SUMMARY
While it is well established that the principal ascending pathways for pain originate in the dorsal horn of the spinal cord and in the medulla, the control and sensitivity to pain may reside in additional neurological loci, especially in the mesolimbic system of the brain (i.e., a reward center), and a number of genes and associated polymorphisms may indeed impact pain tolerance and or sensitivity. It is hypothesized that these polymorphisms associate with a predisposition to intolerance or tolerance to pain. It is further hypothesized that identification of certain gene polymorphisms provides a unique therapeutic target to assist in the treatment of pain. It is hereby proposed that pharmacogenetic testing of certain candidate genes (i.e., mu receptors, PENK etc.) will result in pharmacogenomic solutions personalized to the individual patient, with potential improvement in clinical outcomes.
Background
It is well known that individuals respond differently to medications and certain nutraceuticals, in terms of both toxicity and treatment efficacy [1]. Potential causes for such variability in drug (nutrient) effects include the pathogenesis and severity of the disease being treated: drug (nutrient) interactions; the individual’s age; nutritional status; kidney and liver function; and concomitant illnesses. Despite the potential importance of these clinical variables in determining drug/nutrient effects, it is now recognized that inherited differences in the metabolism and disposition of drugs/nutrients, and genetic variants (polymorphisms) in the targets of drug/nutrient therapy (such as receptors like the dopamine D2 receptor), can have even greater influence on the efficacy and toxicity of medications and of nutraceuticals [2].
Clinical observations of such inherited differences in drug effects were first documented in the 1950s, exemplified by the prolonged muscle relaxation after the drug became known as suxamethonium (an inhibitor of the breakdown of acetylcholine) and by an inherited deficiency in the genes that encode the enzyme responsible for the breakdown of this drug as marked by plasma cholinesterase (the enzyme which breaks down acetylcholine) [3]. The second gene-based drug response was observed when researchers found that certain patients bled to death after they were treated with an anti-malarial therapy, because they carried a gene variant that lowered their blood cell glucose 6-phosphate dehydrogenase activity [4]. Such observations gave rise to the field of ‘pharmacogenetics’, the antecedent to pharmacogenomics, the current topic. However, we now know that individual differences in response to drugs and/or nutrients are not due to single gene variants; rather, they are determined by the interplay of several genes encoding proteins (enzymes, receptors, and transporters) involved in multiple pathways of drug/nutrient metabolism, disposition, and effects [5]. We are embarking on new era where efficacy of any substance is governed by an individual’s inherited genotype to a greater degree than even other non-genetic factors. Understanding structure/function normal physiology, as well as certain observable dysfunctions, may indeed lead to promising nutrient-based targets. However, without the knowledge afforded by accurate DNA-based prescreening (genotyping), subsequent supplementation may be hit-or-miss. Similar to the pharmaceutical industry, the nutraceutical industry can become an equal opportunity player, and begin to initiate ongoing research and development by incorporating these genome-based doctrines as described herein.
Out of the three million unshared DNA bases, individuals could carry gene variants (polymorphisms) that might lead to either an increase or a decrease of certain important drug/nutrient response-related proteins such as receptors, enzymes, cell cycle control, chemical messenger synthesis or catabolism (breakdown), or many other cellular events. As stated earlier, while there is a paucity of molecular studies involving genome-based response in the nutrition field (see below), a plethora of molecular studies have revealed that many genes encoding drug targets exhibit genetic polymorphisms, which in many cases alter their sensitivity to specific medications and/or offer specific targeted therapy.
Pharmacogenetics is the study of the role of genetics in inter-individual variability to drug response and therapy. In this regard, we found more than 200 PubMed reports concerning pharmacogenetic studies for opioid drugs. Opioid analgesics are widely used clinically for pain management, and inter-patient variability with opioid therapy is often reported [6]. Information on genetic polymorphisms in enzymes, receptors, and transporters related to opioid disposition (pharmacokinetics) and pharmacology (pharmacodynamics) is documented [7]. Pharmacogenetics of enzymes, including the cytochrome P450s and uridine diphosphoglucuronosyltransferases, opioid receptors and the ABC family of transporters, are a few examples.
Dopamine and Pain
The principal ascending pathways for pain (e.g., the spinothalamic tract) originate mainly in the dorsal horn of the spinal cord and in the medulla, wherein second order neurons receive synaptic input from primary afferent neurons that supply nociceptors in tissue. The second order neurons of origin are within layer I as well as deep layers (IV–VI) of the dorsal horn [8]. Second order neurons of origin of pain-related pathways are mainly wide-dynamic-range neurons or nociceptive-specific neurons, and these two types of neurons process both exteroceptive and interoceptive information associated with pain. Our cutaneous nociceptive system clearly serves as an exteroreceptive role in signaling potentially dangerous stimuli impinging upon our bodies, so that we can respond appropriately, depending upon the situational context. Our interoreceptive nociceptive system signals tissue disorders (e.g., rheumatoid) that are essentially inescapable, and it calls for responses more obviously in the homeostatic domain.
Pharmacological aspects of pain control
Opioids such as morphine and heroin and psychostimulant drugs such as amphetamine and cocaine are effective pharmacological tools against chronic pain. Interestingly, amphetamine and related drugs relieve cancer pain and sometimes administered as an adjuvant analgesic in the clinical situation, because they potentiate opioid analgesia and counter opioid-related sedation and cognitive disturbances. In support of these clinical findings, studies have shown that, in rats, psychostimulants potentiate the analgesic effect of morphine in an animal model of persistent pain [9]. There is increasing evidence that sites rostral to the brainstem play a critical role in the analgesic effects of opioid and psychostimulant drugs.
It is well known that opioids can inhibit pain by acting at spinal sites and at sites in the brainstem, where they modulate activity in descending brainstem pathways projecting to the spinal cord. A primary site of action is the periacqueductal gray of the brainstem where stimulation of opioid receptors activates, through direct projections, serotonin-containing cells in the nucleus raphe magnus. In turn, the latter cells activate neurons that project, via the dorsolateral funiculus, to the dorsal horns of the spinal cord where they inhibit cells that transmit information about noxious painful stimulation from the periphery to supraspinal sites. The brainstem descending pain-suppression system, however, plays a more important role in the suppression of brief, rapidly rising, transient, and well-localized (i.e., phasic) pain than it does in the suppression of injury-produced persistent (i.e., tonic) and inescapable pain. However, several lines of evidence suggest that the inhibition of the tonic pain requires the activation of neural systems in addition to those require the activation of neural system in addition to those required to inhibit phasic pain [10, 11].
Mesolimbic dopamine in the suppression of tonic pain
There is little information to date concerning the identity of the endogenous pain systems that serve to inhibit tonic pain. The suppression of tonic pain involves systems in addition to those known to suppress phasic pain, and that these systems appear to involve forebrain sites, rostral to the brainstem. A clue to this problem is that both opioids and psychostimulants reduce tonic pain and increase transmission in mesocorticolimbic dopamine neurons known to be activated by natural rewards such as food and sex. These neurons arise from dopamine cell bodies that lie in the ventral tegmental area (VTA), and project to various forebrain sites such as the nucleus accumbens (Nacc), amygdala, and prefrontal cortex. Opioids cause the release of dopamine from these neurons through their indirect activation (see reward cascade Fig. 1), whereas psychostimulant drugs such as amphetamine and cocaine increase dopamine extracellularly by decreasing reuptake and/or inducing release. Moreover, opioids and psychostimulants have both rewarding and analgesic effects in the clinical setting, suggesting that three effects might share common neural substrates [12], and it was found that dopamine–depleting 6-hydroxydopamine lesions of the ventral midbrain, which contains the cell bodies of the neurons that give rise to ascending forebrain projections, block the analgesic effects of systemic morphine and amphetamine in the formalin, but not the tail flick test. Their findings provided the first evidence that mesolimbic dopamine neurons play a role in the suppression of tonic, but not in the phasic pain. In the recent studies, Taylor et al. [13] found that while the D1-selective agonist SKF38393 was without effect at a dose of 0.5 nmol/side, the D2-selective agonist quinpirole dose dependency (0.05–5.0 nmol/side, bilateral) inhibited the persistent phase of formalin-induced nociception. This was blocked by pre-administration of a selective D2-dopaminergic antagonist raclopride. These results indicate that dopamine agonists that activate D2 receptors in the Nacc inhibit inflammatory pain.
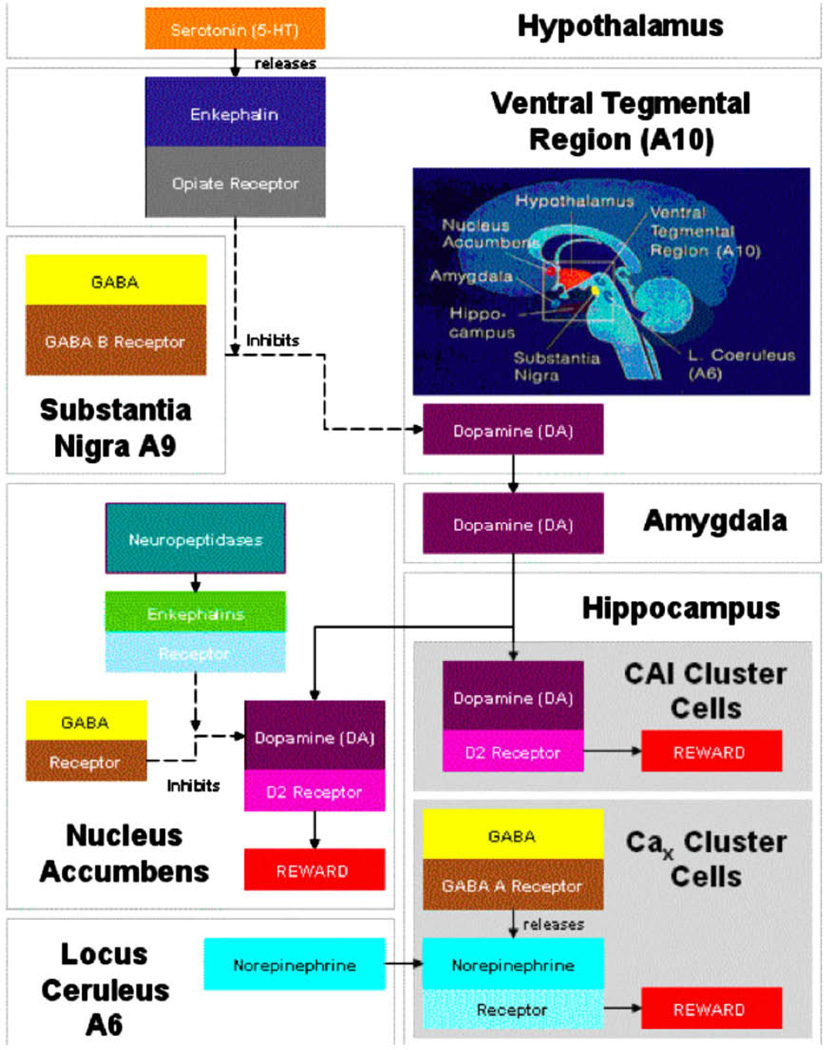
Figure 1.
Brain Reward Cascade. In this cascade, stimulation of the serotonergic system in the hypothalamus leads to the stimulation of delta/mu receptors by serotonin to cause a release of enkephalins. Activation of the enkephalinergic system induces an inhibition of GABA transmission at the substantia nigra by enkephalin stimulation of mu receptors at GABA neurons. Both the endocannabinoid and glutamate receptors influence GABERGIC activity. This inhibitory effect allows for the fine tuning of GABA activity. This provides the normal release of dopamine at the projected area of the n. accumbens (a major reward site of the brain). With permission of Gene Therapy Press [44].
Dopamine D2 receptors and chronic pain
Plastic changes in synaptic neurotransmission in the brain are thought to play a role in chronic pain. Animal studies suggest that striatal and cortical dopaminergic systems participate in pain transmission or modulation. Dopamine D2 receptors have been reported to mediate the inhibitory role of dopamine in animal models for persistent pain [14]. Hagelberg et al. [15] showed in healthy volunteers that high D2 receptor availability in the putamen is associated with low cold pain threshold and a high pain modulation capacity induced by conditioning stimulation. This effect involves mu receptor interaction [16]. Furthermore, decreased [18F] FDOPA uptake and increased D2 receptor availability have been demonstrated in the putamen in a chronic orofacial pain state, the burning mouth syndrome [17].
Moreover, it was found that the increase in D2 receptor availability in the left putamen and the decrease in D1/D2 ratio imply that alterations in the striatal dopamineric system as evaluated by PET may be involved in chronic orofacial pain conditions. In essence, we hypothesize that low or hypodopaminergic function in the brain may predispose individuals to low pain tolerance. Current research would support this concept, and thus carriers of the D2 TaqA1 allele as observed in reward deficiency syndrome (RDS) [18] behaviors may be good candidates for nutrients or bioactive substances designed to enhance dopamine release in the brain.
Stress and pain
The effects of excessive stress in modern life lead to chronic states of fatigue-related depression. According to the American Academy of Family Physicians, about 2/3 of all office visits are related to stress and depression. Therefore, it is important to understand that it is our position that indeed in an individual with chronic pain, the subject is definitely in a stressful condition and has increased neuronal firing. There are numerous examples in the literature to support this contention. Furthermore, if an individual has the DRD2A1 variant, numerous studies have shown that resultant low dopamine D2 receptors caused an inability to cope with stress in the family and as an individual [19–21]. In this regard, it is known that stress could even reduce D2 receptor mRNA message in the substantia nigra, the lateral part of the VTA, the basal ganglia, and especially in the nucleus accumbens [22]. This polymorphism as well as others could not only affect the ability to cope with stress but this in itself can alter one’s pain sensitivity.
Nutrients and anti-stress
This work supports the concept that forebrain dopamine systems are involved in mediating the behavioral effects of chronic mild stress. It further supports the view that in subjects with pain (with chronic mild to moderate stress) with a compromised number of D2 receptor sites and reduced mRNA message, the firing frequency of a catecholaminergic neuron is enhanced and would be quite receptive to l-tyrosine, a dopamine precursor. Moreover, it is also known that neuronal depletion of dopamine could also induce an independent end-product inhibitory state for tyrosine–hydroxylase, which will also respond to l-tyrosine supplementation. In this regard, in order to provide an up-regulation in D2 receptors, we proposed a slow release personalized designed natural solution providing a constant dopamine release, because of the effect of enhanced opioidergic activity ‘via d-phenylalanine (a known enkephalinase inhibitor) on substania nigra GABA neurons. The main point here is that pharmacological manipulation of up-regulation of dopaminergic pathways will ultimately lead to the reduction of stress, since it is well known that the dopamine molecule is considered as the endogenous anti-stress substance.
Stress and dopamine: implications for the pathophysiology of chronic widespread pain
The relationship between stress, endorphins and hypothalmic-pituitary-adrenal (HPA) axis is well researched [23]. Certainly in the world of addiction stress plays a critical role in both the acquisition and relapse. It is known that certain genetic and environmental elements play significant roles in drug dependency and dysregulation of brain reward pathways. In fact, dopamine D2 receptor polymorphisms have been associated with stress-coping mechanisms and posttraumatic stress disorder [24]. Interestingly, either stress can induce a painful condition or it can exacerbate the pain. Exposure to stress also activates dopamine transmission in mesocorticolimbic dopamine neurons [25], and this effect appears to involve opioid mechanisms in the VTA. More specifically, intra-VTA infusions of the opioid receptor antagonist Naltrexone, prevent the stress-induced activation of dopamine metabolism in the NAcc and prefrontal cortex, and exposure to stress causes the release of met-enkephalin into the VTA [26]. These findings combined with those indicating that exposure to stress can inhibit tonic pain and that intra-VTA morphine induces analgesia in the formalin test suggest that the endogenous release of opioids in the VTA might be a mechanism underlying the stress-induced inhibition of tonic pain. This has been supported by the finding that intra-VTA infusions of the opioid receptor antagonist, naltrexone, block stress-induced analgesia in the formalin test [3]. In addition, it has been proposed that release of the tachykinin neuropeptide, substance P (SP), in the VTA might play a similar role in the stress-induced suppression of tonic pain. Moreover, it has been found that activation of midbrain dopamine neurons by SP did indeed inhibit tonic pain in the formalin test [10]. The current data suggest that exposure to stress induces analgesia by causing a release of SP in the VTA, which in turn activates mesocorticolimbic dopamine neurons. Finally, opioids, anphetamine, and SP all share the ability to increase dopamine release in the NAcc. Moreover, opioids administered systemically or into the VTA augment dopamine metabolism and extracellular levels of dopamine in the NAcc.
With that background, it becomes increasingly clear that tonic pain maybe attenuated by dopamine D2 activation. It follows then that in this hypothesis we embrace the concept that supportive research in the area of developing a natural method to cause a preferential release of dopamine in mesocorticolimbic pathways seems warranted. Thus, support of an attenuation of stress has been found with a variant of a complex with dopaminergic activation properties shown in one double-blind placebo-controlled study [27. We further hypothesize herein that unless there is a way of increasing endogenous opioids, which in turn inhibit GABA causing dopamine release in the NAcc, simple neurotransmitter precursors will not be as effective in reducing tonic pain.
Fibromyalgia
One example of how stress and dopamine may interact involves fibromyalgia (FM), which has been called a ‘stress-related disorder’ due to the onset and exacerbation of symptoms on the context of stressful events [28].
The cardinal feature of FM is pain, the experience of which involves both afferent and efferent processes. While exposure to acute stress is known to produce stress-induced analgesia, the induction of which depends on dopamine-containing neurons within the NAcc, rat studies have demonstrated that prolonged exposure to stress eliminates this response, resulting instead in a state of stress-induced hyperalgesia [29]. Chronic stress has been shown to result in the attenuation of dopaminergic activity within the NAcc, and is therefore proposed to contribute to the development of stress-related hyperalgesia.
Interestingly, in FM patients clinical studies have suggested a disruption of dopaminergic function, including but not limited to decreased dopamine metabolites in cerebrospinal fluid [30, 31]. A variety of stressors result in the release of dopamine within the NAcc, including acute psychological stress, a cornerstone symptom of FM [32]. Thus, a vicious cycle occurs whereby stress from the pain further exacerbates the release of dopamine, which in turn results in a hyperalgesia state. Hyperalgesia to both thermal and chemical stimulants persists up to nine days after stress exposure in rats [33]. Moreover, other neurotransmitters are also involved as well. The selective 5-HT reuptake inhibitors clomipramine and fluextine, as well as the 5-HT reuptake precursor tryptophan, blocks the development of hyperalgesia, suggesting that repeated stress produces a long-lasting increase in pain sensitivity. In fact, whereas there is a disruption of both serotonergic and dopaminergic functions that occur within the NAcc following chronic stress, the impact on dopamine outlasts that on 5-HT. In this regard, there are three possibilities which have been proposed: (1) there is regulatory interaction between 5-HT and dopamine during stress-induced analgesia; (2) a disruption of this interaction contributes to the inception of stress-induced hyperalgesia; and (3) dopaminergic dysfunction, which outlasts that of 5-HT, may be responsible for the persistent expression of stress-induced hyperalgesia after serotonergic function has been normalized. This phenomenon may explain why strategies aimed at boosting serotonergic function only on patients with chronic widespread pain have met with limited success insofar as analgesia is concerned. Thus since FM is a stress-related disorder, one would predict that strategies aimed at boosting dopaminergic function within the mesolombic pathway would have superior efficacy. While no one has attempted combining therapies in term of multiple pharmacogenomic targets, and the outcome of such an attempt is unknown, on this provision we are proposing that natural manipulation of the reward signaling and circuitry could become very commercially viable. Breaking of this cycle with a stress-reducing substance, such as passion flower (see below) or the proposed Synaptamine, is clearly warranted.
Genes and opiate addiction: a pharmacogenomic trieste
It has been appreciated for some time now that humans react differently to opioids. A specific opioid such as morphine sulfate may have specific analgesic effects for certain patients with postherpetic neuralgia, whereas in other patients with postherpetic neuralgia it may provide quite different analgesic qualities. Also, in any one individual patient a particular opioid may provide better analgesia than other opioids. Furthermore, these differences are not unique to analgesia; they can also be seen with other opioid effects/toxicities. Although many of the differences can be classified neatly into pharmacokinetic and pharmacodynamic differences, there are certain differences that still remain incompletely understood. Also, clinicians are not yet able to easily predict which patients will respond well or poorly to various opioids. As research unravels the various genetics, biochemical, and receptor interaction differences of opioids in humans, it is hoped that easily obtainable, cost-effective testing will become available to aid clinicians in choosing an optimal opioid analgesic for an individual patient, a process that is currently accomplished via health care provider judgment along with trial and error. In the future, knowledge gained from databases on knockout rodents, pharmacogenetics, and gene polymorphisms may impact on the ability of clinicians to predict patient responses to doses of specific opioids in efforts to individualize optimal opioid analgesic therapy. It is conceivable that eventually information of this type may translate into improved patient care. In the future, armed with data of this type, clinicians may become quite adept at tailoring appropriate opioid therapy as well as optimal opioid rotation strategies. Currently, it is not obvious as to what gene or genes are perfect candidates for gene-directed opioid therapy.
In terms of pain sensitivity, certain candidate genes have been studied. Candidate genes such as those for catechol-O-methyl-transferase, melanocortin-1 receptor, guanosine triphosphate cyclohydrolase, and mu-opioid receptor have been intensively investigated, and associations were found with sensitivity to pain as well as with analgesic requirements in states of acute and chronic pain [34]. In contrast, the impact of genetic variants of drug-metabolizing enzymes on the response to pharmacotherapy is generally well described. Polymorphisms of the cytochrome P450 enzymes influence the analgesic efficacy of codeine, tramadol, tricyclic antidepressants and nonsteroidal antiinflammatory drugs. Together with further candidate genes, they are major targets of ongoing research in order to identify associations between an individual’s genetic profile and drug response (pharmacogenetics). Moreover, sensitivity and tolerance to morphine were determined in two strains of mice, BALB/cBy and C57BL/6By, their reciprocal F1 hybrids and seven of their recombinant inbred strains. Sensitivity was established based on locomotor activity following the administration of saline, 10, or 20 mg/kg of morphine hydrochloride, while tolerance was established according to the ‘hot plate’ method following the single or repeated administration of saline, 5, 10, or 20 mg/kg of morphine hydrochloride. Results indicated that both sensitivity and tolerance to morphine are genotype-dependent, and their inheritance is characterized by dominance or partial dominance [35].
The most common treatment for opioid dependence is substitution therapy with another opioid such as methadone. The methadone dosage is individualized but highly variable, and program retention rates are low due in part to non-optimal dosing resulting in withdrawal symptoms and further heroin craving and use. Methadone is a substrate for the P-glycoprotein transporter, encoded by the ABCB1 gene, which regulates central nervous system exposure. It has been shown that ABCB1 genetic variability influenced daily methadone dose requirements, such that subjects carrying two copies of the wild-type haplotype required higher doses compared with those with one copy and those with no copies (98.3 ± 10.4, 58.6 ± 20.9, and 55.4 ± 26.1 mg/d, respectively; P = 0.029). In addition, carriers of the AGCTT haplotype required significantly lower doses than non-carriers (38.0 ± 16.8 and 61.3 ± 24.6 mg/d, respectively; P = 0.04). Although ABCB1 genetic variability is not related to the development of opioid dependence, identification of variant haplotypes may, after larger prospective studies have been performed, provide clinicians with a tool for methadone dosage individualization [36].
Studies of polymorphisms in the mu opioid receptor gene, which encodes the receptor target of some endogenous opioids, heroin, morphine, and synthetic opioids, have contributed substantially to knowledge of genetic influences on opiate and cocaine addiction. Other genes of the endogenous opioid and monoaminergic systems, particularly genes encoding dopamine β-hydroxylase, and the dopamine, serotonin, and norepinephrine transporters have also been implicated [37].
Moreover, genetically caused inactivity of cytochrome P450 (CYP) 2D6 renders codeine ineffective (lack of morphine formation), slightly decreases the efficacy of tramadol (lack of formation of the active O-desmethyl-tramadol) and slightly decreases the clearance of methadone [38]. MDR1 mutations often demonstrate pharmacogenetic consequences, and since opioids are among the P-glycoprotein substrates, opioid pharmacology may be affected by MDR1 mutations. The single nucleotide polymorphism A118G of the mu opioid receptor gene has been associated with decreased potency of morphine and morphine-6-glucuronide, and with decreased analgesic effects and higher alfentanil dose demands in carriers of the mutated G118 allele [39]. Genetic causes may also trigger or modify drug interactions, which in turn can alter the clinical response to opioid therapy. For example, by inhibiting CYP2D6, paroxetine increases the steady-state plasma concentrations of (R)-methadone in extensive but not in poor metabolisers of debrisoquine/sparteine [38]. So far, the clinical consequences of the pharmacogenetics of opioids are limited to codeine, which should not be administered to poor metabolisers of debrisoquine/sparteine. Genetically precipitated drug interactions might render a standard opioid dose toxic and should, therefore, be taken into consideration. Mutations affecting opioid receptors and pain perception/processing are of interest for the study of opioid actions, but with modern practice of on-demand administration of opioids their utility may be limited to explaining why some patients need higher opioid doses; however, the adverse effects profile may be modified by these mutations. Nonetheless, at a limited level, pharmacogenetics can be expected to facilitate individualized opioid therapy. It has been demonstrated that the muOR 304G variant significantly reduces intrathecal fentanyl ED(50) for labor analgesia, suggesting women with the G variant may be more responsive to opioids and require less analgesic drugs. These findings for intrathecal fentanyl pharmacogenetics may have implications for patients receiving opioids in other settings [28].
The following is a sampling of genes involved in the addictive process that we propose can be informative which relate to opiate addiction and are involved in pain mechanisms and sensitivity: mu opioid receptor, δ-opioid receptor; the metabotropic receptors mGluR6 and mGluR8, nuclear receptor NR4A2 and cryptochrome 1 (photolyase-like), drdgene (D1-D5), Dat1, DBH, proenkephalin (PENK) and prodynorphin (PDYN), CAMKII; GnRH; CYP2D6; BDNF; NT-3 genes; GABA receptor subunit genes on 5q33; GABA(A)gamma2; OPRM1; G-protein alpha subunits; OPRK1; alpha2-adrenoceptor; TTC12; ANKK1; NCAM1; ZCRB1; CYP2B6; CYP2C19; CYP2C9; interleukin-2; RGS-R7; Gbeta5; MAO-A; 287 A/G polymorphism of catechol-O-methyltransferase; serotonin transporter; Ca2+/cAMP responsive element binding protein; CNR1; ABCB1, Pglycoprotein, UGT2B7, and CREB. While there are a number of genes involved in pain mechanisms and in the healing process, the following tables represent a sampling (see Tables 1 and 2).
Table 1.
Genes involved with pain mechanisms
| Gene name | Polymorphism | Pathway(s) | Reference(s) |
|---|---|---|---|
| Human κ opioid receptor gene (OPRK1) | In humans, the 36G > T single nucleotide polymorphism (SNP) on KOR gene | The κ opioid receptor (KOR) system seems to play a role in stress responsivity, opiate withdrawal and responses to psycho stimulants, inhibiting mesolimbic dopamine. KOR gene polymorphisms have been reported to contribute to predisposition to voluntary alcohol-drinking behavior in experimental animals | Gerra G, Leonardi C, Cortese E, D’Amore A, Lucchini A, Strepparola G, et al. Human kappa opioid receptor gene (OPRK1) polymorphism is associated with opiate addiction. Am J Med Genet B Neuropsychiatr Genet 2007;144(6):771–5 |
| Mu opioid receptor | A118G SNP of the mu opioid receptor gene (OPRM1) | Mu opioid receptors are critical for heroin dependence, and A118G SNP of the mu opioid receptor gene (OPRM1) has been linked with heroin abuse. In our population of European Caucasians (n = 118), approximately 90% of 118G allelic carriers were heroin users | Drakenberg K, Nikoshkov A, Horváth MC, Fagergren P, Gharibyan A, Saarelainen K, et al. Mu opioid receptor A118G polymorphism in association with striatal opioid neuropeptide gene expression in heroin abusers. Proc Natl Acad Sci USA 2006;16;103(20):7883–8 |
| D(2) dopamine receptor gene (DRD2) | A haplotype block of 25.8 kb was defined by 8 SNPs extending from SNP3 (TaqIB) at the 5′ end to SNP10 site (TaqIA) located 10 kb distal to the 3′ end of the gene | Within this block, specific haplotype cluster A (carrying TaqIB1 allele) was associated with a high risk of heroin dependence in Chinese patients (P = 1.425 × 10(−22); odds ratio, 52.80; 95% confidence interval, 7.290–382.5 for 8-SNP analysis). A putative recombination ‘hot spot’ was found near SNP6 (intron 6 ins/del G), creating 2 new daughter haplotypes that were associated with a lower risk of heroin dependence in Germans (P = 1.94 × 10(−11) for 8-SNP analysis). Other studies show the relationship of carrying TAq1A1 vs A2 alleles in the treatment outcomes for heroin abuse. The results indicate that DRD2 variants are predictors of heroin use and subsequent methadone treatment outcome, and suggest a pharmacogenetic approach to the treatment of opioid dependence. Others found association between nasal inhalation of opiates and DRD2 promoter - 141 DeltaC polymorphism. Significantly stronger cue-elicited heroin craving was found in individuals carrying D2 dopamine receptor gene (DRD2) TaqI RFLP A1 allele than the non-carriers (P < 0.001) | Xu K, Lichtermann D, Lipsky RH, Franke P, Liu X, Hu Y, et al. Association of specific haplotypes of D2 dopamine receptor gene with vulnerability to heroin dependence in 2 distinct populations. Arch Gen Psychiatry 2004;61(6):597–606 Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, et al. The D(2) dopamine receptor A(1) allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet 2000;96(5):592–8 Li Y, Shao C, Zhang D, Zhao M, Lin L, Yan P, et al. The effect of dopamine D2, D5 receptor and transporter (SLC6A3) polymorphisms on the cue-elicited heroin craving in Chinese. Am J Med Genet B Neuropsychiatr Genet 2006;141(3):269–73 |
| ANKK1 gene | With a non-synonymous G to A transition, rs2734849 produces an amino acid change (arginine to histidine) in C-terminal ankyrin repeat domain of ANKK1 | Since DRD2 expression is regulated by transcription factor NF-κB, we suspect that rs2734849 may indirectly affect dopamine D (2) receptor density. The rs273849 ANNK1 variant alters the expression level of NF-kappaB-regulated genes | Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, et al. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African–American sample. Neuropsychopharmacology; 2008 |
| Catechol-O-methyltransferase (COMT) gene | Val(108/158)met polymorphism of the catechol-O-methyltransferase (COMT) gene | Genotyping 38 Israeli heroin addicts and both parents using a robust family based haplotype relative risk (HRR) strategy. There is an excess of the val COMT allele (likelihood ratio = 4.48, P = 0.03) and a trend for an excess of the val/val COMT genotype (likelihood ratio = 4.97, P = 0.08, 2 df) in the heroin addicts compared to the HRR control group | Horowitz R, Kotler M, Shufman E, Aharoni S, Kremer I, Cohen H, et al. Confirmation of an excess of the high enzyme activity COMT val allele in heroin addicts in a family based haplotype relative risk study. Am J Med Genet 2000;96(5):599–603 Cao L, Li T, Xu K, Liu X. Association study of heroin dependence and -287 A/G polymorphism of catechol-O-methyltransferase gene]. In: Zhonghua Yi, Xue Yi, Chuan Xue, Za Zhi, editors. 2002;19(6):499–501 |
| Proenkephalin gene (PENK) | > or =81 bp allele | Among the subjects with opioid dependence, 66% carried the > or =81 bp allele compared with 40% of subjects with other types of substance abuse (χ2 = 11.31, p < 0.004) and 49% of controls (χ2 = 6.0, p < 0.015). These results are consistent with a role of the PENK gene in opioid dependence. In another study, Heroin abuse was significantly associated with PENK polymorphic 3’ UTR dinucleotide (CA) repeats; 79% of subjects homozygous for the 79-bp allele were heroin abusers. Such individuals tended to express higher PENK mRNA than the 81-bp homozygotes, but PENK levels within the nucleus accumbens (NAc) shell were most strongly correlated to catecholamine-O-methyltransferase (COMT) genotype. Altogether, the data suggest that dysfunction of the opioid reward system is significantly linked to opiate abuse vulnerability and that heroin use alters the apparent influence of heritable dopamine tone on mesolimbic PENK and tyrosine hydroxylase function | Comings DE, Blake H, Dietz G, Gade-Andavolu R, Legro RS, Saucier G, et al. The proenkephalin gene (PENK) and opioid dependence. Neuroreport. 1999;10(5):1133–5 Nikoshkov A, Drakenberg K, Wang X, Horvath MC, Keller E, Hurd YL. Opioid neuropeptide genotypes in relation to heroin abuse: dopamine tone contributes to reversed mesolimbic proenkephalin expression. Proc Natl Acad Sci USA 2008;105(2):786–91 |
| Serotonin transporter (hSERT) | Homozygosity at hSERT (especially 10/10) was associated with early opiate addiction, while genotype 12/10 proved to be protective | Reward system pathway | Galeeva AR, Gareeva AE, Iur’ev EB, Khusnutdinova EK. VNTR polymorphisms of the serotonin transporter and dopamine transporter genes in male opiate addicts. Mol Biol (Mosk). 2002;36(4):593–8 Bonnet-Brilhault F, Laurent C, Thibaut F, Campion D, Chavand O, Samolyk D, et al. Serotonin transporter gene polymorphism and schizophrenia: an association study. Biol Psychiatry 1997;42(7):634–6 |
| Dopamine transporter (DAT1) | In the case of DAT1, genotype 9/9 was associated with early opiate addiction. The combination of hSERT genotype 10/10 with DAT1 genotype 10/10 was shown to be a risk factor of opiate abuse under 16 years of age | Reward system pathway | Galeeva AR, Gareeva AE, Iur’ev EB, Khusnutdinova EK. VNTR polymorphisms of the serotonin transporter and dopamine transporter genes in male opiate addicts. Mol Biol (Mosk). 2002;36(4):593–8 |
| Cannabinoid CB1 (brain) receptor gene (CNR1) | A microsatellite polymorphism (AAT)n at the cannabinoid CB1 (brain) receptor gene (CNR1) consists of 9 alleles. The number of i.v. drugs used was significantly greater for those carrying the > or ≥ or = 5 genotype than for other genotypes (P = 0.005) | Cannabinoid receptors in the modulation of dopamine and cannabinoid reward pathways | Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, MacMurray J. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 2000;5(2):128–30 |
We suggest that the seven genes (shown in Table 2) constitute a sampling of the proposed genes that should be tested prior to treatment of any painful condition because of the potential involvement in pain mechanisms, tissue healing and inflammation.
Table 2.
Genes involved with pain and tissue healing
| Gene | System measured | Medical necessity | Comments |
|---|---|---|---|
| P450 | Drug Metabolism | Pharmacogenomic response tied to narcotic drugs | There are 10 studies relating polymorphisms of this gene and opiate response |
| Mu receptor | Opioid response including endorphins | Pain sensitivity and intolerance as well as pharmacogenomic response to opiates | There are 10 studies relating polymorphisms of this gene and opiate response |
| PENK | Precursor to Enkephalins | Pain sensitivity and intolerance | There are 83 studies relating polymorphisms of this gene and enkephalins and opiate response |
| TNF-α | Inflammation | High risk for development of inflammatory secondary messengers. Required increase in NSAID dosage. | There are 2700 studies relating polymorphisms of this gene and the inflammatory response 3 studies specific to opiate response |
| DRD2 | Dopamine receptors | Dopamine is required for proper pain sensitivity and tolerance. Most opiates work via dopamine to reduce pain. DA is also the anti-stress molecule. | There are 11 studies involving the dopamine d2 receptor and pain mechanisms and 5 studies involving the drd2 gene polymorphisms and pain |
| eNOS | Oxidative stress | Nitric Oxide deficiency leads to oxidative stress | There are 75 studies relating polymorphisms of this gene and oxidative stress |
| VEGF | Angiogenesis Factor-required for proper tissue healing | Slow healing process | There are 3423 studies relating polymorphisms of this gene and angiogenesis |
Pain and addiction
Drug addiction is a serious worldwide problem with strong genetic and environmental influences. It appears that while not all genes associated with pain mechanisms are common to a predisposition of addictive behavior there are, however, similar antecedents. Thus, similar pharamcogenomic treatment solutions are indeed primary therapeutic targets. As we hypothesized, reward circuitry impinges on pain control and associated mechanisms. Thus, in order to be successful in the treatment of pain the clinician should be cognizant that central reward mechanisms and the genes associated with these mechanisms are critical in understanding pain therapeutics (e.g., drd2gene).
Different technologies have revealed a variety of genes and pathways underlying addiction; however, each individual technology can be biased and incomplete. Li et al. [41] integrated 2343 items of evidence from peer-reviewed publications between 1976 and 2006 linking genes and chromosome regions to addiction by single gene strategies, microarray, proteomics, or genetic studies. Li et al. [41] identified 1500 human addiction-related genes and developed KARG (http://karg.cbi.pku.edu.cn), the first molecular database for addiction-related genes with extensive annotations and a friendly Web interface. Li et al. [41] then performed a meta-analysis of 396 genes that were supported by two or more independent items of evidence to identify 18 molecular pathways that were statistically significantly enriched, covering both upstream signaling events and downstream effects. Five molecular pathways significantly enriched for all four different types of addictive drugs were identified as common pathways that may underlie shared rewarding and addictive actions, including two new ones, GnRH signaling pathway and gap junction. They connected the common pathways into a hypothetical common molecular network for addiction. They observed that fast and slow positive feedback loops were interlinked through CAMKII, which may provide clues to explain some of the irreversible features of addiction. Interestingly, the common thread involves dopaminergic and glutaminergic genes.
Pharmacogenetic testing: a diagnostic tool
The importance of pharmacogenetic testing of the above-mentioned genes will provide information related to potential genetic antecedents for a predisposition to not only aberrant pain sensitivity but to an inability to heal properly. This genetic information will ultimately lead to a DNA-directed development of a personalized treatment regimen including a pharmacogenomic resolution. Genetic testing will provide medical evidence for rationale treatment protocols. For example, an electrotherapeutic device known as H-Wave® induces both Nitric oxide-dependent enhancement of blood flow and angiogenesis under chronic utilization, thus, genotyping for the eNOs and VGEF genes will provide a significantly better clinical outcome. In fact clinical outcome, studies have revealed significant benefits from chronic H-wave device stimulation as reported in a recent meta-analysis [42] and rat microcirculation studies [43].
Summary
Based on the findings reviewed herein, we hypothesize that the subsequent coupling of the identified genes as described in this paper, as well as other genes relative to polymorphisms, would allow for additional pharmacologically active substances-based pharmacogenomic mapping. The combination will provide a map that will serve as a platform to derive novel DNA targeted areas, which will link bioactive substances with potential anti-craving actions and pain relief mechanisms. In essence, the linking of known reward genes and other physiological-based endogenous opioid receptors and or other signaling substrates will ensure successful personalized medical treatments for individuals with aberrant inborn pain sensitivity (see Fig. 2).
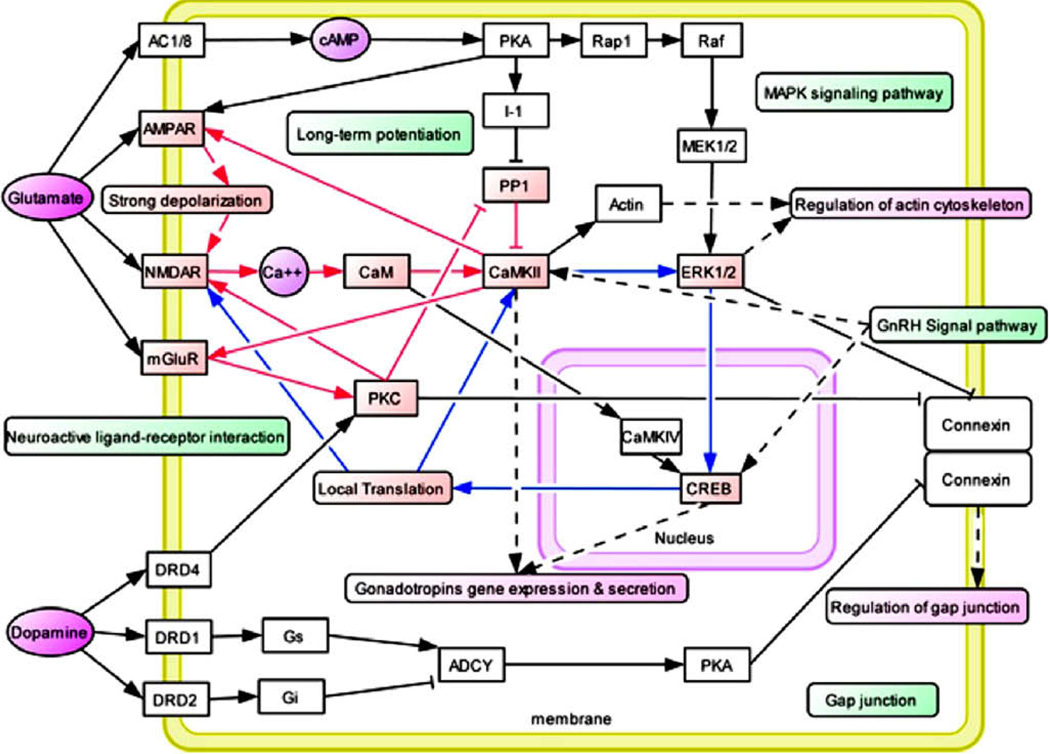
Figure 2.
Hypothetical Common Molecular Network for Drug Addiction (Li et al., 2008 with permission). Most recently Li and his associates [41] developed an addiction gene network that was constructed manually based on the common pathways identified in their 2008 study and protein interaction data. Addiction-related genes were represented as white boxes, while neurotransmitters and secondary massagers were highlighted in purple [29]. The common pathways are highlighted in green boxes. Related functional modules such as “regulation of cytoskeleton”, “regulation of cell cycle”, “regulation of gap junction”, and “gene expression and secretion of gonadotropins” were highlighted in carmine boxes. Several positive feedback loops were identified in this network. Fast positive feedback loops were highlighted in red lines, and slow ones were highlighted in blue lines.
Acknowledgements
The authors want to thank the staff and financial support of Path Research Foundation, New York, Synaptamine Inc., San Antonio, TX, LifeGen Inc., La Jolla, CA, and Electronic Waveform Lab, Huntington, Beach, California.
Footnotes
Conflict of interest
Kenneth Blum, Roger L. Waite, and William B. Downs are officers and stock holder of LifeGen, Inc., worldwide exclusive distributors of PainGen Profile™ genetic testing panels.
References
- 1.Montagna P. Recent advances in the pharmacogenomics of pain and headache. Neurol Sci. 2007;28(Suppl. 2):S208–S212. doi: 10.1007/s10072-007-0778-0. [DOI] [PubMed] [Google Scholar]
- 2.Meshkin B, Chen TJH, Chen ALC, Prihoda TJH, Morrisette H, Braverman ER, et al. Health economics of nutrigenomics in weight management. Gene Ther Mol Biol. 2008;12:25–30. [Google Scholar]
- 3.Gätke MR, Bundgaard JR, Viby-Mogensen J. Two novel mutations in the BCHE gene in patients with prolonged duration of action of mivacurium or succinylcholine during anaesthesia. Pharmacogenet Genomics. 2007;17:995–999. doi: 10.1097/FPC.0b013e3282f06646. [DOI] [PubMed] [Google Scholar]
- 4.Beutler E. Glucose-6-phosphate dehydrogenase deficiency: a historical perspective. Blood. 2008;111:16–24. doi: 10.1182/blood-2007-04-077412. [DOI] [PubMed] [Google Scholar]
- 5.Broekman BF, Olff M, Boer F. The genetic background to PTSD. Neurosci Biobehav Rev. 2007;31:348–362. doi: 10.1016/j.neubiorev.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Stamer UM, Stüber F. Genetic factors in pain and its treatment. Curr Opin Anaesthesiol. 2007;20:478–484. doi: 10.1097/ACO.0b013e3282ef6b2c. [DOI] [PubMed] [Google Scholar]
- 7.Crettol S, Déglon JJ, Besson J, Croquette-Krokar M, Hämmig R, Gothuey I, et al. ABCB1 and cytochrome P450 genotypes and phenotypes: influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–681. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Willis WD. Nociceptive pathways: anatomy and physiology of nociceptive ascending pathways. Philos Trans R Soc Lond B Biol Sci. 1985;308:253–270. doi: 10.1098/rstb.1985.0025. [DOI] [PubMed] [Google Scholar]
- 9.Melzack R. Evolution of the neuromatrix theory of pain. In: The Prithvi Raj Lecture: presented at the third World Congress of World Institute of Pain, Barcelona 2004. Pain Pract. 2005;5:85–94. doi: 10.1111/j.1533-2500.2005.05203.x. [DOI] [PubMed] [Google Scholar]
- 10.Altier N, Stewart J. The tachykinin NK-1 receptor antagonist, RP-67580, infused into the ventral tegmental area prevents stress-induced analgesia in the formalin test. Physiol Behav. 1999;66:717–721. doi: 10.1016/s0031-9384(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 11.Altier N, Stewart JT. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- 12.Morgan MJ, Franklin KB. 6-Hydroxydopamine lesions of the ventral tegmentum abolish D-amphetamine and morphine analgesia in the formalin test but not in the tail flick test. Brain Res. 1990;519:144–149. doi: 10.1016/0006-8993(90)90072-j. [DOI] [PubMed] [Google Scholar]
- 13.Taylor BK, Joshi C, Uppal H. Stimulation of dopamine D2 receptors in the nucleus accumbens inhibits inflammatory pain. Brain Res. 2003;987:135–143. doi: 10.1016/s0006-8993(03)03318-3. [DOI] [PubMed] [Google Scholar]
- 14.Magnusson JE, Fisher K. The involvement of dopamine in nociception: the role of D(1) and D(2) receptors in the dorsolateral striatum. Brain Res. 2000;855:260–266. doi: 10.1016/s0006-8993(99)02396-3. [DOI] [PubMed] [Google Scholar]
- 15.Hagelberg N, Martikainen IK, Mansikka H, Hinkka S, Någren K, Hietala J, et al. Dopamine D2 receptor binding in the human brain is associated with the response to painful stimulation and pain modulatory capacity. Pain. 2002;99:273–279. doi: 10.1016/s0304-3959(02)00121-5. [DOI] [PubMed] [Google Scholar]
- 16.Hagelberg N, Kajander JK, Någren K, Hinkka S, Hietala J, Scheinin HM. Mureceptor agonism with alfentanil increases striatal dopamine D2 receptor binding in man. Synapse. 2002;45:25–30. doi: 10.1002/syn.10078. [DOI] [PubMed] [Google Scholar]
- 17.Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101:149–154. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 18.Chen TJ, Blum K, Waite RL, Meshkin B, Schoolfield J, Downs BW, et al. Gene Narcotic Attenuation Program attenuates substance use disorder, a clinical subtype of reward deficiency syndrome. Adv Ther. 2007;24:402–414. doi: 10.1007/BF02849910. [DOI] [PubMed] [Google Scholar]
- 19.Blum K, Braverman ER, Holder JM, Lubar JF, Monastra VJ, Miller D, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32(Suppl. i–iv):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 20.Elovainio M, Jokela M, Kivimäki M, Pulkki-Råback L, Lehtimäki T, Airla N, et al. Genetic variants in the DRD2 gene moderate the relationship between stressful life events and depressive symptoms in adults: cardiovascular risk in young Finns study. Psychosom Med. 2007;69:391–395. doi: 10.1097/psy.0b013e31806bf365. [DOI] [PubMed] [Google Scholar]
- 21.Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with co-morbid depression, anxiety and social dysfunction in untreated veterans with post-traumatic stress disorder. Eur Psychiat. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Dziedzicka-Wasylewska M. The effect of imipramine on the amount of mRNA coding for rat dopamine D2 autoreceptors. Eur J Pharmacol. 1997;337:291–296. doi: 10.1016/s0014-2999(97)01286-7. [DOI] [PubMed] [Google Scholar]
- 23.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51:23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 24.Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: a study and replication. Biol Psychiat. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- 25.Deutch AY, Clark WA, Roth RH. Prefrontal cortical dopamine depletion enhances the responsiveness of mesolimbic dopamine neurons to stress. Brain Res. 1990;521:311–315. doi: 10.1016/0006-8993(90)91557-w. [DOI] [PubMed] [Google Scholar]
- 26.Kalivas PW, Abhold RE. Enkephalin release into the ventral tegmental area in response to stress: modulation of mesocorticolimbic dopamine. Brain Res. 1987;414:339–348. doi: 10.1016/0006-8993(87)90015-1. [DOI] [PubMed] [Google Scholar]
- 27.Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, Samuels AI, et al. Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: double-blind placebo-controlled study of the nutritional adjunct SAAVE. Alcohol. 1988;5:481–493. doi: 10.1016/0741-8329(88)90087-0. [DOI] [PubMed] [Google Scholar]
- 28.Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62:420–424. doi: 10.1016/j.mehy.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Ninan I, Kulkarni SK. Involvement of dopamine D2 and 5-HT1A receptors in roxindole-induced antinociception. Indian J Exp Biol. 1999;37:234–237. [PubMed] [Google Scholar]
- 30.Wolfe F, Simons DG, Fricton J, Bennett RM, Goldenberg DL, Gerwin R, et al. The fibromyalgia and myofascial pain syndromes: a preliminary study of tender points and trigger points in persons with fibromyalgia, myofascial pain syndrome and no disease. J Rheumatol. 1992;19:944–951. [PubMed] [Google Scholar]
- 31.Legangneux E, Mora JJ, Spreux-Varoquaux O, Thorin I, Herrou M, Alvado G, et al. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology (Oxford) 2001;40:290–296. doi: 10.1093/rheumatology/40.3.290. [DOI] [PubMed] [Google Scholar]
- 32.Kalivas PW, Duffy P. Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res. 1995;675:325–328. doi: 10.1016/0006-8993(95)00013-g. [DOI] [PubMed] [Google Scholar]
- 33.Quintero L, Moreno M, Avila C, Arcaya J, Maixner W, Suarez-Roca H. Longlasting delayed hyperalgesia after subchronic swim stress. Pharmacol Biochem Behav. 2000;67:449–458. doi: 10.1016/s0091-3057(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 34.Limer KL, Nicholl BI, Thomson W, McBeth J. Exploring the genetic susceptibility of chronic widespread pain: the tender points in genetic association studies. Rheumatology (Oxford) 2008;47:572–577. doi: 10.1093/rheumatology/ken027. [DOI] [PubMed] [Google Scholar]
- 35.Mogil JS, Marek P, Flodman P, Spence MA, Sternberg WF, Kest B, et al. One or two genetic loci mediate high opiate analgesia in selectively bred mice. Pain. 1995;60:125–135. doi: 10.1016/0304-3959(94)00098-Y. [DOI] [PubMed] [Google Scholar]
- 36.Coller JK, Barratt DT, Dahlen K, Loennechen MH, Somogyi AA. ABCB1 genetic variability and methadone dosage requirements in opioid-dependent individuals. Clin Pharmacol Ther. 2006;80:682–690. doi: 10.1016/j.clpt.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 37.McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lötsch J, Skarke C, Liefhold J, Geisslinger G. Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet. 2004;43:983–1013. doi: 10.2165/00003088-200443140-00003. [DOI] [PubMed] [Google Scholar]
- 39.Lötsch J, Zimmermann M, Darimont J, Marx C, Dudziak R, Skarke C, et al. Does the A118G polymorphism at the mu-opioid receptor gene protect against morphine-6-glucuronide toxicity? Anesthesiology. 2002;97:814–819. doi: 10.1097/00000542-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 41.Li CY, Mao X, Wei L. Genes and (common) pathways underlying drug addiction. PLoS Comput Biol. 2008;4:e2. doi: 10.1371/journal.pcbi.0040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum K, Chen AL, Chen TJ, Prihoda TJ, Schoolfield J, Dinubile N, et al. The HWave( R) device is an effective and safe non-pharmacological analgesic for chronic pain: a meta-analysis. Adv Ther. 2008 Jul 18; doi: 10.1007/s12325-008-0073-3. [published on line] [DOI] [PubMed] [Google Scholar]
- 43.Smith TL, Blum K, Waite RL, Heaney WJ, Callahan M. Abstract presented at 6th combined meeting of the orthopaedic research societies. Hawaii: Honolulu; 2007. Oct 21, The microvascular and hemodynamic mechanisms for the therapeutic actions of H-Wave muscle stimulation. Abstract #83. [Google Scholar]
- 44.Blum K, et al. Dopamine D2 receptor TaqA1 allele predicts treatment compliance of LG839 in a subset analysis of pilot study in the Netherlands. Gene Therapy & Mol Biol. 2008;12:129–140. [Google Scholar]