD4 T cells was from bacteria that were administered in the CFSE-labeled BMDCs, the results indicate that bacterial antigens were transferred from the infected migratory BMDCs to the resident DCs and macrophages in the MDLN. In these experiments, the CFSE+ BMDCs that were administered intratracheally and recovered from the MDLN were also able to stimulate P25TCR-Th1 CD4 T cells, although they were less effective than the 11chi 11bhi subset (11bhi DCs) of resident lymph node DCs (Figures 4C and 4E). Addition of exogenous peptide 25 or ESAT-63-15 peptide to each of the sorted CFSE+ and CFSE− mononuclear cell subsets increased responses of the respective epitope-specific CD4 T cells (Figures 4D and 4F); the relative efficiency of the individual cell subsets was similar to that observed in the absence of added peptide. Together, these results indicate that bacterial antigens are transferred to resident lymph node cells in the absence of transfer of the bacteria.
Figure 4. Infected BMDCs Transfer Antigen to Recipient Lymph Node Mononuclear Cells In Vivo.
(A–F) Naive wild-type mice received M. tuberculosis-infected, CFSE-labeled BMDCs intratracheally; 48 hr later MDLNs were harvested from 9 mice, pooled, stained with antibodies to CD11c, CD11b, and Gr-1, and sorted by FACS into subsets that were used to stimulate P25TCR-Tg Th1 CD4 cells. Responses were quantitated as IFNg secretion. (A) Flow cytometry plots and sorting strategy for CFSE+, M. tuberculosis-infected (donor) BMDCs, and CFSE− (recipient) MDLN cells after gating out Gr-1hiCD11bhi neutrophils. Cells from mice that received unlabeled infected BMDCs were used to set CFSE+ and CFSE− gates. (B) Quantitation of bacteria in FACS-sorted MDLN cell subsets used to stimulate CD4 T cells in (C)–(F). Sorted cells were serially diluted and plated in triplicate, and colonies were counted 21 days later. (C) IFNg secretion by P25TCR-Tg Th1 CD4 cells (1 APC: 15 T cells) after 4 days of stimulation by sorted CFSE− subsets or by infected CFSE+ BMDC. (D) Same cells and conditions as in (C), with the addition of Ag85B peptide 25 (1 μg/ml). (E) IFNg secretion by ESAT-63-15-specific C7TCRTg Th1 CD4 cells (1 APC: 15 T cells) after 4 days of stimulation by sorted CFSE− subsets or by infected CFSE+ BMDCs. (F) Same cells and conditions as in (E), with the addition of ESAT-63-15 peptide (1 μg/ml). Data are expressed as mean ± SEM of replicates and represent two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001.
Apoptosis and/or Apoptotic Vesicles Do Not Account for Antigen Transfer during Tuberculosis Infection
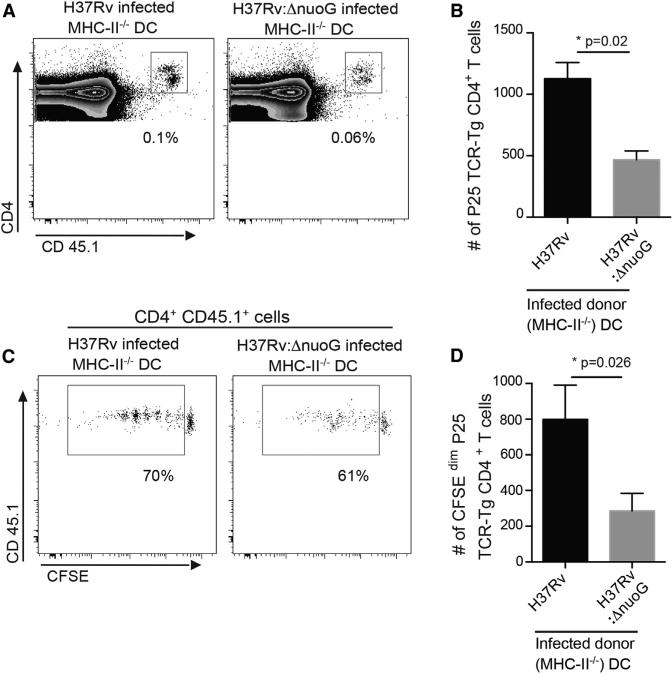
To determine whether apoptosis of infected migratory DCs, followed by uptake of apoptotic cell fragments, accounts for antigen transfer in vivo, we first used fluorescence microscopy examination of sorted resident lymph node cells from the experiment described above to detect cell fragments acquired from the donor CFSE+-infected BMDCs. This revealed that fewer than 1% of the resident cells contained green fluorescent cell fragments. This low frequency was similar in all of the cell subsets and did not correlate with the relative ability of the cells in the individual subsets to activate CD4 T cells (Figures 4C and 4E). To determine whether enhancing apoptosis of infected migratory DCs would increase antigen transfer and T cell activation, we compared activation of P25TCR-Tg CD4 cells in wild-type mice after intratracheal administration of MHC II−/− BMDCs infected with H37Rv or with a well-characterized proapoptotic strain of M. tuberculosis (H37Rv:ΔnuoG) (Blomgran et al., 2012; Velmurugan et al., 2007). Administration of BMDCs infected with the proapoptotic NuoG-deficient mutant resulted in less, not more, proliferation of P25TCR-Tg CD4 T cells than did administration of BMDCs infected with wild-type bacteria (Figures 5A–5D). We confirmed that BMDCs infected with NuoG-deficient M. tuberculosis (H37Rv:ΔnuoG) underwent enhanced apoptosis (Figure S4A) and determined that the reduced ability of BMDCs infected with H37Rv:ΔnuoG to activate naive CD4 T cells was not due to differences in the number of bacteria in the transferred cells (Figure S4B) or the number of bacteria in the MDLN at the time of harvest (Figure S4C). These results suggest that apoptosis contributes little, if at all, to the cell-to-cell antigen transfer that promotes priming of naive CD4 T cells.
Figure 5. Increasing Apoptosis of Migratory DCs Does Not Enhance Antigen-Specific CD4 T Cell Proliferation In Vivo.
(A) Representative FACS plots showing the frequency of adoptively transferred P25TCR-Tg CD4+ T cells in the MDLN of wild-type C57BL/6 mice, 60 hr after intratracheal transfer of MHCII−/− DCs infected with H37Rv or H37Rv:DnuoG (moi 0.5:1; 5 × 105 bacteria/1 × 106 DC/mouse).
(B) Quantitation of total P25TCR-Tg CD4+ T cells in the MDLNs of the groups of mice shown in (A).
(C) Proliferation profile of CFSE-labeled P25TCRTg CD4+ T cells in the MDLNs of groups of mice shown in (A) and (B).
(D) Quantitation of P25TCR-Tg CD4+ T cells that have undergone at least one cycle of proliferation (CFSEdim) in MDLNs of groups of mice shown in (A)–(C). Data are mean ± SEM of three pools of mice (n = 6) per experimental group and represent two independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001. See also Figure S4.
To further assess the potential contribution of apoptosis to antigen transfer, we compared the antigenic activity in conditioned medium from cells infected with the proapoptotic NuoG-deficient strain or with wild-type H37Rv. Unfractionated conditioned medium (CM) from BMDCs infected with H37Rv:ΔnuoG contained significantly less antigenic activity than did CM from BMDCs infected with H37Rv (Figures S4D). This suggests that apoptosis does not account for the release of antigen from infected cells and is consistent with the results of administering BMDCs infected with proapoptotic or wild-type bacteria on activation of naive CD4 T cells in vivo (Figure 5). When we ultracentrifuged CM and examined the antigenic activity in the supernatants and pellets (containing apoptotic vesicles and exosomes), we found that the antigenic activity was most abundant in the supernatant fraction of CM from cells infected with wild-type bacteria. Unfractionated CM from cells infected with the proapoptotic bacteria contained less antigenic activity than CM from cells infected with wild-type bacteria, and even less of the antigenic activity from cells infected with proapoptotic bacteria was present in the supernatant, while a larger proportion appeared in the pellet (Figure S4D). This finding indicates that Ag85B can be shed in apoptotic vesicles, but that antigen released in a soluble form accounts for most of the uptake and presentation to CD4 T cells.
Uninfected Lymph Node Antigen-Presenting Cells Acquire and Present M. tuberculosis Antigen to CD4 T Cells after Aerosol Infection
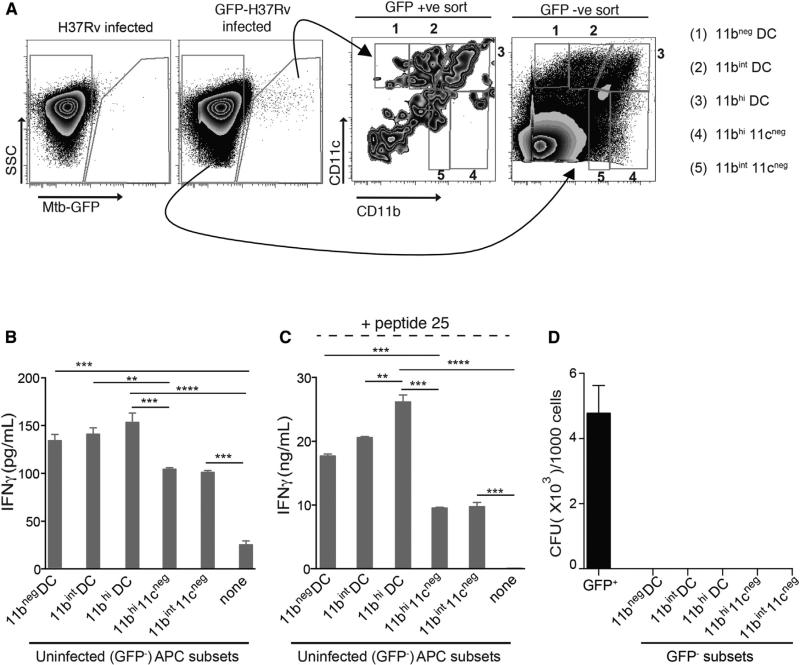
Although our evidence for in vivo M. tuberculosis antigen transfer from infected to uninfected DCs is persuasive, it depended on intratracheal administration of dendritic cells. To determine whether Ag85B is transferred to uninfected lymph node cells after infection by the natural route (aerosol exposure to a low bacterial inoculum), we infected mice with GFP-expressing M. tuberculosis and flow-sorted infected (GFP+) and uninfected (GFP−) live myeloid cells from the MDLNs (Figure 6A). The GFP− fraction was sorted into previously defined CD11c/ CD11b APC subsets (Wolf et al., 2007) and then used to stimulate P25TCR-Th1 CD4 T cells ex vivo without addition of exogenous antigen (Figure 6B). This revealed that the uninfected (i.e., GFP−) MDLN cells activated Ag85B-specific CD4 T cells, and of these, 11bhi DCs, 11bint DCs, and 11bneg DCs were more effective at activating P25TCR-Th1 cells than were GFP− 11cneg11bhi and 11cneg11bint cells (Figure 6B). These results confirm the results obtained by intratracheal transfer of infected BMDCs and indicate that uninfected cells acquire Ag85B in vivo from infected cells in the MDLN of M. tuberculosis-infected mice after low-dose aerosol infection. Acquisition of antigen by uninfected cells did not involve transfer of bacteria, as live bacteria were undetectable in the GFP− cells, while bacteria were readily detected in the sorted GFP+ cells (Figure 6D). Due to the scarcity of GFP+ cells in the lymph node after aerosol infection, we were unable to assay their ability to activate antigen-specific CD4 T cells. Following addition of exogenous peptide 25, the GFP− MDLN cell subsets exhibited a similar trend in activating P25TCR-Th1 cells, albeit with greater effectiveness, implying that these cells were not saturated with antigen in vivo (Figure 6C). Together with our previous finding that M. tuberculosis occupies a small minority of the DCs and macrophages in the MDLN after aerosol infection (Wolf et al., 2007), these results suggest that CD4 T cell activation in the MDLN is driven in large part by uninfected DCs that acquire M. tuberculosis antigens from infected cells and present them to CD4 T cells in the MDLN.
Figure 6. Transfer of Ag85B without Pathogen Transfer to Resident Antigen-Presenting Cells in the MDLN after Aerosol Infection.
(A–D) MDLNs were harvested and pooled from 9–10 mice 15 days post-aerosol infection with GFP-expressing H37Rv (~200 cfu/mouse). Lymph node cells were stained with CD11c, CD11b, and Gr-1-specific antibodies, sorted by FACS, and subsequently used to stimulate P25TCR-Tg Th1 CD4 cells. (A) FACS plot and sorting strategy for GFP-positive (infected) and GFP-negative (uninfected) MDLN cells based on CD11c/CD11b-defined subsets after neutrophils (Gr-1hiCD11bhi) were gated out. (B) IFNg secretion by P25TCR-Tg Th1 CD4 cells (1 APC: 15 T cells) after stimulation by GFP-negative (uninfected) sorted CD11c/ CD11b-defined cell subsets. (C) Same cells and conditions as in (B), with the addition of Ag85B peptide 25 (1 μg/ml). (D) Quantitation of bacteria in MDLN cell subsets used to stimulate CD4 T cells in (B) and (C). Sorted cells were serially diluted and plated in triplicate or quadruplicate; colonies were counted 21 days after plating. Data are expressed as mean ± SEM of replicates. A similar experiment yielded comparable results. *p < 0.05; **p < 0.01; ***p < 0.001. See also Figure S5.
DISCUSSION
Recent studies of Aspergillus, Blastomyces, and M. tuberculosis respiratory infections have clearly established that transport of the pathogen from the lungs to the local draining lymph node is essential for priming pathogen-specific CD4 T cells (Wolf et al., 2008) and have indicated that migratory inflammatory monocytes or DCs cooperate with resident lymph node DCs to initiate CD4 T cell responses (Ersland et al., 2010; Hohl et al., 2009; Samstein et al., 2013; Wüthrich et al., 2012). However, the mechanisms of cooperation between migratory and resident antigen-presenting cells have not been elucidated. Here, we present evidence that migratory DCs release M. tuberculosis antigens as soluble undegraded proteins, for uptake and presentation by resident lymph node cells, to optimize priming of antigen-specific CD4 T cells. We also found that antigen release from infected cells occurs by a mechanism distinct from apoptosis or exosome shedding. Especially notable is our finding that antigen transfer occurs in vivo without transfer of the pathogen. In that regard, the results reported here extend the recently published findings that inflammatory monocytes, which acquire DC characteristics during migration, transport M. tuberculosis from the lungs to the local lymph node, but must cooperate with classical DCs in the lymph node to prime ESAT-6-specific CD4 T cells (Samstein et al., 2013). Our results extend those studies by providing evidence for a mechanism of antigen export that is independent of apoptosis or exosomes, involves transfer of full-length unprocessed antigen, and occurs without transfer of the pathogen.
Studies of pathogen-specific T cell priming have indicated that antigens can be transferred from migratory to resident lymph node DCs, yet the mechanisms of antigen transfer have received less attention. One mechanism of antigen transfer involves apoptosis of pathogen-containing cells, followed by antigen cross-presentation to prime CD8 T cells (Albert et al., 1998). Apoptosis and cross-presentation clearly contribute to the priming of CD8 T cells in the context of vaccination or infection with M. tuberculosis (Divangahi et al., 2010; Hinchey et al., 2007; Schaible et al., 2003; Winau et al., 2006). Apoptosis has also been found to promote priming of CD4 T cells after infection with M. tuberculosis (Divangahi et al., 2010) or Salmonella (Yrlid and Wick, 2000). We considered whether apoptosis could account for antigen transfer in our assays and found that: (1) antigen did not sediment with apoptotic vesicles; (2) compared with wild-type bacteria, infection with a proapoptotic mutant of M. tuberculosis led to a decrease, rather than an increase, in the amount of antigen released; and (3) intratracheal transfer of MHC II−/− BMDCs infected with the proapoptotic bacterial mutant caused less proliferation of Ag85B-specific CD4 T cells in vivo. Although exosome shedding also causes release of antigens from cells infected with mycobacteria (Cheng and Schorey, 2013; Giri and Schorey, 2008), the bulk of antigen released by infected DCs remained soluble under conditions that sediment exosomes. We also examined the possibility that “cross-dressing” involving transfer of preformed MHC II:peptide complexes (Wakim and Bevan, 2011) could contribute to our observations, but were unable to detect evidence of transfer of MHC II to MHC II−/− cells by flow cytometry. While our results do not exclude contributions of apoptosis or exosome shedding to release of antigens by infected cells and presentation by resident DCs, they demonstrate that one or more additional mechanisms contributes to release of antigen for MHC class II presentation by resident lymph node DCs.
Although we have not yet determined the precise mechanism used by M. tuberculosis-infected cells to export soluble antigens, our finding that the antigens are present in conditioned medium as full-length proteins suggests that they transit through a nondegradative cellular compartment en route to the extracellular space. Consistent with previous findings that Ag85B can be detected in intracellular vesicles distinct from phagosomes (Beatty and Russell, 2000; Harth et al., 1996), our observation that multiple secreted bacterial proteins are released intact from infected cells suggests that a vesicular transport mechanism acquires soluble cargo from the phagosome lumen and transports it to the cell exterior, but more investigation is necessary to determine whether this is the dominant mechanism of antigen release. It is noteworthy that the bacterial proteins that we found released from infected dendritic cells are among the immunodominant antigens of M. tuberculosis; whether release from infected cells and uptake and efficient presentation by other cells contributes to their immunodominance warrants consideration, as it may guide efforts to stratify M. tuberculosis antigens for vaccine development. In addition, it will be important to determine whether antigen release from infected cells is restricted to secreted bacterial proteins, or whether somatic bacterial proteins are released with similar efficiency.
The observation that pertussis toxin treatment of infected BMDCs prior to intratracheal administration completely blocked proliferation of Ag85B-specific CD4 T cells in the lymph node indicates that antigen transfer occurs after infected cells migrate to the lymph node, rather than in the lungs prior to migration. If antigen transfer had occurred in the lungs, endogenous lung DCs (i.e., not exposed to pertussis toxin) would have transported it to the lymph node. That antigen transfer occurs after migration of infected cells to the lymph node likely allows for more efficient uptake of the antigen that is released, considering the high density of DCs in the lymph node. That antigen transfer occurs in the lymph node and not the lungs also helps to explain why it is advantageous for migratory monocytes or DCs to transport live bacteria for antigen production in the lymph node, despite the presence of 100-fold more bacteria in the lungs (Samstein et al., 2013; Wolf et al., 2008).
Earlier studies revealed release of mycobacterial lipids from infected macrophages in culture; those lipids include trehalose dimycolate and others with proinflammatory activity (Beatty and Russell, 2000; Bhatnagar and Schorey, 2007; Bhatnagar et al., 2007; Harth et al., 1996). If this process also occurs in migratory DCs and results in transfer of mycobacterial lipids to resident DCs in the lymph node, it can provide potent adjuvants to accompany release of the antigenic proteins that we have detected and further optimize priming of naive M. tuberculosis antigen-specific CD4 T cells. In light of our findings and the results of studies revealing release of adjuvant lipids, the evidence that uninfected cells are the predominant sources of IL-12/23p40 in secondary lymphoid tissues of M. tuberculosis-infected mice (Rothfuchs et al., 2009) implies that the major steps required for priming and differentiation of antigen-specific CD4 T cells in tuberculosis are accomplished by uninfected cells that acquire signals and antigens from infected cells.
Our finding that M. tuberculosis antigens can be transferred to lymph node DCs without transfer of the bacteria is especially important, since there is considerable evidence that M. tuberculosis inhibits MHC class II antigen presentation by infected cells (reviewed in Baena and Porcelli, 2009). While inhibiting presentation of antigens by infected cells may provide an evasion mechanism for the bacteria, antigen transfer to uninfected cells provides a mechanism that allows antigen presentation to naive CD4 T cells to occur without the impediment(s) imposed by intracellular infection. Since uninfected DCs vastly outnumber infected DCs in the MDLN (Wolf et al., 2007), we propose that most or all of the priming of naive M. tuberculosis antigen-specific CD4 T cells is accomplished by uninfected cells that have acquired antigen by antigen transfer. This model is supported by the recent report that migratory inflammatory monocytes or DCs are themselves incapable of priming CD4 T cells during M. tuberculosis infection; in that system, only classical DCs were able to perform this function (Samstein et al., 2013). In this case, the principal, if not the only, role for migratory inflammatory monocyte-derived DCs is to transport bacteria from the lungs to the local lymph node. Thus, transfer of bacterial lipids and antigenic proteins from infected to uninfected cells can overcome the processes that M. tuberculosis uses to inhibit antigen presentation and manipulate cytokine secretion, thereby allowing adaptive immune responses to develop and provide protection to infected individuals.
EXPERIMENTAL PROCEDURES
Mice and Tissue Processing
Mice bred and housed in specific pathogen-free facilities were used according to procedures approved by the NYU School of Medicine IACUC. Following infection, MDLN and lungs were harvested, processed, stained, and analyzed by flow cytometry, and bacteria were quantitated, all as described (Wolf et al., 2007, 2008).
In Vivo Antigen Transfer Assay
BMDCs were infected with M. tuberculosis, washed, treated with amikacin (200 μg/ml, 40 min), washed, and administered intratracheally (0.5–1 × 106/ mouse in 100 ml) to mice 1 day after they received CFSE-labeled naive P25TCR-Tg CD4 cells (1–2 × 106/mouse). Sixty hours after BMDC transfer, MDLN and lungs were harvested and processed. When required, infected BMDCs were treated with pertussis toxin (200 ng/ml, 2 hr, 37°C) prior to intratracheal transfer.
Generation of Conditioned Media and In Vitro Antigen Transfer
BMDCs (106/ml) were infected overnight with the designated strain of M. tuberculosis, washed, treated with amikacin, washed twice, and cultured in fresh BMDC medium (RPMI 1640 with 10% heat-inactivated FBS, 2 mM L-glutamine, 1 mM sodium pyruvate, 1X b-ME, 10 mM HEPES, and 15 ng/ml mouse GM-CSF). This medium was then harvested 20–40 hr later, sterile filtered, and called conditioned medium (CM). The CM was then added to uninfected BMDCs (2 × 105/well) in 24-well plates along with P25TCR-Tg RAG-1-deficient CD4+ Th1 effector cells (1–2 × 106/well) for 60 hr, following which supernatants were collected and quantitated for IFNg by ELISA (BD Biosciences).
Ag85B Immunoblotting
CM was fractionated using 50K (to deplete albumin) and 3K Centricon filters (Amicon Ultra; Millipore). The < 50K filtrates were further concentrated using a 3K filter. Samples were separated on a 10% SDS-PAGE, transferred to nitrocellulose (BioRad), and probed with rabbit anti-Ag85B (1:1,000) prepared by immunization with purified recombinant Ag85B (in house) in Titermax Gold (Sigma). Bound anti-Ag85B was detected using HRP-conjugated goat anti-rabbit IgG (1:10,000).
Cell Sorting and Stimulation of P25TCR-Tg CD4 T Lymphocytes
MDLN cells from mice infected with GFP-expressing H37Rv were pooled; stained with CD11c, CD11b, and Gr-1 antibodies as described (Wolf et al., 2007); and live-sorted using an iCyt Synergy sorter in BSL-3 containment. Neutrophils were gated out, followed by separation of GFP+ cells from GFP− cells. The GFP− set was further sorted based on CD11c and CD11b expression and cultured (~40,000/well) with P25TCR-Tg Th1 effector cells (6 × 105/well) in V-bottom 96-well plates. Supernatants were collected at day 4 and assayed for IFNg by ELISA. For peptide presentation, assay conditions were similar except that 1 μg/ml Ag85B peptide 25 was added. An aliquot of sorted cells (GFP+ and GFP−) was cultured to enumerate bacteria. For cell sorting posttransfer of infected CFSE-labeled DC, the sorting procedure was the same as above where after gating out neutrophils, CFSE+ subset and CFSE− CD11c/CD11b subsets were sorted and used to stimulate either P25TCR-Tg or C7TCR-Tg Th1 effector cells (Gallegos et al., 2008) in the absence or presence of (1 μg/ml) respective peptides. Supernatants were assayed at day 4 for IFNg by ELISA. Aliquots of sorted CFSE− and CFSE+ subsets were plated on 7H11 agar to quantitate bacteria.
Statistical Analysis
Statistical comparisons were performed by unpaired Student's t test using Prism 4 for Macintosh (GraphPad, San Diego, CA). P values < 0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sofia Olmos, Ph.D., for assistance during early stages of this work. We also thank Michael Gregory of the NYU Cancer Institute Cytometry and Cell Sorting Facility (supported by 5P30CA016087-31) for help with cell sorting. This work was supported by NIH grants R01 AI051242 and R01 AI084041.
Footnotes
AUTHOR CONTRIBUTIONS
S.S. and J.D.E. planned the experiments; S.S. performed the experiments; S.S. and J.D.E. interpreted the results and wrote the manuscript.
SUPPLEMENTAL INFORMATION
Supplemental Information includes five figures and Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2014.05.007.
REFERENCES
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Baena A, Porcelli SA. Evasion and subversion of antigen presentation by Mycobacterium tuberculosis. Tissue Antigens. 2009;74:189–204. doi: 10.1111/j.1399-0039.2009.01301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Tato A, León B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat. Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WL, Russell DG. Identification of mycobacterial surface proteins released into subcellular compartments of infected macrophages. Infect. Immun. 2000;68:6997–7002. doi: 10.1128/iai.68.12.6997-7002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc. Natl. Acad. Sci. USA. 2004;101:8670–8675. doi: 10.1073/pnas.0402644101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J. Biol. Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt K, Hickman SP, Salgame P. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J. Immunol. 2004;172:2748–2751. doi: 10.4049/jimmunol.172.5.2748. [DOI] [PubMed] [Google Scholar]
- Blomgran R, Ernst JD. Lung neutrophils facilitate activation of naive antigen-specific CD4+ T cells during Mycobacterium tuberculosis infection. J. Immunol. 2011;186:7110–7119. doi: 10.4049/jimmunol.1100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran R, Desvignes L, Briken V, Ernst JD. Mycobacterium tuberculosis inhibits neutrophil apoptosis, leading to delayed activation of naive CD4 T cells. Cell Host Microbe. 2012;11:81–90. doi: 10.1016/j.chom.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold TD, Banaei N, Wolf AJ, Ernst JD. Suboptimal activation of antigen-specific CD4+ effector cells enables persistence of M. tuberculosis in vivo. PLoS Pathog. 2011;7:e1002063. doi: 10.1371/journal.ppat.1002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Schorey JS. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur. J. Immunol. 2013;43:3279–3290. doi: 10.1002/eji.201343727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS ONE. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divangahi M, Desjardins D, Nunes-Alves C, Remold HG, Behar SM. Eicosanoid pathways regulate adaptive immunity to Mycobacterium tuberculosis. Nat. Immunol. 2010;11:751–758. doi: 10.1038/ni.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersland K, Wüthrich M, Klein BS. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell Host Microbe. 2010;7:474–487. doi: 10.1016/j.chom.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallegos AM, Pamer EG, Glickman MS. Delayed protection by ESAT-6-specific effector CD4+ T cells after airborne M. tuberculosis infection. J. Exp. Med. 2008;205:2359–2368. doi: 10.1084/jem.20080353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri PK, Schorey JS. Exosomes derived from M. Bovis BCG infected macrophages activate antigen-specific CD4+ and CD8+ T cells in vitro and in vivo. PLoS ONE. 2008;3:e2461. doi: 10.1371/journal.pone.0002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harth G, Lee BY, Wang J, Clemens DL, Horwitz MA. Novel insights into the genetics, biochemistry, and immunocytochemistry of the 30-kilodalton major extracellular protein of Mycobacterium tuberculosis. Infect. Immun. 1996;64:3038–3047. doi: 10.1128/iai.64.8.3038-3047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, Chan J, Braunstein M, Orme IM, Derrick SC, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Invest. 2007;117:2279–2288. doi: 10.1172/JCI31947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Turley S, Yamaide F, Iyoda T, Mahnke K, Inaba M, Pack M, Subklewe M, Sauter B, Sheff D, et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 1998;188:2163–2173. doi: 10.1084/jem.188.11.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- Kastenmüller W, Torabi-Parizi P, Subramanian N, Laämmermann T, Germain RN. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell. 2012;150:1235–1248. doi: 10.1016/j.cell.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khader SA, Partida-Sanchez S, Bell G, Jelley-Gibbs DM, Swain S, Pearl JE, Ghilardi N, Desauvage FJ, Lund FE, Cooper AM. Interleukin 12p40 is required for dendritic cell migration and T cell priming after Mycobacterium tuberculosis infection. J. Exp. Med. 2006;203:1805–1815. doi: 10.1084/jem.20052545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan CK, Ernst JD. HIV and tuberculosis: a deadly human syndemic. Clin. Microbiol. Rev. 2011;24:351–376. doi: 10.1128/CMR.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount AM, Smith CM, Kupresanin F, Stoermer K, Heath WR, Belz GT. Multiple dendritic cell populations activate CD4+ T cells after viral stimulation. PLoS One. 2008;3:e1691. doi: 10.1371/journal.pone.0001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos S, Stukes S, Ernst JD. Ectopic activation of Mycobacterium tuberculosis-specific CD4+ T cells in lungs of CCR7−/− mice. J. Immunol. 2010;184:895–901. doi: 10.4049/jimmunol.0901230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Annu. Rev. Pathol. 2012;7:353–384. doi: 10.1146/annurev-pathol-011811-132458. [DOI] [PubMed] [Google Scholar]
- Rothfuchs AG, Egen JG, Feng CG, Antonelli LR, Bafica A, Winter N, Locksley RM, Sher A. In situ IL-12/23p40 production during mycobacterial infection is sustained by CD11bhigh dendritic cells localized in tissue sites distinct from those harboring bacilli. J. Immunol. 2009;182:6915–6925. doi: 10.4049/jimmunol.0900074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. eLife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible UE, Winau F, Sieling PA, Fischer K, Collins HL, Hagens K, Modlin RL, Brinkmann V, Kaufmann SH. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat. Med. 2003;9:1039–1046. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- Velmurugan K, Chen B, Miller JL, Azogue S, Gurses S, Hsu T, Glickman M, Jacobs WR, Jr., Porcelli SA, Briken V. Mycobacterium tuberculosis nuoG is a virulence gene that inhibits apoptosis of infected host cells. PLoS Pathog. 2007;3:e110. doi: 10.1371/journal.ppat.0030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakim LM, Bevan MJ. Cross-dressed dendritic cells drive memory CD8+ T-cell activation after viral infection. Nature. 2011;471:629–632. doi: 10.1038/nature09863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters WR, Palmer MV, Thacker TC, Davis WC, Sreevatsan S, Coussens P, Meade KG, Hope JC, Estes DM. Tuberculosis immunity: opportunities from studies with cattle. Clin. Dev. Immunol. 2011;2011:768542. doi: 10.1155/2011/768542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, Sandhoff K, Brinkmann V, Kaufmann SH, Schaible UE. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24:105–117. doi: 10.1016/j.immuni.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Linas B, Trevejo-Nuñez GJ, Kincaid E, Tamura T, Takatsu K, Ernst JD. Mycobacterium tuberculosis infects dendritic cells with high frequency and impairs their function in vivo. J. Immunol. 2007;179:2509–2519. doi: 10.4049/jimmunol.179.4.2509. [DOI] [PubMed] [Google Scholar]
- Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich M, Ersland K, Sullivan T, Galles K, Klein BS. Fungi subvert vaccine T cell priming at the respiratory mucosa by preventing chemokine-induced influx of inflammatory monocytes. Immunity. 2012;36:680–692. doi: 10.1016/j.immuni.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yrlid U, Wick MJ. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 2000;191:613–624. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.