Abstract
Background
Telomere attrition has been associated with age related diseases although causality is unclear and controversial; low grade systemic inflammation (inflammaging) has also been implicated in age-related pathogenesis. Unpicking the relationship between ageing, telomere length (TL) and inflammaging is hence essential to the understanding of ageing and management of age-related diseases. This longitudinal study explores whether telomere attrition is a cause or consequence of ageing and whether inflammaging explains some of the associations between TL and one marker of ageing, grip strength.
Methods
We studied 253 Hertfordshire Ageing Study participants at baseline and 10 year follow up (mean age at baseline 67.1years). Participants completed a health questionnaire and had blood samples collected for immune-endocrine and telomere analysis at both time points. Physical ageing was characterised at follow-up using grip strength (GS).
Findings
Faster telomere attrition was associated with lower GS at follow-up (β=0.98, p=0.035). This association was completely attenuated when adjusted for inflammaging burden (p=0.86) over the same period. Similarly, greater inflammaging burden was associated with lower GS at follow-up (e.g. interleukin1β (IL-1β): β=−2.18, p=0.001), however, these associations were maintained when adjusted for telomere attrition (IL-1β, p=0.006).
Interpretation
We present evidence that inflammaging may be driving telomere attrition and in-part explains the associations which have previously been reported between TL and grip strength. Thus biomarkers of physical ageing, such as inflammaging, may require greater exploration. Further work is now indicated.
Keywords: Telomere, epidemiology, sarcopenia, inflammation, ageing, osteoporosis, grip strength
Introduction
The deterioration of physical function that occurs with age can include sarcopenia and result in frailty and loss of independence [1;2]. A major healthcare challenge of the 21st century is to identify people at risk of such a decline in physical function and to intervene early. Biomarkers of ageing may facilitate this process and the assessment of telomere length is perhaps the most well known and yet most controversial of such markers.
Telomeres are protein-nucleotide complexes comprising several thousand repetitive DNA sequences located at the end of chromosomes to protect them from deterioration or fusion. They are maintained and lengthened by the enzyme telomerase [3]. Telomere repetitive sequences shorten with each cell division, eventually reaching a critical threshold leading to cellular senescence and death [4]. Telomere length (TL) may therefore be thought of as a biological clock representing cellular age and multiple cohort studies have shown that short telomeres are associated with age-related disease, disease specific mortality and all cause mortality [5;6], although results are not always consistent. However, its predictive value in the assessment future age-related health risks is not known [7]. Uncertainty remains in the literature as to whether TL is a cause or consequence of the ageing process and, increasingly, studies are reporting a lack of association with cognitive, physical and social traits; including grip strength, physical functioning and sarcopenia [8-13]. Despite these poor associations, significant inter-individual variation and wide confidence intervals, several companies now offer commercial testing of TL for the public [14].
Physiological ageing is associated with a chronic sub-clinical state, termed inflammaging, characterised by elevated levels of pro-inflammatory cytokines such as interleukin 1β (IL-1β) and associated with changes to the endocrine axis [15]. Inflammaging (particularly elevations in levels of tumour necrosis factor α [TNFα], IL-6, IL-1β and C-reactive protein [CRP]) is associated with morbidity and mortality in older people. Specifically, pro-inflammatory cytokines have been linked with cardiovascular diseases including atherosclerosis and stroke [16] and similar associations have been found with other age-related disease such as Alzheimer’s dementia [17], type 2 diabetes mellitus [18] and frailty [19]. Furthermore, levels of pro-inflammatory cytokines are noticeably lower in centenarians, a population who can have be deemed to have aged successfully [20].
Cortisol and dehydroepiandosterone sulphate (DHEAS) are both outputs of the hypothalamic-pituitary-adrenal (HPA) axis; cortisol has mainly immunosuppressive actions, whilst DHEAS is immune enhancing [21]. Both change with age, are believed to contribute to the process of immunosenescence, and have been associated with age related diseases including sarcopenia and frailty [19].
There is growing evidence that inflammation is associated with TL although the direction of this relationship is unclear and could be bi-directional. For example, raised IL-6 and TNFα were associated with shorter TL in 1,962 individuals of the Health, Ageing and Body Composition Study [22]. Shorter TL has also been found in cohorts of patients with chronic inflammatory diseases of liver, kidney and lung [23]. Negative relationships with TL have also been demonstrated with CRP [24] and IL-6 [25]. It is thus apparent that the inter-relationships between TL, inflammation and the ageing process are currently unclear and warrant further exploration.
Therefore the objective of this study was to explore these relationships further using grip strength which is associated with age-related morbidity, sarcopenia, frailty, disability and mortality [26-29]. Specifically, we aimed to explore the relationship between TL and the ageing process, asking the question: is TL a cause or consequence of the ageing process and does inflammaging, at least in part, explain the observed associations between TL and ageing?
Methods
The Hertfordshire Ageing Study (HAS) has been described previously [30]. In brief, 717 men and women who were born in Hertfordshire, UK between 1920 and 1930 attended a home interview and clinic in 1994/5 where a wide range of markers of ageing were characterised. In 2003/5, a ten year follow-up was conducted; 359 men and women participated in a home interview. Reasons for attrition to this point were as follows. One hundred and twenty-two had died, 55 were lost to follow-up owing to failure to trace them across the intervening period, and 181 were traced but declined the invitation to take part [30]. 254 attended a clinic for further assessment. All-cause mortality was ascertained between 1994/5 and 2003/5.
At the 1994/5 baseline HAS home interview a trained research nurse, among other things, ascertained smoking habit, alcohol intake and current or most recent full-time occupation and husband’s occupation for ever-married women. Height and weight were measured and a venous blood sample collected and serum stored at −80°C for future analysis. This process was repeated at the 2003/5 follow-up. Grip strength was assessed at 1994/5 baseline and 2003/5 follow-up clinics with a Jamar® handheld hydraulic dynamometer, (Promedics, UK) using the Southampton protocol [31].
Serological and Telomere Analysis
IL-1β and IL-6 are the principle pro-inflammatory cytokines and were selected as markers of inflammaging. They were simultaneously measured using commercially available multiplex luminometry (BioRad Ltd, Hemel Hempstead, UK). Detection of serum cortisol, DHEAS and cytomegalovirus (CMV) IgG antibodies were completed using commercially available enzyme-linked immunosorbent assay kits (IBL International, Germany).
DNA was extracted from full fresh blood (white blood cells, i.e. granulocytes and peripheral blood mononuclear cells (PBMC)) with the QIAmp DNA Maxi kit (Qiagen Ltd, Crawley, UK) and all analyses were performed at the same time. DNA concentration and quality were monitored by agarose gel electrophoresis. Samples were discarded if DNA degradation (smear <20 kb) was visible. TL was measured as the ratio of the starting quantity for telomeres versus the starting quantity for the single copy gene of glyceraldehyde-3-phosphate dehydrogenase (as control) by quantitative real-time polymerase chain reaction (PCR). Measurements were performed in quadruplicate. Three DNA samples with known telomere lengths (3.0, 5.5 and 9.5 kb pairs) were run as internal standards together with each batch of 16 study samples to convert the ratios of starting quality into telomere lengths in base pairs. The intra-assay coefficient of variation for this PCR method in our lab is 2.65% and inter-assay coefficient of variation is 5.12%.
Intra- and inter-observer studies were carried out during the fieldwork. The HAS had ethical approval from the Hertfordshire and Bedfordshire Local Research Ethics Committee and all participants gave written informed consent.
Statistical methods
Percentage changes in biomarkers and TL from baseline to follow-up were calculated by dividing the difference in values (difference = follow-up – baseline values) by the baseline values, multiplying by 100 and then converting to z-scores using a Fisher-Yates transformation. Weight and height were positively correlated at 2003/5 follow-up (rfollow-up=0.58, p<0.001); to avoid multicolinearity problems, standardised residuals of weight adjusted for height were used. Linear regression models were used to explore the associations of biomarkers and TL (expressed as z-scores of % change per year of follow-up) with grip strength at 2003/5 follow-up. Analyses were conducted with adjustment for sex and age, with and without further adjustment for the potential confounding influences of height, weight for height, smoking status, alcohol consumption and social class at 2003/5 follow-up and also CMV seropositivity. Analyses were conducted for both genders combined and repeated separately for men and women given that significant (p<0.05) interactions were identified between gender and percent change in DHEAS and TL as predictors of grip strength at follow-up. A 5% significance level was used to identify statistically significant associations. Data were analysed using Stata version 11.0 (Stata Statistical Software, StataCorp 2009).
Results
In the 1994/5 baseline clinic, 411 men and 306 women participated. Of these, complete data on TL were available for 388 (94.4%) men and 269 (87.9%) women. At 2003/5 follow-up clinic, 208 men and 151 women were successfully followed-up of whom TL data were available for 165 men (79.3%) and 112 (74.2%) women. Analysis was conducted on 158 men and 95 women who had available TL data at both 1994/5 baseline and 2003/5 follow-up.
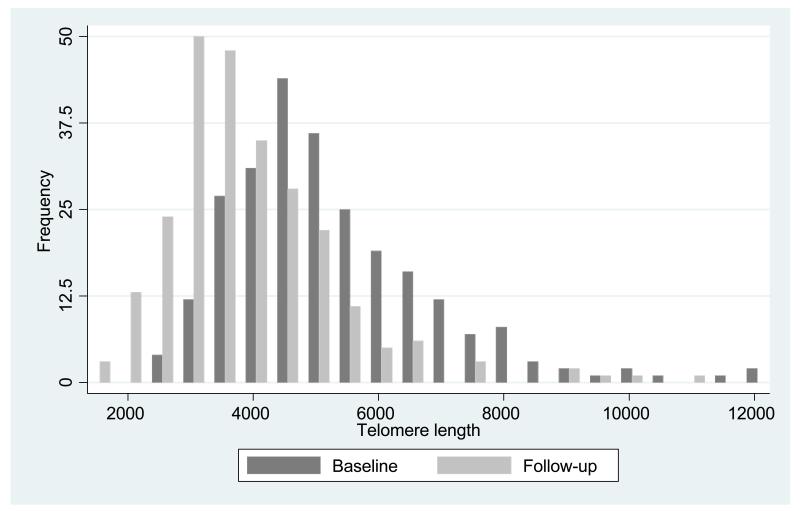
Table 1 shows baseline and follow-up summary characteristics of the 253 subjects in the study. The average age at the 1994/5 baseline clinic was 67.1 years for men and 67.2 years for women. The corresponding average age at follow-up was 76.5 for men and 76.2 years for women. The median follow-up time was 9.3 years. Grip strength was little altered from baseline to follow-up but was significantly higher among men than women at both time points (p<0.001). There was no gender difference in median telomere length at the 1994/5 baseline clinic (TL was 5042 basepairs (bp) in men and 4901 bp in women), nor at the 2003/5 follow-up clinic (TL was 3630 bp in men and 3680 bp in women). Figure 1 demonstrates the significant overall reduction in TL across the two time points (p<0.001).
Table 1.
Baseline (1994/5) and follow-up (2003/5) characteristics
| Baseline: 1994/5 | Follow up: 2003/5 | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Men | Women | Men | Women | |||||
| Age (years) (mean (SD)) | 158 | 67.1 (2.3) | 95 | 67.2 (2.0) | 158 | 76.5 (2.3) | 95 | 76.2 (2.0) |
| Height (cm) (mean (SD)) | 158 | 172.7 (6.2) | 95 | 159.3 (5.3) | 158 | 171.6 (6.4) | 93 | 158.2 (5.5) |
| Weight (kg) (mean (SD)) | 158 | 80.7 (12.4) | 95 | 69.2 (10.7) | 157 | 81.8 (13.7) | 95 | 69.4 (12.6 |
| Current smoker (n(%)) | 20 | 12.7 | 9 | 9.5 | 17 | 10.8 | 7 | 7.4 |
| Moderate/high alcohol (n(%)) | 40 | 25.3 | 9 | 9.5 | 46 | 29.1 | 16 | 16.9 |
| Non-manual social class (n(%)) | 86 | 55.1 | 58 | 61.7 | 86 | 54.4 | 50 | 52.6 |
| BMI (mean (SD)) | 158 | 27 (3.6) | 95 | 27.3 (4.2) | 157 | 27.7 (4.1) | 93 | 27.5 (4.5) |
| Grip (kg) (mean (SD)) | 158 | 39.6 (6.1) | 95 | 22.1 (5.5) | 157 | 38.6 (8.0) | 94 | 23.0 (6.9) |
| IL-1β (pg/ml) (median (IQR)) | 114 | 12.0 (5.6-18.1) | 62 | 14.7 (11.4-24.0) | 111 | 10.9 (6.0-15.0) | 66 | 9.9 (6.7-15.3) |
| IL-6 (pg/ml) (median (IQR)) | 103 | 0.9 (0.2-2.0) | 45 | 1.0 (0.4-2.4) | 150 | 2.8 (1.5-4.3) | 90 | 2.2 (1.5-4.2) |
| Cortisol (nmol/l) (median (IQR)) | 153 | 310.0 (240.8-385.3) | 94 | 270.9 (198.2-351.4) | 151 | 336.0 (288.4-401.6) | 91 | 332.7 (261.8-400.0) |
| DHEAS (nmol/l) (median (IQR)) | 153 | 2138.6 (1680.0-2819.3) | 94 | 1519.2 (788.7-2230.2) | 150 | 1670.1 (963.1-2373.1) | 88 | 1461.7 (563.5-2243.4) |
| Cortisol:DHEAS (median (IQR)) | 153 | 0.1 (0.1-0.2) | 94 | 0.2 (0.1-0.3) | 149 | 0.2 (0.1-0.4) | 87 | 0.2 (0.1-0.6) |
| Telomere length (median (IQR)) | 158 | 5041.7 (4167.4-6210.8) | 95 | 4901.4 (3910.5-5984.7) | 158 | 3629.7 (3080.6-4556.8) | 95 | 3680.1 (3009.6-4777.3) |
IQR = Interquartile range
Height was missing for 2 women at follow up.
Weight was missing for 1 man at follow up.
BMI was missing for 1 man and 2 women at follow up.
Grip strength was missing for 1 man and 1 woman at follow up.
IL-1β data were missing for 44 men and 33 women at baseline and for 47 men and 29 women at follow up.
IL-6 data were missing for 55 men and 53 women at baseline and for 8 men and 5 women at follow up.
Cortisol data were missing for 5 men and 1 woman at baseline and for 7 men and 4 women at follow up.
DHEAS data were missing for 5 men and 1 woman at baseline and for 8 men and 7 women at follow up.
Cortiol:DHEAS ratio data were missing for 5 men and 1 women at baseline and for 9 men and 8 women at follow up.
Fig 1. Distribution of telomere length at two time points.
Table 2 presents the associations between percentage change per year of follow-up (z-scores) in TL (rate of telomere attrition) or inflammatory marker (inflammatory burden), and grip strength at 2003/5 follow-up. Analysis for IL-1β and IL-6 was limited by low volumes of serum in some participants. Among men and women combined, percentage rises per year in follow-up of IL-1β (p=0.005), cortisol (p=0.02) and cortisol:dheas ratio (p=0.042) were all significantly associated with reduced GS at 10 year follow up. Associations were somewhat stronger in men than women with significant interaction between gender and percent change in DHEAS and TL as predictors of grip strength at follow-up (Table 2). Associations were robust for IL-1β (p=0.001) and cortisol (p=0.009) after adjustment for sex, age, height, weight for height, smoking status, alcohol consumption and social class at follow-up. Percentage change in TL over the follow-up period was also significantly associated with GS at 2003/5 follow-up in both unadjusted (p=0.037) and adjusted analyses (p=0.035). Positive associations were found between TL or inflammatory markers at 1994/5 baseline and GS at 2003/5 follow-up although, with the exception of IL-6, these were not statistically significant. All associations remained the same after adjusting for CMV seropositivity (results not shown).
Table 2.
Inflammatory markers and telomere length as predictors of grip strength at 2003/5 follow-up. (All predictor variables are z-scores for percentage changes (follow-up minus baseline) per year of follow-up)
| Predictors | ADJUSTEDa (ALL) | ADJUSTEDa (MEN) | ADJUSTEDa (WOMEN) | ||||||
|
|
|||||||||
| N | Reg coef (95% CI) | p | N | Reg coef (95% CI) | p | N | Reg coef (95% CI) | p | |
|
|
|||||||||
| IL-1β | 130 | −1.90 (−3.22,−0.58) | 0.005 | 85 | −1.68 (−3.39,0.02) | 0.053 | 45 | −2.21 (−4.39,−0.04) | 0.046 |
| IL-6 | 143 | 1.33 (0.09,2.57) | 0.035 | 98 | 1.31 (−0.29,2.92) | 0.108 | 45 | 0.88 (−1.09,2.84) | 0.373 |
| Cortisol | 237 | −1.12 (−2.06,−0.18) | 0.020 | 147 | −1.34 (−2.65,−0.03) | 0.045 | 90 | −0.80 (−2.12,0.52) | 0.234 |
| DHEAS | 233 | 0.83 (−0.15,1.80)c | 0.095 | 146 | 1.75 (0.40,3.11) | 0.012 | 87 | −0.37 (−1.69,0.95) | 0.578 |
| Cortisol:DHEAS | 231 | −0.99 (−1.94,−0.04) | 0.042 | 145 | −1.41 (−2.71,−0.12) | 0.033 | 86 | −0.34 (−1.71,1.03) | 0.626 |
| Telomere length | 251 | 0.99 (0.06,1.92)d | 0.037 | 157 | 1.97 (0.63,3.31) | 0.004 | 94 | −0.20 (−1.42,1.02) | 0.746 |
| ADJUSTEDb (ALL) | ADJUSTEDb (MEN) | ADJUSTEDb (WOMEN) | |||||||
|
|
|||||||||
| N | Reg coef (95% CI) | p | N | Reg coef (95% CI) | p | N | Reg coef (95% CI) | p | |
|
|
|||||||||
| IL-1β | 129 | −2.18 (−3.47,−0.88) | 0.001 | 85 | −2.12 (−3.86,−0.38) | 0.018 | 44 | −2.30 (−4.38,−0.22) | 0.031 |
| IL-6 | 142 | 1.04 (−0.18,2.26) | 0.093 | 97 | 0.94 (−0.74,2.62) | 0.268 | 45 | 1.11 (−0.90,3.11) | 0.270 |
| Cortisol | 234 | −1.23 (−2.14,−0.31) | 0.009 | 146 | −1.42 (−2.70,−0.13) | 0.031 | 88 | −0.79 (−2.06,0.47) | 0.216 |
| DHEAS | 230 | 0.57 (−0.40,1.53) | 0.246 | 145 | 1.49 (0.12,2.85) | 0.033 | 85 | −0.44 (−1.70,0.82) | 0.491 |
| Cortisol:DHEAS | 228 | −0.81 (−1.74,0.13) | 0.090 | 144 | −1.31 (−2.59,−0.03) | 0.045 | 84 | −0.12 (−1.43,1.19) | 0.855 |
| Telomere length | 248 | 0.98 (0.07,1.89) | 0.035 | 156 | 1.71 (0.36,3.06) | 0.014 | 92 | −0.09 (−1.28,1.10) | 0.881 |
Adjusted for age and sex in the combined model;
Adjusted for age, height, weight for height, smoking status, alcohol consumption, social class and sex in the combined model;
p value for interaction with sex=0.025;
p value for interaction with sex=0.023.
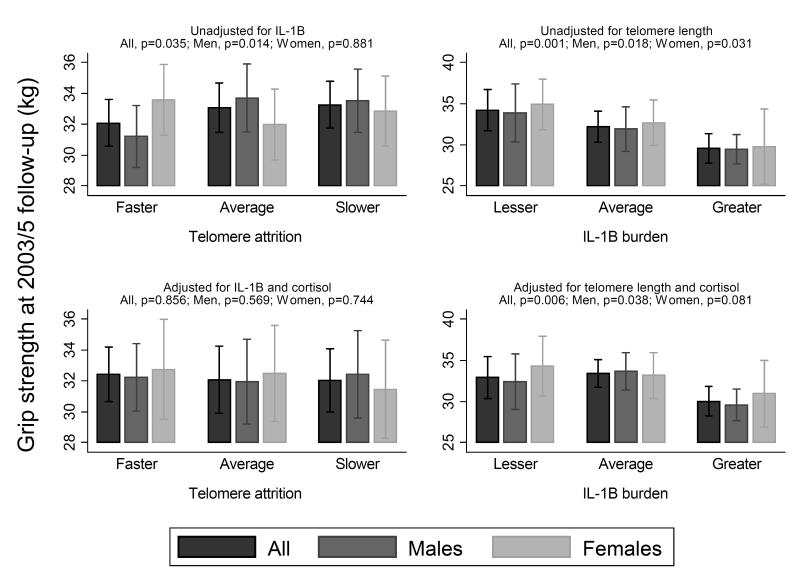
Table 3 presents the associations between percentage change per year in TL (rate of telomere attrition) versus GS at 2003/5 follow-up when adjusted for percentage change per follow-up year in IL-1β and cortisol. After adjustment the association between percentage change per year in TL and GS at 2003/5 follow-up is lost in both sex- and age-adjusted and fully adjusted models. The associations, however, between the percentage changes in the two inflammatory markers, IL-1β and cortisol, and grip strength at 2003/5 follow-up remained significant (both p-values<0.01). These results were seen when adjusted for IL-1β and cortisol alone and combined. When results were assessed for men and women separately, very similar trends were found. However, relationships only reached statistical significance in men after adjustment for anthropometry and lifestyle factors with or without CMV positivity. Figure 2 presents the associations between TL attrition and inflammaging burden over follow-up with grip strength at follow-up.
Table 3.
Mutually adjusted model of telomere length, IL-1β and cortisol (% change/year of follow up) as a predictors of grip strength at 2003/5 follow-up
| ADJUSTEDa (All, N=130 ) | ADJUSTEDa (Men, N=85) | ADJUSTEDa (Women, N=45) | ||||
|
|
||||||
| Reg coef (95% CI) | p | Reg coef (95% CI) | p | Reg coef (95% CI) | p | |
|
|
||||||
| Telomere length | 0.10 (−1.21 to 1.42) | 0.877 | 0.91 (−1.08 to 2.91) | 0.366 | −0.82 (−2.63 to 1.00) | 0.369 |
| IL-1β | −1.56 (−2.86 to −0.26) | 0.019 | −1.48 (−3.15 to 0.19) | 0.082 | −1.92 (−4.15 to 0.30) | 0.088 |
| Cortisol | −1.91 (−3.17 to −0.64) | 0.003 | −1.87 (−3.56 to −0.18) | 0.031 | −1.65 (−3.66 to −0.35) | 0.103 |
|
| ||||||
| ADJUSTEDb (All, N=129) | ADJUSTEDb (Men, N=85) | ADJUSTEDb (Women, N=44) | ||||
|
|
||||||
| Reg coef (95% CI) | p | Reg coef (95% CI) | p | Reg coef (95% CI) | p | |
|
|
||||||
| Telomere length | 0.12 (−1.17 to 1.41) | 0.856 | 0.58 (−1.45 to 2.62) | 0.569 | −0.29 (−2.05 to 1.48) | 0.744 |
| IL-1β | −1.79 (−3.08 to −0.51) | 0.006 | −1.83 (−3.56 to −0.11) | 0.038 | −1.91 (−4.06 to 0.25) | 0.081 |
| Cortisol | −1.80 (−3.05 to −0.56) | 0.005 | −1.83 (−3.54 to −0.12) | 0.036 | −1.55 (−3.49 to 0.38) | 0.111 |
|
| ||||||
| ADJUSTEDc (All, N=128) | ADJUSTEDc (Men, N=85) | ADJUSTEDc (Women, N=43) | ||||
|
|
||||||
| Reg coef (95% CI) | p | Reg coef (95% CI) | p | Reg coef (95% CI) | p | |
|
|
||||||
| Telomere length | 0.14 (−1.16 to 1.44) | 0.836 | 0.59 (−1.49 to 2.67) | 0.572 | −0.29 (−1.94 to 1.36) | 0.720 |
| IL-1β | −1.89 (−3.18 to −0.60) | 0.005 | −1.83 (−3.58 to −0.09) | 0.039 | −2.20 (−4.41 to 0.02) | 0.052 |
| Cortisol | −1.72 (−2.95 to −0.48) | 0.007 | −1.82 (−3.56 to −0.09) | 0.040 | −1.11 (−2.92 to 0.70) | 0.219 |
Adjusted for sex (in the combined sample) and age
Adjusted for sex (in the combined sample), age, height, weight for height, smoking status, alcohol consumption and social class
Adjusted for sex (in the combined sample), age, height, weight for height, smoking status, alcohol consumption, social class and CMV positivity.
Fig 2. Change in telomere length (left column) and IL-1β (right column) vs grip strength at 2003/5 follow-up before and after mutual adjustment.
Each subgroup represents tertiles of percentage change in telomere length or IL-1B over follow-up (z-scores). Grip strength values are means and 95% Confidence Intervals. All values are adjusted for age, sex, height, weight for height, smoking status, alcohol consumption and social class
Additional analyses were run looking at the associations between telomere attrition and other potential biomarkers of ageing at follow-up (timed 6m walk, timed chair rises, self reported physical performance) and no significant associations were found.
Discussion
We have shown that faster telomere attrition over the 10 year follow-up period was associated with lower grip strength at follow-up (β=0.98, p=0.035). These associations were robust in men, and were completely attenuated when adjusted for inflammaging burden over the period (p=0.86). Similarly, greater inflammaging burden over the 10 year period was associated with lower grip strength at follow-up, however conversely, these associations were not lost when also adjusted for telomere attrition (IL-1β, p=0.006).
We observed interesting differences between telomere length and change in grip strength, between men and women. These findings mirror the results of a recent systematic review suggesting that telomere length tends to be greater among women than men, but that these differences do not vary substantially by age or cell type [32]. The differences observed, might contribute to the stronger associations we found between telomere attrition and grip strength, among men. Whichever the case, it was clear that level of inflammation completely accounted for this association.
Our observation that the association between telomere attrition and lower grip strength at 10 year follow-up is accounted for by inflammation is novel and complements cross-sectional analyses that reveal associations between TL and age related diseases including atherosclerosis, hypertension and dementia [33-35]. Longitudinal associations of TL with coronary heart disease, cognitive decline and all cause mortality have also been found [36-39]. Potential mechanisms include telomere attrition causing functional changes within cell populations – for example senescent lymphocytes producing higher amounts of inflammatory cytokines; and also telomere attrition causing a limited cellular repair capacity in stem or progenitor cells – for example worsened endothelial repair accelerating the progression of atherosclerotic plaques [40-41].
However, an alternative explanation is that telomere attrition is a consequence rather than a cause of the ageing process with inflammation being implicated in the aetiology of both. In this study the associations between rate of telomere attrition and lower grip strength at follow-up disappeared after adjustment for markers of inflammatory status (IL-1β and cortisol, figure 2). These findings support the supposition that changes to the immune-endocrine axis are responsible, at least in-part, for the associations seen between TL and age related disease and mortality.
As with previous studies cross-sectional relationships between TL and GS at baseline were not seen and there was no association between TL or inflammation at baseline and GS at 10 year follow-up (data not shown).This is likely to be a consequence of the long follow-up time and a reflection of the multiple internal and environmental influences over that period which are largely immeasurable. These may be more accurately reflected by change in TL and inflammation over the 10 year period; it is therefore unsurprising that this is where we found the significant associations. This observation suggests that it is the environmental burden and immune-endocrine milieu over the follow-up period that is influencing both rate of telomere attrition and GS. For example, an individual at baseline with a short TL but a favourable inflammatory environment over a ten year period is likely to have a slower rate of both telomere attrition and sarcopenic change over that period as compared with an individual with similar TL but less favourable inflammatory environment. This also explains the finding that significant associations were seen between TL and inflammatory markers at follow-up but not baseline – a reflection of the burden of inflammation in the later lifecourse influencing age related disease and TL rather than earlier in the lifecourse at baseline.
This adds to a growing body of epidemiological and mechanistic evidence for the role of inflammation within the ageing process. Pro-inflammatory cytokines have specific receptors on skeletal muscle myocytes that activate intracellular signalling cascades causing myocyte atrophy and apoptosis [42]. Cross-sectional and longitudinal associations between inflammation and sarcopenia have previously been demonstrated and it is thought that inter-individual variations in inflammatory milieu at least in-part explains the different rates at which individuals become sarcopenic [43-44]. Similar mechanisms can be found in relation to other age-related disease processes such as those seen in the cardiovascular, pulmonary, skeletal and central nervous systems [45].
In addition to its effect on skeletal muscle, inflammation may simultaneously accelerate telomere attrition via: direct inhibition of telomerase [46-47]; an increase in oxidative stress leading to decreased telomerase activity; direct oxidative damage to telomere DNA; and promotion of cell turnover and replicative senescence [22;23;48]. Cortisol has been directly associated with reduced telomerase activity in human T-lymphocytes via the reduced transcription of hTERT, the telomerase catalytic component. This relationship may be bi-directional with faster telomere attrition associated with cellular senescence and a greater tendency to secrete pro-inflammatory cytokines.
We have shown that a substantial drop in telomere length was observed from baseline to follow up (an average of 144 base pairs annually). This is similar to rates in cross sectional studies although a more rapid decline has been observed in some longitudinal studies [6;7;9-12;14;34-38;49]. The range of telomere lengths observed in our study was substantial (between 1,300 and 12,300 base pairs). Although considerable attenuation of the variance in telomere length is observed in the very elderly [11] the range observed in our study was comparable with that in other population based samples [6;7;9;10;12;14].
Our study has some limitations. Firstly, we cannot completely exclude the effects of co-existing sub-clinical infections at the time of immune-endocrine analysis. However, participants were presumed fit and able to attend clinic appointments for data collection and results were screened prior to analysis for patterns suggestive of acute infection or haematological malignancy and four results were removed from the data set. Secondly, study participants were lost to follow-up between the 1994/5 baseline and 2003/5 follow-up clinics due to a variety of reasons (including mortality, refusal to participate) and we have previously shown that a healthy participant effect is, unsurprisingly, evident in HAS [19]. However, we have explored internal associations between variables in this analysis. The magnitude of associations that we have identified would only be substantially affected by the presence of a healthy survivor bias if the association between two variables was substantially different among study participants according to follow-up status; this seems unlikely. No further selection effects were evident according to availability of telomere data; specifically there was no difference in GS between participants who were, and were not followed up with reference to TL (data not shown). There are numerous candidate biomarkers of both the ageing process and the immune-endocrine axis which were not included in this study and there is scope to widen this biomarker battery further. However, to avoid complexity and facilitate clinical translation we specifically chose to limit these biomarkers to a small number. Grip strength was used as it is a simple test and easily translatable to the clinical environment. It is a well validated method of assessing muscle strength in older populations [50] and forms part of diagnostic criteria for sarcopenia and physical frailty [1;51]; it is also directly associated with age related outcomes including physical functioning [2]. Furthermore, the use of other markers of ageing such as change in physical performance status is unlikely to yield significant associations in this healthy and active community dwelling population. This was evidenced within this study through the lack of significant associations with timed 6m walk, chair rises and self reported physical performance which may reflect these being less robust than grip strength in a healthy dwelling community population without significant disability. These findings contrast with a multi-cohort analysis undertaken by the Halcyon consortium, which reported telomere attrition as a significant determinant of disability as assessed by chair rises [13]. Finally, our sample size is 253 and therefore confidence intervals are wider and true associations may have been missed; furthermore, as fewer women took part than men this might be another explanation for why significant relationships were only found in the latter in some cases although trends were similar. Whilst the limited study size may increase the validity of the significant findings reported the study needs replicating in other populations with larger numbers and, when appropriate, using other biomarkers of physical ageing.
In conclusion, our data adds to the body of evidence that suggests a raised pro-inflammatory milieu, inflammaging, may influence telomere attrition and at least in-part explain the associations which have previously been reported between TL and age-related outcomes. Unlike TL, simple biomarkers of inflammaging are more readily available, cheaper and have clearly defined reference ranges. This evidence suggests that the use of simple biomarkers of ageing including hand grip strength and markers of the immune-endocrine axis warrant greater exploration and could bring major health and socio-economic benefits at individual and population levels.
Acknowledgements
This work was funded by the Medical Research Council, the Biotechnology and Biological Sciences Research Council and the University of Southampton, United Kingdom. The Hertfordshire Cohort Study was supported the Medical Research Council of Great Britain; Arthritis Research UK; and the International Osteoporosis Foundation. The work herein was also supported by the NIHR Nutrition BRU, University of Southampton and the NIHR Musculoskeletal BRU, University of Oxford. We thank all of the men and women who took part in the Hertfordshire Cohort Study; the HCS Research Staff; Vanessa Cox who managed the data; and Gill Strange who prepared the manuscript.
Footnotes
Conflict of Interest BD, GN, MHE, HES, EMD, DBB and AAS declare no conflict of interest. CC has received honoraria and consulting fees from Amgen, Eli Lilly, Medtronic, Merck, Novartis and Servier. JML has received honoraria from Pfizer and Samsung.
Declaration All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study.
Reference List
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA. Frailty in older adults: evidence for a phenotype. J.Gerontol.A Biol.Sci.Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 2.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, White L. Midlife hand grip strength as a predictor of old age disability. JAMA. 1999;281:558–560. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z, Pan X, Liu L, Liu N. Telomere Length Maintenance, Shortening, and Lengthening. J.Cell Physiol. 2013 doi: 10.1002/jcp.24537. [DOI] [PubMed] [Google Scholar]
- 4.Allsopp RC, Harley CB. Evidence for a critical telomere length in senescent human fibroblasts. Exp.Cell Res. 1995;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- 5.Calado RT, Young NS. Telomere diseases. N.Engl.J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am.J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 7.von Zglinicki T. Will your telomeres tell your future? BMJ. 2012;344:e1727. doi: 10.1136/bmj.e1727. [DOI] [PubMed] [Google Scholar]
- 8.Mather KA, Jorm AF, Milburn PJ, Tan X, Easteal S, Christensen H. No associations between telomere length and age-sensitive indicators of physical function in mid and later life. J.Gerontol.A Biol.Sci.Med.Sci. 2010;65:792–799. doi: 10.1093/gerona/glq050. [DOI] [PubMed] [Google Scholar]
- 9.Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci.Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Mollica L, Fleury I, Belisle C, Provost S, Roy DC, Busque L. No association between telomere length and blood cell counts in elderly individuals. J Gerontol.A Biol.Sci.Med Sci. 2009;64:965–967. doi: 10.1093/gerona/glp065. [DOI] [PubMed] [Google Scholar]
- 11.Den Elzen WP, Martin-Ruiz C, von Zglinicki T, Westendorp RG, Kirkwood TB, Gussekloo J. Telomere length and anaemia in old age: results from the Newcastle 85-plus Study and the Leiden 85-plus Study. Age Ageing. 2011;40:494–500. doi: 10.1093/ageing/afr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris SE, Martin-Ruiz C, von Zglinicki T, Starr JM, Deary IJ. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiol.Aging. 2012;33:1486–1488. doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Gardner MP, Martin-Ruiz C, Cooper R, Hardy R, Sayer AA, Cooper C, Deary IJ, Gallacher J, Harris SE, Shiels PG, Starr JM, Kuh D, von ZT, Ben-Shlomo Y. Telomere length and physical performance at older ages: an individual participant meta-analysis. PLoS.One. 2013;8:e69526. doi: 10.1371/journal.pone.0069526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leslie M. Cell biology. Are telomere tests ready for prime time? Science. 2011;332:414–415. doi: 10.1126/science.332.6028.414. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, Ottaviani E, De BG. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann.N.Y.Acad.Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 16.Haraoui B, Liu PP, Papp KA. Managing cardiovascular risk in patients with chronic inflammatory diseases. Clin.Rheumatol. 2012;31:585–594. doi: 10.1007/s10067-011-1921-0. [DOI] [PubMed] [Google Scholar]
- 17.Franceschi C, Valensin S, Lescai F, Olivieri F, Licastro F, Grimaldi LM, Monti D, De BG, Bonafe M. Neuroinflammation and the genetics of Alzheimer’s disease: the search for a pro-inflammatory phenotype. Aging (Milano.) 2001;13:163–170. doi: 10.1007/BF03351475. [DOI] [PubMed] [Google Scholar]
- 18.Recasens M, Lopez-Bermejo A, Ricart W, Vendrell J, Casamitjana R, Fernandez-Real JM. An inflammation score is better associated with basal than stimulated surrogate indexes of insulin resistance. J Clin.Endocrinol.Metab. 2005;90:112–116. doi: 10.1210/jc.2004-0708. [DOI] [PubMed] [Google Scholar]
- 19.Baylis D, Bartlett DB, Syddall HE, Ntani G, Gale CR, Cooper C, Lord JM, Sayer AA. Immune-endocrine biomarkers as predictors of frailty and mortality: a 10-year longitudinal study in community-dwelling older people. Age (Dordr.) 2012 doi: 10.1007/s11357-012-9396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruunsgaard H, Andersen-Ranberg K, Hjelmborg JB, Pedersen BK, Jeune B. Elevated levels of tumor necrosis factor alpha and mortality in centenarians. Am.J Med. 2003;115:278–283. doi: 10.1016/s0002-9343(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 21.Hazeldine J, Arlt W, Lord JM. Dehydroepiandrosterone as a regulator of immune cell function. J.Steroid Biochem.Mol.Biol. 2010;120:127–136. doi: 10.1016/j.jsbmb.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 22.O’Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Cawthon RM, Opresko PL, Hsueh WC, Satterfield S, Newman AB, Ayonayon HN, Rubin SM, Harris TB, Epel ES. Cumulative inflammatory load is associated with short leukocyte telomere length in the Health, Aging and Body Composition Study. PLoS.One. 2011;6:e19687. doi: 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? Free Radic.Biol.Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Aviv A, Valdes A, Gardner JP, Swaminathan R, Kimura M, Spector TD. Menopause modifies the association of leukocyte telomere length with insulin resistance and inflammation. J Clin.Endocrinol.Metab. 2006;91:635–640. doi: 10.1210/jc.2005-1814. [DOI] [PubMed] [Google Scholar]
- 25.Shiels PG, McGlynn LM, MacIntyre A, Johnson PC, Batty GD, Burns H, Cavanagh J, Deans KA, Ford I, McConnachie A, McGinty A, McLean JS, Millar K, Sattar N, Tannahill C, Velupillai YN, Packard CJ. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS.One. 2011;6:e22521. doi: 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syddall H, Cooper C, Martin F, Briggs R, Aihie SA. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- 27.Gale CR, Martyn CN, Cooper C, Sayer AA. Grip strength, body composition, and mortality. Int.J.Epidemiol. 2007;36:228–235. doi: 10.1093/ije/dyl224. [DOI] [PubMed] [Google Scholar]
- 28.Sayer AA, Syddall HE, Martin HJ, Dennison EM, Roberts HC, Cooper C. Is grip strength associated with health-related quality of life? Findings from the Hertfordshire Cohort Study. Age Ageing. 2006;35:409–415. doi: 10.1093/ageing/afl024. [DOI] [PubMed] [Google Scholar]
- 29.Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. doi: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syddall HE, Simmonds SJ, Martin HJ, Watson C, Dennison EM, Cooper C, Sayer AA. Cohort profile: The Hertfordshire Ageing Study (HAS) Int.J Epidemiol. 2010;39:36–43. doi: 10.1093/ije/dyn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 32.Gardner M, Bann D, Wiley L, Cooper R, Hardy R, Nitsch D, Martin-Ruiz C, et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. doi: 10.1016/j.exger.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benetos A, Gardner JP, Zureik M, Labat C, Xiaobin L, Adamopoulos C, Temmar M, Bean KE, Thomas F, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 34.Panossian LA, Porter VR, Valenzuela HF, Zhu X, Reback E, Masterman D, Cummings JL, Effros RB. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol.Aging. 2003;24:77–84. doi: 10.1016/s0197-4580(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 35.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–473. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 36.Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 37.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 38.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, von ZT. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann.Neurol. 2006;60:174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- 40.Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat.Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spyridopoulos I, Dimmeler S. Can telomere length predict cardiovascular risk? Lancet. 2007;369:81–82. doi: 10.1016/S0140-6736(07)60042-7. [DOI] [PubMed] [Google Scholar]
- 42.Saini A, Faulkner S, Al-Shanti N, Stewart C. Powerful signals for weak muscles. Ageing Res.Rev. 2009;8:251–267. doi: 10.1016/j.arr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Beyer I, Mets T, Bautmans I. Chronic low-grade inflammation and age-related sarcopenia. Curr.Opin.Clin.Nutr.Metab Care. 2012;15:12–22. doi: 10.1097/MCO.0b013e32834dd297. [DOI] [PubMed] [Google Scholar]
- 44.Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, Colbert LH, Pahor M, Rubin SM, Tylavsky FA, Visser M. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J.Gerontol.A Biol.Sci.Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp.Mol.Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Beyne-Rauzy O, Recher C, Dastugue N, Demur C, Pottier G, Laurent G, Sabatier L, Mansat-De M,V. Tumor necrosis factor alpha induces senescence and chromosomal instability in human leukemic cells. Oncogene. 2004;23:7507–7516. doi: 10.1038/sj.onc.1208024. [DOI] [PubMed] [Google Scholar]
- 47.Rahman I, Gilmour PS, Jimenez LA, MacNee W. Oxidative stress and TNF-alpha induce histone acetylation and NF-kappaB/AP-1 activation in alveolar epithelial cells: potential mechanism in gene transcription in lung inflammation. Mol.Cell Biochem. 2002;234:235–239. [PubMed] [Google Scholar]
- 48.von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem.Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- 49.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Kark JD, Aviv A. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J.Gerontol.A Biol.Sci.Med.Sci. 2011;66:312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 51.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]