Abstract
Patients may present with anal incontinence (AI) following repair of a congenital anorectal anomaly years previously, or require total anorectal reconstruction (TAR) following radical rectal extirpation, most commonly for rectal cancer. Others may require removal of their colostomy following sphincter excision for Fournier's gangrene, or in cases of severe perineal trauma. Most of the data pertaining to antegrade continence enema (the ACE or Malone procedure) comes from the pediatric literature in the management of children with AI, but also with supervening chronic constipation, where the quality of life and compliance with this technique appears superior to retrograde colonic washouts. Total anorectal reconstruction requires an anatomical or physical supplement to the performance of a perineal colostomy, which may include an extrinsic muscle interposition (which may or may not be ‘dynamized'), construction of a neorectal reservoir, implantation of an incremental artificial bowel sphincter or creation of a terminal, smooth-muscle neosphincter. The advantages and disadvantages of these techniques and their outcome are presented here.
Keywords: anal incontinence, antegrade continence enema, malone procedure, total anorectal reconstruction
INTRODUCTION
Anal (fecal) incontinence (AI) is characterized by uncontrollable episodes of an involuntary loss of stool at inappropriate times and in socially unacceptable circumstances [1, 2]. Although the incidence varies worldwide, there is a standard reported prevalence of AI in between 4% and 7% of the general population, with a higher estimate (up to 20%) recorded in patients who reside in nursing homes [3, 4]. Evidence would suggest that this symptom seriously impacts on patient-reported standardized quality of life and many aspects of healthy existence where—frequently because of embarrassment—most patients fail to seek specific medical help [5–7]. As a result of these decisions, there is a significant national, annual economic cost of conservative (i.e. non-surgical) care of these patients [8], part of which is influenced by the effect AI has on elderly patient institutionalization [9], as well as the inherent additional costs of anti-diarrheal drugs, healthcare visits, intermittent hospitalizations and patient payment for protective materials and pads. The additive costs of surgical therapies are significant and are impacted by their long-term success rates, the economic impact of procedure-related complications (which are considerable with some of the newer therapies) and the incidence of revisional operative procedures [10]. This article assesses the use and clinical results of antegrade continence enemas, either alone or in combination with total anorectal reconstruction following complete rectal extirpation, as valid surgical alternatives in the management of selected cases of AI.
THE ANTEGRADE CONTINENCE ENEMA OR ‘MALONE' PROCEDURE
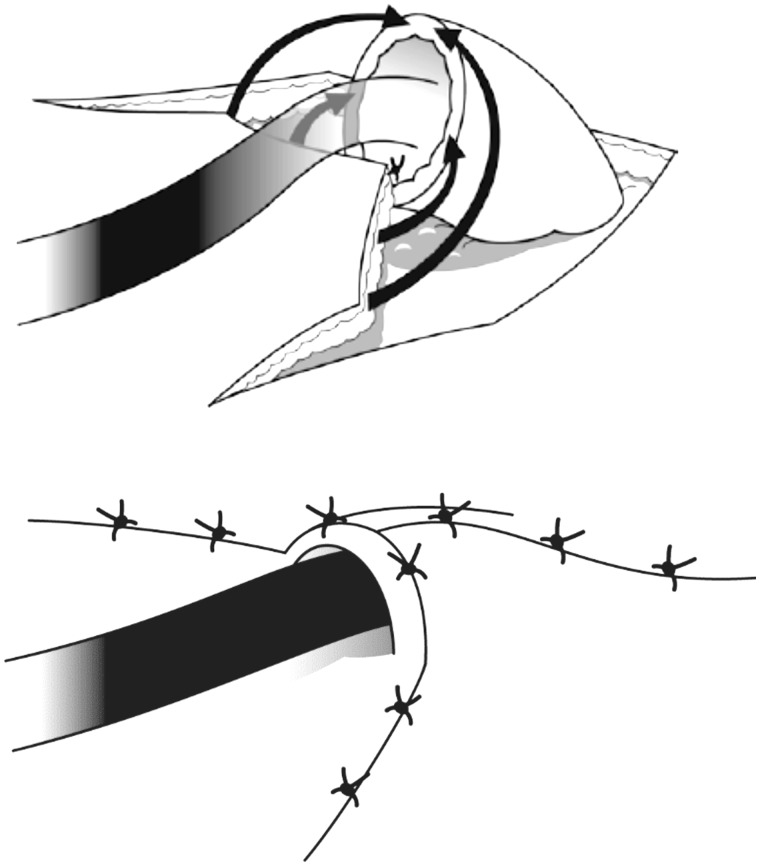
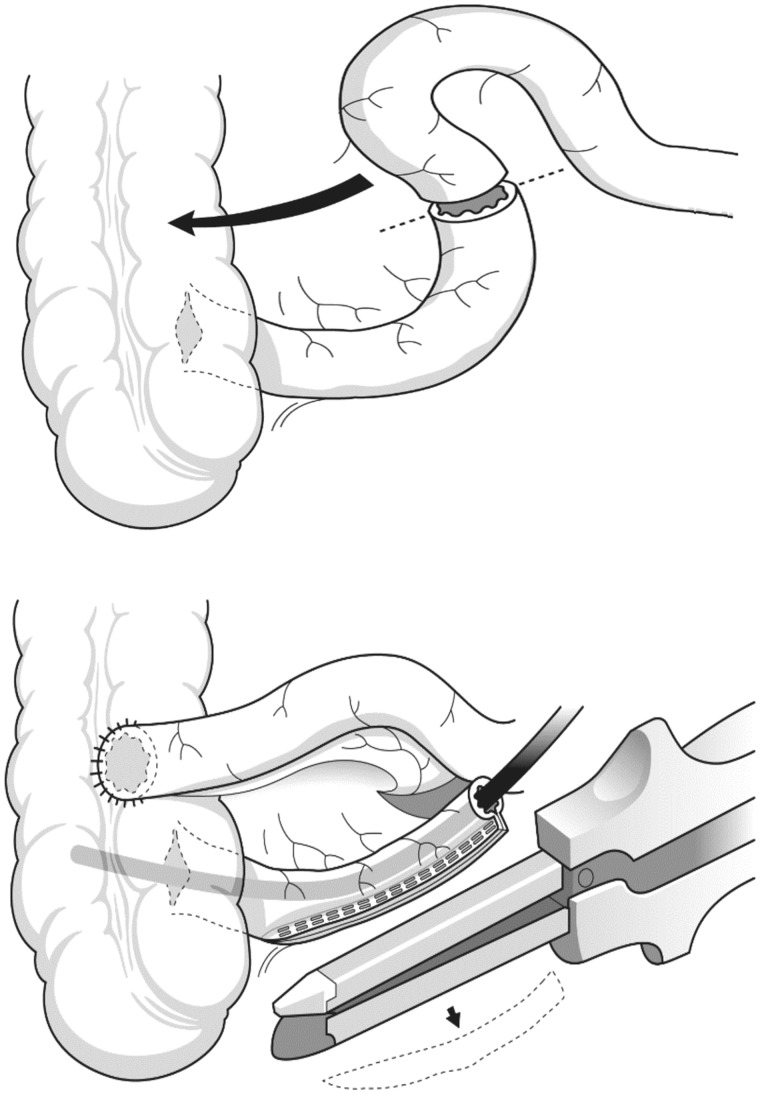
An alternative for the management of various cases of AI that have resisted other more conventional forms of treatment is the operative technique of antegrade continence enema (ACE), rediscovered by Malone [11]. This approach is more popular in Europe, where it was originally reported in 1905 [12]. The basic technique was adapted from the Mitrofanoff procedure used for a continent catheterizable stoma leading to the bladder [13], but using an appendicostomy for antegrade colonic irrigation. Originally, it was used as a cleansing treatment in spina bifida patients presenting with incontinence. The original operative description used the appendix as a continent, catheterizable, abdominal stoma, which was reversed and placed in a submucosal tunnel of the cecum to form a non-refluxing channel. This was modified to a simpler, non-reversed design with or without creation of a definitive anti-reflux mechanism [14]. The Malone procedure may be carried out on the right iliac fossa, employing a V–Y cutaneoplasty with intermittent catheterization, using a Foley's catheter for creation of a continent, usable conduit under the skin (Figure 1). In the event that there has been a prior appendectomy, or where the appendix is atrophic, the cecal wall can be used as a flap, or a flap may be constructed from the terminal ileum, with the latter being the preferred method overall; this is performed by transecting the ileum about 15 cm from the ileocecal valve and turning the vascularized segment outwards as a buried stoma with neo-ileocecal anastomosis (Figure 2) [15–18]. Latterly, part of this procedure may have been laparoscopically assisted [19].
Figure 1.
V–Y ACE procedure. The skin flap is sutured to the wall of either the appendix or a fashioned ileal conduit, with formation of a skin tunnel which covers the stoma. (Reprinted with permission from Christensen P, Laurberg S. The Malone procedure and its variants. In Reconstructive Surgery of the Rectum, Anus and Perineum AP Zbar, RD Madoff and SD Wexner Eds. Springer 2013:273–282).
Figure 2.
An ileocolic anastomosis is fashioned from the proximal ileum and the ascending colon with production of a small-caliber orifice for the stoma preserving the ileo-cecal valve. (Reprinted with permission from Christensen P, Laurberg S. The Malone procedure and its variants. In Reconstructive Surgery of the Rectum, Anus and Perineum AP Zbar, RD Madoff and SD Wexner Eds. Springer 2013:273–282).
Usually after a two-week waiting period—which allows the system to heal and mature—enemas are then progressively increased in volume up to 1 L, with the final regimen determined by trial and error ,as well as by patient tolerance. Results, in both children and adults who are motivated, appear to be acceptable in both the short- and the long terms [20–40]. Long-term quality-of-life data is sparse, where it has been shown that motivation and usage diminishes over time [41]. The results overall appear better in those with neurogenic bowel disability [30, 36]. The principal morbidity of the procedure includes stomal complications, such as stenosis in between one-quarter and one-half of cases during follow-up, and stomal irritation due to refluxing mucus discharge [42]. The complication rate is high but often relatively minor in nature, with fewer problems if the tapered neo-ileal conduit design is used [43].
Latterly the ACE procedure has been performed as a percutaneous, endoscopic colostomy which was originally used in the treatment of intermittent sigmoid volvulus [44, 45]. The comparative functional results appear excellent, although there is a considerable morbidity which, in a small percentage, can be life-threatening [46, 47]. Norman Williams and his group from the London Hospital have used an alternative here, describing a continent colonic conduit with a full-thickness intussuscepted valve, similar to a Kock continent ileostomy [48], with others describing a retubularized ileal segment for this purpose [49]; still others using a retubularized stomach segment [50]. It would appear that antegrade irrigation provides better results than retrograde irrigation [26, 51, 52], although patients should be warned that some symptoms such as bloating—and nausea if there is coincident constipation—may be essentially unaffected.
A range of fluids may be used for irrigation purposes, including phosphate solution, tap water, saline, phosphosoda, polyethylene glycol, liquorice root solution or arachis oil. Caution is advised in small children and fragile, elderly patients, as well as in those with chronic renal failure [53, 54]. Table 1 shows the reported outcomes of ACE-related procedures in a range of disorders that were combined with primary AI.
Table 1.
Antegrade continence enema-related reported outcomes
| Author [Ref] | Indication | Number | Success | Complications |
|---|---|---|---|---|
| Hill [20] | Slow transit | 6 | 6 | 50% |
| Christensen [26] | Neurogenic | 8 | 7 | 38% |
| Rongen [27] | Slow transit | 12 | 8 | 83% |
| Teichman [30] | Neurogenic | 6 | 5 | 67% |
| Lees [31] | Slow transit | 32 | 15 | 88% |
| Hirst [32] | Obstructed defecation syndrome | 20 | 13 | 85% |
| Portier [33] | Mixed | 28 | 28 | 50% |
| Lefevre [35] | Mixed | 22 | 18 | 20% |
| Poirier [36] | Mixed | 18 | 14 | 56% |
| Altomare [37] | Mixed | 11 | 8 | – |
| Koivusalo [38] | Mixed | 27 | 24 | 63% |
| Worsoe [40] | Mixed | 69 | 51 | 38% |
ODS = obstructed defecation syndrome
TOTAL ANORECTAL RECONSTRUCTION
Total anorectal reconstruction (TAR) is a method of neorectal reconstruction following complete rectal and sphincter excision. The concept was first proffered in 1930 by Chittenden, who performed a continent perineal colostomy using a flap of the gluteus maximus as a neosphincter [55], with Margottini reporting a large series of this approach in 1950 [56]. The coincident surgical developments of muscle transfer procedures, techniques of dynamization through electrical field stimulation, artificial implants and myogenic sphincter augmentation techniques have been applied to this approach in the development of TAR. The design makes no real attempt to restore those normal functions that are lost, including an adaptable neorectal reservoir, capability of storage and intermittent discharge, a complex closure (sphincteric) mechanism and a discriminatory sensory apparatus, the arms of which are part of normal continence and, as such, full continence cannot be guaranteed for patients undergoing a TAR.
TAR has been made technically feasible in selected cases by the creation of a neorectal reservoir, along with supplementation using autologous muscle or an artificial sphincter. An additional supplement would be the use of an appendicostomy (or an ileal/colonic conduit) for antegrade (ACE) irrigation, as described above, with the result of a ‘pseudo-continent' status in the patient [57]. Substitution for the rectal functions of storage and sensibility can further be achieved with a segment of descending colon, which has a propulsive function and limited storage capacity, although there is extensive evidence to show that many patients (at least 50%) have a significant ‘low anterior resection syndrome' after low restorative proctectomy, characterized by an increase in the number of daily bowel motions, nocturnal urgency, stool fragmentation, irregular/incomplete defecation and even frank tenesmus and incontinence [58]. In those who have undergone total rectal excision, the lack of sensory receptors in a peri-anal colostomy results in universal passive incontinence whereas, in those with some area of rectal sensation remaining, data from the creation of various forms of neorectal reservoir (such as the colonic 'J' pouch, the side-to-end Baker anastomosis or the coloplasty) suggests that improvements in function over time are due to the reduced action of neorectal motor activity, rather than its role (and capacity) as a true reservoir [59].
In the specific circumstance of TAR, if a pouch is constructed, the shape of the neorectum needs to conform to the anatomical type of reconstruction, where the distal 3–4 cm of the colon will be surrounded by a neosphincter. For this purpose, ‘J' pouch construction has been combined with a gracilis neoanal sphincter in dogs [60], as well as in humans [61–63]. Geerdes et al. [64] described a pouch placed just proximal to a gracilis wrap, opening the colon anti-mesenterically over a length of 15 cm and covering the defect with an isolated patch segment of distal ileum. As an alternative, Williams et al. used a triplicated ileal pouch as 15 cm limbs, combined with a stimulated graciloplasty, for the same purpose [65]. Both of these complex techniques were not, however, associated with particularly good function. A simpler approach is to translate a 6–7 cm long coloplasty above a colo-anal anastomosis, as advocated by Fazio and colleagues [66], or by Devesa, who performed a longitudinal colonic myotomy proximal to a neosphincter, designed to diminish the peristaltic activity of the descending colonic segment [67].
The second approach is that of sphincter substitution, where it is increasingly understood that IAS damage leads to serious continence disturbance in some cases. The issue of IAS implantation and augmentation is discussed elsewhere in this special edition. In this respect, Torres et al. originally described a neo-internal anal sphincter [68], which was wrapped in a spiral configuration around a colonic pull-through similar to that described by others [69, 70]. In this technique, 3–4 cm of distal colon is freed from the pericolic fat and the seromuscular layer is dissected away from the mucosa, creating a smooth-muscle sleeve which is then incised in a spiral fashion. The effect is to construct a pedunculated muscular flap, 1.0–1.5 cm wide and 5–7 cm in length, which is then wrapped around the bowel and fixed to its wall. This creates a cone-shaped smooth muscle cuff attached to the terminal part of the colon. Results of this technique have been variably reported [71–75], with Lorenzi et al. modifying this approach by denuding the mucosa and then everting the last 1.5 cm of the colonic end, which is then anastomosed to the neo-anus in the perineal skin [76]. Physiological studies have shown that these areas distally develop a high pressure zone and a passage pressure gradient. The role of this added approach is unclear, where free grafts obviously lack intrinsic and extrinsic innervation and where they may function more as a biological peri-anastomotic sling and as a barrier to evacuation, than as a true functional neosphincter.
A variety of muscles have been used as translation, for the management of AI, to those patients undergoing TAR, including the gluteus maximus, the adductor musculature and the gracilis.
This technique has been supplemented by Farid of Egypt with fascia lata in very specialized AI patients after reconstruction of congenital anorectal anomaly [77], although the use of a gluteoplasty in adult TAR data is limited [78]. Yuri Shelygin's Moscow group has described success in 82% of patients treated with an adductor longus reconstruction TAR in the only report available [79]. Jacob and colleagues first used a static (adynamic) graciloplasty for the purposes of TAR for a congenital anomaly [80], with Simonsen et al. using the technique after rectal cancer excision [81]. The data here are limited [82–86]; however, the largest series of dynamic graciloplasties for TAR reported by Cavina et al. showed an 87% success rate in 98 patients after 55 months of follow-up, although there was significant morbidity in one-third of cases [83]. The dreaded complication is necrosis of the neo-anus, which appears to occur particularly in the TAR cases [87].
Another approach, by Romano et al., is formal sphincter reinforcement with an artificial anal sphincter with translation to those specialized patients after abdomino-perineal excision [88]. The initially good results seen in his eight cases prompted similar work by Devesa et al. in a small number of cases, but the high rate of complications and the need for explants (as in those patients treated primarily for AI) did not result in extensive use of this technique [67]. The use of an anal sling as a supplement to TAR (a subject covered elsewhere for the management of AI in this special edition) has not been reported.
Others have reported the use of an antegrade continence enema technique for specific use in TAR cases. Chiotasso et al. first reported its use in conjunction with a perineal colostomy [89], where Farroni and colleagues compared the quality-of-life parameters of those with a perineal colostomy and an appendicostomy with those with an abdominal colostomy, concluding that the perineal colostomy with appendicostomy for was a viable option [90]. As per the standard ACE procedure, if the appendix is not available, an ileal neo-appendicostomy, cecal flap or colonic conduit may be fashioned. The advantage of providing ‘pseudo-continence' in these patients is the secondary avoidance of fecal impaction, which can be a very disabling symptom after TAR, particularly where an external sphincter recreation or substitution has also been performed.
Much of the available literature in this specialist group of patients is difficult to interpret, where congenital anomalies that have been reconstructed are mixed with cases where radical rectal extirpation for cancer has been carried out, and where the procedures performed are heterogeneous and combined. Apart from comparing quality-of-life parameters, another way of expressing satisfaction with the procedure might be the comparison of patients' quality of life scores between those with an abdominal stoma and those in whom there is reconversion to a perineal stoma [91]. Such an approach requires a revision of the way in which we assess quality of life in incontinent patients following reconstructive surgery.
Table 2 shows the outcomes of dynamic and adynamic graciloplasty alone for TAR. In this group there is a high morbidity and surgical revision rate, with normal continence reported in only 20% of evaluable patients. At least one year is required to achieve acceptable continence in these cases. There does not appear to be any advantage in ‘dynamizing' the graciloplasty in some series [81, 84, 92], suggesting that the functional results of graciloplasty would be more attributable to the biological ‘cerclage' effect with the gracilis, rather than to the stimulation itself. If this is true, then most of the perineal stomas treated by explantation of the stimulator would have either undergone re-implantation or been reconverted to an abdominal stoma. Further, severe constipation after graciloplasty has almost always been a feature of stimulated cases [93].
Table 2.
Dynamic and adynamic graciloplasty as a supplement to total anorectal reconstruction
| Author [Ref] | Number | Dynamic/ adynamic | Complications | Function |
|---|---|---|---|---|
| Santoro [92] | 14 | 0/14 | 1 converted | 73% pseudocontinuous |
| Mander [61] | 10 | 10/0 | 80% | All wore pads |
| Geerdes [64] | 16 | 16/0 | 4 reconverted | 30% continent |
| Cavina [83] | 98 | 98/0 | 37% | 87% continent |
| Rullier [84] | 15 | 0/15 | 73% | 78% continent |
| Ho [86] | 17 | 17/0 | 40% | 45% continent |
| Simonsen [81] | 24 | 0/24 | 65% | 77% continent |
| Violi [85] | 23 | 15/8 | 37% | 75% continent |
| 87% dynamic | ||||
| 38% adynamic |
Table 3 shows the outcomes of perineal colostomy with a colonic smooth-muscle wrap and colonic irrigations as part of TAR. As patients do better with colonic irrigation, the value of a neosphincter remains somewhat questionable. Table 4 shows the outcomes if an artificial implanted sphincter device is used in TAR, and Table 5 shows the functional outcome if TAR incorporates an ACE procedure in the management. In this latter group, ileal/cecal/colonic conduit procedures are technically more complex and carry a higher morbidity rate than a simple appendicostomy. Late complications are usually related to stomal stenosis, which can be easily managed by a temporary catheter at night or by a surgical V–Y plasty. Stomal leakage and reflux may be prevented by a cecal imbrication—somewhat akin to a Nissen fundoplication [94]. This approach particularly appears very viable for young patients with AI and prior congenital anorectal anomalies [95]. Overall, the ACE procedure contributes to the avoidance of constipation after TAR when external sphincter reconstruction or substitution has been performed, and where it would appear that in all procedures in which ACE was associated, the good functional results are due to colonic irrigation rather than the other more complex aspects of the technique.
Table 3.
Data pertaining to smooth muscle neosphincters combined with colonic irrigation for total anorectal reconstruction
Table 4.
Artificial bowel sphincter use in total anorectal reconstruction
Table 5.
Combined procedures for total anorectal reconstruction with antegrade colonic irrigation
| Author [Ref] | Number | Complications | Functional status |
|---|---|---|---|
| Saunders [100] | 14 Continent colonic conduit + stimulated graciloplasty | 71% | 50% continent |
| Farroni [90] | 13 Malone cecal conduit | Not stated | 85% continent |
| Ardelean [101] | 9 Antegrade continence enema + posterior sagittal rectoplasty | Not reported | All continent and clean |
In summary, the role for TAR (and its preferred technique) is currently unclear. In its use, patients and their families need to be informed that continence will effectively never be perfect. The two main candidate groups for this procedure include those with imperfect continence after surgery for a congenital anomaly as children or infants, and those who request reconstruction after radical rectal extirpation for cancer. Patients must understand the morbidity of any proposed procedure and the reported likelihood of subsequent revisional surgery over time. In cancer, after exclusion of recurrent disease, other factors such as obesity, intra-abdominal adhesions, comorbidity, prior perineal irradiation and even age may be precluding conditions for TAR. The issue of immediate TAR vs delayed TAR is also controversial and somewhat akin to the argument of immediate vs delayed breast reconstruction after mastectomy. It would seem feasible to perform a perineal colostomy and an appendicostomy for ACE at the initial rectal excision in motivated cases, and this may be associated with quite minimal perineal morbidity in early selected cases (those with T1-2N0 tumors) when compared with the known perineal morbidity of primary perineal closure.
Conflict of interest: none declared.
REFERENCES
- 1.Lamah M, Kumar D. Fecal incontinence. Dig Dis Sci. 1999;44:2488–99. doi: 10.1023/a:1026643207180. [DOI] [PubMed] [Google Scholar]
- 2.Wald A. Faecal incontinence in adults. N Engl J Med. 2007;356:1648–55. doi: 10.1056/NEJMcp067041. [DOI] [PubMed] [Google Scholar]
- 3.Teunissen TA, van den Bosch WJ, van den Hoogen HJ, et al. Prevalence of urinary and fecal incontinence among community-dwelling elderly patients in Nijmegen, the Netherlands, January 1999–July 2001. Ned Tijdschr Geneeskd. 2006;150:2430–34. [PubMed] [Google Scholar]
- 4.Harari D. Faecal incontinence in older people. Rev Clin Gerontol. 2009;19:87–101. [Google Scholar]
- 5.Rothbarth J, Bemelman WA, Meijerink WJ, et al. What is the impact of fecal incontinence on quality of life? Dis Colon Rectum. 2001;44:67–71. doi: 10.1007/BF02234823. [DOI] [PubMed] [Google Scholar]
- 6.Deutekom M, Terra MP. Impact of fecal incontinence severity on health domains. Colorectal Dis. 2005;7:263–69. doi: 10.1111/j.1463-1318.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 7.Damon H, Guye O, Seigneurin A, et al. Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol. 2006;30:37–43. doi: 10.1016/s0399-8320(06)73076-7. [DOI] [PubMed] [Google Scholar]
- 8.Deutekom M, Dobben AC, Dijkgraaf MG, et al. Costs of outpatients with fecal incontinence. Scand J Gastroenterol. 2005;40:552–58. doi: 10.1080/00365520510012172. [DOI] [PubMed] [Google Scholar]
- 9.Friedman SM, Steinwachs DM, Rathouz PJ, et al. Characteristics for predicting nursing home admission in the program of all-inclusive care for elderly people. Gerontologist. 2005;45:157–66. doi: 10.1093/geront/45.2.157. [DOI] [PubMed] [Google Scholar]
- 10.Malouf Al Chambers MG, Kamm MA. Clinical and economic evaluation of surgical treatments for faecal incontinence. Br J Surg. 2001;88:1029–36. doi: 10.1046/j.0007-1323.2001.01807.x. [DOI] [PubMed] [Google Scholar]
- 11.Malone PS, Ransley PG, Kiely EM. Preliminary report: the antegrade continence enema. Lancet. 1990;336:1217–18. doi: 10.1016/0140-6736(90)92834-5. [DOI] [PubMed] [Google Scholar]
- 12. Keighley MR, Willams NS. Constipation. In: Keighley MR, Willams NS (ed.) Surgery of the anus, the rectum and colon. 2nd ed. London: W.B.Saunders, 1999, 701–55.
- 13.Mitrofanoff P. Cystostomie continente trans-appendiculire dans le traitement des vessies neurologigues. Chir Pediatr. 1980;21:297–305. [PubMed] [Google Scholar]
- 14.Yang WH. Yang needle tunneling technique in creating antireflux and continent mechanisms. J Urol. 1993;150:830–34. doi: 10.1016/s0022-5347(17)35625-2. [DOI] [PubMed] [Google Scholar]
- 15.Kiely EM, Ade-Ajayi N, Wheeler RA. Caecal flap conduit for antegrade continence enemas. Br J Surg. 1994;81:1215. doi: 10.1002/bjs.1800810847. [DOI] [PubMed] [Google Scholar]
- 16.Marsh PJ, Kiff ES. Ileocaecostomy: an alternative surgical procedure for antegrade colonic enema. Br J Surg. 1996;83:507–8. doi: 10.1002/bjs.1800830423. [DOI] [PubMed] [Google Scholar]
- 17.Shandling B, Chait PG, Richards HF. Percutaneous cecostomy: a new technique in the management of fecal incontinence. J Pediatr Surg. 1996;31:534–37. doi: 10.1016/s0022-3468(96)90490-x. [DOI] [PubMed] [Google Scholar]
- 18.Christensen P, Buntzen S, Krogh K, et al. Ileal neoappendicostomy for antegrade colonic irrigation. Br J Surg. 2001;88:1637–38. doi: 10.1046/j.0007-1323.2001.01959.x. [DOI] [PubMed] [Google Scholar]
- 19.Lynch AC, Beasley SW, Robertson RW, et al. Comparison of results of laparoscopic and open antegrade continence enema procedures. Pediatr Surg Int. 1999;15:343–46. doi: 10.1007/s003830050595. [DOI] [PubMed] [Google Scholar]
- 20.Hill J, Stott S, MacLennan I. Antegrade enemas for the treatment of severe idiopathic constipation. Br J Surg. 1994;81:1490–91. doi: 10.1002/bjs.1800811030. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon CA, Minevich E, Wacksman J, et al. Role of the antegrade continence enema in the management of the most debilitating childhood recto-urogenital anomalies. J Urol. 1997;158:1277–79. doi: 10.1097/00005392-199709000-00161. [DOI] [PubMed] [Google Scholar]
- 22.Malone PS, Curry JI, Osborne A. The antegrade continence enema procedure why, when and how? World J Urol. 1998;16:274–78. doi: 10.1007/s003450050066. [DOI] [PubMed] [Google Scholar]
- 23.Curry JI, Osborne A, Malone PS. How to achieve a successful Malone antegrade continence enema. J Pediatr Surg. 1998;33:138–41. doi: 10.1016/s0022-3468(98)90381-5. [DOI] [PubMed] [Google Scholar]
- 24.Curry JI, Osborne A, Malone PS. The MACE procedure: experience in the United Kingdom. J Pediatr Surg. 1999;34:338–40. doi: 10.1016/s0022-3468(99)90204-x. [DOI] [PubMed] [Google Scholar]
- 25.Yang CC, Stiens SA. Antegrade continence enema for the treatment of neurogenic constipation and fecal incontinence after spinal cord injury. Arch Phys Med Rehabil. 2000;81:683–85. doi: 10.1016/s0003-9993(00)90054-6. [DOI] [PubMed] [Google Scholar]
- 26.Christensen P, Kvitzau B, Krogh K, et al. Neurogenic colorectal dysfunction – use of new antegrade and retrograde colonic wash-out methods. Spinal Cord. 2000;38:255–61. doi: 10.1038/sj.sc.3100991. [DOI] [PubMed] [Google Scholar]
- 27.Rongen MJ, van der Hoop AG, Baeten CG. Cecal access for antegrade colon enemas in medically refractory slow-transit constipation: a prospective study. Dis Colon Rectum. 2001;44:1644–49. doi: 10.1007/BF02234385. [DOI] [PubMed] [Google Scholar]
- 28.Clark T, Pope JC, Adams C, et al. Factors that influence outcomes of the Mitrofanoff and Malone antegrade continence enema reconstructive procedures in children. J Urol. 2002;168:1537–40. doi: 10.1016/S0022-5347(05)64515-6. [DOI] [PubMed] [Google Scholar]
- 29.Dey R, Ferguson C, Kenny SE, et al. After the honeymoon – medium-term outcome of antegrade continence enema procedure. J Pediatr Surg. 2003;38:65–68. doi: 10.1053/jpsu.2003.50012. [DOI] [PubMed] [Google Scholar]
- 30.Teichman JM, Zabihi N, Kraus SR, et al. Longterm results for Malone antegrade continence enema for adults with neurogenic bowel disease. Urology. 2003;61:502–6. doi: 10.1016/s0090-4295(02)02282-3. [DOI] [PubMed] [Google Scholar]
- 31.Lees NP, Hodson P, Hill J, et al. Long-term results of the antegrade continent enema procedure for constipation in adults. Colorectal Dis. 2004;6:362–68. doi: 10.1111/j.1463-1318.2004.00669.x. [DOI] [PubMed] [Google Scholar]
- 32.Hirst GR, Arumugam PJ, Watkins AJ, et al. Antegrade continence enema in the treatment of obstructed defecation with or without faecal incontinence. Tech Coloproctol. 2005;9:217–21. doi: 10.1007/s10151-005-0230-5. [DOI] [PubMed] [Google Scholar]
- 33.Portier G, Ghouti L, Kirzin S, et al. Malone antegrade colonic irrigation: ileal neoappendicostomy is the preferred procedure in adults. Int J Colorectal Dis. 2006;21:458–60. doi: 10.1007/s00384-005-0031-3. [DOI] [PubMed] [Google Scholar]
- 34.Krogsgaard SM, Milling MD, Qvist N. Appendicostomy in the treatment of severe defecation disorders in children. Ugeskr Laeger. 2006;168:692–94. [PubMed] [Google Scholar]
- 35.Lefevre JH, Parc Y, Giraudo G, et al. Outcome of antegrade continence enema procedures for faecal incontinence in adults. Br J Surg. 2006;93:1265–69. doi: 10.1002/bjs.5383. [DOI] [PubMed] [Google Scholar]
- 36.Poirier M, Abcarian H, Nelson R. Malone antegrade continent enema: an alternative to resection in severe defecation disorders. Dis Colon Rectum. 2007;50:22–28. doi: 10.1007/s10350-006-0732-x. [DOI] [PubMed] [Google Scholar]
- 37.Altomare DF, Rinaldi M, Rubini D, et al. Long-term functional assessment of antegrade colonic enema for combined incontinence and constipation using a modified Marsh and Kiff technique. Dis Colon Rectum. 2007;50:1023–31. doi: 10.1007/s10350-006-0863-0. [DOI] [PubMed] [Google Scholar]
- 38.Koivusalo AI, Pakarinen MP, Pauniaho SL, et al. Antegrade continence enema in the treatment of congenital fecal incontinence beyond childhood. Dis Colon Rectum. 2008;51:1605–10. doi: 10.1007/s10350-008-9327-z. [DOI] [PubMed] [Google Scholar]
- 39.Bani-Hani AH, Cain MP, Kaefer M, et al. The Malone antegrade continence enema: single institutional review. J Urol. 2008;180:1106–10. doi: 10.1016/j.juro.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 40.Worsoe J, Christensen P, Krogh K, et al. Long-term results of antegrade colonic enema in adult patients: assessment of functional results. Dis Colon Rectum. 2008;51:1523–28. doi: 10.1007/s10350-008-9401-6. [DOI] [PubMed] [Google Scholar]
- 41.Meurette G, Lehur PA, Coron E, et al. Long-term results of Malone’s procedure with antegrade irrigation for severe chronic constipation. Gastroenterol Clin Biol. 2010;34:209–12. doi: 10.1016/j.gcb.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Driver CP, Barrow C, Fishwick J, et al. The Malone antegrade colonic enema procedure: outcome and lessons of 6 years’ experience. Pediatr Surg Int. 1998;13:370–72. doi: 10.1007/s003830050342. [DOI] [PubMed] [Google Scholar]
- 43.Tackett LD, Minevich E, Benedict JF, et al. Appendiceal versus ileal segment for antegrade continence enema. J Urol. 2002;167:683–86. doi: 10.1016/S0022-5347(01)69125-0. [DOI] [PubMed] [Google Scholar]
- 44.Lynch CR, Jones RG, Hilden K, et al. Percutaneous endoscopic cecostomy in adults: a case series. Gastrointest Endosc. 2006;64:279–82. doi: 10.1016/j.gie.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 45. National Institute for Health and Clinical Excellence. Percutaneous endoscopic colostomy: interventional procedure guidance (IPG161). http://guidance.nice.org.uk/IPG161/Guidance/pdf/English. [22 Mar 2006]
- 46.Rawat DJ, Haddad M, Geoghegan N, et al. Percutaneous endoscopic colostomy of the left colon: a new technique for management of intractable constipation in children. Gastrointest Endosc. 2004;60:39–43. doi: 10.1016/s0016-5107(04)01286-6. [DOI] [PubMed] [Google Scholar]
- 47.Baraza W, Brown S, McAlindon M, et al. Prospective analysis of percutaneous endoscopic colostomy at a tertiary referral centre. Br J Surg. 2007;94:1415–20. doi: 10.1002/bjs.5858. [DOI] [PubMed] [Google Scholar]
- 48.Eccersley AJ, Maw A, Williams NS. Comparative study of two sites of colonic conduit placement in the treatment of constipation due to rectal evacuatory disorders. Br J Surg. 1999;86:647–50. doi: 10.1046/j.1365-2168.1999.01071.x. [DOI] [PubMed] [Google Scholar]
- 49.Monti PR, Lara RC, Dutra MA, et al. New techniques for construction of efferent conduits based on the Mitrofanoff principle. Urology. 1997;49:112–15. doi: 10.1016/S0090-4295(96)00503-1. [DOI] [PubMed] [Google Scholar]
- 50.Bruce RG, el Galley RE, Wells J, et al. Antegrade continence enema for the treatment of fecal incontinence in adults: use of gastric tube for catheterizable access to the descending colon. J Urol. 1999;161:1813–16. [PubMed] [Google Scholar]
- 51.O’Bichere A, Sibbons P, Dore C, et al. Experimental study of faecal continence and colostomy irrigation. Br J Surg. 2000;87:902–8. doi: 10.1046/j.1365-2168.2000.01444.x. [DOI] [PubMed] [Google Scholar]
- 52.Christensen P, Krogh K, Buntzen S, et al. Long-term outcome and safety of transanal irrigation for constipation and fecal incontinence. Dis Colon Rectum. 2009;52:286–92. doi: 10.1007/DCR.0b013e3181979341. [DOI] [PubMed] [Google Scholar]
- 53.Mattsson S, Gladh G. Tap-water enema for children with myelomeningocele and neurogenic bowel dysfunction. Acta Paediatr. 2006;95:369–74. doi: 10.1080/08035250500437507. [DOI] [PubMed] [Google Scholar]
- 54.Mendoza J, Legido J, Rubio S, et al. Systematic review: the adverse effects of sodium phosphate enema. Aliment Pharmacol Ther. 2007;26:9–20. doi: 10.1111/j.1365-2036.2007.03354.x. [DOI] [PubMed] [Google Scholar]
- 55.Chittenden AS. Reconstruction of anal sphincter by muscle slips from glutei. Ann Surg. 1930;92:152–54. [Google Scholar]
- 56.Margottini M. L’amputazione abdomino-perineale del retto con abbassamento del colon al perineo. LII Congr SIC. 1950:181–86. [Google Scholar]
- 57.Penninckx F, D’Hoore A, Vanden Bosch A. Perineal colostomy with antegrade continence enemas as an alternative after abdominoperineal resection for low rectal cancer. Ann Chir. 2005;130:327–30. doi: 10.1016/j.anchir.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 58.Ziv Y, Zbar A, Bar-Shavit Y, et al. Low anterior resection syndrome (LARS): cause and effect and reconstructive considerations. Tech Coloproctol. 2013;17:151–62. doi: 10.1007/s10151-012-0909-3. [DOI] [PubMed] [Google Scholar]
- 59.Fazio VW, Zutshi M, Remzi FH, et al. A randomized multicenter trial to compare long-term functional outcome, quality of life, and complications of surgical procedures for low rectal cancers. Ann Surg. 2007;246:481–90. doi: 10.1097/SLA.0b013e3181485617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hughes SF, Scott SM, Pilot MA, et al. Electrically stimulated colonic reservoir for total anorectal reconstruction. Br J Surg. 1995;82:1321–26. doi: 10.1002/bjs.1800821009. [DOI] [PubMed] [Google Scholar]
- 61.Mander BJ, Abercrombie JF, George BD, et al. The electrically stimulated gracilis neosphincter incorporated as part of total anorectal reconstruction after abdominoperineal excision of the rectum. Ann Surg. 1996;224:702–11. doi: 10.1097/00000658-199612000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rouanet P, Senesse P, Bouamrirene D, et al. Anal sphincter reconstruction by dynamic graciloplasty after abdominoperineal resection for cancer. Dis Colon Rectum. 1999;42:451–56. doi: 10.1007/BF02234165. [DOI] [PubMed] [Google Scholar]
- 63.Vorobiev GI, Odaryuk TS, Tsarkov PV, et al. Resection of the rectum and total excision of the internal anal sphincter with smooth muscle plasty and colonic pouch for treatment of ultralow rectal carcinoma. Br J Surg. 2004;91:1506–12. doi: 10.1002/bjs.4330. [DOI] [PubMed] [Google Scholar]
- 64.Geerdes BP, Zoetmulder FA, Heineman E, et al. Total anorectal reconstruction with a double dynamic graciloplasty after abdominoperineal reconstruction for low rectal cancer. Dis Colon Rectum. 1997;40:698–705. doi: 10.1007/BF02140900. [DOI] [PubMed] [Google Scholar]
- 65.Williams NS, Hallan RI, Koeze TH, et al. Construction of a neorectum and neoanal sphincter following previous proctocolectomy. Br J Surg. 1989;76:1191–94. doi: 10.1002/bjs.1800761124. [DOI] [PubMed] [Google Scholar]
- 66.Fazio VW, Mantyh CR, Hull TL. Colonic. “coloplasty”: novel technique to enhance low colorectal or coloanal anastomosis. Dis Colon Rectum. 2000;43:1448–50. doi: 10.1007/BF02236645. [DOI] [PubMed] [Google Scholar]
- 67.Devesa JM, López-Hervás P, Vicente R, et al. Total anorectal reconstruction: a novel technique. Tech Coloproctol. 2005;9:149–52. doi: 10.1007/s10151-005-0215-4. [DOI] [PubMed] [Google Scholar]
- 68.Torres RA, Gonzalez MA. Perineal continent colostomy. Report of a case. Dis Colon Rectum. 1988;31:957–60. doi: 10.1007/BF02554894. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt E. The continent colostomy. World J Surg. 1982;6:805–9. doi: 10.1007/BF01655382. [DOI] [PubMed] [Google Scholar]
- 70.Fedorov VD, Odaryuk TS, Shelygin YA, et al. Method of creation of a smooth-muscle cuff at the site of perineal colostomy after extirpation of the rectum. Dis Colon Rectum. 1989;32:562–66. doi: 10.1007/BF02554174. [DOI] [PubMed] [Google Scholar]
- 71.Pescatori M, Caracciolo F, Anastasio G. Restoration of intestinal continuity after rectal excision by electrostimulated smooth and striated muscles. BAM. 1991;1:259–62. [Google Scholar]
- 72.Elias D, Lasser P, Leroux A, et al. Colostomies perineales pseudo-continente apres amputacion rectal pour cancer. Gastroenterol Clin Biol. 1993;17:181–86. [PubMed] [Google Scholar]
- 73.Lasser PH, Dubé P, Grillot JM, et al. Colostomie perineale pseudocontinente. J Chir. 1997;134:174–79. [PubMed] [Google Scholar]
- 74.Gamagami RA, Chiotasso P, Lazorthes F. Continent perineal colostomy after abdominoperineal resection: outcome after 63 cases. Dis Colon Rectum. 1999;42:626–31. doi: 10.1007/BF02234140. [DOI] [PubMed] [Google Scholar]
- 75.Portier G, Bonhomme N, Platonoff I, et al. Use of Malone antegrade continence enema with perineal colostomy after rectal resection. Dis Colon Rectum. 2005;48:499–503. doi: 10.1007/s10350-004-0802-x. [DOI] [PubMed] [Google Scholar]
- 76.Lorenzi M, Vernillo R, Garzi A, et al. Experimental internal anal sphincter replacement with demucosated colonic plication. Tech Coloproctol. 2003;7:9–16. doi: 10.1007/s101510300002. [DOI] [PubMed] [Google Scholar]
- 77.Farid M, Moneim HA, Mahdy T, et al. Augmented unilateral gluteoplasty with fascia lata graftin fecal incontinence. Tech Coloproctol. 2003;7:23–28. doi: 10.1007/s101510300004. [DOI] [PubMed] [Google Scholar]
- 78.Cong J, Chen CH, Zhang H, et al. Using the gluteus muscle to reconstruct the anal sphincter for very low rectal cancer. Chin J Clin Oncol. 2007;4:98–102. [Google Scholar]
- 79.Fedorov VD, Shelygin YA. Treatment of patients with rectal cancer. Dis Colon Rectum. 1989;32:138–45. doi: 10.1007/BF02553827. [DOI] [PubMed] [Google Scholar]
- 80.Jacob ET, Shapira Z, Bar-Natan N, et al. Total anorectal reconstruction following congenital anorectal anomaly. Dis Colon Rectum. 1976;19:172–77. doi: 10.1007/BF02590876. [DOI] [PubMed] [Google Scholar]
- 81.Simonsen OS, Stolf NA, Aun F, et al. Rectal sphincter reconstruction in perineal colostomies after abdominoperineal resection for cancer. Br J Surg. 2005;63:389–91. doi: 10.1002/bjs.1800630514. [DOI] [PubMed] [Google Scholar]
- 82.Wong SK, Wee JT. Reconstruction of an orthotopic functional anus after abdominoperineal resection. Aust N Z J Surg. 1984;54:575–78. doi: 10.1111/j.1445-2197.1984.tb05450.x. [DOI] [PubMed] [Google Scholar]
- 83.Cavina E, Seccia M, Chiarugi M. Total anorectal reconstruction supported by electrostimulated gracilis neosphincter. Recent results. Cancer Res. 1998;146:104–13. doi: 10.1007/978-3-642-71967-7_10. [DOI] [PubMed] [Google Scholar]
- 84.Rullier E, Zerbib F, Laurent C, et al. Morbidity and functional outcome after double dynamic graciloplasty for anorectal reconstruction. Br J Surg. 2000;87:909–13. doi: 10.1046/j.1365-2168.2000.01447.x. [DOI] [PubMed] [Google Scholar]
- 85.Violi V, Boselli A, De Bernardinis M, et al. Anorectal reconstruction by electrostimulated graciloplasty as part of abdominoperineal resection. Eur J Surg Oncol. 2005;31:250–58. doi: 10.1016/j.ejso.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 86.Ho KS, Seow-Choen F. Dynamic graciloplasty for total anorectal reconstruction after abdominoperineal resection for rectal tumour. Int J Colorectal Dis. 2005;20:38–41. doi: 10.1007/s00384-004-0622-4. [DOI] [PubMed] [Google Scholar]
- 87.Cera SM, Wexner SD. Muscle transposition: does it still have a role? Clin Colon Rectal Surg. 2005;18:46–54. doi: 10.1055/s-2005-864081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Romano G, La Torre F, Cutini G, et al. Total anorectal reconstruction with the artificial bowel sphincter: report of eight cases. A quality of life assessment. Dis Colon Rectum. 2003;46:730–34. doi: 10.1007/s10350-004-6649-3. [DOI] [PubMed] [Google Scholar]
- 89.Chiotasso P, Schmitt L, Juricic M. Acceptation des stomies perineales. Gastroenterol Clin Biol. 1992;16:200. [Google Scholar]
- 90.Farroni N, Van den Bosch A, Haustermans K, et al. Perineal colostomy with appendicostomy as an alternative for an abdominal colostomy: symptoms, functional status, quality of life, and perceived health. Dis Colon Rectum. 2007;50:817–24. doi: 10.1007/s10350-007-0229-2. [DOI] [PubMed] [Google Scholar]
- 91.Cavina E, Seccia M, Banti P, et al. Quality of life after total anorectal reconstruction: long-term results. Chir Ital. 2000;52:457–62. [PubMed] [Google Scholar]
- 92.Santoro E, Tirelli C, Scutari F, et al. Continent perineal colostomy by transposition of gracilis muscles. Dis Colon Rectum. 1994;37(Suppl):S73–80. doi: 10.1007/BF02048436. [DOI] [PubMed] [Google Scholar]
- 93.Rosen HR, Urbarz C, Novi G, et al. Long-term results of modified graciloplasty for sphincter replacement after rectal excision. Colorectal Dis. 2002;4:266–69. doi: 10.1046/j.1463-1318.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 94. Ransley GP. The VQZ plasty for catheterizable stomas. In: Frank DJ, Gearhart JP, Snyder III HM (ed.) Operative pediatric urology. London/Edinburgh/New York/Philadelphia/St Louis/Sydney/ Toronto: Churchill Livingstone, 2002, 10914. [Google Scholar]
- 95.Ardelean M-A, Bauer J, Schimke C, et al. Improvement of continence with reoperation in selected patients after surgery for anorectal malformation. Dis Colon Rectum. 2009;52:112–18. doi: 10.1007/DCR.0b013e3181972333. [DOI] [PubMed] [Google Scholar]
- 96.Pocard M, Sideris L, Zenasni F, et al. Functional results and quality of life for patients with very low rectal cancer undergoing coloanal anastomosis or perineal colostomy with colonic muscular graft. Eur J Surg Oncol. 2007;33:459–62. doi: 10.1016/j.ejso.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 97.Hirche C, Mrak K, Kneif S, et al. Perineal colostomy with spiral smooth muscle graft for neosphincter reconstruction following abdominoperineal resection of very low rectal cancer: long-term outcome. Dis Colon Rectum. 2010;53:1272–79. doi: 10.1007/DCR.0b013e3181e74c1f. [DOI] [PubMed] [Google Scholar]
- 98.Lirici MM, Ishida Y, Di Paola M, et al. Dynamic graciloplasty versus implant of artificial sphincter for continent perineal colostomy after Miles’ procedure: technique and early results. Minim Invasive Ther Allied Technol. 2004;13:347–61. doi: 10.1080/13645700410006616. [DOI] [PubMed] [Google Scholar]
- 99.Ocares M, Caselli G, Caselli B. Artificial bowel sphincter for anorectal reconstruction. Preliminary report and review of surgical technique. Rev Chil Cir. 2009;61:350–55. [Google Scholar]
- 100.Saunders JR, Williams NS, Eccersley AJP. The combination of electrically stimulated gracilis neoanal sphincter and continent colonic conduit: a step forward for total anorectal reconstruction? Dis Colon Rectum. 2004;47:354–66. doi: 10.1007/s10350-003-0061-2. [DOI] [PubMed] [Google Scholar]
- 101.Ardelean M-A, Bauer J, Schimke C, et al. Improvement of continence with reoperation in selected patients after surgery for anorectal malformation. Dis Colon Rectum. 2009;52:112–18. doi: 10.1007/DCR.0b013e3181972333. [DOI] [PubMed] [Google Scholar]