Abstract
Epidermal TNF expression increases in response to cutaneous permeability barrier disruption and wound healing. TNF signaling is mediated by acid and neutral sphingomyelinases (A- and N-SMase), which generate ceramide, an important regulator of proliferation, differentiation, and apoptosis. In the epidermis, ceramide is known to be an integral part of the extracellular stratum corneum (SC) lipid bilayers that constitute the permeability barrier of the skin. We show here that topical application of TNF after experimental injury to the SC of hairless mice (hr–/–) enhances barrier repair. In TNF receptor p55–deficient (TNF-R55–deficient) mice (hr+/+), cutaneous barrier repair was delayed compared with wild-type (hr+/+) or TNF-R75–deficient (hr+/+) animals. After barrier disruption in hairless (hr–/–) and wild-type (hr+/+), but not in TNF-R55–deficient (hr+/+) mice, the enzymatic activities of both A-SMase and N-SMase were significantly enhanced. Stimulation of SMase activities was accompanied by an increase in C24-ceramide levels. Most A-SMase activity in hairless mice (hr–/–) was found in the outer epidermal cell layers and colocalized in the lamellar bodies with A-SMase and sphingomyelin. Reduction of epidermal A-SMase activity by the inhibitor imipramine resulted in delayed permeability barrier repair after SC injury. Together, these results suggest that TNF-R55 signaling pathways contribute to cutaneous permeability barrier repair through SMase-mediated generation of ceramide.
J. Clin. Invest. 104:1761–1770 (1999).
Introduction
The cutaneous permeability barrier, which is localized in the stratum corneum (SC), allows life in a terrestrial environment. It prevents excessive water loss and the entry of harmful substances into the body (1, 2). A disrupted permeability barrier is present in irritant and allergic contact dermatitis, atopic eczema, and psoriasis, as well as in skin wounds and burns (3–6). Cutaneous ceramides are well-known structural components of SC lipid bilayers in providing the permeability barrier of the skin. Intercellular ceramides are the most abundant lipids within the extracellular domains of the SC, forming multiple lipid-enriched bilayers to prevent excess water loss. The lipid composition of the SC differs markedly from the lipids present in the nucleated layers of the epidermis. The SC consists primarily of a nonpolar mixture of cholesterol, ceramides, and free fatty acids. These lipids are derived from the exocytosis of lamellar body–derived content from stratum granulosum keratinocytes. Because the major lipids in lamellar bodies comprise a rather polar mixture of glucosyl ceramides and phospholipids, including sphingomyelin, secreted lamellar body lipid precursors must be metabolized within the extracellular spaces into more hydrophobic lipids. Lamellar bodies contain hydrolytic enzymes, including triacylglycerol hydrolase, phospholipase A, acid phosphatases, and certain proteases, which are cosecreted with lipids into the extracellular spaces of the SC (for review, see refs. 1, 2). β-glucocerebrosidase enzyme activity in the upper epidermal layers has been described (7, 8). In addition, acid sphingomyelinase (A-SMase) has been localized to the lamellar bodies and SC intercellular domains (9–11).
Apart from their structural importance, ceramides produced by sphingomyelinases have been recognized as an important second messenger in intracellular signaling of various cytokines and growth factors. TNF, through binding to the 55-kDa TNF receptor, rapidly activates 2 distinct types of SMase, an endosomal A-SMase and a cell membrane–associated neutral sphingomyelinase (N-SMase) (ref. 12; for a review, see refs. 13, 14). Ceramides apparently are capable of modulating the activity of several enzymes. Ceramides generated by N-SMase apparently stimulate a protein kinase cascade including CAPK, c-raf-1, and MAP-kinases (for review see refs. 15, 16). The function of ceramides generated by A-SMase is unclear at present. A-SMase and ceramides may act as cofactors for the signaling pathways leading to programmed cell death in select cell types and tissues in response to Fas/APO-1, γ irradiation and ultraviolet (UV) irradiation, TNF, and various stress factors (17–20). The mode of action of ceramides is apparently determined by the subcellular site of their production. The TNF-responsive N-SMase is located at the plasma membrane, whereas the site of A-SMase activation appears to be an endosomal/lysosomal compartment (12).
Previous studies have shown that experimental injury to the SC by acetone treatment or by tape stripping results in permeability barrier disruption (21). During permeability barrier repair an increase in the synthesis of ceramides, cholesterol, and free fatty acids, including an increase in the activity of the rate-limiting enzymes (e.g., serine palmitoyl transferase for ceramide synthesis), has been demonstrated (21–24). In addition, we showed that experimental barrier disruption also results in an increase in epidermal DNA synthesis (25). Recently, we reported that permeability barrier disruption, as was shown for full skin thickness wounds, leads to profound changes in epidermal differentiation. We found induction of cytokeratin K6, K16, and K17, and a premature expression of involucrin (26).
The signals for the increase in lipid synthesis, DNA synthesis, and altered differentiation after barrier disruption are only partially understood. It has been proven already that transepidermal water loss (TEWL), calcium, potassium, and chloride ions are involved in the regulation of permeability barrier repair (27). An important role in cutaneous wound and barrier repair has been suggested for epidermal cytokines, including TNF, Il-1α and Il-β, granulocyte macrophage CSF (GM-CSF), TGF-α and TGF-β, IFN-γ, Il-8, and Il-10 (28–30). In normal epidermis TNF is produced in the upper nucleated layers. After barrier disruption, a more intense staining for TNF throughout all the nucleated epidermal cell layers has been made visible by immunohistochemistry (31). To elucidate the functional role of TNF for cutaneous permeability barrier repair in vivo, we examined here, in normal (hr–/hr–) and (hr+/hr+), TNF-R55–deficient (hr+/+), and TNF-R75–deficient (hr+/+) mice, the involvement of TNF signaling pathways, including sphingomyelinases and ceramides. We also investigated whether sphingomyelinases contribute to ceramide generation as structural components of the SC bilayers. Here we describe that TNF-R55 and A-SMase are crucially involved in early cutaneous barrier repair.
Methods
Animals.
Male hairless mice [Crl: (hr–/hr–) BR] 6–8 weeks of age were supplied by Charles River Wiga (Deutschland) GmbH (Sulzfeld, Germany). TNF-R55–deficient mice (hr+/+) were generated on a C57BL/6 background as described (32) and have been backcrossed to C57BL/6 more than 5 generations. Breeding was then continued by brother-sister mating for a further 5 generations before use. The generation of the TNF-R75–deficient mice (hr+/+) has been described in detail by Peschon et al. (33). Mice on a C57BL/6 background were also generated by successive backcrossing to C57BL/6 for 5 generations. TNF-R55– and TNF-R75–deficient mice (both hr+/+) were bred on a hairy background, as hairless animals (hr–/–) were not available. Therefore, the respective wild-type (hr+/+) mice were used as controls (C57BL/6; Institute of Animal Research, Hannover, Germany). The animals were maintained conventionally under standardized conditions in groups in plastic cages with polyester filter covers. The study protocols were approved by the University of Kiel, Committee of Animal Care.
Biochemicals.
Biochemicals were purchased from Sigma-Aldrich Chemie GmbH (Munich, Germany). Staphylococcal exfoliative toxin was obtained from Toxin Technology (Sarasota, Florida, USA).
Radiochemicals.
[N-methyl-14C]-sphingomyelin (CFA566) (47.0 mCi/mmol) was purchased from Amersham Pharmacia Biotech Europe GmbH (Braunschweig, Germany). The label in [N-methyl-14C]-sphingomyelin was located in the choline residue.
Permeability barrier disruption.
Disruption of the permeability barrier was induced in hairless mice (age 6–12 weeks) by tape stripping (cellophane tape, 6–8 times), which removes cells from the SC, until a 20- to 30-fold increase in TEWL was achieved using a Meeco electronic water analyzer (Meeco Instruments Corp., Warrington, Pennsylvania, USA) or a Tewameter (Courage & Khazaka, Cologne, Germany). In the hairy TNF-R55– and TNF-R75–deficient mice (both hr+/+), the fur was carefully removed using a shaver before barrier disruption. The shaving did not result in irritation or barrier disruption. At different time points after treatment (0–72 hours), skin samples of about 4 cm2 were obtained.
Topical application of TNF, imipramine, or desipramine to normal mouse (hr–/–) skin.
Immediately after barrier disruption, 30 μL of TNF (25,000 U), imipramine (1%), or desipramine (1%) in isopropanol/propylene glycol 3:7 (vol/vol) were applied topically. Vehicle application served as control. TEWL was determined at 1, 3, 5, 7, and 24 hours after barrier disruption.
Isolation of epidermal samples after acute barrier disruption.
Flank skin of the treated or untreated sites were excised and immediately placed epidermal-side down on a covered Petri dish containing crushed ice. The skin pieces were scraped with a scalpel blade to remove excess subcutaneous fat and immersed at 37°C for 30 minutes in 10 mM EDTA in Dulbecco’s PBS, Ca+ and Mg2+ free (0.16 M NaCl, 0.01 M Na2HPO4, 0.03 M KCl, 0.01 M KH2PO4, pH 7.4). Thereafter, the epidermis was peeled off the dermis by gentle scraping with a scalpel blade. Epidermal sheets were minced into small pieces (< 1 mm3) with scissors and stored at –70°C.
Individual epidermal cell layer preparations using staphylococcal exfoliative toxin separation.
Groups of adult hairless mice (3–5 each) were injected intradermally on both flanks with 50 μg staphylococcal exfoliative toxin dissolved in 100 μL PBS. Two hours after injection, the outer epidermis comprised of SC and stratum granulosum, was peeled off by gentle scraping with a scalpel blade. The lower epidermis, comprising stratum basale and stratum spinosum (34, 35), and the dermis was excised, placed on ice, and separated by the EDTA method. Samples of upper and lower epidermis were used for the SMase assays. The staphylococcal exfoliative toxin by itself exhibited no SMase activity.
Isolation of SC samples.
SC was removed by tape stripping. Samples from outer (first strip) and inner SC (fifth strip) were isolated. The tapes were dissolved in the specific homogenization buffers and shaken for 2 hours. Buffer A for A-SMase, containing 0.2% Triton-X 100, or buffer B for B-SMase, containing 0.2% NP-40, 20 mM HEPES, 10 mM NaF, 2 mM EDTA, 10 mM MgCl2, 30 mM p-nitrophenyl-phosphate, 100 μM sodium vanadate, 10 mM β-glycerophosphate, 1 mM phenyl-methyl-sulfonyl-fluoride, 1 mM pepstatin A, leupeptin, antipain, and 750 μM ATP, were used. The presence of the tape neither influenced N-SMase nor A-SMase activity in vitro.
Isolation of epidermal lipids.
The epidermis was separated by the EDTA method and homogenized in a glass homogenizer (500 rpm) with a Potter S (B. Braun Biotech International GmbH, Melsungen, Germany) in 300 μL distilled water, repeatedly squeezed through an 18-gauge needle, and centrifuged (1,000 g) for 10 minutes. The supernatant was taken and protein measurement was performed. The sample was added to 5 mL chloroform/methanol (2:1), washed with this mixture, stirred for at least 12 hours at 48°C, filtered through a cotton wool–stuffed glass pipette, and dried under nitrogen at 48°C.
Lipid extraction.
Lipids were extracted using the method of Bligh and Dyer (36); briefly, the samples were resuspended using 2 × 1 mL H2O and 1.5 mL methanol and sonicated for 5 minutes. Chloroform (1.25 mL) was added, vortex mixed, and centrifuged for 10 minutes at 6,000 g. The supernatants were transferred to a new glass vial, and the pellet was resuspended in 1 mL H2O, 2.5 mL methanol, and 1.25 mL chloroform, repeatedly sonicated, and centrifuged for 5 minutes at 4,000 g. The pooled supernatants were added to 2.5 mL H2O and 2.5 mL chloroform. After vortex mixing, phase separation was performed by centrifugation for 5 minutes at 4000 g. The lower organic phase was transferred into a new glass vial. Chloroform (4 mL) was added to the aqueous phase, vortex mixed, and phase separated. The organic phases were combined with the first and dried under nitrogen. The dried sample was resuspended for separation of neutral lipids and phospholipids with 4 mL hexane/3 mL methanol. Both fractions were dried under nitrogen.
High-performance thin-layer chromatography.
High-performance thin-layer chromatography (HPTLC) silica gel 60 plates (Merck KGaA, Darmstadt, Germany) were pre-run with a solvent system composed of chloroform/methanol (1:1), and the plates were dried at 80°C for 30 minutes. The HPTLC chambers were equilibrated for 1 hour at room temperature with the solvent system consisting of (a) dichloromethane/methanol/acetic acid (100/2/5 vol/vol) for neutral lipids or (b) chloroform/methanol/acetic acid/H2O (100/60/20/5 vol/vol) for phospholipids: 50 μL of each sample and standards suspended in chloroform/methanol (9:1) were applied on HPTLC plates. After chromatography, the HPTLC plate was dried and fixed by heating at 180°C for 10 minutes. At room temperature the HPTLC plate was submerged into a bath of copper sulfate/phosphate acid (10%/8%) for 10 seconds and charred by heating the HPTLC plate to about 160°C. The spots were analyzed by scanning using 2-D laser densitometry (Molecular Dynamics Personal Densitometer; Molecular Dynamics, Sunnyvale, California, USA). Different ceramide species containing C24 versus C16 ester-linked fatty acids were identified by comparison with commercially available standards (Sigma Ceramide Type III; Sigma) as described previously (37–39).
Application of exogenous A-SMases from human placenta to epidermal homogenates.
Epidermal samples were homogenized in a glass homogenizer at 0°C in 0.2% Triton-X 100 as described previously. The homogenate was incubated with exogenous A-SMase from human placenta at 37°C for 2 hours.
In-vitro assay of A-SMase and N-SMase activity.
At different time points after treatment, skin samples (about 4 cm2) were excised. The epidermis or individual epidermal cell layers, respectively, were homogenized in buffer A or B using the glass homogenizer at 0°C at 600 rpm in 400 μL. The cell debris and nuclei were removed by low-speed centrifugation at 420 g for 10 minutes. The supernatants were used for the in vitro assay as described (10). Protein (20 μg) from the supernatants were incubated for 2 hours at 37°C in a reaction buffer C (250 mM sodium acetate and 1 mM EDTA, pH 5.0) or buffer D (20 mM HEPES and 10 mM MgCl2, pH 7.4) for A-SMase and N-SMase (70 μL final volume), respectively, containing 2.25 μL of [N-methyl-14C] sphingomyelin (0.2 μCi/mL, specific activity 56.6 mCi/mol; Amersham) as substrate. The specificity of this assay for A-SMase and N-SMase is based on the different pH values of the buffers and different cofactor requirements of both enzymes (12). The reaction was linear within this time frame, and the amount of [14C]-SM hydrolyzed did not exceed 10% of the total amount of radioactive SM added. The reactions were stopped by the addition of 800 μL chloroform: methanol (2:1, vol/vol) and 250 μL of H2O. [14C] phosphorylcholine, produced from [14C]-SM identified by HPTLC and determined in the aqueous phase by liquid scintillation counting. The results are presented as nanomoles per hour per milligram of protein.
Protein determination.
The protein content in epidermal homogenates was determined by the method of Bradford (40) using BSA as the standard (BCA-protein assay).
Results
Influence of topical application of TNF on permeability barrier repair.
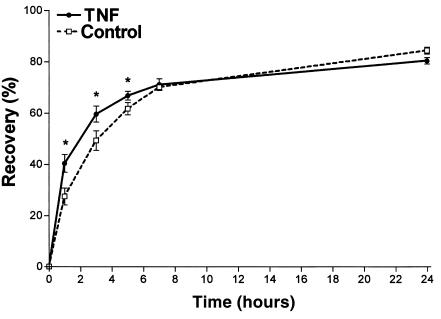
Repeated tape stripping of the skin removed cells from the SC and resulted in a superficial wound. We determined as a marker of barrier disruption an increase in TEWL from 1.8 ± 0.2 to 45.0 ± 15.3 g/m2 per hour. Immediately after treatment, barrier repair commenced. A rapid decrease in TEWL leading to about 60% barrier recovery occurred in hairless mice within 5 hours. This was followed by slower kinetics of barrier recovery within 24 hours (Figure 1). To investigate the possible involvement of TNF in barrier repair, 25,000 U of TNF was applied topically immediately after barrier disruption. As shown in Figure 1, TNF slightly but significantly enhanced permeability barrier repair at 1 hour, 3 hours, and 5 hours, compared with the vehicle-treated control (P < 0.05, n = 5). Seven hours and 24 hours after barrier disruption recovery of TNF-treated mice was indistinguishable from that of untreated control mice.
Figure 1.
Topical application of TNF enhances permeability barrier repair. Acute disruption of the permeability barrier was induced in hairless mice (hr–/–) by tape stripping until a 20- to 30-fold increase in TEWL occurred. Immediately after barrier disruption, TNF (25,000 U) or the vehicle was applied and recovery in TEWL was determined at different time points after treatment. *P < 0.05, n = 5.
Barrier recovery in TNF-R55–deficient mice.
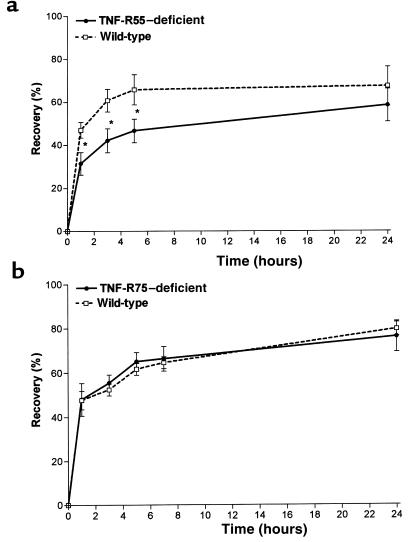
To evaluate the possible role of the p55 TNF receptor, we next examined barrier repair in TNF-R55–deficient mice (hr+/+) after tape stripping. As shown in Figure 2a, TNF-R55–deficient mice exhibited a significant delay in barrier repair at 1–24 hours after treatment. The delay in barrier repair peaked at 3–5 hours (–41% reduced recovery at 3 hours; P < 0.001, n = 7). These data clearly demonstrate the involvement of TNF-R55 in cutaneous barrier repair. In contrast, in TNF-R75–deficient mice (hr+/+), barrier repair was unchanged compared to wild-type animals (n = 5) (Figure 2b).
Figure 2.
TNF-R55 deficiency delays permeability barrier repair. In TNF-R55–deficient, TNF-R75–deficient, and wild-type mice (all hr+/+) the fur was carefully removed and barrier disruption was induced as described in Figure 1. Permeability barrier repair was determined as recovery in TEWL at different time points after treatment for (a) TNF-R55 deficiency (*P < 0.001, n = 7) and (b) TNF-R75 deficiency (NS, n = 6).
Epidermal A- and N-SMase activity after acute barrier disruption in hairless, hairy, and TNF-R55–deficient mice.
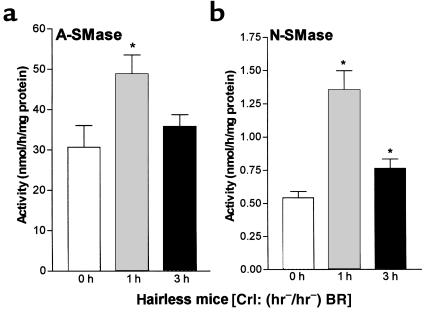
To investigate the involvement of the sphingomyelin metabolism in cutaneous barrier repair, we next assayed the basal enzymatic activities of epidermal sphingomyelinases in hairless mice (hr–/–). A-SMase exerted high basal activity in untreated epidermis (31.1 ± 5.2 nmol/h per milligram of protein). Enzymatic activity of A-SMase was increased at 1 hour after barrier disruption (+59%; P < 0.05, n = 6) and slightly increased at 3 hours (+17%, NS) (Figure 3a). N-SMase activity was 0.55 ± 0.05 nmol/h per milligram of protein in untreated epidermis. After barrier disruption, an increase of the enzyme activity at 1 hour and 3 hours was obtained. A maximum of 149% increase (P < 0.005, n = 8) was noted at 1 hour after treatment. At 3 hours after treatment, the increase in N-SMase activity was lower, but still significant (+41%; P < 0.005, n = 8) (Figure 3b). These results indicate activation of A-SMase as well as N-SMase during barrier repair.
Figure 3.
Barrier disruption results in activation of A- and N-SMase in hairless mice (hr–/–). Immediately (0 hours), 1 hour, and 3 hours after barrier disruption, skin samples were obtained, the epidermis was isolated, and SMases were determined in epidermal homogenates by in vitro mixed micelle enzyme assays using radiolabeled sphingomyelin as substrate. (a) A-SMase (*P < 0.05, n = 6). (b) N-SMase (*P < 0.005, n = 8).
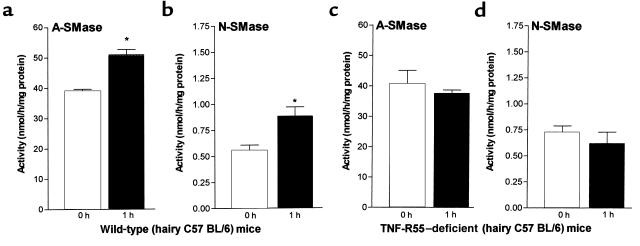
Because TNF-R55-deficient mice (hr+/+)were bred on a hairy background, we determined SMase activities in the respective hairy C57BL/6 mice as controls. Baseline A- and N-SMase activity in C57BL/6 was in the same range as in the hairless mice. At 1 hour after barrier disruption we found a 30% increase in A-SMase (P < 0.05, n = 9) and a 60% increase in N-SMase activity (P < 0.05, n = 9) (Figure 4, a and b). Baseline A- and N-SMase activities in TNF-R55–deficient mice (hr+/+) were nearly identical to the controls. In contrast to hairless mice (hr–/–) and C57BL/6 littermates (hr+/+), barrier disruption in TNF-R55–deficient mice (hr+/+) did not result in an increase in A- and N-SMase activity, indicating a crucial role for TNF-R55 in signaling for activation of both enzymes (Figure 4, c and d).
Figure 4.
TNF-R55 deficiency prevented an activation in A- and N-SMase. Immediately (0 hours) and 1 hour after barrier disruption A- and N-SMase activities were determined in epidermal samples of hairy wild-type (hr+/+) and hairy TNF-R55–deficient (hr+/+) mice as described in Figure 3. Wild-type: (a) A-SMase (*P < 0.05, n = 6) and (b) N-SMase (*P < 0.05, n = 8). TNF-R55–deficient: (c) A-SMase (NS, n = 9) and (d) N-SMase (n = 9, NS).
Epidermal ceramide and sphingomyelin content after acute barrier disruption.
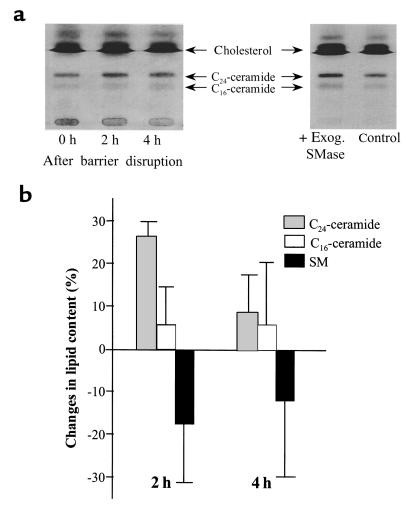
To prove that the enhanced sphingomyelinase activities measured by in vitro assays are also reflected by changes in ceramide mass in vivo, we analyzed the content of ceramide by HPTLC. We detected 2 major fractions for ceramides in accordance with published results (36–38). We found that at 2 hours after barrier disruption, the content in ceramides C16 and C24 increased by 9% and 27%, respectively (P < 0.05, n = 4) (Figure 5). The overall amount of C24 ceramide before and after acetone treatment was 12-fold higher than that of C16 ceramides. In parallel with the increase in ceramides, a decrease in epidermal sphingomyelin was observed (–17% and –12% at 2 hours and 4 hours, respectively). After application of exogenous A-SMase to epidermal homogenates, C24 and C16 ceramides increased by 27% and 34%, respectively (Figure 5). In parallel a reduction in sphingomyelin (–13%) was determined (not shown). Thus, exogenous SMase treatment, leading to hydrolysis of sphingomyelin, results in production of the same ceramide species as in response to experimental barrier disruption, supporting our finding that sphingomyelin hydrolysis rather than ceramide de novo synthesis is involved in the early phase of barrier repair.
Figure 5.
Influence of acute barrier disruption or exogenous sphingomyelinase on ceramide and sphingomyelin content. Barrier disruption and isolation of the epidermis (2 and 4 hours after treatment) were performed as described in Figure 1. Epidermal homogenates were incubated with exogenous sphingomyelinase for 2 hours. Lipids were extracted by the method of Bligh and Dyer (30), separated by HPTLC, shown for ceramides in (a) and analyzed by laser densitometry after charring of the HPTLC plates (b).
Effects of A-SMase inhibition by imipramine on permeability barrier repair.
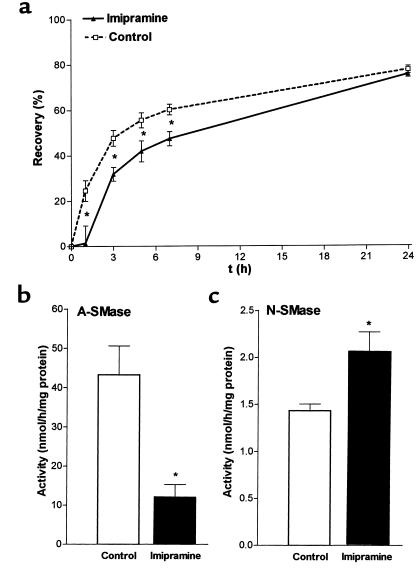
Previous studies have shown that the cyclic antidepressants desipramine and imipramine cause proteolytic degradation of lysosomal sphingomyelinase (A-SMase) in human fibroblasts (41, 42). In our animal model application of imipramine and desipramine resulted in a delay in permeability barrier compared with vehicle treatment. In imipramine-treated mice we found a significant delay in barrier repair at 1, 3, 5, and 7 hours, peaking at 1 hour after barrier disruption (Figure 6a). Delay in barrier repair was less pronounced after desipramine treatment (not shown).
Figure 6.
Topical application of imipramine results in delay of permeability barrier repair and reduction of stimulated A-SMase activity. Barrier disruption in hairless mice and application of imipramine (30 μL, 1% wt/wt) was performed as described in Figure 1. (a) Recovery in TEWL was determined at different time points after treatment (*P < 0.05, n = 5). (b) A-SMase (*P < 0.01, n = 6) and (c) N-SMase (*P < 0.025, n = 6) activities were determined at 1 hour after barrier disruption in the absence (open bars) and presence (filled bars) of imipramine.
To examine if the delay in permeability barrier repair after topical application of imipramine is related to sphingomyelinase we determined the effect of this compound on A- and N-SMase activities. Topical application of imipramine immediately after barrier disruption resulted in a 68% decrease in enzymatic activity 1 hour after treatment (P < 0.01, n = 6) (Figure 6b). In contrast, N-SMase activity was increased after barrier disruption (+42%; P < 0.025, n = 6) (Figure 6c). These results suggest that the delay in permeability barrier repair after topical application of imipramine may be caused by diminished A-SMase activity.
Localization of epidermal A- and N-SMase activity.
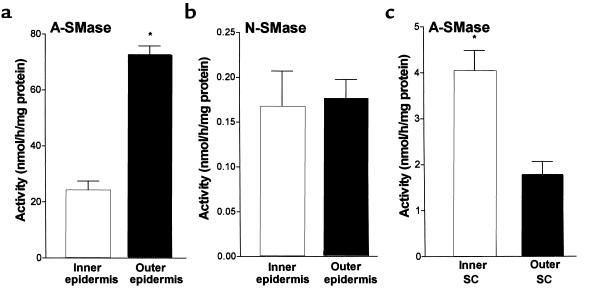
To gain information on possible functional roles of the sphingomyelinases in barrier repair, we next investigated the cutaneous location of both A- and N-SMase in untreated hairless mouse skin. As shown in Figure 7a, A-SMase activity in the outer (SC and stratum granulosum) was 3-fold greater than in the inner epidermal layers (stratum spinosum and stratum basale; P < 0.0001, n = 6). In contrast, N-SMase activity was equally distributed between outer and inner layers (49% and 51% of the total activity, respectively) (Figure 7b). N-SMase activity after staphylococcal exfoliative toxin separation was lower than that without the toxin. This could be related to a certain degree of toxicity against the enzyme. The predominance of A-SMase activity in the outer epidermal layers is most likely related to the localization of A-SMase in the lamellar bodies described previously (9, 10) and points to a possible function for A-SMase in generating ceramides for maintaining barrier function.
Figure 7.
Localization of epidermal A- and N-SMase activities. Hairless mice were injected intradermally with staphylococcal exfoliative. After 2 hours, outer and inner epidermal layers were isolated and (a) A-SMase (*P < 0.0001, n = 6) and (b) N-SMase (NS, n = 6) assays were performed. (c) SC samples were obtained by tape stripping. Cells from the outer (first tape strip) and the inner (fifth tape strip) were eluted in buffer A, and A-SMase activity was determined (P < 0.0001, n = 10).
To examine if A-SMase is still present after exocytosis of lamellar body content we assayed the enzyme activity in cells attached to tapes (cellophane type) obtained from outer and inner SC by different numbers of tape strips. A-SMase activity in the outer SC (first tape strip) was 1.8 nmol/h per milligram of protein and was significantly higher (4.0 nmol/h per milligram of protein; P < 0.0001, n = 10) in the inner SC (fifth tape strip) (Figure 7c). SC A-SMase activity in the inner epidermis was slightly increased at 1 hour (+7.6%; NS, n = 5) and was significantly reduced at 3 hours (not shown). N-SMase activity in the SC of untreated or treated skin isolated by tape strips was very low and did not differ from the basal ground level. These findings show that A-SMase is still active in the lower SC during lamellar body exocytosis and lipid bilayer formation.
Discussion
The cytokine TNF exerts a broad spectrum of biological functions and is involved in cell growth, differentiation, inflammation, and immune function (43, 44). An increase in epidermal TNF after permeability barrier disruption and in wound healing has been described (28–29). In this study we provide evidence that components of the TNF signaling pathway including sphingomyelinase and ceramide are crucially involved in cutaneous barrier repair. In hairless mice it was found that topical application of TNF after experimental injury by tape stripping enhances skin permeability barrier repair at 1–5 hours after treatment. We suggest that accelerated barrier repair in TNF compared with vehicle-treated mice is caused by enhanced concentration of this cytokine, which triggers the release of ceramide from sphingomyelin, which is important for barrier repair. Tape stripping results in endogenous TNF-production, whereas exogenous TNF will act immediately because it does not have to be synthesized de novo.
In TNF-R55–deficient mice barrier repair was significantly delayed compared with the wild-type animals. Because in TNF-R75–deficient mice barrier repair was unchanged, these data suggest a selective involvement of TNF-R55 in epidermal barrier repair. The sphingomyelinase pathway is important for TNF as well as Il-1 signaling (14). Previously, it has been shown that after experimental barrier disruption, an increase in TNF mRNA and protein levels occurs at 1 hour after barrier disruption, whereas an increase in Il-1-α and Il-1-β levels was delayed and occurred at 3 hours after barrier disruption (28). Using several transgenic mice strains that either overexpress or are defective in the Il-1 and Il-1 receptor system we could not find a significant delay in permeability barrier repair in Il-1 receptor 1–deficient mice (45). These results suggest a predominant role of TNF and TNF-R55 for skin barrier repair, whereas the role of the Il-1 seems to be less pronounced.
Previously, several cell-culture studies have documented the important role of N- and A-SMase as well as ceramides in TNF signaling (12, 14, 46, 47). Sphingomyelinases are involved in cell signaling for growth, differentiation, and apoptosis. In this study we found a low activity of N-SMase under basal conditions. Previously, similar values for the specific activities of N-SMase, A-SMase, and alkaline SMase (specific for the gastrointestinal tract) have been described in human colon tissue (48) and have been found in keratinocyte cell culture (E. Proksch, unpublished data). After stimulation by barrier disruption, a 2.5-fold increase in N-SMase activity was observed 1 hour after barrier disruption in this study. At 3 hours there was still a 1.5-fold increase in enzyme activity. The time course of activation was delayed compared with other extracutaneous reports of N-SMase activation by TNF (12). Most of the previous work on the activation of N-SMase has been performed in cell culture with a direct stimulation of receptor-coupled signaling pathways by exogenous TNF. In the in vivo situation in our studies it takes 30 to 60 minutes before the barrier disruption triggers N-SMase because endogenous TNF first must be synthesized.
We propose that N-SMase is responsible for generations of ceramides with signaling functions. Despite an increase during barrier repair the activity of this enzyme is too low to generate a significant amount of ceramides for structural functions in the barrier. However, it was shown previously that increase in epidermal proliferation is important for permeability barrier repair after experimental barrier disruption (25). Very recently we further explored the N-SMase signaling cascade in FAN-deficient mice. FAN is an adapter protein that couples the TNF-receptor p55 to N-SMase (49). We found a delayed permeability barrier repair and a significantly reduced epidermal proliferation in FAN-deficient animals defective in TNF-induced N-SMase activation (50). In TNF-R55–deficient mice we also detected a reduced increase in epidermal proliferation after barrier disruption (E. Proksch, unpublished data). These findings suggest a role of TNF-induced N-SMase activation in the regulation of proliferation during skin barrier repair.
Our data indicate that the TNF pathway through A-SMase generates ceramides with structural functions for the extracellular lipid lamellae in the SC permeability barrier. This assumption is based on the high activity of the enzyme present, the colocalization of A-SMase and sphingomyelin in the lamellar bodies (10, 11, 51, 52), and the increase in predominantly C24 ceramides that are abundant in the SC permeability barrier (37–39). In detail, we found that the activity of A-SMase in the epidermis was 60-fold higher than that of N-SMase. A-SMase activity is in the same range as data published previously for epidermal serine-palmitoyl-transferase (SPT) or hydroxy-methylglutaryl (HMG) CoA reductase activity (22, 23). In keratinocyte culture, where there is no permeability barrier, A-SMase activity is less then one-tenth the activity in the epidermis in vivo (E. Proksch, unpublished data). This implies that in vivo a considerable amount of ceramide for SC structural function is generated by A-SMase. Sublayer analysis in hairless mouse skin revealed that the highest A-SMase activity was localized in the outer epidermal layers (SC/stratum granulosum) where it was 3-fold higher than in the inner layers (stratum spinosum/stratum basale). In the SC a gradient with considerable activity in the inner and low activity in the outer layers became apparent. In contrast, N-SMase was equally distributed in outer and inner epidermal cell layers. Our studies are in accordance with the known colocalization of sphingomyelin and A-SMase in the epidermal lamellar bodies that are restricted to the upper stratum spinosum and the stratum granulosum (10, 11, 51, 52) and a function of A-SMase for degrading sphingomyelin to ceramides for the permeability barrier during the transition from the stratum granulosum to the SC. A significant activity (25% of the total activity) was also determined in the inner epidermis (stratum basale and stratum spinosum) and could represent, in part, the enzyme in the lamellar bodies of the upper stratum spinosum.
The role of the A-SMase for permeability barrier repair was further strengthened by lipid analysis. In parallel with the increase in A-SMase activity, we found a decrease in epidermal sphingomyelin content and an increase in ceramide content after barrier disruption. The values for the increase in ceramide, and especially for the decrease in sphingomyelin, seem to be relatively small. However, in comparison with the large amount of phospholipids, a 15% decrease in sphingomyelin content is significant. A greater loss in sphingomyelin would possibly result in cell destruction. We determined a predominant increase in C24 ceramide after barrier disruption. It was shown previously that C24 ceramide represents about 70% of SC ceramide (53). In addition, we found an increase in C16 ceramide that is also known to be a part of SC lipid bilayers (2, 53). Studies by Madison et al. (37), Vicanova et al. (38), and very recently by Doering et al. (39), have shown that the murine SC contains only 3–4 major ceramide fractions instead of 7 different fractions in human SC. In mouse skin the so-called ceramide 5 is a C16 ceramide containing an α-hydroxy group. Ceramide 2 is a C24 (and C26) ceramide without a hydroxy group. (In addition, in mouse skin there is a second α-hydroxy ceramide [C24], the ceramide 4, and there is the long-chain [C32 and C34] linoleic acid containing ω-hydroxy ceramide 1. The long chain ω-hydroxy ceramide 1 is not obtained by sphingomyelin hydrolysis; refs. 39, 54). Vicanova et al. have shown that ceramide 2 (and 1) can be easily separated from ceramide 5 (and 4) by HPTLC (38). However, ceramide 1 and ceramide 4 may comigrate with ceramide 2 and ceramide 5, respectively. Therefore, we found only 2 ceramide bands in our HPTLC. Application of exogenous A-SMase to epidermal homogenates generated ceramides with a HPTLC pattern comparable to that after experimental barrier disruption. These results suggest that an important amount of epidermal ceramides may be derived from sphingomyelin hydrolysis during permeability barrier repair.
An important novel finding of our study is the previously unrecognized role of A-SMase in the early phase of barrier repair. A-SMase activity after acute barrier disruption was increased already at 1 hour after treatment. It has been reported previously that the synthesis of all the lipids that reside in the SC permeability barrier (cholesterol, free fatty acids, and ceramides) is increased after barrier disruption (21). However, regarding the rate-limiting enzymes, an increase in HMG CoA reductase activity was observed at 2.5–4.5 hours and the increase in fatty acid synthase and acetyl CoA carboxylase was detected at 2–6 hours after barrier disruption (22, 24). The increase in SPT activity for ceramide synthesis was delayed, occurring at 6–12 hours after treatment (23). The amount of ceramides is also regulated by the hydrolytic enzyme β-glucocerebrosidase, which cleaves glucosylceramide. Previously, Holleran et al. showed the important role of β-glucocerebrosidase in skin barrier homeostasis (55–57), and there are parallels between the function of β-glucocerebrosidase and A-SMase in skin barrier repair. β-glucocerebrosidase is also localized in the outer, more differentiated epidermal cell layers (7). Single or repeated treatment by the β-glucocerebrosidase inhibitor bromo-conduritol-B-expoxide delayed barrier recovery or resulted in a chronic disrupted permeability barrier (a model for Gaucher’s disease; ref. 57). However, there are also some differences. The A-SMase inhibitor imipramine only results in a delayed permeability barrier repair; repeated treatment does not induce chronic barrier disruption (E. Proksch, unpublished data). In contrast to early activation of A-SMase, a significant increase in β-glucocerebrosidase activity after barrier disruption did not occur before 8 hours after treatment at the mRNA level and not before 24 hours at the protein level (55). Thus the rapid activation of A-SMase seems to be important for the generation of structural ceramides early during permeability barrier repair, whereas β-glucocerebrosidase exerts a function in late barrier repair and the long-term maintenance of skin barrier homeostasis.
The functional role of A-SMase in barrier repair was further demonstrated in the this report by the employment of the cyclic antidepressants imipramine and desipramine, which cause proteolytic degradation of lysosomal sphingomyelinase (A-SMase) (41, 42). Application of imipramine and desipramine in hairless mice after experimental injury to the skin resulted in a delay in permeability barrier paralleled by a significant reduction in A-SMase activity, whereas the N-SMase level remained unaffected. These results show that inhibition of A-SMase is sufficient to result in a delay in permeability barrier repair. Our findings are in accordance with very recent human study in a family of type B Niemann-Pick disease patients who exhibited a severe reduction in A-SMase activity (58). These patients showed a delay in permeability barrier repair after experimental disruption (59), supporting our findings obtained by A-SMase inhibition with imipramine in mice.
Notably, in TNF-R55–deficient mice no stimulation of either A- and N-SMase activities occurred after barrier disruption. Therefore, the delay in early permeability barrier repair in TNF-R55–deficient mice is very likely related to the abnormal regulation of A- and N-SMase activities. Again, these findings suggest a predominant role of TNF in sphingomyelin hydrolysis for permeability barrier repair.
In summary, our in vivo results suggest a functional role of TNF and the TNF receptor p55 connecting SMases through production of ceramide to cutaneous permeability barrier repair after SC injury. These findings might be important for the development of new treatment modalities to enhance permeability barrier repair in skin diseases and in wound healing.
Acknowledgments
We would like to thank T. Kupper for the opportunity to determine barrier function in TNF-R75–deficient mice. We greatly appreciate the technical assistance of Claudia Neumann. J.-M. Jensen is a recipient of a grant from the European Academy of Dermatology (EADV), Yamanouchi (Europe), and the Deutsche Forschungsgemeinschaft (DFG). This work was supported by the Deutsche Forschungsgemeinschaft (SFB 415).
Footnotes
Jens-Michael Jensen’s current address is: The Harvard Skin Disease Research Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts 02115, USA.Martin Krönke’s current address is: Institute of Medical Microbiology, University of Cologne, 50935 Cologne, Germany.
References
- 1.Elias PM. Epidermal lipids, barrier function, and desquamation. J Invest Dermatol. 1983;80(Suppl.):44S–49S. [PubMed] [Google Scholar]
- 2.Schürer, N.Y., and Elias, P.M. 1991. The biochemistry and function of stratum corneum lipids. In Advances in lipid research (skin lipids). P.M. Elias, editor. Academic Press Inc. San Diego, CA. 27–56. [DOI] [PubMed]
- 3.Wilhelm KP, Surber C, Maibach HI. Effect of sodium lauryl sulfate–induced skin irritation on in vivo percutaneous penetration of four drugs. J Invest Dermatol. 1991;97:927–932. doi: 10.1111/1523-1747.ep12491710. [DOI] [PubMed] [Google Scholar]
- 4.Di Nardo A, Wertz P, Giannetti A, Seidenari S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm Venereol. 1998;78:2–30. doi: 10.1080/00015559850135788. [DOI] [PubMed] [Google Scholar]
- 5.Grice K, Sattar H, Baker H, Sharratt M. The relationship of transepidermal water loss to skin temperature in psoriasis and eczema. J Invest Dermatol. 1975;64:313–315. doi: 10.1111/1523-1747.ep12512258. [DOI] [PubMed] [Google Scholar]
- 6.Suetake T, Sasai S, Zhen YX, Ohi T, Tagami H. Functional analyses of the stratum corneum in scars. Sequential studies after injury and comparison among keloids, hypertrophic scars, and atrophic scars. Arch Dermatol. 1996;132:1453–1458. [PubMed] [Google Scholar]
- 7.Holleran WM, et al. β-glucocerebrosidase activity in murine epidermis: characterization and localisation in relationship to differentiation. J Lipid Res. 1992;33:1201–1209. [PubMed] [Google Scholar]
- 8.Tagaki Y, Kriehuber E, Imokawa G, Elias PM, Holleran WM. β-glucocerebrosidase activity in mammalian stratum corneum. J Lipid Res. 1999;40:861–869. [PubMed] [Google Scholar]
- 9.Menon GK, Grayson S, Elias PM. Cytochemical and biochemical localization of lipase and sphingomyelinase activity in mammalian epidermis. J Invest Dermatol. 1986;86:591–597. doi: 10.1111/1523-1747.ep12355263. [DOI] [PubMed] [Google Scholar]
- 10.Freinkel RK, Traczyk TN. Acid hydrolases of the epidermis: subcellular localization and relationship to cornification. J Invest Dermatol. 1983;80:441–446. doi: 10.1111/1523-1747.ep12555534. [DOI] [PubMed] [Google Scholar]
- 11.Freinkel RK, Traczyk TN. Lipid composition and acid hydrolase content of lamellar granules of fetal rat epidermis. J Invest Dermatol. 1985;85:295–298. doi: 10.1111/1523-1747.ep12276831. [DOI] [PubMed] [Google Scholar]
- 12.Wiegmann K, Schütze S, Machleidt T, Witte D, Krönke M. Functional dichotomy of neutral and acidic sphingomyelinases in tumor necrosis factor signaling. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 13.Hannun YA, Linardic CM. Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta. 1993;1154:223–236. doi: 10.1016/0304-4157(93)90001-5. [DOI] [PubMed] [Google Scholar]
- 14.Kolesnick RN, Golde DW. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 15.Heller RA, Krönke M. Tumor necrosis factor receptor-mediated signaling pathways. J Cell Biol. 1994;126:5–9. doi: 10.1083/jcb.126.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolesnick RN, Krönke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 17.Cifone MG, et al. Multiple pathways originate at the Fas/APO-1 (CD95) receptor: sequential involvement of phosphatidylcholine-specific phospholipase C and acidic sphingomyelinase in the propagation of the apoptotic signal. EMBO J. 1995;14:5859–5868. doi: 10.1002/j.1460-2075.1995.tb00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santana P, et al. Acid sphingomyelinase-deficient human lymphoblasts and mice are defective in radiation-induced apoptosis. Cell. 1996;86:189–199. doi: 10.1016/s0092-8674(00)80091-4. [DOI] [PubMed] [Google Scholar]
- 19.Herr I, Wilhelm D, Bohler T, Angel P, Debatin KM. Activation of CD95 (APO-1/Fas) signaling by ceramide mediates cancer therapy-induced apoptosis. EMBO J. 1997;16:6200–6208. doi: 10.1093/emboj/16.20.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Maria R, Testi R. Fas-FasL interactions: a common pathogenetic mechanism in organ-specific autoimmunity. Immunol Today. 1998;19:121–125. doi: 10.1016/s0167-5699(97)01202-4. [DOI] [PubMed] [Google Scholar]
- 21.Grubauer G, Feingold KR, Elias PM. Relationship of epidermal lipogenesis to cutaneous barrier function. J Lipid Res. 1987;28:746–752. [PubMed] [Google Scholar]
- 22.Proksch E, Elias PM, Feingold KR. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in murine epidermis: modulation of enzyme content and activation state by barrier requirement. J Clin Invest. 1990;85:874–882. doi: 10.1172/JCI114514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holleran WM, et al. Regulation of epidermal sphingolipid synthesis by permeability barrier function. J Lipid Res. 1991;32:1151–1158. [PubMed] [Google Scholar]
- 24.Ottey KA, Wood LC, Grunfeld C, Elias PM, Feingold KR. Cutaneous permeability barrier disruption increases fatty acid synthetic enzyme activity in the epidermis of hairless mice. J Invest Dermatol. 1995;104:401–404. doi: 10.1111/1523-1747.ep12665893. [DOI] [PubMed] [Google Scholar]
- 25.Proksch E, Feingold KR, Elias PM. Barrier function regulates epidermal DNA-synthesis. J Clin Invest. 1991;87:1668–1673. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekanayake-Mudiyanselage S, et al. Expression of epidermal keratins and the cornified envelope protein involucrin is influenced by permeability barrier disruption. J Invest Dermatol. 1998;111:517–523. doi: 10.1046/j.1523-1747.1998.00318.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SH, et al. Calcium and potassium are important regulators of barrier homeostasis in murine epidermis. J Clin Invest. 1992;89:530–538. doi: 10.1172/JCI115617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wood LC, Jackson SM, Elias PM, Grunfeld C, Feingold KR. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90:482–487. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–546. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- 30.Hübner G, et al. Differential regulation of pro-inflammatory cytokines during wound healing in normal and glucocorticoid-treated mice. Cytokine. 1996;8:548–556. doi: 10.1006/cyto.1996.0074. [DOI] [PubMed] [Google Scholar]
- 31.Tsai JC, et al. Permeability barrier disruption alters the localization and expression of TNF-protein in the epidermis. Arch Dermatol Res. 1994;286:242–248. doi: 10.1007/BF00387595. [DOI] [PubMed] [Google Scholar]
- 32.Pfeffer K, et al. Mice deficient for the p55kD tumor necrosis factor receptor are resistant for endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 33.Peschon JJ, et al. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 34.Elias PM, Fritsch P, Tappeiner G, Mittermayer H, Wolff K. Experimental staphylococcal toxic epidermal necrolysis (TEN) in adult humans and mice. J Lab Clin Med. 1974;84:414–424. [PubMed] [Google Scholar]
- 35.Proksch E, Elias PM, Feingold KR. Localization and regulation of epidermal 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity by barrier requirements. Biochim Biophys Acta. 1991;1083:71–79. doi: 10.1016/0005-2760(91)90126-3. [DOI] [PubMed] [Google Scholar]
- 36.Bligh E, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physio. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 37.Madison KC, Swartzendruber DC, Wertz PW, Downing DT. Sphingolipid metabolism in organotypic mouse keratinocyte cultures. J Invest Dermatol. 1990;95:657–664. doi: 10.1111/1523-1747.ep12514333. [DOI] [PubMed] [Google Scholar]
- 38.Vicanova J, et al. Stratum corneum lipid composition and structure in cultured skin substitutes is restored to normal after grafting onto athymic mice. J Investig Dermatol Symp Proc. 1998;3:114–120. doi: 10.1038/jidsymp.1998.24. [DOI] [PubMed] [Google Scholar]
- 39.Doering T, et al. Sphingolipid activator proteins are required for epidermal permeability barrier formation. J Biol Chem. 1999;274:11038–11045. doi: 10.1074/jbc.274.16.11038. [DOI] [PubMed] [Google Scholar]
- 40.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 41.Albouz S, et al. Tricyclic antidepressants induce sphingomyelinase deficiency in fibroblast and neuroblastoma cell cultures. Biomedicine. 1981;35:218–220. [PubMed] [Google Scholar]
- 42.Hurwitz R, Ferlinz K, Sandhoff K. The tricyclic antidepressant desipramine causes proteolytic degradation of lysosomal sphingomyelinase in human fibroblasts. Biol Chem Hoppe Seyler. 1994;375:447–450. doi: 10.1515/bchm3.1994.375.7.447. [DOI] [PubMed] [Google Scholar]
- 43.Ballou LR. Sphingolipids and cell function. Immunol Today. 1992;13:339–341. doi: 10.1016/0167-5699(92)90167-6. [DOI] [PubMed] [Google Scholar]
- 44.Ballou LR, Laulederkind SJ, Rosloniec EF, Raghow R. Ceramide signaling and the immune response. Biochim Biophys Acta. 1996;1301:273–287. doi: 10.1016/0005-2760(96)00004-5. [DOI] [PubMed] [Google Scholar]
- 45.Jensen JM, Kupper TS, Proksch E. Il-1/Il-1 receptor overexpression and knockout constructs in permeability barrier repair of transgenic mice. J Invest Dermatol. 1998;110:499a–(Abstr.). [Google Scholar]
- 46.Schütze S, Pothoff K, Machleidt T, Berkovic D, Wiegmann K, Krönke M. TNF activates NF- B by phosphatidylcholine-specific sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 47.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 48.Hertvig E, Nilsson A, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer. 1997;79:448–453. [PubMed] [Google Scholar]
- 49.Adam-Klages S, et al. FAN, a novel WD-repeat protein, couples the p55 TNF-receptor to neutral sphingomyelinase. Cell. 1996;86:937–947. doi: 10.1016/s0092-8674(00)80169-5. [DOI] [PubMed] [Google Scholar]
- 50.Kreder D, et al. Impaired neutral sphingomyelinase activation and cutaneous barrier repair in FAN-deficient mice. EMBO J. 1999;18:2472–2479. doi: 10.1093/emboj/18.9.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bowser PA, Gray PM. Sphingomyelinase in pig and human epidermis. J Invest Dermatol. 1978;70:331–335. doi: 10.1111/1523-1747.ep12543516. [DOI] [PubMed] [Google Scholar]
- 52.Elias PM, Menon GK, Grayson S, Brown BE. Membrane structural alterations in murine stratum corneum: relationship to the localization of polar lipids and phospholipases. J Invest Dermatol. 1988;91:3–10. doi: 10.1111/1523-1747.ep12463279. [DOI] [PubMed] [Google Scholar]
- 53.Robson KJ, Stewart ME, Michelsen S, Lazo ND, Downing DT. 6-Hydroxy-4-sphingenine in human epidermal ceramides. J Lipid Res. 1994;35:2060–2068. [PubMed] [Google Scholar]
- 54.Yano M, Kishida E, Muneyuki Y, Masuzawa Y. Quantitative analysis of ceramide molecular species by high performance liquid chromatography. J Lipid Res. 1998;39:2091–2098. [PubMed] [Google Scholar]
- 55.Holleran WM, et al. Permeability barrier requirements regulate epidermal β-glucocerebrosidase. J Lipid Res. 1994;35:905–912. [PubMed] [Google Scholar]
- 56.Holleran WM, et al. Processing of epidermal glucosylceramides is required for optimal mammalian cutaneous permeability barrier function. J Clin Invest. 1993;91:1656–1664. doi: 10.1172/JCI116374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Holleran WM, et al. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest. 1994;93:1756–1764. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levade T, Salvayre R, Douste-Blazy L. Sphingomyelinases and Niemann-Pick disease. J Clin Chem Clin Biochem. 1986;24:205–220. [PubMed] [Google Scholar]
- 59.Schmuth M, Weber F, Paschke E, Sepp N, Fritsch P. Delayed epidermal barrier repair in patients with sphingomyelinase deficiency (Niemann Pick Disease) J Invest Dermatol. 1999;112:542a–(Abstr.). [Google Scholar]