Abstract
Recent advances in intestinal microbiota research are the background for the appearance of functional foods. Lactic fermentation products are included in the functional foods and classified into 3 groups based on their mechanisms of action: probiotics, prebiotics and biogenics. Probiotics are viable microorganisms, such as lactobacilli and bifidobacteria, that beneficially affect the host by improving the intestinal bacterial balance. Prebiotics are nondigestible food ingredients, such as oligosaccharides and dietary fiber, that beneficially affect the host by selectively stimulating the growth or activities of beneficial intestinal bacteria in the colon and thus improve the health of the hosts. Biogenics are biologically active peptides, including immunopotentiators (biological response modifier: BRM), plant flavonoids, etc. They act directly or indirectly through modulation of intestinal microbiota on the health of the hosts. Thus, functional foods enhance bioregulation such as stresses, appetite and absorption; biodefence, such as immunity and suppression of allergies; prevent diseases, including diarrhea, constipation, cancer, cholesterolemia and diabetes; and suppress aging through immunostimulation as well as suppression of mutagenesis, carcinogenesis, oxidation processes, intestinal putrefaction, and cholesterolemia.
Keywords: intestinal microbiota, intestinal bacteria, intestinal bacteriology, functional foods
BACKGROUND OF APPEARANCE OF FUNCTIONAL FOODS
Lifestyle-related diseases and enforcement of FOSHU
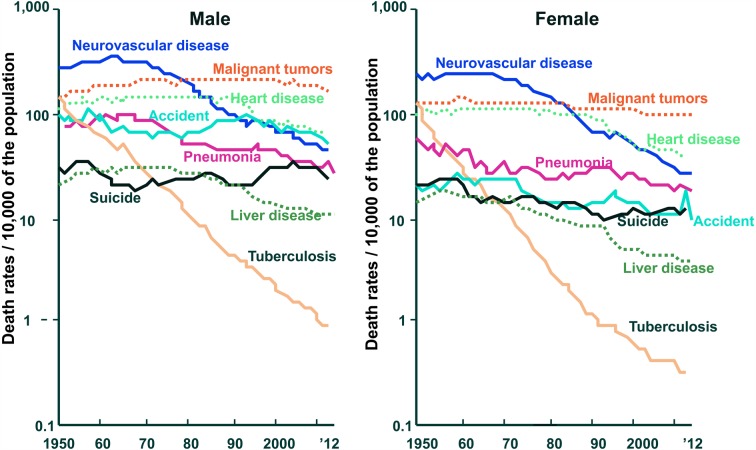
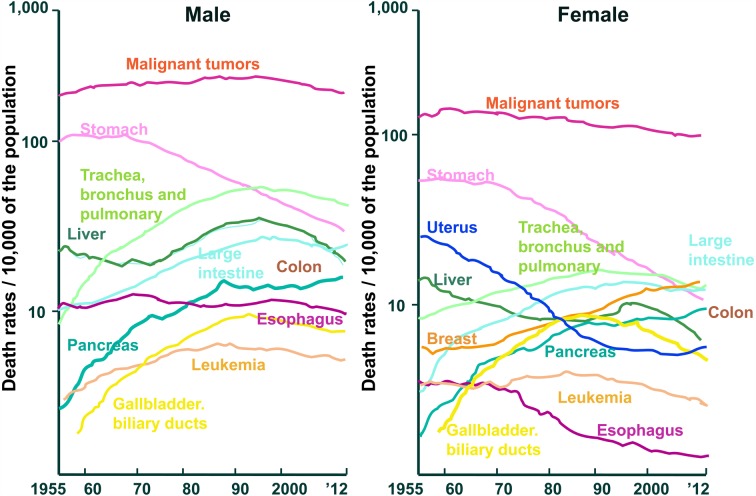
After the 2nd world war, Japanese eating habits westernized. This resulted in the causes of death being more like those in Europe and America. Death from cerebral hemorrhage has decreased, and death from heart disease has increased. Many people live long enough to be troubled by lifestyle-related diseases such as cerebrovascular disease, heart disease, high blood cholesterol, hypertension, osteoporosis, diabetes, and cancer. These diseases occur because of changes in dietary habits. It has become well established that foods and food components contribute to physiological and biological well-being (Fig. 1) [1]. Furthermore, the cancer pattern in the Japanese changed: stomach cancer decreased, and colorectal cancer and breast cancer rapidly increased (Fig. 2) [1].
Fig. 1.
Changes in mortality due to selected causes among the Japanese population (Association for Health and Welfare Statistics).
Fig. 2.
Changes in mortality due to malignant tumors in selected sites of among the Japanese (Association for Health and Welfare Statistics).
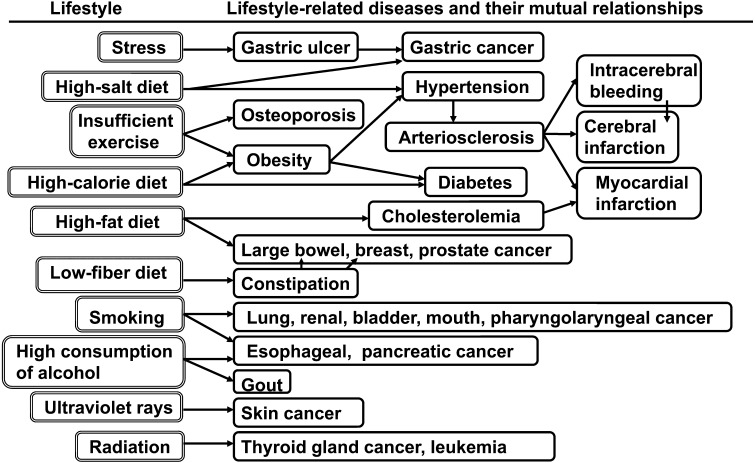
Advances in medical science in the past 5 decades have significantly increased the human life-span. The causes of death in Japan have begun to resemble those in the West. Death from cerebral hemorrhage has decreased, and death from heart disease has increased. Many people live long enough to be troubled by lifestyle-related diseases such as cerebrovascular disease, heart disease, high blood cholesterol, hypertension, osteoporosis, diabetes, liver cirrhosis and cancer. These diseases occur because of changes in dietary habits. It has become well established that foods and food components contribute to physiological and biological well-being (Fig. 3).
Fig. 3.
Lifestyle-related diseases and their mutual relationships.
Recent advances in intestinal flora research are also the background for the appearance of functional foods. Optimal intestinal flora are achieved by a nutritionally well-balanced diet and an active intake of functional foods, such as oligosaccharides, dietary fiber, and fermented milks, which promote beneficial bacteria or suppress harmful bacteria.
In 1991, the Ministry of Health and Welfare started the Food for Specified Health Use (FOSHU) system. This system should help prevent lifestyle-related diseases and reduce their effects, and the foods included in it are equivalent to “functional foods” in Japan. Functional foods promote health by improving well-being (mental and physical conditioning) and reducing the risk of diseases. To be more concrete, these foods are thought to generate such useful effects as bioregulation including central action, peripheral action, appetite and absorption; biodefense including immunostimulation and suppression of allergies; and prophylaxis against hypertension, diabetes, cancer, hypercholesterolemia, anemia, and platelet aggregation. They are also thought to prevent lifestyle-related diseases through a reduction in reactive oxygen species (ROS).
HISTORY OF LACTIC ACID FERMENTATION PRODUCTS
History of fermented milks
Fermented milks made from cow’s milk or other milks are found from Europe to Asia and parts of Africa. They have been viewed as outstanding preservative foods having health effects since prehistoric times. The typical fermented milks in the world include villi (Scandinavia), kefir and koumiss (Russia), yogurt (Eastern Europe), ayrag (Mongolia) and dahi (India and Nepal).
Metchnikoff’s “theory of longevity”
In the early 20th century, Elie Metchnikoff (1845–1916, 1908 Nobel prize winner for physiology) [2] turned his attention to aging as an extension of his work on phagocytes. The basic idea was that aging was caused by chronic intoxication due to toxins produced by intestinal bacteria and to prevent aging and perhaps also arteriosclerosis, it was necessary to remove the harmful bacteria from the intestine. This, he thought, might be achieved by diet therapy, that is, introducing lactic acid bacteria into the intestine. He drew support for this from the fact that longevity was common in a Bulgarian region where yogurt was eaten as a daily food. This is referred to as the “theory of longevity by yogurt”. The concept of “probiotics” had already been proposed by Metchnikoff at this time.
Proposal of acidophilus milk and bifidus milk
When Metchnikoff recommended drinking yogurt, he thought that the lactic acid bacteria in the yogurt would be transplanted into the intestines, would proliferate and would inhibit putrefying bacteria. However, it became clear that the L. bulgaricus in the yogurt did not colonize in the intestine, and the “theory of longevity by yogurt” was temporarily put to the side; subsequently, the intestinal lactobacilli L. acidophilus became the focus of attention. With this providing momentum and based on the idea of making fermented milk using lactic acid bacteria that could multiply in the intestine, Rettger et al. [3] and Henneberg [4] proposed acidophilus milk and Reformjoghurt, respectively. Moreover, Biogurt, Aco-yoghurt, Acidophilus Yoghurt, etc., were also made. However, it was reported that L. acidophilus was not even detected in some fermented milk. It is thought that this might have resulted from the fact that the classification of Lactobacillus was obscure. Moreover, it seems that it was falsely believed that consumed lactic acid bacteria would colonize the intestine.
In the 1960s, it was discovered that Bifidobacterium constituted a predominant lactic acid bacteria in the intestine, not only in babies but also in children, adults and elderly people. Thus, fermented milks and lactic drinks containing bifidobacteria were developed and marketed.
History of fermented milks in Japan
In the 5th century, cow’s milk began to be used in Japan. Techniques to process cow’s milk were brought from Kudara (Korea) to Japan. Around the 6th century, national dairy stock farms were established throughout the country, and goods, such as Raku, So, and Daigo were produced. Raku is considered to have been the yogurt of the day.
In 1919, Unkai Mishima started to sell a pasteurized lactic milk drink, Calpis, after getting the idea from koumis, which is from Mongolia. Such beverages were widely distributed as original Japanese beverages.
On the other hand, Kakutaro Masagaki was influenced by Metchnikoff’s theory of longevity by yogurt, and founded the first yogurt business, Tenjukai in Japan in cooperation with his son, Kazuyoshi Masagaki, in 1914. In 1924, Masagaki brought a rich lactic drink, Elie, to market. It was fermented for 120 hours using L. bulgaricus, L. acidophilus, Lactococcus sp. and yeasts, and the bacterial species used were later increased from 4 kinds to 8 kinds. In 1945, Kazuyoshi Masagaki began developing a lactic fermentation product using 16 kinds of lactic acid bacteria and soy milk instead of cow’s milk under the direction of Kohzui Ohtani, a priest of Honganji sect. Masagaki successfully developed lactic fermentation products and began to sell Chitsuh. Today, there are various kinds of lactic fermentation products available, but the bacterial species used and manufacturing methods are rarely disclosed.
TYPES OF LACTIC ACID FERMENTATION PRODUCTS
Types of lactic acid bacteria fermentation products
Lactic acid bacteria fermentation products (lactic fermentation products) are products fermented with lactic acid bacteria or lactic acid bacteria and yeasts are classified into 4 types as shown in Table 1.
Table 1. Differences among fermented milks, lactic milk drinks, pasteurized lactic milk drinks and lactic acid bacteria products.
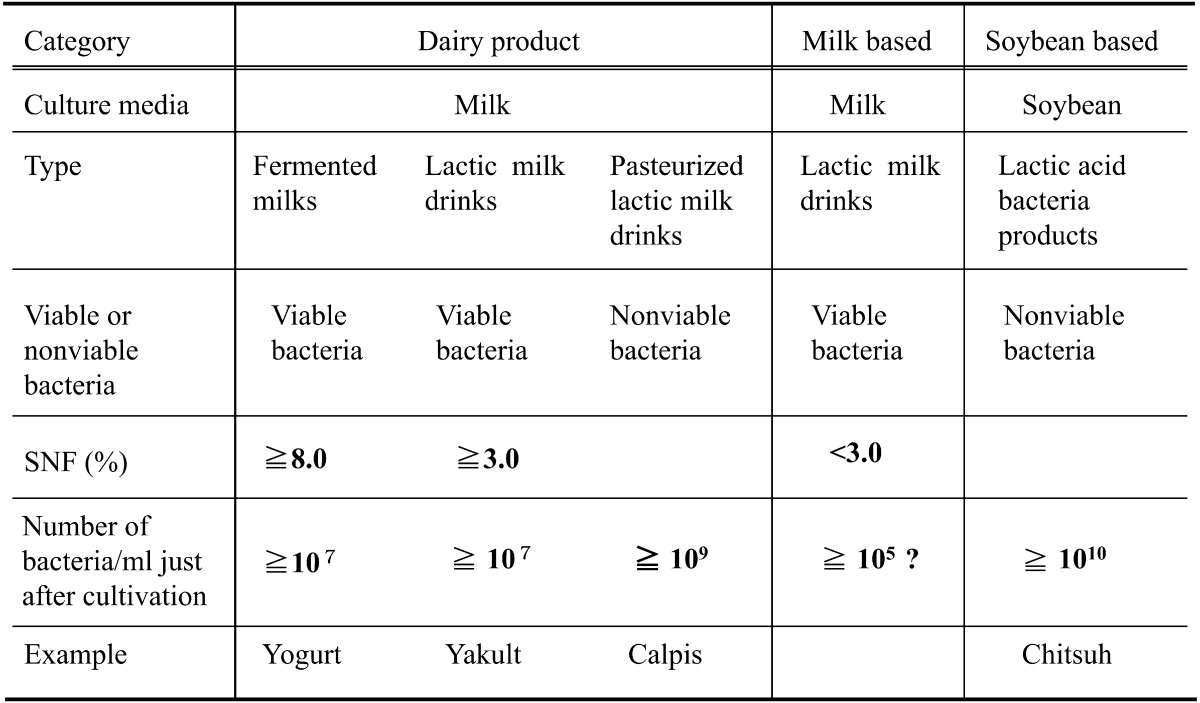
A representative fermented milk product is yogurt, which is manufactured by fermenting milk with lactic acid bacteria. Fermented milks such as kefir containing 1–3% alcohol are made by adding yeasts. The SNF (solids-not-fat) component of fermented milk is more than 8%, the same as in milk. The number of lactic acid bacteria and yeasts in fermented milk is more than 1×107 organisms/ml.
Lactic milk drinks are fermented milk dairy products that have been further processed into drink-type products, with sugar and sometimes flavors added to fermented milk. They are further divided into lactic acid bacteria milk drinks (example: Yakult) and pasteurized lactic acid bacteria milk drinks (example: Calpis). The SNF component of lactic acid drinks is more than 3%. The number of lactic acid bacteria and yeasts in lactic acid drinks is more than 1×107 organisms/ml, the same as in fermented milk.
Lactic milk drinks fermented as dairy products are pasteurized to produce pasteurized lactic acid bacteria drinks and then bottled.
Lactic milk drinks as milk-based foods are processed as lactic milk drinks, but the SNF component of these drinks is less than 3%, and the number of lactic acid bacteria and yeasts is more than 1×105 organisms/ml.
Most lactic acid bacteria products are manufactured by fermenting soybean materials with raw sugar, etc., for a few days using 3–20 kinds of lactic acid bacteria and yeasts and then concentrating the product. Specifications and standards for lactic fermentation products have yet to be established (example: Chitsuh). Since they are manufactured by fermentation of lactic acid bacteria for a few days, the same as pasteurized lactic milk drinks, the numbers of viable and dead bacteria after the completion of fermentation are both more than 109 organisms/ml.
Principal microorganisms used as lactic fermentation products are given in Table 2. The most commonly used strains are strains of lactic acid bacteria: lactobacilli, enterococci and bifidobacteria. However, other microbes and even yeasts have also been used.
Table 2. Microorganisms applied in fermented milks, lactic milk drinks, pasteurized sour milk and lactic fermentation products.
| Lactobacillus | Bifidobacterium | Other species |
|---|---|---|
| L. acidophilus | B. adolescentis | Enterococcus faecalis |
| L. amylovorus | B. animalis | Enterococcus faecium |
| L. casei | B. bifidum | Lactococcus lactis |
| L. crispatus | B. breve | Leuconostoc mesenteroides |
| L. delbrueckii | B. infantis | Sporolactobacillus inulinus |
| ss. bulgaricus | B. lactis (animalis) | Streptococcus thermophilus |
| L. gallinarum | B. longum | Clostridium butyricum |
| L. gasseri | B. thermophilum | Bacillus cereus var. toyoi |
| L. johnsonii | B. pseudolongum | Escherichia coli Nissle |
| L. paracasei | Saccharomyces cerevisiae | |
| L. plantarum | Saccharomyces boulardii | |
| L. reuteri | ||
| L. rhamnosus |
FACTORS AFFECTING PROLIFERATION AND COLONIZATION IN THE INTESTINE OF BIFIDOBACTERIA
In research using laboratory animals and humans, it is practically impossible in most cases for lactobacilli and bifidobacteria administered as probiotics to proliferate and colonize in the intestines. We performed a test to examine the cause of this using laboratory animals and humans.
Relationship between proliferation of bifidobacteria in the intestine and diet
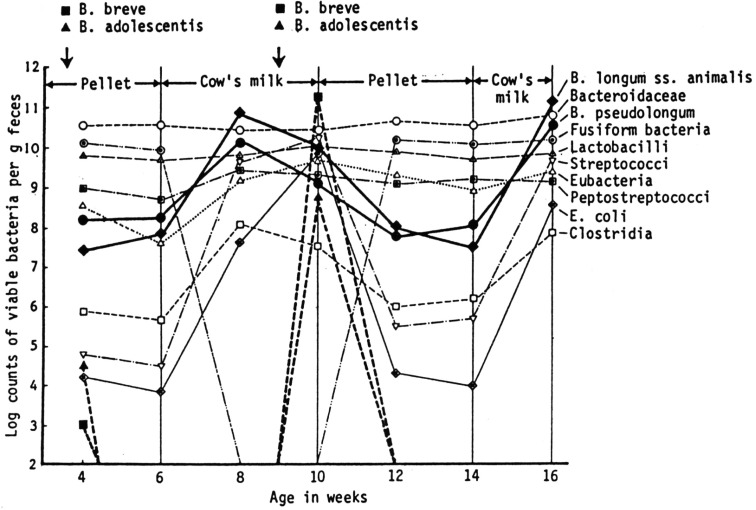
In an attempt to explain the predominance of certain bifidobacteria in infant stool as well as the host specificity of the species or biotypes of bifidobacteria, we examined the influence of feeding human milk or cow’s milk on the bacteria in 14 conventional mice. Some of the mice were inoculated orally with human bifidobacteria and others were not.
In the feces of adult mice receiving commercial pelleted food, bacteroidaceae, fusiform bacteria, and lactobacilli constituted the most predominant organisms of the intestinal microbiota. Peptostreptococci and eubacteria were next in prevalence. Bifidobacteria, including B. pseudolongum and B. longum ss. animalis, were irregularly recovered and usually found in numbers of 107 to 108 organisms/g of feces when present. Streptococci, E. coli and clostridia were isolated in small numbers. When two strains of bifidobacteria of human origin (B. breve BH 1-1 and B. adolescentis KH 1-1) were orally administered to the mice fed commercial pelleted food, they immediately disappeared from the intestine (Fig. 4) [5].
Fig. 4.
Changes in fecal population of conventional mice before and after feeding of cow’s milk and influence of oral incubation of human bifidobacteria on the fecal populations.
After being fed the commercial pelleted food, seven mice received exclusively cow’s milk for 4 weeks, whereas the other seven mice were given human milk. In both experimental groups, no essential differences in bacterial populations were found. In the second week after feeding of cow’s milk or human milk, the levels of murine bifidobacteria (B. pseudolongum and B. longum ss. animalis) increased significantly and reached the maximal levels of approximately 1011 organisms/g of feces. Simultaneously, the levels of clostridia, streptococci and E. coli increased remarkably. By contrast, fusiform bacteria decreased to levels below detection. In the third week after milk feeding, two strains of human bifidobacteria (B. breve BH 1-1 and B. adolescentis KH 1-1) were used again to inoculate the mice orally. Colonization with B. breve BH 1-1 was established, and the maximal population level was 1011 organisms/g of feces within 48 hr after oral inoculation. Colonization with B. adolescentis was also established, but it persisted at a lower population level of approximately 108 organisms/g of feces. These findings indicate that exclusive milk feeding, irrespective of whether human milk or cow’s milk is fed, is responsible for the multiplication of both human and murine types of bifidobacteria.
When commercial pelleted food was substituted for cow’s milk again, two strains of human bifidobacteria apparently disappeared from the intestine, and the level of murine bifidobacteria decreased to approximately 107 to 108 organisms/g, a 100- to 1,000-fold reduction from the cow’s milk feeding levels. After 4 weeks of feeding with pelletted food, mice were fed cow’s milk again. The level of murine bifidobacteria increased to approximately 1011 organisms/gram, but human bifidobacteria did not reappear and could not be isolated from fecal material. These results suggest that the disappearance of human bifidobacteria from the mouse intestine when mice are fed a pelleted diet may result from the effect of microbial antagonisms acting within the intestine.
Effect of oligosaccharides on human intestinal microbiota
Fructo-oligosaccharides are widely distributed in nature in such things as honey, onions, burdock, rye, asparagus, bananas and oats and are prepared from sucrose through fructosyltransferase. Galacto-oligosaccharides are found in human mother’s milk and the colostrum of cow’s milk and are generated from lactose through the transgalactosylation reaction of β-galactosidases from Aspergillus oryzae and Streptococcus thermophilus. Soybean oligosaccharides include raffinose, stachyose, and sucrose. Lactosucrose is prepared from lactose and sucrose through the enzyme of Arthrobacter. Isomalto-oligosaccharides exist in fermented foods such as miso, soy sauce, sake and honey.
To compare the availability in vitro of various oligosaccharides, including isomalto-oligosaccharides, xylo-oligosaccharides, galacto-oligosaccharides, soybean oligosaccharides, fructo-oligosaccharides, lactulose, lactosucrose, raffinose, and palatinose, by intestinal bacteria, 72 strains were used.
Most indigestible oligosaccharides are fermented in vitro by Bifidobacterium species (though not by B. bifidum) and to a limited degree by Lactobacillus salivarius, Mitsuokella multiacida, Megamonas hypermegas, Bacteroides fragilis group, Klebsiella pneumoniae, Enterococcus, Peptosireptococcus species, and some Clostridium species, but they are not fermented by C. perfringens or Escherichia coli (Table 3).
Table 3. Availability of oligosaccharides by various intestinal bacteria.
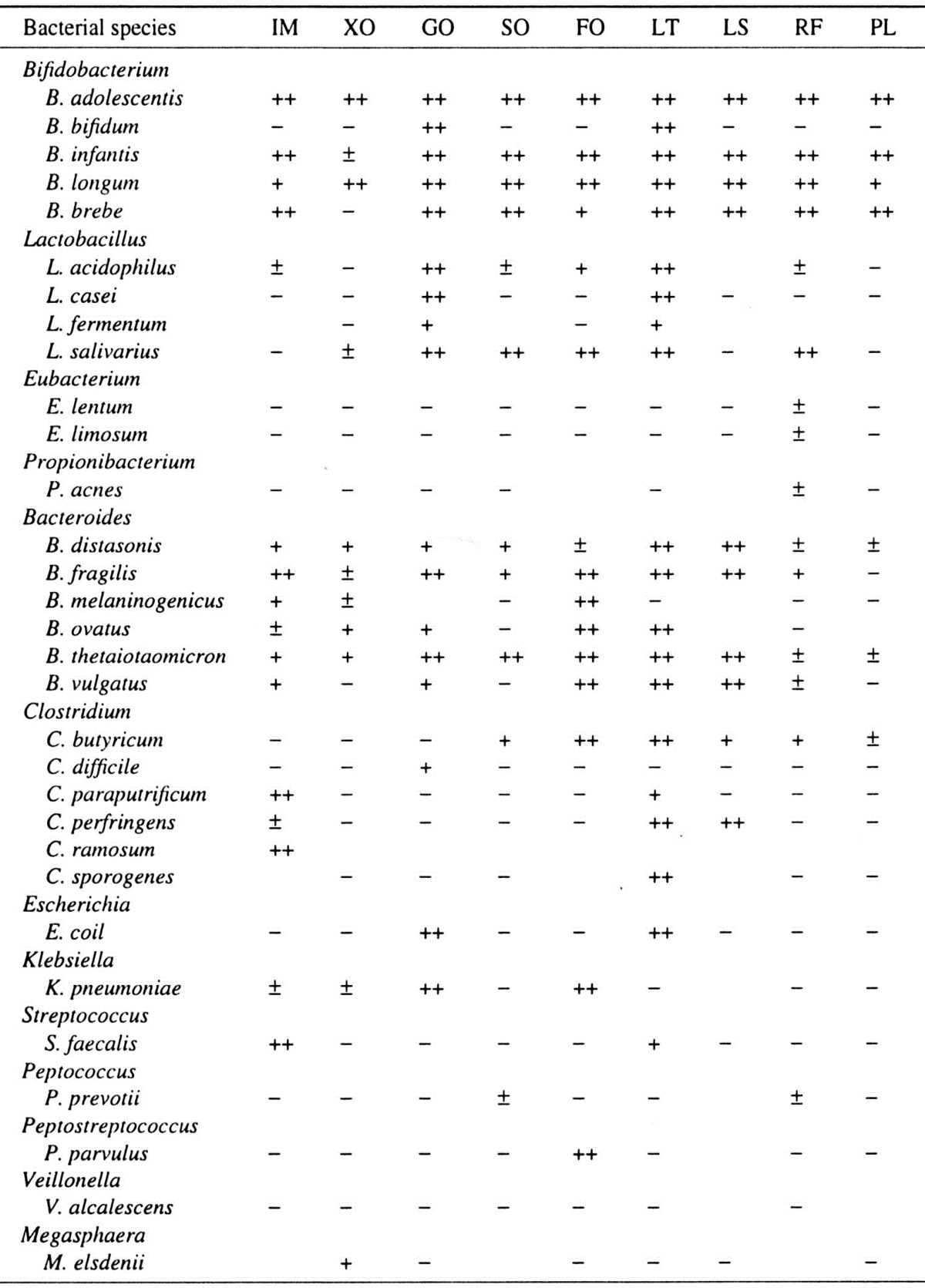
IM, isomalto-oligosaccharides; XO, xylo-oligosaccharides; GO, galacto-oligosaccharides; SO, soybean oligosaccharides; FO, fructo-oligosaccharides; LT, lactulose; LS, lacto-sucrose; RF, raffinose; PL, palatinose.
Human digestive enzymes have little or no effect on oligosaccharides. These substances are hydrolyzed to varying degrees and digested by colonic bacteria with the production of organic acids, mainly volatile fatty acids (acetate, propionate and butyrate) and gas (carbon dioxide and hydrogen). Small amounts of lactic, formic and succinic acids are also produced. Methane may be produced in some people.
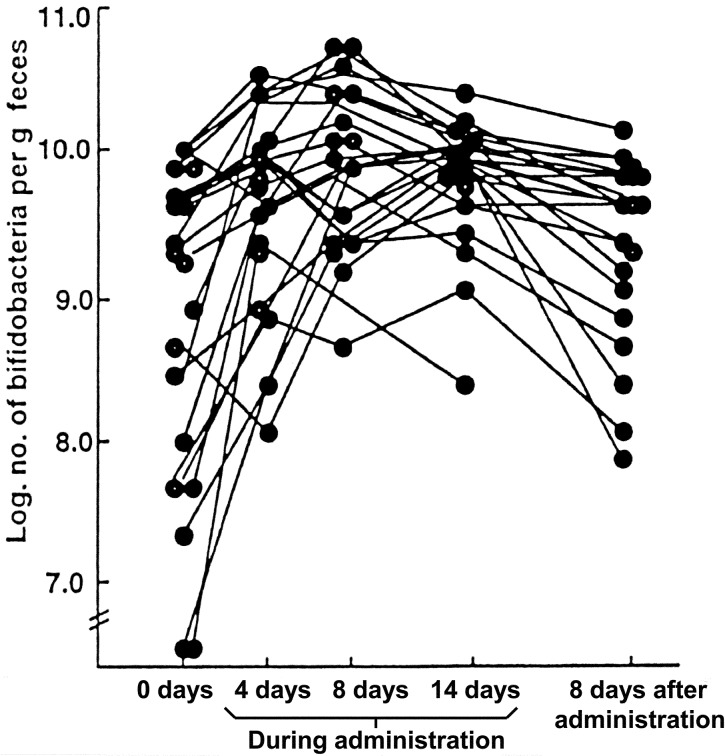
In an experiment with 23 persons with a median age of 73 years, improvement of fecal microbiota was observed by oral administration of 8 g of fructo-oligosaccharides per day for 2 weeks: the population of bifidobacteria in feces increased by about 10 times compared with the levels before administration; the average pH of stool was 0.3 pH units lower than that before administration (Fig. 5) [6].
Fig. 5.
Effect of fructo-oligosaccharides administration on counts of bifidobacteria in feces of elderly patients.
The effect of isomalto-oligosaccharides on human fecal microbiota has also been studied. After administration of 13.5 g of isomalto-oligosaccharides per day for 2 weeks to healthy adults, the level of bifidobacteria was remarkably increased [7].
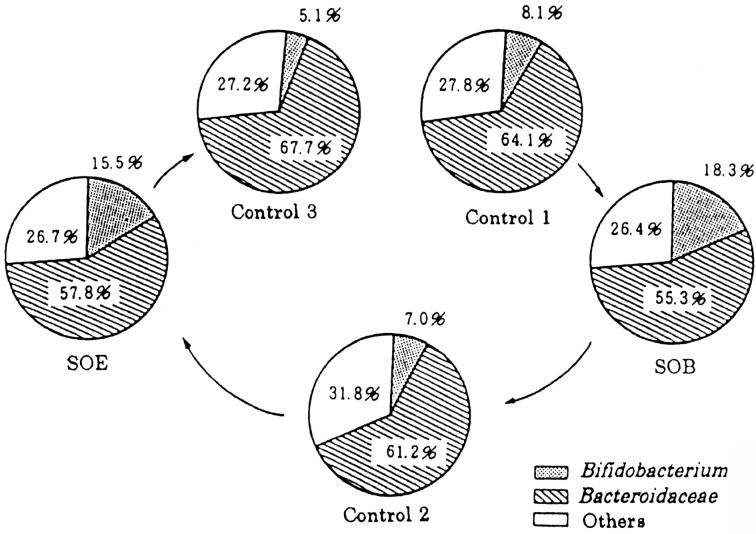
The effect of oral administration of soybean oligosaccharides on the intestinal flora has also been studied. For three weeks, six healthy adult males (28–48 years old) ingested 10 g of a soybean oligosaccharide preparation (SOB diet, including 3 g soybean oligo-saccharide: 23% stachyose and 7% raffinose) daily either independently (SOB diet) or in combination with 6 × 109 colony-forming units of Bifidobacterium longum (SOE diet). The percentage of the total bacteria represented by bifidobacteria increased significantly to 18.3% during ingestion of the SOB diet compared with 8.1% during ingestion of the control diet, and during ingestion of the SOE diet, it increased to 15.5% compared with 7.0% during ingestion of the control diet (Fig. 6) [8]. These results indicate that soybean oligosaccharides stimulate the growth of bifidobacteria in vivo.
Fig. 6.
Changes in the intestinal microbiota of volunteers following administration of soybean oligosaccharide extract (SOE) or soybean oligosaccharide extract with 6 × 109 of Bifidobacterium longum (SOB).
From the results presented here, it may be concluded that oligosaccharides enhance the proliferation of intestinal bifidobacteria, improving the properties of the host’s stool. These bacteria also scavenge the intestine, thereby altering host lipid metabolism in a beneficial way.
Intestinal bacteria that inhibit colonization of human bifidobacteria in the murine intestine
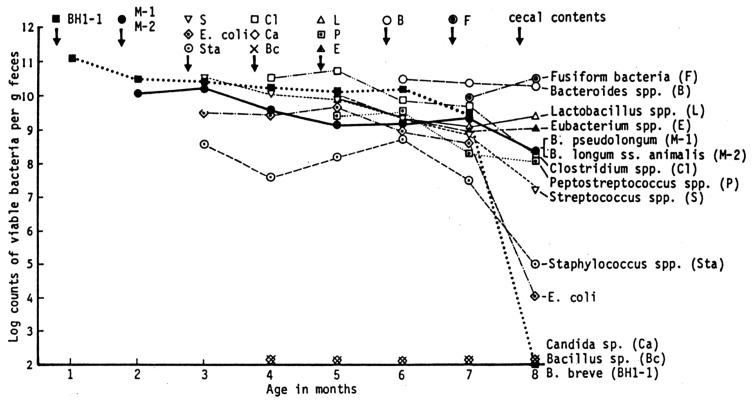
To determine which of the intestinal flora components inhibit human Bifidobacterium multiplication in vivo, germ-free mice fed sterile pelleted food were monoassociated with human bifidobacteria, and then challenged by oral inoculation with 51 bacterial strains isolated from conventional mice fed commercial pelleted foods (Fig. 7) [9]. When germ-free mice were monoassociated with a human Bifidobacterium strain (B. breve BH 1-1), the strain was established within 48 hr and attained the maximal level of approximately 1011 organisms/gram of feces. Then, B. longum ss. animalis M-1 and B. pseudolongum M-2 were inoculated, and these strains were established, with both their levels being 1010 organisms/g of feces. Subsequently, 49 strains including E. coli, Streptococcus, Staphylococcus, Clostridium, Bacillus, Candida, Lactobacillus, Peptostreptococcus, Eubacterium, Bacteroides, and fusiform bacteria were inoculated, one after the other, but none of these organisms was responsible for inhibition of the colonization of human bifidobacteria in the mouse intestine.
Fig. 7.
Changes in the numbers of organisms in the feces of gnotobiotic mice inoculated with various strains.
When Clostridium sp., Bacillus sp. and Candida sp. were inoculated into the mice, the murine bifidobacteria population levels showed 10-fold reductions as compared with their population before inoculation of these organisms. After inoculation of fusiform bacteria, the population of human bifidobacteria slightly decreased, but remained at rather high levels (109 organisms/g).
Finally, cecal contents of conventional mice fed commercial pelleted food were inoculated into gnotobiotic mice. Human bifidobacteria immediately disappeared from the intestine of the mice, and the typical flora of conventional mice was established in the intestine within 1 month. These results indicate that an unknown organism(s) harbored in the ceca of conventional mice is responsible for inhibition of the colonization of human bifidobacteria in the intestine of conventional mice.
HEALTH EFFECTS OF LACTIC FERMENTATION PRODUCTS
Health effects of fermented milks
The health effects of fermented milks reported based on adequate clinical data or animal data include prevention and treatment of diarrheal disease, prevention of systemic infections, management of inflammatory bowel disease, immunomodulation, prevention and treatment of allergies, anticancer effects, treatment of cholesterolemia and alleviation of lactose intolerance.
In our studies in healthy volunteers, it was revealed that common effects of fermented milks and lactic milk drinks on the intestinal environment were an increase in the counts of bifidobacteria and a decrease in the counts of harmful bacteria such as C. perfringens and E coli and that as a result, the levels of, fecal putrefactive products decrease (Figs. 8, 9) [10, 11].
Fig. 8.
Changes in the survival ratio of mice administered with sour milk or whole milk. ○: Control, ■: Whole milk, ▲: Sour milk.
Fig. 9.
Changes in the intestinal microbiota of mice administered with sour milk or whole milk. ○: Control, Δ: Whole milk, ●: Sour milk.
Health effects of pasteurized lactic milk drinks
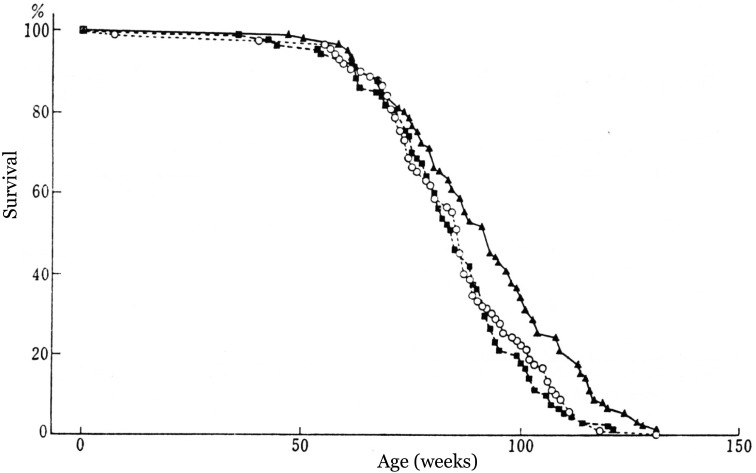
The effects of ingesting pasteurized lactic milk drink (Calpis sour milk) on longevity and transplanted tumors in mice were studied [12]. Three groups of female ICR mice were fed a diet containing 14% pasteurized lactic milk drink or 1.6% whole milk powder or a control diet from 4 weeks of age until the end of life. Figure 10 shows the changes in the survival ratio throughout the experimental period. The average life-spans of the control group, whole milk group and pasteurized lactic milk drink group were 84.9, 84.4 and 91.8 weeks, respectively. The life-span of the pasteurized lactic milk drink group was, on average, 8% longer than that of the control group, while the average life-span of the whole milk group was the same as that of the control group.
Fig. 10.
Effect of administration of yogurt or sour milk to mice on proliferation of Ehrlich ascites tumor cells. ●: Control, ○: Yogurt (pasteurized), Δ: Yogurt (not pasteurized), □: Sour milk (pasteurized).
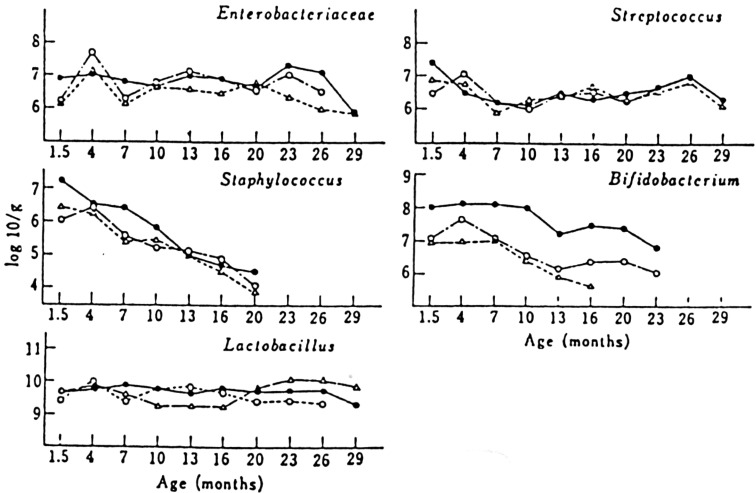
Changes in the intestinal microbiota during the experimental period are shown in Fig. 11. The numbers of Enterobacteriaceae, Streptococcus and Lactobacillus did not significantly differ among the 3 groups. The counts of Bifidobacterium in the intestinal microflora of mice fed sour milk were 10 times higher than those fed either whole milk or the control diet. The effect of sour milk on the life-span of mice might be the result of this modifying effect on the intestinal microbiota.
Fig. 11.
Effect of yogurt (130 g/day) intake on fecal flora and fecal metabolites in healthy adults (21–50 years old).
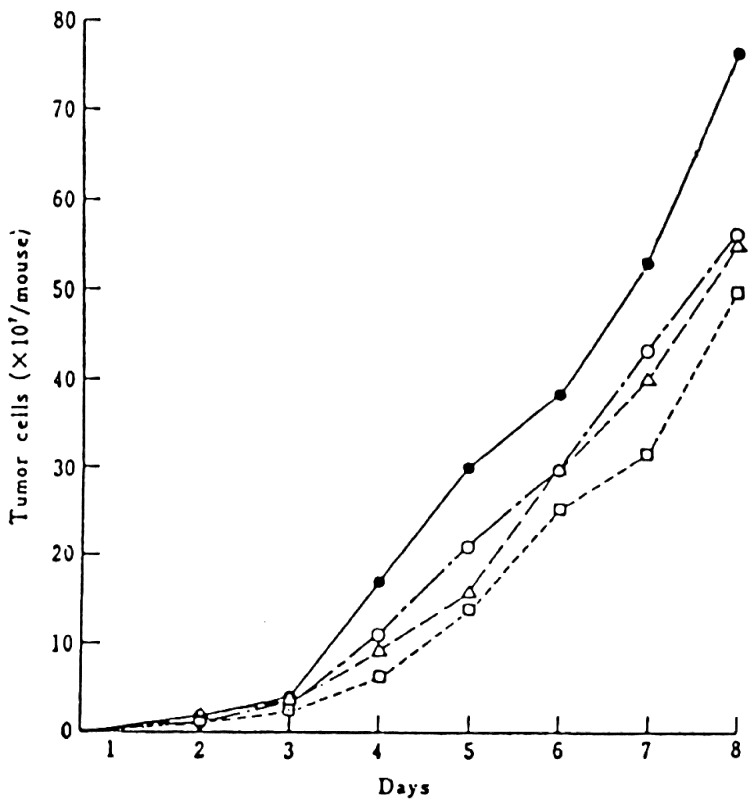
The effect of a pasteurized lactic milk drink on tumors was studied. Female ICR mice at 6 weeks of age were intraperitoneally inoculated with 1 × 106 cells of Ehrlich ascites tumor cells. The mice were given the pasteurized lactic milk drink mixed with the control diet or with drinking water. The ascites tumor growth in the mice was determined at sacrifice.
The pasteurized lactic milk drink was prepared in the same manner as in the longevity experiment. Yogurt was fermented with L. bulgaricus and Streptococcus thermophilus. It was then mixed with water and fed to the mice. The development of tumor cells in the mice given pasteurized sour milk was slower than that in those given the control diet. Both the pasteurized and unpasteurized yogurt inhibited the growth of tumor cells by 28% compared with the control, whereas the sour milk inhibited the growth by 42% compared with the control (Fig. 12).
Fig. 12.
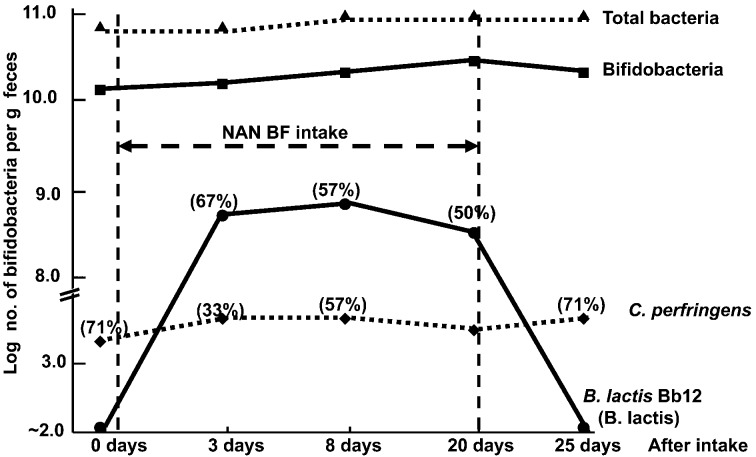
Effect of NAN BF (containing 108.8 B. lactis, 0.14 g galacto-oligosaccharides and 4 g lactose/200 mL) on fecal flora. Figures in parentheses indicate the rate of detection (%). The rate of detection for bacterial groups without parentheses is 100%. Subjects: 9 healthy children aged 12–31 months.
It has been known that the peptidoglycan of bacterial cell walls has adjuvant activity. Anti-tumor glycopeptide has been isolated from the cell wall of L. bulgaricus used for yogurt production. These glycopeptides are also active as an adjuvant when orally administered. Feeding mice yogurt inhibits the growth of transplantable tumor cells, and some components of starter cells have anti-tumor activity. The inhibitory effect of pasteurized sour milk on Ehrlich ascites tumors might be elucidated by the anti-tumor activity of the starter cell component through the host immune system.
Health effects of a heat-killed lactic acid bacteria preparation
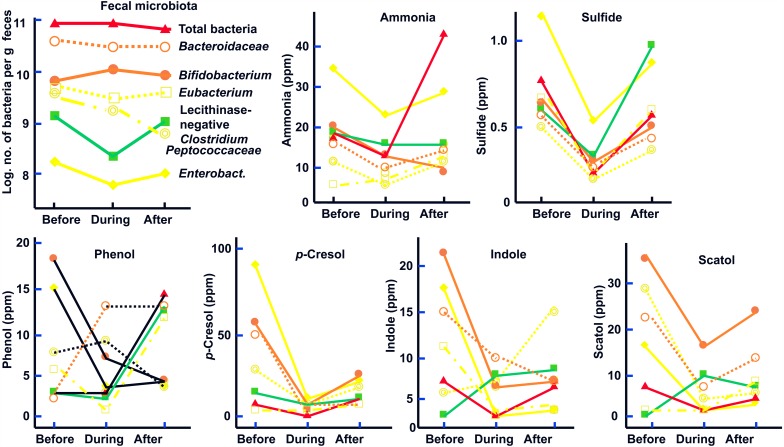
The effects of the consumption (200 mg/day) of a pasteurized Enterococcus faecalis EC-12 preparation (EC-12) for 14 days on fecal microbiota and putrefactive products were studied in eight healthy volunteers (22–26 years of age). During EC-12 consumption, the counts of bifidobacteria were significantly increased, except for one volunteer, whereas the counts and the frequency of occurrence of lecithinase-positive Clostridium, including C. perfringens, significantly decreased when compared with the values before EC-12 consumption and during placebo consumption. A tendency for the Enterobacteriaceae count to decrease was observed during EC-12 consumption compared with the values before EC-12 consumption and during placebo consumption. The fecal concentrations of ammonia, phenol and p-cresol were significantly decreased on day 14 of EC-12 consumption. The fecal levels of sulfide and indole were significantly decreased during EC-12 consumption (Table 4) [13].
Table 4. Effect of administration (1012/day) of heat-killed E. faecalis EC-12 preparation on microbiota and putrefactive products of the feces in healthy adults.
| Bacteria detected / putrefactive products |
Before administration | During administration | After administration | |
|---|---|---|---|---|
| Bifidobacteria | 9.9 ± 0.3a | 10.2 ± 0.1 | ↑c | 10.8 ± 0.1 |
| Enterobacteriaceae | 7.7 ± 1.0 | 6.7 ± 0.6 | ↓ | 7.1 ± 0.8 |
| C. perfringens | 4.7 ± 0.8 | 3.4 ± 0.3 | ↓ | 3.9 ± 0.6 |
| Ammonia | 386.4 ± 164.5b | 263.4 ± 102.8 | ↓ | 353.2 ± 158.6 |
| Sulfide | 8.4 ± 2.8 | 4.2 ± 1.6 | ↓ | 7.1 ± 2.1 |
| Phenol | 21.6 ± 9.2 | 11.6 ± 4.1 | ↓ | 19.3 ± 8.7 |
| p-Cresol | 32.8 ± 11.2 | 16.5 ± 4.2 | ↓ | 30.3 ± 8.4 |
| Indole | 38.3 ± 8.6 | 20.1 ± 6.9 | ↓ | 39.2 ± 9.6 |
aData expressed as means ± SD of log10 numbers per gram feces.
bData expressed as means ± SD of μg/g wet feces.
c ↑: Increase. ↓: Decrease.
Health effects of lactic fermentation products depend upon immune stimulation
The results obtained concerning the health effects of lactic fermentation products may be summarized as follows: The larger the amount of lactic acid bacteria we ingest, regardless of whether they are viable or nonviable, the higher the health effects we receive. Up to now, it has been thought that ingested viable lactic acid bacteria proliferate and colonize in the intestine, even if they could pass through the stomach. However, it is impossible for ingested lactic acid bacteria to proliferate and colonize in the intestine in which indigenous lactic acid bacteria are alredy present. Moreover, it has been found that ingested lactic acid bacteria, irrespective of whether they are viable or nonviable, improve the intestinal microbiota balance. It has also been shown that lactic acid bacteria can stimulate immunity in rodents. Some recent studies in humans have also shown promising results. In animal models, the lactic acid bacteria have been shown to stimulate the production of antibodies (local and systemic), enhance the activity of macrophages, increase γ-interferon levels and increase the concentration of natural killer cells. Thus, it is thought that the main health effect of point of lactic fermentation products is stimulation of the immune system by cell components of lactic acid bacteria.
MECHANISMS OF ACTION OF LACTIC FERMENTATION PRODUCTS
It is now well established that foods and food components contribute to physiologic and biologic well-being. Optimal intestinal flora and intestinal environments are possibly achieved via a nutritionally well-balanced diet and active intake of functional foods, such as oligosaccharides, dietary fiber and fermented milks, which promote useful bacteria or suppress harmful bacteria.
Functional foods promote health in terms of both improving well-being (mental and physical conditioning) and reducing the risk of diseases. To be concrete, these are thought to be such useful effects as bioregulation, including central action, peripheral action, appetite, and absorption; biodefence, including immunostimulation and suppression of allergies; and prophylaxis against hypertension, diabetes, cancer, hypercholesterolemia, anemia, and platelet aggregation as well as prevention of geriatric diseases through reduction of reactive oxygen species (ROS).
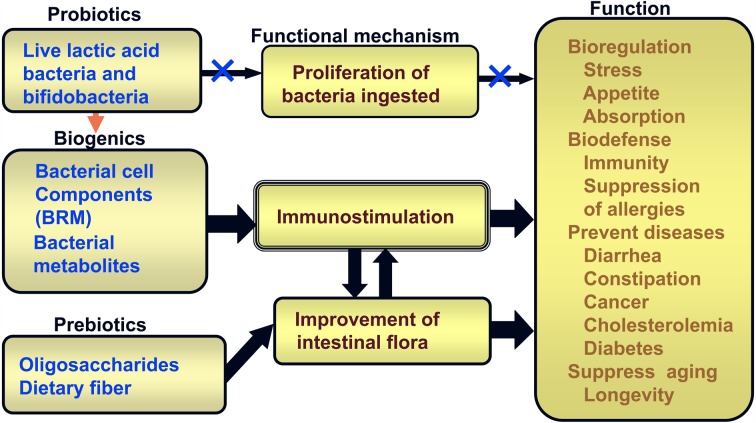
Lactic fermentation products are classified into 3 groups based on their mechanisms of action: probiotics, prebiotics and biogenics (Fig. 13).
Fig. 13.
Functional mechanisms of fermented milks (probiotics), lactic fermentation products (biogenics) and oligosaccharides/dietary fiber (prebiotics).
Probiotics are defined as live microbial food ingredients that beneficially affect the host by improving its intestinal microbial balance; candidates probiotics include lactic acid bacteria, bacilli, yeasts, etc.
Prebiotics are nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth of beneficial bacteria and/or by suppressing that of harmful bacteria in the colon, which have the potential to improve host health; the cadidate prebiotics include oligosaccharides, dietary fiber, resistant starch, etc.
Biogenics are food ingredients that beneficially affect the host by direct immunostimulation, suppression of mutagenesis, tumorigenesis, peroxidation, hyper-cholesterolemia or intestinal putrefaction; candidates biogenic include biological response modifiers (BRM), carotenoids, flavonoids, EPA, DHA, lacto-tripeptide, immunopotentiators, etc.
Probiotics and prebiotics act on the intestinal flora and improve the balance of the flora by enhancing the growth of beneficial intestinal bacteria and/or inhibiting the growth of harmful ones, resulting in scavenging in the intestinal environment.
Up to now, most health effects of lactic acid bacteria contained in yogurt, etc., have been thought to be due to the function of viable lactic acid bacteria that reach the intestine and proliferate there. However, in fact, lactic acid bacteria, when taken orally, can neither proliferate nor readily colonize in the intestine. As such, the effectiveness of probiotics cannot be explained only by the effects of viable lactic acid bacteria. Therefore, the effect of probiotics and the effect due to bacterial cellular components, in other words biogenics, should be taken into consideration. Cellular components of lactic acid bacteria stimulate the intestinal immune system, irrespective whether they are from viable or dead bacteria. That is, probiotics, biogenics and prebiotics improve the balance of the intestinal microbiota by enhancing the growth of bifidobacteria and/or inhibiting the growth of harmful bacteria, resulting in immunostimulation. Immunostimulation enhances bioregulation such as stress, appetite and absorption; biodefenses, such as immunity and suppression of allergies; the prevention of disease, including diarrhea, constipation, cancer, cholesterolemia, hypertension and diabetes; and the suppression of aging.
They also induce production of biogenics such as antibacterial substances and immunopotentiators via proliferation of beneficial intestinal bacteria. Thus, functional foods enhance bioregulation such as stresses, appetite and absorption; biodefenses, such as immunity and suppression of allergies; prevention of diseases, including diarrhea, constipation, cancer, cholesterolemia and diabetes; and suppression of aging through immunostimulation as well as suppression of mutagenesis, carcinogenesis, oxidation processes, intestinal putrefaction and cholesterolemia.
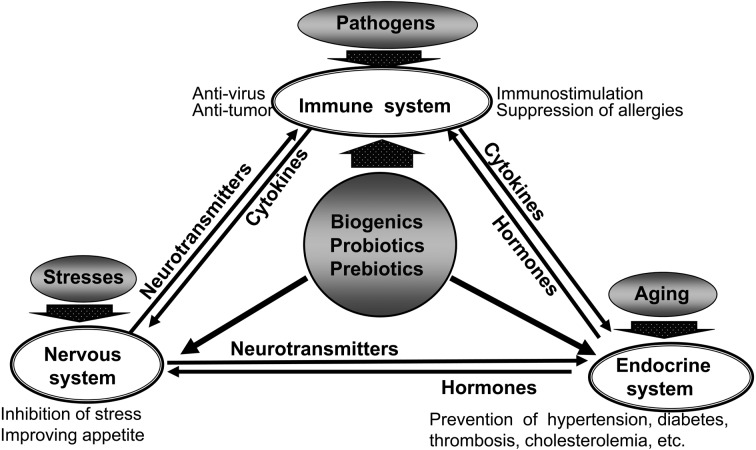
Healthy people naturally have a function that controls the physical condition to maintain bio-homeostasis. The effects exhibited by functional foods occur through modulation of bio-homeostasis (Fig. 14) [14]. In bio-homeostasis, the immune, endocrine and nervous systems constitute separate functional control systems and maintain homeostasis by controlling each other through transmitters.
Fig. 14.
Maintenance of bio-homeostasis by lactic fermentation products.
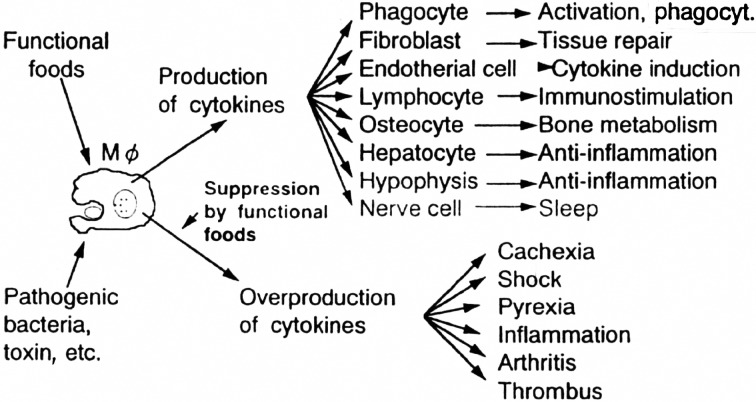
The macrophage cell group refered to as phagocytes seems to work mainly in the mechanism of maintenance of bio-homeostasis (Fig. 15) [14]. These phagocytes play a role in prevention of various pathological conditions in the human body due to abnormality of cells. They are also responsible for spontaneous recovery via various actions, such as anti-cancer and anti-bacterial actions, inflammation and production of antibodies, as well as elimination of foreign cells.
Fig. 15.
Bioregulation by functional foods.
It is very important to maintain the function of phagocytes to maintain proper bio-homeostasis and control of the cytokine network for maintenance of health, especially prophylaxis against geriatric diseases. Therefore, it is necessary to use functional foods actively for this purpose. In an effort to improve human health and to prevent and treat geriatric diseases, numerous studies have recently focused on ways to manipulate the intestinal flora through dietary means.
Given the present knowledge, probiotics are expected to be incorporated into daily eating habits for healthy longevity, whereas biogenics evolved from probiotics are expected to be put into practical use in the area of prevention of lifestyle related diseases and alternative medicine in combination with other biogenics and prebiotics. However, in regard to this, the following aspects need further investigation in humans: 1) Are there any differences in the beneficial effects between lactobacilli and bifidobacteria? 2) Are there any differences between live bacteria and dead bacteria of the same species and strain? 3) Are viable microorganisms necessary for specific health benefits? 4) What dose of live bacteria or dead bacteria should be administrated for a desired health benefit? 5) It is still more important to guarantee the safety of probiotics and biogenics.
CONCLUSIONS
Recent advances in intestinal microbiota research are the background for the appearance of functional foods. The intestinal microbiota is closely related to human health and diseases. The predominance of harmful bacteria in the intestine leads to various disturbances, while the existence of bifidobacteria acts as an effective scavenger in the large intestine. Maintenance of an optimal balance of the intestinal flora is achieved by active intake of functional foods, such as oligosaccharides, dietary fiber and lactic fermentation products, which promote bifidobacteria or suppress harmful bacteria. Daily intake of lactic fermentation products is considered to be one of the best methods to improve both physical condition by intestinal cleaning and the biogenics effect, resulting in prevention, treatment and recovery from various diseases. Although several possible mechanisms of lactic fermentation products have been suggested in the prevention and cure of disease, including lifestyle related diseases, no clear explanation has been reached up to now. However, such mechanisms may be clarified by further detailed clinical studies in the near future. One of the next tasks in the study of healthy longevity is to clarify the relationship of intestinal flora with regard to immunity, allergies and anti-oxidative activity. These subjects are related to the development and evaluation of functional foods through clarification of immunostimulation, prevention of allergic diseases such as eczema and hay fever and elimination of free radicals. In view of the present conditions, functional foods are expected to be put into practical use to prevent lifestyle-related diseases and in alternative medicine.
REFERENCES
- 1.Japanese Ministry of Health and Welfare, editor. Kosei hakusho, Showa Health and white paper (1985) p. 1. Kosei Tokei Kyokai [Association for Health and Welfare Statistics], Tokyo (2013/14). [Google Scholar]
- 2.Metchnikoff II. 1907. The prolongation of life.Optimistic Studies, Springer, New York. [Google Scholar]
- 3.Rettger LF, Levy MN, Weinstein L, Weiss JE. 1935. Lactobacillus acidophilus and its therapeutic application, Yale University Press, New Haven. [Google Scholar]
- 4.Henneberg W. 1934. Zur Kenntnis der stäbchenförmigen Milchsäurebakterienarten. Zbl Bakt Hyg II 91: 102–135 [Google Scholar]
- 5.Mitsuoka T, Kaneuchi C. 1977. Ecology of the bifidobacteria. Am J Clin Nutr 30: 1799–1810 [DOI] [PubMed] [Google Scholar]
- 6.Mitsuoka T, Hidaka H, Eida T. 1987. Effect of fructo-oligosaccharides on intestinal microflora. Nahrung 31: 427–436 [DOI] [PubMed] [Google Scholar]
- 7.Kohmoto T, Fukui F, Takaku H, Machida Y, Arai M, Mitsuoka T. 1988. Effect of isomalto-oligosaccharides on human fecal flora. Bifidobacteria Microflora 7: 61–69 [Google Scholar]
- 8.Hayakawa K, Mizutani J, Wada K, Masasi T, Yoshihara I, Mitsuoka T. 1990. Effects of soybean oligosaccharides on human faecal flora. Microb Ecol Health Dis 3: 293–303 [Google Scholar]
- 9.Mitsuoka T. 2011. History and evolution of probiotics. Jpn J Lactic Acid Bact 22: 26–37 [Google Scholar]
- 10.Hara H, Terada A, Takahashi M, Kaneko T, Mitsuoka T. 1993. Effect of yoghurt consumption on fecal flora and fecal metabolites in healthy adults. Jpn J Food Microbiol 10: 29–34 [Google Scholar]
- 11.Fukushima Y, Li ST, Hara H, Terada A, Mitsuoka T. 1997. Effect of follow-up formula containing bfidobacteria (NAN BF) on fecal flora and fecal metabolites in healthy children. Biosci Microflora 16: 65–72 [Google Scholar]
- 12.Takano T, Arai K, Morota I, Hayakawa K, Mizutani T, Mitsuoka T. 1985. Effects of feeding sour milk on longevity and tumorigenesis in mice and rats. Bifidobacteria Microflora 4: 31–37 [Google Scholar]
- 13.Terada A, Bukawa W, Kan T, Mitsuoka T. 2004. Effect of the Consumption of Heat-killed Enterococcus faecalis EC-12 preparation on microbiota and metabolic activity of the feces in healthy adults. Microb Ecol Health Dis 16: 188–194 [Google Scholar]
- 14.Mitsuoka T. 2000. Significance of dietary modulation of intestinal flora and intestinal environment. Bifidobacteria Microflora 19: 15–25 [Google Scholar]