Abstract
Sigmodontine rats are one of the most diverse components of the Neotropical mammal fauna. They exhibit a wide ecological diversity and a variety of locomotor types that allow them to occupy different environments. To explore the relationship between morphology and locomotor types, we analyzed traits of the postcranial osteology (axial and appendicular skeletons) of 329 specimens belonging to 51 species and 29 genera of sigmodontines exhibiting different locomotor types. In this work, postcranial skeletal characters of these rats are considered in an ecomorphological study for the first time. Statistical analyses showed that of the 34 osteological characters considered, 15 were related to the locomotor types studied, except for ambulatory. However, character mapping showed that climbing and jumping sigmodontines are the only taxa exhibiting clear adaptations in their postcranial osteology, which are highly consistent with the tendencies described in many other mammal taxa. Climbing, digging and swimming rats presented statistically differences in traits associated with their vertebral column and limbs, whereas jumping rats showed modifications associated with all the skeletal regions. Our data suggest that sigmodontine rats retain an all-purpose morphology that allows them to use a variety of habitats. This versatility is particularly important when considering the lack of specialization of sigmodontines for a specific locomotor mode. Another possible interpretation is that our dataset probably did not consider relevant information about these groups and should be increased with other types of characters (e.g. characters from the external morphology, myology, etc.).
Keywords: ecomorphological analysis, locomotion, postcranium, sigmodontine
Introduction
A notable association between modifications of the postcranial anatomy and locomotion has been observed in many mammals, such as primates (Szalay, 1976; Taylor, 1997; Sarmiento, 1998; Shapiro et al. 2005; Manfreda et al. 2006), carnivores (Taylor, 1974; Gál, 1993; Youlatos, 2003; Organ et al. 2009; Samuels et al. 2013), rodents (Hatt, 1932; Lehmann, 1963; Elissamburu & Vizcaíno, 2004; Morgan & Verzi, 2006; Candela & Picasso, 2008; Morgan, 2008; Samuels & Van Valkenburgh, 2008; Hopkins & Davis, 2009), lagomorphs (Reese et al. 2013), marsupials (Elftman, 1929; Argot, 2001, 2002, 2003a,2003b, 2004a,2004b; Szalay & Sargis, 2001; Flores & Díaz, 2009; Ercoli et al. 2012), and scandentia (Sargis, 2001, 2002a,2002b). These studies have found important relationships between the shape of some skeletal structures and different locomotor behaviors of species, identified on the basis of comparative descriptions, morphological indices, or quantitative methods of multivariate analysis.
In rodents, the subfamily Sigmodontinae is one of the most diverse components of the Neotropical mammal fauna (Reig, 1980). This group is mostly distributed in South America, where it is represented by about 84 genera and more than 370 extant species (Musser & Carleton, 2005; D'Elía et al. 2007). These rodents are also very diverse in terms of their ecology and lifestyles, and exhibit a great variety of locomotor behavior, allowing them to occupy a wide range of environments, such as wet and dry forests, steppes, grasslands, savannas, deserts, paramo, and puna (Hershkovitz, 1962; Voss, 1988; Rivas & Linares, 2006; Weksler, 2006; Rivas et al. 2010). Despite this extraordinary ecological diversity, only a few authors have attempted to detect some correlations between morphology and locomotor behavior in sigmodontines (Hershkovitz, 1962; Voss, 1988; Emmons & Feer, 1990; Rivas & Linares, 2006; Rivas et al. 2010). Hershkovitz (1962, 1969, 1972) provided the first detailed study of these relationships. Based on external morphological traits, he mentioned that sigmodontine taxa are specialized primarily in locomotion on the ground, with several groups also presenting functional adaptations related to semiaquatic, fossorial, arboreal, and jumping locomotion. Arboreal rats (e.g. Oecomys,Oligoryzomys, and Rhipidomys) are characterized by the presence of shorter and broader feet, well developed pads and a fifth toe, short claws, and a very long tail; these characters provide a more powerful and extensive grasp. Other remarkable adaptive traits mentioned by Hershkovitz (1962, 1969, 1972) include: the spade-shaped hind feet with the hairy cushion of its sole in jumping rats (e.g. Eligmodontia); stout feet with weak claws and interdigital webbing, characteristic of the semiaquatic forms (e.g. Holochilus,Pseudoryzomys,Zygodontomys); and stout hands with long and strong claws and a short tail in fossorial forms, characteristics that allow them to dig into the ground. Recently, Rivas & Linares (2006) and Rivas et al. (2010) have re-evaluated such morphological characteristics considering new traits of the head, tail, hands and feet of some locomotor types. These authors studied the form–function correspondence using geometric morphometric techniques and multivariate analyses, and explored these characters in relation to the type of locomotion and habitat of these rats.
All those studies have examined the relationships between form and locomotor types in sigmodontines using mainly external morphological traits and observed morphological differentiation among predefined ecological groups. Postcranial traits have seldom been used as evidence in these analyses (Miller & Anderson, 1977; Voss, 1988; Neves, 2003; Carvalho Coutinho et al. 2013). These studies have focused on the analysis the morphometric variation of the appendicular skeleton and its relation to the different types of locomotion, and have revealed a greater association of the appendicular characters with the distinct locomotion modes and ecological habits of sigmodontine rats.
Here we examined qualitative traits of the axial and appendicular skeletons to analyze the relationship between the morphology of postcranial skeleton and locomotor types of representatives of a wider range of sigmodontines. Characters were selected considering that the axial and appendicular skeletons are clearly linked to functional aspects, such as posture, locomotor behavior, and specific capacities of movements of the animals (Slijper, 1946; Hildebrand, 1985; Sargis, 2001; Argot, 2002; Biewener, 2003). For example, it is well-known that saltatory mammals present modifications of the cervical and lumbar vertebrae, and hind limb related to mechanisms and techniques for jumping (Hatt, 1932; Slijper, 1946; Samuels & Van Valkenburgh, 2008; Olivares, 2009). Thoracic and lumbar regions of climbing mammals display traits that increase the rigidity of the vertebral column, and the limbs show attributes that emphasize the abduction and flexion movements, conditions important during climbing (Argot, 2001; Sargis, 2001; Shapiro et al. 2005; Flores & Díaz, 2009; Salton & Sargis, 2009). In the fossorial mammals, the majority of morphological modifications occur in the limbs, which are subject to loads imposed by the action of strong muscle groups and the resistance of the soil (Fernández et al. 2000; Hopkins & Davis, 2009; Carvalho Coutinho et al. 2013). Semiaquatic taxa also exhibit skeletal specializations that improve their performance in the aquatic environment, while preserving mobility on land (Samuels & Van Valkenburgh, 2008); for instance, a long olecranon process, particularly large humeral epicondyles, and increased femoral epicondyle size (Samuels & Van Valkenburgh, 2008). This study is the first to explore the above-mentioned aspects in sigmodontines. Our main objectives were: (i) to investigate the diversity of postcranial osteology among sigmodontine rats exhibiting different locomotor types (ambulatory, swimming, digging, climbing and jumping; and (ii) to assess whether the statistically differences in characters related to the locomotor types have adaptive value.
Material and methods
Material examined
The sample used in this study includes 329 specimens belonging to 51 species and 29 genera. Selected species belong to seven tribes of sigmodontines (Abrotrichini, Akodontini, Oryzomyini, Phyllotini, Reithrodontini, Thomasomyini, and group Incertae sedis) and display a diverse range of locomotor types. Species were identified on the basis of cranial and external morphology characters described in original descriptions and recent literature (e.g. Pearson, 1958; Hershkovitz, 1962; Braun, 1993; Steppan, 1995; Anderson & Yates, 2000; Weksler, 2006; Jayat et al. 2007; Mares et al. 2008). These taxa present a wide range of body sizes, from 111 mm (e.g. Eligmodontia, Salinomys) to 465 mm (e.g. Rhipidomys,Neotomys) (Supporting Information Table S1). All the specimens examined were adults of both sexes, deposited in systematic collections (Supporting Information Appendix S1).
Determination of locomotor types
Although we are aware that the average sigmodontine rodent is capable of a wide range of locomotor modes, we selected the different locomotor types on the basis of their most frequent activity. The assignment of locomotor types in the rats studied was based on information from the literature and on field observations (Table1). Species were classified according to their locomotor types as follows: climbing – species that can occur in trees, bushes, and on rocks (saxicolous); ambulatory – species that usually use the ground to move about and do not have specializations that limit any particular activity; swimming – species that swim for dispersal, escape or foraging; digging – species that regularly dig extensive burrows as shelter or to forage underground; jumping – species in which the progression is composed of a series of leaps in which both limbs extend simultaneously, lifting the body completely off the ground and forward.
Table 1.
Locomotor types of the species examined in this study based on data from the literature (see text).
| Genus | Author | Locomotor type |
|---|---|---|
| Abrothrix | Jayat et al. (2008) | Ambulatory |
| Akodon | Emmons & Feer (1999) | Ambulatory |
| Andalgalomys | Williams & Mares (1978) | Ambulatory |
| Andinomys | Hershkovitz (1962) | Ambulatory |
| Auliscomys | Hershkovitz (1962) | Ambulatory |
| Blarinomys | Lena et al. (2008) | Digger |
| Calomys | Rivas et al. (2010) | Ambulatory |
| Chelemys | Alarcón et al. (2011) | Digger |
| Chinchillula | Hershkovitz (1962) | Climber |
| Eligmodontia | Hershkovitz (1962) | Jumper |
| Euryoryzomys | Weksler (2006) | Ambulatory |
| Euneomys | Patterson et al. (2008) | Ambulatory |
| Graomys | Hershkovitz (1962) | Ambulatory |
| Geoxus | D'Elía et al. (2007) | Swimmer |
| Holochilus | Weksler (2006) | Swimmer |
| Irenomys | Pardiñas et al. (2004) | Climber |
| Juliomys | Hershkovitz (1960) | Climber |
| Loxodontomys | Hershkovitz (1962) | Ambulatory |
| Necromys | Rivas et al. (2010) | Ambulatory |
| Nectomys | Weksler (2006) | Swimmer |
| Oligoryzomys | Weksler (2006) | Climber |
| Oxymycterus | Hershkovitz (1994) | Digger |
| Phyllotis | Hershkovitz (1962) | Ambulatory |
| Reithrodon | Pardiñas et al. (2008a) | Ambulatory |
| Rhipidomys | Carrizo & Díaz (2011) | Climber |
| Salinomys | Braun & Mares (1995) | Jumper |
| Scapteromys | Miller & Anderson (1977) | Swimmer |
| Tapecomys | Anderson & Yates (2000) | Climber |
| Thaptomys | Hershkovitz (1994) | Digger |
Postcranial osteological leftacters
Thirty-four osteological leftacters were recorded for each species, 19 corresponding to the axial skeleton and the remaining ones to the appendicular skeleton (Supporting Information Figs S1-S9 and Appendix S2). Some leftacters are the result of direct observation of the material being studied (leftacters 1, 2, 3, 14, 15, 17, 18, 27, 31, and 32), whereas others were adopted from previous studies (Carleton, 1980; Steppan, 1995; Horovitz & Sánchez-Villagra, 2003; Pacheco, 2003; Weksler, 2006; Flores, 2009). For terminology of bones, foramina, and processes we follow complete descriptions of rodent and other groups of mammals (e.g. Howell, 1926; Hatt, 1932; Evans, 1993; Argot, 2001, 2002, 2003a,2003b; Szalay & Sargis, 2001; Bezuidenhout & Evans, 2005; Flores & Díaz, 2009).
Statistical analysis
To assess the ability of osteological traits (t) to discriminate among the locomotor types (l ), we performed a statistical analysis based on frequencies in different genera of sigmodontine rats (g). In the analyses, each osteological trait is a nominal variable composed of k (> 1) modalities (also called trait attributes), which are assumed to show responses to changes in locomotor types. Consequently, the overall set of attributes to be analyzed has a = ∑ i = 1,t ki elements, where ki denotes the number of modalities exhibited by the osteological trait i. We constructed two tables of study material, namely table L, which represents locomotor types, and table T, osteological traits. Table L is a (g × l ) matrix that codes the set of species in an ecological structure (assigning 0 s and 1 s based on whether species belong to ecological categories or not). The trait table T is a matrix of size (g × a) that denotes the presence (1) or absence (0) of a given trait attribute across the set of biological entities studied. This table was obtained after transforming the original categorical variables into dummy variables. Dummy transformation is a very common step in data analysis in which a nominal variable is broken into as many binary variables as categories have the nominal variable. The next step was to combine the information contained in the previous matrices into a single (a × l ) matrix, hereafter called ecomorphological matrix E = [fij] (Supporting Information Appendix S3a,b). Each entry eij reflects the number of biological entities leftacterized by a locomotor type j and the trait attributes i. The matrix E is essentially a contingency table with counts of the combined occurrence between locomotor type and traits. It can be easily calculated via matrix multiplication of L by the transpose of T, i.e. E = TTL.
We developed a simple quantitative framework to assess the diagnostic value (for locomotor type status) of the traits by using their frequencies in the matrix E (Fig. 1). We consider that a given trait attribute is closely tied to a certain locomotor type if the following pair of conditions are met simultaneously: (i) the chosen attribute is well represented inside the set of biological entities with the target locomotor type and (ii) the chosen attribute is absent or poorly represented in the remaining lifestyle sets. Two calculations can be performed to address these rules numerically. The first parameter is called intrinsic incidence and is estimated through the fraction of observations with the target locomotor type that show the analyzed attribute (Representativeness; Table2). The second parameter is inferred from the odds of intrinsic incidence in relation to the highest extrinsic incidence of the same leftacteristic in items belonging to a locomotor type set other than the target one (Differentiation; Table2). To determine the compliance to both rules, the calculated parameters are checked to be above meaningful thresholds (Supporting Information Appendix S4). These thresholds are the lower tolerance for the intrinsic incidence and the upper tolerance for its odds to the best extrinsic incidence, respectively. We fixed these thresholds at 0.5 and 2 for the first and second parameters, respectively. This setting ensures that the observed trait attribute is unambiguously biased towards the target lifestyle. When translated into a plot of the extrinsic against the intrinsic incidence, this setting divides the domain area into four regions. The southeastern region represents the joint occurrence of incidences beyond the pre-established thresholds, so it represents a theoretical area of distinctiveness where discriminatory (or diagnostic) attribute traits would fall because (i) they occur in most of the items with the target lifestyle and (ii) they occur rarely throughout the items of another lifestyle (Appendix S4). All calculations and figures were made with r statistical software (R Development Core Team, 2011). Scripts are available from the authors upon request.
Figure 1.
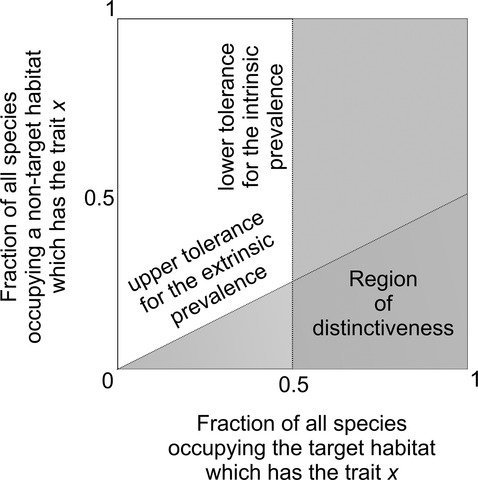
Schematic representation of the quantitative framework used to study the diagnostic value of trait attributes. Attributes that accurately segregate one target locomotor type from another, have (i) a high incidence within the target locomotor types and (ii) a null or low outside incidence. The southeastern region of the plot is the domain of diagnostic trait attributes.
Table 2.
List of characters (with their fit scores) that were diagnostic for a particular locomotive type.
| Character number | Character/state | Rp | df | Locomotortype |
|---|---|---|---|---|
| 19 | cvn/3 | 0.67 | 10.00 | C |
| 2 | atdt/01 | 0.50 | ∞ | J |
| 4 | atcf/01 | 0.50 | ∞ | J |
| 5 | atcrf/1 | 1.00 | 2.00 | J |
| 7 | axnp/0 | 1.00 | ∞ | J |
| 9 | cvpn/0 | 1.00 | 3.00 | J |
| 10 | tvpn/1 | 0.50 | 3.75 | J |
| 18 | tplv/01 | 0.50 | ∞ | J |
| 19 | cvn/2 | 0.50 | 3.75 | J |
| 22 | pdt/1 | 1.00 | 6.00 | J |
| 24 | rhs/01 | 0.50 | 3.75 | J |
| 27 | ltro/2 | 0.50 | 7.50 | J |
| 27 | ltro/01 | 0.50 | ∞ | J |
| 28 | tbs/1 | 1.00 | 3.00 | J |
| 7 | axnp/2 | 1.00 | 3.00 | Sw |
| 19 | cvn/1 | 0.67 | 2.00 | Sw |
| 24 | rhs/1 | 1.00 | 2.00 | Sw |
| 31 | tcl/1 | 0.67 | 2.00 | Sw |
| 17 | npslv/0 | 0.67 | 2.50 | Dg |
| 23 | leop/1 | 0.67 | 2.00 | Dg |
Rp indicates the chance of observing each leftacter within a given locomotive type (representativeness score). df represents the quotient of the previous score vs. the highest probability of observing such leftacter in another locomotor type (differentiation score). C, climbing; Dg, digging; Sw, swimming; J, jumping.
Leftacter mapping
To assess the evolutionary history of the leftacters related to the locomotor types obtained through the statistical analysis, we mapped those leftacters onto a selected phylogeny of sigmodontines with the computer program tree analysis using new technology (TNT; Goloboff et al. 2008). The cladogram used is based on mitochondrial and nuclear markers (Martínez et al. 2012) and was reduced to the taxa used in this analysis. This cladogram included all the genera sampled in this work except Chinchillula.
Results
The leftacters showing the diversity of postcranial osteology of sigmodontines are listed in Appendix S2.
Of the set of leftacters and state leftacters considered, 15 leftacters and 20 states were statistically related to four of the locomotor types studied (Fig. 2A,B; Table2): climbing, digging, swimming, and jumping (Figs 3–5). Ambulatory species showed no association with any particular postcranial osteological traits. Climbing species present 36–40 caudal vertebrae (cvn; leftacter 19 state 3; Fig. 2A). Digging species present lumbar neural processes that are low and long (npslv; leftacter 17 state 0; Fig. 2A), and the olecranon process is curved medially (leop, leftacter 23 state 1; Fig. 2B). Swimming species present a compressed axis neural process (axnp; leftacter 7 state 2; Fig. 2A); 25–30 caudal vertebrae (cvn; leftacter 19 state 1; Fig. 2A); an oval radial head (rhs; leftacter 24 state 1; Fig. 2B), and a long tuber calcaneus (tcl; leftacter 31 state 1; Fig. 2B). Jumping species present a dorsal tubercle of atlas (atdt; leftacter 2 state 01; Fig. 2A); cranial facet dorsally curved of atlas (atcf; leftacter 4 state 01; Fig. 2A); oval shape of the caudal facet of atlas (atcrf; leftacter 5 state 1; Fig 2A); low axis neural process (axnp; leftacter 7 state 0; Fig. 2A); 4–7 cervical vertebrae without neural process (cvpn; leftacter 9 state 0; Fig. 2A); third thoracic vertebra with the highest neural process (tvpn; leftacter 10 state 1; Fig. 2A); transverse processes of the lumbar vertebrae (tplv; leftacter 18 state 01; Fig. 2A); 31–35 caudal vertebrae (cvn; leftacter 19 state 2; Fig. 2A); proximal deltoid tuberosity (pdt, leftacter 22 state 1; Fig. 2B); radius head shape (rhs; leftacter 24 state 01; Fig. 2B); lesser trochanter orientation (ltro; leftacter 27 states 01–2; Fig. 2B), and sigmoid-shaped tibia (tbs; leftacter 28 state 1; Fig. 2B).
Figure 2.
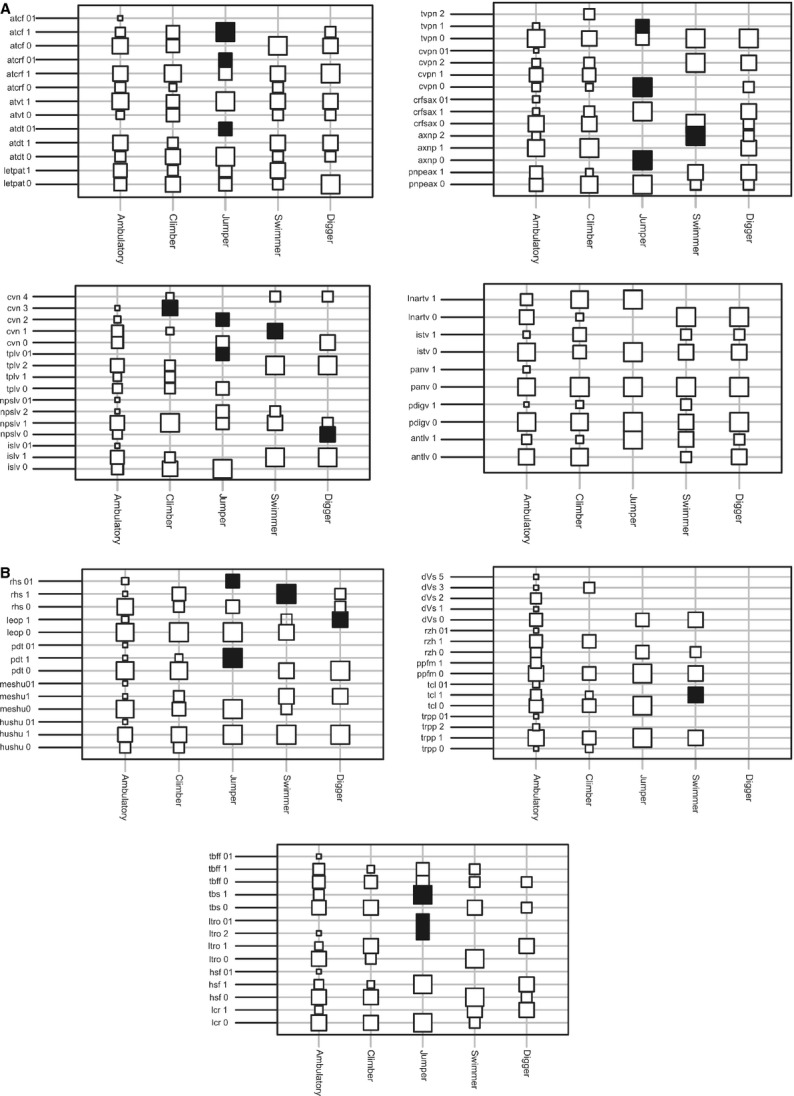
(A) Relative affinities of features across the locomotive types. The size of squares is proportional to the fraction of taxa from each locomotive type in which the respective trait modality occurs. Trait modalities diagnostic for a locomotive type are highlighted with solid squares. Abbreviations correspond to leftacters of vertebral column and are defined in Appendix S2. (B) Relative affinities of features across the locomotive types. The size of squares is proportional to the fraction of taxa from each locomotive type in which the respective trait modality occurs. Trait modalities diagnostic of a locomotive type are represented by solid squares. Abbreviations correspond to leftacters of limbs and are defined in Appendix S2.
Figure 3.
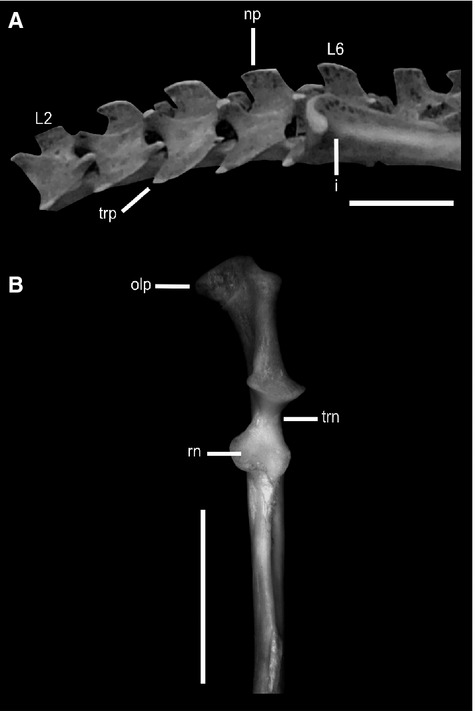
Osteological traits present in digging sigmodontines: (A) lateral view of the lumbar region of Blarinomys breviceps (CM 2640) showing low (dorsoventrally) and long (craniocaudal) neural processes, and (B) cranial view of the ulna of Chelemys macronyx (CNP 440). Note the marked medial curvature of the olecranon process. i, ilium; L2–L6, second to sixth lumbar vertebrae; np, neural process; olp, olecranon process; rn, radial notch; trn, trochlear notch. Scale bars: 5 mm.
Figure 5.
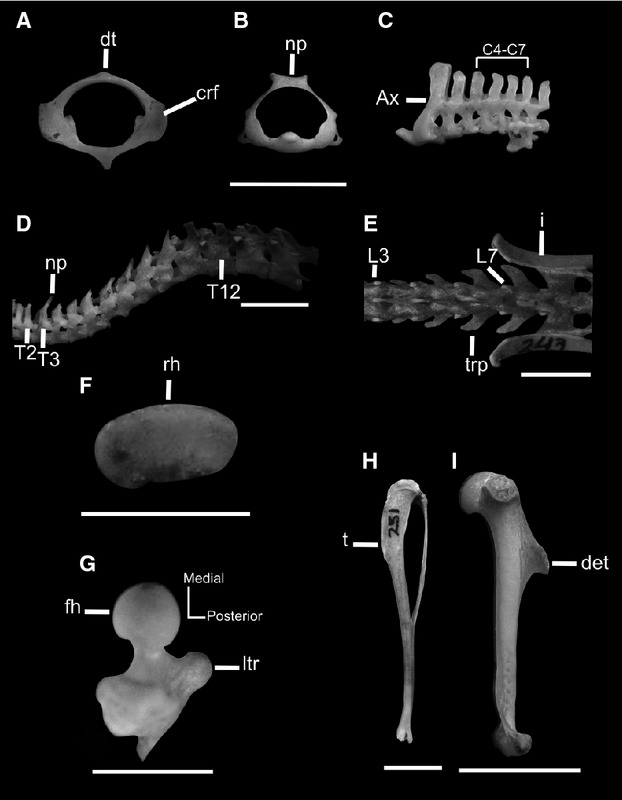
Osteological traits of the jumpers Eligmodontia (CML 8322, 8392, 8397) and Salinomys (CML 3171). (A-C) Cranial and lateral view of vertebrae of the cervical region, illustrating rudimentary or absent neural process and curved cranial facets of the atlas. (D,E) Lateral view of thoracic region and dorsal view of the lumbar region. Note the position of the highest neural process in the third thoracic vertebrae and the elongated shape of transverse processes. (F,G) Proximal view of radius and femur, showing oval shape of radius head and posterior orientation of lesser trochanter of femur. (H,I) Sigmoidal shape of tibia in medial view and position proximal to the deltoid tubercle in a lateral view of humerus. Ax, axis; det, deltoid tuberosity; dt, dorsal tubercle; C4–C7, fourth and seventh cervical vertebrae; crf, cranial facet; fh, femoral head; L3-L7, third to seventh lumbar vertebrae; ltr, lesser trochanter; i, ilium; np, neural process; T2-T12, second to twelfth thoracic vertebrae, trp, transverse process. Scale bars: 5 mm (2 mm in F).
Leftacter mapping
Mapping analyses (Fig. 6) indicated that of the 15 leftacters and 20 states exhibiting diagnostic value, only two leftacters showed clear adaptive trends when their phylogenetic history was considered (Fig. 6). All climber sigmodontines (Irenomys,Juliomys,Oligoryzomys, and Rhipidomys) had 36–40 caudal vertebrae (Fig. 6). Another clear adaptive trait is the presence of a low neural process of the axis (Fig. 6) found in jumping sigmodontines (Eligmodontia and Salinomys).
Figure 6.
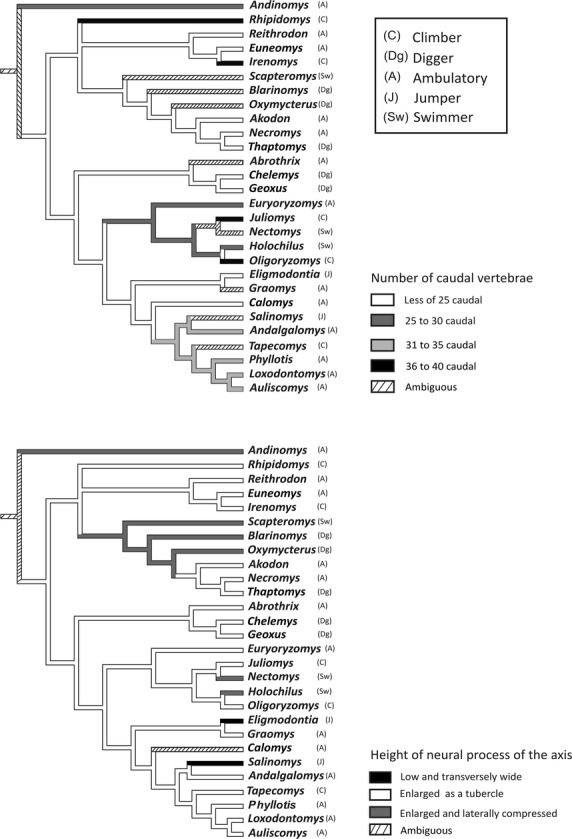
Postcranial leftacters mapped that showed a clear adaptive trend for the phylogeny of the sigmodontine rats (Martínez et al. 2012).
Discussion
The main objective of this work was to relate the postcranial osteological leftacters of sigmodontine rats to their locomotor types and to consider the phylogenetic history of the selected traits. The main outcome of our analysis is the finding of two leftacters showing clear adaptive trends, which are consistent with most of those described for other mammals (e.g. Hatt, 1932; Hildebrand, 1985; Sargis, 2001; Argot, 2003a). We will focus our discussion mainly on those leftacters considered adaptive after the optimization analysis; however, some interesting trends shown by the statistical analysis will be also considered.
The two locomotor types that exhibit those adaptations are climbing and jumping. Indeed, the tail of climbing sigmodontines is composed of 36–40 caudal vertebrae, this being our first adaptive leftacter. Our data support the frequently proposed idea of a correlation between tail length and number of caudal vertebrae (Olds & Anderson, 1989; Steppan, 1995): the longest tails have the highest number of vertebrae. In the climbing taxa studied, Oligoryzomys is a member of the tribe Oryzomyini (Weksler, 2003, 2006), Rhipidomys of the tribe Thomasomyini (Pacheco, 2003), Tapecomys and Chinchillula of the tribe Phyllotini (Steppan, 1995; Anderson & Yates, 2000), and Irenomys and Juliomys are genera currently considered as incertae sedis within Sigmodontinae (Salazar-Bravo et al. 2013). The elongate tails of these taxa would similarly seem to represent convergent traits related to balance (Fig. 6). The prehensile tail of Rhipidomys may possibly have arisen independently from that of Irenomys and Juliomys. According to Essner (2002), the arboreal non-gliding squirrels have the longest tails, followed by gliding and ground-dwelling forms. This pattern was also recovered by our data because the longest tails were found to be present in the climbing sigmodontine rats (Chinchillula,Irenomys,Juliomys,Oligoryzomys,Tapecomys, and Rhipidomys). Tails are functionally critical and versatile, playing primary roles in locomotion, balance, and sexual display (Gillis et al. 2009). Additionally, the tail augments in-air stability during jumping (Gillis et al. 2009), acts as a counterbalance to increase running speed in ambulatory species, and is used for balance while climbing in arboreal species, such as lizards (Ballinger & Tinkle, 1979). The use of the tail in the extremely heterogeneous environment of the forest has often been highlighted (Hatt, 1932; Cartmill, 1985; Sargis, 2001; Youlatos, 2003) and the general trends observed in other mammals are found also among sigmodontine rats. Thus, climbing sigmodontines present a tail which helps them to cope with the balance and safety problems posed by the random disposition of branches in the forest, their often narrow size, and the slippery substrate.
Jumping sigmodontines (Salinomys and Eligmodontia) have a cervical region leftacterized by the curved dorsal portion of the cranial facets of atlas, oval-shaped caudal facet of atlas, rudimentary or absent dorsal tubercle of the atlas, low neural process of the axis (our second adaptive leftacter), and absent neural processes of the cervical vertebrae C4–C7 (Fig.5 A–C). All these leftacters are related to a reduction of the rotational movement of the neck, which ensures stability of the body while jumping. The curved shape of the cranial facet of atlas has been related to a wide range of dorsoventral movements of the skull in the atlas–occipital articulation (Flores & Díaz, 2009). The wide movement possibilities could be related to the occipital condyles, which are less developed posteriorly in lateral view and are present in jumping sigmodontines. This association has been highlighted in arboreal marsupial and arboscansorial canids (Argot, 2003a,b) and seems to be related to their searching behavior adapted to obtain small food. By contrast, ambulatory forms such as Graomys,Calomys, and Phyllotis exhibit condyles better developed craniocaudally (L. V. Carrizo, pers. obs.). The oval shape of the caudal facet is a trait that may be related to greater range of movements of the skull. This facet articulates with the second cervical vertebra, atlanto–axial articulation, and provides for most cranial mobility, forming a pivot joint between the head and the neck (Evans, 1993; Argot, 2003a). Interesting, and although these leftacters also were not recovered as adaptive by the optimization analysis, is the structure of the thoracic region. Jumping sigmodontines present the highest neural process in T3 (Fig. 5D). The neural process of T2 is high in climbing sigmodontines, and its height is similar to that of their posterior thoracic vertebrae. In ambulatory sigmodontines the highest neural process is that of the T2, decreasing its size in the posterior vertebrae. The highest neural process of the thoracic region provides the site of attachment for the nuchal ligament, a stout tendon from which two large muscles (splenius and rhomboideus) of the neck and shoulder regions originate (Rinker, 1954; Voss, 1988). According to Argot (2003a), the dorsoventral shortening of the neural processes of the last thoracic vertebrae is more emphasized in the fast-running terrestrial taxa. This feature is an indicator of the strong flexion and extension of the vertebral column during bounding and running. In the case of jumpers, the neural processes of this region are dorsoventrally short (except in T3), becoming higher towards the posterior part of the thoracic and lumbar region, as in many other rodents that show saltatory behavior (Hatt, 1932; Samuels & Van Valkenburgh, 2008; Olivares, 2009). In the lumbar region, the vertebrae possess elongate transverse processes, oriented downward and forward (Fig. 5E). In swimming (Scapteromys,Nectomys and Holochilus) and digging sigmodontines (Blarinomys,Chelemys and Geoxus) the lumbar transverse processes are short and slightly horizontal. The muscles primarily associated with the modification of the lumbar transverse processes are the longissimus dorsi, quadratus lumborum, and psoas magnus. The functions of these muscles are to extend the vertebral column, support the forward part of the body in bipedal progress, assist in flexing the column, and accompany leaping (Hatt, 1932). Interestingly, jumping sigmodontines also present a high neural process with a marked reduction in the antero-posterior diameters in the lumbar region of the column, a trend that has been described by Hatt (1932), Argot (2003a), and Olivares (2009) in the lumbar vertebrae of other jumping mammals. The increase in length of these processes is associated directly with the increase leverage for the muscle multifidus spine, which supports the fore end of the body (Hatt, 1932). Another trait obtained in the statistical analysis related to this locomotor type is the tail composed of 31–35 vertebrae; this is a long tail, although not as long as that of the typical arboreal rats. The elongate tail is important in reducing torques and pitching during takeoff and landing. When the animal is in the air, the position of the tail also influences the orientation of the body during the jump (Hatt, 1932; Emerson, 1985).
Traits related to the forelimb present in jumping sigmodontines that were recovered by our statistical analysis are the proximally located deltoid tuberosity of the humerus and the oval or subrectangular shape of the radius head (Fig. 5F,I). The deltoid tuberosity is the site of insertion of the deltoid and pectoralis muscles, which contribute to forelimb retraction (Hildebrand, 1985; Fernández et al. 2000; Morgan & Verzi, 2006; Lessa et al. 2008; Olivares, 2009). The proximally located deltoid tuberosity of the humerus in jumping species, would increase the velocity ratio for the limb protracting and retracting muscles and would potentially facilitate rapid take-off when jumping, as these taxa are quadrupedal saltators rather than ricochetal. This would also potentially allow greater ranges of motion, effectively increasing stride length. Regarding the shape of the radius head, a slightly ovoid radius head is also present in other mammals, such as the extant porcupines, probably allowing rotation of the radius, and consequently supination–pronation of the antebrachium (Candela & Picasso, 2008). As a general Bauplan in mammals, a more circular radial head allows a greater rotational mobility of the radius, whereas a more rectangular one limits them (Taylor, 1974; Szalay & Sargis, 2001; Candela & Picasso, 2008). Thus, jumping sigmodontines tend to have forelimbs with greater range of movement.
The leftacters of the hind limb obtained from our statistical analysis and that define the jumping locomotor type are orientation of the lesser trochanter and shape of the tibia (Fig. 5G,H). Jumping sigmodontines can display the lesser trochanter oriented medially, posteromedially and posteriorly (present only in Salinomys). The iliacus and psoas major muscles, as well as the flexors, rotators and protractors of the femur, insert on the lesser trochanter (McEvoy, 1982; Evans, 1993). The orientation of the trochanter has been interpreted as a good indicator of muscle function and locomotor types. The posteromedial and posterior orientations of the trochanter suggest an antero-posterior orientation of the fibers of the iliopsoas complex. This complex acts as a pronator of the femur, allowing the parasagittal movements in terrestrial forms (Argot, 2002, 2003b; Sargis, 2002b; Candela & Picasso, 2008). Argot (2002) showed that arboreal genera are also leftacterized by a lesser trochanter that is much more prominent and oriented more medially than in terrestrial forms; its medial position emphasizes the external rotation and flexion functions of the muscle during the recovery phase of climbing. By contrast, a reduced lesser trochanter, which tends to be posteriorly located in saltatorial forms as well as in the sigmodontine rats surveyed, would indicate a more antero-posterior orientation of the muscles protractor of the femur (iliacus and psoas major), facilitating parasagittal movements (Argot, 2002). In jumping sigmodontines, the tibia is sigmoid in medial view, with the diaphysis exhibiting a proximal convexity. It has been hypothesized that this curvature of the tibial shaft is most likely correlated with the enlarged lateral femoral condyle in marsupials (Argot, 2002; Salton & Sargis, 2009). The sigmoid shape was also attributed to a position of the tibia into the same vertical plane as the fibula, providing the leg with more resistance to load pressure and muscular constraints (Argot, 2002).
The swimming group of sigmodontines, composed of Scapteromys,Nectomys, and Holochilus, is mainly defined by having 25–30 caudal vertebrae, a well developed and laterally compressed axis neural spine, oval shape of the radial head, and a long calcaneus tuber (Fig. 4). Scapteromys is a member of the Akodontini (Fabre et al. 2012), and Holochilus and Nectomys belong to the Oryzomyini (Weksler, 2003, 2006). Similar axial skeleton features and elongate tuber calcanei in these taxa would suggest convergent traits related to locomotor behavior. The high and laterally compressed neural spines provide a robust attachment for the deep musculature of the neck (Argot, 2003a). The more robust neck musculature would stiffen the neck and stabilize the head, helping the animal maintain directional control. Meanwhile, undulation of the tail would help the animal to maintain control rather than in propulsion, such as was described in the muskrat (Ondatra) (Hildebrand, 1985). All these leftacters probably contribute to the drag-based oscillatory mode of swimming present in most rodents, which use a pelvic paddling (Fish, 1996, 2000). The presence of a long tuber calcaneus increases the area of insertion for the gastrocnemius and soleus muscles, which act in plantar flexion of the pes and are involved in the power stroke during swimming (Fish, 1996; Samuels & Van Valkenburgh, 2008). As explained above, a more circular radial head allows more mobility at the elbow joint, facilitating the rotational movements of the radius, which suggests that forelimb movements are important in swimming.
Figure 4.
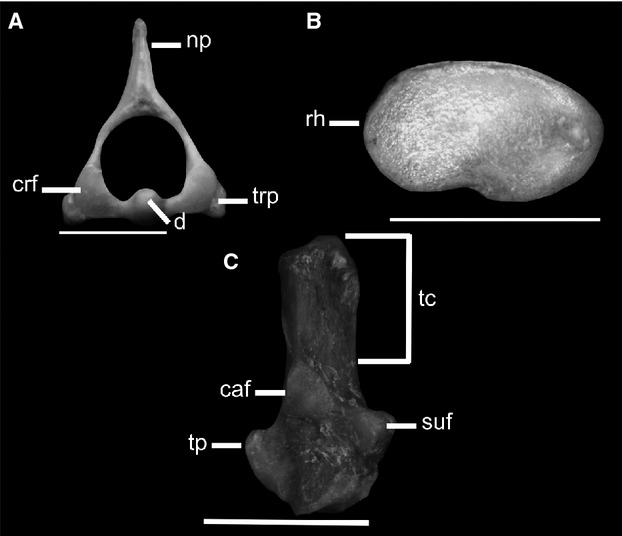
Osteological traits of the swimming: (A-C) Scapteromys aquaticus (ILPLA 052, CNP 711). (A) Cranial view of the atlas showing the neural process very high and laterally compressed and (B) oval shape of the radial head in proximal view. (C) Dorsal view of calcaneus, exhibiting a long calcaneus tuber. caf, calcaneoastragalar facet; crf, cranial facet; d, dens; np, neural process; rh, radial head; suf, sustentacular facet; tc, tuber calcaneus; tp, trochlear process; trp, transverse process. Scale bars: 5 mm.
Digging sigmodontines (Blarinomys,Chelemys,Geoxus,Oxymycterus and Thaptomys) are defined by modifications at the level of the lumbar region and forelimbs: lumbar neural processes dorsoventrally low and long craniocaudally and olecranon process medially curved (Fig. 3). Chelemys and Geoxus are member of the Abrotrichini (D'Elía et al. 2007), and Blarinomys,Oxymycterus, and Thaptomys of the Akodontini (Fabre et al. 2012). This would suggest similar lumbar structure and forelimb morphology, convergent traits related to digging in these taxa. Species examined show a tendency toward linear uniformity in the length of lumbar neural processes: low and long. This morphological pattern has been functionally associated with mobility (or lack of this) of the vertebral column (Sargis, 2001; Argot, 2003a; Shapiro et al. 2005). Gambaryan (1974) has demonstrated that the wide spinous processes restrict vertebral mobility by decreasing intervertebral space. Another leftacter associated to rigidity of the vertebral column is the shortening of the lumbar transverse processes, which probably prevent the ventral flexion of the column (Shapiro, 1993, 1995; Johnson & Shapiro, 1998; Argot, 2003a; Flores & Díaz, 2009). A medially curved olecranon process was also described for other digging mammals (Hildebrand, 1985; Vassallo, 1998; Lessa et al. 2008). The medial surface of the olecranon process is an important point of origin and insertion of the main muscles involved in the movements of the antebrachium (e.g. through the triceps and anconeous muscles), carpus and hand digits (through the flexor digitorum communis and flexor carpi ulnaris muscles), which are very well developed in fossorial animals that use their fore limbs as a tool for digging (Vassallo, 1998; Fernández et al. 2000). This leftacteristic of the ulna, along with others such as the great development of the deltoid tuberosity, related to increased development of stabilizing shoulder muscles, show a fore limb with all the traits clearly adapted to digging (Lehmann, 1963; Argot, 2001; Sargis, 2002a; Morgan & Verzi, 2006).
Distinctive traits for the ambulatory class could not be detected. The circumstance of finding no trait unequivocally restricted to this locomotive type is an interesting outcome of our work. Indeed, two profiles of attribute occurrences preclude us of raising some trait as representative of the ambulatory condition, namely (i) when a given trait was present in the great majority of ambulatory taxa, it was also frequently recorded for items belonging to other locomotive groups, and (ii) when a given trait present in the ambulatory class was rare (or absent) in the out-groups, it showed also a low intragroup incidence, providing inadequate support for an ambulatory locomotive type (Fig. 2A,B).
Our data suggest that sigmodontine rats retain an all-purpose morphology that allows them to use a variety of habitats. This all-purpose morphology may be the basis for the overarching effect of phylogeny observed in our analyses, which left only two adaptive leftacters (see also Carvalho Coutinho et al. 2013). It should be noted that the average sigmodontine rodent is capable of a wide range of locomotor modes, opportunistically shifting habits to facilitate foraging or escape predators. For example, Nowak (1999), based on Barlow (1969), describes Scapteromys as having “excellent swimming ability and agility in climbing onto tall plants” and that “it digs to obtain food but apparently does not excavate burrows”. This versatility is particularly important when considering the lack of specialization of sigmodontines for one locomotor mode or another (Carvalho Coutinho et al. 2013). Furthermore, the locomotive versatility in addition to other attributes such as small body size and high dental diversity can be linked to the broad evolutionary radiation of sigmodontine (see Smith & Patton, 1999; Jansa & Weksler, 2004; Steppan et al. 2004; Fabre et al. 2012). It maybe also the case that conservation of the internal gross morphology may represent a mechanism that is able to accommodate a wide array of environmental challenges by permitting adequacy in all such circumstances (Gans, 1993).
Conclusions
Our study shows that only a few of the leftacters of the postcranial skeleton of sigmodontines exhibit clear adaptive trends that are highly consistent with the trends described in many other mammal taxa. Adaptive traits associated with the climbing and jumping locomotor types were obtained with the mapping analysis. Other selected postcranial leftacters showed a statistically significant association with climbing, swimming, digging and jumping sigmodontines. Since these leftacters are observed among taxa of different tribes, the hypothesis of convergent skeletal morphology associated to the functional performance of locomotive types is sustained. None of the leftacters observed for the ambulatory was enough to define this category. Our data suggest that sigmodontines retain an all-purpose morphology that allows them to use a variety of habitats. Another possible interpretation is that our dataset did not consider relevant information about these groups, and should be increased with other types of leftacters (e.g. leftacters from the external morphology and myology).
Acknowledgments
We thank Ulyses Pardiñas, David Flores, Ricardo Ojeda, Horacio Zeballos, Rubén Barquez and Julieta Vargas for allowing access to mammal collections. We especially thank Pablo Jayat for allowing access to the study material and Pablo Teta for his help with photographs. We also thank Salvador Arias for his cooperation in some of the analyses performed and Guillermo Cassini for his comments, suggestions, and criticism. Our thanks to Mariano S. Sanchez for their great help with the edition of figures. Thanks to Joshua Samuels, and an anonymous reviewer for their valuable comments that improved the quality of this work. L.V.C., M.J.T. and D.A.D.S. were supported by a Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) fellowship.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Taxa and specimens examined.
Data on characters analyzed in this study.
(a,b) Ecomorphological matrix.
Statistical analysis.
Characters of the atlas.
Characters of the axis and posterior cervical vertebrae.
Characters of the thoracic region.
Characters of the lumbar region.
Characters of the humerus.
Characters of the ulna and radius.
Characters of the femur, tibia and fibula.
Characters of calcaneus.
Characters of the pes.
Values of body size.
References
- Alarcón O, D'Elia G, Lessa EP. Phylogeographic structure of the fossorial long-clawed mouse Chelemys macronyx (Cricetidae: Sigmodontinae) Zool Stud. 2011;50:682–688. [Google Scholar]
- Anderson S, Yates T. A new genus and species of phyllotine rodent from Bolivia. J Mamm. 2000;8:18–36. [Google Scholar]
- Argot C. Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene Marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol. 2001;247:51–79. doi: 10.1002/1097-4687(200101)247:1<51::AID-JMOR1003>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Argot C. Functional-adaptive analysis of the hindlimb anatomy of extant Marsupials and Paleobiology of the Paleocene Marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol. 2002;253:76–108. doi: 10.1002/jmor.1114. [DOI] [PubMed] [Google Scholar]
- Argot C. Functional-adaptative anatomy of the axial skeleton of some extant marsupials and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol. 2003a;255:279–300. doi: 10.1002/jmor.10062. [DOI] [PubMed] [Google Scholar]
- Argot C. Functional adaptations of the postcranial skeleton of two miocene Borhyaenoids (Mammalia, Metatheria), Borhyaena and Prothylacinus, from South America. Palaeontology. 2003b;46(part 6):1213–1267. [Google Scholar]
- Argot C. Functional-adaptive features and paleobiologic implications of the postcranial skeleton of the late Miocene sabretooth borhyaenoid Thylacosmilus atrox (Metatheria) Alcheringa. 2004a;28:229–266. [Google Scholar]
- Argot C. Functional-adaptive analysis of the postcranial skeleton of a Laventan borhyaenoid, Lycopsis longirrostris (Marsupialia, Mammalia) J Vert Paleo. 2004b;24:689–708. [Google Scholar]
- Ballinger RE, Tinkle DW. On the cost of tail regeneration to body growth in lizards. J Herpet. 1979;13:374–375. [Google Scholar]
- Barlow JC. Observations on the biology of rodents in Uruguay. In: Wiggens GB, Peterson RL, editors. Life Sciences Contributions. Canada: Royal Ontario Musuem; 1969. pp. 1–59. figs 1–21, vol. 75. [Google Scholar]
- Bezuidenhout AJ, Evans HE. American Society of Mammalogists; 2005. p. 180. Anatomy of woodchuck (Marmota monax)Special publicatión no. 13. [Google Scholar]
- Biewener AA. Animal Locomotion. Oxford: Oxford University Press; 2003. [Google Scholar]
- Braun JK. Systematic Relationships of the Tribe Phyllotini (Muridae: Sigmodontinae) of South America. Norman: Oklahoma Museum of Natural History; 1993. p. 50. [Google Scholar]
- Braun J, Mares M. A new genus and species of phyllotine rodent (Rodentia: Muridae: Sigmodontinae: Phyllotini) from South America. J Mammals. 1995;76:504–521. [Google Scholar]
- Candela A, Picasso MBJ. Functional anatomy of the limbs of Erethizontidae (Rodentia, Caviomorpha): indicators of locomotor behavior in miocene porcupines. J Morphol. 2008;269:552–593. doi: 10.1002/jmor.10606. [DOI] [PubMed] [Google Scholar]
- Carleton MD. Phylogenetic relationships of neotomine-peromyscine rodents (Muroidea) and a reappraisal of the dichotomy within New World Cricetinae. Misc Publ Mus Zool Univ Mich. 1980;157:1–146. [Google Scholar]
- Carrizo LV, Díaz MM. Descripción del postcráneo de Rhipidomys austrinus y Graomys griseoflavus (Rodentia, Cricetidae, Sigmodontinae) Iheringia Ser Zool. 2011;101:207–219. [Google Scholar]
- Cartmill M. Climbing. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge: Belknap Press; 1985. pp. 73–88. [Google Scholar]
- Carvalho Coutinho L, Alves de Oliveira J, Pessoa LM. Morphological variation in appendicular skeleton of Atlantic Forest sigmodontine rodents. J Morphol. 2013;274:779–792. doi: 10.1002/jmor.20134. [DOI] [PubMed] [Google Scholar]
- D'Elía GF, Pardiñas J, Teta P. Definition and diagnosis of a new tribe of sigmodontine rodents (Cricetidae: Sigmodontinae), and a revised classification of the subfamily. Gayana. 2007;71:187–194. [Google Scholar]
- Elftman HO. Functional adaptations of the pelvis in marsupials. Bull Am Mus Nat Hist. 1929;58:189–232. [Google Scholar]
- Elissamburu A, Vizcaíno SF. Limb proportions and adaptations in caviomorph rodents (Rodentia: Caviomorpha) J Zool. 2004;262:145–159. [Google Scholar]
- Emerson SB. Jumping and leaping. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge: Belknap Press; 1985. pp. 58–72. [Google Scholar]
- Emmons L, Feer F. Neotropical Rain Forest Mammals. Chicago: University of Chicago Press; 1990. p. 281. [Google Scholar]
- Emmons L, Feer F. FAN. Una guía de campo. Santa Cruz, Bolivia: Fundación amigos de la naturaleza-Noel Kempff; 1999. Mamíferos de los bosques húmedos de América Tropical; pp. 175–177. Based on revised English 2 nd. [Google Scholar]
- Ercoli DM, Prevosti JP, Álvarez A. Forn and function within a phylogenetic framework: locomotory habits of extant predators and some Miocene Sparassodonta (Metatheria) Zool J Linn Soc. 2012;165:224–251. [Google Scholar]
- Essner RL. Three-dimensional launch kinematics in leaping, parachuting and gliding squirrels. J Exp Biol. 2002;205:2469–2477. doi: 10.1242/jeb.205.16.2469. [DOI] [PubMed] [Google Scholar]
- Evans HE. Miller's Anatomy of the Dog. 3rd edn. Philadelphia: W.B. Saunders; 1993. [Google Scholar]
- Fabre PH, Hautier L, Dimitrov D. A glimpse on the pattern of rodent diversification: a phylogenetic approach. BMC Evol Biol. 2012;12:88. doi: 10.1186/1471-2148-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández ME, Vassallo AI, Zárate M. Functional morphology and palaeobiology of the pliocene rodent Actenomys (Caviomorpha: Octodontidae): the evolution to a subterranean mode of life. Biol J Linn Soc. 2000;71:71–90. [Google Scholar]
- Fish FE. Transitions from drag-based to lift-based propulsion in mammalian swimming. Am Zool. 1996;36:628–641. [Google Scholar]
- Fish FE. Biomechanics and energetics in aquatic and semiaquatic mammals: Platypus to whale. Physiol Biochem Zool. 2000;73:683–698. doi: 10.1086/318108. [DOI] [PubMed] [Google Scholar]
- Flores DA. Phylogenetic analyses of postcranial skeletal morphology in didelphid marsupials. Bull Am Mus Nat Hist. 2009;320:1–81. [Google Scholar]
- Flores DA, Díaz MM. Postcranial skeleton of Glironia venusta (Didelphimorphia, Didelphidae, Caluromyinae): description and functional morphology. Zoosyst Evol. 2009;85:311–339. [Google Scholar]
- Gál JM. Intervertebral lesión experiment and mechanisms of bending resistance. J Exp Biol. 1993;174:281–297. doi: 10.1242/jeb.174.1.281. [DOI] [PubMed] [Google Scholar]
- Gambaryan PP. 1974. How mammals run: anatomical adaptations Translation from Russian edition (Leningrad, 1972) by Hilary Hradin. Halsted (Wiley), Program for Scientific Translations, New York, and Israel, Jerusalem.
- Gans C. On the merits of adequacy. Am J Sci. 1993;293:391–406. [Google Scholar]
- Gillis GB, Bonvini LA, Irschick DJ. Losing stability: tail loss and jumping in the arboreal lizard Anolis carolinensis. J Exp Biol. 2009;212:604–609. doi: 10.1242/jeb.024349. [DOI] [PubMed] [Google Scholar]
- Goloboff PJ, Farris S, Nixon K. T.N.T.: tree analysis using new technologies. Programa y documentación. Cladistics. 2008;24:774–786. [Google Scholar]
- Hatt RT. The vertebral columns of ricochetal rodents. Bull Am Mus Nat Hist. 1932;63:738p. [Google Scholar]
- Hershkovitz P. Mammals of northern Colombia, preliminary report Nº 8: arboreal rice rat, a systematic revision of the Subgenus Oecomys, Genus Oryzomys. Proc US Natl Mus. 1960;110:513–568. [Google Scholar]
- Hershkovitz P. Evolution of Neotropical cricetine rodents (Muridae) with special reference to the phyllotine group. Field Zool. 1962;46:1–524. [Google Scholar]
- Hershkovitz P. The evolution of mammals on South Continents: VI. The recent mammals of the Neotropical region: a zoogeographic and ecological review. Q Rev Biol. 1969;44:1–70. [Google Scholar]
- Hershkovitz P. The recent mammals of the Neotropical region: a zoogeographic and ecological review. In: Keast A, Erk FC, Glass B, editors. Evolution, Mammals and Southern Continents. Albany: State University of New York Press; 1972. pp. 311–431. [Google Scholar]
- Hershkovitz P. The description of a new species of South American Hocicudo, or long-nose mouse genus Oxymycterus (Sigmodontinae, Muroidea), with a critical review of the generic content. Field Zool. 1994;79:1–40. [Google Scholar]
- Hildebrand M. Digging of quadrupeds. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge: Belknap Press of Harvard University; 1985. pp. 90–108. [Google Scholar]
- Hopkins SSB, Davis EB. Quantitative morphological proxies for fossoriality in small mammals. J Mamm. 2009;90:1449–1460. [Google Scholar]
- Horovitz I, Sánchez-Villagra MR. A morphological analysis of marsupial mammal higher-level phylogenetic relationships. Cladistics. 2003;19:181–212. [Google Scholar]
- Howell AB. Anatomy of the Wood Rat. Baltimore: Williams and Wilkins; 1926. p. 262. Monographs of the American Society of Mammalogists. [Google Scholar]
- Jansa S, Weksler M. Phylogeny of muroid rodents: relationships within and among major lineages as determined by IRBP gene sequences. Mol Phylogenet Evol. 2004;31:256–276. doi: 10.1016/j.ympev.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Jayat JP, D'Elia EG, Pardiñas UFJ. A new species of Phyllotis (Rodentia, Cricetidae, Sigmodontinae) from the upper montane forest of the yungas of north western Argentina. In: Kelt DA, Lessa E, Salazar-Bravo JA, Patton JL, editors. The Quintessential Naturalist: Honoring the Life and Legacy of Oliver P. Pearson. Vol. 134. Berkeley: University of California Press; 2007. pp. 775–798. [Google Scholar]
- Jayat J, Pardiñas U, Ojeda R. IUCN 2013. IUCN Red List of Threatened Species. 2008. Abrothrix illuteus. Version 2013.2. Available at http://www.iucnredlist.org. [Google Scholar]
- Johnson SE, Shapiro LJ. Positional behavior and vertebral morphology in atelines and cebines. Am J Phys Anthropol. 1998;105:333–354. doi: 10.1002/(SICI)1096-8644(199803)105:3<333::AID-AJPA4>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Lehmann WH. The forelimb architecture of some fossorial rodents. J Morphol. 1963;113:59–76. doi: 10.1002/jmor.1051130105. [DOI] [PubMed] [Google Scholar]
- Lena G, Berngallo HG, Esbérard CEL. The kariotype of Blarinomys breviceps (Mammalia:Rodentia:Cricetidae) with comments on its morphology and some ecological notes. Zootaxa. 2008;1907:47–60. [Google Scholar]
- Lessa EP, Vassallo AI, Verzi DH. Evolution of morphological adaptations for digging in living and extinct ctenomyid and octodontid rodents. Biol J Linn Soc. 2008;95:267–283. [Google Scholar]
- Manfreda E, Mitteroecker P, Bookstein FL. Functional morphology of the first cervical vertebra in humans and nohuman primates. Anat Rec B New Anat. 2006;2898:184–194. doi: 10.1002/ar.b.20113. [DOI] [PubMed] [Google Scholar]
- Mares MA, Braun JK, Coyner BS. Phylogenetic and biogeographic relationships of gerbil mice Eligmodontia (Rodentia, Cricetidae) in South America, with a description of a new species. Zootaxa. 2008;1753:1–33. [Google Scholar]
- Martínez JJ, Ferro LI, Mollerach MI. The phylogenetic relationships of the Andean swamp rat genus Neotomys (Rodentia, Cricetidae, Sigmodontinae) based on mitochondrial and nuclear markers. Acta Theriol (Warsz) 2012;57:277–287. [Google Scholar]
- McEvoy JS. Comparative myology of the pectoral and pelvic appendages of the North American porcupine (Erethizontidae) and the prehensile-tailed porcupine (Coendou prehensilis. Bull Am Mus Nat Hist. 1982;173:337–421. [Google Scholar]
- Miller LM, Anderson S. Bodily proportions of Uruguayan myomorph rodents. Am Mus Nov. 1977;2615:1–10. [Google Scholar]
- Morgan CC. Geometric morphometrics of the scapula of South American caviomorph rodents (Rodentia: Hystricognathi): form, function and phylogeny. Mamm Biol. 2008;74:497–506. [Google Scholar]
- Morgan CC, Verzi DH. Morphological diversity of the humerus of the South American subterranean rodent Ctenomys (Rodentia, Ctenomyidae) J Mamm. 2006;87:1252–1260. [Google Scholar]
- Musser GM, Carleton MD. Superfamily Muroidea. In: Wilson DE, Reeder DM, editors. Mammal Species of the World: A Taxonomic and Geographic Reference. 3rd. Baltimore: Johns Hopkins University Press; 2005. pp. 894–1531. [Google Scholar]
- Neves RMB. Heterogeneidade Morfológica Escapular e Umeral em Micromamíferos Terrestres (Rodentia: Sigmodontinae): Relacoes com as Estrategias de uso dos Hábitats. Rio de Janeiro: Universidade Federal do Rio de Janeiro, Museu Nacional; 2003. p. 167. [Google Scholar]
- Nowak RM. Walker's Mammals of the World. 6th edn. Baltimore: The Johns Hopkins University Press; 1999. [Google Scholar]
- Olds N, Anderson S. A diagnosis of the tribe Phyllotini (Rodentia, Muridae) In: Redford KH, Eisenberg JF, editors. Adv Neotrop Mammal. Gainesville: Sandhill Crane Press; 1989. pp. 55–74. [Google Scholar]
- Olivares AI. La Plata, Argentina: Universidad Nacional de La Plata; 2009. Anatomía, sistemática y evolución de los roedores caviomorfos sudamericanos del género Eumysops (Rodentia, Echimyidae) [Anatomy, systematics and evolution of the South American caviomorph rodent genus Eumysops (Rodentia, Echimyidae)]PhD Thesis. [Google Scholar]
- Organ JM, Teaford MF, Taylor AB. Functional correlates of fiber architecture of the lateral caudal musculature in prehensile and noprehensile tails of the Platyrrhini (Primate) and procyonidae (Carnivore) Anat Rec B New Anat. 2009;292:827–841. doi: 10.1002/ar.20886. [DOI] [PubMed] [Google Scholar]
- Pacheco VR. 2003. Phylogenetic analyses of the Thomasomyini (Muroidea: Sigmodontinae) based on morphological data.Tesis doctoral inédita. The City University of New York.
- Pardiñas UFJ, Cirignoli S, Laborde J. Nuevos datos sobre la distribución de Irenomys tarsalis (PHILIPPI, 1900) (Rodentia: Sigmodontinae) en Argentina. Mastzool Neotrop. 2004;11:99–104. [Google Scholar]
- Pardiñas UFJ, Jayat P, D'Elia GF. IUCN 2013. IUCN Red List of Threatened Species. 2008a. Reithrodon auritus. Version 2013.2. Available at http://www.iucnredlist.org. [Google Scholar]
- Patterson B, D'Elia GF, Pardiñas UFJ. IUCN 2013. IUCN Red List of Threatened Species. 2008. Euneomys chinchilloides. Version 2013.2. Available at http://www.iucnredlist.org. [Google Scholar]
- Pearson OP. A taxonomic revision of the rodent genus Phyllotis. Zoology. 1958;56:391–496. University of California Publications. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2011. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Reese AT, Lanier HC, Sargis EJ. Skeletal indicators of ecological specialization in Pika (Mammalia, Ochotonidae) J Morphol. 2013;274:585–602. doi: 10.1002/jmor.20127. [DOI] [PubMed] [Google Scholar]
- Reig OA. A new fossil genus of South American cricetid rodents allied to Weidomys, with an assessment of the Sigmodontinae. J Zool Lond (1987) 1980;192:257–281. [Google Scholar]
- Rinker GC. 1954. p. 121. The comparative myology of the mammalian genera Sigmodon, Oryzomys, Neotoma, and Peromyscus (Cricetinae), with remarks on their intergeneric relationships. Miscellaneous Publications Museum of Zoology, University of Michigan, nº 83.
- Rivas BA, Linares OJ. Cambios en la forma de la pata posterior entre roedores sigmodontinos según su locomoción y hábitat. Mastzool Neotrop. 2006;13:205–215. [Google Scholar]
- Rivas BA, D'Elía G, Linares O. Diferenciación morfológica en sigmodontinos (rodentia: cricetidae) de las guayanas venezolanas con relación a su locomoción y hábitat. Mastozool Neotrop. 2010;17:97–109. [Google Scholar]
- Rockwell H, Gaynor Evans F, Pheasant H. The comparative morphology of the vertebrate spinal column its form as related to function. J Morphol. 1938;63:87–117. [Google Scholar]
- Rose KD, Chinnery BJ. The postcranial skeleton of early Eocene rodents. Bull Carnegie Mus Nat Hist. 2004;36:211–244. [Google Scholar]
- Salazar-Bravo JJ, Pardiñas UF, D'Elía G. A phylogenetic appraisal of sigmodontinae (Rodentia, Cricetidae) with emphasis on phyllotine genera: systematics and biogeography. Zool Scrip. 2013;42:250–261. [Google Scholar]
- Salton JA, Sargis E. Evolutionary morphology of the Tenrecoidea (Mammalia) hindlimb skeleton. J Morphol. 2009;270:367–387. doi: 10.1002/jmor.10697. [DOI] [PubMed] [Google Scholar]
- Samuels JX, Van Valkenburgh B. Skeletal indicators of locomotor adaptations in living and extinct rodents. J Morphol. 2008;269:1387–1411. doi: 10.1002/jmor.10662. [DOI] [PubMed] [Google Scholar]
- Samuels JX, Meachen JA, Sakai SA. Postcranial morphology and the locomotor habits of living and extinct carnivorans. J Morphol. 2013;274:121–146. doi: 10.1002/jmor.20077. [DOI] [PubMed] [Google Scholar]
- Sargis EJ. A preliminary qualitative analysis of the axial skeleton of tupaiids (Mammalia, Scandentia): functional morphology and phylogenetic implications. J Zool. 2001;253:473–483. [Google Scholar]
- Sargis EJ. Functional morphology of the forelimb of tupaiids (Mammalia, Scandentia) and its phylogenetic implications. J Morphol. 2002a;253:10–42. doi: 10.1002/jmor.1110. [DOI] [PubMed] [Google Scholar]
- Sargis EJ. Functional morphology of the hindlimb of tupaiids (Mammalia, Scandentia) and its phylogenetic implications. J Morphol. 2002b;254:149–185. doi: 10.1002/jmor.10025. [DOI] [PubMed] [Google Scholar]
- Sarmiento EE. Generalized quadrupeds, committed bipeds and the shift to open habitats: an evolutionary model of hominid divergence. Bull Am Mus Nat Hist. 1998;3250:1–78. [Google Scholar]
- Shapiro LJ. Functional morphology of the vertebral column in primates. In: Gebo D, editor. Poscranial Adaptation in Nonhuman Primates. DeKalb: Northern Illinois University Press; 1993. pp. 121–149. [Google Scholar]
- Shapiro LJ. Functional morphology of indrid lumbar vertebrae. Am J Phys Anthropol. 1995;98:323–342. doi: 10.1002/ajpa.1330980306. [DOI] [PubMed] [Google Scholar]
- Shapiro LJ, Seiffert CV, Godfrey LR. Morphometric analysis of lumbar vertebrae in extinct Malagasy strepsirrhines. Am J Phys Anthropol. 2005;128:823–839. doi: 10.1002/ajpa.20122. [DOI] [PubMed] [Google Scholar]
- Slijper EJ. Comparative biologic-anatomical investigations on the vertebral column and spinal musculature of mammals. Verhandl Kon Ned Akad Wetensch Nat (Tweede Sect.) 1946;42:1–128. [Google Scholar]
- Smith MF, Patton JL. Phylogenetic relationships and the radiation of sigmodontine rodents in South America: evidence from cytochrome b. J Mamm Evol. 1999;6:89–128. [Google Scholar]
- Steppan SJ. Revision of the Tribe Phyllotini (Rodentia: Sigmodontinae), with a phylogenetic hypothesis for the Sigmodontinae. Fieldiana Zool. 1995;80:1–112. [Google Scholar]
- Steppan S, Adkins R, Anderson J. Phylogeny and divergence-date estimates of rapid radiations in muroid rodents based on multiple nuclear genes. Syst Biol. 2004;53:533–553. doi: 10.1080/10635150490468701. [DOI] [PubMed] [Google Scholar]
- Szalay FS. Systematics of the Omomyidae (Tarsiiformes, Primates): taxonomy, phylogeny, and adaptations. Bull Am Mus Nat Hist. 1976;156:157–450. [Google Scholar]
- Szalay FS, Sargis EJ. Model-based analysis of poscranial osteology of marsupials of Palaeocene of Itabora (Brazil) and the phylogenetics and biogeography of Metatheria. Geodiversitas. 2001;23:139–302. [Google Scholar]
- Taylor ME. The functional anatomy of the forelimbs of some African Viverridae (Carnivora) J Morphol. 1974;143:307–336. doi: 10.1002/jmor.1051430305. [DOI] [PubMed] [Google Scholar]
- Taylor AB. Scapula form and biomechanics in gorillas. J Hum Evol. 1997;33:529–553. doi: 10.1006/jhev.1997.0147. [DOI] [PubMed] [Google Scholar]
- Vassallo AI. Functional morphology, comparative behaviour, and adaptation in two sympatric subterranean rodent genus Ctenomys (Rodentia: Octodontidae) J Zool. 1998;244:415–427. [Google Scholar]
- Voss RS. Systematics and ecology of ichthyomyine rodents (Muroidea): patterns of morphological evolution in a small adaptive radiation. Bull Am Mus Nat Hist. 1988;188:259–493. [Google Scholar]
- Weksler M. Phylogeny of Neotropical oryzomyine rodents (Muridae: Sigmodontinae) based on the nuclear IRBP exon. Mol Phylogenet Evol. 2003;29:331–349. doi: 10.1016/s1055-7903(03)00132-5. [DOI] [PubMed] [Google Scholar]
- Weksler M. Phylogenetic relationships of oryzomyine rodents (Muroidea: Sigmodontinae): separate and combined analyses of morphological and molecular data. Bull Am Mus Nat Hist. 2006;296:1–149. [Google Scholar]
- Williams DF, Mares M. A new genus and species of phyllotine rodent (Mammalia: Muridae) from northwestern Argentine. Ann Carn Mus. 1978;47:197–221. [Google Scholar]
- Youlatos D. Osteological correlates of tail prehensility in carnivorans. J Zool (1987) 2003;259:423–430. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Taxa and specimens examined.
Data on characters analyzed in this study.
(a,b) Ecomorphological matrix.
Statistical analysis.
Characters of the atlas.
Characters of the axis and posterior cervical vertebrae.
Characters of the thoracic region.
Characters of the lumbar region.
Characters of the humerus.
Characters of the ulna and radius.
Characters of the femur, tibia and fibula.
Characters of calcaneus.
Characters of the pes.
Values of body size.


