Abstract
The axolotl Ambystoma mexicanum is one of the most used model organisms in developmental and regenerative studies because it is commonly said that it can reconstitute a normal and fully functional forelimb/hindlimb after amputation. However, there is not a publication that has described in detail the regeneration of the axolotl hindlimb muscles. Here we describe and illustrate, for the first time, the regeneration of the thigh, leg and foot muscles in transgenic axolotls that express green fluorescent protein in muscle fibers and compare our results with data obtained by us and by other authors about axolotl forelimb regeneration and about fore-and hindlimb ontogeny in axolotls, frogs and other tetrapods. Our observations and comparisons point out that: (1) there are no muscle anomalies in any regenerated axolotl hindlimbs, in clear contrast to our previous study of axolotl forelimb regeneration, where we found muscle anomalies in 43% of the regenerated forelimbs; (2) during axolotl hindlimb regeneration there is a proximo-distal and a tibio-fibular morphogenetic gradient in the order of muscle regeneration and differentiation, but not a ventro-dorsal gradient, whereas our previous studies showed that in axolotl forelimb muscle regeneration there are proximo-distal, radio-ulnar and ventro-dorsal morphogenetic gradients. We discuss the broader implications of these observations for regenerative, evolutionary, developmental and morphogenetic studies.
Keywords: Ambystoma, anatomy, hindlimb, morphogenesis, muscles, regeneration, urodele amphibians
Introduction
The neotenous axolotl Ambystoma mexicanum (Amphibia: Urodela) is one of the most used model organisms in evolutionary, developmental and regenerative studies, being a particularly powerful regenerative model. This is because it is said that it can reconstitute a fully functional and complete forelimb/hindlimb (Kragl et al., 2009; see also the reviews of Carlson, 2003, 2007; Nacu & Tanaka, 2011; Stocum & Cameron, 2011; Agata & Inoue, 2012). Within the forelimb, amputation anywhere between the shoulder and the hand triggers the formation of a progenitor cell zone (blastema) that regenerates the epidermis, dermis, muscle, nerve, blood vessels and skeletal elements of the regenerated forelimb (Weiss & Walker, 1934; Piatt, 1957; Stephens & Holder, 1987; Kragl et al., 2009). However, there are very few morphological investigations of limb musculature regeneration done in these amphibians. Most were done by Carlson and colleagues (Grim & Carlson, 1974a,b1974b; Carlson, 2003, 2007; and references therein), who suggested that the limb muscles of salamanders, including axolotls, almost always, or always, regenerate normally after amputation (‘epimorphic mode of regeneration’ sensu Carlson, 2003; see also Wigmore & Holder, 1985).
However, in a recent paper we have found that within 23 studied axolotl regenerated forelimbs there were muscle anomalies in 10 (43%) forelimbs (Diogo et al., in press). This was a surprising result of our study because this high percentage contradicted the idea that the axolotl forelimbs that are regenerated after amputation almost always display a muscle configuration that is similar to that of the original limbs. However, we also analyzed our results from a different angle and have shown that the total number of anomalies observed in the 23 regenerated forelimbs was 20, so on average each forelimb had anomalies in only 2.4% of the total (n = 36) number of muscles examined [20/(36 × 23) = 0.024]. However, it should be noted that none of the muscle defects seen in the 23 regenerated limbs was seen in the non-regenerated (i.e. original) limbs analyzed by Diogo & Tanaka (2012).
Interestingly, despite the numerous studies published so far focusing on axolotl limb regeneration, there is no publication that s described in detail the regeneration of the hindlimb muscles of this amphibian. The present paper provides a detailed morphological investigation of the regeneration of the axolotl hindlimb muscles, which is based on analyses of transgenic animals that express green fluorescent protein (GFP) in muscle fibers (Figs 1–5; see Materials and methods). Diogo & Tanaka (2012) recently explained the major advantages of including GFP-transgenic animals in a study such as the present one. For instance, by including GFP-transgenic axolotls and visualizing these animals with and without simultaneous transmission laser light, one can get a more complete and clearer understanding of the exact limit of the fleshy (shown as fluorescent green in GFPs) and tendinous (examined in dissections and also by using the transmission light) parts of the muscles and their specific connections with the skeletal elements.
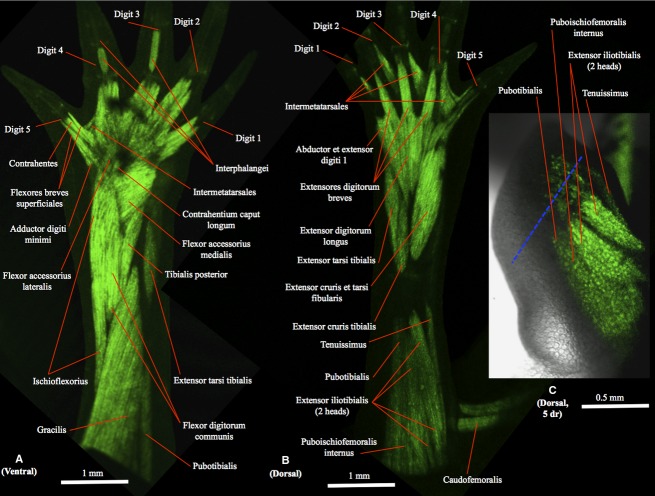
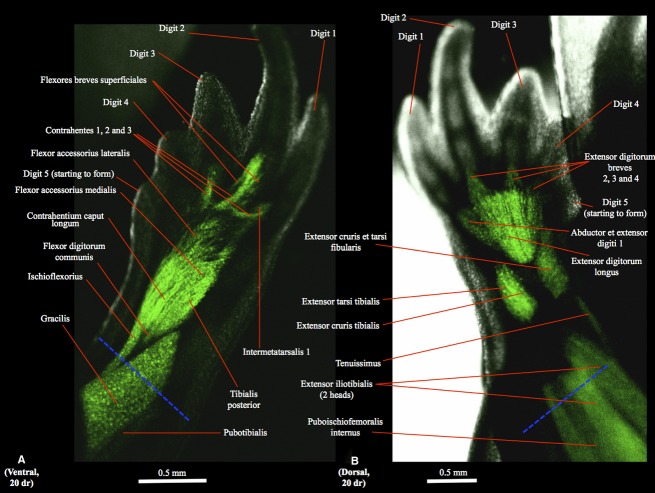
Figure 1.
(A) Ventral (tibial is to the right and distal to the top) and (B) dorsal (tibial is to the left and distal to the top) views of the right hindlimb of GFP-transgenic axolotl CRTD AM125 (10 cm total length) showing a non-amputated limb with a normal muscle configuration, similar to that found in the other non-amputated hindlimbs analyzed for the present study. (C) Dorsal view of the right hindlimb of GFP-transgenic axolotl CRTD AM101 at 5 days of regeneration (dr); tibial is to the left and distal to the top. In this figure and in the next figures the blue dashed line indicates the approximate place of amputation.
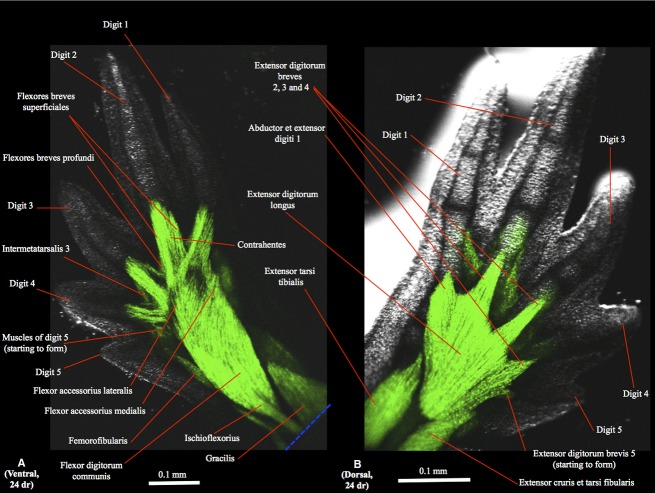
Figure 5.
(A) Ventral (tibial is to the right and distal to the top) and (B) dorsal (tibial is to the left and distal to the top) views of the right hindlimb of GFP-transgenic axolotl CRTD AM101 at 24 days of regeneration (dr).
The main goals of the present publication are therefore: (i) to establish a basis for future regenerative, developmental and morphogenetic studies on the hindlimb of axolotls and other tetrapods, by providing brief, simple anatomical and morphogenetic descriptions of the regeneration of the hindlimb muscles that can be understood by anatomists and non-anatomists, in particular with the aid of photographs of GFP-transgenic animals; (ii) to test whether regeneration of the amputated hindlimb in axolotls leads to a completely normal configuration of the muscles; (iii) to analyze how is muscle is regenerated topologically and morphogenetically, i.e. which muscles develop first and how are they related to each other three-dimensionally; for example, is there a tibio-fibular, a proximo-distal and/or a ventro-dorsal gradient?; (iv) to compare the regeneration of the axolotl hindlimb muscles with that of the axolotl forelimb and with the ontogeny of both limbs in axolotls and other tetrapods.
Materials and methods
A total of 52 Ambystoma mexicanum hindlimbs were examined for this study. Except for two adult wildtype specimens (HU AM1, right hindlimb examined; HU AM2, right hindlimb examined) dissected at the Anatomy Department of Howard University, all the A. mexicanum specimens analyzed were from the CRTD (Center for Regenerative Therapies Dresden), including 25 transgenic animals that express GFP in muscle fibers (under the cardiac-alpha-actin promoter described by Khattak et al. (2013): CRTD AM101-109, 1.5 months post-fertilization, amputation of both limbs at the level of the thigh; CRTD AM110-124, 1.5 months post-fertilization, no amputation; CRTD AM125, 10 cm total length, no amputation). No animal was sacrificed for the purposes of this anatomical study: animals were in general examined alive, and those that were dissected were sacrificed for reasons related to research/work of other individuals (e.g. colony-keeping, experiments: see Diogo & Tanaka, 2012; for more details). All experiments conformed to the relevant regulatory standards. In total, we examined 18 regenerated and 34 non-amputated hindlimbs, the latter being used as controls. The imaging of the GFP-transgenic animals was performed with a Leica TCS LSI confocal microscope at the Light Microscopy Facility of CRTD-BIOTEC. Images were acquired using a 1× zoom objective, the GFP fluorescence being excited with the 488-nm laser line and fluorescence being collected between 500 and 520 nm with the standard photomultiplier (PMT). Simultaneously transmitted laser light was detected with a T-PMT to create a transmitted light image; overlay of both channels was created using las-af software (v 2.6). The nomenclature of the hindlimb muscles follows that of Diogo (in press), which was based on previous works such as Francis (1934) and which takes into account the evolution and homologies of these muscles within all the major tetrapod groups (see also Diogo & Abdala, 2010); in cases in which a synonym has been commonly used in the amphibian literature by other authors, that synonym will be given in the description of the respective muscle in the Results Section. When we refer to the anterior, posterior, dorsal and ventral regions of the body, we therefore do so in the sense the terms are used for pronograde tetrapods (e.g. the forelimb is anterior to the hindlimb, and in each limb the extensor muscles are dorsal to the flexor muscles).
Results
The results of our analyses are shown in detail in Figs 1–5. In this section we will provide a brief textual description of these results, which is divided into five major subsections: ventral thigh muscles, dorsal thigh muscles, ventral leg muscles, dorsal leg muscles and foot muscles (N.B. there are no intrinsic dorsal foot muscles). We report below the regeneration of the muscles of the right hindlimb of specimen CRTD AM101, which illustrates the normal pattern of regeneration seen by us in the other regenerated hindlimbs. In fact, it should be noted that we did not find any differences concerning the order in which the different muscles regenerate or regarding the specific insertion and origin of the muscles among the regenerated hindlimbs analyzed. For each subsection, we provide first a short description of the normal attachments of the adult muscles found in non-regenerated (original) hindlimbs, followed by a description of how the muscles regenerate.
Ventral thigh muscles
The ventral thigh muscles that are affected by the amputations done in our study are the pubotibialis, femorofibularis, gracilis and ischioflexorius. Within these muscles, the gracilis (often also designated as ‘puboischiotibialis’) is the most ventral thigh muscle in non-regenerated adults (Fig. 1A). This muscle has proximal and distal heads (only the latter head can be seen in Fig. 1A) and runs from the ventral midline of the puboischiac plate to the proximal two-thirds of the anteromedial face of the tibia. On the fibular side of this muscle lies the ischioflexorius (Fig. 1A), which runs from the posterolateral corner of the puboischiac plate to the plantar aponeurosis and is divided into proximal and distal portions separated by a tendinous plate at the level of one-third to one-half the distance between the origin and insertion of the muscle. On the tibial side of the gracilis lies the pubotibialis (Fig. 1A), which connects the anterolateral border of the puboischiac plate to the proximal tibia. The femorofibularis lies on the deep ventral side of the thigh and runs from the posteroventral border of the femur, at a point approximately halfway to the knee, to the posterolateral border of the fibula between the insertion of the extensor cruris tibialis and the fibular portion of origin of the flexor digitorum communis.
During the first days of regeneration (dr) the region of these ventral thigh muscles that is just proximal to the amputation site degenerates (Fig. 1C), as is usually the case in axolotl forelimb regeneration (Carlson, 2003, 2007; Diogo et al., in press). Then the muscles start to regenerate, until their normal (original) configuration is basically reconstituted at 16 dr (Fig. 2A), with exception to the most distal attachments of the ischioflexorius and femorofibularis, which are only restored at later stages of regeneration, when the leg muscles are being regenerated (Fig. 5A; see below).
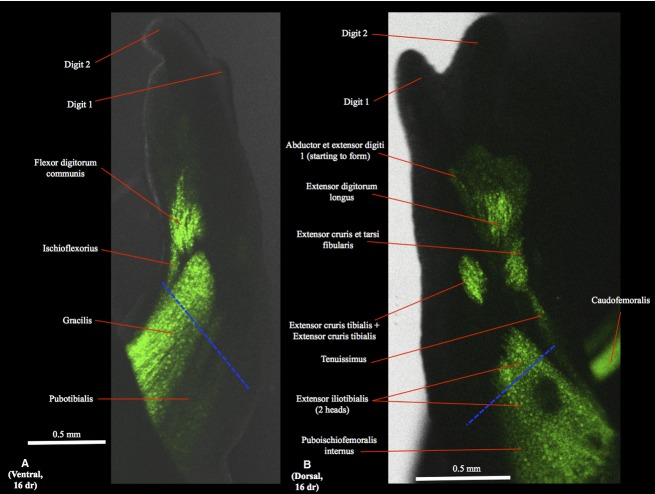
Figure 2.
(A) Ventral (tibial is to the right and distal to the top) and (B) dorsal (tibial is to the left and distal to the top) views of the right hindlimb of GFP-transgenic axolotl CRTD AM101 at 16 days of regeneration (dr).
Dorsal thigh muscles
The dorsal thigh muscles that are affected by the amputations done in our study are the extensor iliotibialis, tenuissimus and puboischiofemoralis internus. Within these muscles, the extensor iliotibialis (often also designated as ‘iliotibialis’) is the most dorsal thigh muscle in non-regenerated adults (Fig. 1B). This muscle is divided into anterior (‘iliotibialis’) and posterior (‘ilioextensorius’) heads and runs from the ilium to a wide tendon inserting onto the crista tibialis and blending with the distal portions of the extensor cruris tibialis and the extensor digitorum longus. On the fibular side of the extensor iliotibialis lies the tenuissimus (often also designated as ‘iliofibularis’) (Fig. 1B), which runs from the tendon of the extensor iliotibialis to the posterior border of the fibula. On the tibial side of the extensor iliotibialis lies the puboischiofemoralis internus (Fig. 1B), which connects the anterior portion of the dorsal midline of the puboischiac plate, ypsiloid cartilage and pubis to the femur.
As described above for the ventral thigh muscles, during the first days of regeneration the region of the dorsal thigh muscles that is just proximal to the amputation site degenerates. Then the muscles start to regenerate, until their normal (original) configuration is reconstituted at 16 dr (Fig. 2B).
Ventral leg muscles
The ventral (flexor) leg muscles found in non-regenerated adults are the flexor digitorum communis, flexor accessorius medialis, flexor accessorius lateralis, contrahentium caput longum, interosseus cruris and tibialis posterior. The most ventral of these muscles is the flexor digitorum communis (Fig. 1A), which originates from the fibular condyle of the femur and sends a broad tendon to the distal phalanges of digits 1–5. The deeper ventral leg muscles can be seen in Fig. 1A because this broad tendon appears as transparent in the figure. The tibialis posterior (often also designated as ‘pronator profundus’) is the most tibial of these deeper muscles (Fig. 1A), running from the medial part of the fibula to the distal portion of the tibia, the tibiale and the base of metatarsal I. On the fibular side of the tibialis posterior lies the flexor accessorius medialis (Fig. 1A), which runs from the distal region of the fibula, the fibulare and intermedium to the plantar fascia, then the contrahentium caput longum (Fig. 1A), which lies deep to the plantar fascia and connects the distal portion of the fibula to the distal tarsal bones and the contrahentes, and then the flexor accessorius lateralis (Fig. 1A), which runs from the fibulare to the plantar fascia. The interosseus cruris is the deeper ventral leg muscle, running from the proximal part of the fibula to the distal portion of the tibia.
In the regenerating hindlimbs the flexor digitorum communis is first found as a well-developed, differentiated muscle at 16 dr (Fig. 2A); at this stage the other, deeper ventral leg muscles are still undifferentiated. At 18 dr the flexor accessorius lateralis, flexor accessorius medialis, contrahentium caput longum and tibialis posterior are already present as differentiated muscles, but are still very poorly developed (Fig. 3A), their normal configuration and attachments being only restored at 20 dr (Fig. 4A) (N.B. the deeper interosseus cruris cannot be seen in the images of the regenerated hindlimbs obtained by us).
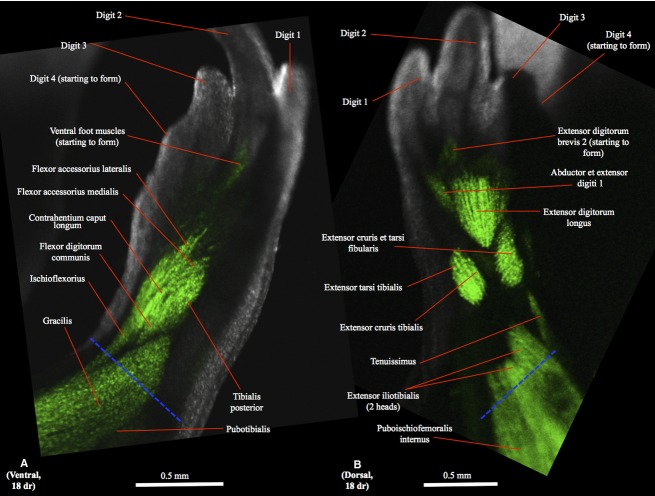
Figure 3.
(A) Ventral (tibial is to the right and distal to the top) and (B) dorsal (tibial is to the left and distal to the top) views of the right hindlimb of GFP-transgenic axolotl CRTD AM101 at 18 days of regeneration (dr).
Figure 4.
(A) Ventral (tibial is to the right and distal to the top) and (B) dorsal (tibial is to the left and distal to the top) views of the right hindlimb of GFP-transgenic axolotl CRTD AM101 at 20 days of regeneration (dr).
Dorsal leg muscles
The dorsal (extensor) leg muscles found in non-regenerated adults are the extensor digitorum longus, extensor tarsi tibialis, extensor cruris tibialis, extensor cruris et tarsi fibularis, extensores digitorum breves and abductor et extensor digiti 1. The most dorsal of these muscles is the extensor digitorum longus (often also designated as “extensor digitorum communis”) (Fig. 1B), which runs from the femoral condyles to the proximal end of metatarsals I–V. On the tibial side of this muscle lies the extensor cruris tibialis (Fig. 1B), running from the tibial epicondyle of the femur to the anteroventral and anterodorsal margins of the tibia, and then the extensor tarsi tibialis (Fig. 1B), running from the tibial epicondyle condyle of the femur to the tibiale bone. On the fibular side of the extensor digitorum longus lies the extensor cruris et tarsi fibularis (Fig. 1B), running from the femoral condyles to the posterodorsal face of the fibula (the posterior part of the muscle) and the fibulare (the anterior part of the muscle). Distally to these four muscles lie the four extensores digitorum breves (Fig. 1B), which connect the distal tarsal bones to the dorsal surface of the proximal end of the distal phalanx of digits 2, 3, 4 and 5 through a long tendon. The extensor digitorum brevis of digit 1 is fused with an abductor muscle to form the abductor et extensor digiti 1 muscle (Fig. 1B), which runs from the distal tarsal bones to the metatarsal I and distal phalanx of digit 1.
In the regenerating hindlimbs the extensor digitorum longus and extensor cruris et tarsi fibularis can first be seen as differentiated muscles at 16 dr (Fig. 2B). At this stage the extensor cruris tibialis and extensor tarsi tibialis are not yet differentiated, forming instead a mainly continuous bundle, and the abductor et extensor digit 1 is just starting to form (Fig. 2B). At 18 dr the extensor cruris tibialis and extensor tarsi tibialis are differentiated and the extensor digitorum brevis 2 is starting to form (Fig. 3B). At 20 dr the extensor digitorum longus, extensor tarsi tibialis, extensor cruris tibialis and extensor cruris et tarsi fibularis have basically restored their normal configuration and attachments, and the extensores digitorum breves 3 and 4 are now present (Fig. 4B). The extensor digitorum brevis 5 starts to form at 22 dr and is still very small at 24 dr (Fig. 5B), its normal configuration and attachments only being restored at 30 dr.
Foot muscles
The intrinsic foot muscles found in non-regenerated adults are the flexores breves superficiales, flexores breves profundi, abductor digiti minimi, contrahentes pedis, flexores digitorum minimi, interphalangei, and intermetatarsales. The most ventral of these muscles are the flexores breves superficiales (Fig. 1A), which run from the dorsal side of the plantar fascia to metatarsals I–V and digits 2–4 (the first muscle only goes to metacarpal I, not to digit 1), and the abductor digiti minimi (Fig. 1A), which runs from the distal end of the fibula to the fibulare, basale V and the base of metatarsal V. The five contrahentes pedis are deep to, and lie between, the flexores breves superficiales (Fig. 1A), connecting the tendon of the contrahentium caput longum and tarsal bones to the proximal phalanx of digits 1–5. Deep to these muscles lie the flexores breves profundi, running from the carpal/metacarpal region to each side of digits 1–5, and then the flexores digitorum minimi, which are deep, small muscles running from the metatarsals to the ventral side of the base of the proximal phalanx of digits 2–5. The four intermetatarsales connect the metatarsals of digits 1–5 (Fig. 1A,B). The interphalangei are the most distal foot muscles (Fig. 1A). Digit 3 has one interphalangeus connecting the metatarsophalangeal and first interphalangeal joints of this digit. Digit 4 has two muscles, one similar to the interphalangeus digiti 3, connecting the metatarsophalangeal and first interphalangeal joints of digit 4, and the other connecting the first and second interphalangeal joints of this digit 4.
In the regenerating limbs the first appearance of intrinsic foot muscles (still mainly undifferentiated) is at 18 dr (Fig. 2B). At 20 dr one can see the intermetatarsalis 1 and the contrahentes and flexores breves superficiales of digits 1, 2 and 3, but not of digits 4 and 5 yet (Fig. 4A). At 22 dr the muscles to digit 4 start to form but are still undifferentiated, and the intermetatarsalis 2 and at least some flexores breves profundi are now present, while at 24 dr the abductor digiti minimi, intermetatarsalis 3 and the muscles of digit 4 are present and differentiated (Fig. 5A) (N.B. the deeper muscles flexores digitorum minimi cannot be seen in the images of the regenerated hindlimbs obtained by us). At 28 dr the muscles of digit 5 are more developed, its flexores breves superficiales being now clearly differentiated and elongated, and the interphalangeus of digit 3 is starting to form. The proximal interphalangeus of digit 4 is first seen at 30 dr, and the distal interphalangeus of this digit is first seen at 34 dr, when all the other foot muscles basically display their normal configuration and attachments.
Discussion
Do the regenerated hindlimb muscles have a normal configuration?
Within the 18 regenerated hindlimbs examined we found no muscle anomalies, i.e. when all muscles were regenerated their configuration and attachments were similar to those found in the non-regenerated (original) hindlimbs examined by us. This clearly contrasts with the results of our previous study of axolotl forelimb regeneration (Diogo et al., in press). As explained above, in that study we found that within 23 regenerated forelimbs that there were muscle anomalies in 10 (43%) forelimbs (all in the left forelimb, which was the only forelimb amputated in those axolotls), contradicting the idea that the axolotl forelimbs that are regenerated after amputation almost always display a muscle configuration that is similar to that of the original limbs (see Introduction). However, the total number of individual anomalies observed in the 23 regenerated forelimbs was 20, and on average each forelimb had anomalies in only 2.4% of the 36 muscles examined (Diogo et al., in press). Moreover, almost all the muscle anomalies found in the regenerated forelimbs concerned a single muscle, the coracoradialis. In normal (non-regenerated; original) forelimbs the coracoradialis has a fleshy origin from the pectoral girdle but at the distal end of this girdle sends a long, thin tendon (which has no muscle fibers attached to it) that extends all the way to attach onto the proximal portion of the forearm. However, in eight of the 23 (35%) regenerated forelimbs the coracoradialis had fleshy fibers at the level of the arm. As suggested in our previous study (Diogo et al., in press), the frequent presence of an anomalous coracoradialis with fleshy fibers at the level of the arm in the regenerated forelimbs might be due to a difference between the ontogenetic and regenerative processes. Specifically, we suggested that the explanation might be that myogenic progenitors end up interacting with the coracoradialis ligament/tendon during regeneration, probably because: (i) during early stages of regeneration the myogenic progenitors end up in the prospective region of the coracoradialis or (ii) during later stage of regeneration, the mature or differentiating muscle fibers from the coracobrachialis or the humeroantebrachialis are attracted to or attract the regenerating coracoradialis tendon.
Our findings of muscle anomalies in the regenerated forelimbs vs. no muscle anomalies in the regenerated hindlimbs may therefore be due simply to the fact that in the hindlimb there are no long tendons (such as the coracoradialis tendon of the forelimb) that would end up with/attract muscle fibers during regeneration. Another possibility is that this may reflect a genuine difference between the regeneration of forelimbs and hindlimbs, thus adding a further difference between the tetrapod forelimbs and hindlimbs to those listed by Diogo et al. (2013) to support their view that these limbs are not serial homologues but instead the result of homoplasy (convergence and/or parallelism: see below). However, more mechanistic studies are clearly needed to test this latter hypothesis. What seems to be clear, based on the results of the present study, is that the axolotl hindlimb provides a good model for regenerative studies of limb muscle regeneration in the sense that the muscles of the regenerated limbs are effectively similar to those of the original limbs.
Morphogenesis, development and evolution
The regeneration of urodele forelimbs is a classic case study for the investigation of the morphogenesis of both hard and soft tissues (Carlson, 2007). In our previous study of axolotl forelimb regeneration the tempo and mode of the morphological events observed during regeneration were similar to those reported by other authors, i.e. the formation and differentiation of the muscles followed a proximo-distal and a radio-ulnar gradient (Grim & Carlson, 1974b). However, apart from these two morphogenetic gradients described in the literature, the results of that study indicated that there is also a marked ventro-dorsal gradient during the regeneration of at least some axolotl forearm muscles.
This contrasts with the results of the present study of axolotl hindlimb regeneration, in which we found proximo-distal and tibio-fibular morphogenetic gradients but not a ventro-dorsal gradient. The proximo-distal gradient is clearly seen in Figs 2–5: the thigh muscles are the first to regenerate, followed by the leg muscles and then by the intrinsic foot muscles. The tibio-fibular gradient corresponds to the radio-ulnar gradient of the forelimb, and as in the forelimb it is associated with a similar gradient of skeletal formation, e.g. the most radial/tibial digits form first then the most ulnar/fibular digits (Figs 2–5; N.B. the most tibial digit is digit 1, the most fibular one is digit 5). For instance, the abductor et extensor digit 1 starts to regenerate before the extensor digitorum brevis 2, followed by the extensor digitorum brevis 3 and then by the extensor digitorum brevis 4, the extensor digitorum brevis 5 being the last muscle to regenerate. The flexores breves superficiales and contrahentes of digits 1, 2 and 3 also start to regenerate before those of digits 4 and 5, the interphalangeus of digit 3 starts regenerating before the interphalangei of digit 4, and the order of regeneration of the four intermetatarsales is also from digit 1 to 4. The lack of a ventro-dorsal gradient in hindlimb regeneration is also clearly seen in Figs 2–5: for instance, when the ventral leg muscles start to regenerate and differentiate, the dorsal leg muscles are also regenerating and differentiating (Fig. 2 at 16 dr). This clearly contrasts what we found in forelimb regeneration, where the regeneration and differentiation of the ventral forearm muscles was in general earlier than those of the dorsal forearm muscles (Diogo et al., in press). It is therefore important to note that when we refer to ventro-dorsal gradient we refer to cases in which the ventral/flexor musculature of a certain region of a limb (for example zeugopod, i.e. leg or forearm) starts differentiating before the dorsal/extensor musculature of that same region. This gradient should thus not be confused with a superficial to deep gradient, as seen for instance during both regeneration and ontogeny of the axolotl leg in which the superficial flexor digitorum communis becomes a well-developed, differentiated muscle while the deeper ventral/flexor muscles are still undifferentiated (see Results). It should also be explained that the ventro-dorsal gradient seen in the regeneration of the forelimb refers to the zeugopod (forearm) and not to the stylopod (arm) or autopod; during regeneration there is no clear ventro-dorsal gradient in any region of the hindlimb.
Our recent studies of axolotl limb muscle ontogeny, using transgenic animals that express GFP in muscle fibers (Diogo & Tanaka, 2014), revealed that in forelimb ontogeny there are proximo-distal, radio-ulnar and ventro-dorsal gradients as seen in axolotl forelimb regeneration; in hindlimb ontogeny there are only proximo-distal and tibio-fibular gradients (i.e. there is no ventro-dorsal gradient) as seen in axolotl hindlimb regeneration. The occurrence of proximo-distal and radio-ulnar/tibio-fibular gradients in both limbs and both in ontogeny and regeneration of axolotls indicates that muscle patterning is probably dependent upon patterning of connective tissue. This is because in both axolotl development and regeneration there are many markers and patterning genes that have been extensively implicated in the patterning of limb connective tissue and that are upregulated in proximo-distal (e.g. Hox,FGFs, RA) and radio-ulnar and/or tibio-fibular fashion (e.g. Shh) (Carlson, 2007). It is therefore possible that either these molecules may have direct impact on muscle patterning or can influence muscle patterning via patterning of connective tissue.
Regarding the occurrence of a ventro-dorsal gradient in the regeneration and ontogeny of the forelimb, but not of the hindlimb, muscles, this could be seen as another potential genuine difference between the forelimbs and hindlimbs. That is, this could potentially be added to the list of differences between the tetrapod forelimbs and hindlimbs provided by Diogo et al. (2013), who as explained above proposed that these limbs are not serial homologues. However, it should be noted that a dorso-ventral gradient was reported in Kardon's (1998) study of the ontogeny of the hindlimb muscles of chickens. Moreover, our recent ontogenetic study of the frog Eleutherodactylus coqui (Diogo & Ziermann, 2014) has shown that both limbs display a proximo-distal muscle morphogenetic gradient and that the hindlimb displays a dorso-ventral gradient, as reported in chickens by Kardon (1998). Also, instead of a radio-ulnar/tibio-fibular gradient as seen in the regeneration and ontogeny of the musculature of both limbs of axolotls, there is mainly a ulno-radial/fibulo-tibial gradient in the ontogeny of the musculature of the fore-and hindlimbs of this frog. This makes sense in view of what is known about the ontogeny of the skeletal structures of the hand of E. coqui: the first phalanges to form are those of digit 4, then 5, then 3, and only then 2, i.e. the more central/ulnar digits form before the more radial ones (Hanken et al., 2001). The ulno-radial muscle morphogenetic gradient observed in frogs is thus more similar to the ulno-radial gradient seen during the ontogenesis of the limb skeletal structures in other non-urodele tetrapod groups and also during the ontogenesis of limb muscles seen in at least some of these groups (e.g. chickens; Carlson, 2007).
The existence of different morphogenetic gradients of muscle formation and differentiation in the same limbs of different taxa (e.g. tibio-fibular in axolotl hindlimb regeneration and ontogeny vs. fibulo-tibial in the ontogeny of the hindlimb of the E. coqui frog) and in different limbs of the same taxon (e.g. ventro-dorsal gradient in axolotl forelimb, but not in axolotl hindlimb, regeneration and ontogeny) shows a remarkable plasticity regarding the morphogenesis of tetrapod limbs. In fact, Alberch & Gale (1985) described reversal of patterns of toe reduction in frogs vs. salamanders, which clearly shows how plastic these gradient features are. Moreover, the fact that some of these gradients (e.g. ventro-dorsal in axolotl forelimb regeneration and ontogeny) are only now being discovered and reported, emphasizes how much still remains to be done to have a more integrative and comprehensive understanding of tetrapod limb regeneration, development and evolution. The major goal of the present study is precisely to contribute to this understanding, and stimulate and to pave the way for further studies that will also increase knowledge about the fascinating and highly diverse pectoral and pelvic appendages of tetrapods and of vertebrates in general.
Acknowledgments
We would like to thank Beate Gruhl and Hella Hartmann for providing the CRTD specimens and for helping with the CRTD-BIOTEC microscope and imaging system, respectively. R.D. was supported by a Howard University start-up package, and E.M.T. and P.M. by DFG grants DFG TA274/3-2, The Volkswagen Foundation, and funds from the DFG Research Center for Regenerative Therapies.
References
- Agata K, Inoue T. Survey of the differences between regenerative and non-regenerative animals. Dev Growth Differ. 2012;54:143–152. doi: 10.1111/j.1440-169X.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- Alberch P, Gale E. A developmental analysis of an evolutionary trend: digital reductions in amphibians. Evolution. 1985;39:8–23. doi: 10.1111/j.1558-5646.1985.tb04076.x. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Muscle regeneration in amphibians and mammals: passing the torch. Dev Dyn. 2003;226:167–181. doi: 10.1002/dvdy.10223. [DOI] [PubMed] [Google Scholar]
- Carlson BM. Principles of Regenerative Biology. Amsterdam: Elsevier; 2007. [Google Scholar]
- Diogo R. Comparative anatomy, evolution and homologies of the tetrapod hindlimb muscles, comparisons with the forelimb muscles, and deconstruction of the forelimb-hindlimb serial homology myth. Anat Rec. doi: 10.1002/ar.22919. (In press), in press. [DOI] [PubMed] [Google Scholar]
- Diogo R, Abdala V. Muscles of Vertebrates – Comparative Anatomy, Evolution, Homologies and Development. Oxford: Taylor & Francis; 2010. [Google Scholar]
- Diogo R, Tanaka EM. Anatomy of the pectoral and forelimb muscles of wildtype and GFP-transgenic axolotls and comparison with other tetrapods including humans: a basis for regenerative, evolutionary and developmental studies. J Anat. 2012;221:622–635. doi: 10.1111/j.1469-7580.2012.01567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diogo R, Tanaka EM. Development of fore-and hindlimb muscles in GFP-transgenic axolotls: Morphogenesis, the tetrapod bauplan, and new insights on the forelimb-hindlimb Enigma. J Exp Zool B. 2014;322:106–127. doi: 10.1002/jez.b.22552. [DOI] [PubMed] [Google Scholar]
- Diogo R, Ziermann JM. Development of fore-and hindlimb muscles in frogs: morphogenesis, homeotic transformations, digit reduction, and the forelimb-hindlimb enigma. J Exp Zool B Mol Dev Evol. 2014;322:86–105. doi: 10.1002/jez.b.22549. [DOI] [PubMed] [Google Scholar]
- Diogo R, Linde-Medina M, Abdala V. New, puzzling insights from comparative myological studies on the old and unsolved forelimb/hindlimb enigma. Biol Rev. 2013;88:196–214. doi: 10.1111/j.1469-185X.2012.00247.x. [DOI] [PubMed] [Google Scholar]
- Diogo R, Nacu E, Tanaka EM. Is salamander limb regeneration perfect? Anatomical and morphogenetic analysis of forelimb muscle regeneration in GFP-transgenic axolotls as a basis for regenerative, development and evolutionary studies. Anat Rec. doi: 10.1002/ar.22906. (In press), in press. [DOI] [PubMed] [Google Scholar]
- Francis ETB. The Anatomy of the Salamander. Oxford: Clarendon Press; 1934. [Google Scholar]
- Grim M, Carlson BM. A comparison of morphogenesis of muscles of the forearm and hand during ontogenesis and regeneration in the axolotl (Ambystoma mexicanum). I. Anatomical description of muscles of the forearm and hand. Z Anat Entwicklungsgesch. 1974a;145:137–148. doi: 10.1007/BF00519725. [DOI] [PubMed] [Google Scholar]
- Grim M, Carlson BM. A comparison of morphogenesis of muscles of the forearm and hand during ontogenesis and regeneration in the axolotl (Ambystoma mexicanum). II. The development of muscular pattern in the embryonic and regenerating limb. Z Anat Entwicklungsgesch. 1974b;145:149–167. doi: 10.1007/BF00519726. [DOI] [PubMed] [Google Scholar]
- Hanken J, Carl TF, Richardson MK. Limb development in a ‘non-model’ vertebrate, the direct-developing frog Eleutherodactylus coqui. J Exp Zool B Mol Dev Evol. 2001;291:375–388. doi: 10.1002/jez.1136. [DOI] [PubMed] [Google Scholar]
- Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- Khattak S, Schuez M, Richter T. Germline transgenic methods for tracking cells and testing gene function during regeneration in the axolotl. Stem Cell Reports. 2013;1:90–103. doi: 10.1016/j.stemcr.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragl M, Knapp D, Nacu E. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature. 2009;460:60–65. doi: 10.1038/nature08152. [DOI] [PubMed] [Google Scholar]
- Nacu E, Tanaka EM. Limb regeneration: a new development? Annu Rev Cell Dev Biol. 2011;27:409–440. doi: 10.1146/annurev-cellbio-092910-154115. [DOI] [PubMed] [Google Scholar]
- Piatt J. Studies on the problem of nerve pattern. III. Innervation of the regenerated forelimb in Ambystoma. J Exp Zool. 1957;136:229–247. doi: 10.1002/jez.1401360203. [DOI] [PubMed] [Google Scholar]
- Stephens N, Holder N. Reformation of the pattern of neuromuscular connections in the regenerated axolotl hindlimb. Development. 1987;99:221–230. doi: 10.1242/dev.99.2.221. [DOI] [PubMed] [Google Scholar]
- Stocum DL, Cameron JA. Looking proximally and distally: 100 years of limb regeneration and beyond. Dev Dyn. 2011;240:943–968. doi: 10.1002/dvdy.22553. [DOI] [PubMed] [Google Scholar]
- Weiss P, Walker R. Nerve pattern in regenerated urodele limbs. Proc Soc Exp Biol Med. 1934;31:810–812. [Google Scholar]
- Wigmore P, Holder N. Regeneration from isolated half limbs in the upper arm of the axolotl. J Embryol Exp Morphol. 1985;89:333–347. [PubMed] [Google Scholar]