Abstract
Background
Vandetanib is an oral tyrosine kinase inhibitor of VEGFR-2/3, EGFR and RET, which has demonstrated clinical activity as a single agent and in combination with taxanes. We explored the efficacy, safety and toxicity of docetaxel and vandetanib in women with recurrent ovarian cancer (OC).
Methods
Women with refractory or progressive OC were randomized 1:1 to docetaxel (75 mg/m2, IV)+vandetanib (100 mg daily, PO, D+V) or docetaxel (75 mg/m2,D). Up to 3 additional cytotoxic regimens for recurrence and prior anti-angiogenic agents (as primary therapy) were allowed. The primary endpoint was progression free survival (PFS). The study had 84% power to detect a PFS hazard ratio of 0.65, using a one-sided P of 0.1. This corresponds to an increase in median PFS from 3.6 months to 5.6 months. Patients progressing on D were allowed to receive single agent vandetanib (D→V).
Results
131 patients were enrolled; 2 were excluded. 16% had received prior anti-angiogenic therapy. The median PFS estimates were 3.0 mos (D+V) vs. 3.5 (D); HR:0.99 (80% CI:0.79-1.26). 61 patients on D+V were assessable for toxicity; 20(33%) had treatment-related Grade (G) 4 events, primarily hematologic. Similarly, 17(27%) of 64 patients receiving D had G4 events, primarily hematologic. 27 evaluable patients crossed-over to V. 1/27(4%) experienced a G4 event. G3 diarrhea was observed in 4% D→V patients. Median OS was 14 mos (D+V) vs. 18 mos(D→V); HR(OS):1.25 (80% CI:0.93-1.68). Crossover vandetanib response was 4%(1/27 evaluable patients). High plasma IL-8 levels were associated with response to D+V.
Conclusions
Combination docetaxel+vandetanib did not prolong PFS relative to docetaxel alone in OC patients. No unexpected safety issues were identified.
Keywords: Ovarian Cancer, Primary Peritoneal Cancer, Fallopian Tube Cancer, Epithelial Cancer, Chemotherapy, Taxanes, Vandetanib, Clinical Trial, Randomized phase II
Introduction/Background
Ovarian cancer is the leading cause of death from gynecologic malignancy and the fifth leading cause of cancer death in American women.1 Despite improvements in surgical techniques, adjuvant chemotherapy, and biological therapy, the major cause of death from ovarian cancer is due to metastases that are resistant to therapy. The progressive growth of primary tumor and metastases is dependent on an adequate blood supply (angiogenesis) and can occur by sprouting, vessel cooption, vascular mimicry or vascular mosiacism.2-5
Vascular endothelial growth factor (VEGF) plays a pivotal role in developmental, physiological, and pathological neovascularization.6 VEGF mediates its effects by interacting with two high-affinity transmembrane tyrosine kinase receptors (VEGFR-1 and VEGFR-2), which promote endothelial survival and angiogenesis. Elevated tumor VEGF expression and serum VEGF levels are associated with poor overall survival in ovarian cancer patients.7-10 Clinical investigations of therapeutic agents targeting VEGF or its receptors have proved promising in ovarian cancer. Single agent efficacy for anti-ligand strategies (e.g., bevacizumab, aflibercept) in ovarian cancer patients prompted phase III investigation (bevacizumab) in primary and recurrent disease patients. Each of these trials met their primary endpoint (PFS) when receiving a novel chemotherapy-based combination.11-13 Nevertheless, recurrence was common and overall survival was not improved; observations, which underscore the exploration of alternative targets.14
On the basis of EGFR's role in potentially blunting the efficacy of anti-VEGF agents, dual VEGFR/EGFR approaches were considered.15 AEE788 (an oral VEGFR/EGFR TKI, Novartis Pharmaceuticals, Basel, Switzerland) demonstrated potent tumor growth inhibition in mouse models of ovarian and other cancers, especially in combination with taxanes.16 However, AEE788 was not developed further clinically. For the current trial, vandetanib (inhibits VEGFR-2,3, EGFR and RET; Caprelsa; ZD6474; AstraZeneca, Macclesfield, United Kingdom)17,18 was considered due to a relatively similar kinase profile and superior clinical tolerability. It is currently FDA approved for medullary thyroid cancer, and has shown clinical efficacy in phase II/III studies, as a single agent and in combination with chemotherapy, in a number of other solid tumors.19-23 Here, we demonstrate that vandetanib was well tolerated, but did not increase PFS or OS in women with recurrent ovarian, primary peritoneal or fallopian tube cancer. To our knowledge this study represents the first randomized phase II trial of vandetanib in this population.
Methods: Patients
Eligible patients had histologically confirmed ovarian epithelial, fallopian tube, or primary peritoneal carcinoma, which was recurrent, refractory, or progressive/persistent and had measurable or non-measurable evaluable disease by imaging. They were to have received 1 prior platinum-based chemotherapy regimen for management of primary disease and were allowed ≤ 3 additional cytotoxic regimens for recurrent disease. Patients were to have a performance status of Zubrod 0-2, adequate hematological function (ANC ≥1,500/mcl, Platelet count ≥100,000/mcl), renal function (creatinine clearance ≥30 mL/min, urine protein:creatinine ratio <1), normal liver function (bilirubin ≤1.5 times upper limit of normal (ULN), AST or ALT ≤2.5 times ULN, alkaline phosphatase ≤2.5 times ULN). Important exclusions included pre-existing neuropathy ≥grade 2, active infection, significant cardiovascular disease, including uncontrolled hypertension (i.e., systolic blood pressure [BP] >140 mm Hg or diastolic BP >90 mm Hg) within the past 28 days, myocardial infarction, or NYHA class II-IV heart disease within the past 3 months, QTc with Bazett's correction ≥480 msec, history of symptomatic arrhythmia requiring treatment or asymptomatic sustained ventricular tachycardia. Atrial fibrillation controlled by medication was permitted. While patients were excluded for prior vandetanib use, treatment with other anti-VEGF targeted therapy was allowed. Patients were allowed to receive docetaxel as long as its use was limited to treatment of primary disease and a 6-month window between treatment discontinuation and study registration had been documented. The trial was approved by the institutional ethics and scientific review committees of each participating center and conducted in accordance with the Declaration of Helsinki, Good Clinical Practice policy. Each patient provided written informed consent.
Study Design and Treatment
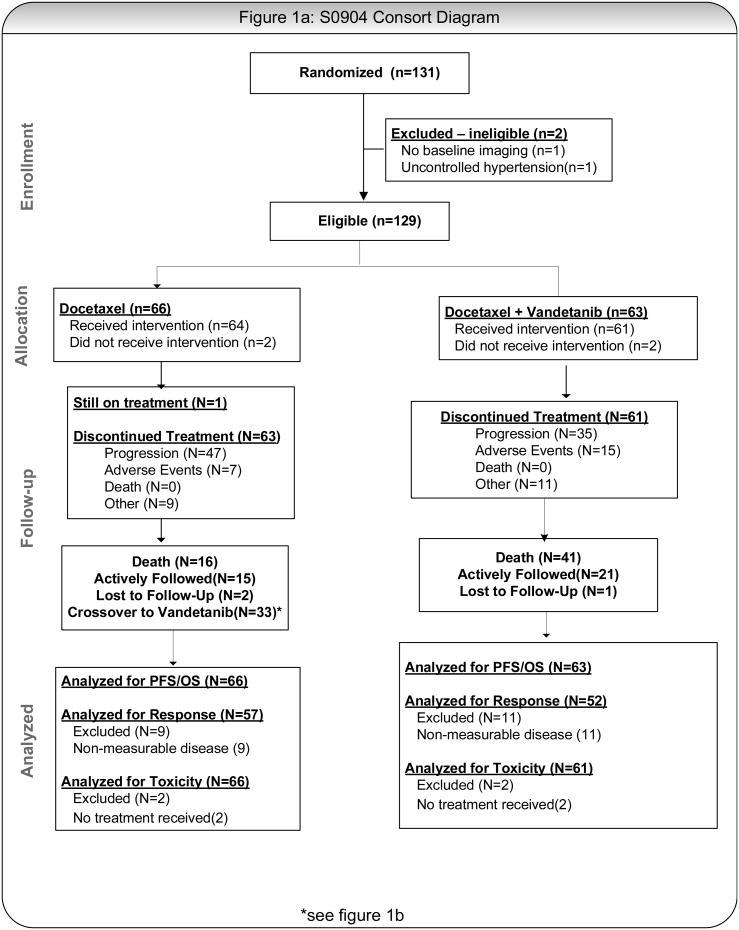
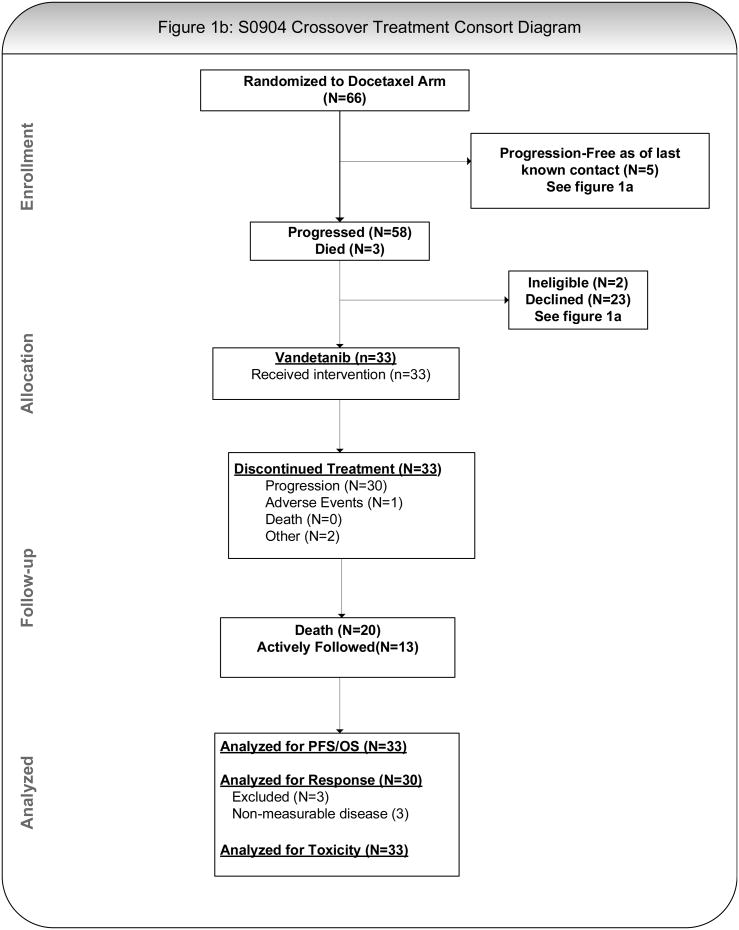
Figures 1A and 1B present the general study schema, which included up to 2 registrations for patients randomized to single agent docetaxel (cross-over arm). Patients were randomized centrally 1:1 using a dynamic balancing algorithm with stratification at the SWOG Statistical Center to docetaxel (75 mg/m2 IV) with or without vandetanib (100 mg PO daily; D+V or D); each cycle was 21 days.24 Patients randomized to single agent docetaxel were allowed to crossover to single agent vandetanib (100 mg PO daily) upon documented progression (D→V). Treatment in both arms was continued until disease progression, unacceptable toxicity or withdrawal of consent.
Figure 1.
A: CONSORT Diagram, Randomized Population
B: CONSORT Diagram for Crossover Treatment From Docetaxel to Vandetanib
Assessment
Objective tumor assessments were conducted using the Response Evaluation Criteria in Solid Tumors (RECISTv1.0).25 Imaging was obtained at baseline and every 6 weeks thereafter until progression or withdrawal of consent. The same imaging technique was to be used for defined “target” lesions. Adverse events were monitored before each treatment cycle and graded according to CTCAEv3. 12-lead ECGs were obtained at baseline, weeks 1, 2, 4, 7, 10 and 13, and every 12 weeks thereafter while taking vandetanib. Patients experiencing significant QTc prolongation as previously described had treatment withheld until resolution.
Quantification of plasma markers by multiplex ELISA assay
Plasma was collected from 74 available patients treated with this regimen at the following time points: prior to treatment (baseline), 48 hours after the first dose of docetaxel, and at the beginning of cycles 2, 4, 6, 8 and 10. Plasma IL-8, IL-6, VEGF, sVEGFR1, sVEGFR3 and sVEGFR2 were measured by multiplex ELISA assays (Luminex xMAP® platform and MILLIPLEX® MAG Human Cytokine/Chemokine panel (Millipore Corporation, Billerica, MA)) based on the manufacturer's instruction. All plasma samples were run in triplicate.
Statistical Considerations
This randomized Phase II trial was designed to provide evidence of the potential benefit of adding vandetanib to docetaxel in women with platinum-resistant, refractory, recurrent or persistent ovarian, fallopian tube or primary peritoneal cancer. Because vandetanib is an anti-angiogenesis agent, progression-free survival (PFS) was considered the most relevant intermediate endpoint to assess its potential benefit. Vandetanib crossover was allowed to estimate single-agent toxicity and preliminary response information. This design allowed patients in both arms the opportunity to have vandetanib therapy while preserving a valid comparison of PFS with and without vandetanib. Secondary clinical endpoints included response for each treatment (D, D+V, D→V), PFS considering CA-125 progression (D+V vs. D) and overall survival (OS) (D+V vs. D→V). Translational studies examined the effects of vandetanib on surrogate markers (IL-6, IL-8, sVEGFR1-3). In addition, the association between plasma VEGF, IL-6, IL-8 and sVEGFR1-3 concentrations and response or PFS was assessed.
Sample size Considerations
Based on historical data indicating an expected median PFS of 3.6 months in the docetaxel group, 120 evaluable patients would provide 84% power to detect a hazard ratio of 0.65 (corresponding to a PFS of 5.6 months in the D+V group based on a one-sided 0.1-level test.26 This level was chosen to provide supportive evidence to justify additional investigation. For the primary analyses PFS, defined as the time from randomization until objective tumor progression or death, was compared using a log-rank test based on the intent-to-treat principle, with stratification on prior anti-angiogenesis treatment. A similar analysis was planned for OS but because the docetaxel alone arm was offered vandetanib after documented progression, the OS comparison assesses two strategies–simultaneous (D+V) versus sequential (D→V) docetaxel plus vandetanib. The comparison of response rates was based on exact methods. The endpoints of toxicity, response (complete and partial) and PFS for single agent vandetanib were observational, intended to provide preliminary data and would come from a treatment pool of less than 60 patients. Estimated proportions for clinical response (RECIST criteria) and toxicity and Kaplan-Meier estimates of time-to-treatment failure were calculated. The primary analysis compared change from baseline in each biomarker between treatment groups over time. Power calculations were based on a comparison of the two treatment groups at a specific post-treatment time point.
Results
Over a 17.5 month enrollment period ending in August 2011, 131 patients were registered (Table 1). Two patients were ineligible: no radiographic evidence of disease at baseline (1), uncontrolled hypertension (1). Thus, 129 eligible patients are included in the intent-to-treat analysis. Baseline demographics were similar between the cohorts including measurable disease (D: 85%, D+V: 79%) and prior anti-angiogenesis therapy (D: 18%, D+V: 14%). The primary reason for treatment discontinuation was disease progression (64%, Table 2). At this time, one patient remains on protocol treatment having received 35 cycles of docetaxel over 25 months. This patient achieved a confirmed partial response.
Table 1. Demographics for the Study Population (n=129).
| Demographic | Docetaxel | Docetaxel + Vandetanib | ||
|---|---|---|---|---|
| (n=66) | (n=63) | |||
| AGE | ||||
| Median (Range) | 61.7 | 32.6-80 | 61.9 | 34.3-82.5 |
| HISPANIC | ||||
| Yes | 2 | 3% | 4 | 6% |
| No | 64 | 97% | 58 | 92% |
| Unknown | 0 | 0% | 1 | 2% |
| RACE | ||||
| White | 58 | 88% | 56 | 89% |
| Black | 4 | 6% | 4 | 6% |
| Asian | 4 | 6% | 2 | 3% |
| Unknown | 0 | 0% | 1 | 2% |
| PRIOR ANTIANGIOGENIC THERAPY | ||||
| Yes | 12 | 18% | 9 | 14% |
| No | 54 | 82% | 54 | 86% |
| PRIMARY SITE | ||||
| Fallopian Tube | 3 | 5% | 4 | 6% |
| Ovary | 56 | 85% | 53 | 84% |
| Peritoneal | 7 | 11% | 6 | 10% |
| PERFORMANCE STATUS | ||||
| 0 | 37 | 56% | 33 | 52% |
| 1 | 27 | 41% | 26 | 41% |
| 2 | 2 | 3% | 4 | 6% |
| BASELINE CA-125 | ||||
| ≤ 35 | 17 | 26% | 10 | 16% |
| > 35 | 49 | 74% | 53 | 84% |
| Median (Range) | 111 | (1 -6,019) | 160 | (5 -4,113) |
| Prior treatment with platinum for recurrent disease* | 34 | 52% | 28 | 44% |
All patients were considered platinum resistant or refractory; this category refers to the number and proportion of patients who were secondarily platinum-refractory/resistant having progressed on or within 6 months of platinum-based therapy for recurrent disease. Between-group proportions for this variable are not significant (P=0.48)
Table 2. Current Status and Reasons for Removal from Protocol Treatment.
| Initial Registration | Crossover Treatment with Vandetanib following Progression on Docetaxel Arm | |||
|---|---|---|---|---|
| Treatment Result | TOTAL | Docetaxel | Docetaxel + Vandetanib | Vandetanib |
| NUMBER ON PROTOCOL TREATMENT | 1 | 1 | 0 | 0 |
| NUMBER OFF PROTOCOL TREATMENT | 128 | 65 | 63 | 33 |
| REASON OFF TREATMENT | ||||
| Adverse events or side effects | 22 | 7 | 15 | 1 |
| Refusal unrelated to adverse event | 13 | 8 | 5 | 1 |
| Progression/relapse | 82 | 47 | 35 | 30 |
| Death | 0 | 0 | 0 | 0 |
| Other-not protocol specified | 11 | 3 | 8 | 1 |
| MAJOR PROTOCOL DEVIATIONS | 4 | 2 | 2 | 0 |
Efficacy Analysis
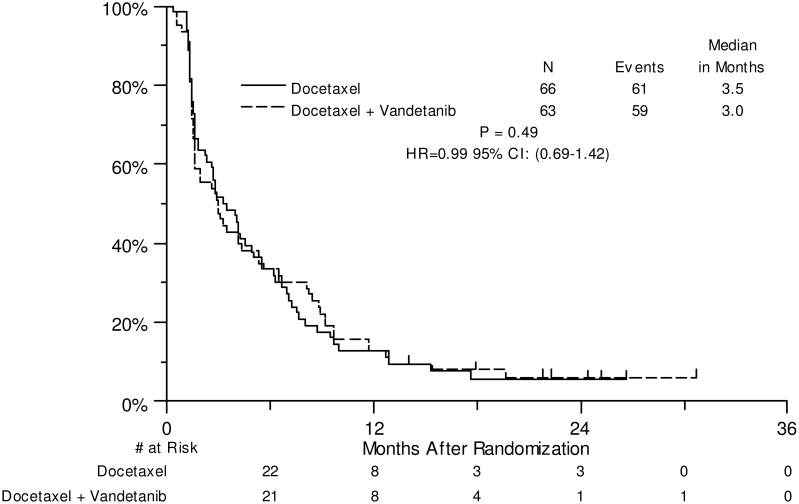
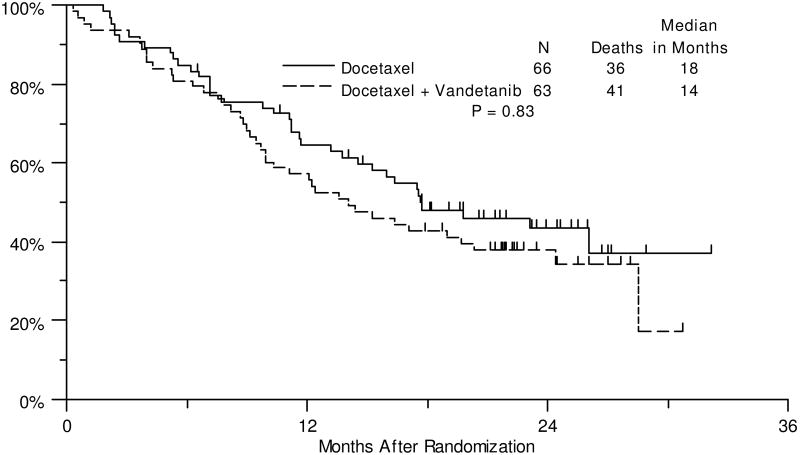
Matching preliminary estimates, the median PFS for docetaxel was 3.5 months and compared to a median PFS of 3.0 months in the combination arm (HR: 0.99, 80% CI: 0.79-1.26, P=0.49, Figure 2). No significant differences were seen for the secondary endpoints of clinical efficacy: PFS by CA125 progression criteria, OS and response rate (Supplemental Figure 1, Figure 3 and Table 3, respectively). Median OS for docetaxel was 18 months and compared to 14 months in the combination arm (HR: 1.25, 80% CI: 0.93-1.68, P=0.83).
Figure 2. Progression-Free Survival.
Biomarker Assessment
Plasma biomarker assessment was available in 74 evaluable patients. Quantitative changes from baseline were compared at each time point using a Wilcoxon Rank-Sum test. The only significant change from baseline to Cycle 2 was sVEGFR2 in the combination arm (Supplemental Table 1). Univariate Cox regression models were fit to assess the prognostic value of the baseline marker levels for progression-free-survival (PFS). Due to the skewed distribution of the data, a log transformation was applied to the original lab values, however, none of the markers were significantly related to either clinical outcome at the P<0.05 level. (Supplemental Table 2) To further evaluate the possible predictive value biomarkers for PFS and response, a treatment interaction term was added to each Cox model. Due to the low power for testing the treatment interaction term, a p-value of 0.15 was considered worthy of future investigation. In the PFS model for IL-8, the treatment interaction term had a p=0.13, and a hazard ratio of 0.66, suggesting that patients with high baseline values of IL-8 may have a better PFS on the combination arm. To investigate this further, analyses looking at each arm separately suggested that among patients on the single agent docetaxel arm, those with high baseline levels of IL-8 had worse PFS than patients with low baseline IL-8 levels (HR: 1.67, p=0.02). Finally, the change in marker values from baseline to the beginning of cycle 2 and the beginning of cycle 4 was compared between patients with a documented response versus patients without a response. This analysis was exploratory, and results were combined across both arms due to the low number of responders in each arm. Comparisons were made using a two-sided Wilcoxon Rank-Sum test. No p-values <0.05 were observed (Supplemental Table 3).
Vandetanib Monotherapy
Thirty-three of 47 patients who discontinued single agent docetaxel due to progression elected to participate in the crossover registration; 30 had measurable disease at the time they started vandetanib monotherapy and 27 were evaluable for response. The median time to progression in this cohort was 1.4 months following progression on study treatment (Supplemental Figure 2) and 1 patient experienced an unconfirmed partial response (4%). Six patients had stable disease at 12 weeks producing a clinical benefit rate (CR+PR+SD) of 26% (90% CI: 13%-43%)(Supplemental Table 4).
Safety and Tolerability
Table 4 presents the observed treatment-related adverse events in the treated population. In general, both regimens were well tolerated and outside of expected myelosuppression, grade 3 or higher adverse events were uncommon. One treatment related death occurred on the combination arm attributed as chemotherapy related myelosuppression. Importantly, QTc prolongation was registered in just 2 patients; both receiving vandetanib and both grade 1. As anticipated, maculo-papillary rash (37% all grade, 7% grade 3-4), acneiform rash (22% all grade, 2% grade 3-4), and hypertension (13% all grade, 0% grade 3-4) were observed more frequently in the combination arm. Significant neuropathy (grade 3-4) was observed infrequently (D: 5%, D+V: 0%) consistent with similar number of cycles administered in each arm of the trial (D: 4 cycles, D+V: 3 cycles, median). Adverse events observed in the cross-over arm (D-vandetanib) are listed in Table 4.
Table 4. Observed Toxicities by Treatment Arm (Randomized Population) and in the Crossover Treatment Population.
| Observed Toxicities by Treatment Arm (Randomized Population) | Docetaxel (N=64) Grade | Docetaxel + Vandetanib (N=61) Grade | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | ||
| Hematologic | |||||||||||
| Anemia | 29 | 16 | 1 | 0 | 0 | 17 | 9 | 2 | 0 | 0 | |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Leukocytosis | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Neutrophil count decreased | 0 | 3 | 17 | 15 | 0 | 0 | 3 | 8 | 20 | 0 | |
| Platelet count decreased | 6 | 0 | 0 | 0 | 0 | 5 | 2 | 1 | 0 | 0 | |
| White blood cell decreased | 4 | 10 | 17 | 3 | 0 | 2 | 4 | 17 | 3 | 0 | |
| Non-Hematologic | |||||||||||
| Allergic reaction | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Alopecia | 12 | 31 | 0 | 0 | 0 | 5 | 27 | 0 | 0 | 0 | |
| Anorexia | 5 | 10 | 1 | 0 | 0 | 10 | 5 | 2 | 0 | 0 | |
| Constipation | 15 | 7 | 0 | 0 | 0 | 10 | 4 | 1 | 0 | 0 | |
| ECG QT corrected interval prolonged | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| Edema | 9 | 2 | 1 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | |
| Elevated Liver Function Tests | 8 | 0 | 0 | 0 | 0 | 13 | 0 | 0 | 0 | 0 | |
| Fatigue/Malaise | 25 | 17 | 6 | 0 | 0 | 12 | 19 | 5 | 0 | 0 | |
| Hand-Foot syndrome | 4 | 4 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |
| Hypertension | 0 | 0 | 0 | 0 | 0 | 1 | 7 | 0 | 0 | 0 | |
| Hypocalcemia | 4 | 1 | 2 | 0 | 0 | 6 | 3 | 0 | 1 | 0 | |
| Hypokalemia | 8 | 0 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | |
| Hypomagnesemia | 11 | 3 | 0 | 1 | 0 | 19 | 2 | 1 | 1 | 0 | |
| Mucositis oral | 15 | 5 | 0 | 1 | 0 | 8 | 9 | 1 | 0 | 0 | |
| Myalgia | 3 | 7 | 0 | 0 | 0 | 10 | 4 | 0 | 0 | 0 | |
| Nail disorders | 14 | 4 | 0 | 0 | 0 | 9 | 1 | 1 | 0 | 0 | |
| Nausea/Vomiting | 22 | 7 | 5 | 0 | 0 | 19 | 8 | 3 | 0 | 0 | |
| Neuropathy | 25 | 5 | 3 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | |
| Proteinuria | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rash | 8 | 3 | 0 | 0 | 0 | 13 | 11 | 6 | 0 | 0 | |
| Thromboembolic event | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Treatment-related secondary malignancy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Observed Toxicities during Crossover Treatment with Vandetanib | Vandetanib (N=33) Grade | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Hematologic | |||||
| Anemia | 9 | 2 | 0 | 0 | 0 |
| Neutrophil count decreased | 1 | 0 | 0 | 0 | 0 |
| White blood cell decreased | 2 | 0 | 0 | 0 | 0 |
| Non-Hematologic | |||||
| Alopecia | 2 | 6 | 0 | 0 | 0 |
| Anorexia | 2 | 1 | 1 | 0 | 0 |
| Constipation | 5 | 4 | 0 | 0 | 0 |
| Elevated Liver Function Tests | 5 | 1 | 0 | 0 | 0 |
| Fatigue/Malaise | 11 | 5 | 1 | 0 | 0 |
| Hand-Foot syndrome | 1 | 1 | 0 | 0 | 0 |
| Hypertension | 1 | 0 | 2 | 0 | 0 |
| Hypokalemia | 1 | 0 | 0 | 1 | 0 |
| Hypomagnesemia | 5 | 1 | 0 | 0 | 0 |
| Mucositis oral | 0 | 1 | 0 | 0 | 0 |
| Myalgia | 4 | 1 | 1 | 0 | 0 |
| Nail disorders | 2 | 0 | 0 | 0 | 0 |
| Nausea/Vomiting | 5 | 1 | 1 | 0 | 0 |
| Neuropathy | 8 | 0 | 0 | 0 | 0 |
| Proteinuria | 1 | 0 | 0 | 0 | 0 |
| Rash | 0 | 1 | 0 | 0 | 0 |
Discussion
This study was designed to assess the effectiveness of combination docetaxel and vandetanib compared to docetaxel alone in women with previously treated, recurrent and platinum-resistant ovarian cancer. The rationale was based on preclinical observations demonstrating at least additive activity of anti-VEGFR and EGFR agents with taxanes in multiple tumor models, including ovarian cancer. In the current trial, although the addition of vandetanib was well tolerated, there was no evidence of benefit on PFS, or the secondary outcomes of ORR or OS.
Vandetanib is an inhibitor of VEGFR-2/3, EGFR and RET tyrosine kinases. It was selected for this trial since it was one of the few clinically available approaches for targeting the VEGFR/EGFR pathways. Proof-of-concept studies have been conducted in several tumor types with non-small cell lung cancer and advanced medullary thyroid cancer demonstrating clinical activity measured in objective response and PFS. In non-small cell lung cancer (NSCLC) patients, a randomized phase II and phase III trial has been conducted with the docetaxel and vandetanib combination. Dosed similar to the current trial (docetaxel 80 mg/m2, vandetanib 100 mg PO daily), the phase II trial demonstrated a HR of 0.76, one-sided P=0.098, corresponding to a difference of 24 weeks (combination) vs. 23 weeks (single agent).27 This met the proscribed level of significance to warrant further investigation and served as the primary support for the phase III trial. In the latter study, docetaxel/vandetanib improved objective response (17%vs.10%, P= 0.0001), and PFS (HR:0.79, 97.58%CI: 0.7-0.9, P<0.0001, median: 4 vs. 3.2 months) in the 1391 intent-to-treat population; the results among female patients were similar.28 There was no improvement in OS in this trial. Recently, vandetanib (100 mg PO daily) was studied in combination with pegylated liposomal doxorubicin (PLD, 50 mg IV, every 28 days) in patients with platinum-resistant ovarian cancer patients. Although 1 PR and 4 SD's were observed among 10 evaluable patients, the combination was not well tolerated, with toxicity leading to treatment discontinuation in 4 of 14 patients (29%).29
Single agent activity of vandetanib has also been previously examined in solid tumors producing response rates of 0 to 18%.30 In a small ovarian cancer trial of 12 patients, 8 of whom underwent paired tumor biopsies, no objective responses were observed but 4 patients (33%) had stable disease (≥16 weeks) as a best response. Tissue analysis demonstrated serial decrease in EGFR phosphorylation, but inconsistent effects on VEGFR phosphorylation or in plasma VEGF concentration.31 Similarly, vandetanib was studied in the BATTLE-1 trial producing a disease control rate at 8 weeks of 33%.32 In light of these observations, we allowed patients randomized to docetaxel to crossover to single agent vandetanib upon progression; half of the patients elected to do so. While objective response was similarly low (4%), 26% of patients had response or stable disease at 12 weeks. Other VEGFR and EGFR agents have been used for mono-therapy in patients with ovarian cancer. Experience with small molecule tyrosine receptor antagonists targeting EGFR such as erlotinib and gefitinib have been disappointing, producing single digit responses with stable disease rates of 15% at 6 months.33,34 While overexpression of EGFR is a common event in epithelial ovarian tumors, activating mutations are rare (<4%) likely explaining the poor clinical efficacy as single agents in this disease. Moreover, kinase-independent functions of EGFR could also explain lack of efficacy of some the current drugs.35 Other EGF receptor targets such as ErbB3, however, are entering clinical investigation.36,37 In contrast, agents targeting VEGF:VEGFR have been more promising with anti-VEGF based ligand approaches demonstrated merit in numerous phase II and phase III trials. VEGFR TKI's have had a more limited experience although several agents have undergone phase II and Phase III investigation. Notably, pazopanib and nintedanib, agents targeting VEGFR, PDGFR and FGFR have demonstrated single-agent and combination activity and have completed phase III investigation (pazopanib for primary maintenance, nintedanib in combination with paclitaxel and carboplatin for primary therapy followed by maintenance) in ovarian cancer patients.38 In addition, Cediranib, a VEGFR TKI was recently shown to increase both PFS and OS in combination with platinum-based chemotherapy among patients with platinum-sensitive recurrent disease.39 To date, phase III comparisons of VEGF-R TKI's to ligand-targeted monoclonal antibodies have not been done in this setting.
Our correlative studies of dynamic change in plasma marker values over time showed the VEGFR2 levels were significantly decreased at the beginning of Cycle 2 in patients treated with the combination of two drugs, which may be explained by the high affinity of vandetanib to VEGFR2 at low dose (IC50=40nM). Other vandetanib targets were not changed by the treatment, which might reflect lack of full engagement of other targets at the dose used here; this is consistent with the results from the isolated enzyme assays that at higher doses, other targets may be inhibited by vandetanib.
Notably, among all the markers tested, we found that baseline IL-8 levels appeared to carry prognostic inference with high levels associated with more favorable outcomes in patients receiving vandetanib combination and poorer prognosis in patients receiving single agent docetaxel. The mechanism by which IL-8 interacts with VEGF signaling is not yet fully understood. It has been reported that IL-8 can be induced under the hypoxic environment in HIF-1 dependent or independent manner.40 Whether baseline IL-8 can be used as a predictive marker to this treatment needs to be validated in a larger correlative study.
In summary, vandetanib added to docetaxel did not significantly improve objective response, PFS or OS in this cohort of recurrent platinum-resistant ovarian cancer patients. No new safety signals emerged from the trial supporting the tolerance of the combination. Alternative chemotherapy strategies such as metronomically-dosed paclitaxel or topotecan may provide additional avenues of investigation given their effects on non-overlapping angiogenesis targeting.
Supplementary Material
Figure 3. Overall Survival.
Table 3. Best Response to Therapy by Randomization Arm (Intent-to-Treat Population, Patients with Measurable Disease per RECIST at Baseline).
| Docetaxel (N=57) | Docetaxel + Vandetanib (N=52) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Complete Response | 0 | 0% | 0 | 0% |
| Partial Response | 5 | 9% | 6 | 12% |
| Unconfirmed Complete Response | 1 | 2% | 0 | 0% |
| Unconfirmed Partial Response | 2 | 4% | 2 | 4% |
| Stable/No Response | 19 | 33% | 17 | 33% |
| Increasing Disease | 23 | 40% | 20 | 38% |
| Symptomatic Deterioration | 0 | 0% | 1 | 2% |
| Assessment Inadequate | 7 | 12% | 5 | 10% |
Acknowledgments
This investigation was supported by the Marcus Foundation, the Investigator-Sponsored Study Program of AstraZeneca, Wilmington, DE, and in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA105409, CA35431, CA45560, CA20319, CA13612, CA46441, CA45808, CA45461, CA67575, CA58882, CA35128, CA37981, CA46282, CA35421, CA76132, CA58723, CA16385 and CA42777; as well as P50 CA083639, OCRP-OC0931146, and the Blanton-Davis Ovarian Cancer Research Program. AKS is supported by the Betty Anne Asche Murray Distinguished Professorship. Research support to RLC is by the Ann Rife Cox Chair in Gynecology. The authors thank Yunjie Sun in the Department of Gynecologic Oncology and Reproductive Medicine at MD Anderson Cancer Center for performing the quantitative multiplex ELISA assay.
This trial was registered at clinicaltrials.gov (NCT00872989)
Footnotes
Author Contribution: Conception and design: R Coleman, G Anderson, S Chambers, A Sood, M Markman,
Collection and assembly of data: R Coleman, J Moon, A Sood, W Hu, A Bonebrake
Data analysis and interpretation: R Coleman, J Moon, G Anderson, A Sood, W Hu, J Delmore,
Manuscript writing: R Coleman, J Moon, G Anderson, S Chambers, A Sood, W Hu, J Delmore, M Markman
Final approval of manuscript: R Coleman, J Moon, G Anderson, S Chambers, A Sood, W Hu, J Delmore, M Markman, A Bonebrake
Financial support: R Coleman, A Sood
Administrative support: R Coleman, A Sood
Provision of study material or patients: R Coleman, A Sood
Author Disclosure Declaration: Employment or leadership position: none
Consultant or advisory role: R Coleman has served as an uncompensated scientific advisor to AstraZeneca for developmental programs not involving vandetanib
Stock ownership: none
Honoraria: none
Research funding: none
Expert testimony: none
Patents, licenses or royalties: none
Other remuneration: none
No COIs to Report: J Moon, G Anderson, S Chambers, A Sood, W Hu, J Delmore, J Delmore, A Bonebrake
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. discussion 35-6. [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Medicine. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 4.Carmeliet P. Developmental biology. One cell, two fates. Nature. 2000;408:43, 45. doi: 10.1038/35040684. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P. VEGF gene therapy: stimulating angiogenesis or angiomagenesis? Nat Med. 2000;6:1102–3. doi: 10.1038/80430. [DOI] [PubMed] [Google Scholar]
- 6.Senger DR, Brown LF, Claffey KP, et al. Vascular permeability factor, tumor angiogenesis and stroma generation. Invasion Metastasis. 1994;14:385–94. [PubMed] [Google Scholar]
- 7.Cooper BC, Ritchie JM, Broghammer CL, et al. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clinical Cancer Research. 2002;8:3193–7. [PubMed] [Google Scholar]
- 8.Paley PJ, Staskus KA, Gebhard K, et al. Vascular endothelial growth factor expression in early stage ovarian carcinoma. Cancer. 1997;80:98–106. doi: 10.1002/(sici)1097-0142(19970701)80:1<98::aid-cncr13>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.So J, Wang FQ, Navari J, et al. LPA-induced epithelial ovarian cancer (EOC) in vitro invasion and migration are mediated by VEGF receptor-2 (VEGF-R2) Gynecol Oncol. 2005;97:870–8. doi: 10.1016/j.ygyno.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Wang FQ, So J, Reierstad S, et al. Vascular endothelial growth factor-regulated ovarian cancer invasion and migration involves expression and activation of matrix metalloproteinases. Int J Cancer. 2006;118:879–88. doi: 10.1002/ijc.21421. [DOI] [PubMed] [Google Scholar]
- 11.Aghajanian C, Blank SV, Goff BA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England journal of medicine. 2011;365:2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 13.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. The New England journal of medicine. 2011;365:2484–96. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 14.Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:4026–34. doi: 10.1200/JCO.2012.41.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–101. [PubMed] [Google Scholar]
- 16.Kamat AA, Kim TJ, Landen CN, Jr, et al. Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281–8. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 17.Wedge SR, Ogilvie DJ, Dukes M, et al. ZD6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–55. [PubMed] [Google Scholar]
- 18.Carlomagno F, Vitagliano D, Guida T, et al. ZD6474, an orally available inhibitor of KDR tyrosine kinase activity, efficiently blocks oncogenic RET kinases. Cancer Res. 2002;62:7284–90. [PubMed] [Google Scholar]
- 19.Miller KD, Trigo JM, Wheeler C, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2005;11:3369–76. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 20.Arnold AM, Seymour L, Smylie M, et al. Phase II study of vandetanib or placebo in small-cell lung cancer patients after complete or partial response to induction chemotherapy with or without radiation therapy: National Cancer Institute of Canada Clinical Trials Group Study BR.20. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4278–84. doi: 10.1200/JCO.2007.12.3083. [DOI] [PubMed] [Google Scholar]
- 21.Hsu C, Yang TS, Huo TI, et al. Vandetanib in patients with inoperable hepatocellular carcinoma: a phase II, randomized, double-blind, placebo-controlled study. Journal of hepatology. 2012;56:1097–103. doi: 10.1016/j.jhep.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Wells SA, Jr, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Boer RH, Arrieta O, Yang CH, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: a randomized, double-blind phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:1067–74. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 24.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–15. [PubMed] [Google Scholar]
- 25.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 26.Rose PG, Blessing JA, Ball HG, et al. A phase II study of docetaxel in paclitaxel-resistant ovarian and peritoneal carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88:130–5. doi: 10.1016/s0090-8258(02)00091-4. [DOI] [PubMed] [Google Scholar]
- 27.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–7. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Sun Y, Eberhardt WE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. The lancet oncology. 2010;11:619–26. doi: 10.1016/S1470-2045(10)70132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harter P, Sehouli J, Kimmig R, et al. Addition of vandetanib to pegylated liposomal doxorubicin (PLD) in patients with recurrent ovarian cancer. A randomized phase I/II study of the AGO Study Group (AGO-OVAR 2.13) Invest New Drugs. 2013;31:1499–504. doi: 10.1007/s10637-013-0011-3. [DOI] [PubMed] [Google Scholar]
- 30.Heymach JV. ZD6474--clinical experience to date. Br J Cancer. 2005;92(Suppl 1):S14–20. doi: 10.1038/sj.bjc.6602604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Annunziata CM, Walker AJ, Minasian L, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010;16:664–72. doi: 10.1158/1078-0432.CCR-09-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsao AS, Liu S, Lee JJ, et al. Clinical and biomarker outcomes of the phase II vandetanib study from the BATTLE trial. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8:658–61. doi: 10.1097/JTO.0b013e31828d08ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirte H, Oza A, Swenerton K, et al. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149) Gynecol Oncol. 118:308–12. doi: 10.1016/j.ygyno.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Schilder RJ, Sill MW, Chen X, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11:5539–48. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Besteiro MI, Somoza-Digon J, Einoder-Moreno M, et al. Health care quality perceived by the patients in an urban health centre in a coruna. Enferm Clin. 2012;22:182–90. doi: 10.1016/j.enfcli.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 36.Jia Y, Zhang Y, Qiao C, et al. IGF-1R and ErbB3/HER3 contribute to enhanced proliferation and carcinogenesis in trastuzumab-resistant ovarian cancer model. Biochemical and biophysical research communications. 2013 doi: 10.1016/j.bbrc.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Sheng Q, Liu X, Fleming E, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.From ASCO-gynaecological cancer: Pazopanib in ovarian cancer delays progression. Nature reviews Clinical oncology. 2013;10:367. doi: 10.1038/nrclinonc.2013.109. [DOI] [PubMed] [Google Scholar]
- 39.Ledermann JA, Perren T, Raja FA, et al. European Society for Medical Oncology. Amsterdam, The Netherlands: 2013. Randomised double-blind phase III trial of cediranib (AZD 2171) in relapsed platinum sensitive ovarian cancer: Results of the ICON6 trial. [Google Scholar]
- 40.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nature medicine. 2005;11:992–7. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.