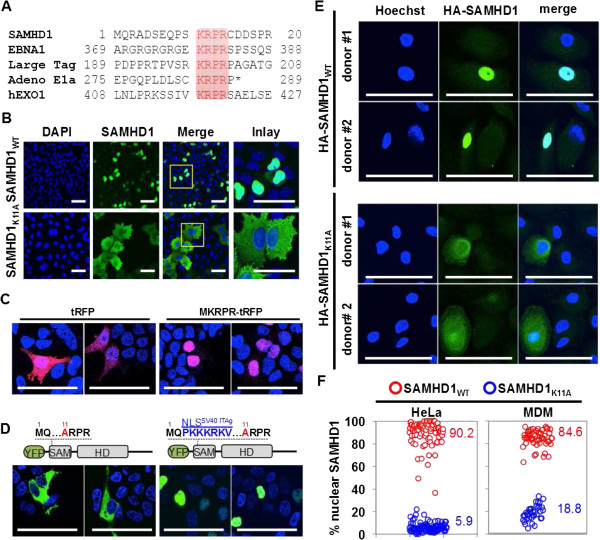
Figure 1.
Determination of the SAMHD1 nuclear localization signal (NLS). A) Comparison of the SAMHD1 amino acid sequence with known nuclear localization signals (NLS) from EBNA1, polyoma virus large T antigen, adenovirus E1a and human EXO1 identifies an N-terminal four amino acid stretch as a putative NLS. B) Substitution of a critical lysine residue K11 with alanine abrogates SAMHD1 nuclear localization and causes cytoplasmic accumulation. HeLa cells transduced with HA-SAMHD1WT or HA-SAMHD1K11A expressing MLV vectors were subjected to immunofluorescence using a HA-specific antibody and nuclei were stained with DAPI. C) Expression of turbo red fluorescence protein (tRFP) fused to amino-terminal KRPR sequence in 293 T cells causes redistribution of the protein from a pan-cellular to a nuclear localization. Nuclei were stained with DAPI and tRFP was detected by autofluorescence. D) Fusion of the SV40 large T antigen NLS is sufficient to rescue nuclear localization of YFP-SAMHD1K11A. 293 T cells were transfected with YFP-SAMHD1K11A (left panels) or YFP-SAMHD1K11A fused to the NLS of the SV40 large T antigen (right panels) and autofluorescence was measured 48 h after transfection. Nuclei were stained with DAPI. E) Primary monocyte-derived macrophages from two independent donors were transduced with HA-tagged wild type or K11A mutant SAMHD1 expressing HIV-1 lentiviral vectors and HA-immunostaining was performed to detect SAMHD1 three days later. F) Quantification of the percentage of nuclear SAMHD1 for HA-SAMHD1WT and HA-SAMHD1K11A expressing cells comparing HeLa cells with primary monocyte derived macrophages (MDM). Each circle represents one cell and numbers indicate average percentages of cells shown. Scale bars, 50 μm.