Correspondence to: You-Lin Qiao, Department of Cancer Epidemiology, Cancer Hospital/Institute, Chinese Academy of Medical Sciences, 17 South Panjiayuan Lane, Beijing 100021, PR China (qiaoy@cicams.ac.cn); and Philip R Taylor, Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, 6120 Executive Blvd, Rm 7006, Rockville, Maryland, 20852-7236, USA (ptaylor@mail.nih.gov)
Although substantial numbers of people worldwide take multivitamin supplements, including an estimated 40% or more of US adults, their effectiveness remains unclear. Recent reports from the Physicians’ Health Study (PHS) II, a randomized trial of daily multivitamins, found fewer total cancers in multivitamin recipients, but no effect on overall or cause-specific mortality 1, 2 in a Western population that was well nourished. However, few multivitamin trials have been conducted in under-nourished populations where the potential for benefit is most likely.
In 1985, we initiated the Linxian Dysplasia Nutrition Intervention Trial (NIT) to evaluate the effect of multivitamin supplements on cancer incidence and mortality in Linxian, China, a region with extremely high rates of esophageal and gastric cardia cancer and multiple vitamin and mineral deficiencies. Individuals with a previous cytological diagnosis of esophageal squamous dysplasia were randomized to receive multivitamin supplementation or placebo for six years.3 Results after the six-year intervention period showed no statistically significant benefit on mortality.4 However, an additional 20 years of active follow-up after cessation of the intervention gave us the opportunity to examine the long-term effects of supplementation.
The purpose of this report is to update results of the Linxian Dysplasia NIT after 26 years of follow-up to provide data informative on the effect of multivitamin supplementation on mortality in an under-nourished population. Our findings should be helpful for clinical practice and public health recommendations.
Methods
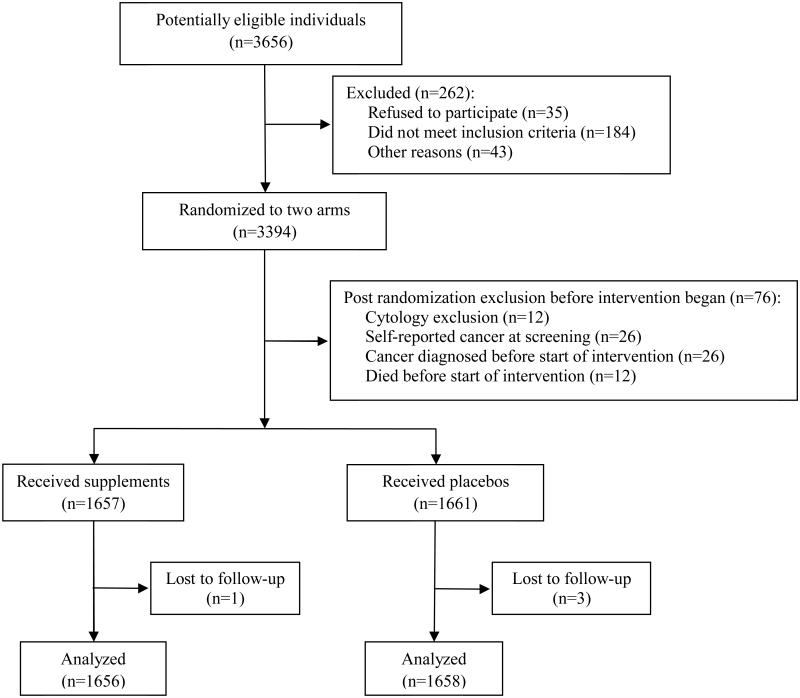
The Linxian Dysplasia NIT was a randomized, double-blind, placebo-controlled trial of multivitamins conducted in 1985-1991 in northern China in an under-nourished population of 3318 persons aged 40-69 years who had a previous cytologic diagnosis of esophageal squamous dysplasia. Participants were followed for 20 additional years after cessation of supplementation. Methods 3 and results 4 for the intervention phase of this trial were previously published and are further detailed in eMethods, eFigure 1, and eTable 1.
efigure 1.
CONSORT flow diagram of the Linxian Dysplasia Nutrition Intervention Trial
eTable 1.
Daily dose and types of nutrients in supplements in the Linxian Dysplasia Nutrition Intervention Trial
| Vitamin/mineral | As | Dose |
|---|---|---|
| Beta carotene | 15 mg | |
| Vitamin A | Acetate | 10000 IU |
| Vitamin E | dl-alpha-Tocopheryl acetate | 60 IU |
| Vitamin C | Ascorbic acid | 180 mg |
| Folic acid | 800 μg | |
| Vitamin B1 | Thiamine mononitrate | 5 mg |
| Vitamin B2 | Riboflavin | 5.2 mg |
| Niacinamide | 40 mg | |
| Vitamin B6 | Pyridoxine HCl | 6 mg |
| Vitamin B12 | Cyanocobalamin | 18 μg |
| Vitamin D | 800 IU | |
| Biotin | 90 μg | |
| Pantothenic acid | Calcium pantothenate | 20 mg |
| Calcium | Dibasic calcium phosphate | 324 mg |
| Phosphorus | Dibasic calcium phosphate | 250 mg |
| Iodine | Potassium iodide | 300 μg |
| Iron | Ferrous fumarate | 54 mg |
| Magnesium | Magnesium oxide | 200 mg |
| Copper | Cupric oxide | 6 mg |
| Manganese | Manganese sulfate | 15mg |
| Potassium | Potassium chloride | 15.4 mg |
| Chloride | Potassium chloride | 14 mg |
| Chromium | Chromium chloride | 30 μg |
| Molybdenum | Sodium molybdate | 30 μg |
| Selenium | Sodium selenate | 50 μg |
| Zinc | Zinc sulfate | 45 mg |
Results
Baseline characteristics are summarized in eTable 2. Participant characteristics, including age, sex, smoking, drinking, family history of esophageal and gastric cancers, and body mass index were similar between the supplementation and placebo groups.
eTable 2.
Baseline demographic characteristics and risk factors in the Linxian Dysplasia Nutrition Intervention Trial
| All participants | Placebo | Active | |
|---|---|---|---|
| No. of participants (N, %) | 3314 (100.0) | 1658 (50.0) | 1656 (50.0) |
| Age group (N, %) | |||
| <50 | 1081 (32.6) | 536 (32.3) | 545 (32.9) |
| ≥50 to <60 | 1458 (44.0) | 742 (44.8) | 716 (43.2) |
| ≥ 60 | 775 (23.4) | 380 (22.9) | 395 (23.9) |
| Sex (N, %) | |||
| Female | 1854 (56.0) | 927 (55.9) | 927 (56.0) |
| Male | 1460 (44.0) | 731 (44.1) | 729 (44.0) |
| Smoking a (N, %) | |||
| Non-smoker | 2344 (71.1) | 1173 (71.1) | 1171 (71.1) |
| Smoker | 954 (28.9) | 477 (28.9) | 477 (28.9) |
| Alcohol drinking b (N, %) | |||
| Non-drinker | 2683 (81.3) | 1356 (82.2) | 1327 (80.5) |
| Drinker | 615 (18.7) | 294 (17.8) | 321 (19.5) |
| Family history of esophageal or stomach cancer (N, %) |
|||
| No | 1974 (59.9) | 990 (60.0) | 984 (59.7) |
| Yes | 1324 (40.1) | 660 (40.0) | 664 (40.3) |
| Body Mass Index (Mean, SD) | 20.35±2.29 | 20.36±2.30 | 20.33±2.28 |
Ever smoking cigarettes for six or more months.
Ever drinking any alcoholic beverage in the last 12 months.
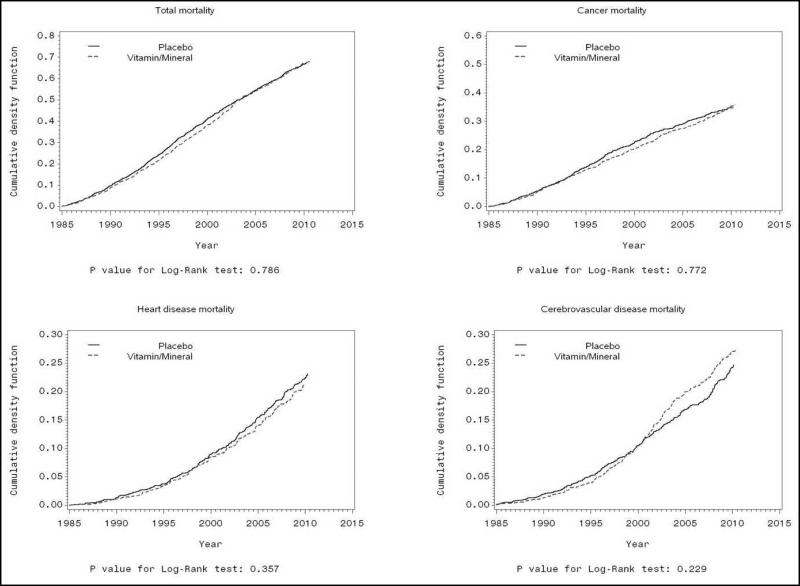
A total of 2239 deaths occurred during follow-up (1985-2010), including 42% from cancer, 21% from heart disease, 25% from cerebrovascular disease, and 12% due to other causes. Cumulative mortality for all causes, cancer, heart disease, and cerebrovascular disease for all participants is shown in eFigure 2. Results from Cox models were similar to the cumulative mortality graphs (Table). Overall, multivitamin supplements had no effect on total mortality or mortality from any of the specific causes of death examined (including cancer mortality) among all participants.
efigure 2.
Effects of multivitamin supplements on mortality from all causes, cancer, heart disease, and cerebrovascular disease for all participants, as shown by cumulative mortality in Kaplan-Meier plots
Table.
Hazard ratios and 95% confidence intervals for death by cause stratified by genders and age in the Linxian Dysplasia Nutrition Intervention Trial
| Cause of death (1985- 2010) |
All | Men | Women | Age<55 years | Age≥55 years | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| N | HR (95% CI)a | n | HR (95% CI)b | n | HR (95% CI)b | n | HR (95% CI)c | n | HR (95% CI)c | |
| Total deaths | 2239 | 0.98 (0.90-1.06) | 1090 | 0.90 (0.80-1.01) | 1149 | 1.06 (0.95-1.19) | 885 | 1.04 (0.91-1.19) | 1354 | 0.94 (0.84-1.04) |
| Cancer | 935 | 0.97 (0.85-1.10) | 489 | 0.92 (0.77-1.09) | 446 | 1.03 (0.86-1.24) | 446 | 0.90 (0.75-1.09) | 489 | 1.03 (0.86-1.23) |
| Esophageal | 491 | 0.98 (0.82-1.16) | 241 | 0.87 (0.68-1.12) | 250 | 1.09 (0.85-1.40) | 247 | 0.93 (0.73-1.20) | 244 | 1.01 (0.79-1.30) |
| Gastric | 327 | 0.91 (0.73-1.13) | 188 | 0.88 (0.66-1.17) | 139 | 0.96 (0.69-1.33) | 141 | 0.76 (0.55-1.06) | 186 | 1.05 (0.79-1.40) |
| Cardia | 265 | 0.91 (0.72-1.16) | 157 | 0.86 (0.63-1.18) | 108 | 1.00 (0.68-1.45) | 113 | 0.77 (0.53-1.11) | 152 | 1.04 (0.75-1.43) |
| Noncardia | 62 | 0.91 (0.55-1.49) | 31 | 0.98 (0.49-1.99) | 31 | 0.82 (0.41-1.67) | 28 | 0.74 (0.35-1.56) | 34 | 1.10 (0.56-2.16) |
| Esophageal/cardia | 756 | 0.95 (0.83-1.10) | 398 | 0.87 (0.71-1.05) | 358 | 1.06 (0.87-1.31) | 360 | 0.88 (0.71-1.08) | 396 | 1.02 (0.84-1.24) |
| Other cancer | 117 | 1.13 (0.79-1.63) | 60 | 1.29 (0.77-2.15) | 57 | 0.97 (0.58-1.63) | 58 | 1.20 (0.72-2.02) | 59 | 1.06 (0.63-1.76) |
| Cerebrovascular | 565 | 1.10 (0.93-1.30) | 247 | 0.92 (0.72-1.18) | 318 | 1.25 (1.00-1.56) d | 203 | 1.42 (1.07-1.88) d | 362 | 0.96 (0.78-1.18) |
| Heart | 463 | 0.90 (0.75-1.08) | 212 | 0.73 (0.56-0.96) d | 251 | 1.08 (0.85-1.39) | 125 | 1.28 (0.90-1.82) | 338 | 0.79 (0.64-0.98) d |
| Other | 276 | 0.90 (0.71-1.14) | 142 | 1.04 (0.75-1.45) | 134 | 0.78 (0.55-1.10) | 111 | 0.83 (0.57-1.21) | 165 | 0.95 (0.70-1.29) |
HR=hazard ratio; 95% CI=95% confidence intervals.
Adjusted for age, sex and commune.
Adjusted for age and commune.
Adjusted for age, sex and commune.
Statistical significance (P < 0.05).
When results were examined by subgroups defined by gender and age (Table), heart disease deaths were reduced in supplemented men (HR=0.73, 95% CI=0.56-0.96) and cerebrovascular disease deaths were increased in supplemented women (HR=1.25, 95% CI=1.00-1.56, P=0.047). Heart disease deaths were also decreased in older supplemented participants (HR=0.79, 95% CI=0.64-0.98) and cerebrovascular disease deaths were increased in younger supplemented participants (HR=1.42, 95% CI=1.07-1.88).
Comment
In the Linxian Dysplasia NIT, after six years of supplementation and nearly 20 years of additional follow-up, multivitamin supplementation had no effect on total or cause-specific mortality. Both beneficial and adverse effects on heart disease and stroke mortality were observed among subgroups defined by gender and age.
Most prior micronutrient intervention trials tested only one or two supplements. Among those that tested three or more vitamins/minerals, supplements reduced total mortality in two trials.5, 6 However, only one previously-reported micronutrient trial was truly comparable to the Linxian Dysplasia NIT in terms of testing an existing commercially available multivitamin and mineral supplement formulation: the PHS II supplemented with Centrum Silver (31 vitamins/minerals), whereas the Linxian Dysplasia NIT supplemented with two Centrum tablets (26 vitamins/minerals). Poorly-nourished populations should benefit most from multivitamin supplementation, making the current study a strong test of their potential beneficial effects. However, like the well-nourished PHS II population, no benefit of multivitamins for total mortality was observed in our study.
Our results show differences in the effect of supplementation on heart disease and stroke mortality in men and women. Multivitamin trials in well-nourished Western populations have not shown reduced heart disease in supplemented men or women. For stroke, Western trials in women either showed no effect 6-8 or suggested a benefit. 9 For heart disease in men, PHS II indicated no effect.2 The different cardiovascular disease results observed in Linxian compared to other multivitamin trials may be due to differences in nutritional status, trial design, or chance.
In summary, during six years of multivitamin supplementation and 20 years of post-intervention follow-up, we observed no effect of multivitamins on total or cause-specific mortality in a nutrient-deficient population. Together with data from previous trials, these results demonstrate little benefit of multivitamin supplementation on mortality in either well- or poorly-nourished populations.
Supplementary Material
Acknowledgements
The authors thank: (1) Dr. Sanford M Dawsey for his contributions to study concept and design, analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; (2) Dr. Neal D Freedman for his contributions to the analysis and interpretation of data, drafting of the manuscript, and critical revision of the manuscript for important intellectual content; and (3) the citizens of Linxian who have faithfully participated in these studies over the past 26 years.
Funding/Supporting: This study was sponsored in part by National Cancer Institute contracts (N01-SC-91030 and N01-RC-47701) to the Cancer Hospital/Institute, Chinese Academy of Medical Sciences); and by the Intramural Research Program of Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health.
Footnotes
Author contributions: Drs. Qiao and Taylor had access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Qiao, Taylor. Acquisition of data: Wang, Fan. Analysis and interpret of data: Wang, Abnet, Qiao, Taylor. Drafting of the manuscript: Wang, Abnet, Qiao, Taylor. Critical revision of the manuscript for important intellectual content: Abnet, Taylor. Statistical analysis: Wang, Fan. Administrative, technical, or material support: Fan, Qiao. Study supervision: Qiao, Taylor.
Conflict of Interest Disclosures:
All authors declare no conflicts of interest.
Role of the Sponsor: Sponsors or funders did not influence the study in any way, including design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Trial registration The trial is registered with ClinicalTrials.gov, NCT00342654. The trial registry name is Nutrition Intervention Trials in Linxian Follow-up Study, and the URL for the registry is http://www.clinicaltrials.gov/ct2/show/NCT00342654?term=NCT00342654&rank=1.
References
- 1.Gaziano JM, Sesso HD, Christen WG, et al. Multivitamins in the prevention of cancer in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2012;308(18):1871–1880. doi: 10.1001/jama.2012.14641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sesso HD, Christen WG, Bubes V, et al. Multivitamins in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2012;308(17):1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li B, Taylor PR, Li JY, et al. Linxian nutrition intervention trials. Design, methods, participant characteristics, and compliance. Ann Epidemiol. 1993;3(6):577–585. doi: 10.1016/1047-2797(93)90078-i. [DOI] [PubMed] [Google Scholar]
- 4.Li JY, Taylor PR, Li B, et al. Nutrition intervention trials in Linxian, China: multiple vitamin/mineral supplementation, cancer incidence, and disease-specific mortality among adults with esophageal dysplasia. J Natl Cancer Inst. 1993;85(18):1492–1498. doi: 10.1093/jnci/85.18.1492. [DOI] [PubMed] [Google Scholar]
- 5.Qiao YL, Dawsey SM, Kamangar F, et al. Total and cancer mortality following supplementation with vitamins and minerals: Follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101(7):507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med. 2004;164(21):2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 7.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lancet. 2002;360(9326):23–33. doi: 10.1016/S0140-6736(02)09328-5. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.