Abstract
Objectives
Major burn triggers immune dysfunction, which is associated with wound healing complications. Gamma delta T-cells have been shown to be important in post-burn inflammation and wound healing; however their cytokine phenotype at the burn wound site is unknown.
Methods
C57BL/6 male mice were subjected to a major burn (25% TBSA, 3rd degree) or sham treatment. At 3 h, 3 days and 7 days thereafter, skin samples were collected and subjected to dispase and trypsin digestion to isolate single cells. The cells were phenotyped and evaluated for cytokine profiles by flow cytometry. Th-1 cells were defined as IFNγ positive, Th-2 cells were defined IL-10 positive and Th-17 cells were defined as IL-17 positive.
Results
At 7 days after burn a shift towards Th-2 and Th-17 positive T-cells at the wound site was observed. Further analysis revealed that at 3 h post-injury the percentage of γδ T-cells positive for IFNγ, IL-10 and IL-17 where comparable between sham and burn skin samples. At 3 days and 7 days post-injury the percentage of cells positive for each cytokine increased; however, the increase was significantly greater for IL-10 and IL-17, as compared with IFNγ (i.e., 9-20 fold vs. 3-fold). Skin αβ T-cells preferentially produced IFNγ (~20%), which was unaffected by burn injury.
Conclusions
These data demonstrate that burn wound γδ T-cells are activated for enhanced cytokine production and display a shift towards a Th-2 and/or Th-17 phenotype. In contrast, burn wound αβ T-cells were not activated for enhanced cytokine production.
Keywords: Injury, Cytokines, Inflammation
INTRODUCTION
Burn related morbidity and mortality can in part be attributed to immune dysfunction and wound healing complications.1 These conditions enhance the risk of infections leading to the development of sepsis, and multiple organ failure (MOF).2,3 It is also well established that inflammation plays a major role in wound healing involving a wide range of immune cells.4,5
In this regard, T-cells regulate the activity of other immune cells by releasing T-cell cytokines and thereby shaping the immune response.6 T helper 1 (Th1) cells release IL-2, IFNγ and TNFβ and initiate a cell-mediated immune response, whereas Th2 cells secrete IL-4, IL-5, IL-10 and IL-13 and support humoral immune responses.6 Therefore, the induction of an appropriate Th1/Th2 response is crucial to control infections. In this regard, major burns in experimental models and in patients have been shown to decrease the Th1 (IFNγ) response and increase a Th2 (IL-10) response. This altered immune response was correlated with increased septic events in patients and decreased resistance to infection in the experimental systems.7-9 A recently defined Th cell population, termed as Th17 cells, which secrete IL-17, are described as key players in the chronic inflammation and autoimmunity.10,11 Th17 cells also play a critical role in maintaining barrier integrity of the skin, lung and gut.12
Our recent studies have demonstrated the induction of a Th17 response after burn.13,14 Gamma delta T-cells are major producers of the IL-17 cytokines and play a major role during inflammation and wound healing.15,16 Our studies have also clearly demonstrated that γδ T-cells, are central in the response to burn injury.17,18 Nonetheless, the relationship of γδ T-cells to T-cell cytokine responses at the injury site after burn remains unidentified.
METHODS
Mice
C57BL/6 male mice weighing 18-25 g (the Jackson Laboratory, Bar Harbor, ME) were used for all experiments. Mice were allowed to acclimatize for at least one week prior to experimentation and maintained in ventilated cages under specific pathogen-free conditions. Animals were randomly assigned into either sham or burn group. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Health Science Center at San Antonio and were performed in accordance with the National Institutes of Health guidelines for the care and handling of laboratory animals.
Burn procedure
Mice received a scald burn as described previously.13 Briefly, the mice were anesthetized by intraperitoneal (i.p.) injection of ketamine/xylazine, and the dorsal surface was shaved. The anesthetized mouse was placed in a custom insulated mold exposing 12.5% of their total body surface area (TBSA) along the right dorsum. The mold was immersed in 70°C water for 10 sec to produce a 3rd degree burn. The burn procedure was repeated on the left dorsal side yielding a total burn size to be 25% TBSA. Previous studies have verified this injury to be a full thickness burn with damage to the epidermal, dermal and sub-dermal layers. 13,19 The mice were then resuscitated with 1 ml of Ringer’s lactate solution administered by intraperitoneal injection and returned to their cages. The cages were placed on a heating pad until the mice were fully awake, at which time they were returned to the animal facility. Sham treatment consisted of anesthesia and resuscitation only.
Skin tissue collection and processing
At 3 h, 3 d or 7 d after burn or sham procedure, skin samples were collected and wet weight was measured. Normal non-inujured skin was collected from sham and injured skin including the wound margin was collected from burn mice. The burn injured skin was excised, down to the level of the musculofascia, including the submucosal layer by sharp dissection and processed to isolate single cells for flow cytometry.
Skin tissue digestion and single cell isolation
Full thickness skin tissues were collected and washed in PBS with 50 U/ml penicillin and 50 μg/ml streptomycin (GIBCO). Skin tissues were collected in a 60 mm petri dish (Corning) and minced with scissors into small pieces of approximately 2-3 mm in size and put into dispase II (0.05%, Roche) medium for overnight digestion at 4°C on orbital rocker. The next day, skin samples were further minced into smaller pieces and then digested by agitating in trypsin-GNK (0.3%, Glucose/dextrose, NaCl and KCl buffer, Sigma) for 30 min at 37°C in water bath shaker. Heat inactivated Fetal Bovine Serum (FBS, GIBCO) was added to stop the digestion reaction. The dissociated cells were sieved through a 100 μm mesh. The cell suspension was collected and centrifuged at 400 g for 10 min at 4°C. The cell pellet was resuspended in RPMI containing 10% heat-inactivated FBS (GIBCO), 50 μM of 2-Mercaptoethanol (Sigma-Aldrich), 2 mM of L-glutamine (GIBCO), 1 mM of sodium pyruvate (GIBCO), 100 μM Non-essential amino acids (GIBCO), 50 U/ml penicillin and 50 μg/ml streptomycin (GIBCO) supplemented with 10 U/ml murine recombinant IL-2 (BD Biosciences). Cells were counted and cultured at a density of 1×106/ml in a 12-well plate for overnight. The next day, the cells were collected after passing through a 70 μm mesh and used for flow cytometry.
Flow cytometry
The isolated skin cells were washed in staining buffer (PBS with 0.2 % BSA and 0.09% NaN3) and treated with Fc-blocking antibody (anti-CD16/CD32, BD Biosciences) for 15 min. The cells were then stained with the following directly conjugated antibodies: anti-CD3 (PE or APC-Cy7) in combination with anti-β TCR (PerCPCy5.5) and anti-δ TCR (FITC). After 30 min of incubation on ice, the cells were washed and resuspended in staining buffer.
For intracellular cytokine staining, cells were stimulated first for 4 h with phorbol-12-myristate-13-acetate (PMA, 1 μg/ml, Calbiochem) and Ionomycin (1 μg/ml, Calbiochem) and then for 2 h with Brefeldin A (GolgiPlug, 1 μl/ml, BD Biosciences). After surface staining cells were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) for 20 min in the dark. After washing twice with permeabilization buffer (BD Biosciences), intracellular cytokines were stained using anti-IFNγ (Pacific blue), anti-IL-10 (PE) or anti-IL-17 (PE) antibodies. After 30 min on ice, the cells were washed and resuspended in staining buffer.
Appropriate isotype controls were used for all staining. All data were acquired using a LSRII (BD Biosciences) and analyzed using FlowJo (Tree Star) software. A minimum of 50,000 events was collected and live cells were gated according to forward- and side-scatter properties. Total cell number was calculated as % cells × total number of cells per gram of wet weight of skin tissue/100.
Statistical analyses
Data are expressed as mean ± SEM. Comparisons were analyzed using ANOVA and student’s t-test was used for comparisons between two groups. A p value of < 0.05 was considered to be statistically significant for all analyses.
RESULTS
Wound T-cell phenotype
A profound influx of total CD3+ T-cells was observed at the wound site at 3 h, 3 days and 7 days after burn (Table 1). The absolute numbers of T-cells were significantly elevated as early as 3 h post-burn (1.7-fold) and remained elevated up to 7 days (3.9-fold) in comparison with the T-cells numbers in skin from sham mice (Table 1).
Table 1.
Quantification of Skin T-cells (# of cells/g wet weight of skin tissue)
| Time Post-Burn
|
||||
|---|---|---|---|---|
| 3 h | 3 d | 7 d | ||
| T-Cells | Sham | 4.6 ± 0.7 a | 3.7 ± 0.5 | 2.1 ± 0.4 |
| Burn | 7.9 ± 2.4 b | 11.8 ± 3.4 b, c | 8.2 ± 3.2 b | |
|
| ||||
| γδ T-Cells | Sham | 3.7 ± 0.5 | 2.3± 0.3 | 1.7± 0.3 |
| Burn | 0.5 ± 0.1 b | 0.5 ± 0.0 | 0.2 ± 0.0 b | |
|
| ||||
| αβ T Cells | Sham | 0.4 ± 0.1 | 0.5± 0.1 | 0.3± 0.1 |
| Burn | 7.4 ± 2.3 b | 10.5 ± 2.9 b, c | 7.6 ± 3.1 b | |
Data are expressed as mean ± SEM (n = 3-8 mice/group).
p < 0.05 vs. respective sham,
p<0.05 vs. day 3 and day 7 burn.
The majority of T-cells in the uninjured skin of sham mice were of the γδ T-cell receptor lineage (80-90%) (Table 1). At 3 h and 7 d post-burn, γδ T-cell numbers were significantly reduced as compared with sham skin (Table 1). The absolute number of γδ T-cells at the wound site; however, did not significantly change between 3 h and 7 d. In contrast, the overall percentage of γδ T-cells of the CD3+ T-cell population was reduced by 70 - 95 % at the wound site as compared as compared to sham skin, due to the influx of large numbers of αβ T-cells. Both, the percentages (data not shown) and he absolute numbers of αβ T-cells at the wound site was significantly increased at all time points after burn, contributing to approximately 95% of the total T-cells (Table 1). Alpha beta T-cell numbers increased by 19-fold as early as 3 h post-burn and remained elevated at 3 and 7 d (20-25-fold) post injury (Table 1).
Th profiles after the burn injury
Skin cells were stained with T-cell and cytokine markers to investigate their cytokine profile. IFNγ, IL-10 and IL-17 were used as the representative cytokines for the Th1, Th2 and Th17 profiles, respectively. The gating strategy is shown in Fig 1A. Representative dot plots from 3 h, 3 d and 7 d post-burn are shown in Fig 1B. The percentages of the γδ T-cells positive for different cytokines after sham procedure remained comparable at all time points (data not shown). Representative dot plots for sham group at the 3 h time point are shown (Fig. 1B).
Figure 1.
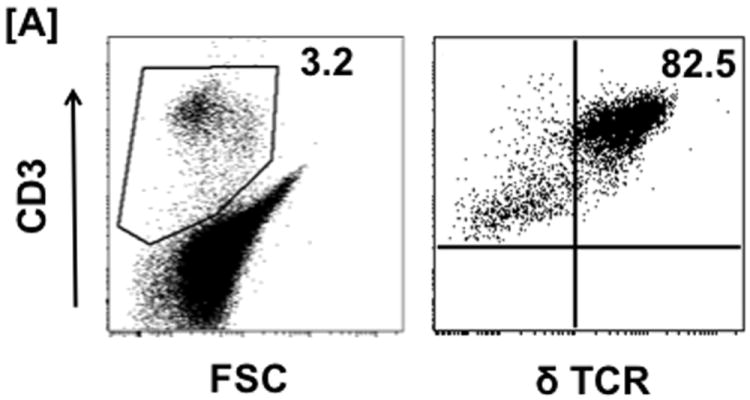
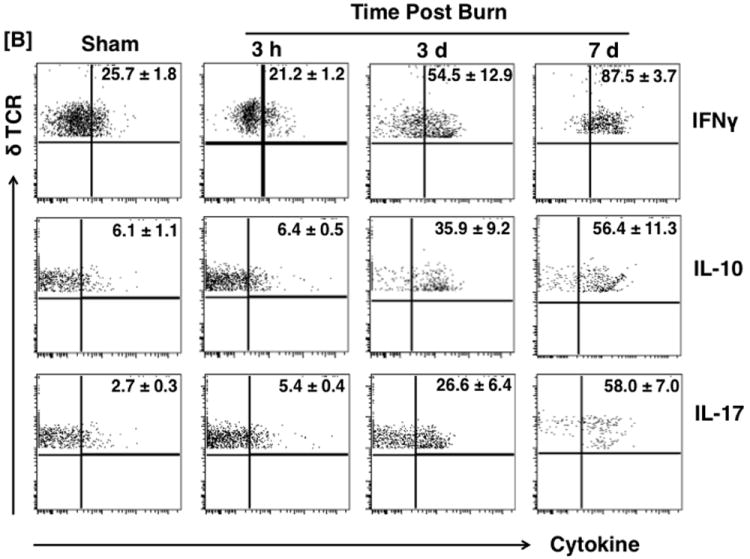

Th profiles during burn injury. Three Hours, 3 and 7 days after sham or burn procedure, skin cells were prepared and Th profiles [Th1 (IFNγ), Th2 (IL-10) and TH17 (IL-17)] of γδ and αβ T-cells were determined by means of flow cytometry. [A] Gating strategy. [B] Gating strategy and represents the percentages of γδ T-cells positive for IFNγ, IL-10 and IL-17 cytokines; population shown in the upper right quadrants of the dot plots. The numbers in the dot plots indicate the mean ± SEM of respective population. [C] Graphs demonstrate the Percentage (upper panel) and MFI (lower panel) of the γδ T-cells positive for different cytokines. Data are mean ± SEM for 3-8 mice/group; *p < 0.05 vs. respective sham.
In the sham group, the majority of the γδ T-cells did not produce cytokines. In the cytokine producing γδ T-cells the majority were IFNγ+ (~26%) as compared with IL-10+ (~6%) and IL-17+ (~3%; Fig. 1B). After burn, there was a progressive increase in the percentage of cytokine producing cells with time, and by 7 days post-injury ~90% of the cells produced IFNγ, and ~60% of the cells produced either IL-10 or IL-17 (Fig. 1B and 1C). While, this represents a significant 3-fold increase in IFNγ+ γδ T-cells after burn, the increase in IL-10+ γδ T-cells was 9-fold and IL-17+ γδ T-cells were increased by more than 20-fold, representing a shift from a Th1 profile in sham γδ T-cells to a mixed IL-10/IL-17 profile in burn γδ T-cells (Fig. 1B and 1C).
In addition to changes in the percentage of γδ T-cells positive of a given cytokine, MFI (Median Fluorescence Intensity) was also measured (Fig 1C). MFI measures the shift in the fluorescence intensity of a population of cells. In this case MFI is the measurement of the expression levels of different cytokines associated with the T-cells, whereas an increase in MFI would be indicative of that cell producing more of a specific cytokine. The IFNγ MFI value remained comparable at 3 h post-burn, but was enhanced significantly at 3 and 7 d post-burn (1.8 and 2.7 -fold respectively) when compared with the sham group. While, γδ T-cells from sham skin produced very little IL-10 and IL-17, the MFI values increased significantly after burn. IL-10 MFI values remained unchanged at 3 h post-burn but were significantly enhanced by approximately 2.7-fold at 3 d post burn and remained elevated at 7 d (2.4-fold) after burn as compared to the shams (Fig 1C). IL-17 MFI values were increased significantly at 3 h (1.5-fold), 3 d (2.3-fold) and 7 d (2-fold) (Fig 1C).
Similar to γδ T-cells, the majority of the αβ T-cells did not produce cytokines in the sham group. In the cytokine producing αβ T-cells, the majority were IFNγ+ (~40%) at 3 h time point as compared with IL-10+ (~6%) and IL-17+ -αβ T-cells (~6%) (Table 2) in both sham and burn group. These values remained comparable at 3 days post-burn. A significant increase in the percentage of αβ T-cells positive for IFNγ, IL-10 and IL-17 was observed at 7 d post-burn. However, unlike skin αβ T-cells, no preference for Th1 (IFNγ), Th2 (IL-10) or Th17 (IL-17) profile was evident (Table 2). MFI values for IFNγ, IL-10 or IL-17 did not change after burn as compared with shams (Table 3).
Table 2.
αβ T-cell Cytokine Phenotypes (% positive).
| Time Post-Burn
|
||||
|---|---|---|---|---|
| 3 h | 3 d | 7 d | ||
| IFNγ | Sham | 38.7 ± 2.9 a | 25.3 ± 2.2 | 28.6 ± 2.0 |
| Burn | 22.2 ± 6.8 | 19.9 ± 7.3 | 55.9 ± 13.1 b, c | |
|
| ||||
| IL-10 | Sham | 6.2 ± 1.8 | 3.9 ± 0.8 | 14.0 ± 1.4 |
| Burn | 10.9 ± 2.2 | 4.2 ± 1.5 | 39.1 ± 13.0 b, c | |
|
| ||||
| IL-17 | Sham | 6.2 ± 0.4 | 7.5 ± 1.0 | 12.4 ± 0.6 |
| Burn | 8.6 ± 2.7 | 6.7 ± 2.1 | 38.5 ± 12.8 b, c | |
Data are expressed as mean ± SEM (n = 3-8 mice/group).
p < 0.05 vs. respective sham,
p<0.05 vs. 3 h and 3 d burn.
Table 3.
αβ T-cell Cytokine Phenotypes (MFI)
| Time Post-Burn
|
||||
|---|---|---|---|---|
| 3 h | 3 d | 7 d | ||
| IFNγ | Sham | 874.4 ± 48.1 a | 832.6 ± 20.3 | 1023.6 ± 33.5 |
| Burn | 1054.2 ± 104.7 | 755.0 ± 16.3 | 1054.2 ± 104.7 | |
|
| ||||
| IL-10 | Sham | 766.9 ± 164.7 | 1370.3 ± 48.3 | 766.9 ± 164.7 |
| Burn | 749.3 ± 93.0 | 1354.5 ± 53.6 | 683.8 ± 31.0 | |
|
| ||||
| IL-17 | Sham | 652.2 ± 20.4 | 866.2 ± 119.0 | 652.2 ± 20.4 |
| Burn | 725.8 ± 7.8 | 687.8 ± 26.6 | 800.6 ± 30.8 | |
Data are expressed as mean ± SEM (n = 3-8 mice/group).
DISCUSSION
Considering the implication of Th profile in the wound healing process, the current study was performed to assess the cytokine phenotype of epidermal γδ T-cells at the burn wound site. The finding demonstrate the development of a mixed Th2 and Th17 response at the burn wound site, which likely plays an important role in subsequent inflammation and wound healing complications. Specifically, our findings show an increase in the Th2 cytokine, IL-10+ and Th17, IL-17+ γδ T-cells after burn. While, a significant increase in Th1 cytokine, IFNγ+ γδ T-cells was also evident after the injury, the increase in Th2 and Th17 positive γδ T-cells was far greater (by more than 10 to 20-folds for Th2 and Th17 respectively) than Th1 positive γδ T-cells. These changes represent a shift from a Th1 profile in sham γδ T-cells to a mixed IL-10/IL-17 profile in burn γδ T-cells. In contrast, wound αβ T-cells cytokine profile did not change.
T-cells are the critical component of the immune system and Th1 and Th2 subtypes are the most recognized T-cell population as first described in 1980s.20 While, IL-2 and IFNγ are the signature cytokines produced by Th1 cells, IL-4 and IL-10 are the hallmark cytokines produced by Th2 cells.6 Development of a distinct Th1 or Th2 profile influences susceptibility and outcomes from various diseases. Studies by different groups have shown that in allergic diseases, such as atopic dermatitis and allergic asthma, allergen-specific T cells acquired the Th2 phenotype and produced IL-4, IL-5, IL-6, IL-10 and IL-13.21-23 These findings suggest a Th2-bias in the development of allergic diseases. In contrast, Th1-directed responses have been shown to be involved in the pathogenesis of organ-specific autoimmune disorder (rheumatoid arthritis), psoriasis and graft versus host disease (GVDH).24-27
Gamma-delta T-cells are functionally specialized and are involved in different disease processes.4,28,29 With regard to trauma, studies have shown the presence of activated γ δ T-cells in the circulation of patients with severe SIRS.30 Studies from our laboratory have established an important role of γ δ T-cells in burn-induced immunopathology influencing macrophage function, distal organ injury and wound repair.17,18,31-33 Importantly, we observed that γδ T-cells are important in the recruitment of inflammatory cells to the injury site after burn, as γ δ T-cell deficient mice displayed a significant reduction in the cellular filtrate.31 While in the study herein we have observed that the total number of T-cells at the burn wound site increases and are predominantly α β T-cells, concurrent findings have shown that the α β T-cell influx was dependent upon the presence of γ δ T-cells, as this T-cell infiltration of the wound site was not evident in mice deficient in γ δ T-cells.34 Moreover, the absolute numbers of γδ T-cells in the burn wound were comparable to that of uninjured skin, but were activated with increased expression of TLR2, TLR4 and CD69.34,35 Thus, while γ δ T-cells are not the predominant T-cell at the wound site they are essential in the initiation and propagation of the inflammatory infiltration.
Th1/Th2 polarization has also been shown in the area of trauma research. Major injury has been documented to induce T-cell specific changes in the immune system.36 The disruptions of the natural and adaptive immunity after traumatic and burn injuries are related to the increased susceptibility to SIRS, sepsis and MOF.8 Furthermore, there is evidence that suggest that trauma induces a polarized Th2 lymphocyte response and the suppression of the Th1 response that may further predispose patients to SIRS, sepsis and MOF.8,37-41 Different studies have been conducted to elucidate those cytokines and Th profiles and inflammatory markers that occur in traumatic and burn injury through animal models and patient populations. In this regard, Lyons et al. showed an elevated Th2 response (IL-10) in PBMC of burn and trauma patients as compared to PBMC from healthy volunteers at 7 to 14 days post-injury. A significant correlation between increased IL-10 and subsequent septic events was found in the first 10 days after injury.9 In a yearlong longitudinal study, Tredget et al. have also demonstrated a polarized Th2 cytokine response in patients with hypertrophic scarring after burn.42 The study demonstrates that circulating lymphocytes shift their cytokine response away from an IFNγ-mediated Th1 response to a predominantly Th2 response, which persisted for more than 12 months.42 The present study herein is consistent with these findings showing a shift towards a Th2 response. We demonstrated the presence of Th2 polarization by the wound γδ T-cells at the injury site early post-injury (i.e., 7 days), when the immune system is still in the inflammatory phase rather than in remodeling phase. In another study Neidhardt et al. found a correlation between IL-10 levels in plasma and injury severity score (ISS).43 Increased levels of IL-10 have been associated with MODS and sepsis in trauma patients.44-46 Yeh et al. demonstrated a significant difference between the survival of burn patients and the levels of serum IL-10, which were further, correlated to patients with TBSA of greater or less than 50%.47 Zhijun et al. showed that burn injury favors antigen-driven Th2 response in vivo in the mouse burn model.7 Taking all these studies together, our present study further confirms the presence of a Th2 bias after burn injury.
A recently defined Th17 response involving T-cells that produce IL-17 has been shown to play a pivotal role in chronic inflammation and autoimmune disorders.10,11 IL-17 acts on different cell types such as neutrophils, fibroblasts, epithelial cells and endothelial cells.48,49 Different sources of IL-17 have been identified including CD8 T-cells, natural killer (NK) cells, γδ T-cells and neutrophils.50 Based on these observations, an important and unidentified role of Th17 response in the development of immune complications following injury may exist. Gamma-delta T-cells, which are important in post-burn inflammation and wound healing have also been shown to be a major source of IL-17.18,51,52 Recent studies have demonstrated a causative relationship between IL-17, γδ T-cells and survival following sepsis.49
Studies by Finnerty et al. have shown the increased levels of circulating IL-17 in pediatric burn patients, as well as in mouse burn model.53,54 Other studies have also documented elevated levels of IL-17 during pulmonary complications.55,56 and in sepsis models.49,57 These models demonstrated a role of IL-17 in recruiting immune cells, such as neutrophils and propagating inflammation.56,57 Recently, we have demonstrated the increased expression of IL-17 in different tissue beds, such as cardiac and skin tissue as early as 3 h after burn injury. 13,14 The early activation in cardiac and skin IL-17 levels is consistent with our present findings. We have shown in the present study the association between IL-17 levels and γδ T-cells present at the wound site as early as 3 h post-injury that was maintained at 7 days post-injury. This is in agreement with the others studies that have shown that γδ T-cells are an important source of IL-17.52 Therefore, we speculate that the elevation in the skin tissue levels of IL-17 after burn is due to the activation of resident γδ T-cells.
An important question is whether this mixed IL-10 and IL-17 response after burn injury is beneficial or detrimental to the healing response. Recent findings by Rutz et al. have reviewed the co-existence of IL-10 and IL-22 (cytokine produced by Th17 cells) during inflammation.58 Another study by Lemaitre et al. have shown the co-existence of Th2 and Th17 in a post-transplant obliterative airway disease.59 They showed in a mouse trachea transplant model, Cyclosporin A treatment favored Th2 and Th17 responses as co-existing pathways mediating chronic rejection of the tracheal allograft.59 Therefore, the Th2 or Th17 response appears to be detrimental.
In conclusion, T-cells of the γδ TCR lineage have a significant role in the burn wound healing process through the development of a mixed Th2 and Th17 response at the injury site. Specific elevation of these cytokines suggests that they may serve as unique targets for therapeutic manipulation to improve wound healing.
Acknowledgments
MR was responsible for experimental design, animal and flow-cytometry experiments, data analysis, scientific interpretation and drafted the manuscript. QZ was responsible for the animal experiments. MGS was responsible for scientific conception, design and helped to draft the manuscript. All authors read and approved the final manuscript. The opinions or assertions contained herein are the private views of the author and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Support: Support was provided by National Institutes of Health Grant GM079122.
Footnotes
Conflict of Interests: None
The authors declare that they have no competing interests.
References
- 1.Park MS, Salinas J, Wade CE, et al. Combining early coagulation and inflammatory status improves prediction of mortality in burned and nonburned trauma patients. J Trauma. 2008;64(2 Suppl):S188–94. doi: 10.1097/TA.0b013e318160a5a3. [DOI] [PubMed] [Google Scholar]
- 2.Schwacha MG. Macrophages and post-burn immune dysfunction. Burns. 2003;29(1):1–14. doi: 10.1016/s0305-4179(02)00187-0. [DOI] [PubMed] [Google Scholar]
- 3.Ipaktchi K, Mattar A, Niederbichler AD, et al. Attenuating burn wound inflammatory signaling reduces systemic inflammation and acute lung injury. J Immunol. 2006;177(11):8065–71. doi: 10.4049/jimmunol.177.11.8065. [DOI] [PubMed] [Google Scholar]
- 4.Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol. 2010;184(10):5423–8. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evers LH, Bhavsar D, Mailander P. The biology of burn injury. Exp Dermatol. 2010;19(9):777–83. doi: 10.1111/j.1600-0625.2010.01105.x. [DOI] [PubMed] [Google Scholar]
- 6.Romagnani S. T-cell subsets (Th1 versus Th2) Ann Allergy Asthma Immunol. 2000;85(1):9–18. doi: 10.1016/S1081-1206(10)62426-X. quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 7.Guo Z, Kavanagh E, Zang Y, et al. Burn injury promotes antigen-driven Th2-type responses in vivo. J Immunol. 2003;171(8):3983–90. doi: 10.4049/jimmunol.171.8.3983. [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–90. doi: 10.1097/00000658-199522240-00006. discussion 90-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons A, Kelly JL, Rodrick ML, et al. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226(4):450–8. doi: 10.1097/00000658-199710000-00006. discussion 58-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouser LA, Wright JF, Dunussi-Joannopoulos K, et al. Th17 cytokines and their emerging roles in inflammation and autoimmunity. Immunol Rev. 2008;226:87–102. doi: 10.1111/j.1600-065X.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 11.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–76. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 12.Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol. 2012;92(3):529–38. doi: 10.1189/jlb.0212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sasaki JR, Zhang Q, Schwacha MG. Burn induces a Th-17 inflammatory response at the injury site. Burns. 2011;37(4):646–51. doi: 10.1016/j.burns.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oppeltz RF, Zhang Q, Rani M, et al. Increased expression of cardiac IL-17 after burn. J Inflamm (Lond) 2010;7:38. doi: 10.1186/1476-9255-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Z, Burns AR, Miller SB, et al. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. Faseb J. 2011;25(8):2659–68. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray EE, Suzuki K, Cyster JG. Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol. 2011;186(11):6091–5. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwacha MG, Ayala A, Chaudry IH. Insights into the role of gammadelta T lymphocytes in the immunopathogenic response to thermal injury. J Leukoc Biol. 2000;67(5):644–50. [PubMed] [Google Scholar]
- 18.Schwacha MG. Gammadelta T-cells: potential regulators of the post-burn inflammatory response. Burns. 2009;35(3):318–26. doi: 10.1016/j.burns.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwacha MG, Knoferl MW, Chaudry IH. Does burn wound excision after thermal injury attenuate subsequent macrophage hyperactivity and immunosuppression? Shock. 2000;14(6):623–8. doi: 10.1097/00024382-200014060-00009. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann TR, Cherwinski H, Bond MW, et al. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–57. [PubMed] [Google Scholar]
- 21.Hansbro PM, Kaiko GE, Foster PS. Cytokine/anti-cytokine therapy - novel treatments for asthma? Br J Pharmacol. 2011;163(1):81–95. doi: 10.1111/j.1476-5381.2011.01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larche M. Immunoregulation by targeting T cells in the treatment of allergy and asthma. Curr Opin Immunol. 2006;18(6):745–50. doi: 10.1016/j.coi.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 23.Levine SJ, Wenzel SE. Narrative review: the role of Th2 immune pathway modulation in the treatment of severe asthma and its phenotypes. Ann Intern Med. 2010;152(4):232–7. doi: 10.1059/0003-4819-152-4-201002160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovese MC. Biologic therapies in clinical development for the treatment of rheumatoid arthritis. J Clin Rheumatol. 2005;11(3 Suppl):S45–54. doi: 10.1097/01.rhu.0000166625.65114.5f. [DOI] [PubMed] [Google Scholar]
- 25.Kupetsky EA, Mathers AR, Ferris LK. Anti-cytokine therapy in the treatment of psoriasis. Cytokine. 2013 doi: 10.1016/j.cyto.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 26.Greenblatt MB, Vbranac V, Tivey T, et al. Graft versus host disease in the bone marrow, liver and thymus humanized mouse model. PLoS One. 2012;7(9):e44664. doi: 10.1371/journal.pone.0044664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang EY, Kolumam GA, Yu X, et al. Targeted depletion of lymphotoxin-alpha-expressing TH1 and TH17 cells inhibits autoimmune disease. Nat Med. 2009;15(7):766–73. doi: 10.1038/nm.1984. [DOI] [PubMed] [Google Scholar]
- 28.Laggner U, Di Meglio P, Perera GK, et al. Identification of a novel proinflammatory human skin-homing Vgamma9Vdelta2 T cell subset with a potential role in psoriasis. J Immunol. 2011;187(5):2783–93. doi: 10.4049/jimmunol.1100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopez RD. Human gammadelta-T cells in adoptive immunotherapy of malignant and infectious diseases. Immunol Res. 2002;26(1-3):207–21. doi: 10.1385/IR:26:1-3:207. [DOI] [PubMed] [Google Scholar]
- 30.Matsushima A, Ogura H, Fujita K, et al. Early activation of gammadelta T lymphocytes in patients with severe systemic inflammatory response syndrome. Shock. 2004;22(1):11–5. doi: 10.1097/01.shk.0000129203.84330.b3. [DOI] [PubMed] [Google Scholar]
- 31.Daniel T, Thobe BM, Chaudry IH, et al. Regulation of the postburn wound inflammatory response by gammadelta T-cells. Shock. 2007;28(3):278–83. doi: 10.1097/shk.0b013e318034264c. [DOI] [PubMed] [Google Scholar]
- 32.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44(3):328–34. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oppeltz RF, Rani M, Zhang Q, et al. Gamma delta (gammadelta) T-cells are critical in the up-regulation of inducible nitric oxide synthase at the burn wound site. Cytokine. 2012 doi: 10.1016/j.cyto.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rani M, Zhang Q, Oppeltz RF, et al. Burn induces activation of γ δ T-cells at the injury site. Shock. 2011;35(Suppl 1):47. [Google Scholar]
- 35.Rani M, Zhang Q, Oppeltz RF, et al. γ δ T-cells are activated at the wound site early after burn. Journal of Burn Care and Research. 2011;32(Suppl):79. [Google Scholar]
- 36.Miller AC, Rashid RM, Elamin EM. The “T” in trauma: the helper T-cell response and the role of immunomodulation in trauma and burn patients. J Trauma. 2007;63(6):1407–17. doi: 10.1097/TA.0b013e31815b839e. [DOI] [PubMed] [Google Scholar]
- 37.Menges T, Engel J, Welters I, et al. Changes in blood lymphocyte populations after multiple trauma: association with posttraumatic complications. Crit Care Med. 1999;27(4):733–40. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 38.Mack VE, McCarter MD, Naama HA, et al. Dominance of T-helper 2-type cytokines after severe injury. Arch Surg. 1996;131(12):1303–8. doi: 10.1001/archsurg.1996.01430240057007. discussion 08-9. [DOI] [PubMed] [Google Scholar]
- 39.Kilani RT, Delehanty M, Shankowsky HA, et al. Fluorescent-activated cell-sorting analysis of intracellular interferon-gamma and interleukin-4 in fresh and frozen human peripheral blood T-helper cells. Wound Repair Regen. 2005;13(4):441–9. doi: 10.1111/j.1067-1927.2005.130412.x. [DOI] [PubMed] [Google Scholar]
- 40.Marik PE, Flemmer M. The immune response to surgery and trauma: Implications for treatment. J Trauma Acute Care Surg. 2012;73(4):801–8. doi: 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- 41.De AK, Kodys KM, Pellegrini J, et al. Induction of global anergy rather than inhibitory Th2 lymphokines mediates posttrauma T cell immunodepression. Clin Immunol. 2000;96(1):52–66. doi: 10.1006/clim.2000.4879. [DOI] [PubMed] [Google Scholar]
- 42.Tredget EE, Yang L, Delehanty M, et al. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res. 2006;26(3):179–89. doi: 10.1089/jir.2006.26.179. [DOI] [PubMed] [Google Scholar]
- 43.Neidhardt R, Keel M, Steckholzer U, et al. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997;42(5):863–70. doi: 10.1097/00005373-199705000-00017. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 44.Seekamp A, Jochum M, Ziegler M, et al. Cytokines and adhesion molecules in elective and accidental trauma-related ischemia/reperfusion. J Trauma. 1998;44(5):874–82. doi: 10.1097/00005373-199805000-00022. [DOI] [PubMed] [Google Scholar]
- 45.Giannoudis PV, Smith RM, Perry SL, et al. Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med. 2000;26(8):1076–81. doi: 10.1007/s001340051320. [DOI] [PubMed] [Google Scholar]
- 46.von Heymann C, Langenkamp J, Dubisz N, et al. Posttraumatic immune modulation in chronic alcoholics is associated with multiple organ dysfunction syndrome. J Trauma. 2002;52(1):95–103. doi: 10.1097/00005373-200201000-00017. [DOI] [PubMed] [Google Scholar]
- 47.Yeh FL, Lin WL, Shen HD. Changes in circulating levels of an anti-inflammatory cytokine interleukin 10 in burned patients. Burns. 2000;26(5):454–9. doi: 10.1016/s0305-4179(99)00174-6. [DOI] [PubMed] [Google Scholar]
- 48.Dong C. Diversification of T-helper-cell lineages: finding the family root of IL-17-producing cells. Nat Rev Immunol. 2006;6(4):329–33. doi: 10.1038/nri1807. [DOI] [PubMed] [Google Scholar]
- 49.Flierl MA, Rittirsch D, Gao H, et al. Adverse functions of IL-17A in experimental sepsis. Faseb J. 2008;22(7):2198–205. doi: 10.1096/fj.07-105221. [DOI] [PubMed] [Google Scholar]
- 50.Weaver CT, Hatton RD, Mangan PR, et al. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–52. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 51.Korn T, Oukka M, Kuchroo V, et al. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19(6):362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roark CL, Simonian PL, Fontenot AP, et al. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20(3):353–7. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finnerty CC, Herndon DN, Przkora R, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26(1):13–9. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 54.Finnerty CC, Przkora R, Herndon DN, et al. Cytokine expression profile over time in burned mice. Cytokine. 2009;45(1):20–5. doi: 10.1016/j.cyto.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo Re S, Dumoutier L, Couillin I, et al. IL-17A-producing gammadelta T and Th17 lymphocytes mediate lung inflammation but not fibrosis in experimental silicosis. J Immunol. 2010;184(11):6367–77. doi: 10.4049/jimmunol.0900459. [DOI] [PubMed] [Google Scholar]
- 56.Liu J, Feng Y, Yang K, et al. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol Med Microbiol. 2011;61(2):179–88. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 57.Kasten KR, Prakash PS, Unsinger J, et al. Interleukin-7 (IL-7) treatment accelerates neutrophil recruitment through gamma delta T-cell IL-17 production in a murine model of sepsis. Infect Immun. 2010;78(11):4714–22. doi: 10.1128/IAI.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rutz S, Ouyang W. Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr Opin Immunol. 2011;23(5):605–12. doi: 10.1016/j.coi.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 59.Lemaitre PH, Vokaer B, Charbonnier LM, et al. Cyclosporine A Drives a Th17- and Th2-Mediated Posttransplant Obliterative Airway Disease. Am J Transplant. 2013 doi: 10.1111/ajt.12067. [DOI] [PubMed] [Google Scholar]


