Abstract
The renin-angiotensin system (RAS) is a key regulator of vascular tone and blood pressure. In addition, angiotensin II also has a number of cellular effects that may contribute to disease pathogenesis. Using Agtr1a–/– mice, which lack AT1A receptors for angiotensin II, we have identified a novel function of the RAS to modulate the immune system. We find that angiotensin II, acting through type 1 (AT1) receptors on immune cells, triggers the proliferation of splenic lymphocytes. These actions contribute to the vigor of cellular alloimmune responses. Within lymphoid organs, sufficient components of the RAS are present to activate AT1 receptors during an immune response, promoting cell growth. These actions require activation of calcineurin phosphatase. In an in vivo model of cardiac transplantation, the absence of AT1 signaling accentuates the immunosuppressive effects of the calcineurin inhibitor cyclosporine. We conclude that inhibition of AT1 receptor signaling should be useful as an anti-inflammatory and immunosuppressive therapy. Furthermore, the actions of the RAS to promote lymphocyte activation may contribute to inflammation that characterizes a number of diseases of the heart and the vascular system.
J. Clin. Invest. 104:1693–1701 (1999).
Introduction
A prominent role for the renin-angiotensin system (RAS) in blood pressure and fluid homeostasis has been long recognized (1, 2). However, angiotensin II, the major biologically active peptide produced by the RAS, also has nonhemodynamic actions that may mediate end-organ injury in diseases such as hypertension and congestive heart failure (1, 2). For example, angiotensin II stimulates growth and hypertrophy of vascular smooth muscle cells and cardiac myocytes (3). Accordingly, in addition to their hemodynamic benefits, the effectiveness of pharmacologic antagonists of the RAS in cardiovascular diseases has been attributed, in part, to inhibition of these cellular actions of angiotensin II (1, 2). Virtually all the classically recognized actions of the RAS, including the nonhemodynamic actions just listed, are mediated by angiotensin II signaling through type 1 (AT1) receptors (1, 2). Although humans have only a single AT1 receptor isoform, mice have 2 AT1 receptor isoforms, AT1A and AT1B, that are encoded by distinct genes (Agtr1a and Agtr1b) (4, 5). The murine AT1A receptor is the predominantly expressed isoform in most tissues (6, 7) and is the closest homologue to the human AT1 receptor.
In addition to its effects to promote cell growth and proliferation, emerging evidence has suggested that the RAS may also have potent proinflammatory effects that may contribute to disease pathogenesis. For example, in cell-culture and whole-animal experiments, angiotensin II, acting through AT1 receptors, stimulates the production of inflammatory mediators such as TNF-α, TGF-β, and MCP-1 (8). In some cell types, stimulation of AT1 receptors is coupled to activation of JAK/STAT kinase systems (9–11). These signaling pathways are also used by T-cell cytokines such as IL-2 and γ-IFN (12). Although previous studies have suggested that the RAS may influence certain immunological functions, their results are conflicting and evidence for such actions is largely indirect (13–19). Moreover, a number of recent studies have demonstrated protective effect of RAS antagonists in immunologically mediated diseases such as myocarditis and chronic allograft rejection and in antiglomerular basement membrane nephritis (20–25). However, the mechanism of the beneficial actions of RAS inhibitors to prevent immunological injury in these models is not clear.
Various components of the RAS are expressed on inflammatory cells, and AT1 receptors are present on T cells and macrophages (26–28). To explore the effects of the RAS acting through AT1 receptors to regulate immune responses, we used mice in which the gene encoding the AT1A receptor (Agtr1a) had been disrupted by gene targeting (29). Our studies suggest that angiotensin II functions as an autocrine factor in alloimmune responses, promoting T-cell proliferation.
Methods
Animals.
Mice lacking AT1A receptors for angiotensin II were generated by homologous recombination in embryonic stem cells as described previously (29). To avoid the potential confounding effect of heterogeneous genetic background on the interpretation of our experiments, the Agtr1a mutation was backcrossed for more than 10 generations onto the C57BL/6 background. Agtr1a genotypes, designated (+) for the wild-type allele, and (–) for the targeted allele, were determined by Southern blot analysis of DNA isolated from tail biopsies as described elsewhere (29). Inbred C57BL/6 Agtr1a+/+ or Agtr1a–/– mice were used in all the experiments. Animals were bred and maintained in the animal facility of the Durham VA Medical Center under National Institutes of Health guidelines.
[125I]Angiotensin II receptor autoradiography.
Angiotensin II binding in whole spleen was determined by receptor autoradiography as we have described previously (29). Spleens were removed from Agtr1a+/+ and Agtr1a–/– mice, and the tissues were embedded in OTC compound (Miles Inc., Elkhart, Indiana, USA) and frozen in liquid nitrogen-chilled isopentane. Tissue blocks were sectioned serially (15 μm) on a cryostat and thaw-mounted on glass microscope slides (FisherPlus; Fisher Scientific, Pittsburgh, Pennsylvania, USA). The sections were incubated with buffer containing 200 pM [125I]angiotensin II. Excess concentrations (10 μM) of unlabeled angiotensin II, losartan (AT1 receptor antagonist), or PD123319 (AT2 receptor antagonist) were added to some sections to define specificity of binding. After incubation for 1 hour, the slides were washed, dried overnight, and then exposed to high-resolution x-ray film (Hyperfilm-betamax; Amersham International, Little Chalfont, Buckinghamshire, United Kingdom).
Radioligand binding studies using [125I]angiotensin II.
Angiotensin II binding in splenocytes was assessed by radioligand binding as described previously (30), using [125I]angiotensin II. Splenocyte suspensions were prepared from Agtr1a+/+ and Agtr1a–/– mice by gently grinding the spleen between glass slides. The cells were washed once in PBS and then resuspended in ice-cold PBS containing 10 mM Tris HCl (pH 7.4), 1% BSA. Aliquots of 5 × 105 splenocytes were incubated in triplicate with 20 pM [125I]angiotensin II for 30 minutes at 37°C with or without excess concentrations of unlabeled angiotensin II, losartan, or PD123319. The incubation mixtures were filtered through Whatman GF/C glass fiber filters (VWR Scientific, Westchester, Pennsylvania, USA). After washing, cell-bound labeled counts were determined using a gamma counter, and specific binding was calculated using standard formulas (30).
Identification of AT1A receptor mRNA by RT-PCR.
Expression of AT1A receptor mRNA was assessed by RT-PCR as described previously (7). Splenocytes were prepared from Agtr1a+/+ and Agtr1a–/– mice as already described here. Splenic T cells were isolated using a commercial separation column (R&D Systems Inc., Minneapolis, Minnesota, USA), splenic B cells were isolated by panning using a polyvalent anti-mouse IgG antibody, and splenic macrophages were isolated by plastic adherence. Total RNA was isolated from these cell preparations using Tri-reagent (Sigma Chemical Co., St. Louis, Missouri, USA), and 0.5 μg was reverse-transcribed using oligo-dT primers. AT1A receptor cDNA was amplified in PCR reactions using the following primers: sense 5′-GCATCATCTTTGTGGTGGG-3′ and antisense 5′-ATCAGCACATCCAGGAATG-3′. The PCR products were size fractionated on 1.8% agarose in TBE gels, stained with ethidium bromide, and photographed.
Cytofluorometric survey of lymphoid organs in Agtr1a+/+ and Agtr1a–/– mice.
Flow cytometry on splenocytes and thymocytes was performed as described previously (31). Briefly, spleen and thymus were isolated from Agtr1a+/+ and Agtr1a–/– mice, and cell suspensions were obtained by gently grinding the tissue between sterile glass slides. Cells were counted and resuspended (2 × 106 cells/mL) in cold FACS buffer (PBS/2% FBS/0.05% NaN3). Aliquots of resuspended cells (100 μL) were incubated with optimal concentrations of antibody for 30 minutes at 4°C and were washed 3 times in FACS buffer. The antibodies used were as follows: RM2-5 (anti-CD2), 145-2C11 (anti-CD3), GK 1.5 (anti-CD4), 53-6.7 (anti-CD8), 30-H122 (anti-Thy 1.2), H57-597 (anti-αβTCR), PK136/DX5 (anti-NK1.1), RA36B2 (anti-B220), and M1/70 (anti-CD11b) (all from PharMingen, San Diego, California, USA). An irrelevant isotypic antibody R35-95 (rat IgG2a; PharMingen) was used as a control for nonspecific staining. After final washing, cells were fixed with PBS containing 2% formalin and were analyzed within 72 hours. Analyses and sorting were performed in a FACscan (Becton Dickinson, Mountain View, California, USA). Forward scatter threshold was set to exclude dead cells and debris from acquisition. At least 1 × 104 cells were analyzed for each antibody or antibody combination.
Effects of angiotensin II on proliferation of splenocytes.
Single-cell suspensions of splenocytes were prepared from Agtr1a+/+ and Agtr1a–/– mice as already described here. The cells were incubated in complete RPMI containing 1% FBS and rested in media containing 1 μM of the ACE-inhibitor enalapril and 1 μM indomethacin at 37°C for 12 hours. A range of concentrations of angiotensin II was added to the cells with or without the AT1 receptor antagonist losartan or the calcineurin phosphatase inhibitor cyclosporine. After 18 and 30 hours of culture, 0.5 μCi of [3H]thymidine was added to each well, and the cells were incubated for an additional 12 hours at 37°C. Cell proliferation was assessed by the amount of [3H]thymidine incorporated by cells. To calculate specific [3H]thymidine incorporation, 3H counts per minute from wells without angiotensin II were subtracted from the wells containing angiotensin II.
Mixed lymphocyte responses.
Primary 1-way mixed lymphocyte responses (MLRs) were performed as described previously (31). Single-cell suspensions of responder splenocytes were reconstituted at various concentrations and were mixed with irradiated stimulator splenocytes at the indicated ratios. Fifty microliters of each cell suspension were added to individual wells of a 96-well plate with or without pharmacologic inhibitors. After varying periods of incubation, cells were pulsed with 0.5 μCi of [3H]thymidine per well for the final 18 hours of culture. The cells were harvested, and cell-associated [3H]thymidine content was determined by scintillation counting. Values are expressed as specific counts per minute (counts from wells with responders only were subtracted from counts from wells with responders and stimulators), with each point measured in triplicate or quadruplicate samples.
Mouse heart transplantation.
Heterotopic cardiac transplants in mice were performed as described previously (31). The recipient C57BL/6 (H-2b) Agtr1a+/+ and Agtr1a–/– mice were anesthetized with isoflurane and prepared by separating the aorta and vena cava between the renal vessels and the bifurcation of the iliacs. The donor heart was harvested from an MHC-disparate (DBA/2 × BALB/c)F1 (H-2d) mouse, and an end-to-side anastomosis was created between the recipient aorta and the ascending aorta from the donor heart. A similar anastomosis was then created between the recipient vena cava and the superior vena cava of the donor heart. The total ischemic time averaged 15 minutes and was not different between the groups. Surgical mortality of the recipients was less than 5%. All transplant recipients were treated with subtherapeutic doses (10 or 20 mg/kg by subcutaneous injection) of cyclosporine beginning on the day of transplant and continuing for 7 days. Allograft survival was monitored by direct palpation of the heartbeat through the abdominal wall, and graft failure was defined as the cessation of palpable heartbeat.
To examine further the effect of the Agtr1a mutation on the character of rejection, we evaluated the histopathology of cardiac allografts in the experimental groups. In additional groups of Agtr1a+/+ (n = 7) and Agtr1a–/– (n = 6) animals, cardiac transplants were performed as already described here and the recipients were treated with 20 mg/kg per day of cyclosporine by subcutaneous injection. On day 5 after transplantation, the allografts were removed and placed in 10% buffered formalin. After formalin fixation, the hearts were sectioned, stained with hematoxylin and eosin, and the slides were reviewed by a pathologist (P. Ruiz) who was masked to the treatment groups. The severity of rejection, interstitial infiltrates, myocyte injury, and vascular abnormalities were each graded separately using a semiquantitative scale where 0 was no abnormality and 1, 2, and 3 represented mild, moderate, and severe abnormalities, respectively.
Statistical analysis.
The values for each parameter within a group are expressed as the mean ± SEM. For comparisons between Agtr1a+/+ and Agtr1a–/– groups, statistical significance was assessed using an unpaired t test for normally distributed data. A paired t test was used for comparisons within groups. For nonparametric analyses, a Mann-Whitney U test was used.
Results
To determine whether the RAS might have direct effects on the immune system, we first characterized angiotensin receptors on lymphoid tissue by receptor autoradiography (29). As shown in Figure 1a, binding of [125I]angiotensin II was easily detected throughout the parenchyma of the spleen. The vast majority of [125I]angiotensin II binding was specific and was displaced by the addition of excess concentrations of unlabeled angiotensin II (data not shown). To differentiate between the 2 classes of angiotensin II receptors (AT1 and AT2), we determined the proportion of [125I]angiotensin II binding that could be displaced by specific antagonists for the receptor subtypes. In mouse spleen, most of the [125I]angiotensin II binding was displaced by the AT1 receptor antagonist losartan (Figure 1b). The AT2 receptor antagonist PD123319 had no effect on [125I]angiotensin II binding in spleen (data not shown).
Figure 1.
Angiotensin binding in mouse spleen. (a) Total [125I]angiotensin II binding in a section of normal mouse spleen. (b) [125I]Angiotensin II binding in the presence of excess concentrations of the AT1 receptor antagonist losartan.
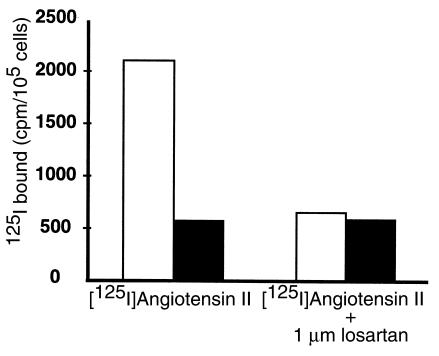
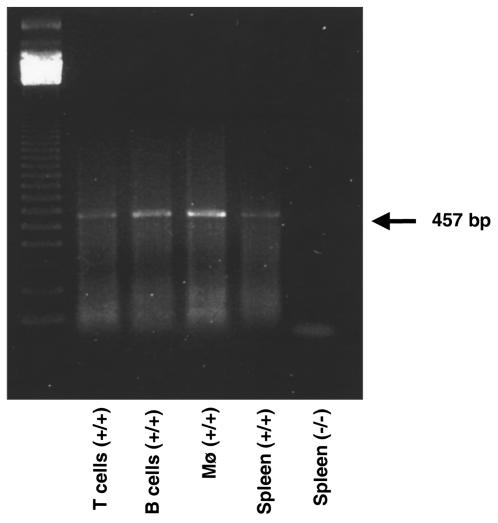
To characterize further angiotensin II binding sites in splenic lymphocytes, we performed radioligand binding studies in single-cell suspensions prepared from spleens of C57BL/6 Agtr1a+/+ and Agtr1a–/–mice (31). Specific [125I]angiotensin II binding was readily detected in splenocytes from wild-type mice, and the major portion of this binding was displaced by losartan (Figure 2). In contrast, total and losartan-displaceable binding of [125I]angiotensin II to AT1A receptor-deficient cells was not different from background. We used an RT-PCR assay to determine which cell populations within the spleen expressed AT1A receptor mRNA. As depicted in Figure 3, AT1A receptor mRNA could be amplified from RNA prepared from populations of T cells, B cells, or macrophages that were isolated from spleens of wild-type C57BL/6 mice. Thus, the AT1A receptor is, by far, the predominant angiotensin II receptor expressed by immune cells in the spleen.
Figure 2.
[125I]Angiotensin II radioligand binding in mouse splenocytes. [125I]Angiotensin II radioligand binding in cell suspensions prepared from whole spleen from Agtr1a+/+ (open bars) and Agtr1a–/– (filled bars) mice. Total binding is shown in the left, and binding in the presence of the AT1 receptor antagonist losartan is shown on the right.
Figure 3.
AT1A receptor mRNA expression in cell populations isolated from spleen. AT1A receptor mRNA expression in spleen assessed by RT-PCR. Total RNA was isolated, and RT-PCR was performed using primers specific for the AT1A receptor (7). The PCR products were size fractionated on an agarose gel that was stained with ethidium bromide. The 457-bp PCR product is easily seen in samples from isolated splenic T cells (lane 2), B cells (lane 3), and macrophages (lane 4), or unseparated splenocytes (lane 5) from a wild-type mouse. No product was detected in RNA isolated from splenocytes of an AT1A receptor–deficient mouse (lane 6).
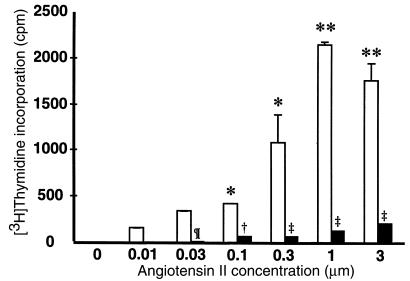
In other cell types such as vascular smooth muscle cells, angiotensin II stimulates cell growth and proliferation (3, 32, 33). To examine the function of angiotensin receptors expressed by inflammatory cells, we tested whether angiotensin II could induce proliferation of splenic lymphocytes. After incubation with 1 μM concentrations of an angiotensin-converting enzyme (ACE) inhibitor to block endogenous angiotensin II synthesis, splenocytes from wild-type and Agtr1a–/– mice were exposed to increasing concentrations of angiotensin II peptide. As shown in Figure 4, angiotensin II alone was sufficient to cause proliferation of splenic lymphocytes from Agtr1a+/+ animals. The magnitude of the response was dose dependent, and the maximal effect was observed at angiotensin II concentrations of 1–3 μM. The actions of angiotensin II to stimulate proliferation of wild-type cells were completely blocked by the AT1 receptor antagonist losartan (data not shown). Moreover, as shown in Figure 4, splenocytes from AT1A receptor–deficient mice do not proliferate in response to angiotensin II, indicating that these actions are mediated by AT1A receptors.
Figure 4.
Angiotensin II stimulates splenocyte proliferation. Concentrations of angiotensin II in the range of 0.01–3 μM were added to single-cell suspensions of splenocytes from wild-type (open bars) and Agtr1a–/– mice (filled bars). Proliferation was assessed 48 hours later as [3H]thymidine incorporation. *P < 0.001 versus 0 μM angiotensin II; **P < 0.0001 versus 0 μM angiotensin II; ¶P < 0.03 versus Agtr1a+/+; †P < 0.008 versus Agtr1a+/+; ‡P < 0.001 versus Agtr1a+/+.
To examine whether angiotensin II receptors contribute to proliferation during an ongoing immune response, we measured alloantigen-induced proliferation of splenic lymphocytes isolated from C57BL/6 (H-2b) Agtr1a+/+ and Agtr1a–/– mice in a 1-way MLR. After 5 days in culture with irradiated, allogeneic (H-2d) stimulators, proliferative responses were approximately 50% lower in AT1A receptor–deficient cells than controls across a range of stimulator cell concentrations (Figure 5a). This was not simply a difference in the kinetics of the response, as proliferation by AT1A receptor–deficient cells was also reduced after 3, 4, and 5 days in MLRs (Figure 5b). When purified T cells were used as responders, significantly less proliferation was once again observed in the T cells lacking AT1A receptors compared with controls (5,807 ± 1,437 vs. 2,568 ± 760; P = 0.0013). In contrast, there was no difference in the level of proliferation in reverse MLR using Agtr1a+/+ and Agtr1a–/– cells as stimulators (data not shown). When anti-CD3 antibody was used as a stimulus, proliferation of AT1A receptor–deficient cells was also significantly reduced compared with controls (Figure 5c). However, proliferative responses after exposure to the nonspecific mitogen concanavalin A were not different between the groups. Therefore, stimulation of the AT1A receptor occurs as a part of normal cellular immune responses promoting lymphocyte proliferation. The actions of AT1A receptors to enhance splenocyte proliferation are most prominent when activation is triggered through stimulation of the T-cell receptor/CD3 complex.
Figure 5.
MLRs and responses to mitogens by splenocytes from Agtr1a+/+ and Agtr1a–/– mice. (a) MLR using splenocytes from C57BL/6 (H-2b) Agtr1a+/+ and Agtr1a–/– mice as responders and irradiated splenocytes from (BALB/c × DBA/2)F1 (H-2d) mice as stimulators. [3H]Thymidine incorporation is significantly reduced in splenocytes from Agtr1a–/– mice (filled bars) compared with Agtr1a+/+ controls (open bars) across a wide range of stimulator concentrations. *P < 0.001 versus Agtr1a–/–. (b) Proliferative responses in MLR were measured after 3, 4, and 5 days in culture comparing C57BL/6 wild-type (open bars) and Agtr1a–/– (filled bars) responder cells. *P < 0.04 versus Agtr1a+/+; †P < 0.0001 versus Agtr1a+/+. (c) The effects of concanavalin A (left) and anti-CD3 antibodies (right) on proliferation of splenocytes isolated from Agtr1a+/+ (open bars) and Agtr1a–/– mice (filled bars). Proliferation is once again measured as [3H]thymidine incorporation. *P < 0.01 versus Agtr1a+/+; †P < 0.002 versus Agtr1a+/+.
To determine whether the Agtr1a mutation might produce gross abnormalities in development of the immune system, we examined the size and cellular constitution of the major lymphoid organs in Agtr1a+/+ and Agtr1a–/– mice. As depicted in Table 1, there were no differences between the AT1A receptor–deficient mice and controls. To confirm that the defect in proliferation was due to reduced angiotensin II signaling and not to a subtle developmental abnormality in the AT1A receptor–deficient mice, 1-way MLR experiments were performed in the presence of the ACE inhibitor enalapril or the AT1 receptor antagonist losartan. Both agents caused dose-dependent reductions in proliferative responses when added to MLR cultures of Agtr1a+/+ cells (Figure 6). Moreover, the maximal inhibition induced by the 2 pharmacologic RAS inhibitors was approximately 50% of control values, similar to the diminution seen in MLRs with Agtr1a–/– cells. The actions of the ACE inhibitor to suppress proliferation in the MLR were reversed by the simultaneous administration of 10 μM angiotensin II on day 0 of culture (control MLR: 6,678 ± 276 cpm; enalapril alone: 2,884 ± 192 cpm; P < 0.0001 versus control; enalapril + angiotensin II: 7,664 ± 427; P < 0.0001 versus enalapril alone). Taken together, these data demonstrate that angiotensin II acts as an autocrine growth factor to augment T-cell proliferation in MLRs. They also suggest that ample components of the RAS are present in mixed lymphocyte culture to produce angiotensin II in sufficient quantities to induce cellular effects.
Table 1.
Analysis of lymphoid organs
Figure 6.
The effects of RAS inhibitors on lymphocyte proliferation. A range of concentrations of the ACE inhibitor enalapril or the AT1 receptor antagonist losartan were added to a 1-way MLR. Proliferation was measured as specific incorporation of [3H]thymidine. Both losartan (open bars) and enalapril (filled bars) caused significant, dose-dependent reductions in proliferation of wild-type splenocytes in MLR. *P < 0.005 versus no drug.
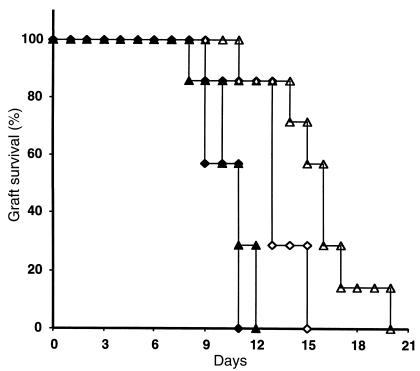
Because of the important role of calcineurin phosphatase in T-cell activation in alloimmune responses (34–36), along with recent studies demonstrating activation of calcineurin in cardiac myocytes by angiotensin II (37–39), we considered the possibility that calcineurin might play a role in the stimulation of lymphocyte proliferation by AT1 receptors. To examine the role of the pathway of calcineurin and a family of transcription factors named nuclear factor of activated T cells (NFATs) in angiotensin II–induced lymphocyte growth, we incubated splenic lymphocytes with 1 μM angiotensin II in the presence of increasing concentrations of the calcineurin inhibitor cyclosporine. Cyclosporine caused a dose-proportional inhibition of angiotensin II–stimulated lymphocyte proliferation (Figure 7), and at the highest dose (3 ng/mL), the effect of angiotensin II to promote splenocyte proliferation was completely blocked. To determine whether these actions have any relevance to an in vivo immune response, we used a heterotopic cardiac allograft model (31). C57BL/6 Agtr1a+/+ and Agtr1a–/– mice were transplanted with H-2 disparate cardiac allografts and were treated with subtherapeutic doses (10 or 20 mg/kg) of cyclosporine for 7 days. Allograft survival was monitored by direct palpation of the heartbeat through the abdominal wall. Without treatment, survival of cardiac allografts in this aggressive model of acute rejection was similar in Agtr1a+/+ and Agtr1a–/–recipients (9 ± 1 and 10 ± 2 days, respectively). As shown in Figure 8, administration of 10 or 20 mg/kg per day of cyclosporine had little effect on allograft survival in the wild-type animals (10 ± 1 and 11 ± 1 days). In contrast, graft survival was significantly prolonged in the AT1A receptor–deficient animals (13 ± 1 days with 10 mg/kg and 16 ± 1 days with 20 mg/kg; P < 0.014 versus wild-type), demonstrating that simultaneous inhibition of AT1 and calcineurin signaling have cooperative effects to ameliorate allograft rejection.
Figure 7.
The effects of calcineurin inhibition on angiotensin II–stimulated lymphocyte proliferation. Splenocyte suspensions from C57BL/6 wild-type mice were cultured with 1 μM angiotensin II along with 0.3 or 3 ng/mL cyclosporine, or its vehicle alone; after 24 hours [3H]thymidine incorporation was determined. Baseline proliferation, in the absence of angiotensin II, was similar between the groups: 2,094 ± 125 cpm for vehicle; 1,791 ± 168 cpm for 0.3 ng/mL cyclosporine; and 1,789 ± 245 cpm for 3 ng/mL cyclosporine. *P < 0.02 versus vehicle.
Figure 8.
Enhanced effect of cyclosporine to prolong allograft survival in AT1A receptor-deficient recipients. Heterotropic cardiac allografts were placed in Agtr1a+/+ (filled symbols) and Agtr1a–/– (open symbols) mice. Recipient animals received cyclosporine for 7 days at 2 doses, 10 mg/kg per day (diamonds) and 20 mg/kg per day (triangles). These subtherapeutic doses of cyclosporine had no significant effect on graft survival in the Agtr1a+/+ mice. Cyclosporine caused a modest but significant prolongation of survival in the Agtr1a–/– animals (P < 0.014 versus wild-type).
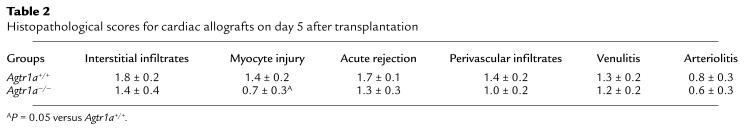
To determine whether the absence of AT1A receptors altered the character of intragraft inflammation, allograft histopathology was examined on day 5 after transplantation in additional groups of Agtr1a+/+ and Agtr1a–/–mice that had been treated with 20 mg/kg per day of cyclosporine. As shown in Table 2, the severity of each abnormality tended to be less in the Agtr1a–/– recipients than controls. In particular, the degree of myocyte injury was reduced in the AT1A receptor–deficient recipients (P = 0.05 versus wild-type).
Table 2.
Histopathological scores for cardiac allografts on day 5 after transplantation
Discussion
Accumulating evidence over the past few years has suggested that angiotensin II might be produced by and act directly on immune cells. For example, Gomez and associates reported that rat leukocytes express angiotensinogen mRNA and synthesize angiotensinogen protein (28). Moreover, angiotensin peptides are released by rat alveolar macrophages (39), mouse lymphocytes, and granuloma cells in murine schistosomiasis (14, 40). Addition of angiotensinogen or angiotensin I peptides to immune cell preparations can augment the release of angiotensin II (14), implying that these cells possess the requisite enzymatic machinery to perform the complete conversion of angiotensinogen substrate to active angiotensin II. Although some studies suggest that these pathways may be independent of renin and/or ACE (41), ACE activity and mRNA expression have been documented in macrophages and T cells (42–47), and ACE expression may be upregulated in inflammatory conditions (42, 43). Enhanced expression of ACE in inflammatory cells has been linked to diseases such as sarcoidosis (42, 43), although its pathophysiological role has not been defined. Local accumulation of ACE has also been identified in atherosclerotic lesions, particularly in regions of inflammatory cell infiltration (48). In these settings, it has been suggested that local expression and accumulation of ACE may contribute to enhanced production of angiotensin II in tissues.
The potential for angiotensin II to directly modulate inflammatory cell functions was suggested by the observation that human mononuclear leukocytes express specific binding sites for angiotensin II (27). Tsutsumi and associates subsequently demonstrated large numbers of binding sites for angiotensin II in rat spleen and found that these sites were primarily AT1 receptors (26). This is consistent with our receptor autoradiography findings of diffuse AT1-specific binding in mouse spleen, depicted in Figure 1a. Our studies further show that these are predominantly AT1A receptors and that AT1A receptors are expressed in a variety of splenocyte populations including T cells, macrophages, and B cells.
Effects of angiotensin II to modulate T-cell functions have also been suggested by previous studies. In a preliminary report, Vance and Kelly reported that proliferation of a nephritogenic T-cell clone was significantly augmented by the addition of angiotensin II and that this effect seemed to be mediated by AT1 receptors (49). In contrast, Simon and associates reported inhibition of PHA-induced proliferation by angiotensin II (50). Thus, although the data are conflicting, there are several reports suggesting that angiotensin II modulates immune functions and that this peptide may have direct effects on T-cell proliferation. In the experiments described here, we find that exposure of splenocytes to angiotensin II, with no other exogenous stimulus, is sufficient to trigger proliferation. This response is blocked by specific AT1 receptor antagonists and it is absent in cells from Agtr1a–/– mice, indicating that these effects are mediated by AT1A receptors.
To determine whether the actions of angiotensin II to stimulate lymphocyte proliferation play a role in modulating immune responses, we used the MLR as a model of the cellular alloimmune response. The MLR is designed to mimic the conditions that might occur in a transplanted organ when recipient immune cells are activated by recognition of foreign MHC antigens expressed on the donor tissue. When the responder cell population came from Agtr1a–/– mice that lack AT1A receptors, we found that proliferative responses in MLR were significantly reduced, by approximately 50%. Proliferation was also reduced when purified T cells from Agtr1a–/– mice were used as responders. Because AT1 receptors are also normally expressed on antigen-presenting cells, we tested whether the response would be affected if AT1A receptors were absent from stimulator cells that provide the antigenic stimulus to trigger the MLR. In this case, proliferation was similar to that induced by wild-type stimulators. Taken together, these data suggest that reduced proliferation of AT1A receptor–deficient cells in MLR is due to the absence of direct effects of angiotensin II on responder cell populations, including T cells.
We have extensively analyzed the lymphoid organs of Agtr1a–/– mice by size, cell numbers, and cell constituents and have found no discernible differences compared with wild-type animals (see Table 1). Nonetheless, to ensure that the differences in MLR were not due to a subtle developmental defect caused by the absence of AT1A receptors, we also performed MLR experiments in wild-type cells in the presence of an ACE inhibitor or an AT1 receptor antagonist. Both inhibition of angiotensin II production and blockade of AT1 receptors produced a dose-dependent inhibition of the proliferative response. The level of inhibition achieved by pharmacologic blockade, approximately 50% of normal, was similar to that seen in the genetic experiments. The similarity between the effects of ACE inhibition and AT1 receptor blockade suggests that virtually all the effects of angiotensin II to modulate T-cell proliferation are mediated by AT1 receptors. These experiments also suggest that adequate components of the RAS are present in cultured splenocytes to produce angiotensin II in sufficient quantities to induce cellular effects. Therefore, these studies identify a “tissue RAS” in lymphoid organs that contributes to the regulation of cellular immune responses.
The differential effects of the Agtr1a mutation on proliferation induced by anti-CD3 antibody compared with the nonspecific mitogen concanavalin A suggest that the actions of AT1A receptors to modulate T-cell proliferation may be specific for stimulation through the TCR/CD3 complex. Engagement of the T-cell receptor with antigen in the setting of appropriate costimulation causes a brisk increase in intracellular calcium (51). This leads to activation of calcineurin, a calcium- and calmodulin-dependent phosphatase (34) that dephosphorylates NFATs (34–36, 52–54). Once these proteins are dephosphorylated by calcineurin, they translocate to the nucleus where they directly activate transcription of genes that promote T cell activation and proliferation (55). AT1 receptor stimulation is coupled to increased intracellular calcium levels in a number of systems (1) including human PBMCs (56). Accordingly, we considered the possibility that stimulation of AT1 receptors on lymphocytes leads to an increase in intracellular calcium concentration and that this AT1-mediated calcium signal triggers calcineurin and NFAT activation. In support of this possibility, recent studies have demonstrated that AT1 receptor stimulation is sufficient to activate the calcineurin-NFAT pathway in cardiac myocytes and this activation leads to alterations of cell phenotype that can be inhibited by cyclosporine. In the heart, this pathway seems to be an important cause of myocardial hypertrophy (37, 38).
To examine the role of the calcineurin-NFAT pathway in the angiotensin II–mediated lymphocyte proliferation, we used cyclosporine, a specific inhibitor of calcineurin phosphatase (52). Cyclosporine completely blocked the ability of angiotensin II to induce proliferation of cultured splenic lymphocytes, demonstrating that calcineurin is required for stimulation of lymphocyte proliferation by AT1 receptors. To determine whether this pathway is operative during an immune response in an intact animal, we studied the effects of the Agtr1a mutation on cyclosporine responses in a mouse model of cardiac transplant rejection. In this model, donor and recipient are completely mismatched at the MHC locus, resulting in a very aggressive acute cellular rejection response to the allograft. Using these strain combinations, doses of cyclosporine of approximately 100 mg/kg per day are generally required to achieve prolongation of graft survival. In wild-type animals, doses of cyclosporine that were an order of magnitude below this therapeutic level had no effect on graft survival. In contrast, these small doses of cyclosporine caused a significant prolongation of graft survival in mice lacking AT1A receptors on their lymphocytes. This result demonstrates the relationship between AT1 receptor signaling and calcineurin activation in an alloimmune response in vivo and suggests that these pathways cooperate to promote allograft injury in this model. Because AT1A receptor expression is normal in the donor heart, these actions result from the absence of AT1A receptor signaling in recipient tissues.
Although these transplant studies provide proof of the concept that AT1 receptor signaling on immune cells contributes to in vivo alloimmune responses, the effect in this powerful model of acute rejection is relatively modest. However, it is possible that the relative contribution of this pathway may be more significant in more indolent alloimmune responses, such as in chronic allograft rejection or in patients who are being treated with therapeutic doses of immunosuppressive agents. In this regard, a number of studies in animal models have demonstrated an important role for the renin-angiotensin system in the pathogenesis of chronic allograft rejection. For example, several groups have demonstrated that AT1 receptor blockade significantly ameliorates kidney injury in rat models of chronic renal allograft rejection and that these effects are not due to reduction in systemic blood pressure (22–24). In a model of chronic rejection of cardiac allografts, AT1 receptor blockade significantly ameliorates intimal proliferation of coronary arteries, the pathological lesion that characterizes chronic rejection (20). The efficacy of RAS antagonists in immunological diseases has largely been attributed to effects on vasculature and production of inflammatory mediators. Our studies suggest that blockade of direct actions of angiotensin II to promote the T-cell response may also contribute to these beneficial effects.
In summary, these studies identify potent effects of the RAS to modulate the immune system. Because inflammation is critical to the pathogenesis of diseases such as atherosclerosis (57), the effects of angiotensin II on immune cell activation may contribute to the pathological effects of RAS dysregulation in cardiovascular disease. The enhanced sensitivity of AT1A receptor–deficient mice to the immunosuppressive actions of cyclosporine suggests a rationale for the use of RAS antagonists in patients with organ transplants who are being treated with calcineurin inhibitors. In this setting, antagonism of the RAS may potentiate the immunosuppressive effects of calcineurin inhibition reducing the doses required to achieve adequate immunosuppression and therefore limiting dose-related toxicity.
Acknowledgments
We thank K. Snow and R. Griffiths for expert technical assistance and N. Turner for secretarial and administrative help. This work was supported by grants from the National Institutes of Health (DK-38103, HL-56122, and HL-49277) and the Research Service of the Department of Veterans Affairs. R.B. Mannon is supported by a Clinical Scientist Development Award (AI01389).
References
- 1.Griendling K, Lassegue B, Alexander R. Angiotensin receptors and their therapeutic implications. Annu Rev Pharmacol Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- 2.Timmermans P, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 3.Owens G. Control of hypertrophic versus hyperplastic growth of vascular smooth muscle cells. Am J Physiol. 1989;257:H1755–H1765. doi: 10.1152/ajpheart.1989.257.6.H1755. [DOI] [PubMed] [Google Scholar]
- 4.Sasamura H, et al. Cloning, characterization, and expression of two angiotensin receptor (AT-1) isoforms from the mouse genome. Biochem Biophys Res Commun. 1992;185:253–259. doi: 10.1016/s0006-291x(05)80983-0. [DOI] [PubMed] [Google Scholar]
- 5.MacTaggart T, Ito M, Smithies O, John S. Mouse angiotensin receptor genes Agtr1a and Agtr1b map to chromosomes 13 and 3. Mamm Genome. 1997;8:294–295. doi: 10.1007/s003359900419. [DOI] [PubMed] [Google Scholar]
- 6.Llorens-Cortes C, Greenberg B, Huang H, Corvol P. Tissular expression and regulation of type 1 angiotensin II receptor subtypes by quantitative reverse transcriptase-polymerase chain reaction analysis. Hypertension. 1994;24:538–548. doi: 10.1161/01.hyp.24.5.538. [DOI] [PubMed] [Google Scholar]
- 7.Burson J, Aguilera G, Gross K, Sigmund C. Differential expression of angiotensin receptor 1A and 1B in mouse. Am J Physiol. 1994;267:E260–E267. doi: 10.1152/ajpendo.1994.267.2.E260. [DOI] [PubMed] [Google Scholar]
- 8.Klahr S, Morrissey J. Angiotensin II and gene expression in kidney. Am J Kidney Dis. 1998;31:171–176. doi: 10.1053/ajkd.1998.v31.pm9428470. [DOI] [PubMed] [Google Scholar]
- 9.Marrero M, et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature. 1995;375:247–250. doi: 10.1038/375247a0. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein K, Ali M, Sayeski P, Semeniuk D, Marrero M. New insights into the cellular signaling of seven transmembrane receptors: the role of tyrosine phosphorylation. Lab Invest. 1998;78:3–7. [PubMed] [Google Scholar]
- 11.Duff J, et al. Angiotensin II signal transduction and the mitogen-activated protein kinase pathway. Cardiovasc Res. 1995;30:511–517. [PubMed] [Google Scholar]
- 12.Liu K, Gaffen S, Goldsmith M. JAK/STAT signalling by cytokine receptors. Curr Opin Immunol. 1998;10:271–278. doi: 10.1016/s0952-7915(98)80165-9. [DOI] [PubMed] [Google Scholar]
- 13.Weinstock J, Kassab J. Chemotactic response of splenic mononuclear cells to angiotensin II in murine schistosomiasis. J Immunol. 1986;137:2020–2024. [PubMed] [Google Scholar]
- 14.Weinstock J, Blum A. Granuloma macrophages in murine Schistosomiasis mansoni generate components of the angiotensin system. Cell Immunol. 1984;89:39–45. doi: 10.1016/0008-8749(84)90195-3. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Berliner J, Nadler J. Angiotensin II increases monocyte binding to endothelial cells. Biochem Biophys Res Commun. 1996;226:862–868. doi: 10.1006/bbrc.1996.1441. [DOI] [PubMed] [Google Scholar]
- 16.Foris G, Dezso B, Medgyesi G, Fust G. Effect of angiotensin II on macrophage functions. Immunology. 1983;48:529–535. [PMC free article] [PubMed] [Google Scholar]
- 17.Kunert-Radek J, Stepien H, Komorowski J, Pawlikowski M. Stimulatory effect of angiotensin II on the proliferation of mouse spleen lymphocytes in vitro is mediated via both types of angiotensin II receptors. Biochem Biophys Res Commun. 1994;198:1034–1039. doi: 10.1006/bbrc.1994.1147. [DOI] [PubMed] [Google Scholar]
- 18.Simon M, Weinstock J. Angiotensin II inhibition of mitogen- and antigen-induced human blood mononuclear cell thymidine uptake. J Clin Lab Immunol. 1985;18:141–144. [PubMed] [Google Scholar]
- 19.Yeung J. A comparison of the effects of angiotensin-converting enzyme inhibitors with bradykinin, angiotensin II and their specific antagonists on concanavalin A-induced proliferation of mouse T-lymphocytes. Methods Find Exp Clin Pharmacol. 1994;16:163–172. [PubMed] [Google Scholar]
- 20.Furukawa Y, Matsumori A, Hirozane T, Sasayama S. Angiotensin II receptor antagonist TCV-116 reduces graft coronary artery disease and preserves graft status in a murine model. Circulation. 1996;93:333–339. doi: 10.1161/01.cir.93.2.333. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka A, Matsumori A, Wang W, Sasayama S. An angiotensin II receptor antagonist reduces myocardial damage in an animal model of myocarditis. Circulation. 1994;90:2051–2055. doi: 10.1161/01.cir.90.4.2051. [DOI] [PubMed] [Google Scholar]
- 22.Amuchastegui S, et al. Chronic allograft nephropathy in the rat is improved by angiotensin II receptor blockade but not by calcium channel antagonism. J Am Soc Nephrol. 1998;9:1948–1955. doi: 10.1681/ASN.V9101948. [DOI] [PubMed] [Google Scholar]
- 23.Benediktsson H, Chea R, Davidoff A, Paul L. Antihypertensive drug treatment in chronic renal allograft rejection in the rat. Effect on structure function. Transplantation. 1996;62:1634–1642. doi: 10.1097/00007890-199612150-00018. [DOI] [PubMed] [Google Scholar]
- 24.Mackenzie H, et al. Candesartan cilexetil reduces chronic renal allograft injury in Fisher→Lewis rats. J Hypertens Suppl. 1997;15:S21–S25. doi: 10.1097/00004872-199715066-00005. [DOI] [PubMed] [Google Scholar]
- 25.Hisada Y, et al. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsutsumi K, Stromberg C, Saavedra J. Characterization of angiotensin II receptor subtypes in the rat spleen. Peptides. 1992;13:291–296. doi: 10.1016/0196-9781(92)90111-f. [DOI] [PubMed] [Google Scholar]
- 27.Shimada K, Yazaki Y. Binding sites for angiotensin II in human mononuclear leucocytes. J Biochem (Tokyo) 1978;84:1013–1015. doi: 10.1093/oxfordjournals.jbchem.a132183. [DOI] [PubMed] [Google Scholar]
- 28.Gomez R, et al. Leukocytes synthesize angiotensinogen. Hypertension. 1993;21:470–475. doi: 10.1161/01.hyp.21.4.470. [DOI] [PubMed] [Google Scholar]
- 29.Ito M, et al. Regulation of blood pressure by the type IA receptor for angiotensin II. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spurney R, Onorato J, Ruiz P, Pisetsky D, Coffman T. Characterization of glomerular thromboxane receptors in murine lupus nephritis. J Pharmacol Exp Ther. 1993;264:584–590. [PubMed] [Google Scholar]
- 31.Mannon R, et al. Downregulation of T cell receptor expression by CD8+ lymphocytes in kidney allografts. J Clin Invest. 1998;101:2517–2527. doi: 10.1172/JCI1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ray P, Bruggeman L, Horikoshi S, Aguilera G, Klotman P. Angiotensin II stimulates human fetal mesangial cell proliferation and fibronectin biosynthesis by binding to AT1 receptors. Kidney Int. 1994;45:177–184. doi: 10.1038/ki.1994.21. [DOI] [PubMed] [Google Scholar]
- 33.Wolf G, Neilson E. Angiotensin II induces cellular hypertrophy in cultured murine proximal tubular cells. Am J Physiol. 1990;259:F768–F777. doi: 10.1152/ajprenal.1990.259.5.F768. [DOI] [PubMed] [Google Scholar]
- 34.Clipstone N, Crabtree G. Identification of calcineurin as a key signaling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 35.McCaffrey P, et al. Isolation of the cyclosporin-sensitive T cell transcription factor NFATp. Science. 1993;262:750–754. doi: 10.1126/science.8235597. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber S, Crabtree G. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 37.Sussman M, et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;28:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 38.Molkentin J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dezso B, Jacobsen J, Poulsen K. Evidence for the presence of angiotensins in normal, unstimulated alveolar macrophages and monocytes. J Hypertens. 1989;7:5–11. doi: 10.1097/00004872-198901000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Weinstock J, Kassab J. Functional angiotensin II receptors on macrophages from isolated liver granulomas of murine Schistosoma mansoni. J Immunol. 1984;132:2598–2602. [PubMed] [Google Scholar]
- 41.Tonnesen M, Klempner M, Austen K, Wintroub B. Identification of a human neutrophil angiotensin II–generating protease as cathepsin G. J Clin Invest. 1982;69:25–30. doi: 10.1172/JCI110437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silverstein E, Pertschuk L, Friedland J. Immunofluorescent localization of angiotensin converting enzyme in epithelioid and giant cells of sarcoidosis granulomas. Proc Natl Acad Sci USA. 1979;76:6646–6648. doi: 10.1073/pnas.76.12.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okabe T, Susuki A, Ishukawa H, Hotsumoto H, Ohsawa N. Cells originating from sarcoid granulomas in vitro. Am Rev Respir Dis. 1981;124:608–612. doi: 10.1164/arrd.1981.124.5.608. [DOI] [PubMed] [Google Scholar]
- 44.Rohrbach M, Conrad A. Comparison of the T lymphocyte–dependent induction of angiotensin-converting enzyme and leucine aminopeptidase in cultured human monocytes. Clin Exp Immunol. 1991;83:510–515. doi: 10.1111/j.1365-2249.1991.tb05670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryu J, Vuk-Pavlovic Z, Rohrbach M. Monocyte heterogeneity in angiotensin-converting enzyme induction mediated by autologous T lymphocytes. Clin Exp Immunol. 1992;88:288–294. doi: 10.1111/j.1365-2249.1992.tb03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazarus D, Aschoff J, Fanburg B, Lanzillo J. Angiotensin converting enzyme (kininase II) mRNA production and enzymatic activity in human peripheral blood monocytes are induced by GM-CSF but not by other cytokines. Biochem Biophys Acta. 1994;1226:12–18. doi: 10.1016/0925-4439(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 47.Costerousse O, Allegrini J, Lopez M, Alhenc-Gelas F. Angiotensin I-converting enzyme in human circulating mononuclear cells: genetic polymorphism of expression in T-lymphocytes. Biochem J. 1993;290:33–40. doi: 10.1042/bj2900033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diet F, et al. Increased accumulation of tissue ACE in human atherosclerotic coronary artery disease. Circulation. 1996;94:2756–2767. doi: 10.1161/01.cir.94.11.2756. [DOI] [PubMed] [Google Scholar]
- 49.Vance B, Kelly C. A nephritogenic T cell clone expresses components of the renin-angiotensin system and is responsive to angiotensin II. J Am Soc Nephrol. 1994;5:772. [Google Scholar]
- 50.Simon M, Engel D, Weinstock J, Roi L. The effect of angiotensin II on human mononuclear cell reactivity: suppression of PHA-P-induced thymidine incorporation. Immunol Invest. 1985;14:389–400. doi: 10.3109/08820138509047607. [DOI] [PubMed] [Google Scholar]
- 51.Weiss A, Littman D. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 52.Foor F, et al. Calcineurin mediates inhibition by FK506 and cyclosporin of recovery from a-factor arrest in yeast. Nature. 1992;360:682–684. doi: 10.1038/360682a0. [DOI] [PubMed] [Google Scholar]
- 53.Harding M, Galat A, Uehling D, Schreiber S. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 54.Kay J, Benzie C, Goodier M, Wick C, Doe S. Inhibition of T-lymphocyte activation by the immunosuppressive drug FK-506. Immunology. 1989;67:473–477. [PMC free article] [PubMed] [Google Scholar]
- 55.Rao A, Luo C, Hogan P. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 56.Lijnen P, Fagard R, Petrov V. Cytosolic calcium changes induced by angiotensin II in human peripheral blood mononuclear cells are mediated via angiotensin II subtype 1 receptors. J Hypertens. 1997;15:871–876. doi: 10.1097/00004872-199715080-00011. [DOI] [PubMed] [Google Scholar]
- 57.Mach F, Schonbeck U, Sukhova G, Atkinson E, Libby P. Reduction of atherosclerosis in mice by inhibition of CD40 signaling. Nature. 1998;394:200–203. doi: 10.1038/28204. [DOI] [PubMed] [Google Scholar]