Summary
Oxysterols are cholesterol metabolites that serve multiple functions in lipid metabolism, including as liver X receptor (LXR) ligands. 27-hydroxycholesterol (27HC) is an abundant oxysterol metabolized by CYP7B1. How 27HC impacts vascular health is unknown. We show that elevations in 27HC via cyp7b1 deletion promote atherosclerosis in apoe−/− mice without altering lipid status; furthermore, estrogen-related atheroprotection is attenuated. In wild-type mice, leukocyte-endothelial cell adhesion is increased by 27HC via estrogen-receptor (ER)-dependent processes. In monocyte/macrophages 27HC upregulates proinflammatory genes and increases adhesion via ERα. In endothelial cells 27HC is also proadhesive via ERα, and in contrast to estrogen which blunts NF-κB activation, via Erk1,2- and JNK-dependent IκBα degradation 27HC stimulates NF-κB activation. Whereas 27HC administration to apoe−/− mice increases atherosclerosis, apoe−/−;erα−/− are unaffected. Thus, 27HC promotes atherosclerosis via novel proinflammatory processes mediated by ERα, and it attenuates estrogen-related atheroprotection. Strategies to lower 27HC may complement approaches targeting cholesterol to prevent vascular disease.
Introduction
Oxysterols are metabolites of cholesterol that classically perform multiple functions in lipid metabolism. They attenuate the expression of transcription factors necessary for the activation of genes within cholesterol supply pathways, they serve as ligands for the liver X receptors (LXR) α and β, they are substrates for the synthesis of bile acids, and they function in reverse cholesterol transport (RCT) to deliver sterols from peripheral tissues to the liver. The most abundant oxysterol is 27-hydroxycholesterol (27HC), which is synthesized by sterol 27-hydroxylase (CYP27A1) and metabolized by oxysterol 7α-hydroxylase (CYP7B1) (Brown and Jessup, 1999; Li-Hawkins et al., 2000a; Russell, 2000a; Tontonoz and Mangelsdorf, 2003).
The consideration of a potential impact of oxysterols on vascular disease was initially prompted by the observation that they are concentrated in atherosclerotic lesions. The most prevalent oxysterol in lesions is 27HC. Whereas circulating levels of 27HC in humans range from 150 to 730 nM, 27HC levels in atherosclerotic lesions are two orders of magnitude higher(Brown and Jessup, 1999). Circulating 27HC concentrations are predictably elevated with hypercholesterolemia, and they also increase with age(Brown and Jessup, 1999), particularly after the age of 30(Burkard et al., 2007). However, studies of 27HC in vascular health and disease, including work in animal models as well as in humans, have yielded equivocal results(Tontonoz and Mangelsdorf, 2003), and how 27HC influences vascular well-being remains unknown.
In the present investigation we sought to determine how 27HC impacts atherosclerosis. Recognizing that prior studies have been difficult to interpret because of the close correlation between 27HC and total cholesterol concentrations(Tontonoz and Mangelsdorf, 2003), we have employed cyp7b1−/− mice, which have elevations in 27HC in both the circulation and in tissues but entirely normal plasma cholesterol and triglyceride levels(Li-Hawkins et al., 2000a). To segregate the actions of cholesterol and 27HC, we have crossed apolipoprotein E−/− (apoe−/−) and cyp7b1−/− mice to generate models in which there is differing 27HC abundance in the setting of normocholesterolemia, differing cholesterol abundance in the setting of comparable 27HC levels, or differing 27HC abundance in the setting of comparable hypercholesterolemia. We have discovered that without altering lipid status, elevations in 27HC promote atherosclerosis. This is initially surprising because of the known roles of oxysterols in RCT and as ligands for LXR, whose activation affords atheroprotection(Michael et al., 2012; Im and Osborne, 2011). Having previously identified 27HC to be an ER ligand(Umetani and Shaul, 2011), the hypothesis was then tested that 27HC impacts atherosclerosis via ER-dependent processes. The cellular targets of 27HC potentially operative in the promotion of atherosclerosis by the oxysterol were also investigated, and the underlying mechanisms were delineated.
Results
Impact of 27HC on Atherosclerosis
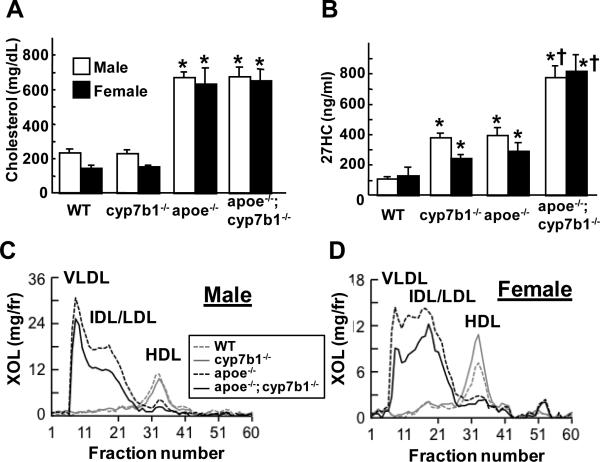
To determine how an increase in endogenous 27HC impacts atherosclerosis, lesions were evaluated at 12 months of age in intact male and female apoe+/+;cyp7b1+/+ wild-type, cyp7b1−/−, apoe−/−, and apoe−/−;cyp7b1−/−littermates. Total cholesterol was similar in cyp7b1−/− mice versus wild-type mice, and predictably plasma 27HC was elevated in cyp7b1−/− (Figure 1A, B). Lipid profiles were normal in cyp7b1−/− mice (Figure 1C, D). In apoe−/−, predictably both plasma cholesterol and 27HC were elevated compared to wild-type, and the lipid profiles displayed elevated VLDL, IDL/LDL fractions and decreased HDL cholesterol. In apoe−/−;cyp7b1−/−, total plasma cholesterol was similar to that in apoe−/−, and plasma 27HC was greater than in either cyp7b1−/− or apoe−/− single knockout mice. Plasma triglyceride was predictably elevated by apoe deletion, and similar in apoe−/− and apoe−/−;cyp7b1−/− mice (Table S1). Interestingly, lipid profiles in apoe−/−;cyp7b1−/− mice revealed lower IDL/LDL cholesterol than in apoe−/− mice. Thus, comparisons between wild-type and cyp7b1−/− determine the impact of 27HC in the setting of normal cholesterol status, and comparisons between apoe−/− and apoe−/−;cyp7b1−/− indicate how 27HC influences atherosclerosis in the setting of comparable hypercholesterolemia.
Figure 1.
Lipid and 27HC status of wild-type (WT), cyp7b1−/−, apoe−/−, and apoe−/−;cyp7b1−/−male and female mice. At 12 months of age plasma cholesterol (A) and 27HC concentrations (B) were measured in intact mice. Values are mean±SEM, n=5-10, *p<0.05 vs WT, †p<0.05 vs apoe−/−. Lipid profiles were also evaluated in samples pooled from 2 male (C) or 2 female mice (D).
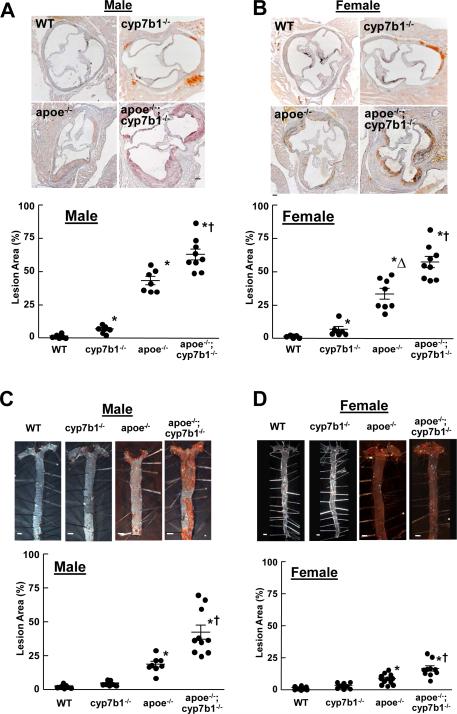
In the aortic root at 12 months of age, there was increased lipid deposition in the medial layer in cyp7b1−/− compared with wild-type mice (Figure 2A,B), and predictably atherosclerotic lesions were prevalent in apoe−/−. Revealing the impact of 27HC in the setting of hypercholesterolemia, atherosclerotic lesions were larger in apoe−/−;cyp7b1−/− than in apoe−/− mice. In comparisons between sexes, in apoe−/− males had greater lesions than females, mimicking the previous observation of gender differences likely related to protective actions of estrogen(Nofer, 2012). In contrast, in apoe−/−;cyp7b1−/−, lesion size was comparable in males and females. In analyses of aortic root lesions at 6 months of age (Figure S1), lesions were greater in apoe−/−;cyp7b1−/− versus apoe−/− in both males and females despite lower plasma cholesterol in double knockouts.
Figure 2.
27HC increases atherosclerotic lesion formation. Representative images and summary data for aortic root lesions in intact 12 month-old wild-type (WT), cyp7b1−/−, apoe−/−, and apoe−/−;cyp7b1−/− male and female mice are shown in A and B, respectively. Findings for atherosclerotic lesions in the aortas of male and female mice are provided in C and D, respectively. Summary data are provided in scatter plots. Mean values are indicated by the long horizontal line, and SEM by the short horizontal line. Related data are provided in Table S1. *p<0.05 vs WT, †p<0.05 vs apoe−/−, Δp<0.05 vs. males. See also Figure S1.
Lesion abundance in the aorta was evaluated at 12 months of age (Figure 2C, D), and paralleling the findings in the aortic root, en face lesions were larger in apoe−/−;cyp7b1−/− than in apoe−/− both in males and females. The cumulative findings at 6 and 12 months of age indicate that under normocholesterolemic conditions, elevations in 27HC cause increased lipid deposition in the media of the proximal aorta, and that in the setting of hypercholesterolemia and independent of adverse impact on circulating lipids, elevated 27HC causes a progressive, marked increase in atherosclerotic lesion development. In addition, they reveal that the capacity of 27HC to promote atherosclerosis is comparable in male and female mice.
Impact of 27HC on Estrogen-related Atheroprotection
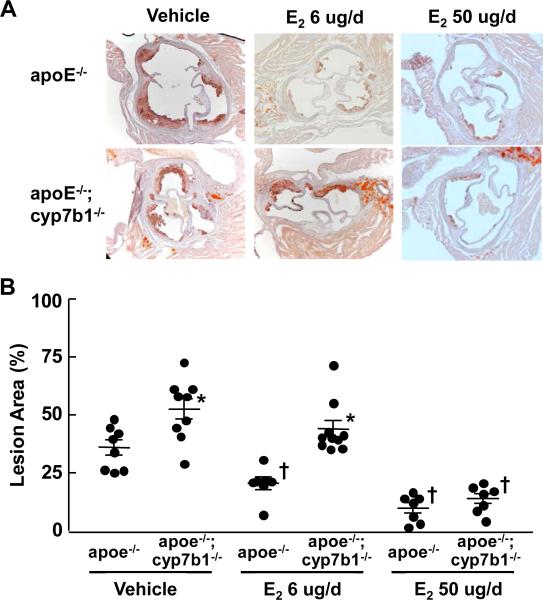
Since oxysterols participate in RCT and are ligands for LXR, and both mechanisms are atheroprotective(Michael et al., 2012; Im and Osborne, 2011), the worsening of atherosclerosis by 27HC does not likely entail these processes. Having previously discovered 27HC to be an ER ligand(Umetani et al., 2007), we next evaluated how 27HC impacts estrogen-related atheroprotection in ovariectomized female apoe−/− and apoe−/−;cyp7b1−/− mice. Lipid analyses revealed that in vehicle and E2-treated mice receiving 6 ug/d, total cholesterol (Table S3) and IDL and LDL cholesterol (Figure S2) were actually lower in apoe−/−;cyp7b1−/− versus apoe−/− mice. In apoe−/−, E2 treatment lowered plasma 27HC, whereas there was no impact of E2 on plasma 27HC in apoe−/−;cyp7b1−/− (Table S3). This is consistent with the previous observation that E2 upregulates hepatic CYP7B1 expression(Yamamoto et al., 2006). In vehicle-treated mice, aortic root lesion area was greater in apoe−/−;cyp7b1−/− versus apoe−/− mice (Figure 3). In apoe−/−, 6 ug/d E2 treatment caused an approximately 50% decrease in lesion area, and 50 ug/d E2 treatment caused a further decline in lesion area. In contrast, in apoe−/− ;cyp7b1−/−, 6 ug/d E2 was not atheroprotective, and lesion size following 6 ug/d E2 remained far greater in apoe−/−;cyp7b1−/− than in apoe−/−. However, E2 at 50 ug/d provided atheroprotection in apoe−/−;cyp7b1−/−, such that lesion areas were similar following 50 ug/d E2 in apoe−/−;cyp7b1−/− and apoe−/− mice. Since 27HC and E2 compete for ER binding(Umetani et al., 2007), the dose-related capacity of E2 to reverse the exaggerated atherosclerosis caused by elevated endogenous 27HC suggests that the atherogenic effects of 27HC are related to its function as an ER ligand. In addition, since the 27HC-associated worsening of atherosclerosis is evident in ovariectomized females and also in males, the adverse impact of the oxysterol occurs via the direct modulation of ER function rather than through the antagonism of estrogen action.
Figure 3.
27HC attenuate E2-related atheroprotection. A. Representative images of aortic roots in female apoe−/− versus apoe−/−;cyp7b1−/− mice ovariectomized at 12 weeks of age, fed a western diet and treated with vehicle, 6 ug/d E2 or 50 ug/d E2 for 8 weeks. B. Summary data are provided in scatter plots. Mean values are indicated by the long horizontal line, and SEM by the short horizontal line. *p<0.05 vs apoe−/−, †p<0.05 vs vehicle. See also Figures S2 and Table S3.
Impact of 27HC on Vascular Inflammation In Vivo
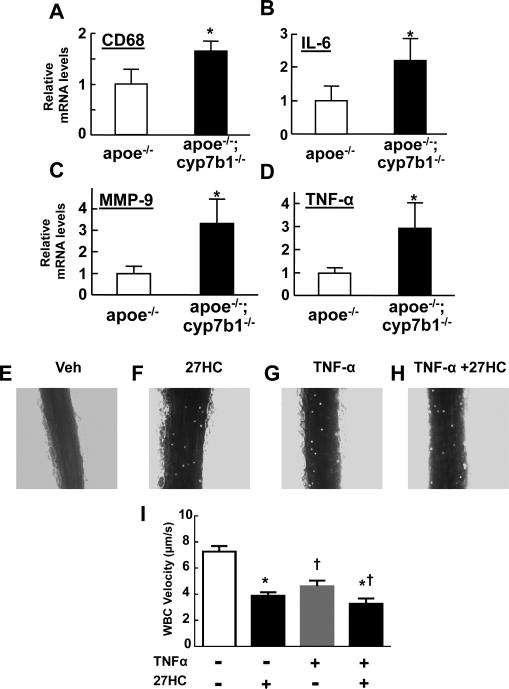
Since inflammation is critically involved in atherogenesis(Galkina and Ley, 2009; Libby, 2012) and ER modulate inflammatory processes(Straub, 2007; Arnal et al., 2009), the impact of 27HC on inflammation in the atherosclerotic lesions was evaluated. Mac-3 staining of aortic root lesions (Figure S3) revealed greater areas of macrophage accumulation in apoe−/−;cyp7b1−/− versus apoe−/− mice (Figure S3A,B). There was also a directional change in lesion size relative to macrophage abundance (p=0.11) (Figure S3C), perhaps suggesting greater macrophage-driven lesion development in apoe−/−;cyp7b1−/− versus apoe−/−. In aortae from 12 month-old males, macrophage infiltration assessed by quantifying CD68 transcript abundance was greater in apoe−/−;cyp7b1−/− versus apoe−/− mice (Figure 4A). Steady-state mRNA levels for IL-6, MMP-9 and TNF-α normalized to CD68 mRNA were 2.2-, 3.3-, and 2.5-fold greater, respectively, in the aortas of apoe−/−;cyp7b1−/− versus apoe−/− mice (Figure 4B-D). Plasma TNF-α was also increased in apoe−/−;cyp7b1−/− versus apoe−/− mice (Figure S4A). Thus, an increase in 27HC in the setting of hypercholesterolemia causes greater macrophage infiltration and exaggerated of inflammatory cytokine gene activation by the macrophages or other cell types in the vascular wall.
Figure 4.
27HC promotes vascular inflammation. A. Macrophage infiltration was compared in the aortas of 12 month-old male apoe−/− versus apoe−/−;cyp7b1−/− mice by quantifying transcript abundance for CD68. B-D. Steady-state mRNA levels for IL-6 (A), MMP-9 (B) and TNF-α (C) normalized to CD68 expression were also compared. In A-D, values are mean±SEM, n=4-6, *p<0.05 vs apoe−/−. E-I. 27HC promotes leukocyte-endothelial cell adhesion in vivo. Male C57BL/6 mice were treated with vehicle or 27HC, followed by vehicle or TNF-α, and intravital microscopy was then performed to evaluate leukocyte-endothelial cell adhesion in the mesenteric microcirculation. Representative still images are shown for mice treated with vehicle (Veh, E), 27HC (F), TNF-α (G), or TNF-α plus 27HC (H). Summary data are provided in I. Values are mean±SEM, n=7-9, *p<0.05 vs no 27HC, †p<0.05 vs no TNF-α. See also Figure S3, S4A and Movies S1-S4.
To directly evaluate the initiating events by which 27HC potentially drives vascular inflammation in vivo, intravital microscopy was performed to quantify leukocyte-endothelial adhesion in the mesenteric microcirculation. Male C57BL/6 mice were treated daily for 3 days with vehicle or 27HC. On the following day they received vehicle or TNF-α to promote inflammation, endogenous leukocytes were fluorescently labeled by the injection of Rhodamine-6G, and intravital microscopy was done to visualize the leukocytes and quantify their velocity. Treatment with TNF-α predictably lowered leukocyte velocity, and 27HC caused a comparable decrease in velocity (Figure 4, Movies S1-4). 27HC also lessened velocity in mice that received TNF-α co-treatment. These collective findings indicate that 27HC promotes pro-inflammatory processes in the vasculature, including causing marked enhancement of leukocyte-endothelial adhesion under normocholesterolemic conditions.
Impact of 27HC on Monocytes/macrophages and Endothelial Cells
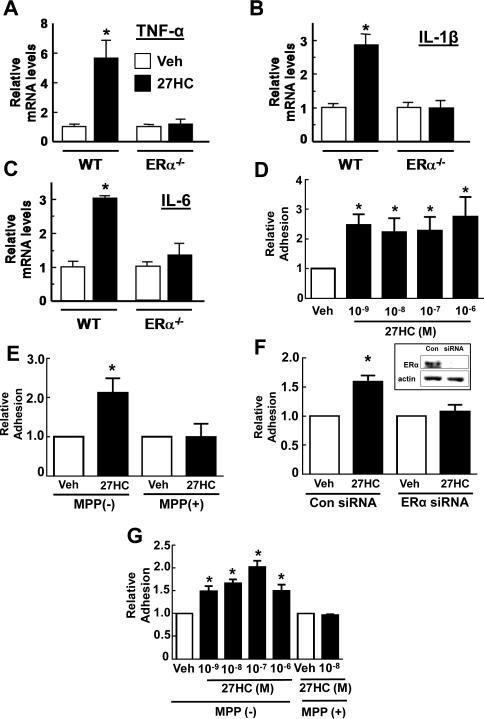
To determine the cellular targets of 27HC participating in its promotion of vascular inflammation, experiments were performed in cell culture. In peritoneal macrophages from wild-type mice, 27HC upregulated TNF-α mRNA by almost 6-fold and IL-1β mRNA and IL-6 mRNA by 3-fold (Figure 5A-C); these responses did not occur in macrophages devoid of ERα, and they were present in macrophages lacking both LXRα and LXRβ (Figure S4B-D). The changes in cytokine transcript abundance were associated with increases in cytokine production (Figure S4E-G). Thus, 27HC promotes the expression of pro-inflammatory genes in macrophages through ERα-dependent, LXR-independent processes.
Figure 5.
27HC has direct proinflammatory actions on monocytes/macrophages and endothelial cells mediated by ERα. A-C. Peritoneal macrophages from wild-type (WT) or erα−/− mice were treated with vehicle (Veh) or 27HC for 20h, and transcript abundance for TNF-α (A), IL-1β (B) or IL-6 (C) was evaluated. n=3. D-F. Bovine aortic endothelial cells were treated with vehicle or 27HC, and the adhesion of added U937 cells was evaluated. The dose-response to 27HC was assessed (D, n=7), and the involvement of endothelial cell ERα was determined using the selective ERα antagonist MPP (E, n=4) or siRNA knockdown of the receptor (F, n=4). Effective loss of ERα protein with siRNA knockdown is shown in the inset. G. The impact of 27HC on adhesion-promoting mechanisms in monocytes was determined in U937 cells treated 18h with vehicle or 27HC, with or without MPP added, prior to their addition to endothelial cells (n=6). Values are mean±SEM expressed relative to vehicle treatment, *p<0.05 vs vehicle. See also Figure S4B-G.
To assess direct 27HC action on endothelial cells, bovine aortic endothelial cells (BAEC) were treated for 24h and endothelial cell-monocyte adhesion assays were then performed. 27HC promoted monocyte-endothelial cell adhesion (Figure 5D), and the increase in adhesion was fully prevented by the ERα antagonist methyl-piperidino-pyrazole (MPP) (Figure 5E), or by siRNA-based knockdown of endothelial cell ERα (Figure 5F).
To determine if 27HC also has direct action on monocytes/macrophages that promotes adhesion, additional experiments were performed following 24h treatment of the monocytes with 27HC. Again 27HC promoted monocyte-endothelial cell adhesion, and MPP blunted the response (Figure 5G). Thus, in both endothelial cells and monocyte/macrophages, ERα modulation by 27HC in the absence of estrogen enhances diverse pro-inflammatory processes.
27HC Activation of NF-κB
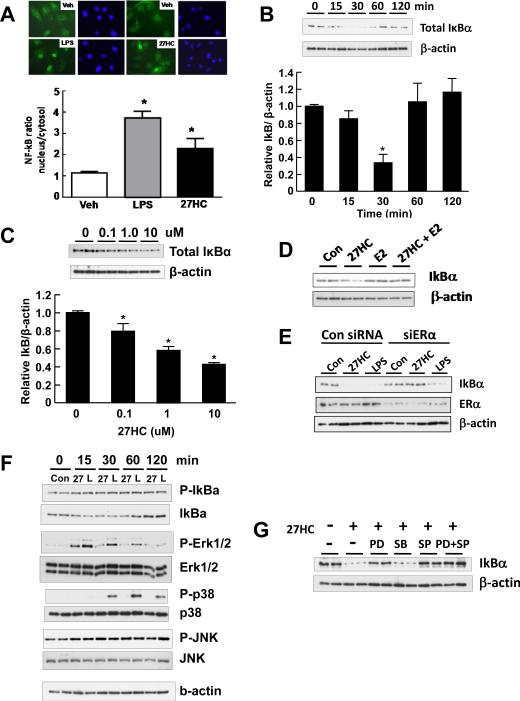
To further interrogate the proinflammatory actions of 27HC and compare the effects of 27HC with those of E2, NF-κB activation in endothelial cells was evaluated. Although the overall impact of NF-κB activation on atherosclerosis is complex, studies in mice have demonstrated that NF-κB activation in endothelial cells plays a major role in the pathogenesis of the disorder(Gareus et al., 2008). Under quiescent conditions, inactive NF-κB is bound to IκBα in the cytoplasm, and inflammatory stimuli induce the phosphorylation of IκBα at Ser32 leading to its ubiquitination and proteasomal degradation, releasing NF-κB to translocate to the nucleus to drive the expression of target genes(Mercurio and Manning, 1999). E2 inhibits NF-κB activation in both endothelial and nonendothelial cells(Simoncini et al., 2000; Ghisletti et al., 2005), and a number of mechanisms have been implicated, including direct protein-protein interactions between ER and NF-κB, sharing of coactivators, inhibition of NF-κB DNA binding, increased expression of IκBα and decreased IκBα Ser32 phosphorylation(Stein and Yang, 1995; Ray et al., 1994; Ray et al., 1997; Deshpande et al., 1997; Sun et al., 1998; Harnish et al., 2000; Speir et al., 2000; Simoncini et al., 2000). Using LPS as a positive control, we evaluated NF-κB activation in endothelial cells by immunocytochemical localization of the p65 subunit, and found that 27HC caused NF-κB activation (Figure 6A). In contrast, E2 abrogated LPS-induced p65 nuclear translocation (Figure S5). Thus, the two ER ligands, 27HC and E2, have entirely opposite effects on the activation of NF-κB.
Figure 6.
27HC promotes IκBα degradation and activates NF-κB in endothelial cells via ERα and Erk1,2- and JNK-dependent processes. A. Using immunofluorescence, the subcellular distribution of the NF-kB subunit p65 was evaluated in endothelial cells treated with vehicle, LPS (100 nM) or 27HC (10 uM). p65 shown in green, nuclei stained with Dapi. Values are mean±SEM, n=10-18, *p<0.05 vs vehicle. B-C. The impact of 27HC on IκBα abundance was evaluated in cells treated for 0-120 min with 10 uM 27HC (B), or in cells treated for 30 min with 0-10uM 27HC (C). In B and C, values are mean±SEM, n=6-9 and *p<0.05 vs vehicle. D. The effects of 27HC (10 uM), E2 (10 nM), or the combination of 27HC and E2 on IκBα abundance were compared over 30 min. E. The effects of 27HC (10 uM) or LPS (100 ng/ml) on IκBα abundance were compared over 30 min in cells previously transfected with control siRNA or siRNA targeting ERα. F. MAPK activation in response to 27HC (10 uM) or LPS (100 ng/ml) treatment for 0-120 min was evaluated by immunoblot analyses for phosphorylated and total Erk1,2, p38MAPK and JNK. IκBα Ser32 phosphorylation and total protein abundance were also assessed. G. The effect of 27HC (10 uM) on IκBα abundance was compared over 30 min in cells coincubated with vehicle or the MAPK inhibitors PD98059 (1 uM), SB203580 (10 uM), SP600125 (10 uM), or PD98059 plus SP600125. See also Figure S5.
To determine how 27HC activates NF-κB in endothelial cells, its impact on IκBα protein abundance was evaluated. 27HC treatment caused IκBα abundance to fall to 30% of basal levels by 30 min (Figure 6B). Dose-response studies revealed that the effect is apparent at a threshold concentration of 27HC of 0.1 uM (Figure 6C). In contrast to 27HC, E2 did not cause a fall in IκBα abundance, and its addition to 27HC-treated cells prevented the 27HC-induced decline in IκBα (Figure 6D). As importantly, whereas LPS-induced IκBα degradation was not prevented by ERα deletion, the degradation caused by 27HC was fully reversed by silencing of the receptor (Figure 6E). Thus, 27HC uniquely activates NF-κB in endothelial cells, and this is related to ERα-dependent promotion of IκBα degradation.
Recognizing that NF-κB activation is often modulated by mitogen-activated protein kinases (MAPK)(Kaminska, 2005; Hoesel and Schmid, 2013), to elucidate the basis for 27HC action on IκBα, the relative levels of activating phosphorylation of extracellular signal-regulating kinase (Erk)1,2, p38MAPK and c-Jun N-terminal kinase (JNK) were evaluated in 27HC-treated endothelial cells. Responses to LPS were studied in comparison. Both 27HC and LPS caused a decline in IκBα and also an increase in IκBα Ser32 phosphorylation (Figure 6F). Both 27HC and LPS also caused an increase in Erk1,2 phosphorylation and an increase in JNK phosphorylation, whereas only LPS yielded an increase in p38MAPK phosphorylation. To determine the potential role of MAPK activation by 27HC in the actions of the oxysterol on IκBα, MAPK inhibitors were employed. Whereas the MEK inhibitor PD98059 and the JNK inhibitor SP600125 prevented the decline in IκBα abundance caused by 27HC, either alone or in combination, the p38MAPK inhibitor SB203580 had no effect (Figure 6G). Thus, Erk1,2 and JNK activation are required for 27HC engagement of ERα in endothelial cells to promote the degradation of IκBα and thereby activate the NF-κB pathway.
ER and 27HC Exacerbation of Inflammation and Atherosclerosis
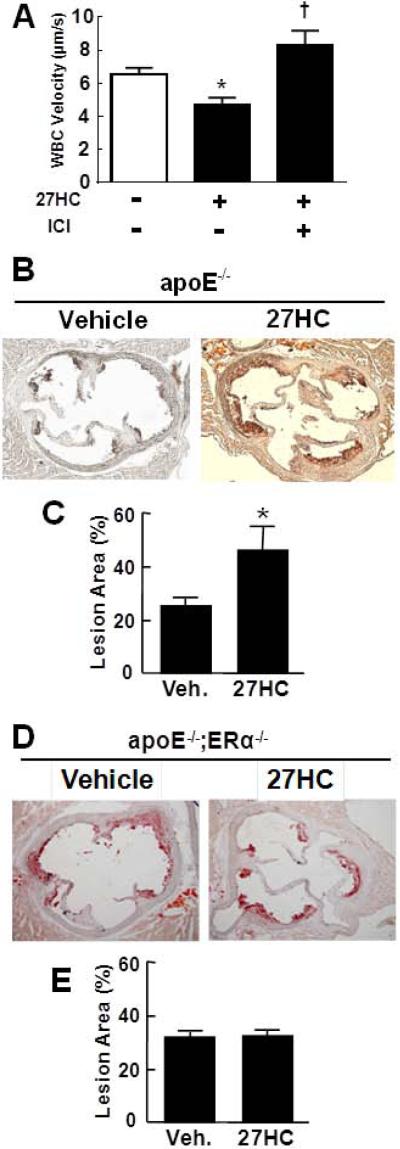
Having determined that ERα uniquely modulates the direct pro-inflammatory actions of 27HC on cultured monocytes/macrophages and endothelial cells, the role of ER in 27HC-induced enhancement of leukocyte-endothelial cell adhesion was studied in vivo by intravital microscopy (Figure 7A) using the ER-specific antagonist ICI 182,780(Chambliss et al., 2010). The treatment of healthy male mice with ICI 182,780 caused a decrease in leukocyte velocity (Figure S6A), indicating that ER antagonize adhesion in vivo under basal conditions. A dose of ICI 182,780 below the threshold that promotes adhesion was then employed in studies of male mice administered 27HC. The decline in leukocyte velocity indicative of an enhancement in adhesion by 27HC was fully prevented by ICI 182,780 (Figure 7A). When combined with the observations made in cell culture (Figure 5), these in vivo findings indicate that the proinflammatory actions of 27HC on the vascular wall occur via ER-dependent mechanisms, that the effects of 27HC are not merely via antagonism of estrogen action, and that instead the oxysterol is directly modifying both leukocyte and endothelial cell functions independent of steroid hormone action.
Figure 7.
ER mediate the pro-inflammatory and pro-atherosclerotic actions of 27HC in vivo. A. Leukocyte-endothelial adhesion was evaluated by intravital microscopy in wild-type male mice administered vehicle or 27HC without or with cotreatment with ICI 182,780. Values are mean±SEM, n=7-11, *p<0.05 vs no 27HC, †p<0.05 vs no ICI 182,780. B-E. The impact of 27HC administration on atherosclerosis was evaluated in apoe−/− (B, C) or apoe−/−;erα−/− male mice (D,E). Representative aortic root lesions for vehicle or 27HC-treated mice are shown in B and D, and summary data (mean±SEM) are provided in C (n= 12-13) and E (n= 7), *p<0.05 vs vehicle. See also Figure S6.
Whereas ERα classically mediates anti-atherosclerotic processes(Ribas et al., 2011; Billon-Gales et al., 2009), the potential pro-atherosclerotic role of the receptor was then interrogated in studies of apoe−/− versus apoe−/−;erα−/− male mice administered vehicle or 27HC for 6 weeks. Serum 27HC levels in the vehicle versus 27HC-treated apoe−/− mice were 1022±68 and 1840±188 ng/ml, respectively (p<0.05), and in vehicle versus 27HC-treated apoe−/−;erα−/− mice they were 955±133 and 2228±332 ng/ml, respectively (p<0.05). The lipid profiles in both genotype groups were unaffected by 27HC (Figure S6B,C). Whereas 27HC administration caused a doubling in lesion area in apoe−/− (Figure 7B,C), it had no impact in apoe−/−;erα−/− mice (Figure 7D,E). These findings parallel those obtained with genetic deletion of cyp7b1 (Figure 2), and they demonstrate that the pro-atherosclerotic processes are mediated by 27HC action on ERα. Thus, in contrast to the E2-ERα tandem, the ligand-receptor partnership between ERα and its non-aromatized ligand 27HC is uniquely proatherogenic.
Discussion
Circulating levels of oxysterols are predictably elevated with hypercholesterolemia, and oxysterols are concentrated in atherosclerotic lesions(Brown and Jessup, 1999). How oxysterols, including the most abundant oxysterol 27HC, impact vascular disease has been enigmatic because of the close correlation between 27HC and cholesterol abundance(Tontonoz and Mangelsdorf, 2003). In the present work, we employed both genetic and pharmacologic manipulations in mice to segregate the actions of cholesterol and 27HC. We discovered that atherosclerosis is promoted in both males and females by elevations in 27HC independent of changes in cholesterol, and that the proatherogenic actions of 27HC are uniquely mediated by ERα. Thus, in addition to the adverse impact of 27HC on bone mineralization and breast cancer(Wu et al., 2013; Nelson et al., 2013), it is now apparent that the only other known endogenous ER ligand besides estrogen negatively affects cardiovascular health.
Inflammation is critically involved in atherogenesis(Galkina and Ley, 2009; Libby, 2012), and we discovered that 27HC invokes proinflammatory processes in vascular cells both in vitro and in vivo. Macrophage accumulation in the vascular wall was increased by 27HC and proinflammatory genes were upregulated, and plasma TNF-α was elevated. Importantly, we demonstrated that leukocyte-endothelial cell adhesion, which is a key early event in the pathogenesis of atherosclerosis(Libby et al., 2006), is potently promoted in vivo by elevations in 27HC even in the setting of normocholesterolemia. Studies of cultured monocytes/macrophages and endothelial cells then revealed that both cell types are targets of the proinflammatory actions of 27HC, and that these effects are ERα-dependent. In parallel, ER loss-of-function prevented the in vivo enhancement of leukocyte-endothelial cell adhesion by 27HC and also the worsening of atherosclerosis by the oxysterol. Although the impact of NF-κB activation on atherosclerosis is complex, it has been convincingly demonstrated in apoe−/− mice that NF-κB activation in endothelial cells plays a major role in atherogenesis(Gareus et al., 2008). We therefore focused further mechanistic studies of the basis for 27HC activation of NF-κB on endothelial cells, and found that 27HC promotes NF-κB nuclear translocation whereas E2 is inhibitory, and that the activation of NF-κB by 27HC is related to ERα-dependent, Erk1,2- and JNK-dependent IκBα protein degradation. The biochemical basis for 27HC-ERα modulation of MAPKs and other processes that lead to IκBα degradation can now be pursued.
In addition to demonstrating proinflammatory actions of 27HC directly mediated by ERα in the absence of E2 in cell culture, and pro-atherogenic, ERα-dependent effects of 27HC in male mice, competing actions of the oxysterol and estrogen on atherosclerosis were observed in ovariectomized female mice. 27HC potently antagonized the atheroprotection afforded by E2 when the hormone was replaced at physiologic concentrations, and it was only at superphysiologic levels that E2 was atheroprotective in the setting of elevated 27HC. These findings lend further support to the conclusion that the proatherosclerotic actions of 27HC are ER-dependent. In addition, they have potentially important clinical implications regarding cardiovascular health and its management in women. The risk of coronary heart disease in women increases dramatically after menopause(Lloyd-Jones et al., 2009), and there is considerable evidence that hormone replacement therapy (HRT) with estrogen is not beneficial and instead is possibly harmful when given to women years after they have gone through menopause(Mendelsohn and Karas, 2005; Clarkson et al., 2013). Although numerous mechanisms may underlie the change in response to HRT with age, existing atherosclerosis likely plays a critical role(Mendelsohn and Karas, 2005; Karas, 2004; Wagner and Clarkson, 2005; Dubey et al., 2005). Since 27HC levels rise with age and 27HC is abundant in atherosclerotic lesions(Brown and Jessup, 1999; Burkard et al., 2007), our current findings suggest that the potential vascular benefits of either endogenous or exogenous estrogen may be attenuated in older women by the actions of 27HC.
Now knowing that 27HC promotes atherosclerosis independent of changes in cholesterol, the processes that govern 27HC abundance can potentially be targeted to provide therapeutic opportunities independent of the multiple strategies aimed at cholesterol lowering. The enzyme that synthesizes 27HC, CYP27A1, is normally abundant in the liver and also constitutively expressed in normal arterial wall, and it is upregulated and abundant in atherosclerotic lesions(Crisby et al., 1997; Shanahan et al., 2001; Russell, 2000b). The cellular or tissue source(s) of 27HC that is important to the contribution of the oxysterol to atherogenesis is yet to be identified. This consideration, as well as the numerous other roles of CYP27A1 and 27HC such as in bile acid synthesis and RCT(Brown and Jessup, 1999; Li-Hawkins et al., 2000a; Russell, 2000a; Tontonoz and Mangelsdorf, 2003), need to be carefully addressed in order to optimally contemplate a mode of CYP27A1 loss-of-function as a strategy for possible atheroprotection. CYP7B1, which metabolizes 27HC, is also normally highly abundant in the liver(Russell, 2003). Complementary to possibly targeting CYP27A1 to lower 27HC synthesis, whether enhancing its metabolism by CYP7B1 lessens atherosclerosis in the setting of hypercholesterolemia now also deserves investigation.
In addition to lowering 27HC abundance for therapeutic gain, impacting its mechanisms of action can be contemplated. Since we previously showed that 27HC binding cause novel changes in ERα conformation(DuSell et al., 2008), the ER-dependent actions of the oxysterol can potentially be selectively inhibited to lessen its adverse influence on vascular disease while retaining potential beneficial effects of ER activation by estrogen.
Strategies to lower cholesterol such as the use of statins have had a dramatic impact on cardiovascular health. However, there continues to be a need for complementary approaches to combat atherosclerosis, and these have been somewhat limited in number or are accompanied by complications or unresolved questions, as is the case with HDL-targeted therapies and HRT(Phan and Toth, 2013; Larach et al., 2012; Santen et al., 2010). At the same time that novel insights have been gained about the biology of oxysterols and nuclear receptors, the discovery that 27HC promotes atherosclerosis through unique ERα-mediated mechanisms may afford new opportunities for therapy development.
Experimental Procedures
Animal and cell culture models
Wild-type, cyp7b1−/−, apoe−/−, and apoe−/−;cyp7b1−/− littermates were generated by crossing cyp7b1−/− mice (originally from David Russell, Department of Molecular Genetics, UT Southwestern)(Li-Hawkins et al., 2000b) with apoe−/− mice (Jackson Labs). All four genotype groups were fed standard chow. E2-related atheroprotection was studied in apoe−/− and apoe−/−;cyp7b1−/− female mice ovariectomized at 12 weeks of age and then placed on a western diet (21% milk fat, 0.2% cholesterol, Teklad 88137) and treated with vehicle or E2 at 6ug/d or 50ug/d using subcutaneously-implanted pellets (Innovative Research of America). Atherosclerotic lesions were evaluated 8 weeks later. To determine the effect of exogenous 27HC on atherogenesis and the role of ERα, 12 week-old male apoe−/− or apoe−/−;erα−/− mice were treated with vehicle or 27HC (20 mg/kg body weight) by subcutaneous injection every 2 days for 6 weeks, and lesions were then evaluated. The 27HC was dissolved in 30% (2-hydroxypropyl)-β-cyclodextrin solution, and the same solution served as the control treatment. All animal studies were approved by the Institutional Animal Care and Use Committee at UT Southwestern.
To investigate processes in monocytes/macrophages, studies were performed in either macrophages isolated from the mouse peritoneum using thioglycolate(Venkateswaran et al., 2000), or the monocyte cell line U937. Mice used for macrophage isolation were wild-type, era−/− or lxrα−/−;lxrβ−/− (Ishikawa et al., 2013). Processes in endothelial cells were interrogated in primary bovine aortic endothelial cells (BAEC)(Chambliss et al., 2010). For in vitro studies, 27HC was diluted from a 10 mM ethanol solvent stock directly into assay medium, and identical concentrations of ethanol were present under control conditions.
Cholesterol, triglyceride, 27HC and estradiol analyses
Plasma total cholesterol, 27HC and E2 concentrations were evaluated as previously described(Umetani et al., 2007). Plasma triglyceride levels were measured by colorimetric enzymatic assay (Roche Diagnostics). Aorta cholesterol and triglyceride content were assessed following ether lipid extraction(Kalaany et al., 2005). Plasma lipid profiles were obtained by column fractionation and measurements of fraction cholesterol content on pooled samples from 2 or 3 mice (Kawashiri et al., 2001).
Quantitative RT-PCR
Transcript abundance for IL-6, IL-1β, MMP-9, TNFα, CD68 or ERα was evaluated in mouse aorta or cultured cells by quantitative RT-PCR using previously-described approaches(Bookout and Mangelsdorf, 2003; Umetani et al., 2007).
Cytokine measurements
Plasma TNF-α levels and TNF-α, IL-6 and IL-1β concentrations in cell supernatants following vehicle versus 27HC treatment were measured using the DuoSet ELISA System (R&D Systems).
Assessment of atherosclerosis
Atherosclerotic lesions were evaluated in a blinded fashion(Bourassa et al., 1996). Mice were anesthetized with avertin and killed, blood was obtained for analyses and perfusion fixation was performed. The heart and aorta were removed, the heart and proximal aorta were embedded in OCT, serial frozen sections (10 um) of the aortic root were obtained, and 4 to 6 sections per aorta were processed. To evaluate atherosclerotic lesions in en face preparations of the aorta, using a dissecting microscope the adventitial fat was removed and the aorta was opened longitudinally and pinned onto a silicon bed. Lipid staining was performed with Sudan IV(Bourassa et al., 1996). To evaluate macrophage infiltration in the aortic root, immunohistochemical analysis was done with anti-Mac-3 antibody (BD Pharmingen). Images were captured and areas were determined using Image Pro Plus software(Tangirala et al., 1999; Reddick et al., 1994).
Intravital microscopy for quantification of leukocyte-endothelial adhesion
Leukocyte-endothelial adhesion was evaluated as described previously(Ramesh et al., 2011). Briefly, 5 to 7 week-old male mice were subcutaneously-injected with vehicle or 27HC (20 mg/kg body weight) daily for 3 days, and on the following day they received an IP injection of vehicle or TNF-α (0.3ug). The dose of 27HC used yields serum 27HC concentrations of 150-200 ng/ml. Vehicle versus TNF-α treatment was employed to evaluate the impact of 27HC on adhesion under basal conditions and also in the setting of elevated adhesion. Four hours after vehicle or TNF-α treatment, the mice were prepared for intravital microscopy. Endogenous leukocytes were fluorescence-labeled by injection of the mice with Rhodamine-6G (100 ul of 0.05% solution given by optic vascular plexus), and the mesentery was exposed for the observation and recording of images of leukocyte adhesion and rolling using a Regita digital camera (X200 magnification; QImaging). The velocity of leukocyte rolling was calculated using Image-Pro V6.2(Wang et al., 2012). In additional studies, mice were administered vehicle or 27HC, and also vehicle or the ER antagonist ICI 182,780 (5ug) given subcutaneously 48h, 24h and 4h prior to study.
Endothelial cell-monocyte adhesion assays, NF-κB activation
The adhesion of U937 monocytes to monolayers of BAEC was evaluated as previously reported(Umetani et al., 2000). Select experiments were done in the absence versus presence of the selective ERα antagonist methyl-piperidinopyrazole (MPP, 10−6M)(Sun et al., 2002). In additional studies U937 cells, and not BAEC, were treated with vehicle versus 27HC for 24h prior to inclusion in the adhesion assay.
To evaluate NF-κB activation in endothelial cells, NF-κB p65 subunit intracellular localization was assessed by immunocytochemistry(Simoncini et al., 2000; Noursadeghi et al., 2008). The relative distribution of p65 in nucleus versus cytoplasm was quantified by image analysis.
Immunoblot Analyses
ERα protein abundance in endothelial cells was assessed by immunoblot analysis using mouse monoclonal antibody F-10 (sc-8002, Santa Cruz Biotechnology). Total IκBα abundance and relative IκBα Ser32 phosphorylation in endothelial cells were evaluated by immunoblot analyses with anti-IκBα antibody (Cell Signaling), anti-IκBα phospho-Ser32 antibody (Cell Signaling), and anti-actin antibody (Santa Cruz Biotechnology). In selected experiments cells were treated with PD98059 (1 uM), SB203580 (10 uM), or SP600125 (10 uM, Sigma-Aldrich) to evaluate the roles of Erk1,2, p38MAPK or JNK, respectively, in the modulation of IκBα. MAPK activation was evaluated by immunoblotting using antibodies against phospho-Thr202/Tyr204 Erk1,2, phospho-THr180/Tyr182 p38MAPK, or phospho-Thr183/Tyr185 JNK or the respective total proteins (Cell Signaling).
Statistical Analysis
All data are expressed as mean±SEM. Two-tailed Student's t test or ANOVA was used to assess differences between 2 groups or among more than 2 groups, respectively, with Newman-Keuls post-hoc testing following ANOVA. P values less than 0.05 were considered significant.
Supplementary Material
Highlights.
- 27HC promotes atherosclerosis and prevents estrogen-related atheroprotection.
- 27HC actions via ER increase leukocyte-endothelial cell adhesion in vivo in mice.
- The 27HC-ERα tandem has proinflammatory actions in macrophages.
- The 27HC-ERα tandem also has proinflammatory actions in endothelial cells.
Acknowledgements
This work was supported by National Institutes of Health grants HL087564 (P.W.S.), P03DK079328 (M.U.) and T32 HL098040 (P.G.), American Diabetes Association Grant 7-11-JF-46 (M.U.), and by the Associates First Capital Corporation Distinguished Chair in Pediatrics (P.W.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnal JF, Laurell H, Fontaine C, Billon A, Calippe B, Lenfant F, Gourdy P. Estrogen receptor actions on vascular biology and inflammation: implications in vascular pathophysiology. Climacteric. 12 Suppl. 2009;1:12–17. doi: 10.1080/13697130902820006. [DOI] [PubMed] [Google Scholar]
- Billon-Gales A, Fontaine C, Douin-Echinard V, Delpy L, Berges H, Calippe B, Lenfant F, Laurell H, Guery JC, Gourdy P, Arnal JF. Endothelial estrogen receptor-alpha plays a crucial role in the atheroprotective action of 17beta-estradiol in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:2567–2576. doi: 10.1161/CIRCULATIONAHA.109.898445. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Mangelsdorf DJ. Quantitative real-time PCR protocol for analysis of nuclear receptor signaling pathways. Nucl. Recept. Signal. 2003;1:e012. doi: 10.1621/nrs.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourassa PA, Milos PM, Gaynor BJ, Breslow JL, Aiello RJ. Estrogen reduces atherosclerotic lesion development in apolipoprotein E-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- Burkard I, von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis. 2007;194:71–78. doi: 10.1016/j.atherosclerosis.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J. Clin. Invest. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TB, Melendez GC, Appt SE. Timing hypothesis for postmenopausal hormone therapy: its origin, current status, and future. Menopause. 2013;20:342–353. doi: 10.1097/GME.0b013e3182843aad. [DOI] [PubMed] [Google Scholar]
- Crisby M, Nilsson J, Kostulas V, Bjorkhem I, Diczfalusy U. Localization of sterol 27-hydroxylase immuno-reactivity in human atherosclerotic plaques. Biochim. Biophys. Acta. 1997;1344:278–285. doi: 10.1016/s0005-2760(96)00152-x. [DOI] [PubMed] [Google Scholar]
- Deshpande R, Khalili H, Pergolizzi RG, Michael SD, Chang MD. Estradiol down-regulates LPS-induced cytokine production and NFkB activation in murine macrophages. Am. J. Reprod. Immunol. 1997;38:46–54. doi: 10.1111/j.1600-0897.1997.tb00275.x. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Imthurn B, Barton M, Jackson EK. Vascular consequences of menopause and hormone therapy: importance of timing of treatment and type of estrogen. Cardiovasc. Res. 2005;66:295–306. doi: 10.1016/j.cardiores.2004.12.012. [DOI] [PubMed] [Google Scholar]
- DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol. Endocrinol. 2008;22:65–77. doi: 10.1210/me.2007-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareus R, Kotsaki E, Xanthoulea S, van d.M., I, Gijbels MJ, Kardakaris R, Polykratis A, Kollias G, De Winther MP, Pasparakis M. Endothelial cell-specific NF-kappaB inhibition protects mice from atherosclerosis. Cell Metab. 2008;8:372–383. doi: 10.1016/j.cmet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol. Cell Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnish DC, Scicchitano MS, Adelman SJ, Lyttle CR, Karathanasis SK. The role of CBP in estrogen receptor cross-talk with nuclear factor-kappaB in HepG2 cells. Endocrinology. 2000;141:3403–3411. doi: 10.1210/endo.141.9.7646. [DOI] [PubMed] [Google Scholar]
- Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SS, Osborne TF. Liver x receptors in atherosclerosis and inflammation. Circ. Res. 2011;108:996–1001. doi: 10.1161/CIRCRESAHA.110.226878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Yuhanna IS, Umetani J, Lee WR, Korach KS, Shaul PW, Umetani M. LXRbeta/estrogen receptor-alpha signaling in lipid rafts preserves endothelial integrity. J. Clin. Invest. 2013;123:3488–3497. doi: 10.1172/JCI66533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta. 2005;1754:253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Karas RH. Current controversies regarding the cardiovascular effects of hormone therapy. Clin. Obstet. Gynecol. 2004;47:489–499. doi: 10.1097/00003081-200406000-00024. [DOI] [PubMed] [Google Scholar]
- Kawashiri M, Zhang Y, Usher D, Reilly M, Pure E, Rader DJ. Effects of coexpression of the LDL receptor and apoE on cholesterol metabolism and atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 2001;42:943–950. [PubMed] [Google Scholar]
- Larach DB, deGoma EM, Rader DJ. Targeting high density lipoproteins in the prevention of cardiovascular disease? Curr. Cardiol. Rep. 2012;14:684–691. doi: 10.1007/s11886-012-0317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol. Chem. 2000a;275:16536–16542. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J. Biol. Chem. 2000b;275:16536–16542. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Aikawa M, Jain MK. Vascular endothelium and atherosclerosis. Handb. Exp. Pharmacol. 285-306. 2006 doi: 10.1007/3-540-36028-x_9. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Manning AM. Multiple signals converging on NF-kappaB. Curr. Opin. Cell Biol. 1999;11:226–232. doi: 10.1016/s0955-0674(99)80030-1. [DOI] [PubMed] [Google Scholar]
- Michael DR, Ashlin TG, Buckley ML, Ramji DP. Liver X receptors, atherosclerosis and inflammation. Curr. Atheroscler. Rep. 2012;14:284–293. doi: 10.1007/s11883-012-0239-y. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofer JR. Estrogens and atherosclerosis: insights from animal models and cell systems. J. Mol. Endocrinol. 2012;48:R13–R29. doi: 10.1530/JME-11-0145. [DOI] [PubMed] [Google Scholar]
- Noursadeghi M, Tsang J, Haustein T, Miller RF, Chain BM, Katz DR. Quantitative imaging assay for NF-kappaB nuclear translocation in primary human macrophages. J. Immunol. Methods. 2008;329:194–200. doi: 10.1016/j.jim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan BA, Toth PP. Is the future of statins aligned with new novel lipid modulation therapies? Curr. Atheroscler. Rep. 2013;15:300. doi: 10.1007/s11883-012-0300-x. [DOI] [PubMed] [Google Scholar]
- Ramesh S, Morrell CN, Tarango C, Thomas GD, Yuhanna IS, Girardi G, Herz J, Urbanus RT, de Groot PG, Thorpe PE, Salmon JE, Shaul PW, Mineo C. Antiphospholipid antibodies promote leukocyte-endothelial cell adhesion and thrombosis in mice by antagonizing eNOS via beta2GPI and apoER2. J. Clin. Invest. 2011;121:120–131. doi: 10.1172/JCI39828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE, Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J. Biol. Chem. 1994;269:12940–12946. [PubMed] [Google Scholar]
- Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409:79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arterioscler. Thromb. 1994;14:141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor {alpha} deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proc. Natl. Acad. Sci. U. S. A. 2011;108:16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta. 2000a;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- Russell DW. Oxysterol biosynthetic enzymes. Biochim. Biophys. Acta. 2000b;1529:126–135. doi: 10.1016/s1388-1981(00)00142-6. [DOI] [PubMed] [Google Scholar]
- Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- Santen RJ, Allred DC, Ardoin SP, Archer DF, Boyd N, Braunstein GD, Burger HG, Colditz GA, Davis SR, Gambacciani M, Gower BA, Henderson VW, Jarjour WN, Karas RH, Kleerekoper M, Lobo RA, Manson JE, Marsden J, Martin KA, Martin L, Pinkerton JV, Rubinow DR, Teede H, Thiboutot DM, Utian WH. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2010;95:s1–s66. doi: 10.1210/jc.2009-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan CM, Carpenter KL, Cary NR. A potential role for sterol 27-hydroxylase in atherogenesis. Atherosclerosis. 2001;154:269–276. doi: 10.1016/s0021-9150(00)00473-1. [DOI] [PubMed] [Google Scholar]
- Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, De Caterina R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ. Res. 2000;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- Speir E, Yu ZX, Takeda K, Ferrans VJ, Cannon RO., III Competition for p300 regulates transcription by estrogen receptors and nuclear factor-kappaB in human coronary smooth muscle cells. Circ. Res. 2000;87:1006–1011. doi: 10.1161/01.res.87.11.1006. [DOI] [PubMed] [Google Scholar]
- Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol. Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr. Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS. Antagonists selective for estrogen receptor alpha. Endocrinology. 2002;143:941–947. doi: 10.1210/endo.143.3.8704. [DOI] [PubMed] [Google Scholar]
- Sun WH, Keller ET, Stebler BS, Ershler WB. Estrogen inhibits phorbol ester-induced I kappa B alpha transcription and protein degradation. Biochem. Biophys. Res. Commun. 1998;244:691–695. doi: 10.1006/bbrc.1998.8324. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Mangelsdorf DJ. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 2003;17:985–993. doi: 10.1210/me.2003-0061. [DOI] [PubMed] [Google Scholar]
- Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007;13:1185–1192. doi: 10.1038/nm1641. [DOI] [PubMed] [Google Scholar]
- Umetani M, Nakao H, Doi T, Iwasaki A, Ohtaka M, Nagoya T, Mataki C, Hamakubo T, Kodama T. A novel cell adhesion inhibitor, K-7174, reduces the endothelial VCAM-1 induction by inflammatory cytokines, acting through the regulation of GATA. Biochem. Biophys. Res. Commun. 2000;272:370–374. doi: 10.1006/bbrc.2000.2784. [DOI] [PubMed] [Google Scholar]
- Umetani M, Shaul PW. 27-Hydroxycholesterol: the first identified endogenous SERM. Trends Endocrinol. Metab. 2011;22:130–135. doi: 10.1016/j.tem.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran A, Repa JJ, Lobaccaro JM, Bronson A, Mangelsdorf DJ, Edwards PA. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J. Biol. Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- Wagner JD, Clarkson TB. The applicability of hormonal effects on atherosclerosis in animals to heart disease in postmenopausal women. Semin. Reprod. Med. 2005;23:149–156. doi: 10.1055/s-2005-869482. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Nigro P, World C, Fujiwara K, Yan C, Berk BC. Thioredoxin interacting protein promotes endothelial cell inflammation in response to disturbed flow by increasing leukocyte adhesion and repressing Kruppel-like factor 2. Circ. Res. 2012;110:560–568. doi: 10.1161/CIRCRESAHA.111.256362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA, Umetani M, Euhus DM, Xie Y, Shaul PW. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013;5:637–645. doi: 10.1016/j.celrep.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J. Biol. Chem. 2006;281:16625–16631. doi: 10.1074/jbc.M602723200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.