Abstract
Decitabine improves overall survival (OS) and reduces risk of progression to acute myeloid leukemia (AML) in myelodysplastic syndromes (MDS). In this retrospective analysis of data from 2 decitabine studies (n = 162), hemoglobin level > 10 g/dL, platelet count > 50 × 103/μL, and lack of chromosome 5 or 7 abnormalities predicted longer OS. Identifying potential prognostic factors for survival may guide decitabine treatment decisions and improve outcomes.
Background
Myelodysplastic syndromes (MDS) progress to acute myeloid leukemia (AML) in approximately 30% of patients. Identification of risk factors for progression to AML and overall survival (OS) would help guide treatment decisions.
Patients and Methods
We investigated prognostic factors for progression to AML and survival in 163 patients with MDS treated with decitabine 15 mg/m2 over 3 hours every 8 hours for 3 days every 6 weeks (n = 74) or 20 mg/m2 over 1 hour daily for 5 days every 4 weeks (n = 89).
Results
Multivariate analysis of pooled baseline data revealed that only study effect was associated with progression to AML. A hemoglobin value at least 10 g/dL, platelet count at least 50 × 103/μL, and lack of chromosome 5 or 7 abnormalities were associated with longer OS.
Conclusions
Patients with certain prognostic factors should be considered for other interventions in addition to decitabine treatment.
Keywords: Acute myeloid leukemia, Decitabine, Myelodysplastic syndromes, Myelomonocytic leukemia, chronic, Risk factors
Introduction
Myelodysplastic syndromes (MDS) encompass an array of bone marrow stem cell disorders that involve bone marrow dysfunction, proliferative abnormalities in hematopoietic cells, genomic instability, and a progressive increase in bone marrow blast cells (blasts).1
A characteristic pathogenic factor in MDS is irregular DNA hypermethylation, with associated silencing of host tumor suppressor genes.2,3 Decitabine (5-aza-2’-deoxycytidine), a cytosine analog that induces DNA hypomethylation, is indicated for the treatment of all primary and secondary French-American-British (FAB) subtypes of MDS and International Prognostic Scoring System (IPSS) intermediate-1, intermediate-2, and high-risk disease stratifications including de novo and previously treated MDS.4 The clinical benefit of decitabine in MDS is thought to arise from 2 potential mechanisms of action—direct cytotoxicity and/or DNA hypomethylation—resulting in terminal differentiation, reduced proliferative potential, and increased apoptotic activity.2,4-6
Baseline characteristics in patients with MDS may predict response to therapy. Several key baseline factors, including longer duration of disease and previous MDS therapy, have been identified as poor prognostic factors for complete response (CR) to decitabine.7 Factors predictive of poor prognosis for survival include chromo-some 5 or 7 abnormalities, older age, and previous MDS treatment, excluding treatment with growth factors.7
The current analysis of data from 2 studies of decitabine was conducted to investigate potential prognostic factors in patients with MDS that may be associated with progression to acute myeloid leukemia (AML) after treatment with either a 3-day or a 5-day dosage regimen.
Patients and Methods
Study Design and Patients
This was a retrospective comparison of baseline data from the cohorts of 2 studies of decitabine, D-0007 (NCT00043381) and DACO-020 (NCT00260065), as well as an analysis of pooled data. Both studies were conducted at multiple sites in the United States and Canada in patients with de novo or secondary MDS. Written informed consent was obtained from all patients before study participation.
D-0007 was an open-label randomized phase III study that evaluated a 3-day decitabine dosage regimen in patients with de novo or secondary MDS of any FAB subtype, including chronic myelomonocytic leukemia (CMML) with a white blood cell count < 12 × 103 cells/μL and an IPSS score of ≥ 0.5, equivalent to intermediate- or high-risk disease. It was conducted between July 2001 and January 2004, and the results have been published.8 Patients were randomly assigned to receive decitabine (15 mg/m2 intravenously over 3 hours every 8 hours for 3 consecutive days every 6 weeks) plus supportive care or supportive care alone.8 Patients could receive up to 10 cycles of treatment, depending on clinical response or toxicity. Treatment response was based on percentage of myeloblasts and the profile of other cell lines quantified by the pathologist in each bone marrow sample, as well as the differential finding of types and percentages of individual cell lines identified in the weekly complete blood count. Response was evaluated according to the 2000 MDS International Working Group criteria.9 Primary endpoints were overall response rate (ORR, defined as CR + partial response [PR]) and time to AML or death; secondary endpoints were overall response improvement (ORI; CR + PR + hematologic improvement [HI]), survival, transfusion requirements, quality of life, and cytogenetic response.8
DACO-020 was an open-label nonrandomized phase II study that evaluated a 5-day decitabine dosage regimen in patients with characteristics similar to those of patients in the phase III study, namely, diagnosis of de novo or secondary MDS of any FAB subtype, including CMML, and a white blood cell count <12 × 103 cells/μL and an IPSS score of ≥ 0.5. The study commenced in June 2005 and was completed in 2009. The results as of March 2008 have been published.10 All patients received decitabine 20 mg/m2 intravenously over 1 hour once daily for 5 consecutive days every 4 weeks; there was no comparator arm. Treatment was continued until the occurrence of any of the following: death, disease progression, unacceptable toxicity, intercurrent illness preventing further treatment, patient withdrawal, or lack of clinical benefit after four treatment cycles.10 Responses were evaluated according to the 2000 and 2006 MDS International Working Group criteria.9,11 The primary endpoint was ORR, but ORI was also calculated; secondary endpoints were overall survival (OS), time to AML or death, HI, transfusion requirements, cytogenetic response, and safety.10
Both studies allowed administration of supportive care, including blood products and prophylactic antibiotics, and treatment of serious infection or sepsis with granulocyte colony-stimulating factor.8,10
In the current pooled analysis, the response criteria for the D-0007 trial were reclassified according to the International Working Group 2006 criteria11 to maintain consistency between the 2 study populations. Patients from both studies were stratified based on progression to AML. Patients with ≥ 20% marrow blasts were excluded from the analysis. Efficacy endpoints were assessed according to an intention-to-treat analysis.
Study Evaluations
Patient data from both studies were pooled and then divided into those who progressed to AML and those who did not. Baseline patient variables were recorded for each group and differences in values and response rates compared to determine factors associated with progression to AML. Baseline patient variables were age (> 70 vs. ≤ 70 years), race (white vs. nonwhite), IPSS risk score (intermediate-1, intermediate-2, or high), chromosome 5 and/or 7 abnormalities, FAB classification (CMML vs. MDS), Eastern Cooperative Oncology Group (ECOG) performance status (0-1 vs. > 1), time from MDS diagnosis (< 3 vs. ≥ 3 months), hemoglobin level (< 10 vs. ≥ 10 g/dL), platelet count (< 50 vs. ≥ 50 × 103 cells/μL), neutro-phil count (< 150 vs. ≥ 150 cells/μL), MDS type (de novo vs. secondary), previous therapy for MDS, growth factor use only, previous chemotherapy, and decitabine dosage regimen (3-day vs. 5-day schedule). Treatment outcomes were measured and reported in terms of ORR (CR + PR), ORI (CR + PR + HI), cytogenetic response, and OS. Safety was recorded as frequency and severity of adverse events.
Statistical Analysis
Risk factor analyses were conducted to evaluate prognostic factors associated with AML progression in these patients receiving decitabine. Each baseline variable was evaluated as a possible prognostic factor for progression to AML using χ2 testing for final logistic regression analysis. Factors that were significant in univariate analyses (P < .05) were included in multivariate analyses. Regression analysis was conducted to determine factors responsible for progression to AML. Differences in baseline characteristics and response rates by progression status were compared between cohorts using χ2 analysis.
Risk factor analyses were conducted using a log-rank test to evaluate prognostic factors associated with survival outcomes with decitabine therapy. Factors that were significant in these univariate analyses (P < .05) were included in a multivariate analysis using a Cox proportional hazards model.
Results
Patients
A total of 163 patients were included in this analysis, 74 from the D-0007 study and 89 patients from the DACO-020 study.
Progression to AML
Baseline characteristics of patients who received decitabine in the D-0007 and DACO-020 studies are summarized in Table 1 according to those whose disease progressed to AML and those whose disease did not. Patients whose disease progressed to AML (n = 37) had a significantly (P < .05) shorter time since the MDS diagnosis, were less likely to have received growth factor support, and were more likely to have a FAB classification of refractory anemia with excess blasts (RAEB), higher IPSS scores, and no previous therapy for MDS compared with patients whose disease did not progress. Cytogenetic abnormalities were not associated with progression to AML in the pooled analysis (Table 1).
Table 1.
Baseline Characteristics According to AML Progression Status for Patients in the Pooled Analysis Receiving Decitabine in Studies D-0007 and DACO-020
| Pooled Analysis | |||
|---|---|---|---|
| Parameter | Progressed n = 37 | Not Progressed n = 126 | P Valuea |
| Age, y | NS | ||
| Mean | 71 | 69 | |
| SD | 6.6 | 10.3 | |
| Sex, n (%) | NS | ||
| Female | 9(24) | 44 (35) | |
| Male | 28 (76) | 82 (65) | |
| Time from Diagnosis, d | .013 | ||
| Mean | 330 | 633 | |
| SD | 549.4 | 873.5 | |
| White Race, n (%) | 31 (84) | 113(90) | NS |
| Previous Treatment for MDS, n (%) | 5(14) | 46 (37) | .008 |
| IPSS Cytogenetic Status, n (%) | NS | ||
| Good | 16(43) | 62 (49) | |
| Intermediate | 3 (8) | 23 (18) | |
| Poor | 12 (32) | 36 (29) | |
| Unknown | 6 (16) | 5 (4) | |
| IPSS Score, % | .017 | ||
| High | 11 (30) | 16(13) | |
| Intermediate-1 | 11 (30) | 68 (54) | |
| Intermediate-2 | 15(41) | 41 (33) | |
| Low | 0 | 1 (1) | |
| FAB Classification, n (%) | .008 | ||
| RA | 3 (8) | 29 (23) | |
| RARS | 2 (5) | 22 (18) | |
| RAEB | 29 (78) | 58 (46) | |
| RAEB-tb | 1 (3) | 2 (2) | |
| CMML | 2 (5) | 15(12) | |
| Type of MDS, n (%) | NS | ||
| De novo | 34 (92) | 111 (88) | |
| Secondary | 3 (8) | 15(12) | |
| Cytogenetic Anomalies, n (%) | |||
| del(5) | 6 (16) | 16(13) | NS |
| del(20) | 4 (11) | 6 (5) | NS |
| del(Y) | 1 (3) | 5 (4) | NS |
| del(7) | 8 (22) | 23 (18) | NS |
| White Blood Cells, x103/μL | NS | ||
| Mean | 6 | 4 | |
| SD | 9.5 | 3.6 | |
| Platelets, x103/μL | NS | ||
| Mean | 90 | 115 | |
| SD | 92.2 | 136.1 | |
| Pooled Analysis | |||
| Variable | Progressed n = 37 | Not Progressed n = 126 | P Valuea |
| Hemoglobin, g/dL | NS | ||
| Mean | 9 | 9 | |
| SD | 1.5 | 1.4 | |
| Independent RBC Transfusion, n (%) | 11 (30) | 39 (31) | NS |
| Independent Platelet Transfusion, n (%) | 28 (76) | 09 (87) | NS |
| Growth Factor, n (%) | 13(35) | 49 (39) | NS |
Abbreviations: AML = acute myeloid leukemia; CMML = chronic myelomonocytlc leukemia; del = deletion; FAB = French-American-British; IPSS = International Prognostic Scoring System; MDS = myelodysplastic syndromes; NR = not reported; NS = not significant; RA = refractory anemia; RAEB = refractory anemia with excess blasts; RAEB-t = refractory anemia with excess blasts in transformation; RARS = refractory anemia with ring sideroblasts; RBC = red blood cell; SD = standard deviation.
Fisher's exact test for categorical variables; ttest for continuous variables.
Three patients originally classified as having RAEB-t were included in the analysis because they were found to have < 20% baseline marrow blasts.
Treatment Outcomes According to Progression to AML
In the D-0007 study (decitabine 3-day dosage regimen), the median follow-up was 391 days, and patients received a median of 3 cycles of decitabine (range, 0-9 cycles).8 In the DACO-020 study (decitabine 5-day dosage regimen), the median follow-up was 566 days, and patients received a median of 5 cycles of decitabine.10 In the pooled analysis, response rates were similar between patients whose disease progressed to AML and those whose disease did not (Table 2). The ORR was 27% in both patient subgroups, and the ORI was 35% among patients whose disease progressed to AML and 44% among those whose disease did not. Median OS was significantly longer in patients whose disease did not progress to AML compared with those whose disease did (566 vs. 422 days, respectively; P = .022).
Table 2.
Response Rates and Survival Duration According to AML Progression Status for Patients in the Pooled Analysis Receiving Decitabine in the D-0007 and DACO-020 Studies
| Variable | Pooled Analysis | ||
|---|---|---|---|
| Progressed n = 37 | Not Progressed n = 126 | P Valuea | |
| ORR (CR + PR),b n (%) | 10(27) | 34 (27) | NS |
| ORI (CR + PR + HI),c n (%) | 13(35) | 55 (44) | NS |
| Cytogenetic Response,d n (%) (Major + Minor) | 2 (5.4) | 17(13.5) | NS |
| Median OS, d (95% CI) | 422 (384, 506) | 566 (391, 627) | .022 |
Abbreviations: CI = confidence interval; CR = complete response; HI = hematologic Improve ment; NS = not significant; OS = overall survival; ORI = overall response improvement; ORR = overall response rate; PR = partial response; OS = overall survival.
Fisher’s exact test for ORR, ORI, and cytogenetic response; log-rank test for OS.
In DACO-020, ORR included marrow CR (mCR).
In DACO-020, ORI included mCR.
In DACO-020, cytogenetic response = complete cytogenic response + partial cytogenic response.
Prognostic Factors Associated With Progression to AML
Prognostic factors for progression to AML were evaluated using patient baseline data from a pooled population of 159 of 163 patients from either the D-0007 or the DACO-020 study. Four factors that were significantly (P < .05) associated with AML progression—<3 months since MDS diagnosis, no previous therapy for MDS, baseline hemoglobin levels < 10 g/dL, and study effect from the 3-day decitabine study (D-0007 study) (Table 3)—were included in the multivariate analysis.
Table 3.
Univariate Analysis of Baseline Characteristics of the Pooled Population (n = 159) for Progression to AML With Decitabine Therapy
| Baseline Characteristic | P Valuea |
|---|---|
| Age Group | |
| > 70/≤ 70 y | .573 |
| ECOG Performance Status | |
| 0-1/> 1 | .126 |
| FAB Classification | |
| CMML/MDS | .780 |
| MDS Type | |
| De novo/secondary | .390 |
| IPSS Risk Group | |
| Intermediate-1/intermediate-2/high | .268 |
| Time Since Diagnosis of MDS | |
| < 3 mo/≥ 3 mo | .008 |
| Any Previous Therapy for MDS | |
| Yes/no | .0006 |
| Previous Chemotherapy | |
| Yes/no | .073 |
| Chromosome 5 and/or 7 Abnormalities | |
| Yes/no | .769 |
| Hemoglobin Level | |
| < 10/≥ 10 g/dL | .002 |
| Platelet Count | |
| < 50/≥ 50 X103cells/μL | .828 |
| Neutrophil Count | |
| < 1.5/≥1.5 X 103 cells/μL | .320 |
Abbreviations: AML = acute myeloid leukemia; CMML = chronic myelomonocytic leukemia; ECOG = Eastern Cooperative Oncology Group; FAB = French-American-British; IPSS = Inter national Prognostic Scoring System; MDS = myelodysplastic syndromes.
Fisher's exact test.
Prognostic Factors Associated With Survival
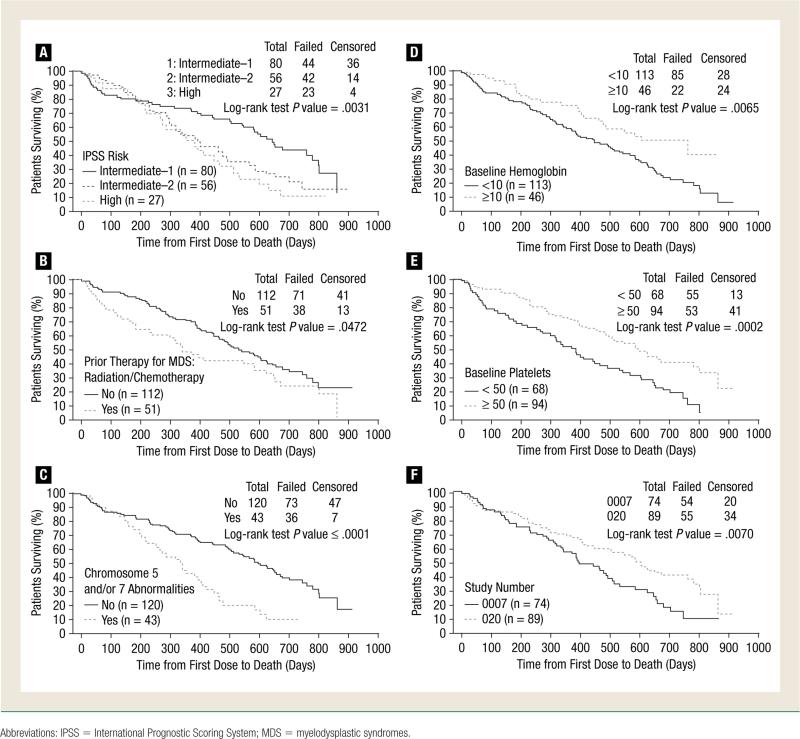
Prognostic factors associated with survival were evaluated using patient baseline data from a pooled population of 158 of 163 patients from either the D-0007 or the DACO-020 study. Seven factors were identified that were significantly (P < .05) associated with longer OS: IPSS risk group intermediate-1, IPSS risk group intermediate-2, no previous radiotherapy/chemotherapy, no chromosome 5 or 7 abnormalities, a hemoglobin level ≥10 g/dL, a platelet count ≥50 × 103 cells/μL, and study effect from the 5-day decitabine study (DACO-020) (Table 4). Differences in survival in cohorts with and without these baseline factors are depicted in Figure 1. These 7 variables were included in the multivariate analysis.
Table 4.
Univariate Analysis of Baseline Characteristics of the Pooled Population (n = 158) for Overall Survival With Decitabine Therapy
| Baseline Characteristic | P Valuea |
|---|---|
| Age Group | |
| > 70/≤ 70 y | .665 |
| ECOG Performance Status | |
| 0-1/> 1 | .669 |
| FAB Classification | |
| CMML/MDS | .236 |
| MDS Type | |
| De novo/secondary | .198 |
| IPSS Risk Group | |
| Intermediate-1/intermediate-2/high | 003 |
| Time Since Diagnosis of MDS | |
| < 3/≥ 3 mo | .388 |
| Any Previous Therapy for MDS | |
| Yes/no | .192 |
| Previous Radiotherapy/Chemotherapy | |
| Yes/no | .047 |
| Growth Factor Use Only | |
| Yes/no | .549 |
| Chromosome 5 and/or 7 Abnormalities | |
| Yes/no | < .0001 |
| Hemoglobin Level | |
| < 10/≥ 10 g/dL | .005 |
| Platelet Count | |
| < 50/≥ 50 X 103 cells/μL | .0002 |
| Neutrophil Count | |
| < 1.5/≥ 1.5 X 103 cells/μL | .159 |
| Study Effect | |
| DACO-0007/DACO-020 | .007 |
Abbreviations: CMML = chronic myelomonocytic leukemia; ECOG = Eastern Cooperative Oncology Group; FAB = French-American-British; IPSS = International Prognostic Scoring System; MDS = myelodysplastic syndromes.
Log-rank test.
Figure 1.
Kaplan-Meier Analysis of Overall Survival According to Factors Predictive of Extended Survival in Univariate Analysis. Survival Over Time is Depicted by the Following Cohorts From the D-0007 Study or the DACO-020 Study. (A) IPSS Risk Group; (B) Previous Radiotherapy/Chemotherapy; (C) Chromosome 5 or 7 Abnormalities; (D) Baseline Hemoglobin Levels; (E) Baseline Platelet Counts; and (F) Study Effect
Multivariate Analysis
In the multivariate analysis for prognostic factors associated with progression to AML, only study effect was found to be significant (P = .005), and in the multivariate analysis for prognostic factors associated with OS, lack of chromosome 5 or 7 abnormalities, elevated hemoglobin levels, and elevated platelet counts were significantly (P < .02) associated with longer survival (Table 5).
Table 5.
Multivariate Analysis of Prognostic Factors for Progression to AML and for Improved Overall Survival With Decitabine Therapy
| Variable | χ 2 | OR (95% CI) | P Value |
|---|---|---|---|
| For Progression to AML a | |||
| Prognostic factor | |||
| Time from MDS diagnosis (< 3/≥3 mo) | 1.7288 | 1.779(0.754, 4.196) | .189 |
| Any previous therapy for MDS (no/yes) | 0.1995 | 1.206 (0.530, 2.747) | .655 |
| Hemoglobin level < 10 g/dL (< 10/≥10 g/dL) | 0.2561 | 0.800 (0.337, 1.897) | .613 |
| Study effect (DACO-0007/DACO-020) | 7.9702 | 3.132 (1.418, 6.918) | .005 |
| For Improved Overall Survival b | |||
| Prognostic factor | |||
| IPSS risk group (intermediate-1/intermediate-3) | 0.4402 | 0.809(0.434, 1.511) | .507 |
| IPSS risk group (intermediate-2/intermediate- 3) | 0.0009 | 1.009(0.570, 1.786) | .976 |
| Previous radiotherapy/chemotherapy (no/yes) | 2.0319 | 0.740(0.490, 1.119) | .154 |
| Chromosome 5 and/or 7 abnormalities (no/yes) | 10.4011 | 0.446 (0.273, 0.728) | .001 |
| Hemoglobin level (< 10/≥ 10 g/dL) | 5.1986 | 1. 780 (1.084, 2.921) | .023 |
| Platelet count (< 50/≥ 50 x 103 cells/μL) | 6.2605 | 1. 709 (1.123, 2.599) | .012 |
| Study effect (DACO-0007/DACO-020) | 2.4863 | 1.406(0.921, 2.146) | .115 |
Abbreviations: AML = acute myelogenous leukemia; CI = confidence interval; HR = hazard ratio; IPSS = International Prognostic Scoring System; MDS = myelodysplastic syndromes.
Logistic regression model.
Cox proportional hazards model.
Discussion
Decitabine treatment on either a 3-day (D-0007 study) or a 5-day (DACO-020 study) schedule is associated with favorable outcomes in patients with MDS.8,10 Response rates and survival outcomes were particularly favorable with the lower intensity 5-day decitabine regimen; however, this observation has not been verified in a head-to-head comparison of the 3- and 5-day decitabine schedules.
In the univariate analyses of pooled data from the D-0007 and DACO-020 studies, the characteristics associated with both progression to AML and shorter OS were baseline hemoglobin levels (reduced levels associated with progression and shorter survival), previous treatment for MDS (no previous therapy was associated with progression to AML, yet previous radiotherapy/chemotherapy was associated with shorter survival), and study effect of the 3- or 5-day decitabine studies (the 5-day schedule was associated with longer survival). These results support those of other analyses of prognostics factors for survival in patients with MDS treated with decitabine. A lower hemoglobin level was found to be an independent adverse prognostic factor for survival in univariate and multivariate analyses of 1915 patients with MDS,12 and a hemoglobin level of <10 g/dL was reported to be a prognostic factor for shorter survival in 856 patients with low-risk or intermediate-1–risk MDS.13 A history of previous chemotherapy or radiotherapy could suggest treatment-resistant or relapsed MDS, thus explaining, at least in part, the association with shorter survival. Understanding the association between lack of previous therapy and progression to AML might require a more detailed disease history than that provided in the trial at baseline.
In multivariate analyses, only study effect was independently associated with progression to AML.
Lack of chromosome 5 or 7 abnormalities, hemoglobin level ≥ 10 g/dL, and platelet count ≥50 × 103 cells/μL were independently predictive of longer survival. The longer survival in patients without chromosome 5 or 7 abnormalities is in agreement with the study by Haase et al, who reported the effects on survival of different karyo-types and the degree of complexity of karyotypes in patients with MDS.14 In their analysis of 2124 patients with MDS, median sur vival was longer in those who had normal karyotypes compared with those who had ≥ 1 abnormality. We suggest that patients with chromosome 5 or 7 abnormalities should be treated with caution and considered for suitable clinical trials when enrollment is available.
Shortened OS and increased rates of transformation to AML in patients with low hemoglobin levels (<10 g/dL) were previously described in a patient registry of 572 CMML cases.15 In addition, although not evaluated in our current study, the effects of anemia on outcomes may be the result of red blood cell (RBC) transfusion dependency and subsequent secondary iron overload. These factors have been described as predictors of shorter OS in patients with MDS.16 Iron overload as a result of chronic RBC transfusions can impair vital hepatic, cardiac, and endocrine functions and may lead to greater genomic instability because of an increased presence of oxygen-free radicals.17,18 The significant predictive factors for longer OS (lack of chromosome 5 or 7 abnormalities, hemoglobin level ≥10 g/dL, and platelet count ≥50 × 103 cells/μL) complement those reported by Kantarjian et al, who identified prognostic factors associated with poorer CR (previous MDS therapy and longer duration of disease) and poorer survival (chromosome 5 or 7 abnormality, older age, and previous MDS therapy) in patients with MDS after treatment with decitabine.7 Furthermore, in a recent study of decitabine in patients with MDS who were aged 60 years or older, multivariate analyses indicated that IPSS high risk, poor cytogenetic factors, shorter time since MDS diagnosis, and ECOG performance status (PS) of 1 or 2 were all independent prognostic factors for poorer outcome for OS, progression-free survival, and AML-free survival.19
Our data for patients receiving decitabine complement recently published analyses on prognostic factors for response and survival in patients with MDS receiving azacitidine, another hypomethylating agent. In the analysis of 282 patients with intermediate-2–risk or high-risk MDS and high ECOG PS, univariate analysis identified a higher risk IPSS category, secondary MDS, presence of peripheral blasts, higher RBC trans-fusion requirement, low baseline platelet count, and intermediate- or poor-risk karyotypes as being predictive of shorter survival. A multivariate analysis identified high ECOG PS, intermediate- and high-risk IPSS category, presence of peripheral blasts, and higher RBC transfusion requirement as independent poor prognostic factors.20
There were some potential limitations in the original studies from which the current analyses were done. First, it has been suggested that patients in the phase III study of 3-day decitabine were under-treated, which resulted in response and survival rates that were lower than anticipated. Second, although the 2 studies had similar patient populations, they used different decitabine dosing regimens. To address this issue in the current study, therapy was included in the statistical analyses. An area for further research may be to undertake these analyses in a broader population, with a longer period of follow-up so that results could be applicable to the wider population of patients with MDS.
Conclusions
Lower hemoglobin levels and lower platelet counts were associated with lower OS. The findings of our analysis and those from studies with other hypomethylating agents suggest that prognostic factors for poor outcomes in patients treated with these agents are the same as the known prognostic factors associated with poor outcomes in patients with MDS. Consequently, these patients should be offered treatment options in addition to decitabine, such as participation in clinical trials of investigational agents and/or investigational combination therapies, and/or earlier allogeneic transplantation because of the risks for earlier progression. Because the decitabine dosage regimen of 20 mg/m2 daily for 5 days was associated with high response rates, prolonged OS, and a lower risk of progression to AML, this regimen appears to be preferred for the treatment of patients with de novo or secondary MDS.
Clinical Practice Points.
The progress of MDS to AML occurs in approximately 30% of patients.
Decitabine improves OS and reduces risk of progression to AML in patients with MDS, but identification of baseline characteristics that may be independent risk factors for progression to AML and OS could help improve treatment decisions.
In a retrospective analysis, we investigated prognostic factors for progression to AML and survival in 163 patients with MDS treated with 2 different dosage regimens of decitabine.
Multivariate analysis of pooled baseline data revealed that hemoglobin levels ≥ 10 g/dL, platelet counts ≥ 50 × 103/μL, and lack of chromosome 5 or 7 abnormalities predicted longer OS.
Based on these results, it is our opinion that patients with these prognostic factors should be considered for other interventions in addition to decitabine treatment.
Acknowledgments
The authors thank Yvonne E. Yarker, PhD, CMPP, of Peloton Advantage LLC, for providing medical writing and editorial assistance during the preparation of this manuscript, which was funded by EISAI Inc.
Angela Teng, MS, of EISAI Inc, conducted the statistical analyses. Dr Jabbour has received honoraria from Bristol-Myers Squibb and Novartis. Dr Ravandi has received research grants from Bayer, Bristol-Myers Squibb, Cephalon, Incyte, and Sunesis, and has performed formal advisory activities for Bayer, Cephalon, and Sunesis. Dr Cortes has received research grants from Celgene and EISAI Inc. Dr Kantarjian has received research grants from Celgene and EISAI Inc. The other authors have stated that they have no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
This study was supported by research funding from EISAI Inc. Medical writing, editorial, and graphics assistance was provided by Peloton Advantage, LLC, and was funded by EISAI Inc.
References
- 1.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines on Oncology: Myelodysplastic Syndromes. doi: 10.6004/jnccn.2011.0005. Available at: http://www.nccn.org. Accessed: March 2, 2011. [DOI] [PMC free article] [PubMed]
- 2.Jabbour E, Issa JP, Garcia-Manero G, et al. Evolution of decitabine development: accomplishments, ongoing investigations, and future strategies. Cancer. 2008;112:2341–51. doi: 10.1002/cncr.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leone G, Teofili L, Voso MT, et al. DNA methylation and demethylating drugs in myelodysplastic syndromes and secondary leukemias. Haematologica. 2002;87:1324–41. [PubMed] [Google Scholar]
- 4.Dacogen [package insert] Eisai Inc.; Woodcliff Lake, NJ.: 2010. [Google Scholar]
- 5.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 6.Pinto A, Zagonel V. 5-aza-2’-deoxycytidine (decitabine) and 5-azacytidine in the treatment of acute myeloid leukemias and myelodysplastic syndromes: past, present and future trends. Leukemia. 1993;7(suppl 1):51–60. [PubMed] [Google Scholar]
- 7.Kantarjian HM, O'Brien S, Shan J, et al. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–73. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 8.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 9.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–4. [PubMed] [Google Scholar]
- 10.Steensma DP, Baer MR, Slack JL, et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol. 2009;27:3842–8. doi: 10.1200/JCO.2008.19.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–25. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 12.Kantarjian H, O'Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original international prognostic scoring system. Cancer. 2008;113:1351–61. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–43. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 14.Haase D, Germing U, Schanz J, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 15.Such E, Cervera J, Nomdedeu B, et al. A new prognostic scoring system including transfusion dependency and cytogenetic abnormalities for patients with chronic myelomonocytic leukemia. Blood. 2009;114 abstract 1750. [Google Scholar]
- 16.Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 17.Farquhar MJ, Bowen DT. Oxidative stress and the myelodysplastic syndromes. Int J Hematol. 2003;77:342–50. doi: 10.1007/BF02982641. [DOI] [PubMed] [Google Scholar]
- 18.Hershko C, Link G, Cabantchik I. Pathophysiology of iron overload. Ann N Y Acad Sci. 1998;850:191–201. doi: 10.1111/j.1749-6632.1998.tb10475.x. [DOI] [PubMed] [Google Scholar]
- 19.Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol. 2011;29:1987–96. doi: 10.1200/JCO.2010.30.9245. [DOI] [PubMed] [Google Scholar]
- 20.Itzykson R, Thépot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2011;117:403–11. doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]