Abstract
Hypomethylating agents such as 5-azacytidine or decitabine have been a major breakthrough in the treatment of patients with myelodysplastic syndromes (MDS). They have been shown to improve transfusion requirements and change the natural history of the disease. However, with increasing cumulative clinical experience, it has become apparent that these agents are not curative and have their own shortcomings. There are a subgroup of patients who do not respond to frontline therapy and a large, growing cohort of patients that lose response or progress while on hypomethylating agent-based therapy. There are no standard treatment options in this arena and is therefore a focus of significant research interest. Since the mechanisms of resistance to hypomethylating agents are not known, selection of therapy is largely empiric, but must take into account the age, comorbidities, and performance status of the patient as well as the characteristics of the disease at the time treatment failure. Higher intensity approaches and allogeneic stem cell transplant can yield high response rates and long term disease control, but should be limited to a selected cohort of patients who can tolerate the treatment related morbidities. For the majority of patients who will likely be better candidates for lower intensity therapy, several novel, investigational approaches are becoming available. Among these include newer nucleoside analogues, inhibitors of protein tyrosine kinases, molecules that interact with redox signaling within the cell, immunotherapy approaches, and others. Patients with MDS whose disease has failed hypomethylating agent therapy should be referred for clinical trials when available. As we learn more about the patterns and mechanisms of failure, the next challenge will be determining which therapies would be suitable for each individual patient.
Keywords: decitabine, 5-azacytidine, MDS
Introduction
Myelodysplastic syndromes (MDS) are a heterogeneous group of malignant clonal disorders that are characterized by ineffective hematopoiesis, morphologic dysplasia in the bone marrow, cytopenias, and the increased potential to transform into acute myeloid leukemia. MDS is a generally a disease of older adults, with a median age at diagnosis of about 70 years.1–4 MDS remains a lethal disease, with allogeneic bone marrow transplantation being the only potentially curative therapy, a therapy that is difficult to deliver in an elderly population with age-related comorbidities. Until recently, there has been no effective, MDS-specific therapy other than supportive care and growth factor supplementation. However, the recent approval of agents such as lenalidomide5,6, 5-azacytidine7, and decitabine8 confirm their proven activity in MDS and the potential to change the natural history of this disease.
Two of these drugs, 5-azacytidine (5-aza) and decitabine are both DNA-methyltransferase inhibitors or so-called “hypomethylating” agents at low doses and represent among the first examples of epigenetic therapy in cancer. Both are widely used currently in the treatment of MDS and are generally well tolerated. Recent data demonstrating an overall survival (OS) benefit for patients treated with 5-aza suggest that this approach may be changing the natural history of the disease.9 However, there are still a significant proportion of patients with MDS who do not respond to therapy with hypomethylating agents and patients who lose response or progress on therapy. Since there are limited therapeutic options in this setting, this represents a challenging cohort of patients and constitutes important area of research.
In this review, we will briefly summarize the treatment of MDS with hypomethylating agents, including data from clinical trials of 5-aza and decitabine. A more detailed review of these agents is available in other sections of this issue. We will then review the patterns of failure to hypomethylating agent-based therapy and highlight some of the challenges in treating these patients. Finally, we will explore some of the newer therapies and strategies that are being studied in this population.
5-azacytidine
5-aza is a nucleoside analog of the naturally occurring cytidine in which the 5-carbon of the pyrimidine ring is replaced with a nitrogen. Since it is a ribonucleoside, it becomes phosphorylated and incorporated into newly created strands of RNA.7 There, it can disrupt RNA metabolism and protein synthesis, leading to cytotoxicity. However, 5-aza is also a substrate of ribonucleotide reductase, which converts it into a dexoribonucleoside. This can then become phosphorylated and incorporated into DNA – leading to inhibition of DNA synthesis or inhibition of DNMT1 (DNA methyltransferase −1) leading to cytotoxicity or hypomethylation, respectively.7
Initial studies in MDS were conducted by the Cancer and Leukemia Group B (CALGB) and demonstrated the safety and significant clinical activity of intravenous (IV) or subcutaneous (SQ) administration of 5-aza. After demonstrating efficacy in 2 single arm trials (CALGB 8421, 8921) a randomized controlled phase III multicenter clinical trial was conducted comparing 5-aza treatment to best supportive care.10,11 The trial enrolled 191 patients with MDS who had cytopenias or transfusion requirement. The patients were randomized to either 5-aza given at a dose of 75 mg/m2/d SQ daily for 7 days every 28 days vs. best supportive care, with the primary efficacy endpoint of overall response (OR). The trial was designed to allow for patients to crossover from the observation arm to treatment with 5-aza if they met prespecified criteria of disease progression. The study demonstrated an OR rate (ORR) of 60% in the treatment arm (including 7% complete remission [CR], 16% partial remission [PR], and 37% hematological improvement [HI]) compared to an ORR of 5% (no CR or PR) in the supportive arm. There was also an improved time to leukemic transformation or death in the treatment arm, as well as a suggestion of possible survival benefit. Given the cross over design of the trial and lack of power, a statistically significant survival benefit was not shown. This study (CALBG 9221) led to the FDA approval of 5-aza for the treatment of MDS in the U.S.10
A second, large randomized trial was performed to study the effect of 5-aza on overall survival.9 This was a Phase III international, multicenter, randomized prospective trial designed to demonstrate the superiority of 5-aza + best supportive care (BSC) in prolonging overall survival compared to so called “conventional care regimens” (CCR) + BSC. Conventional care regimens were defined as: 1) best supportive care, 2) low dose ara-C (LDAC, 20mg/m2/d × 14d, q28d), or 3) standard chemotherapy including induction/consolidation. Investigators preselected patients with high or int-2 risk patients into one of the 3 CCRs prior to them being randomized. The trial did not allow use of erythropoietin and analysis was by intent to treat principle. A total of 358 patients with higher risk MDS were randomized between the two arms. The 5-aza was well tolerated among the treated patients and its safety profile was consistent with previous reports. Most notably, the study demonstrated a significant survival benefit for patients in the 5-aza arm compared to those in the CCR arm (HR 0.58; 95% CI: 0.43, 0.77). The Kaplan-Meier estimated overall survival was 24.4 months for 5-aza compared to 15 months with CCR (p=0.0001). The 2 year probability of survival was 50.8% vs 26.2% (p<0.0001) in favor of the azacytidine arm.9 This trial confirms previous observations of 5-aza in MDS and establishes its role as the standard of care in this population.
Decitabine
Decitabine (5-aza-2’-deoxycytidine), much like 5-aza, is a nucleoside analog of cytidine in which the 5 –carbon of the pyrimidine ring is replaced by a nitrogen. The difference lies in the carbohydrate backbone, which is a deoxyribose sugar in decitabine – allowing it become incorporated directly into DNA after being phosphorylated by cellular kinases.12 There is no requirement for conversion by ribonucleotide reductase and decitabine is not incorporated into RNA. Once incorporated into DNA, decitabine covalently binds to DNA-methyltransferase and traps it, leading to its irreversible inhibition.12 At high doses, decitabine causes DNA crosslinking and DNA synthesis arrest, emulating a cytotoxic agent. However, at lower doses, the cell survives with depleted levels of DNA-methyltransferase and consequent DNA hypomethylation.13
Following promising phase I and II clinical trial data demonstrating significant activity of low dose decitabine in MDS, a multi-center randomized phase III trial of decitabine vs. best supportive care was conducted in the U.S.8 In this trial, decitabine was given at a dose of 15 mg/m2 IV over 3 hours, every 8 hours daily for 3 consecutive days once every 6 weeks. Supportive care, including transfusions, antibiotics, and hematopoietic growth factors, was provided according to generally accepted guidelines to patients on both arms. A total of 170 patients representing all FAB (French American British classification) subtypes of MDS were enrolled on the trial – 89 randomized to decitabine, and 81 to supportive care alone. The ORR of patients on the decitabine arm was 17% (15/89) including 8 CRs (9%) and 7 PRs (8%). The supportive care arm had no responses. The time to first response was 3.3 months (2 – 9.7 months) with a median duration of response of 10.3 months (4.1 – 13.9 months). The rate of HI on the decitabine arm was 13% vs. 7% on the supportive care arm. This translated into an overall improvement rate (CR+PR+HI) of 30% vs. 7% in favor of the treatment arm (p<0.001). There was a trend towards longer time to progression to acute myeloid leukemia (AML) or death for the decitabine arm (12.1 months vs. 7.8months, p=0.16) but it was not statistically significant. Of the patients who were evaluable for cytogenetic response, 35% vs. 10% achieved complete cytogenetic remission in favor of the decitabine cohort. Decitabine was relatively well tolerated in this population, with the expected myelosuppression being the most common side effect. Results from this and previous studies led to the FDA approval of decitabine for patients with MDS.
Further work to define the optimal dose and schedule of decitabine to exploit its hypomethylating effects was conducted by investigators at MD Anderson Cancer Center (MDACC). Two separate trials studied lower dose and prolonged exposure of decitabine.14,15 In a phase II adaptively randomized trial of low dose decitabine in advanced leukemia a 5 day intravenous schedule (20 mg/m2) was chosen as the most optimal, demonstrating a CR rate of 32%, compared with 21% in the SQ arm and 24% in the 10 day IV arm.15 Correlative studies on the trial also showed a more pronounced degree of hypomethylation achieved with the 5 day IV schedule.
Outcomes after Hypomethylating Agent-Based Therapy
Based on these data and subsequent FDA approval, treatment with the hypomethylating agents 5-aza and decitabine has become the standard of care for patients with MDS who require therapy. The treatments are well tolerated even in the elderly population and are most commonly administered in an outpatient setting, reducing hospital days and improving quality of life. Studies have shown overall response rates of 28% to 48% with complete remission rates 6% to 34%. In responding patients, the median duration of response is between 8 and 10 months with improved overall survival and decreasing transfusion requirements. In those patients who do not respond to hypomethylating therapy and those who relapse or progress after an initial response, the prognosis is poor. These patients often have a resistant-disease phenotype and generalized deconditioning associated with disease progression after chemotherapy. The paucity of active agents in this setting creates a challenging situation and an opportunity for further research.
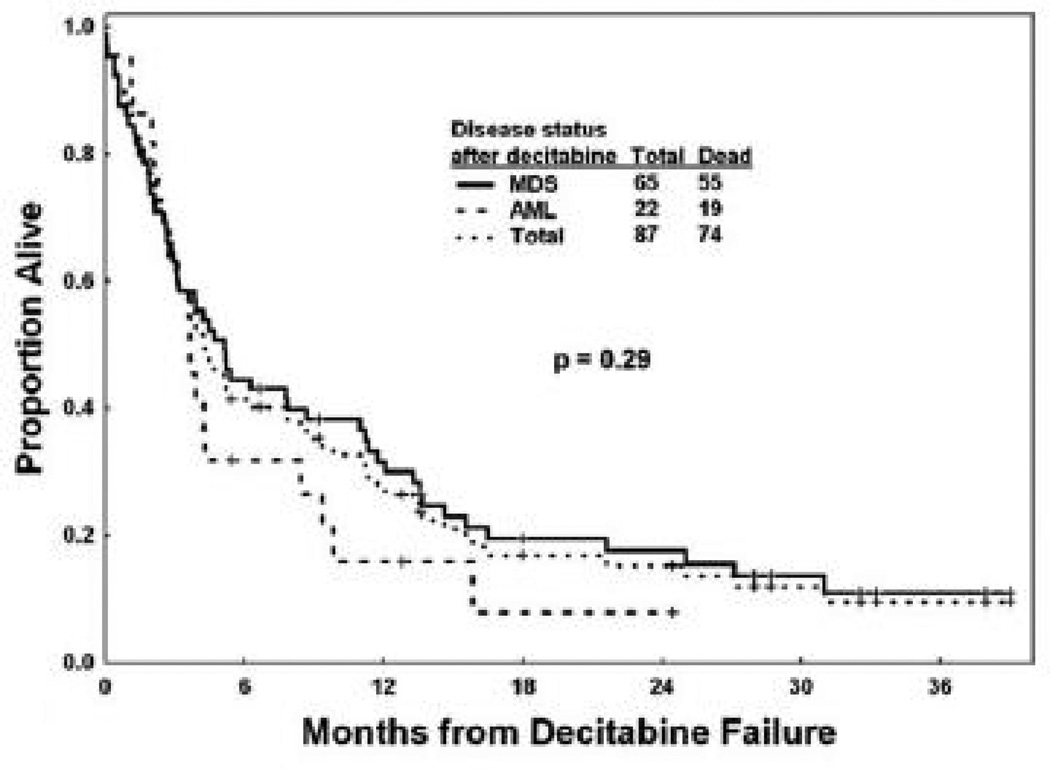
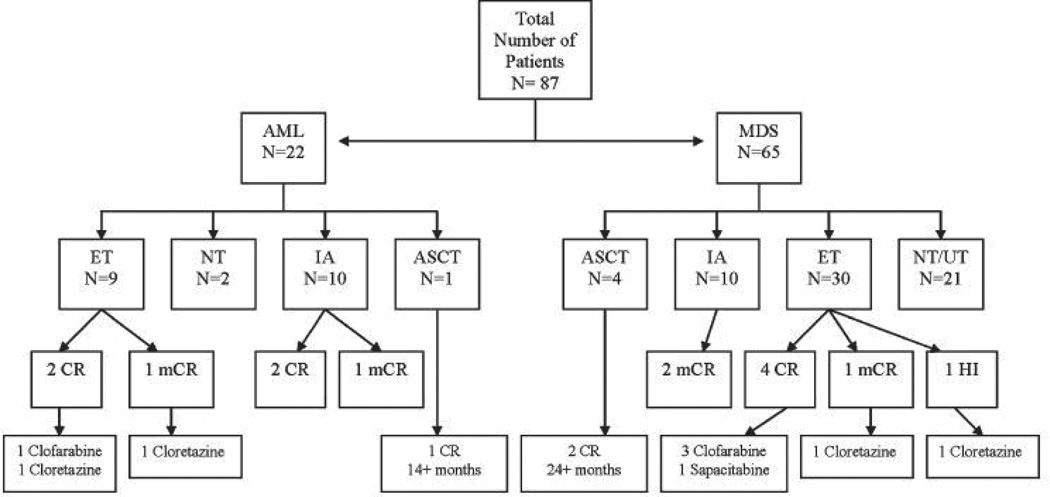
Retrospective studies following the natural history of patients in whom hypomethylating agents have failed allow us to frame the problem and identify the patterns of failure. For example, investigators at MDACC recently reviewed their experience with patients with MDS after failure of decitabine therapy.16 Data from 87 patients with MDS and chronic myelomonocytic leukemia who received decitabine were retrospectively analyzed. The best response in this cohort included CR in 21 (24%), PR in 2 (2%), marrow CR in 6 (7%), and HI in 21 (24%) patients, for an overall response rate of 57%. With a median followup of 21 months, the median survival after decitabine failure was only 4.3 months and the estimated 12-month survival rate was 28%. (Figure 1) The patterns of failure in this cohort included 25% (22 patients) who progressed to AML, and 75% (65 patients) who had persistent MDS.(Figure 2)
Figure 1.
Overall survival after decitabine failure in patients with myelodysplastic syndrome (MDS), acute myeloid leukemia (AML) and the total population. (Adapted with permission from Jabbour, et. al.16).
Figure 2.
Tree diagram outlining outcome after decitabine failure. AML, acute myeloid leukemia. MDS, myelodysplastic syndrome. ET, experimental therapies. NT, no therapy. IA, idarubicin and cytarabine. ASCT, allogeneic stem cell transplantation. UT, unknown therapy. CR, complete remission. mCR, bone marrow complete remission. HI, hematologic improvement.(Adapted with permission from Jabbour, et. al.16)
Among the 22 patients who evolved to AML: 10 received intense chemotherapy (IC), 2 of whom achieved a CR with a median duration of 6 months, and 1 marrow CR with a response duration of >5 months; 9 patients received lower intensity investigational agents, 2 of whom who achieved a CR of 3 and 11 months with clofarabine and cloretizine respectively, and 1 achieved a marrow CR of 8 months after cloretazine; 1 patient received an allogeneic stem cell transplant (SCT) with a CR > 14months.
Of the 65 patients who remained with MDS, 10 received IC: 2 of whom achieved a marrow CR with a median duration of 7 months; 30 patients received investigational agents: 3 of whom achieved a CR with median duration of 5 months on clofarabine, 1 a CR of 4 months with sapacitabine, and 2 with bone marrow CR and hematologic improvement of 3 months and 2 months respectively on clofarabine; and 4 patients received an allogeneic SCT: 2 of whom achieved a sustained CR of > 24months. So in summary, overall response rates to subsequent therapy after failure of decitabine were 20% to 30% for intensive chemotherapy and 20% to 33% for lower intensity investigational agents. For the small number of patients who were offered (and who were candidates for) a SCT it remained a good option with durable responses.
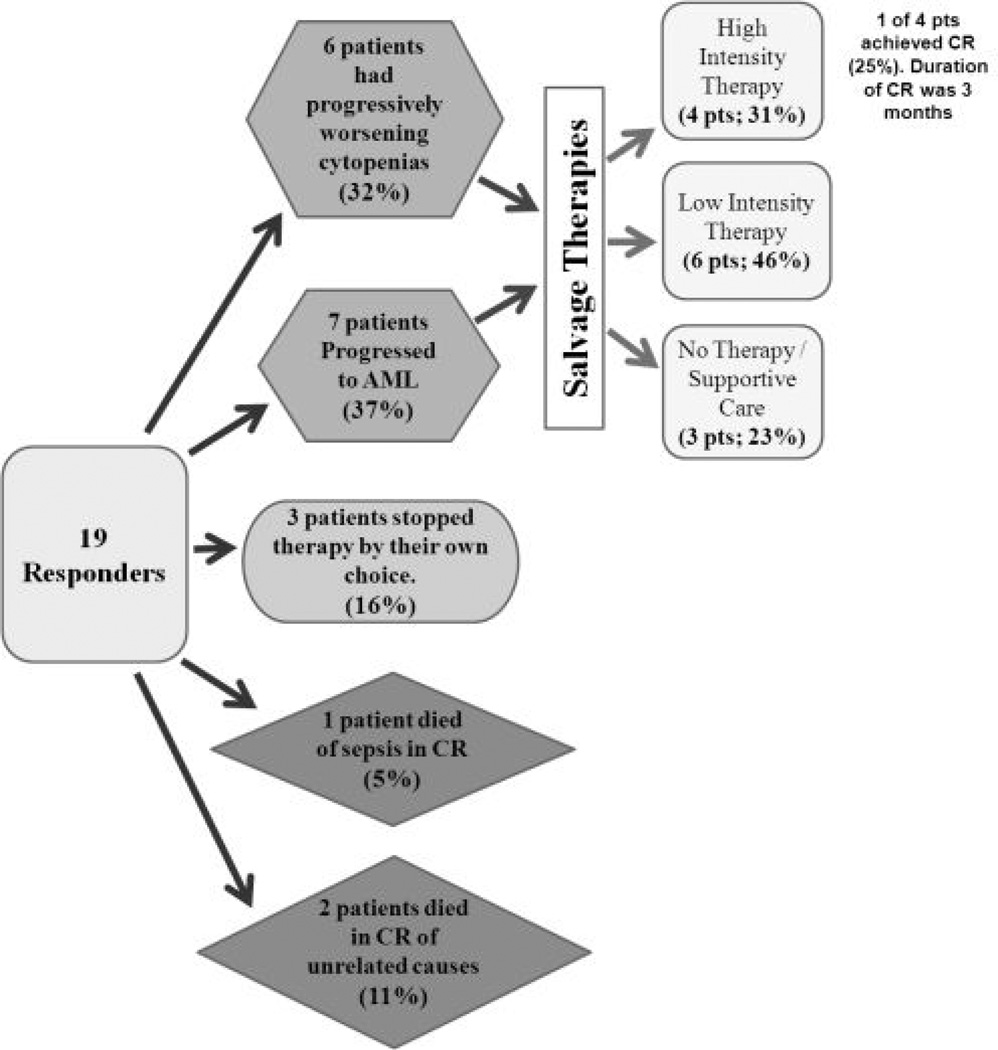
In a separate study, investigators examined the long term outcomes and patterns of failure of hypomethylating agent-based therapy with either 5-aza or decitabine in combination with valproic acid on 2 completed clinical trials involving patients aged >60 years with high risk MDS or AML.17 Of 46 evaluable patients, there were 19 responses (41%), including 16 CR (35%), 1 CRp (CR without platelet recovery, 2%), and 2 PR (4%). The median duration of response was 36 weeks and the median overall survival of the entire group was 39 weeks (58 weeks in the responding patients). Among the responding patients, the patterns of failure included: 7 (37%) with progressive AML, 6 (32%) with prolonged cytopenias/persistent MDS unable to receive further therapy, 3(16%) stopped by patient choice, 1 (5%) died of infection while in CR, and 2 (11%) died in CR of unrelated causes.(Figure 3) Of the 13 responding patients who were candidates for further therapy, salvage regimens were: IC in 4 (31%), low intensity investigational therapy (oral 5-azacytidine, lenalidomide, sapacitabine, clofarabine+low dose cytarabine,vorinostat) in 6 (46%), and no further therapy or supportive care in 3 (23%). Only 1 of the 4 patients (25%) receiving IC responded and had a CR duration of 3 months. The authors concluded that patients who fail epigenetic therapy have a poor prognosis, low response rate to IC and should be considered for investigational approaches.
Figure 3.
Outcome after failure of hypomethylating agent + valproic acid-based combination therapy. AML, acute myeloid leukemia. CR, complete remission. (Adapted with permission from Kadia, et. al.17)
From these and other clinical experiences, it is apparent that the typical failures to hypomethylating agent-based therapies can be generally divided into 3 categories: about one-third of patients progress to AML; about one-third have persistent, progressive MDS with worsening cytopenias; and about one-third refuse further therapy or die from complications. Patients within the first 2 groups are focus of the ensuing discussion regarding further therapy. Given their age and performance status following progression after chemotherapy, many of these patients are not eligible for intensive chemotherapy or allogeneic stem cell transplant. In those patients who are eligible, especially those who progress to AML, intensive chemotherapy can be a reasonable option as long as the treatment-related mortality and overall survival rates associated with these approaches is made clear. For the majority of patients who need more lower-intensity approaches, there is no clear standard of care and there are several lines of clinical investigation that are ongoing. Since we do not yet know the clear mechanism of resistance to hypomethylating agents nor the biology associated with progressive disease, choosing the next therapy at this stage is largely empirical and based on availability and predicted tolerance of the therapy in the individual patient.
Mechanism of Resistance
Although clinical experience with these agents has outlined patterns of resistance and failure of hypomethylating agents, there are no formal clinical studies that establish mechanisms of resistance to these drugs. There are many possibilities that may contribute to resistance in different patients, ranging from varied pharmacokinetics within individual patients to biochemical alterations of the drug target (DNA methyltransferase). Using a panel of various cancer cell lines Qin et. al.18 attempted to investigate the mechanisms of resistance of decitabine in vitro. Since decitabine (DAC) is a nucleoside analogue that is needs to be phosphorylated and incorporated into DNA to exert its therapeutic effect, the investigators hypothesized that alterations of proteins involved in the transport, phosphorylation, and catabolism of decitabine could explain some mechanisms of resistance. The investigators first determined the IC50 of decitabine among the different cell lines to establish ‘sensitive’ and ‘resistant’ lines. They determined the levels of baseline LINE methylation among these cell lines and found that the baseline difference in methylation did not explain different levels of resistance. Cytarabine and decitabine are similarly handled by cellular transport and metabolism, unlike 5-azacytidine. Using this information, the investigators examined the sensitivity of the different cell lines to the 3 drugs and found that the sensitivity to decitabine correlated directly with sensitivity to cytarabine and had no relation to that of 5-aza. This suggests that the mechanisms of resistance to decitabine are similar to those that are known of cytarabine, namely alterations in transport proteins (hENT1, hENT2), deoxycytidine kinase (dCK), and catabolizing enzymes such as cytidine deaminase (CDA). Using this as a lead, the investigators next interrogated this pathway of nucleoside metabolism in the different cell lines. The found indeed, that the IC50 of decitabine correlated inversely with the mRNA expression of dCK, and tended to inversely correlate with hENT1 and hENT2 mRNA. In other words, the resistant cell lines expressed lower levels of the proteins required to transport decitabine into the cell as well as lower levels of the enzyme (dCK) needed to phosphorylate and thereby activate decitabine. In addition, the more resistant cell lines expressed higher levels of CDA mRNA, the enzyme responsible for inactivating decitabine. These series of experiments suggest that the ‘natural’ or baseline resistance to decitabine among cancer cell lines occurs as a result of mechanisms that reduce the ability of decitabine to become incorporated into DNA. Next, the investigators aimed to determine mechanism of induced resistance to decitabine. They first selected out and propagated decitabine-resistant HL60 cell lines (HL60R) and compared them to de novo HL60 lines. They found that induced resistance to DAC in HL60R correlated with markedly lower levels of dCK compared to HL60. In their search for a mechanism of dCK loss in the HL60R cells, they found a sporadically occurring heterozygous point mutation in codon 98 (ACA to AGA) in some of the cell lines. Among the HL60R cells, they found a loss of heterozygosity leading to a loss of the wild type allele and two copies of the mutant DCK gene. This mutation was shown to be responsible for the lower dCK levels and a mechanism of resistance. The authors concluded that preexisting and DAC-induced genetic instability may lead to a generation of resistant clones such as this example that could result in a growth advantage. Presumably, other spontaneously occurring mutations in key proteins involved with drug metabolism and transport could select out resistant clones and confer resistance. Further investigation into these pathways may lead to development of strategies employing non-cross-resistant drug combinations to overcome these mechanisms.
Approaches to Treating Patients after Hypomethylating Agent Failure
While there have been significant advances in the treatment of MDS with the introduction of hypomethylating agents, it is clear that these are not curative therapies. Although they are associated with excellent tolerance, improvement in transfusion requirements, and improvement in survival, continued clinical experience with these agents highlight their limitations. Treatment with hypomethylating agents is usually indefinite until progression of disease, resistance, poor tolerance or patient decision. There is a clear need for the development of newer therapies and continued enrollment on clinical trials to generate advances at this stage of treatment. In this arena, there are several promising approaches which show signals of activity and should be studied further.
Ezatiostat (TLK199)
Ezatiostat (TLK199) is a synthetic tripeptide analog of glutathione that has been shown in preclinical models to stimulate the proliferation of myeloid precursors.19 Once ingested in the form of orally bioavailable tablets, ezatiostat is converted to its active metabolite TLK117. TLK117 is an inhibitor of glutathione S-transferase P1-1 (GST P1-1). GST P1-1 is overexpressed in cancer and is known to inhibit the function of Jun-N-terminal kinase (JNK) – which itself is an important promoter of growth and maturation of normal hematopoietic precursors and a inducer of apoptosis in leukemia cell lines. TLK117 can lead to the dissociation of GSTP1-1 from JNK, releasing its inhibition and allowing the promotion of maturation and growth of normal hematopoietic precursors.19 In preclinical models, ezatiostat has been shown to induce multilineage differentiation and maturation. In a phase I study utilizing an intravenous form of ezatiostat, the drug was found to be well tolerated in humans with evidence of hematologic improvement.
This, coupled with preclinical data led to a phase I dose-escalation trial of ezatiostat hydrochloride tablets in patients with MDS.19 Ten dose levels were tested, ranging from 200mg to 6000mg daily given on a day 1–7 schedule of a 21 day cycle. Forty-five patients with low, intermediate-1, or intermediate-2 MDS were enrolled. Patients had received a median of 1 prior therapy (range 0 –5) with 33% having had received prior chemotherapy and 11% having received a prior investigational agent. The drug was very well tolerated with no DLTs (dose-limiting toxicities) and no MTD (maximum tolerated dose). The most common adverse events were gastrointestinal, including nausea, diarrhea, vomiting, and abdominal pain. Pharmacokinetics confirmed a dose-related elevation in the active metabolite. There were 17 patients out of 45 (38%) who achieved HI, with 11 of the responses occurring at dose levels between 4000mg and 6000mg daily, suggesting a dose response. Three of the responders had bilineage improvement in blood counts and there was one complete cytogenetic response. Hematologic responses occurred in all cell lines and there was evidence of decrease in transfusion requirements in >50% of responders. Patients also reported increased energy, feeling of well-being, and improved daily activities. The authors concluded that oral ezatiostat was well tolerated with promising hematologic activity in patients with MDS.19 Advanced single-agent and combination trials are underway to confirm the activity of this interesting compound.
ON 01910.Na
ON 01910.Na is small molecule multi-tyrosine kinase inhibitor that has demonstrated both preclinical and clinical anticancer activity. It exerts its anticancer activity through a variety of mechanisms of action including modulation of the PI3K-Akt-mTOR pathway and disruption of mitosis through modulation of the polo-like kinase (PLK) pathway.20 Multiple early phase clinical trials in solid tumors and hematologic malignancies are currently ongoing and confirm the safety and tolerability of in ON 01910.Na patients. In a first-in-man Phase I study, ON 01910.Na was studied in 20 patients with advanced solid tumors.21 The agent was administered twice weekly for 3 weeks on a 28 day cycle and the dose was escalated from 80mg up to 4370 mg. The dose of 3120 mg was found to be the MTD. The drug was well tolerated. Toxicities included skeletal pain, abdominal pain, tumor pain, nausea, and fatigue. One patient on that initial trial with refractory ovarian cancer had an objective response and remained progression free for 2 years. Preclinical work demonstrating cytotoxic activity against leukemic cell lines22 in vitro and in ex vivo samples from patients with MDS23 led to the development of several ongoing clinical trials in AML and MDS. These studies are early in accrual but have already demonstrated promising signals of activity in patients with high risk MDS and AML, including those who have been previously treated with hypomethylating agent-based therapy.24 In a preliminary report of a Phase I/II trial, ON 01910.Na was studied in patients with advanced MDS.25 The drug was administered as a 48 hour continuous infusion weekly for 3 weeks on a 4 week cycle at either 800 mg/m2/day (10 patients) or 1500 mg/m2/day (3 patients). Adverse events included thrombocytopenia, neutropenia, anemia, fatigue, and nausea and the drug was found to be well tolerated. Early data on responses included 5 patients with stable disease, 2 with decrease in bone marrow blasts, and 2 patients with HI (erythroid and neutrophil responses). Other studies using ON 01910.Na in MDS and AML are ongoing and have not yet reported. However, responses in MDS including decrease in bone marrow blasts and cytogenetic responses have been noted (data not published). The activity of this well tolerated multikinase inhibitor in patients who have been treated with hypomethylating agents is an important advance and needs to be built upon.
Sapacitiabine
Sapacitabine is an N4-palmitoyl derivative of CNDAC which is orally bioavailable and resistant to deamination and consequent inactivation.26–28 CNDAC (2’-C-Cyano-2’-deoxy-β-D-arabino-pentofuranosylcytosine), the active component of sapacitabine, is a deoxycytidine analog much like cytarabine with a unique mechanism of action. Upon phosphorylation to the nucleotide and incorporation into actively synthesized DNA, replication is not immediately inhibited in a cytotoxic fashion like cytarabine, fludarabine, or clofarabine.26–28 Instead, as a result of the cyano group within the ring, rearrangement of the nucleotide while it is incorporated in the DNA, creates a single strand DNA break. This is then converted to a double stranded break after a round of DNA replication, which leads to cell death. This may explain the effect it has on actively dividing tissue (eg. hematopoietic cells) and the observation that responses and myelosuppression are more profound with successive courses of therapy.
After demonstrating broad preclinical activity in multiple human tumor cells, including leukemia cell lines, a phase I study was done in solid tumors.29,30 The major DLT was myelosuppression and this led to further interest of this agent in hematologic malignancies. A phase I trial27 was conducted in patients with relapsed/refractory acute leukemia and MDS to determine the DLTs and MTD of sapacitabine given in 2 different schedules: (A) orally twice daily (BID) for 7 days every 3 to 4 weeks, or (B) orally BID on days 1–3 and 8–10 every 3 to 4 weeks. A total of 47 patients were treated (42 AML, 4 MDS, 1 ALL, acute lymphoblastic leukemia) using a classical 3+3 dose escalation design with each schedule. The DLTs on both schedules were gastrointestinal in nature, including diarrhea, abdominal pain, neutropenic colitis, and small bowel obstruction. The MTD for schedule A was 325mg BID and the MTD for schedule B was 425mg BID. The most common non-hematologic adverse events were gastrointestinal (anorexia, nausea, vomiting, diarrhea, abdominal pain) and fatigue. Grade 3 or 4 myelosuppression was common and 14 patients (30%) had febrile episodes associated with myelosuppression. Thirteen patients (28%) achieve an objective response, including 4 CR (9%), 2 CRp (4%), and 7 CRi (CR with incomplete recovery of blood counts, 15%). Only 2 patients died in the first 4 weeks of this therapy, leading to an estimated 4-week mortality of 4%. The response rate and low 4-week mortality in this heavily pretreated population warrants further study of this orally bioavailable agent.
Clofarabine
Clofarabine is a purine nucleoside analog that is currently approved for the treatment of pediatric relapsed or refractory ALL that has progressed after at least 2 prior therapies.31 Studies have also shown its activity in myeloid malignancies.32 Upon action by intracellular kinases, clofarabine becomes phosphorylated to its nucleotide form and exerts its cytotoxic effect through multiple mechanisms, including: inhibition of DNA synthesis through incorporation, disruption of mitochondrial activity, and though the inhibition of ribonucleotide reductase.31,32 Although the current FDA approved formulation for clofarabine is intravenous, both oral and intravenous (IV) clofarabine have been investigated extensively in patients with MDS and AML.
In a recently published phase II study33, single-agent IV clofarabine was tested in the treatment of elderly patients (≥60 years) with AML and unfavorable prognostic factors – precisely the population of patients that represent a challenge in clinical practice. Clofarabine was administered at a dose of 30 mg/m2/day IV for 5 days during induction and 20 mg/m2/day IV for 5 days during consolidation cycles. In 112 evaluable patients, the ORR was 46% including 38% CR and 8% CRp. This high ORR was preserved between 32% and 54% when analyzed by unfavorable subgroups including age ≥70, performance status of 2, history of an antecedent hematologic disorder (AHD), intermediate karyotype, and unfavorable karyotype. The median disease free survival was 37 weeks, with a median CR duration of 56 weeks. The OS was 41 weeks for the entire cohort and 72 weeks for those in CR. The 30 day mortality was 9.8%.33 Overall, clofarabine was well tolerated in this elderly cohort with unfavorable prognostic factors with the most common toxicities being nausea, neutropenic fever, vomiting, diarrhea, rash, and fatigue.
The safety and tolerability of the oral formulation of clofarabine was also studied in older patients with higher risk MDS.34 Due to the substitution of fluorine a the C-2’ position of the carbohydrate ring, clofarabine has improved stability in gastric acid and therefore approximately 50% oral bioavailability. Thirty-two patients were treated on the trial, of whom 22 had intermediate-2 or high risk disease by the IPSS.34 Three dose levels of oral clofarabine were tested: 40 mg/m2/day, 30 mg/m2/day, and 20 mg/m2/day for 5 days every 4 to 8 weeks. Nine patients had secondary MDS and 20 patients had prior therapy failure with hypomethylating agents. The overall response rate was 43% with 8 CR (25%), 3 (9%) HI, and 3 (9%) clinical benefit (CB). In patients who had previous hypomethylating agent failure, the ORR was 30% with 2 CR (10%), 2 HI (10%), and 2 with CB (10%).
Switching Hypomethylating Agent
Although both 5-azacytidine and decitabine are inhibitors of DNA-methyltransferase and therefore active as hypomethylating agents, they each have their own structure and slightly different mechanisms-of-action. This lends the possibility that these agents may not be entirely “cross-resistant” – that is to say, that failure of one drug may not preclude clinical activity of the other. Therefore, the use of an alternative hypomethylating agent in patients whose disease has failed one is an important option that should be considered. In small prospective study, Borthakur et. al.35 investigated the use of decitabine in patients who had been previously treated with 5-azacytidine and had stopped due to failure, lack of response, or intolerance. To qualify for the study, patients must have been treated with 5-azacytidine and had either (1) no response after at least 3 cycles of 5-aza, (2) progression of disease on 5-aza, or (3) grade 3–4 non-hematologic toxicity while on 5-aza. Fourteen patients were reported in the interim analysis with 3 CRs (21%) and 1 patient with hematologic improvement (7%), for an overall response rate of 28%. Of the responders, 1 had stopped prior 5-aza due to disease progression, 2 for lack of response, and 1 for severe skin toxicity. Of these responders, 3 out of 4 had receive at least 4 cycles of 5-aza prior to being treated with decitabine. The median number of courses of decitabine to response was 3 (range 1–5). Although a small preliminary study, it suggests that changing hypomethylating therapy from 5-aza to decitabine may have some clinical utility. Although there is no firm data documenting the use of 5-aza in decitabine failures, clinical experience with these agents suggests a similar small benefit.
Alemtuzumab
Alemtuzumab (CAMPATH-1H) is a humanized monoclonal antibody directed against CD52, a cell surface protein expressed at high density on most normal and malignant lymphocytes, B-cells, and T-cells but not on hematopoietic stem cells.36–38 Alemtuzumab is FDA approved for the treatment of patients with B-chronic lymphocytic leukemia (CLL).39 It is a potent immunosuppressive agent that can efficiently lead to marked lymphocyte depletion. There is a subset of patients with MDS who are responsive to immunosuppressive therapy, suggesting that autoreactive cytotoxic T-cells may have a pathogenic role in suppressing hematopoietic progenitors. Several investigators have demonstrated the presence of clonal T-cell populations in the bone marrows of patients with MDS, similar to those in aplastic anemia or T-LGL leukemia.40–42 In such cases of MDS, T-cell suppressive agents such as anti-thymocyte globulin and/or cyclosporine have been used with measured success.43–45 Alemtuzumab has a number of advantages over these therapies and may play an important role in the chronic treatment of these diseases. Compared to ATG, alemtuzumab leads to more potent T-cell depletion, has a longer half-life of activity, and is associated a much lower incidence of infusion reactions, serum sickness, or renal dysfunction. Although there are no good, validated biomarkers that predict for response to immunosuppressive therapy in MDS, investigators at the NIH have developed a clinical model describing patients that may respond. In general, the model includes patients who are young, with a low IPSS score, HLA-DR15 positive, and who have a low transfusion requirement.46 Using a score derived from this model to select patients, Sloand et. al. conducted a Phase I/II study of alemtuzumab in patients with MDS.47 Thirty-two selected patients were treated with alemtuzumab at a flat dose of 10 mg IV daily for 10 days for one cycle. In patients with Int-1 MDS by IPSS, there were 17/22 (77%) responses. In patients with Int-2 MDS, the response rate was 57% (4/7 patients). The median time to response was 3 months and the responses were durable with significant improvement in transfusion dependence. In fact, out of 7 evaluable patients with cytogenetic abnormalities prior to treatment, 4 had normal cytogenetics at 12 months. In selected patients with MDS which has progressed on or failed to respond to hypomethylating agents, such immunosuppressive approaches may be opportunities for response. Selecting the appropriate patients for immunosuppressive therapy with alemtuzumab will be important. Detection of aberrant, clonal populations of cytotoxic T-cells in bone marrow specimens by flow cytometry or molecular techniques may provide a basis for predictive testing that needs to be validated going forward.
Stem Cell Transplant
Allogeneic SCT is known to be able to produce long term remissions in patients with MDS and remains the only potentially curative therapy for this disease. However, it also carries significant treatment-related morbidity and mortality that is especially pronounced in older patients with comorbidities.48,49 Various approaches have been explored in an effort to safely treat patients with a SCT, including appropriate patient selection as well as reduced intensity approaches. The first consideration should be to appropriately choose the population of patients who would benefit the most from an allogeneic SCT, given its inherent toxicity. Cutler et. al.50 attempted to answer this question by retrospectively analyzing a large cohort of patients and developing a decision-making model. Should patients with MDS be considered for a transplant: 1) at diagnosis of MDS, 2) at the time of progression to AML, or 3) at some point prior to progression to AML. The investigators found, that in patients with low or intermediate-1 risk MDS (by IPSS), delayed transplant (although prior to transformation to AML) maximized the overall survival benefit. Among patients less than 40 years of age in this cohort, the OS was even greater. In patients, with intermediate-2 or high risk MDS, performing a bone marrow transplant at diagnosis maximized the overall survival benefit. Higher risk patients, therefore, should be considered for a SCT if they are ‘fit’ - especially if they relapsed or refractory to hypomethylating agent based therapy. Additional factors to consider would be age, comorbidities, performance status, IPSS score, availability of a donor, and surrounding social/family support.
After appropriate selection of patients and counseling of the patients about the risk and commitment of an allogeneic SCT, the next consideration should involve discussion about the intensity of conditioning. Higher intensity conditioning is associated with better disease control and lower risk of relapse, but with a higher risk of non-relapse related mortality (NRM). In this population of MDS patients with a median age > 60 years and associated comorbidities, the NRM becomes an important issue. Non-myeloablative or reduced intensity conditioning (RIC) is therefore an important option. Alyea, et. al.51 performed a retrospective analysis of 152 patients older than 50 years with mainly leukemia or MDS who had received either non-myeloablative (fludarabine + IV busulfan, 71 pts) or myeloablative (cyclophosphamide + total body irradiation [TBI], 81 pts) conditioning to examine the outcomes. In their cohort, patients in the non-myeloablative cohort were more likely to have had unrelated donors (58% vs. 36%, p=0.009); history of a prior transplant (25% vs. 4%, p<0.0001); and active disease going into transplant (85% vs. 59%, p<0.001). Despite having a significantly higher proportion of patients with these known adverse prognostic factors in the non-myeloablative cohort there was a higher overall survival in this cohort at 1 year (51% vs. 39%) and 2 years (39% vs. 29%, p=0.056). The NRM was lower in the non-myeloablative cohort (32% vs. 50%, p=0.01), but the relapse rate was higher (46% vs. 30%, p=0.052). The authors concluded that, in an older cohort of patients, RIC was at least as good as myeloablative therapy and was associated with lower treatment related toxicity. In a separate multicenter study defining the outcomes of older patients (median age 59 years) with AML or MDS undergoing an allogeneic SCT after RIC, the results are similar.52 The report outlines 148 patients who underwent conditioning with either low dose TBI (200 cGy) alone or in combination with fludarabine (30 mg/m2/day on days -4 to -2). The cohort had approximately equal numbers of match-related and unrelated donor transplants. There was no difference in acute or chronic graft-versus-host-disease (GVHD) among these 2 groups. The 3-year relapse free survival and overall survival was 27%, with a 41% incidence of relapse. The 3-year NRM was 32%. Factors associated with a lower risk of relapse in this study were development of chronic GVHD, the presence of low or intermediate-1 risk disease in de novo MDS patients.
Even within the category of RIC, there are different levels of intensity that are offered to older patients that may carry their own characteristics of toxicity and disease control. De Lima and colleagues53 explored this question in their retrospective analysis of 94 patients with MDS and AML who were treated with reduced intensity conditioning regimens. The investigators divided the cohort into those who received a truly nonmyeloablative regimen (fludarabine 120 mg/m2 + AraC 4 g/m2 + idarubicin 36 mg/m2, FAI) and those who received a more myelosuppressive RIC (fludarabine 100–150 mg/m2 + melphalan 140–180 mg/m2, FM). At baseline, the FAI group had a higher proportion of patients in CR (44% vs. 16%, p=0.006); a higher number of patients who were in their first CR (28% vs. 3%, p=0.008); and a higher number with HLA-matched sibling donors (81% vs. 40%, p=0.001). FM was associated with a higher degree of engraftment, a higher incidence of treatment-related mortality (TRM, p=0.36), and a lower relapse-related mortality (p=0.29). The relapse rate after FAI and FM was 61% and 30%, but the 3-year survival was 30% and 35% respectively. The authors concluded that the more myelosuppressive nature of the regimen is important for disease control in MDS and AML, and that more non-myeloablative approaches may be more useful in cases where there is low burden or minimal residual level of disease.
Although SCT remains the only potentially curative option in patients with MDS, these and other studies provide a glimpse of the complexity in decision-making and commitment involved with these approaches. Allogeneic transplant with RIC is a useful approach in selected patients, but the level of intensity and timing needs to tailored to the individual patient – taking into account age, comorbidities, performance status, burden of disease, source of donor, and treatment goal.
Summary
The discovery and development of hypomethylating agents and their activity in MDS has been a major breakthrough in the treatment of this disease. Many patients have benefitted with improved overall survival, reduced transfusion requirements, and control of their disease. However, as we accumulate more clinical experience with these medicines, it is clear that these agents are not curative and further therapy is needed when patients progress. As clinicians treat more MDS patients with 5-aza and decitabine, we are beginning to accumulate patients in our practice whose disease has failed hypomethylating agent therapy. The patterns of failure are familiar: progression to AML, persistent cytopenias/MDS, or death. In patients who desire and are candidates for further therapy, there are limited standard options, including allogeneic SCT. However, there are ongoing clinical research efforts focused on this population of patients with some promising leads. It is important that patients in this situation who are not eligible for intense chemotherapy be referred for clinical trials. In addition to finding newer and active therapies, the challenge will be how to appropriate choose the appropriate therapy for this heterogeneous population.
References
- 1.Aul C, Giagounidis A, Germing U. Epidemiological features of myelodysplastic syndromes: results from regional cancer surveys and hospital-based statistics. Int J Hematol. 2001;73:405–410. doi: 10.1007/BF02994001. [DOI] [PubMed] [Google Scholar]
- 2.Germing U, Strupp C, Kundgen A, et al. No increase in age-specific incidence of myelodysplastic syndromes. Haematologica. 2004;89:905–910. [PubMed] [Google Scholar]
- 3.Iglesias Gallego M, Sastre Moral JL, Gayoso Diz P, Garcia Costa A, Ros Forteza S, Mayan Santos JM. Incidence and characteristics of myelodysplastic syndromes in Ourense (Spain) between 1994–1998. Haematologica. 2003;88:1197–1199. [PubMed] [Google Scholar]
- 4.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 5.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 6.List A, Kurtin S, Roe DJ, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- 7.Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10:176–182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 11.Silverman LR, McKenzie DR, Peterson BL, et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421, 8921, and 9221 by the Cancer and Leukemia Group B. J Clin Oncol. 2006;24:3895–3903. doi: 10.1200/JCO.2005.05.4346. [DOI] [PubMed] [Google Scholar]
- 12.Momparler RL. Pharmacology of 5-Aza-2'-deoxycytidine (decitabine) Semin Hematol. 2005;42:S9–S16. doi: 10.1053/j.seminhematol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Santini V, Kantarjian HM, Issa JP. Changes in DNA methylation in neoplasia: pathophysiology and therapeutic implications. Ann Intern Med. 2001;134:573–586. doi: 10.7326/0003-4819-134-7-200104030-00011. [DOI] [PubMed] [Google Scholar]
- 14.Issa JP, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2'-deoxycytidine (decitabine) in hematopoietic malignancies. Blood. 2004;103:1635–1640. doi: 10.1182/blood-2003-03-0687. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]
- 16.Jabbour E, Garcia-Manero G, Batty N, et al. Outcome of patients with myelodysplastic syndrome after failure of decitabine therapy. Cancer. 116:3830–3834. doi: 10.1002/cncr.25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadia T, Estrov Z, Ravandi F, et al. Long Term Followup and Patterns of Failure in Patients with Acute Myeloid Leukemia (AML) and High Risk Myelodysplastic Syndrome (MDS) Treated On Studies Combining a Hypomethylating Agent and the Histone Deacetylase Inhibitor (HDACi) Valproic Acid. ASH Annual Meeting Abstracts. 2009;114:2074. [Google Scholar]
- 18.Qin T, Jelinek J, Si J, Shu J, Issa JP. Mechanisms of resistance to 5-aza-2'-deoxycytidine in human cancer cell lines. Blood. 2009;113:659–667. doi: 10.1182/blood-2008-02-140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raza A, Galili N, Smith S, et al. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood. 2009;113:6533–6540. doi: 10.1182/blood-2009-01-176032. [DOI] [PubMed] [Google Scholar]
- 20.Prasad A, Park IW, Allen H, et al. Styryl sulfonyl compounds inhibit translation of cyclin D1 in mantle cell lymphoma cells. Oncogene. 2009;28:1518–1528. doi: 10.1038/onc.2008.502. [DOI] [PubMed] [Google Scholar]
- 21.Jimeno A, Li J, Messersmith WA, et al. Phase I study of ON 01910.Na, a novel modulator of the Polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–5510. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skidan I, Zinzar S, Holland JF, Reddy R, Reddy EP, Silverman LR. Toxicology of a novel small molecule ON1910Na on human bone marrow and leukemic cells in vitro. AACR Meeting Abstracts. 2006;2006:309-a. [Google Scholar]
- 23.Sloand EM, Pfannes L, Reddy R, Reddy P, Groopman JS, Young NS. Suppression of Cyclin D1 by on 01910.Na Is Associated with Decreased Survival of Trisomy 8 Myelodyplastic Bone Marrow Progenitors: A Potential Targetted Therapy. ASH Annual Meeting Abstracts. 2007;110:822. [Google Scholar]
- 24.Silverman LR, Odchimar-Reissig R, Navada SC, et al. Effects of a Novel Benzyl Styryl Sulfone Derivative ON 01910.Na On the Myelodysplastic Syndrome (MDS) Derived Clone in Patients Relapsing Following Response to Azacitidine (AzaC) Therapy. ASH Annual Meeting Abstracts. 2009;114:4839. [Google Scholar]
- 25.Raza A, Galili N, Ali MS, et al. Initial Evaluation of a 48-h Continuous Intravenous Infusion Weekly Regimen of ON 01910.Na in Advanced Myelodysplastic Syndrome (MDS) ASH Annual Meeting Abstracts. 2009;114:3815. [Google Scholar]
- 26.Hanaoka K, Suzuki M, Kobayashi T, et al. Antitumor activity and novel DNA-self-strand-breaking mechanism of CNDAC (1-(2-C-cyano-2-deoxy-beta-D-arabino-pentofuranosyl) cytosine) and its N4-palmitoyl derivative (CS-682) Int J Cancer. 1999;82:226–236. doi: 10.1002/(sici)1097-0215(19990719)82:2<226::aid-ijc13>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Garcia-Manero G, O'Brien S, et al. Phase I clinical and pharmacokinetic study of oral sapacitabine in patients with acute leukemia and myelodysplastic syndrome. J Clin Oncol. 28:285–291. doi: 10.1200/JCO.2009.25.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuda A, Nakajima Y, Azuma A, Tanaka M, Sasaki T. Nucleosides and nucleotides. 100. 2'-C-cyano-2'-deoxy-1-beta-D-arabinofuranosyl-cytosine (CNDAC): design of a potential mechanism-based DNA-strand-breaking antineoplastic nucleoside. J Med Chem. 1991;34:2917–2919. doi: 10.1021/jm00113a034. [DOI] [PubMed] [Google Scholar]
- 29.Delaunoit T, Burch PA, Reid JM, et al. A phase I clinical and pharmacokinetic study of CS-682 administered orally in advanced malignant solid tumors. Invest New Drugs. 2006;24:327–333. doi: 10.1007/s10637-006-5392-0. [DOI] [PubMed] [Google Scholar]
- 30.Gilbert J, Carducci MA, Baker SD, Dees EC, Donehower R. A Phase I study of the oral antimetabolite, CS-682, administered once daily 5 days per week in patients with refractory solid tumor malignancies. Invest New Drugs. 2006;24:499–508. doi: 10.1007/s10637-006-8219-0. [DOI] [PubMed] [Google Scholar]
- 31.Gandhi V, Plunkett W. Clofarabine and nelarabine: two new purine nucleoside analogs. Curr Opin Oncol. 2006;18:584–590. doi: 10.1097/01.cco.0000245326.65152.af. [DOI] [PubMed] [Google Scholar]
- 32.Kantarjian HM, Gandhi V, Kozuch P, et al. Phase I clinical and pharmacology study of clofarabine in patients with solid and hematologic cancers. J Clin Oncol. 2003;21:1167–1173. doi: 10.1200/JCO.2003.04.031. [DOI] [PubMed] [Google Scholar]
- 33.Kantarjian HM, Erba HP, Claxton D, et al. Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol. 28:549–555. doi: 10.1200/JCO.2009.23.3130. [DOI] [PubMed] [Google Scholar]
- 34.Faderl S, Garcia-Manero G, Estrov Z, et al. Oral clofarabine in the treatment of patients with higher-risk myelodysplastic syndrome. J Clin Oncol. 28:2755–2760. doi: 10.1200/JCO.2009.26.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borthakur G, Ahdab SE, Ravandi F, et al. Activity of decitabine in patients with myelodysplastic syndrome previously treated with azacitidine. Leuk Lymphoma. 2008;49:690–695. doi: 10.1080/10428190701882146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilleece MH, Dexter TM. Effect of Campath-1H antibody on human hematopoietic progenitors in vitro. Blood. 1993;82:807–812. [PubMed] [Google Scholar]
- 37.Hale G, Xia MQ, Tighe HP, Dyer MJ, Waldmann H. The CAMPATH-1 antigen (CDw52) Tissue Antigens. 1990;35:118–127. doi: 10.1111/j.1399-0039.1990.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 38.Treumann A, Lifely MR, Schneider P, Ferguson MA. Primary structure of CD52. J Biol Chem. 1995;270:6088–6099. doi: 10.1074/jbc.270.11.6088. [DOI] [PubMed] [Google Scholar]
- 39.Demko S, Summers J, Keegan P, Pazdur R. FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia. Oncologist. 2008;13:167–174. doi: 10.1634/theoncologist.2007-0218. [DOI] [PubMed] [Google Scholar]
- 40.Epling-Burnette PK, Painter JS, Rollison DE, et al. Prevalence and clinical association of clonal T-cell expansions in Myelodysplastic Syndrome. Leukemia. 2007;21:659–667. doi: 10.1038/sj.leu.2404590. [DOI] [PubMed] [Google Scholar]
- 41.Kook H, Zeng W, Guibin C, Kirby M, Young NS, Maciejewski JP. Increased cytotoxic T cells with effector phenotype in aplastic anemia and myelodysplasia. Exp Hematol. 2001;29:1270–1277. doi: 10.1016/s0301-472x(01)00736-6. [DOI] [PubMed] [Google Scholar]
- 42.Maciejewski JP, Risitano A, Kook H, Zeng W, Chen G, Young NS. Immune pathophysiology of aplastic anemia. Int J Hematol. 2002;76(Suppl 1):207–214. doi: 10.1007/BF03165246. [DOI] [PubMed] [Google Scholar]
- 43.Garg R, Faderl S, Garcia-Manero G, et al. Phase II study of rabbit anti-thymocyte globulin, cyclosporine and granulocyte colony-stimulating factor in patients with aplastic anemia and myelodysplastic syndrome. Leukemia. 2009;23:1297–1302. doi: 10.1038/leu.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molldrem JJ, Leifer E, Bahceci E, et al. Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med. 2002;137:156–163. doi: 10.7326/0003-4819-137-3-200208060-00007. [DOI] [PubMed] [Google Scholar]
- 45.Yazji S, Giles FJ, Tsimberidou AM, et al. Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia. 2003;17:2101–2106. doi: 10.1038/sj.leu.2403124. [DOI] [PubMed] [Google Scholar]
- 46.Saunthararajah Y, Nakamura R, Wesley R, Wang QJ, Barrett AJ. A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood. 2003;102:3025–3027. doi: 10.1182/blood-2002-11-3325. [DOI] [PubMed] [Google Scholar]
- 47.Sloand EM, Olnes MJ, Shenoy A, et al. Alemtuzumab Treatment of Intermediate-1 Myelodysplasia Patients Is Associated With Sustained Improvement in Blood Counts and Cytogenetic Remissions. J Clin Oncol. doi: 10.1200/JCO.2010.29.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gergis U, Wissa U. High-risk myelodysplastic syndromes: chemotherapy, transplantation, and beyond. Curr Hematol Malig Rep. 5:1–8. doi: 10.1007/s11899-009-0035-0. [DOI] [PubMed] [Google Scholar]
- 49.McClune BL, Weisdorf DJ. Reduced-intensity conditioning allogeneic stem cell transplantation for older adults: is it the standard of care? Curr Opin Hematol. 17:133–138. doi: 10.1097/MOH.0b013e3283366ba4. [DOI] [PubMed] [Google Scholar]
- 50.Cutler CS, Lee SJ, Greenberg P, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004;104:579–585. doi: 10.1182/blood-2004-01-0338. [DOI] [PubMed] [Google Scholar]
- 51.Alyea EP, Kim HT, Ho V, et al. Comparative outcome of nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation for patients older than 50 years of age. Blood. 2005;105:1810–1814. doi: 10.1182/blood-2004-05-1947. [DOI] [PubMed] [Google Scholar]
- 52.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]