Abstract
Ligaments have limited regenerative potential and as a consequence, repair is protracted and results in a mechanically inferior tissue more scar-like than native ligament. We previously reported that a single injection of interleukin-1 receptor antagonist (IL-1Ra) delivered at the time of injury, decreased the number of M2 macrophage-associated inflammatory cytokines. Based on these results, we hypothesized that IL-1Ra administered after injury and closer to peak inflammation (as would occur clinically), would more effectively decrease inflammation and thereby improve healing. Since IL-1Ra has a short half-life, we also investigated the effect of multiple injections. The objective of this study was to elucidate healing of a medial collateral ligament (MCL) with either a single IL-1Ra injection delivered one day after injury or with multiple injections of IL-1Ra on days 1, 2, 3, and 4. One day after MCL injury, rats received either single or multiple injections of IL-1Ra or PBS. Tissue was then collected at days 5 and 11. Both single and multiple IL-1Ra injections reduced inflammatory cytokines, but did not change mechanical behavior. A single injection of IL-1Ra also reduced the number of myofibroblasts and increased type I procollagen. Multiple IL-1Ra doses provided no additive response and, in fact, reduced the M2 macrophages. Based on these results, a single dose of IL-1Ra was better at reducing the MCL-derived inflammatory cytokines compared to multiple injections. The changes in type I procollagen and myofibroblasts further suggest a single injection of IL-1Ra enhanced repair of the ligament but not sufficiently to improve functional behavior.
Keywords: ligament, healing, scar, IL-1Ra, immunohistochemistry, rat, mechanical testing
Introduction
Ligament and tendon repair involves a complex cascade of coordinated events involving various cell types, cytokines and extracellular matrix (ECM) factors. The repair process ranges from months to years and results in scar tissue mechanically inferior to native tissue. Normal ligament healing after injury undergoes inflammation, proliferation, and remodeling. Neutrophils, macrophages, and T-lymphocytes initially infiltrate the wound followed by fibroblasts, myofibroblasts, endothelial cells and additional macrophages.(1, 2) These cells form granulation tissue which expands to remodel residual ECM, reorganizing intact portions of the ligament and contributing to an inefficient healing response.(1) As healing continues, type I procollagen decreases, while myofibroblasts and type III collagen increases.(1) Myofibroblasts serve in early wound contraction and scar formation. The newly formed type III collagen serves as a weak transient connection for the injured ligament and is an indicator of scar formation. As remodeling progresses, the scar matures and the ratio of type I to type III collagen normalizes, improving the tensile strength of the compromised region, but never fully returning to its original state.(3, 4) In an improved healing scenario, granulation tissue would develop within the confines of the injury, collagen type I would regenerate rapidly, myofibroblasts numbers and type III collagen production would be minimal, and native tissue would regenerate to demonstrate pre-damage organization, laxity and strength. Numerous approaches to stimulate a regenerative scenario have been attempted, but none have resulted in complete regeneration. Previous research from our lab identified a number of cellular, vascular, and molecular components integral to early healing.(1, 5, 6) Specifically, interleukin-1β (Il1b) and interleukin-1 receptor antagonist (Il1ra) were found to be significantly up-regulated in the healing medial collateral ligament (MCL) 3 days post-transection compared to the intact ligament.(7) Interleukin-1 (Il1) gene expression levels decreased at day 7 but remained significantly higher than the intact tissue.(7) The M1 (classically activated) and M2 (alternatively activated) macrophages have also been reported to peak between days 3 and 5.(1) The M1 macrophages secrete pro-inflammatory mediators, such as IL-1, and participate in the activation of various cytotoxic processes, including the respiratory burst, which creates extensive collateral damage and aberrant inflammation.(8-10) In contrast to the M1 inflammatory macrophages, the M2 macrophages secrete potent anti-inflammatory cytokines, such as interleukin-10 (IL-10), and may play important roles in wound healing and restoration of tissue homeostasis.(11, 12) Past research indicated that M1 macrophages can be phenotypically converted into M2 anti-inflammatory macrophages. The M2 macrophages are then able to induce myofibroblast apoptosis, serve as antigen presenting cells, and decrease the magnitude and duration of inflammation to promote wound healing.(13, 14) Controlling these early inflammatory mediators and cell types may therefore modulate subsequent healing and diminish scar formation.
IL-1 is a pleiotropic inflammatory cytokine, exerting distinct effects on various cell types involved in all phases of healing. Receptors for IL-1 are found on inflammatory cells, including neutrophils, macrophages, T and B lymphocytes, which allow IL-1 to modulate immune-cell dominated inflammation.(10) IL-1 receptors are also localized to fibroblasts, allowing for myofibroblast/matrix deposition-controlled fibrogenesis.(10) IL-1 produced within a wound regulates production of multiple inflammatory mediators, including chemokines that direct inflammatory cell infiltration and pain.(15) In skin wound models, IL-1 receptor knockout mice showed reduced inflammatory cell infiltration and fibrosis, suggesting that IL-1 inhibition could provide therapeutic value in attenuating scar formation.(16)
IL-1Ra is an endogenous antagonist that binds to the IL-1 receptor with comparable affinity as IL-1, but requires a 10-100 molar excess to inhibit IL-1 activity.(17, 18) Mice with Il1ra gene deletion were found to have an enhanced response to IL-1, were more susceptible to infections, and were more likely to develop spontaneously occurring inflammatory arthritis.(19-21) We previously reported that perioperative administration of IL-1Ra reduced the MCL-derived inflammatory cytokines and concomitantly increased the M2 macrophages when administered to a rat ligament healing model, indicating a potential therapeutic role for IL-1Ra.(7) Indeed, the recombinant form of IL-1Ra, Anakinra (Swedish Orphan Biovitrum, Stockholm, Sweden), has been FDA approved for treatment of rheumatoid arthritis and has a good safety record.(22-24) However, our previous results also indicated no significant improvement in ligament mechanical behavior after one injection of IL-1Ra during injury. Based on these results, we first hypothesized that IL-1Ra administered after injury and closer to peak inflammation, would improve healing in a therapeutic (i.e. more clinically relevant) manner. We then hypothesized that multiple injections of IL-1Ra administered after injury would provide an additive healing response compared to one IL-1Ra injection. The objective of this study was to therefore elucidate the effects of single IL-1Ra injection delivered one day after injury or the effects of multiple IL-1Ra injections delivered at post injury days 1, 2, 3, and 4, on rat medial collateral ligament (MCL) healing.
Methods
IL-1Ra Experimental Model
Experimental procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee. In order to identify the influence of IL-Ra on MCL healing, 38 skeletally mature male Wistar rats (275-299 g) were randomly divided into 4 groups and subjected to bilateral MCL transections. The MCLs were transected, rather than torn, to create uniform defects for healing. Skin was incised (1 cm) over the medial aspect of the left and right stifles, exposing each gracilis muscle and underlying MCL. The mid-point of each MCL was completely transected. The transected edges were then were positioned back to their natural state. The muscular, subcutaneous and subdermal tissue layers were each repaired with 4-0 Dexon suture. Animals were allowed unhindered cage movement immediately after surgery. Animals were then randomly divided into “single injection” or “multiple injections” groups. For the single injection experiment, rats were treated with either a single 600 ng injection of rat recombinant IL-1Ra (R&D Systems, Minneapolis, MN) or a single injection of phosphate buffered saline (PBS; vehicle control for IL-1Ra), SC over each MCL (n=16 rats) at 18-24 hours post-injury. For the multiple injection experiment, animals (n= 16) received daily injections of 600 ng IL-1Ra (n=8) or PBS (vehicle control for IL-1Ra; n=8) SC over each MCL, starting at 18-24 hours post-injury and continuing on and until day 4 post-injury. For both experiments, MCLs were collected at 5 and 11 days post-injury and used for IHC/multiplex analysis and mechanical testing, respectively. A day 5 collection was chosen since our previous work showed macrophage infiltration and granulation tissue formation peaks at this time and we were interested in the effects of IL-1Ra on macrophage response and granulation tissue formation.(1) A day 11 collection was chosen for mechanical testing since ligaments are substantially compromised earlier making it more difficult to obtain meaningful data. Ligaments used for IHC for both single and multiple injection experiments were carefully dissected and immediately embedded longitudinally in optimal cutting temperature (O.C.T.) medium for liquid nitrogen flash freezing. Tissue used for multiplex analysis was carefully dissected and immediately snap-frozen. Animals used for mechanical testing, were sacrificed and limbs were stored in toto at -70C until used.
Immunohistochemistry
In order to identify cellular and ECM changes within the healing MCL after IL-1Ra treatment, IHC and histology were performed on day 5 MCLs. Longitudinally positioned cryospecimens were sectioned (5 μm thickness), mounted on Colorfrost Plus (Fisher Scientific, Pittsburgh, PA) microscope slides and maintained at −70 C. Cryosections were fixed in acetone, exposed to 3% hydrogen peroxide to remove endogenous peroxidase activity, blocked with Background Buster (Innovex Biosciences, Richmond, CA) and incubated with mouse primary antibodies. Mouse monoclonal antibodies to CD68 (1:100, Abcam-Serotec, Raleigh, NC), CD163 (1:100, Abcam-Serotec, Raleigh, NC), CD31 (1:100, Abcam-Serotec, Raleigh, NC), α-smooth muscle actin (SMA; no dilution, Abcam-Serotec, Raleigh, NC), and Ki-67 (1:100, Dako, Carpinteria, CA) were utilized to identify the classically activated macrophages (M1), alternatively activated macrophages (M2), endothelial cells, myofibroblasts and proliferating cells, respectively. Mouse antibodies were also used to identify type I procollagen (straight; SP1.D8; Developmental Hybridoma, Iowa City, Iowa) and the scar indicator, type III collagen (1:8000, Sigma-Aldrich, St. Louis, MO). After primary antibody inbubation, sections were incubated with biotin, and streptavidin-conjugated to horseradish peroxidase using the Stat Q IHC staining kit (Innovex Biosciences, Richmond, CA). The bound antibody was then visualized using diaminobenzidine (DAB). Stained sections were dehydrated, cleared, cover-slipped and visualized using light microscopy.
After staining, images of each IHC marker were collected using a camera assisted microscope (Nikon Eclipse microscope, model E6000 (Mellville, NY) with an Olympus camera, model DP79 (Center Valley, PA)). Within each IHC-stained tissue section, five images were obtained; three images were captured within the granulation tissue (granulation tissue proximal edge, granulation tissue distal edge, center of granulation tissue) and two images were captured away from the granulation tissue (proximal end and distal end of the MCL; Fig. 1a). Images were obtained from 3 sections per rat for a total of 15 images. Pictures captured for measurement of endothelial cells, myofibroblasts, type I procollagen, and type III collagen were quantified using Image J (National Institutes of Health, NIH, Bethesda, MD). Briefly, images were first converted to 8-bit files. The total area of each ligament within each captured image was then measured. A set threshold to identify DAB staining was applied (the threshold was adjusted specifically for the IHC protein of interest). Particle size was then adjusted (based on the protein of interest). The resulting output was recorded, adjusted based on the ligament area, and expressed as density/mm^2. Images of blood vessel lumen, proliferating cells, M1 and M2 macrophages were quantified manually. For all images, the resulting output was recorded based on the area the image was captured (ex: granulation tissue, proximal end, etc) to later determine any spatial influence of IL-1Ra within the MCL.
Figure 1. Representative images of measurement techniques.
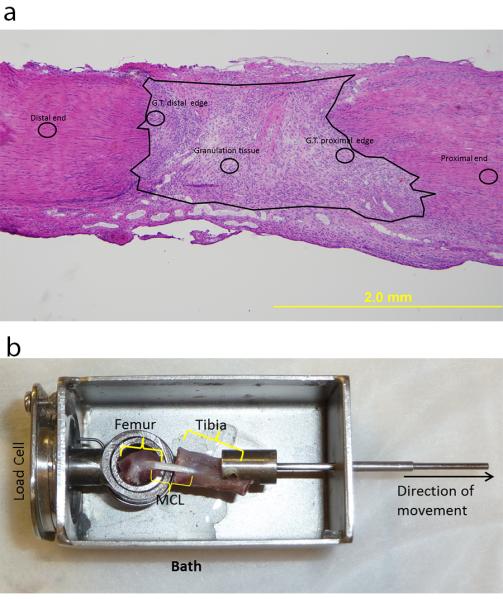
Low magnification H&E image of the day 5 MCL demonstrating the regions where high magnification images were captured (a). The circles indicate regions on the MCL where representative images were collected. The regions consist of the granulation tissue (G.T.), G.T. distal edge, G.T. proximal edge, distal end, and proximal end of the ligament. The large black circle encircles the granulation tissue. Image of the mechanical testing device (bath) containing the gripped MCL prior to mechanical testing (b).
Histology
MCL sections were H&E stained to observe general morphology of the healing ligaments. Tissue was observed for any changes in cell distribution, collagen organization, and granulation tissue size between treatment groups. To measure granulation tissue size, images of the MCL were captured using a camera assisted microscope (Nikon Eclipse microscope, model E6000 with an Olympus camera, model DP79) and the granulation tissue area was measured using Image J (National Institutes of Health, NIH, Bethesda, MD; Fig. 1a). Size of the granulation tissue was normalized to the total MCL area and expressed as the percent normalized granulation tissue.
Multiplex Assay
To identify the influence of IL-1Ra on MCL cytokine production, a rat 10-plex Luminex assay (Life Technologies, Grand Island, NY) was performed using the day 5 MCLs. MCLs were rinsed in Cell Wash Buffer (Bio-Rad, Hercules, CA) and placed in Navy Bead Lysis Kit tubes containing a 0.9-2.0 mm stainless steel bead blend, 3.2 mm stainless steel balls (Next Advance, Averill Park, NY) and Lysing Solution (Bio-Rad, Hercules, CA). Samples were homogenized for 10 minutes using a Bullet Blender (Next Advance, Averill Park, NY). Supernatant was collected and used for Bicinchoninic acid (BCA), to determine total protein concentration, and subsequent multiplex analysis. Multiplex cytokine assays (Life Technologies, Grand Island, NY) were performed according to the manufacturer’s instructions. Diluted magnetic bead solution was vortexed, sonicated and added to each well. Standards, MCLs samples, spleen (serving as positive control), and lysis buffer (serving as negative control) were added to the wells and incubated in the dark, overnight at 4 C on a plate shaker. The following day, samples were washed, incubated with biotinylated detector antibody, and streptavidin-RPE solution. After washing and re-suspension in working wash solution, samples were quantified on a Luminex 200 instrument (Luminex, Austin TX). Ten proteins were included in the assay, including interleukin-1α (IL-1α), IL-1β, IL-10, interleukin-2 (IL-2), interleukin-12 (IL-12), interleukin-4 (IL-4), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and granulocyte macrophage-colony stimulating factor (GM-CSF). Assessment of the multiplex assay was performed by verifying each standard curve point was within 80-120% recovery, and 2 standard deviations above background. Cytokine concentrations were normalized to protein concentration and expressed as ng of cytokine per mg of tissue (ng/mg).
Mechanical Testing
In order to assess the functional behavior of the healing MCL after treatment with IL-1Ra, day 11 ligaments were mechanically tested. Pull-to-failure analysis was performed as previously described.(25-27) After rat sacrifice the MCL was removed with both femoral and tibial (FMT) insertion sites intact, and the surrounding tissue was carefully excised with special care taken to avoid damaging the insertion sites. During preparation, the FMT complex remained hydrated using PBS. The width and thickness of the MCL was measured optically and the cross-sectional area for the ligament was estimated assuming an elliptical cross section. The hydrated femur-MCL-tibia complex was mounted in a custom made testing bath and mechanical testing machine (Fig. 1b). Optical markers (silicon gel with graphite) were applied to the ligament on the insertion sites and the displacement was recorded optically. After pre-loading to 0.1 newtons (N), dimension measurements were recorded and the ligament was preconditioned (cyclically loaded to 1% strain for 10 cycles). The MCL was then pulled to failure at a rate of 10% strain per second. Failure force was documented as the highest load prior to failure of the ligament. The slope of the linear portion of the line relating stress to elongation was used to calculate stiffness.
Statistical Analysis
For the IHC results, MCL regions were subgrouped (granulation tissue, outside of granulation tissue, and total MCL) to identify any potential spatial differences of each factor measured. Student’s T-tests were then used to examine treatment differences for both IHC and mechanical results. For the multiplex data, analysis of variance (ANOVA) was used to examine treatment differences. If the overall p-value for the F-test in ANOVA was significant, Fisher’s least significant difference (LSD) post-hoc comparisons were performed. Experimental results are presented as the means ± standard error of the mean (S.E.M) If p < 0.050, data were considered statistically significant. Computations were performed using KaleidaGraph, version 4.03 (Synergy Software, Inc., Reading, PA).
Results
H&E staining
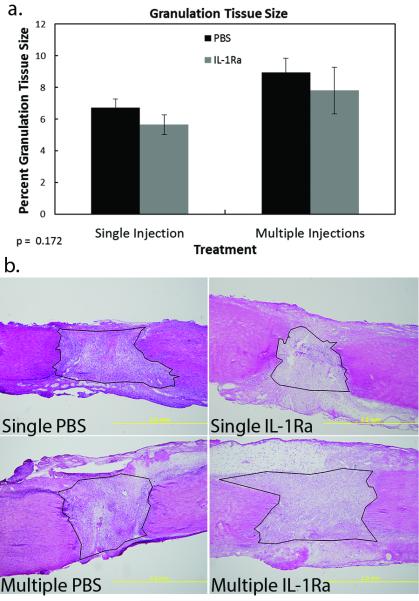
To first determine if IL-1Ra modulated general ligament morphology, H&E staining of the tissue was performed. No changes in cell distribution, cell number, or collagen organization were observed between treatment groups. Granulation tissue size normalized to the total MCL area also did not differ (p > 0.050) between treatments, regardless of the number of IL-1Ra injections (Fig. 2a-b).
Figure 2. Size of granulation tissue of the MCL after PBS or IL-1Ra treatment.
Graph demonstrating the effect of single or multiple treatments of PBS or IL-1Ra on granulation tissue size (a). No significant differences were noted. Data was considered significant when p< .05. Values are expressed as mean cell numbers ± S.E.M. Representative micrographs of H&E stained day 5 MCLs after single or multiple treatments of PBS or IL-1Ra (b). Black lines encircling the granulation tissue represent examples of how the granulation tissue was measured.
Immunohistochemistry
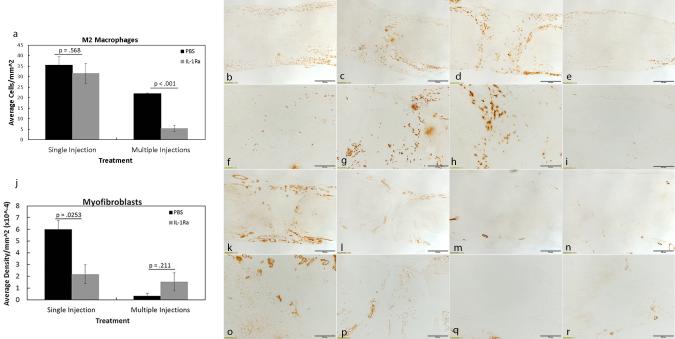
Components of inflammation and granulation tissue formation, including the M1 and M2 macrophages, proliferating cells, endothelial cells, blood vessel lumen, and myofibroblasts, were examined since we previously reported significant regulation by IL-1Ra when administered perioperatively. No significant changes were observed in M1 macrophage numbers after single (p = 0.325) or multiple (p= 0.617) injections of IL-1Ra (Table 1). In contrast, multiple injections with IL-1Ra significantly reduced (p < 0.001; 5.50 ± 1.50 cells/mm2; Figs. 3a-i) the granulation tissue-localized M2 macrophages compared to PBS (22.00 ± 0.19 cells/mm2). No significant changes (p = 0.568) were observed in M2 macrophage numbers after a single injection of IL-1Ra (PBS, 35.53 ± 4.04 cells/mm2 vs. IL-1Ra, 31.61 ± 4.83 cells/mm2). Myofibroblasts were decreased (p = 0.025) with a single injection of IL-1Ra (2.19 ± 0.80 × 10−4 density/mm2) throughout the entire ligament compared to PBS (6.00 ± 0.75 × 10−4 density/mm2; Figs. 3j-r) whereas no treatment differences (p = 0.211) were noted after multiple IL-1Ra treatments (PBS, 0.35 ± 0.22 × 10−4 density/mm2 vs. IL-1Ra, 1.54 ± 0.77 × 10−4 density/mm2). Regardless of injection number, proliferating cells, endothelial cells, and blood vessel lumen were not significantly (p > 0.050) different after IL-1Ra treatment (Table 1). To further assess whether IL-1Ra improved healing and reduced scar, type I procollagen and type III collagen were measured. Type I procollagen significantly (p =0.029) increased after a single injection of IL-1Ra (PBS, 1.48 ± 0.38 × 10−3 density/mm2 vs. IL-1Ra, 8.15 ± 1.62 × 10−3 density/mm2; Figs. 4a-j). Procollagen also tended to increase (p = 0.069) after daily IL-1Ra injections (PBS, 0.19 ± 0.09 × 10−3 density/mm2 vs. IL-1Ra, 3.42 ± 1.02 × 10−3 density/mm2). No changes were noted between treatments in the scar-indicator, type III collagen (Table 1).
Table 1.
Immunohistochemistry results of MCL cellular and ECM factors after treatment with IL-1Ra
| IHC Factor | P- value |
Single PBS | Single IL-1Ra | P- Value |
Multiple PBS | Multiple IL-1Ra |
|---|---|---|---|---|---|---|
| M1 Macrophages (Ave. cells/mm^2) |
0.325 | 86.34 ± 13.35 | 71.18 ± 2.22 | 0.617 | 57.55 ± 4.28 | 53.06 ± 7.09 |
| Proliferating Cells (Ave. cells/mm^2) |
0.676 | 31.92 ± 3.38 | 28.71 ± 6.27 | 0.368 | 45.58 ± 11.22 | 60.48 ± 9.48 |
| Endothelial Cells (Ave. density/mm^2) |
0.250 | 2.54 ± 0.10 × 10 −3 | 3.38 ± 0.62 × 10 −3 | 0.139 | 4.09 ± 0.54 × 10 −3 | 5.2 ± 0.26 × 10 −3 |
| Blood Vessel Lumen (Ave. lumen number/mm^2) |
0.294 | 2.38 ± 0.56 | 4.06 ± 1.27 | 0.215 | 6.82 ± 0.94 | 4.60 ± 1.18 |
| Type III Collagen (Ave. density/mm^2) |
0.363 | 6.53 ± 0.16 × 10 −2 | 6.28 ± 0.19 × 10 −2 | 0.794 | 5.31 ± 0.25 × 10 −2 | 5.39 ± 0.12 × 10 −2 |
Figure 3. Immunohistochemistry results of significant cellular factors regulated by single or multiple injections of IL-1Ra.
Graphs and representative images showing M2 macrophages (a-i) and myofibroblasts (j-r), 5 days post-injury after PBS or IL-1Ra treatment. A single injection of IL-1Ra was ineffective at modulating M2 macrophage numbers whereas multiple IL-1Ra injections significantly reduced the M2 macrophages (a). A single IL-1Ra injection significantly reduced myofibroblasts whereas multiple IL-1Ra injections were ineffective (b). Representative micrographs show M2 macrophage IHC after single injection (low magnification b-c; high magnification f-g) and multiple IL-1Ra (low magnification d-e; high magnification h-i) injections. Representative micrographs are also shown for the myofibroblasts after single (low magnification k-l; high magnification o-p) and multiple (low magnification m-n; high magnification q-r) IL-1Ra injections. Values above bars in graphs (a, j) indicate p-values resulting from Student’s t-tests. Data was considered significant when p< .05. Values are expressed as mean cell numbers ± S.E.M.
Figure 4. Immunohistochemistry results of significant ECM factors regulated by single or multiple injections of IL-1Ra.
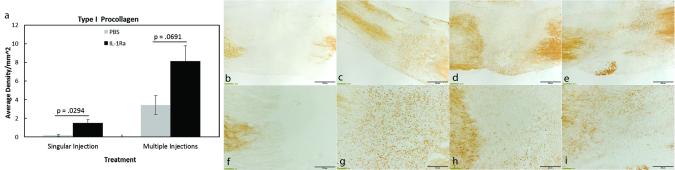
Graphs and representative images showing type I procollagen (a-i) 5 days post-injury after PBS or IL-1Ra treatment. A single injection of IL-1Ra significantly increased type I procollagen (a). Multiple IL-1Ra injections Type I procollagen tended to increase after administration of multiple IL-1Ra injections (a). Representative micrographs show type I procollagen IHC after single injection (low magnification b-c; high magnification f-g) and multiple IL-1Ra (low magnification d-e; high magnification h-i) injections. Values above bars in graphs (a) indicate p-values resulting from Student’s t-tests. Data was considered significant when p< .05. Values are expressed as mean cell numbers ± S.E.M.
Multiplex Results
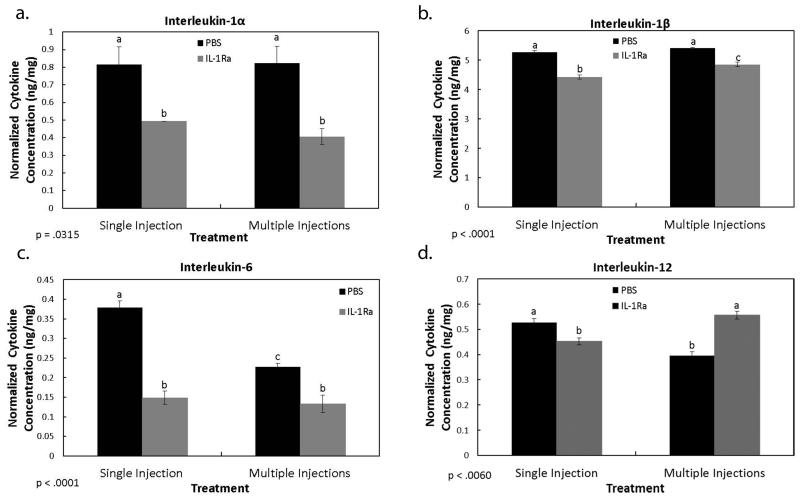
The IHC results identified specific cells and ECM factors influenced by IL-1Ra treatment during MCL healing. This set of experiments examined the influence of IL-1Ra on MCL-derived cytokine production. As Figure 5a-b indicates, both IL-1 α (single PBS, 0.82 ± 0.10 ng/mg vs. single IL-1Ra, 0.49 ± 0.00 ng/mg; and multiple PBS, 0.82 ± 0.10 ng/mg vs. multiple IL-1Ra, 0.41 ± 0.05 ng/mg) and IL-1 β (single PBS, 5.26 ± 0.06 ng/mg vs. single IL-1Ra, 4.43 ± 0.07 ng/mg; and multiple PBS, 5.40 ± .03 ng/mg vs. multiple IL-1Ra, 4.85 ± 0.07 ng/mg) were significantly down-regulated after single and multiple injections of IL-1Ra. Similarly, IL-6 (Fig. 5c) was also reduced after single (PBS, 0.65 ± 0.02 ng/mg vs. IL-1Ra, 0.36 ± 0.02 ng/mg) and multiple (PBS, 0.48 ± 0.01 ng/mg vs. IL-1Ra, 0.37 ± .02 ng/mg) injections of IL-1Ra. In contrast, IL-12 (Fig. 5d) was up-regulated by multiple (PBS, 0.40 ± .02 ng/mg vs. IL-1Ra, 0.56 ± .02 ng/mg) doses of IL-1Ra but decreased by a single dose (PBS, 0.53 ± 0.02 ng/mg vs. IL-1Ra, 0.45 ± 0.01 ng/mg). The anti-inflammatory cytokine, IL-10, was not significantly regulated by IL-1Ra treatment (p= 0.195). Levels of GM-CSF, TNF-α, IFN-γ, IL-2, and IL-4 were below the detectable sensitivity range.
Figure 5. Multiplex results of cytokines regulated by IL-1Ra.
Graphs show the effects of PBS and IL-1Ra on MCL-derived IL-1α (a), IL-1β (b), IL-6 (c), and IL-12 (d) production. Blockade of the IL-1 activation resulted in a decrease of IL-1α (a), IL-1β (b), and IL-6 (c) after single and multiple IL-1Ra injections. A single injection of IL-1Ra significantly decreased IL-12 whereas multiple IL-1Ra injections increased IL-12(d). Beneath graph, p value indicates ANOVA results. a,b,c indicates within a graph, bars without a common superscript letter differ (results of Fisher’s LSD post-hoc pairwise analysis, p < .05). Values are expressed as normalized cytokine concentration (ng cytokine/mg tissue weight) ± S.E.M.
Mechanical Testing
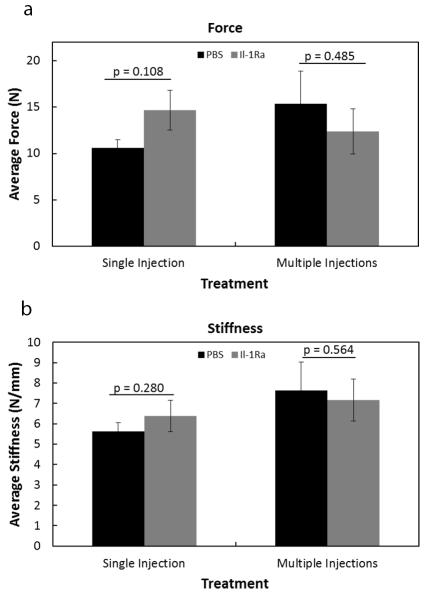
Ligament failure force and stiffness were measured using day 11 healing MCLs. A single injection of IL-1Ra, 1 day after injury, tended to increase force (p = 0.108; Fig. 6a), but had no significant effect on stiffness (p = 0.280; Fig. 6b) when compared to the control specimens. Multiple injections of IL-1Ra provided no additional mechanical influence on failure force (p = 0.485; Fig. 6a) or stiffness (p = 0.564; Fig. 6b) when compared to the PBS controls data. Mechanical results show that IL-1Ra treatment did not significantly alter the behavior of the healing ligaments nor inhibit their functional recovery at day 11.
Figure 6. Mechanical results of the healing MCL after IL-1Ra treatment.
Graphs demonstrating failure force (a), and stiffness (b) of the MCL 11 days post-injury after single or multiple injections of PBS or IL-1Ra treatment. A single injection of IL-1Ra tended to increase failure. No other significance was observed in any tested parameter (p >.05). Results are expressed as mean ± S.E.M.
Discussion
The goal of this study was to further elucidate the temporal and spatial dependence of exogenous IL-1Ra on ligament healing. We previously reported improved ligament healing after perioperative (30 minutes prior and 3 hours post-injury) intraperitoneal (IP) injection of IL-1Ra. (7) However, because ligament ruptures are not typically treated immediately during injury, the current study was performed to evaluate the influence of IL-1Ra administered in a more therapeutic manner, one day after injury. Results demonstrated that a single IL-1Ra injection administered subcutaneously over the MCL reduced the number of myofibroblasts and the pro-inflammatory cytokines (IL-1α, IL-1β, IL-12 and IL-6), increased type I procollagen, and tended to increase tensile strength. Multiple IL-1Ra injections initiated one day after injury likewise decreased IL-1α, IL-1β, and IL-6 but increased IL-12, decreased the number of M2 macrophages, and had no significant influence on myofibroblasts or tensile strength. These results further support the concept that exogenous IL-1Ra can modulate the granulation tissue and scar forming components of the healing MCL and have no detrimental effect on mechanical function.
IL-1 induces production of a number of inflammatory mediators, including nitric oxide, prostaglandins, TNF-α and IL-6. The pro-inflammatory and leukocyte chemotactic functions of IL-1 suggest it contributes to the pathogenesis of acute injury. Therefore, interruption of IL-1 activity via IL-1Ra could significantly alter tissue response after injury. The decrease in IL-1α and IL-1β suggests IL-1Ra directly inhibited the IL-1 mediated pathway and/or suppressed infiltration of inflammatory cells that secrete IL-1. Previous studies have shown that IL-1 blockade inhibited IL-6 production (28-30) and cell infiltration (28) in the murine wound. Based on the decrease in IL-6, the current results suggest that IL-1Ra directly modulated the IL-1 pathway rather than inflammatory cell infiltration since macrophages were not significantly (in the case of the M1 macrophages) or consistently (in the case of the M2 macrophages) regulated by IL-1Ra. These results are in contrast to our prior report indicating that IL-1Ra treatment increased day 5 IL-1α, and did not alter IL-6 or IL-1β levels; by 11 days post-injury both IL-1α and IL-1β had decreased. The SC injection of IL-1Ra over the MCL in the current study may have elicited a quicker response by targeting MCL-derived IL-1 and IL-6 compared to IP IL-1Ra administration.
Circulating monocytes differentiate into macrophages that are broadly subgrouped as classically activated M1 or alternatively activated M2 macrophages. The M2 macrophage phenotype, characterized by a cytokine profile of IL-12low, IL-10high, IL-1Rahigh, IL-1βlow and increased angiogenic mediators, are involved in tissue remodeling, reducing acute inflammation and regulating tissue homeostasis. (31-33) Thus an upregulation of the M2 phenotype within the wound may accelerate healing. Our previous work demonstrated a significant increase in the M2 macrophages, angiogenesis, IL-10 and a decrease in IL-12 after IL-1Ra treatment perioperatively.(7) In the current study, IL-1Ra did not increase the M2 macrophages or angiogenic factors. The addition of multiple IL-1Ra doses also did not upregulate the M2 phenotype, but rather, decreased the M2 macrophages and increased IL-12. After injury, monocytes migrate from the circulation, extravasate through the endothelium and differentiate into macrophages with an M1 or M2 phenotype, based on their environmental cues. IL-1Ra administered SC directly over the MCL would likely target the monoctyes/macrophages localized to the MCL, especially if IL-1Ra injection was administered one day post injury. In contrast, an IP injection of IL-1Ra would provide a more systemic delivery mechanism which could potentially influence a greater number of monocytes traveling to the wound. Compared to our previous study in which IL-1Ra was delivered IP the day of injury, the single SC dose of IL-1Ra localized to the MCL had less influence on the circulating monocytes/macrophages to induce M2 polarization and subsequent M2-induced angiogenesis and cytokine production.
The decrease in M2 macrophages and increase in IL-12 after multiple IL-1Ra injections may have resulted from the delivery method chosen for this study. A study by Jensen compared the ligament inflammatory cell infiltrate of commonly used prolotherapies versus needlestick only. (34) The needlestick alone resulted in increased inflammatory cell infiltrate, similar to the prolotherapies. The multiple needle sticks in the current study could then have influenced the inflammatory response, which may explain the decrease in the anti-inflammatory M2 macrophages and the increase IL-12.
The increase in type I procollagen, decrease in myofibroblasts and tendency to increase tensile strength after a single IL-1Ra injection, suggests that IL-1Ra is capable of reducing scar formation within the healing ligament. Despite these improvements, IL-1Ra did not recover the mechanical properties of the tissue to its native state. The lack of true significance in tensile load suggests that although type I procollagen production was increased by IL-1Ra, the formation of type I collagen fibers had not yet occurred. Perhaps a mechanical improvement would manifest later in healing, since biologically, IL-1Ra elicited a more favorable response. Taken together, these results suggest that a concomitant improvement in scar formation and tensile strength may be possible by optimizing the timing and localization of IL-1Ra in order to modulate the necessary inflammatory and ECM factors necessary for healing.
To compensate for the short half-life of IL-1Ra (4-6 hours), multiple IL-1Ra injections were also included as a treatment group and were found to provide no additional improvements in healing. Multiple IL-1Ra injections may have even reduced the ability to control scar formation based on the lack of change in myofibroblasts and tensile strength and only a trend increase in type I procollagen. The lack of improvement between single and multiple injections may suggest that a small window of opportunity is available for IL-1Ra to influence both inflammation and early scar formation. These results also suggest that other biological factors likely play a pertinent role in controlling scar formation.
Conclusions
Overall, IL-1Ra administration SC after injury reduced selected pro-inflammatory components and scar-modulating factors, but despite an increase in type I procollagen, IL-1Ra did not significantly improve ligament mechanics. The modulation of inflammation and ECM factors appears to be dependent on the time of intervention and may involve cross-talk between immune and connective tissue cells. Clinically, IL-1Ra may be appropriate as an anti-inflammatory agent during surgical repair of the tendon or ligament or as a downstream modulator to scar formation although detailed behaviors and mechanisms remain to be elucidated.
Acknowledgements
The authors acknowledge immunohistochemistry, image capturing, and cell counting contributions by Kevin I. Rolnick, David G. Sterken, Michael Stitgen, and Bryan Roberts.
Footnotes
Declaration of Interest
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR059916. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Authors have no potential conflicts of interest to report.
References
- 1.Chamberlain CS, Crowley E, Vanderby R. The spatio-temporal dynamics of ligament healing. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(2):206–15. doi: 10.1111/j.1524-475X.2009.00465.x. Epub 2009/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank C, Schachar N, Dittrich D. Natural history of healing in the repaired medial collateral ligament. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1983;1(2):179–88. doi: 10.1002/jor.1100010209. Epub 1983/01/01. [DOI] [PubMed] [Google Scholar]
- 3.Levenson SM, Geever EF, Crowley LV, Oates JF, 3rd, Berard CW, Rosen H. The Healing of Rat Skin Wounds. Annals of surgery. 1965;161:293–308. doi: 10.1097/00000658-196502000-00019. Epub 1965/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. Journal of biomechanics. 2004;37(6):865–77. doi: 10.1016/j.jbiomech.2003.11.005. Epub 2004/04/28. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain CS, Brounts SH, Sterken DG, Rolnick KI, Baer GS, Vanderby R. Gene profiling of the rat medial collateral ligament during early healing using microarray analysis. Journal of applied physiology. 2011;111(2):552–65. doi: 10.1152/japplphysiol.00073.2011. Epub 2011/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain CS, Crowley EM, Kobayashi H, Eliceiri KW, Vanderby R. Quantification of collagen organization and extracellular matrix factors within the healing ligament. Microscopy and microanalysis: the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2011;17(5):779–87. doi: 10.1017/S1431927611011925. Epub 2011/09/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamberlain CS, Leiferman EM, Frisch KE, Brickson SL, Murphy WL, Baer GS, Vanderby R. TheInfluence of Interleukin-1 Receptor Antagonist on Ligament Healing. PloS one. 2014 in press. [Google Scholar]
- 8.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS pathogens. 2010;6(8):e1001045. doi: 10.1371/journal.ppat.1001045. Epub 2010/08/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TN/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19(1):59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 10.Nathan C, Ding AH. Nonresolving Inflammation. Cell. 2010;140(6):871–82. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nature Reviews Immunology. 2004;4(8):583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao W, Hong H, Kawakami Y, Lowell CA, Kawakami T. Regulation of myeloproliferation and M2 macrophage programming in mice by Lyn/Hck, SHIP, and Stat5. The Journal of clinical investigation. 2008;118(3):924–34. doi: 10.1172/JCI34013. Epub 2008/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11(10):889–96. doi: 10.1038/ni.1937. Epub 2010/09/22. [DOI] [PubMed] [Google Scholar]
- 14.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. The Journal of experimental medicine. 2007;204(5):1057–69. doi: 10.1084/jem.20070075. Epub 2007/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinarello CA. Blocking interleukin-1 receptors. International journal of clinical & laboratory research. 1994;24(2):61–79. doi: 10.1007/BF02593903. Epub 1994/01/01. [DOI] [PubMed] [Google Scholar]
- 16.Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, Albina JE. Disruption of interleukin-1 signaling improves the quality of wound healing. The American journal of pathology. 2009;174(6):2129–36. doi: 10.2353/ajpath.2009.080765. Epub 2009/04/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–147. [PubMed] [Google Scholar]
- 18.Kim DH, Lee SH, Kim KT, Yu SD. Association of interleukin-1 receptor antagonist gene polymorphism with response to conservative treatment of lumbar herniated nucleus pulposus. Spine. 2010;35(16):1527–31. doi: 10.1097/BRS.0b013e3181e4efb6. Epub 2010/06/29. [DOI] [PubMed] [Google Scholar]
- 19.Hirsch E, Irikura VM, Paul SM, Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11008–13. doi: 10.1073/pnas.93.20.11008. Epub 1996/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. The Journal of experimental medicine. 2000;191(2):303–12. doi: 10.1084/jem.191.2.303. Epub 2000/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, Ikuse T, Asano M, Iwakura Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. The Journal of experimental medicine. 2000;191(2):313–20. doi: 10.1084/jem.191.2.313. Epub 2000/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Kiefer P, Zhou L, Bevirt T, Appleton B. Results from a double blind, placebo-controlled, multicenter trial of a single intra-articular injection of anakinra (kineret (R)) in patients with osteoarthritis of the knee. Arthritis and rheumatism. 2005;52(9):S507–S. [Google Scholar]
- 23.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, Loeuille D, Kivitz AJ, Silver D, Appleton BE. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis and rheumatism. 2009;61(3):344–52. doi: 10.1002/art.24096. Epub 2009/02/28. [DOI] [PubMed] [Google Scholar]
- 24.Goupille P, Mulleman D, Chevalier X. Is interleukin-1 a good target for therapeutic intervention in intervertebral disc degeneration: lessons from the osteoarthritic experience. Arthritis research & therapy. 2007;9(6):110–1. doi: 10.1186/ar2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano PP, Heisey D, Hayashi K, Lakes R, Vanderby R. Subfailure damage in ligament: astructural and cellular evaluation. Journal of applied physiology. 2002;92(1):362–71. doi: 10.1152/jappl.2002.92.1.362. [DOI] [PubMed] [Google Scholar]
- 26.Provenzano PP, Martinez DA, Grindeland RE, Dwyer KW, Turner J, Vailas AC, Vanderby R. Hindlimb unloading alters ligament healing. Journal of applied physiology. 2003;94(1):314–24. doi: 10.1152/japplphysiol.00340.2002. [DOI] [PubMed] [Google Scholar]
- 27.Provenzano PP, Rueden CT, Trier SM, Yan L, Ponik SM, Inman DR, Keely PJ, Eliceiri KW. Nonlinear optical imaging and spectral-lifetime computational analysis of endogenous and exogenous fluorophores in breast cancer. J Biomed Opt. 2008;13(3):031220. doi: 10.1117/1.2940365. [DOI] [PubMed] [Google Scholar]
- 28.Yamada J, Dana MR, Sotozono C, Kinoshita S. Local suppression of IL-1 by receptor antagonist in the rat model of corneal alkali injury. Experimental eye research. 2003;76(2):161–7. doi: 10.1016/s0014-4835(02)00293-2. Epub 2003/02/05. [DOI] [PubMed] [Google Scholar]
- 29.Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, Dill D, Shi X, Qiao Y, Yeomans D, Carvalho B, Angst MS, Clark JD, Peltz G. The role of interleukin-1 in wound biology. Part I: Murine in silico and in vitro experimental analysis. Anesthesia and analgesia. 2010;111(6):1525–33. doi: 10.1213/ANE.0b013e3181f5ef5a. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 30.Hu Y, Liang D, Li X, Liu HH, Zhang X, Zheng M, Dill D, Shi X, Qiao Y, Yeomans D, Carvalho B, Angst MS, Clark JD, Peltz G. The role of interleukin-1 in wound biology. Part II: In vivo and human translational studies. Anesthesia and analgesia. 2010;111(6):1534–42. doi: 10.1213/ANE.0b013e3181f691eb. Epub 2010/10/05. [DOI] [PubMed] [Google Scholar]
- 31.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. Epub 2003/01/04. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5. Epub 2002/10/29. [DOI] [PubMed] [Google Scholar]
- 33.Na YR, Yoon YN, Son DI, Seok SH. Cyclooxygenase-2 Inhibition Blocks M2 Macrophage Differentiation and Suppresses Metastasis in Murine Breast Cancer Model. PloS one. 2013;8(5):e63451, 1–11. doi: 10.1371/journal.pone.0063451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen KT, Rabago DP, Best TM, Patterson JJ, Vanderby R., Jr. Early inflammatory response of knee ligaments to prolotherapy in a rat model. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2008;26(6):816–23. doi: 10.1002/jor.20600. Epub 2008/02/02. [DOI] [PMC free article] [PubMed] [Google Scholar]