Abstract
Background
Elevated plasma fibrinogen associates with arterial thrombosis in humans and promotes thrombosis in mice by increasing fibrin formation and thrombus fibrin content. Fibrinogen is composed of six polypeptide chains: (Aα, Bβ, and γ)2. Alternative splicing of the γ chain leads to a dominant form (γA/γA) and a minor species (γA/γ’). Epidemiologic studies have detected elevated γA/γ’ fibrinogen in patients with arterial thrombosis, suggesting this isoform promotes thrombosis. However, in vitro data show that γA/γ’ is anticoagulant due to its ability to sequester thrombin, and suggest its expression is upregulated in response to inflammatory processes.
Objective
To determine whether γA/γ’ fibrinogen is prothrombotic in vivo.
Methods
We separated γA/γA and γA/γ’ fibrinogen from human plasma-purified fibrinogen and determined effects on in vitro plasma clot formation, and in vivo thrombus formation and circulating thrombin-antithrombin complexes in mice.
Results and Conclusions
Both γA/γA and γA/γ’ fibrinogen were cleaved by murine and human thrombin and were incorporated into murine and human clots. When γA/γA or γA/γ’ was spiked into plasma, γA/γA increased the fibrin formation rate to a greater extent than γA/γ’. In mice, compared to controls, γA/γA infusion shortened the time to carotid artery occlusion, whereas γA/γ’ infusion did not. Additionally, γA/γ’ infusion led to lower levels of plasma thrombin-antithrombin complexes following arterial injury, whereas γA/γA infusion did not. These data suggest that γA/γ’ binds thrombin in vivo, and decreases prothrombotic activity. Together, these findings indicate that elevated levels of γA/γA fibrinogen promote arterial thrombosis in vivo, whereas γA/γ’ does not.
Keywords: Fibrinogen, Thrombosis, Fibrin, Thrombin, Animal Models
INTRODUCTION
Fibrinogen is a 340 kDa glycoprotein that circulates in plasma at 2–4 mg/mL, but during acute inflammation can exceed 7 mg/mL. Fibrinogen is composed of two sets of three polypeptide chains: Aα, Bβ, and γ. Alternative splicing of the main γA chain leads to the γ’ chain. Molecules containing the γ’ chain circulate as a heterodimer with the γA chain (2Aα, 2Bβ, and γA/ γ’) and comprise 8–15% of total fibrinogen in healthy individuals [1, 2]. Elevated fibrinogen levels are associated with increased risk of arterial thrombosis [3–5], and we previously showed that when mice are infused with unfractionated human fibrinogen (~90% γA/γA and 10% γA/γ’) and subjected to FeCl3-mediated carotid artery injury, elevated plasma fibrinogen shortens the time to vessel occlusion [6]. These findings suggest elevated fibrinogen is a causative, etiologic agent in arterial thrombosis. However, the specific contributions of γA/γA and γA/γ’ fibrinogen isoforms to thrombosis in vivo are unknown.
In vitro studies to define the biochemical role of the γ’ chain have shown that clots made with purified γA/γ’ fibrinogen polymerize at a slower rate than clots made with purified γA/γA fibrinogen [7]. Additionally, the γ’ chain supports high affinity binding to thrombin exosite II [8, 9], and studies have shown that thrombin binding to the γ’ chain competitively inhibits thrombin-mediated platelet activation [10] and reduces thrombin-mediated FpB cleavage [7], and factor VIII [11] and V [12] activation. These properties suggest γA/γ’ fibrinogen has anticoagulant activity in vitro. Conversely, the γ’ chain does not inhibit thrombin-mediated cleavage of FpA [7, 13], and has been reported to support higher affinity binding of FXIII than the γA chain [14], although more recent studies suggest only slightly tighter [14], or even similar [15], binding of FXIII to the γA/γ’ isoform compared to the γA/γA isoform. Additional studies in purified systems report contradictory effects of the γ’ chain on clot structure and mechanical properties, demonstrating that the γ’ chain induces the formation of alternately smaller [7, 13, 16] or larger [17] pores, and stiffer [18] or less stiff [17] clots. These conflicting observations make it difficult to predict the role of γA/γ’ fibrinogen under physiologic conditions in thrombosis in vivo.
The role of the human γ’ chain in thrombosis has previously been tested in two in vivo studies. Since the murine γ’ chain does not contain the thrombin-binding sequence found on the human γ’ chain, Mossesson et al. developed a transgenic mouse that replaced the murine γ’ chain with the human γ’ chain [19]. Following electrolytic injury to the femoral vein, there was no difference in thrombus volume between mice containing the human γ’ chain and wild type (WT) controls, although the presence of the human γ’ chain reduced thrombus volume in mice that were also heterozygous for the factor V Leiden mutation [19]. However, interpretation of these findings is complicated by the higher total fibrinogen in WT mice compared to mice expressing the human γ’ chain. In a baboon model in which an arteriovenous shunt was placed between the femoral artery and vein, an 18 amino acid peptide mimicking the γ’ chain C-terminus (γ’ 410–427) inhibited fibrin-rich thrombus formation [11]. These studies suggest the γ’ chain reduces fibrin accumulation and is antithrombotic during venous thrombosis.
Given these findings, it is interesting that retrospective epidemiological studies have correlated elevated γA/γ’ fibrinogen levels with increased incidence of coronary artery disease [20], myocardial infarction [21], and stroke [22–24]. In particular, the finding that some patients have an increased γ’-to-total fibrinogen ratio [22–25] indicates γA/γ’ fibrinogen is not merely a biomarker of increased total fibrinogen, and suggests a specific role for γA/γ’ in arterial thrombosis. However, these studies do not and cannot demonstrate causality of γ’ chain-containing fibrinogen in thrombosis. The objective of our study was to determine the contribution of γA/γA and γA/γ’ fibrinogen to arterial thrombosis.
METHODS
Proteins and Materials
Polyclonal rabbit anti-human fibrinogen antibody was from DAKOCytomation (Carpinteria, CA). Monoclonal anti-fibrin(ogen) antibody (59D8) was a generous gift of Drs. Marschall Runge (University of North Carolina [UNC]), Charles Esmon (Oklahoma College of Medicine), and Rodney Camire (University of Pennsylvania). Mouse anti-human γ’ chain-specific antibody (2.G2.H9) was from Millipore (Temecula, CA). Biotinylated secondary antibodies were from Vector Laboratories (Burlingame, CA). The AlexaFluor-488 protein labeling kit and 10% pre-cast Tris-glycine gels were from Invitrogen (Carlsbad, CA). Human α-thrombin and murine thrombin were from Enzyme Research Laboratories (South Bend, IN). Lipidated tissue factor (TF, Innovin) was from Siemens (Newark, DE). Phospholipid vesicles (phosphatidylserine/phosphatidylcholine/phosphatidylethanolamine) were prepared as described [26]. Bovine serum albumin was from Sigma-Aldrich (St. Louis, MO). Peroxidase substrate was from KPL (Gaithersburg, MD).
Plasma preparation
Contact-inhibited human normal pooled plasma (hNPP) was prepared from 40 healthy subjects (50% female, 68% nonwhite) as described [27], in a protocol approved by the UNC Institutional Review Board. γA/γ’ fibrinogen levels in hNPP were measured by ELISA, as described [28]. Murine normal pooled plasma (mNPP) was prepared by collecting blood from 49 female C57Bl/6 mice by inferior vena cava (IVC) venipuncture into 3.2% sodium citrate (1:9 ratio sodium citrate:blood). Pooled whole blood was centrifuged (4000xg, 20 minutes), and platelet-poor plasma was aliquoted and frozen at −80°C.
Isolation of γA/γA and γA/γ’ fibrinogen
The γA/γA and γA/γ’ fibrinogen variants were separated from human plasminogen-, von Willebrand Factor-, and fibronectin-depleted human fibrinogen (Enzyme Research Laboratories Ltd., Swansea, UK), based on the method described previously [7]. After purification, variants were concentrated using Vivaspin 20 MWCO 100,000 columns (GE Healthcare, Uppsala, Sweden) and dialyzed into 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (pH 7.4) containing 150 mM NaCl (HBS). Fibrinogen concentration was determined by absorbance at 280 nm using an extinction coefficient of 1.51 mL/(mg/cm). Both variants were functionally active (>95%) in a standard clotability assay.
SDS-PAGE and western blotting
Fibrinogen preparations were assessed by 10% SDS-PAGE and Coomassie Brilliant Blue staining or western blotting for total fibrinogen or fibrinogen γ’ chain. For western blots, membranes were blocked with Tris-buffered saline with 1% Tween containing 5% milk, washed, and probed sequentially with mouse-anti human γ’-specific primary antibody and AlexaFluor-488 conjugated anti-mouse secondary antibody. Fluorescent signal was detected on a Typhoon 900 FLA fluorescent scanner.
Clot formation with purified fibrin(ogen)
Purified fibrinogen, thrombin, and CaCl2 (0.5 mg/mL, 5 nM, and 10 mM, final, respectively) were combined in 96-half-well plates and polymerization was monitored by turbidity at 405 nm using SpectraMax Plus340 plate reader (Molecular Devices, Sunnyvale, CA).
Clot formation in plasma
hNPP or mNPP was spiked with HBS (Control), or γA/γA or γA/γ’ fibrinogen, and clotting was initiated with TF (1:30,000 dilution of Innovin, final), 10 mM CaCl2, and 4 µM phospholipid vesicles in 96-well plates. Clot formation was monitored by turbidity at 405 nm.
Intravital microscopy
Procedures were approved by the UNC Institutional Animal Care and Use Committee. Laser-induced thrombosis to cremaster muscle venules was performed as described [29]. Briefly, 6–8 week old male C57Bl/6 mice (Charles River Laboratories, Wilmington, MA) were anesthetized and laser injuries were induced with an Ablate! photoablation system equipped with an attenuatable 532 nm pulse laser (Intelligent Imaging Innovations). Five minutes before injury, mice were injected via the retro-orbital plexus with AlexaFluor 595-labeled anti-glycoprotein IX antibody (0.3 mg/g body weight; Emfret, Eibelstadt, Germany), and AlexaFluor 647-labeled murine anti-fibrin antibody (0.2 mg/g body weight), and trace amounts (5% of total fibrinogen) of AlexaFluor 488-labeled γA/γA or γA/γ’ fibrinogen. Five venules maximum were studied per mouse.
FeCl3 thrombosis model
FeCl3 injury to carotid arteries was performed as described [6]. Briefly, 6–8 week old male C57Bl/6 mice were anesthetized, and human fibrinogen or vehicle (HBS) was administered through the left saphenous vein cannula on a per-weight basis 5 minutes before injury. The right common carotid artery was exposed, dried and treated with FeCl3 (10% on 0.5×1.0-mm filter paper) for 2 minutes. We specifically titrated the conditions to perform these experiments at a threshold at which some mice do not form thrombi, to allow for sensitivity to both increased and decreased procoagulant activity. Blood flow was monitored by Doppler ultrasonic flow probe, and the time to occlusion (TTO) was defined as the time between FeCl3 administration and lack of flow for 60 consecutive seconds, as previously described [6].
Measurement of circulating TAT complexes
TAT levels were measured by ELISA (Enzygnost TAT micro ELISA, Siemens) using plasma prepared from IVC blood draws from mice subject to FeCl3 carotid artery thrombosis. Samples showing hemolysis were excluded.
Statistical Methods
Descriptive statistics (mean, median, standard deviation [SD], standard error of the mean [SEM]) were calculated. Groups were compared using Student’s t-tests (normally-distributed data determined by Lilliefors test for normality) or Wilcoxon-Mann-Whitney Rank Sum Tests (non-normally distributed data) in Kaleidagraph v4.1.3. Correlations were performed using SAS 9.2 (SAS Inc., Cary, NC). P<0.05 was considered statistically significant.
RESULTS
γA/γA fibrinogen increases the fibrin polymerization rate to a greater extent than γA/γ’ fibrinogen
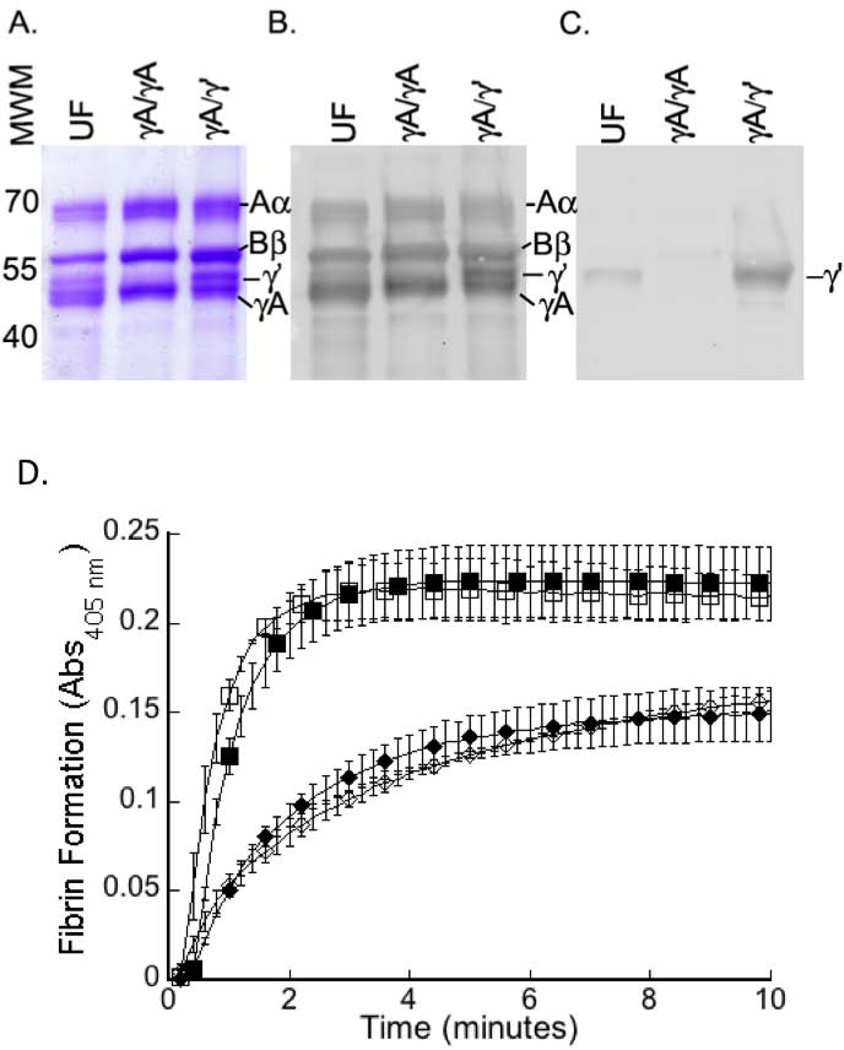
Purified γA/γA fibrinogen contained all three fibrinogen chains (Aα, Bβ, and γ) at expected molecular weights (Figures 1A-B). No γ’ chain was detected in γA/γA fibrinogen (Figure 1C), whereas purified γA/γ’ fibrinogen showed equal intensities of γA and γ’ bands (Figures 1A–B). We first clotted purified fibrinogens with purified human thrombin and followed clotting by turbidity. Although fibrinogen γA/γA and γA/γ’ isoforms were not explicitly depleted of FXIII, Allen et al. previously showed that the presence or absence of FXIII does not affect differences in polymerization between γA/γA and γA/γ’ fibrinogen [17]. Indeed, consistent with previous reports [7, 13, 17], purified γA/γA exhibited a faster polymerization rate (2.7-fold, P<0.05) and higher final turbidity (1.5-fold, P<0.05) than purified γA/γ’ (Figure 1D, Table 1). Findings were similar when murine thrombin was used (Figure 1D, Table 1), showing murine thrombin can convert human fibrinogen to fibrin.
Figure 1. Purified fibrinogen contains all three chains (Aα, Bβ, and γA and/or γ’) at the expected molecular weights and is equally cleaved by human and mouse thrombin.
Unfractionated (UF), or purified γA/γA, or γA/γ’ fibrinogen were reduced and separated by 10% SDS-PAGE and detected by: A) Coomassie Brilliant Blue staining, B) polyclonal anti-fibrin(ogen) antibody, or C) 2.G2.H9 antibody against the γ’ chain. D) Purified human γA/γA (squares) or γA/γ’ (diamonds) fibrinogen was clotted in the presence of CaCl2 and human (closed symbols) or murine (open symbols) thrombin. Data show mean±SD, for experiments with human (n=3) and mouse (n=2) thrombin.
Table 1.
Polymerization of Purified Fibrinogen Isoforms by Human and Murine Thrombin
| Human Thrombin | Murine Thrombin | |||||
|---|---|---|---|---|---|---|
| Lagtime (seconds) |
Change in Turbidity (OD) |
Vmax (mOD/min) |
Lagtime (seconds) |
Change in Turbidity (OD) |
Vmax (mOD/min) |
|
| γA/γA | 14.2±4.7 | 0.222±0.022 | 179.6±16.5 | 8.25±4.9 | 0.214±0.024 | 208.7±38.4 |
| γA/γ’ | 13.7±5.0 | 0.149±0.016# | 66.3±6.5# | 6.25±6.2 | 0.164±0.012# | 61.1±14.7# |
Mean±SD,
P<0.05 versus γA/γA
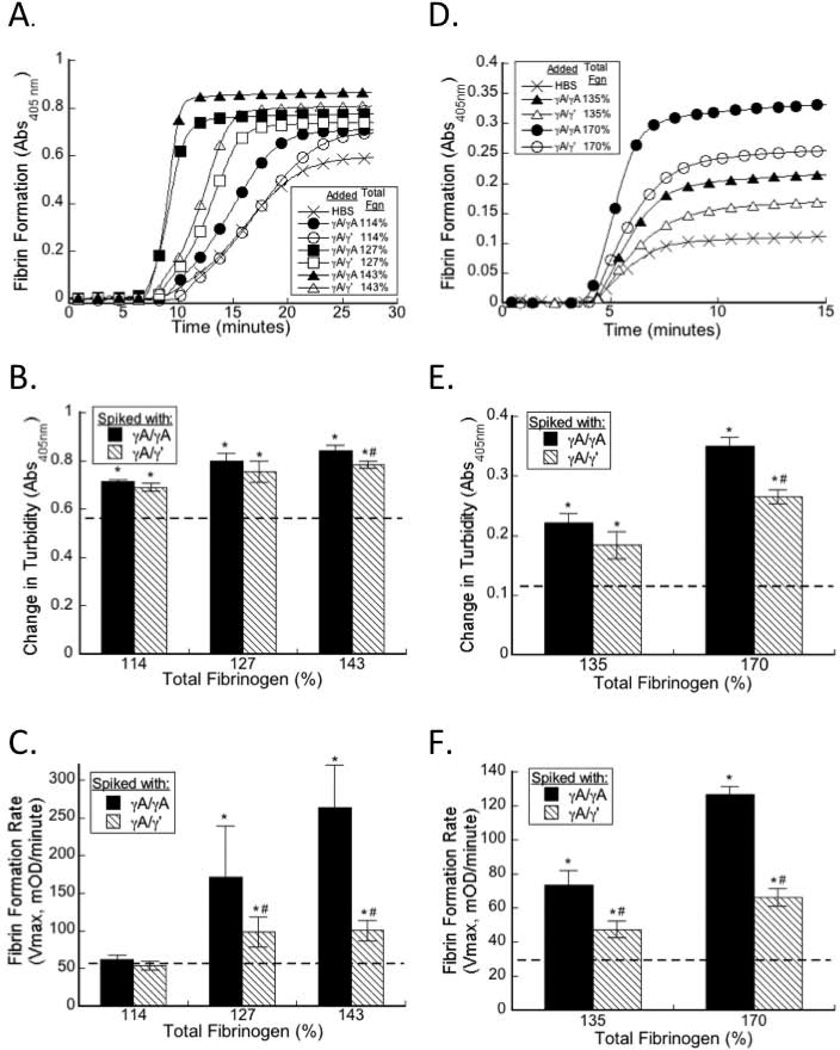
To determine the effect of elevated γA/γA and γA/γ’ fibrinogen on plasma clot formation during in situ thrombin generation, we spiked purified γA/γA, γA/γ’, or HBS (control) into hNPP. The concentration of fibrinogen in hNPP was 3.1±0.1 mg/mL (100%) and baseline concentration of γA/γ’ fibrinogen in hNPP was 0.42 mg/mL (13.5% of total fibrinogen). We increased the total fibrinogen concentration to 3.5 (114%), 3.9 (127%), or 4.4 (143%) mg/mL by spiking in purified γA/γA or γA/γ’, so that the γA/γ’-to-total fibrinogen ratios ranged from 9.6–40.1% (Table 2). These levels span the range of γA/γ’ levels measured in healthy individuals and patients with thrombosis [23–25, 30, 31]. Elevating either γA/γA or γA/γ’ fibrinogen increased final clot turbidity compared to plasma spiked with HBS (Figure 2B, Table 2). When total fibrinogen was raised to 114%, neither γA/γA nor γA/γ’ fibrinogen increased the clot formation rate. However, elevating total fibrinogen to 127% or 143% with γA/γA or γA/γ’ significantly and dose-dependently increased the clot formation rate versus baseline (HBS). Notably, at each concentration, elevating total fibrinogen with γA/γA increased the clot formation rate to a significantly greater extent than elevating total fibrinogen with γA/γ’ (Figure 2C, Table 2). Linear regression analysis showed that the clot formation rate correlated positively with elevated total fibrinogen (r=0.667, P<0.001) and negatively with the γ’-to-total fibrinogen ratio (r=−0.0245, P=0.17), although the relationship between γ’-to-total and clot formation rate did not reach significance. Moreover, the level of γA/γA isoform correlated strongly with the clot formation rate (r=0.795, P<0.001) whereas the level of γA/γ’ did not.
Table 2.
Effect of Fibrinogen Isoforms on Human Plasma Clotting
| Total Fibrinogen (mg/mL [%]) |
Fibrinogen/Buffer Infused |
Human γA/γ’ Final (mg/mL) |
Human γ’-to- Total Ratio (%) |
Lagtime (minutes) |
Change in Turbidity (OD) |
Vmax (mOD/min) |
|---|---|---|---|---|---|---|
| 3.1 (100%) | HBS | 0.4 | 13.5 | 9.7±3.0 | 0.587±0.034 | 54.8±9.3 |
| 3.5 (114%) | γA/γA | 0.4 | 11.9 | 9.7±1.5 | 0.715±0.007* | 62.5±5.7 |
| 3.5 (114%) | γA/γ’ | 0.8 | 23.9 | 8.7±0.6 | 0.690±0.016* | 54.3±5.8 |
| 3.9 (127%) | γA/γA | 0.4 | 10.7 | 8.7±1.9 | 0.789±0.032* | 171.7±67.1* |
| 3.9 (127%) | γA/γ’ | 1.3 | 32.0 | 10.0±3.4 | 0.755±0.043* | 98.2±19.9*,# |
| 4.4 (143%) | γA/γA | 0.4 | 9.6 | 8.5±1.1 | 0.844±0.022* | 263.9±56.6* |
| 4.4 (143%) | γA/γ’ | 1.8 | 40.1 | 10.1±13.5 | 0.784±0.016*,# | 100.4±13.5*,# |
Mean±SD,
P<0.05 versus HBS;
P<0.04 versus γA/γA (at same total fibrinogen)
Figure 2. Both γA/γA and γA/γ’ fibrinogen accelerate clotting in human and mouse plasma.
A–C) hNPP was spiked with γA/γA or γA/γ’ to increase total fibrinogen to 114%, 127%, or 143% of normal (symbols appear in figure legend), and clot formation was triggered by addition of TF and CaCl2. D-F) mNPP was spiked with human γA/γA or γA/γ’ to increase total fibrinogen to 135% or 170% of normal (symbols appear in figure legend) and clot formation was triggered by addition of TF and CaCl2. A, D) Polymerization was monitored by turbidity; for clarity, only a subset of points is shown. B, C, E, F) The contribution of increasing total fibrinogen with γA/γA (solid bars) or γA/γ’ (striped bars) on final turbidity (B, E) and fibrin formation rate (C, F) in human (B, C) and mouse (E, F) plasma. Dashed lines represent final turbidity and clot formation rate of HBS controls. Data show means, n=3. *p<0.05 versus HBS; #p<0.05 versus γA/γA.
Spiking purified human γA/γA, γA/γ’, or HBS (Control) into mNPP produced similar results. For these experiments, the fibrinogen concentration in mNPP was 2.4±0.2 mg/mL (100%), and we spiked mNPP to 3.2 (135%) and 4.1 mg/mL (170%) with γA/γA or γA/γ’, yielding human γ’-to total fibrinogen ratios ranging from 0–41.2%. Consistent with previous observations [6], the final turbidity of murine plasma clots was lower than that of human plasma clots, likely reflecting increased fibrin density of murine fibrin networks versus human networks (unpublished observation). As in human plasma, both γA/γA and γA/γ' increased the clot formation rate, but γA/γA increased the rate to a greater extent than γA/γ' at each concentration tested (P<0.02, Figure 2F, Table 3). These findings suggest that during in situ thrombin generation, both elevated γA/γA and γA/γ’ fibrinogen promote clot formation, but γA/γA does so to a greater extent.
Table 3.
Effect of Fibrinogen Isoforms on Mouse Plasma Clotting
| Total Fibrinogen (mg/mL [%]) |
Fibrinogen/Buffer Infused |
Human γA/γ’ Final (mg/mL) |
Human γ’-to- Total Ratio (%) |
Lagtime (minutes) |
Change in Turbidity (OD) |
Vmax (mOD/min) |
|---|---|---|---|---|---|---|
| 2.4 (100%) | HBS | 0 | 0 | 4.0±0.3 | 0.111±0.003 | 28.0±2.5 |
| 3.2 (135%) | γA/γA | 0 | 0 | 3.5±0.3 | 0.222±0.016* | 73.3±8.9* |
| 3.2 (135%) | γA/γ’ | 0.8 | 25.9 | 4.0±0.4 | 0.184±0.023* | 47.4±5.0*,# |
| 4.1 (170%) | γA/γA | 0 | 0 | 4.0±0.3 | 0.350±0.015* | 126.7±4.6* |
| 4.1 (170%) | γA/γ’ | 1.7 | 41.2 | 4.1±0.2 | 0.265±0.012*,# | 66.2±5.2*,# |
Mean±SD,
P<0.03 versus HBS;
P<0.02 versus γA/γA (at same total fibrinogen)
Both γA/γA and γA/γ’ fibrinogen are incorporated into murine thrombi in vivo
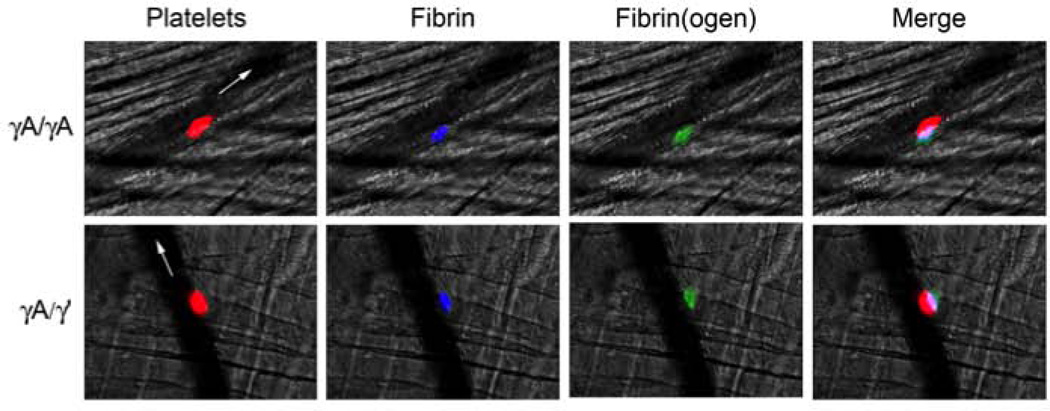
Drouet et al. previously suggested that an increased γ’-to-total fibrinogen ratio is detected in patient plasmas because γA/γA is incorporated into platelet thrombi, whereas γA/γ’ is not [25]. Therefore, we determined whether γA/γ’ was incorporated into thrombi in vivo. We infused mice with AlexaFluor 594-labeled anti-platelet (anti-GPIX) antibody, AlexaFluor 647-labeled antibody against fibrin(ogen) (59D8), and trace amounts (5% of total fibrinogen) of fluorescently-labeled γA/γA or γA/γ’ fibrinogen. We then triggered vascular injury to the cremaster vessels and detected γA/γA or γA/γ’ fibrinogen within thrombi using intravital microscopy. We initially performed this experiment with arterioles, but observed substantial vessel constriction in response to the injury. However, the venule provided a reasonable alternative that enabled us to avoid the issue of vasoconstriction while observing platelet and fibrin(ogen) accumulation at the injury site in vivo. Figure 3 shows that both γA/γA and γA/γ’ isoforms were incorporated into murine thrombi in vivo.
Figure 3. Intravital microscopy shows both γA/γA and γA/γ’ isoforms are incorporated into murine thrombi.
Venules were visualized in the cremaster muscle of mice infused with HBS (control) or AlexaFluor 594-labeled anti-platelet (anti-GPIX) antibody, AlexaFluor 647-labeled anti-fibrin antibody, and purified γA/γA or γA/γ’ directly labeled with AlexaFluor 488. Thrombosis was triggered via laser injury. Flow is indicated by white arrows. Colors are: platelets (red), fibrin(ogen) (green), and fibrin (blue). In the merged image, colors are: platelets plus fibrin(ogen) (pink), platelets plus fibrin (purple), and fibrin(ogen) plus fibrin (teal). Images show representative thrombi from 3–4 mice with 14–20 injuries total.
Following FeCl3 injury, γA/γA, but not γA/γ’, fibrinogen shortens the time to artery occlusion
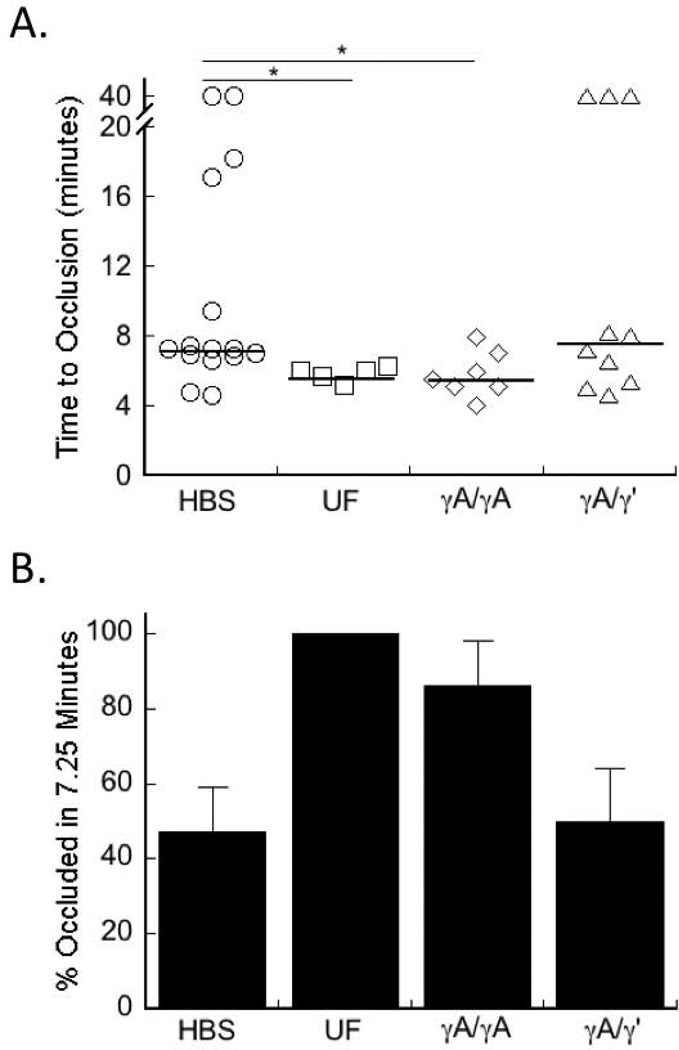
To determine the effect of elevated circulating γA/γA and γA/γ’ fibrinogen on arterial thrombosis, we infused mice with HBS or purified human γA/γA, γA/γ’, or unfractionated fibrinogen and induced thrombosis via FeCl3 application to the carotid artery. Both human and mouse fibrinogen can be cleaved by human and murine thrombin, cross-linked by factor XIIIa, and digested by plasmin [32]. Additionally, human fibrinogen circulates in mouse plasma, and is incorporated into murine thrombi (Figure 1D, [6, 33–35]). For these experiments, we obtained total fibrinogen levels of 135% and 170% of normal levels, with human-γ’-to-total fibrinogen ratios of 0%, 25.9%, and 41.2%, consistent with ratios found in normal and pathological conditions [23–25, 30, 31, 36].
Consistent with previous findings, following FeCl3 injury, there was no significant difference in TTO between control mice or mice infused to 135% mg/mL total fibrinogen with either γA/γA or γA/γ’ (data not shown) [6]. When total fibrinogen was raised to 170% with γA/γA fibrinogen, the median TTO was faster than that of mice infused with HBS (5.48±0.50 versus 7.25±3.03 minutes [median±SEM], P<0.05, Figure 4A), similar to that seen in mice infused with unfractionated fibrinogen. However, raising the level of fibrinogen to 170% with γA/γ’ fibrinogen did not shorten the median TTO compared to controls (Figure 4A). Moreover, 7.25 minutes after FeCl3 injury, 100% and 86% of mice infused with unfractionated or γA/γA fibrinogen, respectively, had an occluded vessel, whereas only 50% of mice infused with γA/γ’ fibrinogen developed vessel occlusion (Figure 4B). Together, these data indicate that elevated γA/γA fibrinogen promotes arterial thrombosis, whereas elevated γA/γ’ does not.
Figure 4. γA/ γA fibrinogen shortens the time to vessel occlusion after arterial injury, but γA/γ’ does not.
Mice were infused with HBS, unfractionated (UF), γA/γA, or γA/γ’ fibrinogen to 170%, total fibrinogen. Thrombosis was induced by FeCl3 application to the carotid artery and TTO was determined by Doppler flow probe. In vessels that did not occlude, the TTO was recorded as 40 minutes. A) Each point represents a separate mouse. Lines indicate median values, *p<0.05 versus HBS. B) Percent of mice occluded at 7.25 minutes (the median TTO of HBS-infused mice), using the data from (A); 100%, 86%, and 50% of UF-, γA/γA-, and γA/γ’-infused mice, respectively, had occluded vessels at this time.
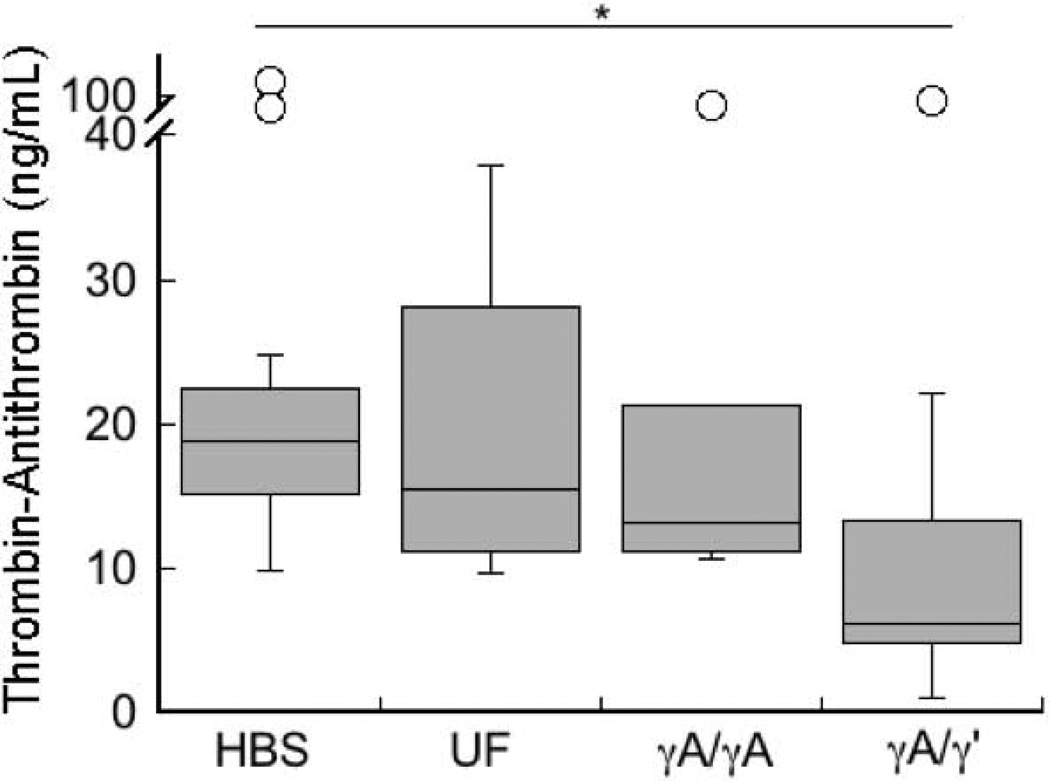
Following FeCl3 injury, mice infused with γA/γ’ fibrinogen have lower circulating TAT complexes than mice infused with γA/γA fibrinogen
The γ’ chain supports high affinity binding to thrombin exosite II [8, 9], and prior studies have shown that γA/γ’ fibrinogen has anticoagulant properties (antithrombin I activity) in vitro [10–12]. To determine the effect of γA/γ’ on procoagulant activity in vivo, we measured TAT complexes in murine plasma following FeCl3 injury and stable vessel occlusion. Whereas mice infused with unfractionated or γA/γA fibrinogen had similar circulating TAT complexes as HBS-infused mice, mice infused with γA/γ’ had significantly lower circulating TAT complexes (6.2±8.4 versus 18.9±10.9 ng/mL [median±SEM] for γA/γ’ and HBS-infused mice, respectively, P<0.01, Figure 5), consistent with the concept that thrombin binding to γA/γ’ fibrinogen sequesters thrombin [10–12, 37] and protects it from inhibition by antithrombin. These findings suggest γA/γ’ fibrinogen binds and sequesters thrombin in vivo and limits thrombin activity following vascular injury.
Figure 5. Following arterial injury, mice infused with γA/γ’ fibrinogen have reduced circulating TAT complexes.
TAT levels were measured in plasmas collected from mice subjected to the FeCl3 carotid artery thrombosis. Box plots indicate medians and upper and lower quartiles, *p<0.05 versus HBS.
DISCUSSION
Although epidemiologic studies have associated elevated plasma fibrinogen with arterial thrombosis [3–5], the operant pathogenic mechanisms have been controversial. We previously showed that increased total plasma fibrinogen directly promotes arterial thrombosis in mice [6]. Herein, we separately tested the role of γA/γA and γA/γ’ fibrinogen and show that both elevated γA/γA and γA/γ’ increased the plasma clot formation rate, but that γA/γA increased the rate to a greater extent than γA/γ’. Although both γA/γA and γA/γ’ fibrinogen were incorporated into murine clots, γA/γA fibrinogen shortened the TTO, whereas γA/γ’ did not. Interestingly, compared to controls, mice infused with γA/γ’ fibrinogen had lower levels of circulating plasma TAT complexes following arterial injury, whereas mice infused with γA/γA did not, suggesting that γA/γ’ fibrinogen binds and sequesters thrombin in vivo. Together, our data indicate that γA/γ’ fibrinogen is not prothrombotic in vivo and may even have a protective role in preventing elevated total fibrinogen levels from promoting thrombosis.
Our data support the premise that γA/γ’ fibrinogen has both procoagulant and anticoagulant properties and exhibits both of these activities during thrombosis in vivo. Similar to γA/γA fibrinogen, γA/γ’ increased the fibrin formation rate and final turbidity, though to a lesser extent than γA/γA. Consequently, increased total fibrinogen levels, via either increased γA/γA or γA/γ’, would be expected to promote fibrin formation. However, unlike γA/γA, γA/γ’ fibrinogen exhibits antithrombin I activity in vitro [10–12, 37] and in vivo (Figure 5). Thus, our finding that elevated γA/γA fibrinogen shortened the TTO, but elevated γA/γ’ did not, suggests that the net effect of γA/γ’ fibrinogen’s opposing procoagulant and anticoagulant activities yielded no change in the TTO. These data suggest that a peptide representing the C-terminus of the γ’ chain would have strong anticoagulant effects in vivo, since the procoagulant properties of the full length fibrinogen molecule would not be present, whereas the thrombin binding properties of the γ’ chain would decrease circulating thrombin. Indeed, this effect was previously demonstrated during in vivo thrombosis, in which Lovely et al. saw decreased platelet and fibrin accumulation in the presence of γ’ chain peptide [11].
Although previous studies have compared isolated γA/γA and γA/γ’ fibrinogens in purified systems, only one has done so during in situ thrombin generation in plasma. Using plasmas from apparently healthy Black South Africans, Pieters et al. correlated total fibrinogen levels, γA/γ’ fibrinogen levels, and the γ’-to-total fibrinogen ratio with the plasma clot formation rate and turbidity change [38]. Their data suggest that the clot formation rate increases with total fibrinogen, but decreases with elevated γ’-to-total fibrinogen ratio. Our data extend these findings in a system that enabled us to precisely control fibrinogen isoform levels and avoid variability between donor plasmas. Consistent with Pieters et al., we found the clot formation rate correlated positively with elevated total fibrinogen. Importantly, the level of γA/γA isoform correlated strongly with the clot formation rate, whereas the level of γA/γ’ did not, suggesting the increase in clot formation rate caused by elevated total fibrinogen is due to γA/γA fibrinogen.
Two prior studies evaluated the effect of the γ’ chain on thrombosis in vivo. Those studies were limited by differences in the total fibrinogen level expressed by WT and human γ’-expressing mice [19] and use of isolated γ’ peptide rather than full length γA/γ’ fibrinogen [11]. Moreover, Mosesson et al. [19] evaluated γA/γ’ fibrinogen in a venous thrombosis model, and although the arteriovenous shunt model used by Lovely et al. [11] included aspects of arterial thrombosis, it did not recapitulate endothelial denudation and subendothelial exposure associated with plaque rupture and arterial thrombus formation. Consequently, our study supports and extends the prior findings in several important ways. First, our infusion strategy enabled us to tightly-control the level of circulating γA/γA and γA/γ’ fibrinogen, allowing us to specifically attribute effects to the levels of isoform and total fibrinogen. Second, our study demonstrated the antithrombin I properties of the full-length form of the γ’ chain. Third, our findings extend previous data from venous thrombosis to arterial pathology. This extension is important since the role of γA/γ’ in arterial thrombosis has been controversial. Our findings provide important evidence that γA/γA fibrinogen is causative in the etiology of arterial thrombosis, whereas γA/γ’ fibrinogen is not.
Given our findings showing that γA/γ’ fibrinogen does not promote arterial thrombosis, it remains unclear why epidemiological studies find a positive association between elevated γA/γ’ fibrinogen and arterial thrombosis. Previous studies have suggested that clots formed from γA/γ’ fibrinogen are more resistant to lysis, and conflicting studies report abnormal structure and mechanical stability in γ’-chain containing clots [7, 17, 18]. Thus, γA/γ’ fibrinogen may produce clots with increased stability that are detected because they persist longer than clots that contain γA/γA. Interestingly, hypofibrinolysis is correlated with increased risk of arterial thrombosis in young (<~50) [39, 40], but not older (>~50) individuals [41, 42], suggesting abnormal clot stability explains some, but not all, of the mechanisms leading to arterial thrombosis. Future studies are warranted to determine the effect of the γA/γ’ isoform on arterial clot stability.
Interestingly, Rein-Smith et al. recently showed interleukin-6 preferentially up-regulates hepatocyte production of γA/γ’ fibrinogen compared to γA/γA [43]. These data suggest γA/γ’ (“antithrombin I”) expression is upregulated to limit endogenous procoagulant activity triggered by inflammation. Indeed, C-reactive protein is elevated in patients with a history of arterial thrombosis [23], reflecting the proinflammatory pathology. Increased γA/γ’ levels detected in patients after arterial thrombosis are likely a consequence of disease rather than cause, and reflect an innate, antithrombotic response to inflammation. Although our fibrinogen infusion/acute thrombosis model enabled us to isolate and investigate the immediate, direct effects of elevated γA/γA and γA/γ’ on thrombus formation, it did not recapitulate the inflammatory process associated with atherosclerosis. Consequently, long-term exposure to circulating γA/γ’ fibrinogen may have additional effects on plaque formation and/or stability. Notably, however, Mosesson et al. did not report evidence of chronic inflammation or atherosclerosis in their model of chronically-elevated fibrinogen γ’ levels [19] suggesting even chronic exposure to elevated γA/γ’ fibrinogen levels does not cause thrombosis.
In summary, our results show that both γA/γA and γA/γ’ fibrinogen increased the fibrin formation rate in plasma, but γA/γA fibrinogen accelerated the rate to a greater extent than γA/γ’ fibrinogen. After arterial injury, γA/γA fibrinogen promoted thrombosis, whereas γA/γ’ did not. Mice infused with γA/γ’ had lower levels of circulating TAT complexes, suggesting that following vascular injury, γA/γ’ fibrinogen binds thrombin in vivo and limits thrombin activity. Our data establish independent roles of fibrinogen γA/γA and γA/γ’ in arterial thrombosis, and suggest γA/γA fibrinogen promotes thrombosis, whereas γA/γ’ sequesters thrombin and protects against procoagulant processes induced by inflammation.
ACKNOWLEDGEMENTS
The authors thank Dr. Dougald M. Monroe and Leana LeFrapper for phospholipid vesicle preparations.
This study was supported by research funding from the NIH (R01HL094740 to A. S. Wolberg, R01HL094594 to W. Bergmeier, and T32HL069768 to the University of North Carolina and B. L. Walton), American Heart Association (12GRNT11840006 to A. S. Wolberg), and the Howard Hughes Medical Institute (#56005708 to the UNC Program in Translational Medicine and B. L. Walton).
Footnotes
CONTRIBUTIONS
B. L. Walton designed and performed experiments, analyzed results and wrote the manuscript. T. M. Getz performed experiments and reviewed the manuscript. W. Bergmeier supervised experiments and reviewed the manuscript. F.-C. Lin performed statistical analysis and reviewed the manuscript. S. Uitte de Willige provided important material and reviewed the manuscript. A. S. Wolberg supervised experiments and wrote the manuscript.
DISCLOSURE OF CONFLICT OF INTERESTS
REFERENCES
- 1.Francis CW, Marder VJ, Martin SE. Demonstration of a large molecular weight variant of the gamma chain of normal human plasma fibrinogen. J Biol Chem. 1980;255:5599–5604. [PubMed] [Google Scholar]
- 2.Wolfenstein-Todel C, Mosesson MW. Human plasma fibrinogen heterogeneity: evidence for an extended carboxyl-terminal sequence in a normal gamma chain variant (gamma') Proc Natl Acad Sci U S A. 1980;77:5069–5073. doi: 10.1073/pnas.77.9.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 4.Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G. Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med. 1984;311:501–505. doi: 10.1056/NEJM198408233110804. [DOI] [PubMed] [Google Scholar]
- 5.Kannel WB, Wolf PA, Castelli WP, D'Agostino RB. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258:1183–1186. [PubMed] [Google Scholar]
- 6.Machlus KR, Cardenas JC, Church FC, Wolberg AS. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood. 2011;117:4953–4963. doi: 10.1182/blood-2010-11-316885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper AV, Standeven KF, Ariens RA. Fibrinogen gamma-chain splice variant gamma' alters fibrin formation and structure. Blood. 2003;102:535–540. doi: 10.1182/blood-2002-10-3150. [DOI] [PubMed] [Google Scholar]
- 8.Meh DA, Siebenlist KR, Brennan SO, Holyst T, Mosesson MW. The amino acid sequence in fibrin responsible for high affinity thrombin binding. Thromb Haemost. 2001;85:470–474. [PubMed] [Google Scholar]
- 9.Fredenburgh JC, Stafford AR, Leslie BA, Weitz JI. Bivalent binding to gammaA/gamma'-fibrin engages both exosites of thrombin and protects it from inhibition by the antithrombin-heparin complex. J Biol Chem. 2008;283:2470–2477. doi: 10.1074/jbc.M707710200. [DOI] [PubMed] [Google Scholar]
- 10.Lovely RS, Rein CM, White TC, Jouihan SA, Boshkov LK, Bakke AC, McCarty OJ, Farrell DH. gammaA/gamma' fibrinogen inhibits thrombin-induced platelet aggregation. Thromb Haemost. 2008;100:837–846. [PubMed] [Google Scholar]
- 11.Lovely RS, Boshkov LK, Marzec UM, Hanson SR, Farrell DH. Fibrinogen gamma' chain carboxy terminal peptide selectively inhibits the intrinsic coagulation pathway. Br J Haematol. 2007;139:494–503. doi: 10.1111/j.1365-2141.2007.06825.x. [DOI] [PubMed] [Google Scholar]
- 12.Omarova F, Uitte de Willige S, Ariens RA, Rosing J, Bertina RM, Castoldi E. Inhibition of thrombin-mediated factor V activation contributes to the anticoagulant activity of fibrinogen gamma'. J Thromb Haemost. 2013;9:1669–1678. doi: 10.1111/jth.12354. [DOI] [PubMed] [Google Scholar]
- 13.Gersh KC, Nagaswami C, Weisel JW, Lord ST. The presence of gamma' chain impairs fibrin polymerization. Thromb Res. 2009;124:356–363. doi: 10.1016/j.thromres.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moaddel M, Farrell DH, Daugherty MA, Fried MG. Interactions of human fibrinogens with factor XIII: roles of calcium and the gamma' peptide. Biochemistry. 2000;39:6698–6705. doi: 10.1021/bi000098u. [DOI] [PubMed] [Google Scholar]
- 15.Gersh KC, Lord ST. An Investigation of Factor XIII binding to recombinant g'/g' and gA/g' fibrinogen. Blood. 2006;108:1705. [Google Scholar]
- 16.Siebenlist KR, Mosesson MW, Hernandez I, Bush LA, Di Cera E, Shainoff JR, Di Orio JP, Stojanovic L. Studies on the basis for the properties of fibrin produced from fibrinogen-containing gamma' chains. Blood. 2005;106:2730–2736. doi: 10.1182/blood-2005-01-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allan P, Uitte de Willige S, Abou-Saleh RH, Connell SD, Ariens RA. Evidence that fibrinogen gamma' directly interferes with protofibril growth: implications for fibrin structure and clot stiffness. J Thromb Haemost. 2012;10:1072–1080. doi: 10.1111/j.1538-7836.2012.04717.x. [DOI] [PubMed] [Google Scholar]
- 18.Collet JP, Nagaswami C, Farrell DH, Montalescot G, Weisel JW. Influence of gamma' fibrinogen splice variant on fibrin physical properties and fibrinolysis rate. Arterioscler Thromb Vasc Biol. 2004;24:382–386. doi: 10.1161/01.ATV.0000109748.77727.3e. [DOI] [PubMed] [Google Scholar]
- 19.Mosesson MW, Cooley BC, Hernandez I, Diorio JP, Weiler H. Thrombosis risk modification in transgenic mice containing the human fibrinogen thrombin-binding gamma' chain sequence. J Thromb Haemost. 2009;7:102–110. doi: 10.1111/j.1538-7836.2008.03213.x. [DOI] [PubMed] [Google Scholar]
- 20.Lovely RS, Falls LA, Al-Mondhiry HA, Chambers CE, Sexton GJ, Ni H, Farrell DH. Association of gammaA/gamma' fibrinogen levels and coronary artery disease. Thromb Haemost. 2002;88:26–31. [PubMed] [Google Scholar]
- 21.Mannila MN, Lovely RS, Kazmierczak SC, Eriksson P, Samnegard A, Farrell DH, Hamsten A, Silveira A. Elevated plasma fibrinogen gamma' concentration is associated with myocardial infarction: effects of variation in fibrinogen genes and environmental factors. J Thromb Haemost. 2007;5:766–773. doi: 10.1111/j.1538-7836.2007.02406.x. [DOI] [PubMed] [Google Scholar]
- 22.Cheung EY, Uitte de Willige S, Vos HL, Leebeek FW, Dippel DW, Bertina RM, de Maat MP. Fibrinogen gamma' in ischemic stroke: a case-control study. Stroke. 2008;39:1033–1035. doi: 10.1161/STROKEAHA.107.495499. [DOI] [PubMed] [Google Scholar]
- 23.Cheung EY, Vos HL, Kruip MJ, den Hertog HM, Jukema JW, de Maat MP. Elevated fibrinogen gamma' ratio is associated with cardiovascular diseases and acute phase reaction but not with clinical outcome. Blood. 2009;114:4603–4604. doi: 10.1182/blood-2009-08-236240. [DOI] [PubMed] [Google Scholar]
- 24.van den Herik EG, Cheung EY, de Lau LM. gamma'/total fibrinogen ratio is associated with short-term outcome in ischaemic stroke. Thromb Haemonst. 2011;105:430–434. doi: 10.1160/TH10-09-0569. [DOI] [PubMed] [Google Scholar]
- 25.Drouet L, Paolucci F, Pasqualini N, Laprade M, Ripoll L, Mazoyer E, Bal dit Sollier C, Vanhove N. Plasma gamma'/gamma fibrinogen ratio, a marker of arterial thrombotic activity: a new potential cardiovascular risk factor? Blood Coagul Fibrinolysis. 1999;10(Suppl 1):S35–S39. [PubMed] [Google Scholar]
- 26.Hope MJ, Bally MB, Webb G, Cullis PR. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim Biophys Acta. 1985;812:55–65. doi: 10.1016/0005-2736(85)90521-8. [DOI] [PubMed] [Google Scholar]
- 27.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114:4886–4896. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovely RS, Kazmierczak SC, Massaro JM, D'Agostino RB, Sr, O'Donnell CJ, Farrell DH. Gamma' fibrinogen: evaluation of a new assay for study of associations with cardiovascular disease. Clin Chem. 2010;56:781–788. doi: 10.1373/clinchem.2009.138347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stolla M, Stefanini L, Roden RC, Chavez M, Hirsch J, Greene T, Ouellette TD, Maloney SF, Diamond SL, Poncz M, Woulfe DS, Bergmeier W. The kinetics of alphaIIbbeta3 activation determines the size and stability of thrombi in mice: implications for antiplatelet therapy. Blood. 2011;117:1005–1013. doi: 10.1182/blood-2010-07-297713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander KS, Madden TE, Farrell DH. Association between gamma' fibrinogen levels and inflammation. Thromb Haemost. 2011;105:605–609. doi: 10.1160/TH10-09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Herik EG, Cheung EY, de Lau LM, den Hertog HM, Leebeek FW, Dippel DW, Koudstaal PJ, de Maat MP. Fibrinogen gamma' levels in patients with intracerebral hemorrhage. Thromb Res. 2012;129:807–809. doi: 10.1016/j.thromres.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 32.Lijnen HR, van Hoef B, Beelen V, Collen D. Characterization of the murine plasma fibrinolytic system. Eur J Biochem. 1994;224:863–871. doi: 10.1111/j.1432-1033.1994.00863.x. [DOI] [PubMed] [Google Scholar]
- 33.Krystofiak E, Oliver J. Human fibrinogen supports normal hemostatic function in a mouse platelet system. J Thromb Haemost. 2009;7(suppl 2):PP-MO-039. [Google Scholar]
- 34.Krohn KA, DeNardo SJ, Wheeler DW, DeNardo GL. I-fibrinogen as an oncophilic radiodiagnostic agent: distribution kinetics in tumour-bearing mice. Br J Cancer. 1977;36:227–234. doi: 10.1038/bjc.1977.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jirouskova M, Smyth SS, Kudryk B, Coller BS. A hamster antibody to the mouse fibrinogen gamma chain inhibits platelet-fibrinogen interactions and FXIIIa-mediated fibrin cross-linking, and facilitates thrombolysis. Thromb Haemost. 2001;86:1047–1056. [PubMed] [Google Scholar]
- 36.Farrell DH. Pathophysiologic roles of the fibrinogen gamma chain. Curr Opin Hematol. 2004;11:151–155. doi: 10.1097/01.moh.0000131440.02397.a4. [DOI] [PubMed] [Google Scholar]
- 37.Mosesson MW. Antithrombin I. Inhibition of thrombin generation in plasma by fibrin formation. Thromb Haemost. 2003;89:9–12. [PubMed] [Google Scholar]
- 38.Pieters M, Kotze RC, Jerling JC, Kruger A, Ariens RA. Evidence that fibrinogen gamma' regulates plasma clot structure and lysis and relationship to cardiovascular risk factors in black Africans. Blood. 2013;121:3254–3260. doi: 10.1182/blood-2012-12-471482. [DOI] [PubMed] [Google Scholar]
- 39.Guimaraes AH, de Bruijne EL, Lisman T, Dippel DW, Deckers JW, Poldermans D, Rijken DC, Leebeek FW. Hypofibrinolysis is a risk factor for arterial thrombosis at young age. Br J Haematol. 2009;145:115–120. doi: 10.1111/j.1365-2141.2008.07568.x. [DOI] [PubMed] [Google Scholar]
- 40.Zorio E, Castello R, Falco C, Espana F, Osa A, Almenar L, Aznar J, Estelles A. Thrombin-activatable fibrinolysis inhibitor in young patients with myocardial infarction and its relationship with the fibrinolytic function and the protein C system. Br J Haematol. 2003;122:958–965. doi: 10.1046/j.1365-2141.2003.04549.x. [DOI] [PubMed] [Google Scholar]
- 41.Meltzer ME, Doggen CJ, de Groot PG, Rosendaal FR, Lisman T. Reduced plasma fibrinolytic capacity as a potential risk factor for a first myocardial infarction in young men. Br J Haematol. 2009;145:121–7. doi: 10.1111/j.1365-2141.2008.07569.x. [DOI] [PubMed] [Google Scholar]
- 42.Meade TW, Ruddock V, Stirling Y, Chakrabarti R, Miller GJ. Fibrinolytic activity, clotting factors, and long-term incidence of ischaemic heart disease in the Northwick Park Heart Study. Lancet. 1993;342:1076–1079. doi: 10.1016/0140-6736(93)92062-x. [DOI] [PubMed] [Google Scholar]
- 43.Rein-Smith CM, Anderson NW, Farrell DH. Differential regulation of fibrinogen gamma chain splice isoforms by interleukin-6. Thromb Res. 2013;131:89–93. doi: 10.1016/j.thromres.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]