Abstract
The young of marsupials and monotremes are all born in an immature state, followed by prolonged nurturing by maternal lactation in either a pouch or nest. Nevertheless, the level of locomotor ability required for newborn marsupials and monotremes to reach the safety of the pouch or nest varies considerably: some are transferred to the pouch or nest in an egg (monotremes); others are transferred passively by gravity (e.g. dasyurid marsupials); some have only a horizontal wriggle to make (e.g. peramelid and didelphid marsupials); and others must climb vertically for a long distance to reach the maternal pouch (e.g. diprotodontid marsupials). In the present study, archived sections of the inner ear and hindbrain held in the Bolk, Hill and Hubrecht collections at the Museum für Naturkunde, Berlin, were used to test the relationship between structural maturity of the vestibular apparatus and the locomotor challenges that face the young of these different mammalian groups. A system for staging different levels of structural maturity of the vestibular apparatus was applied to the embryos, pouch young and hatchlings, and correlated with somatic size as indicated by greatest body length. Dasyurids are born at the most immature state, with the vestibular apparatus at little more than the otocyst stage. Peramelids are born with the vestibular apparatus at a more mature state (fully developed semicircular ducts and a ductus reuniens forming between the cochlear duct and saccule, but no semicircular canals). Diprotodontids and monotremes are born with the vestibular apparatus at the most mature state for the non-eutherians (semicircular canals formed, maculae present, but vestibular nuclei in the brainstem not yet differentiated). Monotremes and marsupials reach the later stages of vestibular apparatus development at mean body lengths that lie within the range of those found for laboratory rodents (mouse and rat) reaching the same vestibular stage.
Keywords: brain evolution, dasyurids, didelphids, diprotodontids, echidna, peramelids, platypus, reticulospinal pathway, vestibulospinal pathway
Introduction
The marsupials and monotremes are distinguished by the immature state in which their young are born, with the bulk of development occurring in a maternal pouch or nest (Griffiths, 1968, 1978; Tyndale-Biscoe, 2005). Although all the marsupials and the monotremes (platypus and echidna) spend a considerable period of time in either a pouch or nest supported by maternal lactation, there are significant differences in body size and degree of locomotor co-ordination and climbing ability at birth between the various marsupial groups (e.g. didelphids such as the opossums; dasyurids such as the quolls; peramelids such as bandicoots; and diprotodontids like the koala, wombats, possums, wallabies and kangaroos) and the monotremes (platypus and echidna). Some marsupial young are very small at birth (e.g. dasyurids, about 10–15 mg body weight and < 5 mm body length; Nelson et al. 2003) and have only a poor ability to perform co-ordinated movement, whereas others are much larger at birth (e.g. diprotodontids such as wallabies and kangaroos, body weight of 200 mg to several g) and are much more proficient at motor co-ordination (Tyndale-Biscoe, 2005).
In previous studies, several authors proposed a role for the vestibular apparatus in the locomotor control and/or guidance of newborn marsupials (grey short-tailed opossum – Pflieger & Cabana, 1996; Adadja et al. 2013; tammar wallaby – McCluskey et al. 2008). In the grey short-tailed opossum and the tammar wallaby, the vestibular apparatus has connections with the brainstem at birth (Pflieger & Cabana, 1996; McCluskey et al. 2008) and at least in the case of the opossum, there may be a direct projection from the vestibular nuclei to the cervical spinal cord (Adadja et al. 2013). Nevertheless, nothing is currently known about how vestibular apparatus development compares between the monotremes, many types of marsupials (e.g. dasyurids, peramelids, non-macropod diprotodontids like the koala, wombats and possums) and eutherians. The aim of the present study was to compare vestibular apparatus development both between the monotremes and the four major groups of marsupials (didelphids, dasyurids, peramelids and diprotodontids) as well as altricial eutherians such as the laboratory mouse and rat, and humans. Marsupials exhibit precocious development of the forelimbs and associated somitic structures (Hill & Hill, 1955; Weisbecker et al. 2008; Keyte & Smith, 2010, 2012), as well as advanced maxillo-mandibular development (Smith, 2006). Our hypothesis was that marsupials and monotremes would show similarly advanced vestibular apparatus development relative to body length, when compared with laboratory rodents and humans.
Materials and methods
Specimens
Most of the data for this study was obtained using sections of the inner ear and brainstem in marsupials and monotremes from the Hubrecht, Bolk and Hill collections held at the Museum für Naturkunde (MfN) in Berlin. Data for the laboratory rat were obtained from sections prepared previously for published studies in those species (Ashwell, 1986; Ashwell & Zhang, 1998; Song et al. 2000; Ashwell & Paxinos, 2007). The Supporting Information table contains the full list of specimens.
The marsupial and monotreme specimens from MfN had been embedded in paraffin and sectioned at 8, 10 or 35 μm thickness in the transverse plane, before being stained with haematoxylin, haematoxylin and eosin, or alcian blue and nuclear red. The dataset for monotremes (18 platypuses, Ornithorhynchus anatinus; nine echidnas, Tachyglossus aculeatus) has been published in several studies previously (Ashwell et al. 2012). The marsupial dataset from MfN includes two specimens of dasyurid (newborn quolls, Dasyurus), three didelphids (one specimen of Didelphis virginiana; two specimens of Didelphis aurita), 17 peramelids (Isoodon obesulus – 11; Perameles nasuta – 6), 35 phalangerids (all Trichosurus vulpecula), 13 specimens of koala (Phascolarctos cinereus), six common wombats (Vombatus ursinus), six diprotodontid macropods (mixture of Macropus rufogriseus – two specimens; Macropus dorsalis – one specimen; Bettongia gaimardi – one specimen; Petrogale penicillata – one specimen; Thylogale thetis – one specimen). Note that the MfN records use discarded synonyms for several species (e.g. Macropus ruficollis for the red-necked wallaby Macropus rufogriseus,Phascolomys mitchelli for the common wombat Vombatus ursinus,Macropus thetidis for the pademelon wallaby Thylogale thetis). All MfN specimens had been serially sectioned. Sections through the inner ear were examined at intervals ranging from 30 μm for small embryos (5.0 mm body length), up to 70–105 μm for larger size young (10–33 mm body length). Additional specimens of a young macropod (tammar wallaby, Macropus eugenii – eight specimens) were available from previous studies by our group (Ashwell et al. 1996; McCluskey et al. 2008). These are the heads of pouch young that had been fixed by perfusion with buffered paraformaldehyde, embedded in paraffin, sectioned at 7–15 μm thickness and stained with either haematoxylin and eosin or Holmes silver stain. All these specimens had been obtained from a breeding colony with the approval of the Animal Ethical Committee of the Australian National University and had been treated according to the guidelines of the National Health and Medical Council of Australia.
Sections through the inner ears of 27 outbred laboratory rats at ages ranging from E11 to E19 (E0 denotes the day of finding a sperm-positive vaginal smear) and birth (P0) were also examined and ranked for stage of vestibular development. These specimens were from previous studies by the author (Ashwell, 1986; Ashwell & Zhang, 1998; Song et al. 2000; Ashwell & Paxinos, 2007). They had been obtained with the approval of the Animal Ethical Committees of the Universities of Sydney and New South Wales and had been treated according to the guidelines of the National Health and Medical Council of Australia. The developmental ages of unborn rats were converted to body length using a graph in a Taconic information sheet for laboratory rats. For E11 rat embryos, body length is 5.0 mm, rising to 42 mm at birth. These rat specimens had been fixed in 4% paraformaldehyde (in 0.1 m phosphate buffer at pH 7.4), completely dehydrated in ethanol and embedded in either Historesin® or paraffin, before being sectioned at 5 μm (historesin blocks) or 7 μm (paraffin blocks) in either the sagittal, horizontal or transverse plane and stained with haematoxylin and eosin.
Photography
The marsupial and monotreme material was photographed with the aid of either a Zeiss Axioplan2® fitted with an AxioCam MRc5® camera, or with a Leica M420® macroscope fitted with an Apozoom® 1 : 6 lens and Leica DFC490® camera. All images were calibrated by photographing a scale bar at the same magnification.
Statistics
The aim of the statistical analysis was to compare the association between body length and stage of development across species. The analysis compared the means of body length within each stage of development across the groups of animals. We used SPSS V21 and applied Bootstrapping to calculate the 95% confidence intervals (95%CI) of the mean body length within each stage (Sjhao & Dongsheng, 1995).
Results
Staging of events in vestibular development
To convert the description of vestibular development in the various mammals to a format that could be quantitatively presented and analysed, a simple scheme of stages of vestibular apparatus development was applied (Table1, Figs 1 and 2). As the goal of the study was to compare the development of functionally significant structures between mammals, the focus was on the development of neurosensory structures (e.g. changes in the shape of the otocyst, formation of utricle, saccule and semicircular ducts) rather than mesenchymal features (e.g. the condensation of mesenchyme around inner ear elements). The landmark stages were chosen because they are anatomical features that are clearly recognisable even in routine and thick histological sections, and the sequence covers the range of body lengths available in the MfN collection. Initially, a pre-stage a (formation of otic placode, but before invagination of the otic pit) was considered, but this was not seen consistently in the available developmental series and the stage was removed. Other hypothetical intermediate steps between stages h and i (e.g. the first appearance of discrete vestibular and cochlear ganglia and the first appearance of maculae of the utricle and saccule) were not seen consistently in the material, presumably because the vestibular apparatus progresses rapidly through those milestones. The 12 stages can also be applied to published photomicrographs of inner ear development in eutherians (Kaufman, 1992; Müller & O'Rahilly, 1988, 2011; O'Rahilly & Müller, 2007, 2008, 2010; Streeter, 1905, 1906, 1908; see below).
Table 1.
Staging events in mammalian vestibular development.
| Stage a: Otic pit forms by invagination of the otic placode, but a communication with the amniotic cavity is still present. |
| Stage b: Otocyst is separated from the surface ectoderm, but the facioacoustic (pre)ganglion has not yet condensed. |
| Stage c: First appearance of facioacoustic (pre)ganglion, but endolymphatic diverticulum of the otocyst is not present. |
| Stage d: Otocyst develops a dorsal diverticulum (future endolymphatic duct), but no ventral diverticulum (future cochlear duct) is present. |
| Stage e: Otocyst develops a ventral diverticulum (future cochlear duct), but the facioacoustic ganglion is unseparated. |
| Stage f: Separation of facial and acoustic components of facioacoustic (pre)ganglion, but a discrete vestibular part of the otocyst is not present. |
| Stage g: First appearance of vestibular portion of otocyst because the endolymphatic and cochlear ducts have formed clear lumens, but further subdivision of the vestibular otocyst into utricle and saccule is not present. |
| Stage h: First appearance of discrete utricle, saccule and cochlea, but semicircular ducts are not present. |
| Stage i: First appearance of semicircular ducts, but communication between the saccule and the cochlear duct is un-narrowed. |
| Stage j: Narrowing of ductus reuniens between cochlear duct and saccule, but mesenchyme around the semicircular ducts is still darkly staining. |
| Stage k: Semicircular ducts are now surrounded by semicircular canals, but discrete vestibular nuclei in the brainstem cannot be distinguished. |
| Stage l: Differentiation of vestibular nuclei with expansion of neuropil, so that different vestibular nuclei can be distinguished. |
Figure 1.
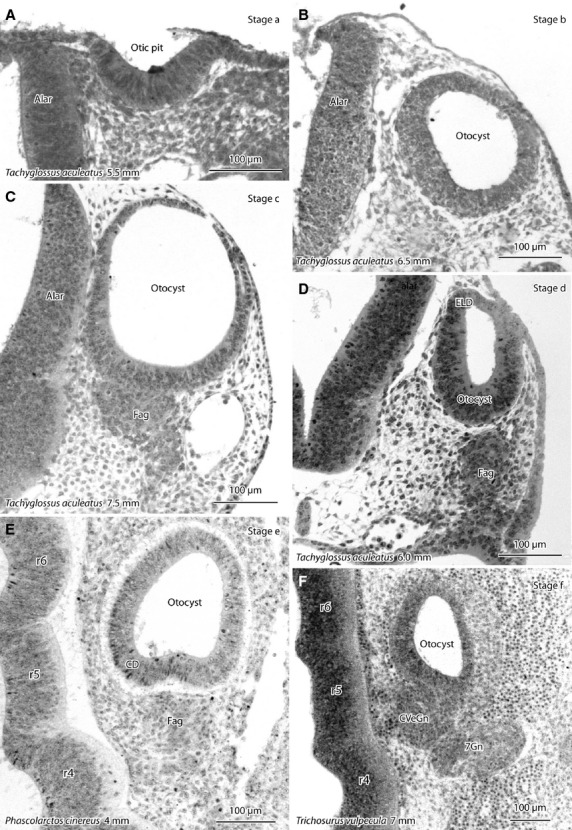
Photomicrographs of the otocyst/inner ear of developing echidna (Tachyglossus aculeatus) and marsupials (koala – Phascolarctos cinereus; brush-tailed possum – Trichosurus vulpecula) showing stages a–f of vestibular apparatus development (see Table1). Sections are in the frontal plane unless otherwise specified. (A) Invagination of the otic placode to form an otic pit, (B) otocyst has separated from the ectoderm, (C) facioacoustic (pre)ganglion/neural crest complex (fag) has condensed ventral to the otocyst, (D) a dorsal diverticulum of the otocyst (the future endolymphatic duct-ELD) appears, (E) a ventral diverticulum of the otocyst (the future cochlear duct – CD) appears (horizontal section), (F) the fag separates into a cochlear/vestibular ganglion (CVeGn) medially and a geniculate ganglion (7Gn) laterally (horizontal section). Species and greatest (body) length of the developing monotreme or marsupial are shown for each image. alar, alar plate of hindbrain neuroepithelium; r4, r5, r6, rhombomeres of brainstem.
Figure 2.
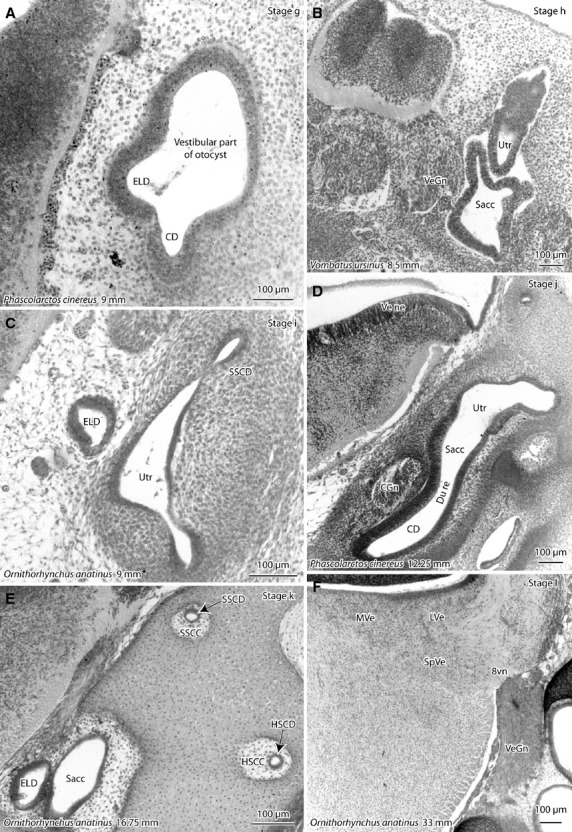
Photomicrographs of the otocyst/inner ear of developing platypus (Ornithorhynchus anatinus) and marsupials (koala – Phascolarctos cinereus; wombat – Vombatus ursinus) showing stages g–l of vestibular apparatus development (see Table1). Sections are in the frontal plane unless otherwise specified. (A) First appearance of the vestibular portion of the otocyst; (B) first appearance of discrete utricle (Utr), saccule (Sacc) and cochlea (lying out of plane of this sagittal section); (C) first appearance of semicircular ducts (SSCD); (D) narrowing of the ductus reuniens (du re) between the cochlear duct and saccule; (E) semicircular ducts are now surrounded by semicircular canals; (F) differentiation of vestibular nuclei with expansion of neuropil, so that different vestibular nuclei can be distinguished. Species and greatest (body) length of the developing monotreme or marsupial are shown for each image. 8vn, vestibular nerve; CD, cochlear duct; CGn, cochlear ganglion; ELD, endolymphatic duct; HSCC, horizontal semicircular canal; HSCD, horizontal semicircular duct; LVe, lateral vestibular nucleus; MVe, medial vestibular nucleus; SSCC, superior semicircular canal; SSCD, superior semicircular duct; SpVe, spinal vestibular nucleus; VeGn, vestibular ganglion; Ve ne, vestibular neuroepithelium.
State of the vestibular apparatus at the time of hatching in monotremes
Hatching in the monotremes occurs at about 14–15 mm greatest body length (GL; Griffiths, 1968, 1978). The monotreme specimens in the Hill collection include a short-beaked echidna immediately before hatching (M158; 12.5 mm GL; Fig. 3A–C) and a platypus immediately after hatching (M44; 16.75 mm GL; Fig. 3D–F). At the time of hatching in both species, the vestibular apparatus is of a similar developmental stage (stage k), with the formation of semicircular ducts, separation of the cochlear and vestibular ganglia, discrete utricle and saccule and narrowing of the passage between the saccule and cochlear duct to form a ductus reuniens. The mesenchymal tissue around the semicircular ducts has also undergone a reduction in ground substance to produce semicircular canals (Fig. 3F). The boundaries of the semicircular canals are slightly better defined in the platypus than in the echidna, but the vestibular apparatuses of the two species are otherwise very similar at this developmental point. Thickened regions in the utricle and maccule mark the sites of the future maculae (particularly in the older platypus; see asterisks in Fig. 3D,E), semicircular canal ampullae are present (Amp in Fig. 3D,E) and fibres from the vestibular ganglion have penetrated the brainstem, but the vestibular nuclei (Ve) are undifferentiated. There is no separation of distinct vestibular nuclei as would be seen at stage l; that feature does not appear until about 33 mm GL (cf. Fig. 2F).
Figure 3.
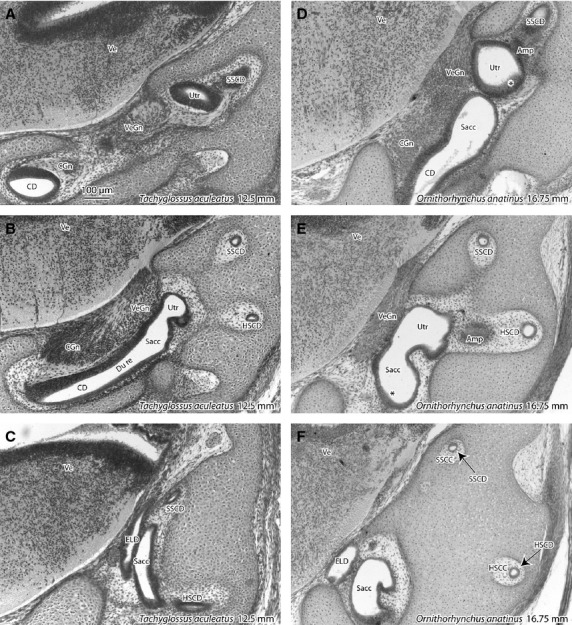
Photomicrographs of frontal sections through the vestibular apparatus in the short-beaked echidna immediately before hatching (A-C), and the platypus immediately after hatching (D,E). Each series of images is in a rostrocaudal sequence. Around the time of hatching (at a size around 14–15 mm greatest length), both species are at stage k of vestibular development (please refer to Table1). (E,F) Asterisks indicate developing maculae. Amp, ampulla of semicircular duct; Ve, developing vestibular nuclei. Other abbreviations are the same as for previous figures.
State of the vestibular apparatus at birth in marsupials
There is a striking difference between the newborn of the dasyurids and other marsupials in the structural maturity of the vestibular apparatus (Fig. 4). In both newborn dasyurids (5.5 and 6.5 mm GL), the otocyst is very primitive (Fig. 4A) in that the only diverticulum of the otocyst is the dorsal endolymphatic duct primordium, and the facioacoustic ganglion has yet to separate into geniculate and vestibulocochlear components (stage d in Table1). Furthermore, there are no axons running between the facioacoustic ganglion and the hindbrain, and very few postmitotic neurons are present in the sensory area of the hindbrain (not shown).
Figure 4.
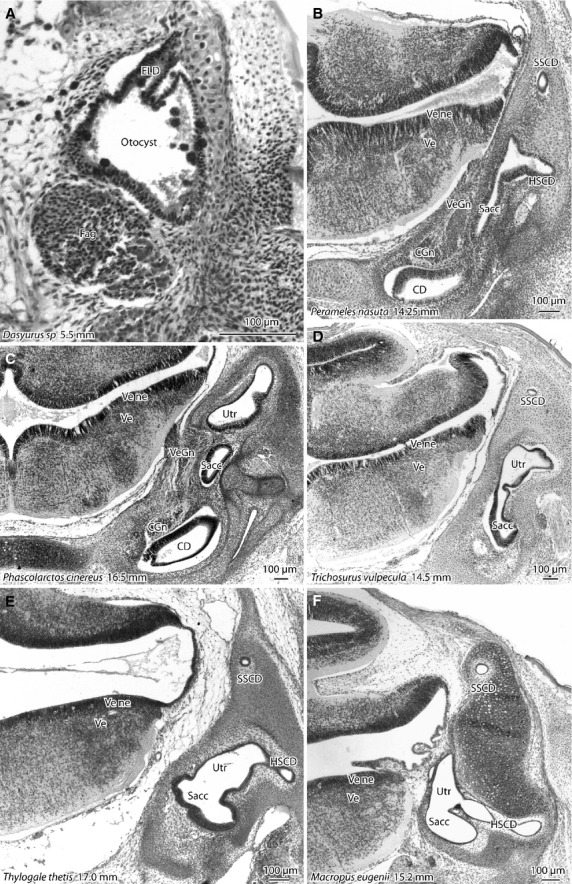
Photomicrographs of frontal sections through the vestibular apparatus in marsupials at the time of birth. The body length of the newborn is shown for each specimen. In the newborn dasyurid (A) the inner ear is very simple in structure (stage d) with a condensed facioacoustic ganglion and a dorsal diverticulum (endolymphatic duct) arising from the otocyst. The newborn peramelid (B) has a much more developed inner ear (stage j), with clearly separated semicircular ducts and a narrowed ductus reuniens. The vestibular apparatus of the newborn koala (C) and brush-tailed possum (D) are at late stage j to early stage k, with semicircular canals beginning to appear around the semicircular ducts. Newborn macropods such as the pademelon (E) and tammar wallaby (F) have vestibular apparatuses that are similar to those of the newborn koala and possum, but the semicircular canals are much more defined, as the extracellular matrix around the semicircular ducts becomes much looser and less cellular. Ve, developing vestibular nuclei. All other abbreviations are the same as for previous figures.
By contrast, all the other newborn marsupials available for study have much more mature vestibular apparatuses at the time of their birth. For the peramelids (bandicoots), the vestibular apparatus is at stage j at birth, with fully developed semicircular ducts and a ductus reuniens forming between the cochlear duct and saccule. The vestibular apparatuses of diprotodontids are slightly more mature at birth. The koala, wombat and possum are at late stage j to early stage k, in that the ductus reuniens is present as a distinct narrowing but the semicircular canals are only beginning to form around the semicircular ducts. The vestibular apparatuses of the newborn macropod diprotodontids (i.e. pademelons, wallabies, kangaroos; Fig. 4E,F) are slightly more developed still, because the semicircular canals are clearer, even though the utricle and saccule are not particularly different in structure from those of the non-diprotodontids. Nevertheless, in none of the available newborn marsupials are the vestibular nuclei differentiated; in other words, it is impossible to distinguish discrete medial, spinal and lateral vestibular nuclei. That particular stage of development was not reached until 23 or 24 mm GL in the diprotodontids.
Comparison of vestibular apparatus development in monotremes, marsupials and eutherians
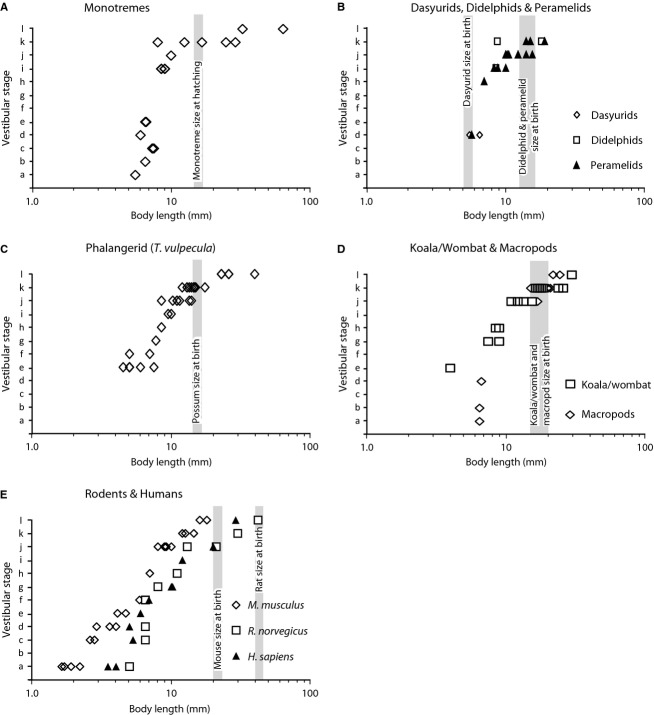
To compare vestibular development in different mammalian groups, the stage of vestibular development for individual specimens was plotted against greatest body length (Fig. 5). A logarithmic scale was used for body length to accommodate the large range of values. The monotremes progress from stage a at a body length of 5.0–6.0 mm, to stage l at about 30–40 mm (Fig. 5A). The dataset for dasyurids and didelphids is limited, so these animals have been plotted along with the peramelids (Fig. 5B). All three of these mammalian groups appear to conform to a similar trajectory of progression through the vestibular stages relative to body length (i.e. stage d at 5.0–6.0 mm body length to stage k at about 17 or 18 mm body length). Significantly, the dasyurid and bandicoot are at a similar stage of vestibular development at 5.0–6.0 mm body length, even though the dasyurid is newly born but the bandicoot will not be born until it reaches 14.0–15.0 mm body length. The phalangerid (brush-tailed possum; Fig. 5C), koala, wombat and assorted macropods (Fig. 5D) have a very similar passage through the vestibular stages, progressing from early otocyst stages (stages a–d) at 4.0–7.0 mm body length, to stage l at a body length of more than 20 mm.
Figure 5.
Graphical presentation of the progression of different mammal groups through the 12 stages of vestibular apparatus development (see Table1 for details). The stages for individual animals have been plotted against body length in mm on a logarithmic scale for monotremes (A), dasyurids, didelphids and peramelids (B), three types of diprotodontids (C – the phalangerid Trichosurus vulpecula; D – koala/common wombat and macropods); rodents and humans (E). Vertical grey bars show the body length at which birth or hatching occurs for each relevant group, except humans, which are born at a body size beyond the abscissa of graph E.
To extend the comparison to altricial eutherians, the stages of vestibular development of 50 embryonic rodents and 11 embryonic humans have been plotted against body length (Kaufman, 1992; O'Rahilly & Müller, 2006). The vestibular stages were diagnosed using the same criteria applied to the marsupials and monotremes from photomicrographs and illustrations of different developmental stages (Kaufman, 1992; Müller & O'Rahilly, 1988, 2011; O'Rahilly & Müller, 2006, 2007, 2008, 2010; Streeter, 1905, 1906, 1908). The data for the laboratory mouse (E) shows steady progression through the vestibular stages relative to log10 of body length, from stage a at about 2.0 mm body length, to stage l at about 16–17 mm. The laboratory rat and human progress through the vestibular stages at a similar pace but with a delay of about 1.5–2.0 mm of body length for a given stage. Significantly, the trajectories of vestibular development relative to body length for the rat and human appeared to be intermingled (Fig. 5E). Both the mouse and rat reach stage l by the time of birth (a little over 20 and 40 mm body length, respectively) and humans reach this stage at about 30–40 mm body length, well before birth.
Statistical analysis of the body length at which vestibular development stages are reached
The available data are mainly drawn from archived specimens, so the dataset for quantitative analysis is, in essence, patchy. Vestibular stages also represent categories rather than continuous variables, therefore we have confined our statistical analysis to comparing the body length at the particular stages reached for the various mammal groups (Table2). Dasyurids and didelphids are represented by only a few specimens, so those marsupials have been excluded from the statistical analysis.
Table 2.
Analysis of body length reached for each vestibular stage.
| Stage | Species | Body length (mm) | |||
|---|---|---|---|---|---|
| Mean | 95%CI low | 95%CI high | n | ||
| a | Monotremes | 5.5 | – | - | 1 |
| Peramelids | 7.9 | 7.4 | 8.5 | 4* | |
| Macropods | 6.5 | – | – | 1 | |
| Laboratory mouse | 1.9 | 1.6 | 2.2 | 4* | |
| Laboratory rat | 5.0 | – | – | 1 | |
| Human | 3.8 | 3.5 | 4.0 | 2 | |
| All specimens | 4.9 | 3.5 | 6.2 | 13 | |
| b | Monotremes | 6.5 | – | – | 1 |
| Macropods | 6.5 | – | – | 1 | |
| All specimens | 6.5 | 6.5 | 6.5 | 2 | |
| c | Monotremes | 7.4 | 7.3 | 7.5 | 2 |
| Laboratory mouse | 2.7 | 2.6 | 2.8 | 2 | |
| Laboratory rat | 6.5 | – | – | 1 | |
| Human | 5.3 | – | – | 1 | |
| All specimens | 5.3 | 3.8 | 6.9 | 6 | |
| d | Monotremes | 6.0 | 1 | ||
| Dasyurids | 6.0 | 5.5 | 6.5 | 2 | |
| Peramelids | 5.7 | – | – | 1 | |
| Macropods | 6.7 | – | – | 1 | |
| Laboratory mouse | 3.5 | 2.9 | 4.0 | 3 | |
| Laboratory rat | 6.5 | – | – | 1 | |
| Human | 5.0 | – | – | 1 | |
| All specimens | 5.2 | 4.4 | 6.0 | 10 | |
| e | Monotremes | 6.6 | 6.5 | 6.6 | 2 |
| Peramelids | 9.4 | – | – | 1 | |
| Phalangerids | 5.4 | 4.8 | 6.3 | 7 | |
| Koala/wombat | 4.0 | – | – | 1 | |
| Laboratory mouse | 4.4 | 4.1 | 4.7 | 2 | |
| Human | 6.0 | – | – | 1 | |
| All specimens | 5.7 | 5.0 | 6.5 | 14 | |
| f | Phalangerids | 6.0 | 5.0 | 7.0 | 2 |
| Laboratory mouse | 5.9 | – | – | 1 | |
| Laboratory rat | 6.5 | 6.5 | 6.5 | 6 | |
| Human | 6.9 | – | – | 1 | |
| All specimens | 6.4 | 6.0 | 6.7 | 10 | |
| g | Phalangerids | 7.8 | – | – | 1 |
| Koala/wombat | 8.3 | 7.5 | 9.0 | 2 | |
| Laboratory rat | 8.0 | 8.0 | 8.0 | 2 | |
| Human | 10.1 | 10.0 | 10.2 | 2 | |
| All specimens | 8.6 | 7.9 | 9.4 | 7 | |
| h | Peramelids | 7.0 | – | – | 1 |
| Phalangerids | 8.5 | – | – | 1 | |
| Koala/wombat | 8.8 | 8.5 | 9.0 | 2 | |
| Laboratory mouse | 7.0 | – | – | 1 | |
| Laboratory rat | 11.0 | 11.0 | 11.0 | 4 | |
| All specimens | 9.3 | 8.2 | 10.3 | 9 | |
| i | Monotremes | 8.8 | 8.6 | 8.9 | 8* |
| Didelphids | 8.5 | 1 | |||
| Peramelids | 9.0 | 8.3 | 10.0 | 3* | |
| Phalangerids | 9.8 | 9.5 | 10.0 | 2 | |
| Human | 12.0 | 1 | |||
| All specimens | 9.1 | 8.7 | 9.7 | 15 | |
| j | Monotremes | 10.0 | 1 | ||
| Peramelids | 11.8 | 10.5 | 13.5 | 8* | |
| Phalangerids | 11.4 | 10.1 | 12.8 | 7* | |
| Koala/wombat | 13.5 | 11.9 | 15.0 | 6* | |
| Macropods | 17.0 | 1 | |||
| Laboratory mouse | 9.0 | 8.4 | 9.6 | 5* | |
| Laboratory rat | 17.0 | 13.0 | 21.0 | 6* | |
| Human | 20.0 | 20.0 | 20.0 | 1 | |
| All specimens | 12.9 | 11.8 | 14.0 | 35 | |
| k | Monotremes | 18.3 | 11.0 | 27.2 | 5 |
| Didelphids | 13.4 | 8.7 | 18.0 | 2 | |
| Peramelids | 15.8 | 14.0 | 19.0 | 4* | |
| Phalangerids | 14.2 | 13.3 | 15.2 | 10* | |
| Koala/wombat | 19.6 | 17.3 | 22.8 | 7* | |
| Macropods | 18.2 | 16.7 | 19.7 | 8* | |
| Laboratory mouse | 13.0 | 12.0 | 14.5 | 3* | |
| Laboratory rat | 30.0 | 30.0 | 30.0 | 3* | |
| All specimens | 17.5 | 16.0 | 19.2 | 42 | |
| l | Monotremes | 79.4 | 55.3 | 101.3 | 6* |
| Phalangerids | 27.6 | 23.0 | 34.8 | 5* | |
| Koala/wombat | 30.0 | – | – | 1 | |
| Macropods | 23.3 | 22.0 | 24.6 | 2 | |
| Laboratory mouse | 17.0 | 16.0 | 18.0 | 2 | |
| Laboratory rat | 42.0 | 42.0 | 42.0 | 3* | |
| Human | 29.0 | – | – | 1 | |
| All specimens | 44.0 | 32.7 | 57.4 | 20 | |
Asterisks indicate mammal groups/stages with sufficient n to allow comparison with other groups at that stage.
95%CI were calculated using bootstrapping (Sjhao & Dongsheng, 1995).
The analysis was undertaken using a bootstrapping approach with spss V21, i.e. multiple re-sampling of the data, to generate means as well as low and high 95% confidence intervals for the means for each mammal group, within each stage. Comparisons between mammal groups for a given stage were considered viable only when three or more specimens were available for that stage; the asterisks in Table2 indicate the number of specimens with sufficient n to allow comparison with other groups.
A consistent observation for several stages (a, c, j, k, l) is that the laboratory mouse reaches those vestibular stages at a shorter body length than the other mammals. This conclusion was based on the range of low to high 95%CI for the mean body length for the mouse lying outside those values for the other mammal groups. This appears to be statistically significant for vestibular stage a (comparison of mouse – mean of 1.9 mm body length, range of CI from 1.6 to 2.2 mm; with peramelids – mean of 7.9 mm body length, range of CI 7.4–8.5 mm) and vestibular stage l (comparison of mouse – mean of 17.0 mm, range of CI 16.0–18.0 mm; with both monotremes – mean of 79.4 mm, range of CI 55.3–101.3 mm; and phalangerids – mean of 27.6 mm, range of CI 23.0–34.8 mm). For the marsupials and monotremes, the means of body length at which a given vestibular stage is reached lie within the range of CI for the other mammals (see stages i, j and k). The exception to this would be that the monotremes appear to reach stage l at a significantly larger body length (mean of 79.4 mm) than the phalangerids (mean of 27.6 mm).
Discussion
Limitations and benefits of using archived material
The Bolk, Hubrecht and Hill collections at the Museum für Naturkunde in Berlin include a range of marsupial and monotreme specimens that would be very difficult, if not impossible, to obtain in the modern world. Nevertheless, these specimens were collected and processed in the late 19th and early 20th centuries and this leads to some significant technical limitations. The tissues were embedded in paraffin and sectioned relatively thickly (8 μm and greater), before being stained with histological pigments available at the time (mainly haematoxylin and eosin). No tissue is available to stain with modern immunochemicals and it is not possible to trace the projections of individual vestibular ganglion cells in the available sections. In the present study we have adopted an approach to the analysis of the material that extracts information on vestibular development using staging criteria that are easy to assess objectively in thick-sectioned material. It is not possible to make conclusions concerning the fine-scale development of vestibular connections, although large-scale development of the vestibular nerve is easy to judge. Without the opportunity to sample electrical activity in the vestibular system, conclusions concerning the functional activity of the sensory areas of the utricle, saccule and semicircular ducts can only be speculative. Nevertheless, it is possible to reach some significant conclusions concerning vestibular development in the different mammalian groups that were studied.
Comparison of vestibular development between different mammalian groups
Of all the mammalian groups available to study, the dasyurids (quolls, i.e. marsupial carnivores) have the distinction of being born with the most immature vestibular apparatus (Gemmell & Nelson, 1992; Nelson et al. 2003). In fact, the otocyst of a newborn quoll is little more than a simple disc-shaped or spherical cavity with an endolymphatic diverticulum. The (pre)ganglion complex is so immature that discrete geniculate and vestibulocochlear components have yet to appear, no axonal connections have been made by the vestibular apparatus with the hindbrain, and neurons of the vestibular nuclei are yet to be generated.
The monotremes and non-dasyurid marsupials share a rather similar level of vestibular maturity at hatching or birth (stages j–k). The newborn peramelids (bandicoots) are slightly less structurally mature (stage j) than the diprotodontids (late stage j to early k) and the inner ears of newborn macropods (pademelons, wallabies, kangaroos) look slightly more mature than those of non-macropod diprotodontids (koala, common wombat, brush-tailed possum). The available monotreme specimens are limited in number but the material suggests that vestibular structural development in monotreme hatchlings (whether echidna or platypus) is roughly similar to that in macropod diprotodontids.
The statistical analysis did not find any significant difference between marsupials and monotremes in the body length at which most vestibular stages (e.g. h, i, j and k) was reached, with the possible exception of the body length at which the monotremes reached stage l (see Table2 and above). Furthermore, although the eutherian dataset is limited to a few species, the statistical analysis did not reveal any difference between the monotremes/marsupials and the laboratory rat in the body length at which vestibular stage j was reached (a stage at which most of the marsupials and monotremes are born or hatched). There does not appear to be any basis for arguing that marsupials and monotremes have a precocious development of the vestibular apparatus relative to body size when compared with laboratory rodents.
Vestibular maturity at the time of birth: correlation with newborn behaviour
Although marsupials and monotremes are all born in an immature state, there are significant differences between the various groups in their level of locomotor ability and behavioural requirements at the time of birth or hatching. Dasyurids are born very small (the size of a grain of rice – less than 5 mm long) and are transferred passively from the urogenital sinus to the pouch (actually a hollow pit on the maternal abdomen) by gravity, often while still within the amniotic sac (Huston, 1976; Tyndale-Biscoe & Renfree, 1987; Nelson, 1992; Nelson et al. 2003; Tyndale-Biscoe, 2005). Once in the pouch, the young break through the amniotic sac and move about using their forelimbs alone to find a teat and attach. Often there are more young born (up to 18 in some species; Van Dyck & Strahan, 2008) than there are teats available, and any newborns that fail to find a teat will quickly dehydrate and die. The remarkable immaturity of the vestibular apparatus at birth in the dasyurids is consistent with the passive transfer from the urogenital sinus to the pouch. These newborns are clearly unable to orientate against a gravitational field, presumably because the vestibular apparatus is apparently unconnected with the brainstem, so the newborn must be relying on other sensory cues to locate the teat.
Peramelids (bandicoots and the bilby) have backward-facing pouches. During birth, the mother lies on her side with the urogenital sinus slightly above or level with the pouch. The young slither horizontally or slightly downwards to the pouch with a wriggling movement for a distance of about 1 cm (Gemmell et al. 1999). Young peramelids therefore do not have to climb against gravity, but they face a more demanding challenge to reach the pouch than dasyurids do. Newborn peramelids have a vestibular apparatus that is evidently more developed than that in dasyurids but clearly not fully mature. Without the opportunity to trace pathways or use electrophysiological techniques it is difficult to reach firm conclusions about vestibular function in newborn peramelids, but the vestibular apparatus is very similar anatomically to that of the newborn grey short-tailed opossum (Monodelphis domestica, Pflieger & Cabana, 1996). In the newborn opossum, the vestibular part of the vestibulocochlear nerve distributes to the vestibular nuclei, which in turn project to the spinal cord (Pflieger & Cabana, 1996; Adadja et al. 2013). Even the vestibular apparatus of embryonic rats (E13; vestibular stage h) has vestibular ganglion axons innervating both the maculae and cristae of the vestibular apparatus as well as penetrating the brainstem (Ashwell & Zhang, 1998). It is therefore likely that although the peramelid vestibular apparatus is not fully mature at birth, it may be able directly or indirectly to influence forelimb locomotor function.
In diprotodontid marsupials (e.g. possums, the koala, wombats, wallabies, kangaroos) the pouch is usually forward-facing (except for the burrowing wombat, where a forward-facing pouch would fill with dirt). Birth in this group often involves the mother sitting on her tail or lower back so that the urogenital sinus is positioned below the pouch. The newborn climbs the intervening distance (perhaps as much as 150 mm in larger macropod diprotodontids like the red kangaroo) up the external wall of the pouch to its rim, before turning downwards into the pouch interior (Beek, 1955; Poole & Pilton, 1964; Sharman & Calaby, 1964; Sharman & Pilton, 1964; Sharman, 1972; Tyndale-Biscoe & Renfree, 1987; Renfree et al. 1989; Veitch et al. 2000). Our group has already shown that the vestibular apparatus of the newborn tammar (a macropod diprotodontid) has connections with both ipsi-and contralateral vestibular nuclei (McCluskey et al. 2008). Although no direct vestibulospinal connection was found in the newborn tammar, the vestibular nuclei may nevertheless be able to influence spinal cord function through reticulospinal connections.
Young monotremes face a rather different set of challenges to the marsupials. The monotreme mother transfers the egg to either a vegetation nest (the platypus) or the maternal pouch (the short-beaked echidna; Griffiths, 1968, 1978), so there is no need for the young to navigate from the birth canal to the pouch or nest. Nevertheless, the young must break through the reproductive membranes of the egg (by repeated flexion and extension of the neck to use the egg tooth and os caruncle to tear the membranes), as well as being able to move about on the maternal abdomen and locate the milk source. Since monotremes do not have teats, the young monotreme is unable to attach semi-permanently (as the marsupials do) and must repeatedly locate the milk source every time it feeds. Newly hatched monotremes have a vestibular apparatus at a similar stage of structural maturity (stage k) to macropods. Given this structural similarity, one would expect the monotreme vestibular apparatus to have a similar level of functional ability to that in the tammar wallaby. One might therefore predict that the newly hatched monotreme has a vestibular apparatus where the sensory areas (maculae and cristae) receive peripheral processes of vestibular ganglion cells, and where the central projections of the vestibular nerve contact neurons of the vestibular nuclei. It is also possible that the vestibular system is able directly or indirectly to influence function of the spinal cord as the young monotreme moves around to locate the milk source.
Precocity of systems development in the newborn vs. maternal behaviour
Monotremes and marsupials reach vestibular stages j and k (when they are hatched or born) at body lengths that lie within the range of those found for the laboratory mouse and rat at the same vestibular stage (see Table2). This stands in contrast to the precocious development of the forelimbs and associated somitic structures in marsupials (Hill & Hill, 1955; Weisbecker et al. 2008; Keyte & Smith, 2010, 2012), as well as the craniofacial neural crest required for advanced maxillo-mandibular development of marsupials (Smith, 2006). Precocity in these structures is considered an adaptation to survival of the newborn marsupial in that those anatomical components that are crucial for development and survival immediately after birth develop early, whereas other non-essential components have relatively delayed development (Keyte & Smith, 2010).
Concluding remarks
The findings indicate that monotremes and marsupials reach the later stages of vestibular apparatus development at body lengths that lie within the range of those found for laboratory rodents at the same stage. This suggests that marsupials and monotremes are neither precocious nor delayed in vestibular apparatus development relative to body length, when compared with the available eutherians. In other words, body size, not taxon, appears to be the major determinant of vestibular system maturity, at least for the mammals studied here.
Acknowledgments
This work was supported by a grant from the Alexander von Humboldt Foundation. We would like to thank Dr Peter Giere of the Museum für Naturkunde, Berlin, for access to the collections and for all his kind help during the work. We would also like to thank Dr Lauren Marotte of the Australian National University for access to her collection of tammar wallaby material. There is no conflict of interest associated with this work.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Specimen number, species, greatest length (GL) and vestibular stage.
References
- Adadja T, Cabana T, Pflieger J-F. Cephalic sensory influence on forelimb movement in newborn opossums, Monodelphis domestica. Neuroscience. 2013;228:259–270. doi: 10.1016/j.neuroscience.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Ashwell KWS. The University of Sydney; 1986. Alterations to the structure of the rat visual system following prenatal exposure to a cytotoxic agent PhD Thesis. [Google Scholar]
- Ashwell KWS, Paxinos G. Atlas of the Developing Rat Nervous System. 3rd edn. San Diego: Elsevier Academic; 2007. [Google Scholar]
- Ashwell KWS, Zhang L. Prenatal development of the vestibular ganglion and vestibulocochlear fibres in the rat. Anat Embryol. 1998;198:149–161. doi: 10.1007/s004290050173. [DOI] [PubMed] [Google Scholar]
- Ashwell KWS, Waite PME, Marotte L. Ontogeny of the projection tracts and commissural fibres in the forebrain of the wallaby (Macropus eugenii): a comparison of timing with other mammals. Brain Behav Evol. 1996;47:8–22. doi: 10.1159/000113225. [DOI] [PubMed] [Google Scholar]
- Ashwell KW, Hardman CD, Giere P. Distinct development of peripheral trigeminal pathways in the platypus (Ornithorhynchus anatinus) and short-beaked echidna (Tachyglossus aculeatus. Brain Behav Evol. 2012;79:113–127. doi: 10.1159/000334469. [DOI] [PubMed] [Google Scholar]
- Beek DM. Observations on the birth of the grey kangaroo (Macropus ocydromus. West Aust Nat. 1955;5:9. [Google Scholar]
- Gemmell RT, Nelson J. Development of the vestibular apparatus and auditory system in the northern native cat, Dasyurus hallucatus. Anat Rec. 1992;234:136–143. doi: 10.1002/ar.1092340115. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Veitch C, Nelson J. Birth in the northern brown bandicoot, Isoodon macrourus (Marsupialia: Peramelidae) Aust J Zool. 1999;47:517–528. [Google Scholar]
- Griffiths M. Echidnas. London: Pergamon; 1968. [Google Scholar]
- Griffiths M. The Biology of the Monotremes. New York: Academic; 1978. [Google Scholar]
- Hill JP, Hill WC. The growth stages of the pouch young of the native cat (Dasyurus viverinnus) together with observations on the anatomy of the newborn young. Trans Zool Soc Lond. 1955;28:349–453. [Google Scholar]
- Huston GD. Grooming behaviour and birth in the dasyurid marsupial Dasyuroides byrnei. Aust J Zool. 1976;24:277–282. [Google Scholar]
- Kaufman MH. The Atlas of Mouse Development. London: Elsevier Academic; 1992. [Google Scholar]
- Keyte AL, Smith KK. Developmental origins of precocial forelimbs in marsupial neonates. Development. 2010;137:4283–4294. doi: 10.1242/dev.049445. [DOI] [PubMed] [Google Scholar]
- Keyte AL, Smith KK. Heterochrony in somitogenesis rate in a model marsupial, Monodelphis domestica. Evol Dev. 2012;14:93–103. doi: 10.1111/j.1525-142X.2011.00524.x. [DOI] [PubMed] [Google Scholar]
- McCluskey SU, Marotte LR, Ashwell KWS. Development of the vestibular apparatus and central vestibular connections in a wallaby (Macropus eugenii. Brain Behav Evol. 2008;71:271–286. doi: 10.1159/000127047. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The development of the human brain from a closed neural tube at stage 13. Anat Embryol. 1988;177:203–224. doi: 10.1007/BF00321132. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The initial appearance of the cranial nerves and related neuronal migration in staged human embryos. Cells Tissues Organs. 2011;193:215–238. doi: 10.1159/000320026. [DOI] [PubMed] [Google Scholar]
- Nelson J. Developmental staging in a marsupial Dasyurus hallucatus. Anat Embryol. 1992;185:333–354. doi: 10.1007/BF00188546. [DOI] [PubMed] [Google Scholar]
- Nelson J, Knight RM, Kingham C. Perinatal sensory and motor development in marsupials with special reference to the northern quoll Dasyurus hallucatus. In: Jones M, Dickman C, Archer M, editors. Predators with Pouches. The Biology of Carnivorous Marsupials. Collingwood: CSIRO Publishing; 2003. pp. 205–217. [Google Scholar]
- O'Rahilly R, Müller F. The Embryonic Human Brain. An Atlas of Developmental Stages. 3rd edn. Hoboken: J. Wiley & Sons; 2006. [Google Scholar]
- O'Rahilly R, Müller F. The development of the neural crest in the human. J Anat. 2007;211:335–351. doi: 10.1111/j.1469-7580.2007.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. Significant features in the early prenatal development of the human brain. Ann Anat. 2008;190:105–118. doi: 10.1016/j.aanat.2008.01.001. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. Developmental stages in human embryos: revised and new measurements. Cells Tissue Organs. 2010;192:73–84. doi: 10.1159/000289817. [DOI] [PubMed] [Google Scholar]
- Pflieger J-F, Cabana T. The vestibular primary afferents and vestibulospinal projections in the developing and adult opossum, Monodelphis domestica. Anat Embryol. 1996;194:75–88. doi: 10.1007/BF00196317. [DOI] [PubMed] [Google Scholar]
- Poole WE, Pilton PE. Reproduction in the grey kangaroo, Macropus cangaru, in captivity. CSIRO Wildl Res. 1964;9:218–234. [Google Scholar]
- Renfree MB, Fletcher TP, Blanden DR. Physiological and behavioural events around the time of birth in macropodid marsupials. In: Jarman P, Hume ID, Grigg G, editors. Kangaroos, Wallabies and Rat-kangaroos. Sydney: Surrey Beatty and Sons; 1989. pp. 323–327. [Google Scholar]
- Sharman GB. Australia: 1972. Birth of the red kangaroo. A film produced by the CSIRO Film Unit and the Division of Wildlife Research. [Google Scholar]
- Sharman GB, Calaby JH. Reproductive behaviour in the red kangaroo, Megaleia rufa, in captivity. CSIRO Wildl Res. 1964;9:58–85. [Google Scholar]
- Sharman GB, Pilton P. The life history and reproduction of the red kangaroo (Megalaia rufa. Proc Zool Soc Lond. 1964;1432:29–48. [Google Scholar]
- Sjhao J, Dongsheng T. The Jackknife and Bootstrap. New York: Springer; 1995. [Google Scholar]
- Smith KK. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Dev Dyn. 2006;235:1181–1193. doi: 10.1002/dvdy.20676. [DOI] [PubMed] [Google Scholar]
- Song A, Ashwell KW, Tracey DJ. Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat Embryol. 2000;202:159–177. doi: 10.1007/s004290000096. [DOI] [PubMed] [Google Scholar]
- Streeter GL. The development of the cranial and spinal nerves in the occipital region of the human embryo. Am J Anat. 1905;4:83–116. [Google Scholar]
- Streeter GL. On the development of the membranous labyrinth and the acoustic and facial nerves in the human embryos. Am J Anat. 1906;6:139–165. [Google Scholar]
- Streeter GL. The peripheral nervous system in the human embryo at the end of the first month (10 mm) Am J Anat. 1908;8:285–301. [Google Scholar]
- Tyndale-Biscoe H. Life of Marsupials. Collingwood: CSIRO Publishing; 2005. [Google Scholar]
- Tyndale-Biscoe H, Renfree M. Reproductive Physiology of Marsupials. Cambridge: Cambridge University Press; 1987. [Google Scholar]
- Van Dyck S, Strahan R. The Mammals of Australia. 3rd edn. Sydney: New Holland Publishers; 2008. [Google Scholar]
- Veitch CE, Nelson J, Gemmell RT. Birth in the brushtail possumTrichosurus vulpecula(Marsupialia: Phalangeridae) Aust J Zool. 2000;48:691–700. [Google Scholar]
- Weisbecker V, Goswami A, Wroe S. Ossification heterochrony in the therian postcranial skeleton and the marsupial-placental dichotomy. Evolution. 2008;62:2027–2041. doi: 10.1111/j.1558-5646.2008.00424.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Specimen number, species, greatest length (GL) and vestibular stage.