Abstract
Study Objectives:
To investigate the contribution of sleep duration and quality to age-related changes in brain structure and cognitive performance in relatively healthy older adults.
Design:
Community-based longitudinal brain and cognitive aging study using a convenience sample.
Setting:
Participants were studied in a research laboratory.
Participants:
Relatively healthy adults aged 55 y and older at study commencement.
Interventions:
N/A.
Measurements and Results:
Participants underwent magnetic resonance imaging and neuropsychological assessment every 2 y. Subjective assessments of sleep duration and quality and blood samples were obtained. Each hour of reduced sleep duration at baseline augmented the annual expansion rate of the ventricles by 0.59% (P = 0.007) and the annual decline rate in global cognitive performance by 0.67% (P = 0.050) in the subsequent 2 y after controlling for the effects of age, sex, education, and body mass index. In contrast, global sleep quality at baseline did not modulate either brain or cognitive aging. High-sensitivity C-reactive protein, a marker of systemic inflammation, showed no correlation with baseline sleep duration, brain structure, or cognitive performance.
Conclusions:
In healthy older adults, short sleep duration is associated with greater age-related brain atrophy and cognitive decline. These associations are not associated with elevated inflammatory responses among short sleepers.
Citation:
Lo JC, Loh KK, Zheng H, Sim SK, Chee MW. Sleep duration and age-related changes in brain structure and cognitive performance. SLEEP 2014;37(7):1171-1178.
Keywords: aging, brain structure, cognitive performance, sleep duration, sleep quality
INTRODUCTION
With increasing life expectancy in many societies, cognitive decline accompanying aging or neurodegenerative disease has become a growing problem. Changes in brain structure can precede the appearance of clinical symptoms of cognitive decline or dementia by several years.1,2 Accordingly, there is considerable interest in identifying modifiable risk factors that exert negative effects on brain structure and cognitive performance.3
Recent epidemiological findings from middle-aged and older adults point to an important role of sleep in moderating cognitive aging. Poorer cognitive function is associated with short sleep,4–10 long sleep,4,7,8,10–12 and changes (both increases13,14 and decreases13) in sleep duration. Self-reported poor sleep quality has also been linked to decline in cognitive performance.7,15–24 Importantly, sleep duration and quality at midlife can influence cognitive functioning 20 y later.25
Although neuropsychological tests are instrumental in identifying cognitive decline, they are associated with considerable measurement variability. Contrastingly, volumetric assessment of brain structure has been shown to predict cognitive outcome with considerably lower measurement variability.26 No brain region is spared from age-related atrophy, even among persons with minimal cognitive decline.27 However, there are significant regional differences in the severity of atrophy28 that are also evident when comparing persons at very low, average, and high risk of dementia.27
Among brain regions showing age-related atrophy, the hippo-campus is sensitive to a wide variety of insults.3 Hippocampal volume at baseline and an elevated rate of hippocampal volume reduction have both been validated as risk markers for cognitive decline as well as subsequent Alzheimer disease.2 The lateral ventricles are also affected by multiple medical conditions29–32 and have been validated as a biomarker for cognitive decline in older adults.1 Finally, age-related changes in many important higher cognitive functions have been linked to the volume of the lateral prefrontal cortices.33
Only a few imaging studies have examined the effect of reduced sleep on alterations in brain structure in healthy individuals with no known sleep disorder. In one study, right temporal lobe volume was reduced in flight attendants exposed to sleep disruption from frequent transmeridian travel.34 Reduced hippocampal gray matter volume has been reported in healthy children and adolescents who slept less on weekdays.35 These studies suggest that short sleep duration might affect brain structure in younger persons and motivate our study on how short sleep modulates longitudinal changes in brain structure and cognition in older adults.
The mechanisms of how sleep loss may result in altered brain structure and cognition remain unknown. Investigated here is the relationship between sleep loss and increased inflammation.36,37 Elevated inflammatory cytokine levels increase risk of cognitive decline38–40 and have been associated with increased white matter hyperintensities41 as well as reduced gray matter volume.41,42
The current study involved relatively healthy, community-dwelling older adult participants of an ongoing longitudinal brain and cognitive aging study.43 We hypothesized that self-reported short sleep duration and poor global sleep quality at baseline would be associated with greater longitudinal changes in hippocampal, ventricular, and prefrontal volumes as well as a more pronounced decline in neuropsychological test performance over a 2-y interval. We also explored whether any significant contribution of sleep to cognitive aging was mediated through its effect on brain atrophy. Additionally, we measured high-sensitivity C-reactive protein (hs-CRP) to explore associations between inflammation, sleep, brain structure, and cognition.
METHODS
Participants
Participants were healthy, community-dwelling, ethnically Chinese older adults of the Singapore-Longitudinal Aging Brain Study recruited from healthy aging clubs and by word of mouth.43 They were aged 55 y and older in 2005 when the study started. Exclusion criteria for all phases include (1) history of significant vascular events (i.e., myocardial infarction, stroke, or peripheral vascular disease); (2) history of malignant neoplasia of any form; (3) a history of cardiac, lung, liver, or kidney failure; (4) active or inadequately treated thyroid disease; (5) active neurological or psychiatric conditions; (6) a history of head trauma with loss of consciousness; (7) a Mini-Mental State Examination (MMSE44) score < 26; or (8) a 15-item Geriatric Depression Scale (GDS45) score > 5.
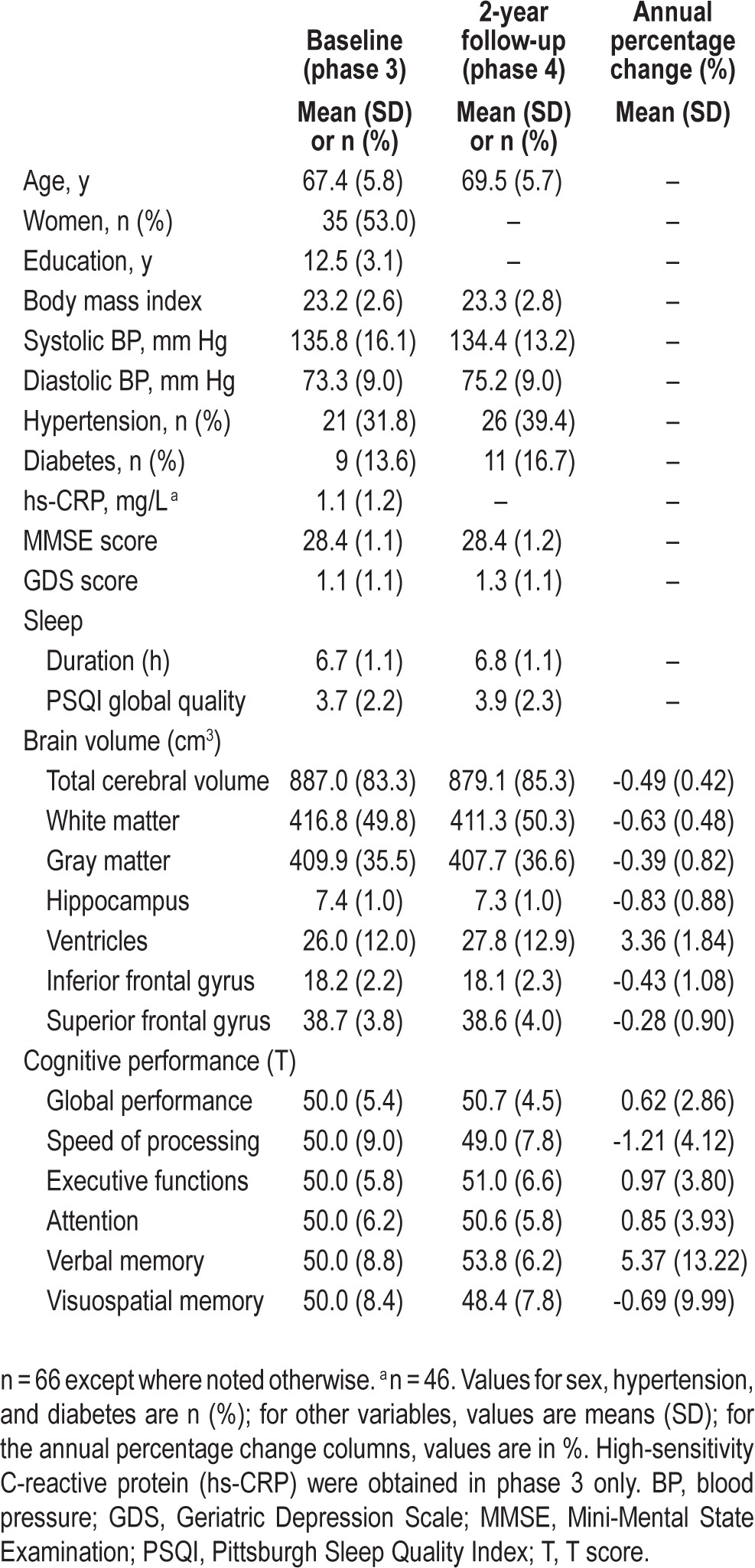
Midway through phase 3 (November 2009–January 2011), the Pittsburgh Sleep Quality Index (PSQI46) was introduced for sleep assessment and was filled in by a subset of 119 participants. In phase 4 (October 2011–April 2013), this subset dropped to 66 (mean age ± standard deviation [SD]: 69.5 ± 5.7 y; 31 males) for the following reasons: loss of contact and withdrawal (n = 27), heart condition or use of blood thinner (n = 8), GDS score > 5 (n = 7), MMSE < 26 (n = 7), thyroid problems (n = 2), brain hemorrhage (n = 1), and cancer (n = 1). The characteristics of these 66 participants are summarized in Table 1.
Table 1.
Sample characteristics.
Experimental Design
In the Singapore-Longitudinal Aging Brain Study, structural magnetic resonance brain scans and cognitive assessments were performed at approximately 2-y intervals. At phase 3, blood samples were collected in a subset of participants (n = 47) for measuring their hs-CRP levels. Assessment of subjective sleep duration and quality began midway in phase 3.
Brain Imaging
High-resolution images of the brain were acquired using a T1-weighted multi-echo magnetization prepared rapid acquisition gradient echo (MEMPRAGE) sequence with a 3T Siemens Tim Trio system (Siemens, Erlangen, Germany). There were 192 contiguous sagittal slices with the following scanning parameters: repetition time (TR) = 2530 ms, TI = 1200 ms, flip angle = 7°, field of view (FOV) 256 mm × 256 mm, 256 × 256 matrix, isotropic voxel dimensions of 1.0 mm, 6 min 3 sec acquisition time. Automated measurements of brain volumes were performed using FreeSurfer 5.1.0 (http://surfer.nmr.mgh.harvard.edu/). Here, we report findings regarding total cerebral, gray, and white matter, hippocampal, and ventricular volumes, as well as the volumes of inferior frontal gyrus and the superior frontal gyrus.43 Total intracranial volume was estimated (eTIV43,47) and used as a covariate in statistical analyses involving brain structures to compensate for differences in head size across participants.
Cognitive Assessments
Cognitive performance was assessed with a battery of 11 neuropsychological tests that evaluated performance in five cognitive domains—speed of processing, executive functions, attention, verbal memory, and visuospatial memory. Speed of processing was evaluated with the Symbol Digit Modalities Test,48 the Symbol Search Task in the Wechsler Memory Scale– Third Edition (WMS-III),49 and the Trail Making Test A.50 Executive functions were assessed with the Categorical Verbal Fluency Test,51 the Design Fluency Test in the Delis-Kaplan Executive Function System,52 and the Trail Making Test B.50 Attention was measured with the Digit Span Test and the Spatial Span Test in WMS-III.49 Verbal memory was assessed with the Verbal Paired Associates Test in WMS-III49 (dropped in phase 4) and the Rey Auditory Verbal Learning Test.51 Visuospatial memory was evaluated with the Visual Reproduction Test in WMS-III49 (dropped in phase 4) and a Visual Paired Associates Test (refer to supplemental materials for the details of each test).
We used T scores to indicate participants' performance in each cognitive domain. T transformation is commonly used in studies involving multiple cognitive measures.13 This is to enable tasks that use different scales to have a mean of 50 and SD of 10 after transformation. It was thus possible to combine tasks within the same domain so as to give a more robust measure of performance as well as to minimize the problem of multiple comparisons. Like z transformation, T transformation is a standard data transform in neuropsychological studies.53 Here, for each cognitive domain, participants' scores in the respective tests within each phase were transformed to T scores (mean = 50 and SD = 10 at phase 3) and then averaged for computation of composite scores (see reference 43 for details). Additionally, we averaged the T scores of the five cognitive domains in each phase to derive a measure of global cognitive performance.
Sleep Assessments
The PSQI46 was used to assess sleep duration and quality. Sleep duration was indicated by participants' response to item 4 in the questionnaire: “During the past month, how many hours of actual sleep did you get at night?” The wording of this question was similar to the sleep duration item used in previous epidemiological studies on sleep and cognitive aging.6,13,14 Global sleep quality was indicated by the global PSQI score.
High-Sensitivity C-reactive Protein
Venous blood samples were drawn between 08:30 and 09:30 and tested for hs-CRP. Measurements were made using quality controlled procedures (Synchron LX Systems, CA, US), with a sensitivity of 0.02 mg/L. One participant's data were excluded from all the analyses concerning hs-CRP because his hs-CRP level was more than 3 SDs from the group mean.
Statistical Analyses
Statistical analyses were performed with SPSS 20.0 (IBM, Chicago, IL, USA). To quantify the annual change in brain volume and cognitive performance from baseline to the 2-y follow-up (i.e. between phase 3 and phase 4), for each participant, we first calculated the annual percentage change (APC) of each brain and cognitive measure using the following formula:
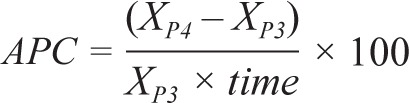 |
where XP3 and XP4 are the brain or cognitive measures at phase 3 and phase 4 respectively, and time is the interval in years between the two phases. We used multiple regression analyses to determine whether sleep duration at baseline, i.e., in phase 3, significantly predicted the APC of brain volume or cognitive performance over the following 2 y after controlling for the effects of age, sex, education, and body mass index (BMI). In these analyses, we included in model 1 sleep duration in phase 3 as a predictor (and eTIV as a covariate for brain measures). We added age and BMI in phase 3, as well as sex and years of education into model 2.
To examine whether sleep quality at baseline contributed to the age-related changes in brain structures and cognitive performance, similar analyses were performed.
As a result of the significant effects of baseline sleep duration on the APC of total ventricular volume and global cognitive performance (see Results for more details), we determined whether sleep's effect on global cognitive aging was mediated by its effect on ventricular expansion. We did so by adding the APC of total ventricular volume into model 3 for global cognitive performance.
To investigate whether hs-CRP accounted for any significant contribution of sleep to longitudinal changes in brain structure and cognitive performance, we used Pearson correlational analyses to ascertain for association between hs-CRP, sleep, and APC. Because none of these associations were statistically significant (refer to the Results section for further details), we did not include hs-CRP into the regression models that would dramatically reduce the sample size from 66 to 46.
Similarly, correlation between sleep and age at baseline was computed to investigate whether age-related alterations in sleep might confound the interpretation of potential effects of sleep on brain and cognitive aging. One participant was excluded from all the analyses concerning total ventricular volume because his corresponding APC was 3 SDs below the group mean.
RESULTS
Effects of Sleep at Baseline on Longitudinal Changes in Brain Structures
The longitudinal changes in total cerebral volume, gray matter volume, white matter volume, hippocampal volume, total ventricular volume, inferior frontal gyrus volume, and superior frontal gyrus volume in our cohort (Table 1) were comparable to or slightly smaller than those reported in other samples of healthy older adults.26,28,54
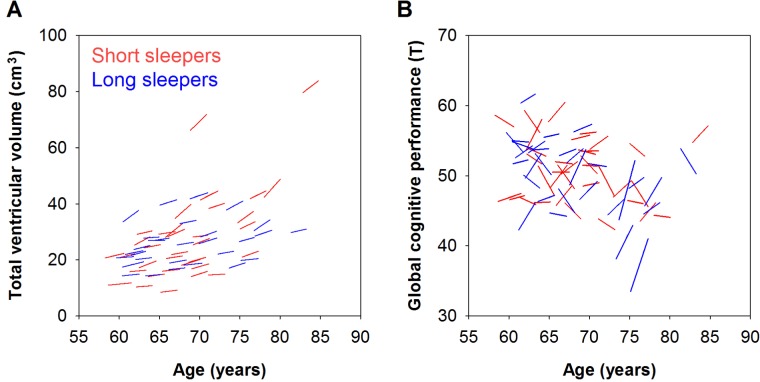
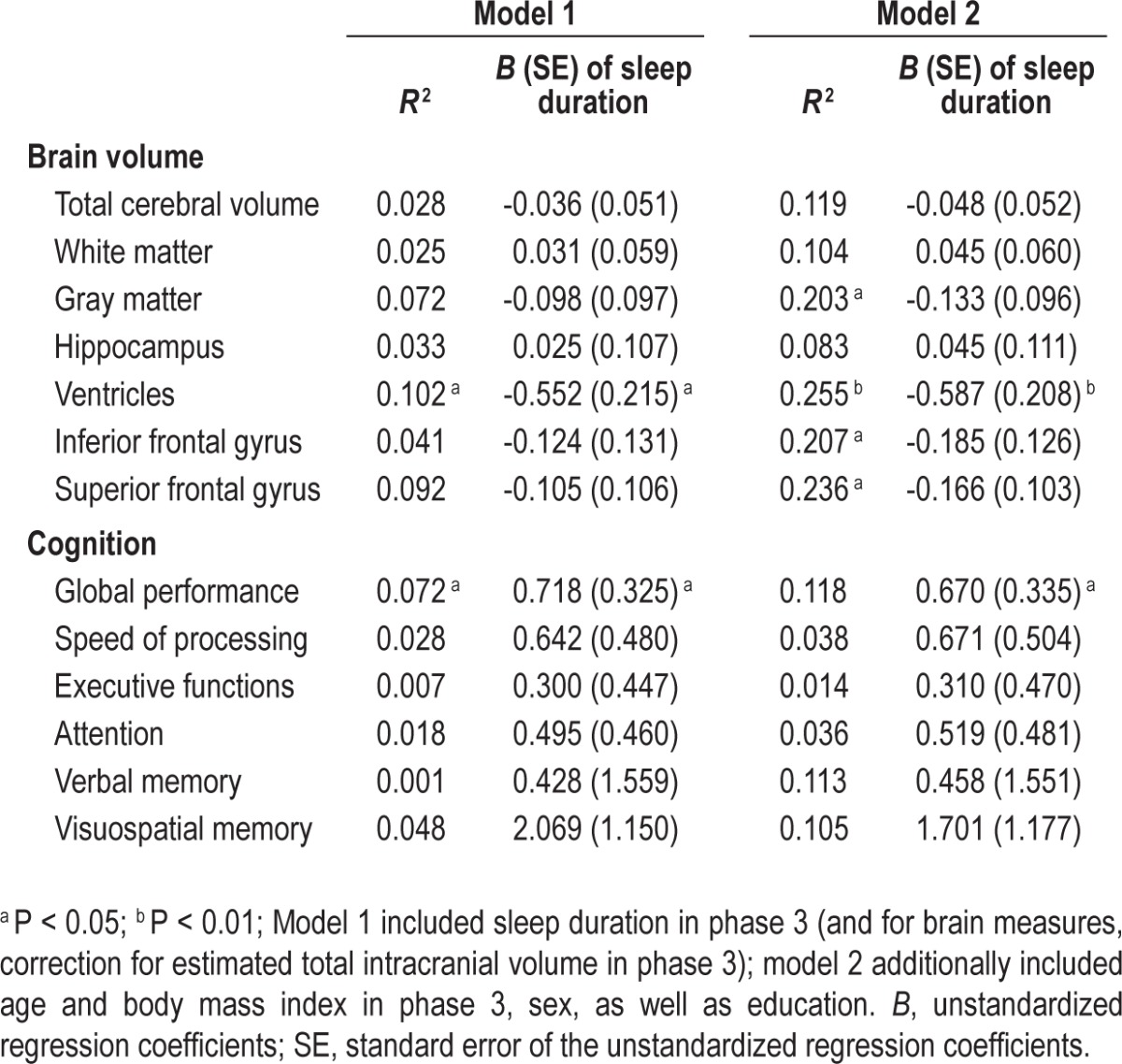
Participants varied in their rate of ventricular expansion, with some showing no noticeable change, and others showing a maximum APC of 8.35% (Figure 1A). Approximately 10.2% of the variance of ventricular APC was accounted for by sleep duration at baseline (P = 0.039). After controlling for the effect of eTIV, each 1-h decrease in sleep duration at baseline elevated the annual expansion of the ventricles by 0.55% (unstandardized coefficient: B = -0.552, P = 0.013; Table 2). After further controlling for the effects of age, sex, education, and BMI, the effect of baseline sleep duration remained statistically significant. Each 1-h decrease in sleep duration at baseline predicted a 0.59% increase in the annual ventricular expansion rate (B = -0.587, P = 0.007; Table 2). In addition, even after excluding the two participants whose total ventricular volume was more than 3 SDs from the group mean in both phases (Figure 1A), we still found a significant contribution of baseline sleep duration to the rate of ventricular expansion (B = -0.616, P = 0.005). This effect of sleep duration was not observed for any of the other brain measures (P > 0.148; Table 2).
Figure 1.
The contribution of sleep duration to brain and cognitive aging. Compared with long sleepers (median sleep duration ≥ 6.8 h; blue lines), short sleepers (sleep duration < 6.8 h; red lines) showed faster age-related (A) expansion in the ventricles and (B) decline in global cognitive performance. The interval between baseline and the 2-y follow-up ranged from 1.8 to 2.9 y, contributing to the slight differences in line lengths. Note also that although most participants showed increased ventricular volume over time, there were considerable interindividual differences in the longitudinal global cognitive changes, with some participants showing noticeable improvement in performance, probably because of practice effect.
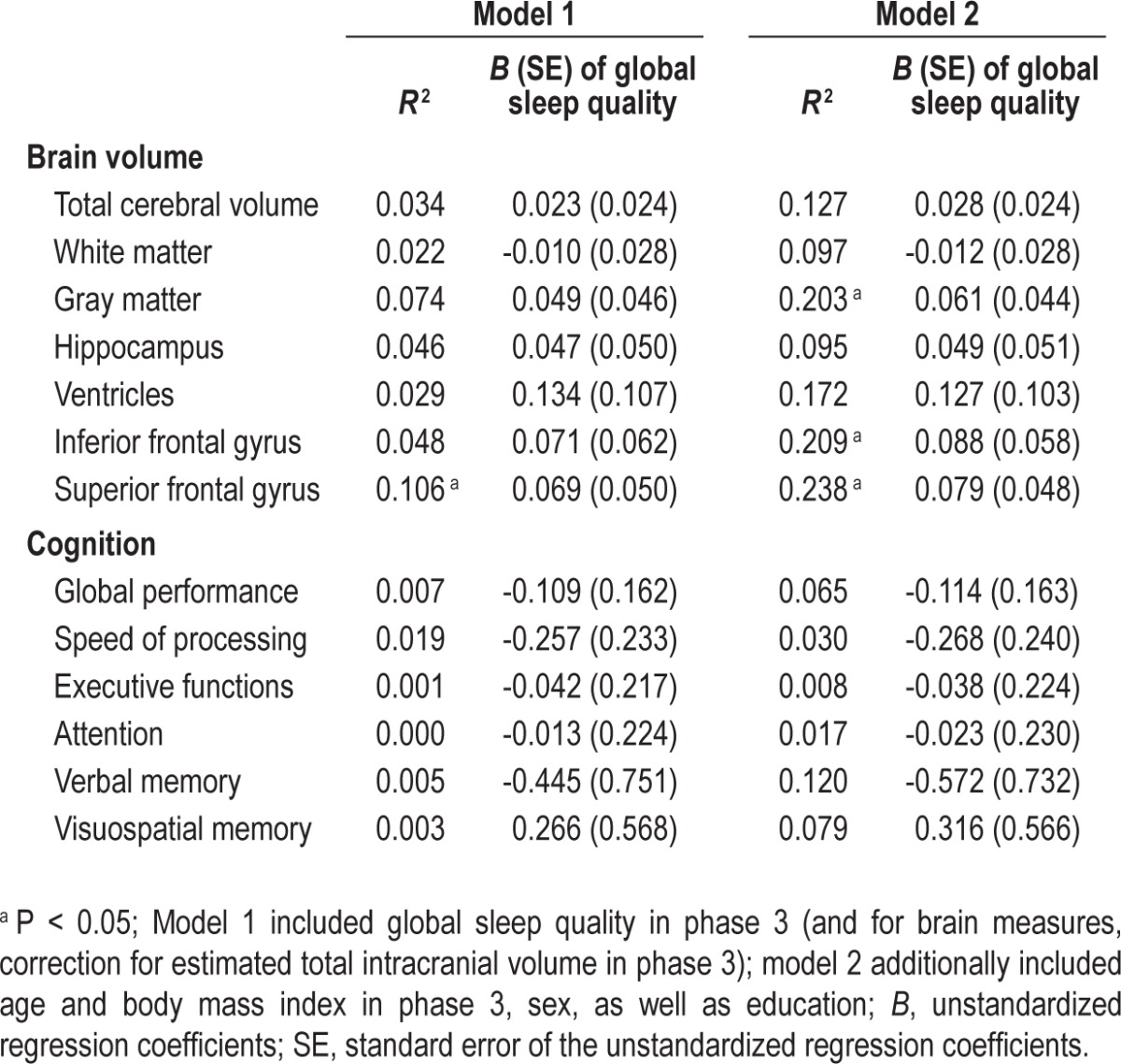
Table 2.
Associations of longitudinal changes in brain structures and cognitive performance with sleep duration
In contrast to sleep duration, global sleep quality at baseline (range = 0–11) did not modulate the longitudinal change in any of the brain morphometric measures (P > 0.104; Table 3).
Table 3.
Associations of longitudinal changes in brain structures and cognitive performance with global sleep quality
Effects of Sleep at Baseline on Longitudinal Changes in Cognitive Performance
Relative to the brain measures, there was greater interindividual variability in the longitudinal change of global cognitive performance. Some participants showed cognitive decline over time, while in others, performance improved as a result of practice effects (Figure 1B). Approximately 7.2% of the variance of the rate of global cognitive changes was determined by sleep duration (P = 0.031). Every 1-h decrease in sleep duration at baseline incremented the annual decline in global cognitive performance by 0.72% (B = 0.718, P = 0.031; Table 2). After controlling for age, sex, education, and BMI, this effect remained statistically significant and every 1-h decrease in sleep duration at baseline predicted a 0.67% increase in annual decrement in global cognitive performance (B = 0.670, P < 0.050; Table 2). Sleep duration at baseline did not predict performance decrement in any of the individual cognitive domains (P > 0.077; Table 2).
Contrary to the effects of sleep duration, global sleep quality at baseline did not exert a significant effect on cognitive change over a 2-y period (P > 0.269; Table 3).
In light of the effects of short sleep in elevating the rates of ventricular expansion and global cognitive decline, we added the APC of total ventricular volume into the regression model for the APC of global cognitive performance to determine whether the effect of baseline sleep duration on cognitive changes was mediated by the effect of sleep on brain aging. The APC of total ventricular volume was not a significant predictor of the APC of global cognitive performance (B = -0.120, P = 0.594), and hence, not a significant mediator. Thus, the contribution of sleep duration to cognitive aging appeared unrelated to its effect on ventricular expansion.
Absence of Association Among hs-CRP, Sleep Duration, Brain Structure, and Cognition
hs-CRP was not correlated with sleep duration at baseline (r = -0.061, P = 0.685), or the APC of brain structures and cognitive performance over 2 y (P > 0.088; Table S1, supplemental material). Also, there was no significant association between baseline sleep duration and age (r = 0.037, P = 0.767). Hence, the contribution of sleep duration at baseline to the longitudinal changes in total ventricular volume and global performance could not be attributed to increased hs-CRP or older age among the short sleepers.
DISCUSSION
In our longitudinal study of healthy older adults, self-reported short sleep duration at baseline was associated with faster ventricular expansion and faster decline in general cognitive performance in the subsequent 2 y. These effects remained significant after controlling for the effects of age, sex, education, and BMI, and were not related to hs-CRP, a marker of inflammation. Global sleep quality at baseline did not influence any of the variables of interest.
Effects of Sleep Duration on Age-Related Brain Atrophy
Among the brain structures studied, the ventricles were most sensitive to the effects of sleep duration. Ventricles can expand as a result of a variety of brain insults,29 schizophrenia,31 bipolar disorder,32 and neurodegenerative disorders30 in addition to aging. Notwithstanding, an increased rate of expansion in older adults is a reliable marker for risk of cognitive impairment.1 Intriguingly, although the medial temporal structures, particularly the hippo-campus, might be expected to be more sensitive to cognitive decline with age, the relatively unrefined measure of ventricular volume is in fact an equally, if not a more sensitive measure.1,26 The neurobiological basis for this is unclear but compared to smaller structures or those with less well-defined boundaries, ventricles are more reliably measured,30,55 even in lower resolution scans.1
Accelerated ventricular expansion may be evident years prior to the clinical symptoms of mild cognitive impairment (MCI).1 The contribution of short sleep duration to the development of neurodegenerative disorders has received preliminary empirical support.56 In addition, recent studies have shown that fragmented sleep may increase the risk of Alzheimer disease,57 and that sleep deprivation reduces the clearance of β-amyloid in brain interstitial fluid.58
The absence of an effect of baseline sleep duration on hippocampal atrophy was slightly surprising, given the sensitivity of this structure to a diverse set of risk factors ranging from post-traumatic stress disorder to obesity.3 However, to date, there has only been a single report on the association between short sleep duration and smaller hippocampal volume in children and adolescents.35
Our data suggest that sleep duration at baseline does not modulate age-related atrophy in the lateral prefrontal cortex (the inferior and the superior frontal gyri). Although frontal changes occur in healthy aging,28,54 it is important to note that unlike hippocampal and ventricular volume changes that occur years before diagnosis of MCI and Alzheimer disease,1,2 accelerated frontal atrophy is a later feature in MCI.59
The frequent co-occurrence of ventricular enlargement and cerebral atrophy has led to the notion that ventricular enlargement may predict neuronal loss.60 However, ventricular expansion without reduced volume in brain parenchyma has been observed recently and attributed to fluid redistribution.61 Thus, although unusual, the current findings regarding short sleep duration and ventricular enlargement without accompanying gray and white matter atrophy are not without precedent. Future studies would do well to elucidate the underlying mechanisms of this combination of findings.
Effects of Sleep Duration on Age-Related Cognitive Decline
Corroborating a recent study on a much larger group of older adults assessed only with a single general cognitive test,62 and with a far more comprehensive battery of neuropsychological tests, we found that short sleep duration at baseline was associated with faster decline in overall cognitive performance. Every 1-h reduction in sleep duration at baseline was associated with an increase in global cognitive decline by 0.67%. However, the relatively smaller standardized regression coefficient of sleep duration on global cognitive changes (0.250 versus 0.337 for ventricular expansion), together with the absence of an association between sleep and decline of any specific cognitive domain, suggest that the effect on cognition was small over the relatively short observation period of the current study.
Indeed, the effect of sleep duration on cognition may require a decade or two to be observable.25,62 A temporal lag between when clinical symptoms of cognitive impairment are detected relative to the detection and evolution of structural brain changes is now well documented in MCI and Alzheimer disease1,2 and could account for why the ventricular volume changes observed here did not predict concurrent changes in global cognitive scores. Further research would be needed to determine if our findings regarding ventricular volume and short sleep duration portend to greater risk of later cognitive impairment.
Unlike many earlier studies,7,15–24 we did not find an association between sleep quality and cognitive performance despite our relatively healthy participants reporting a broad range of PSQI global scores (range 0–11). We speculate that the inclusion of participants with physical, mental, and sleep-related conditions in many previous studies might have concurrently affected sleep quality and cognitive functions. When only healthy older adults were studied, significant association between poor sleep quality and poor performance was found in only one of the many tasks within an extensive neuropsychological test battery.22
Absence of Association Between Sleep Duration and hs-CRP in Healthy Subjects
Experimental sleep deprivation has been shown to elevate inflammatory markers including hs-CRP, interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α).36,37 Epidemiological surveys have linked elevated hs-CRP and other inflammatory cytokines with negative effects on cognitive performance.38–40 Elevated cytokine levels in older adults have been associated with increased white matter hyperintensities and reduced gray matter volume.41,42 However, effects on brain structure may be restricted to persons with high hs-CRP values (e.g., > 4.24 mg/L for reduced gray matter volume41). In the current sample, only three participants' hs-CRP exceeded this value. In relatively healthy older adults such as those in the current study, hs-CRP does not appear to be a contributory mechanism through which shorter sleep exerts negative effects on ventricular enlargement and cognitive decline.
Limitations and Future Studies
Our data were collected from relatively healthy older adults who had been followed up for a minimum of 4 y, excluding those with MCI or dementia symptoms. Our sample included only a few participants with long sleep duration (maximum = 9.25 h) and excluded persons who had significant co-morbid medical conditions. Such persons are typically underrepresented in convenience samples used in cognitive aging studies.63 We were thus unable to comment on the effects of long sleep on brain atrophy and cognitive decline.
Age-related reductions in sleep duration and quality are associated with changes in the macrostructure and microstructure of sleep,64,65 both of which potentially influence cognitive performance.66 Reduction in slow wave sleep, slow wave activity, and spindle activity are the most pronounced age-related changes,64,65 all of which can negatively affect memory consolidation.67 Although one recent cross-sectional study linked age-related reduction in medial prefrontal gray matter volume with attenuated slow wave activity and poorer memory retention,68 it remains of interest to examine how longitudinal changes in the macrostructure and microstructure of sleep affect brain and cognitive aging.
CONCLUSION
Even in relatively healthy older adults and over a short interval of 2 y, self-reported short sleep at baseline predicts faster ventricular enlargement. The effect of short sleep duration at baseline on accelerating decline in cognitive performance was more variable and modest and should be regarded as preliminary. It remains to be investigated if sleep duration plays a more important role in predicting brain and cognitive aging in less healthy older adults.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was approved by the Institutional Review Board of the National University of Singapore (08-332). All participants provided written, informed consent. Financial support was provided by Biomedical Research Council, Singapore: BMRC 04/1/36/19/372 A*STAR: SRP R-913-200-004-263 and NMRC/STaR/0004/2008. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank A/Prof Woon Puay Koh for her advice on statistical analyses.
SUPPLEMENTAL MATERIAL
Pearson correlations between high-sensitivity C-reactive protein, sleep duration at baseline, and the annual percentage change in brain structures and cognitive performance (n = 46).
COGNITIVE ASSESSMENTS
Cognitive performance was assessed with a battery of 11 neuropsychological tests that evaluated performance in five cognitive domains—speed of processing, executive functions, attention, verbal memory, and visuospatial memory.
Speed of Processing
Symbol Digit Modalities Test (SDMT)1
Participants were presented with a symbol-digit key in which each of the nine symbols was matched uniquely to a digit between 1 and 9. Participants were given a list of symbols below the key and were required to indicate the corresponding number. They had 90 sec to match as many symbols as they could. Each participant completed a written and an oral version of SDMT. The SDMT score was determined by averaging the number of correct responses across the two versions.
Symbol Search Task in the Wechsler Memory Scale–Third Edition (WMS-III)2
In each of the 60 questions, participants were required to indicate whether any of the five symbols shown in the middle of the page matched with either of the two symbols on the left side. Participants responded by putting a tick in either the ‘yes’ or the ‘no’ box on the right side of the page. They were instructed to complete as many questions as they could within 2 min. Performance was indicated by the total number of correct responses subtracted by the total number of incorrect responses.
Trail Making Test A3
Participants were presented with 25 numbers (from 1 to 25) scattered on a piece of paper and were instructed to draw lines to connect the numbers in an ascending order as quickly and accurately as they could. A shorter time taken to complete the task suggests faster speed of processing. The time taken to complete the task was transformed to speed for the computation of composite score (see below, for details).
Executive Functions
Categorical Verbal Fluency Test4
Participants were given 60 sec to list as many exemplars of each of the three categories (animals, vegetables, and fruits) as possible. Performance was indicated by the average number of correct exemplars named in the three categories.
Design Fluency Test in the Delis-Kaplan Executive Function System5
This task consisted of three parts. In the first part, participants were shown sets of five filled circles, and they were required to use four straight lines to connect the circles in each set to create different designs. For the next two parts of the task, participants were shown sets of five filled and five open circles, and they used four straight lines to connect either only the five open circles in the set (in the second part) or alternate between filled and open circles (in the third part). Each part consisted of 35 sets, and participants were given 60 sec to create as many unique designs as possible. Performance in the Design Fluency Test was indicated by the total number of unique designs created in all the three parts.
Trail Making Test B3
Participants were presented with 13 numbers (1–13) and 12 letters (A–L) scattered on a piece of paper. They were required to draw lines to connect the numbers and the letters in an ascending order while switching between the two stimulus categories (i.e., 1, A, 2, B, 3, and so on). Participants were instructed to complete the task as quickly and accurately as they could. The time taken to complete the task was transformed to speed for the computation of composite score (see subsequent text for details).
Attention
Digit Span Test in WMS-III2
Both the forward span and the backward span of the participants were tested. In the forward and the backward span tests, a series of single digits (0–9) were read aloud to the participants and they needed to orally repeat the digits in either the same or the reverse order. Each test started with a two-digit block, and task difficulty increased by adding one more digit in each subsequent block. Each block consisted of two trials. Participants were allowed to proceed to the next block if they were correct in at least one of the trials. The maximum number of digits presented was 12. Performance in each test was indicated by the total number of trials in which participants provided the correct sequence.
Spatial Span Test in WMS-III2
This task consisted of a forward and a backward span test. Each test had 11 blocks with two trials each. Nine blue squares were shown on a computer screen with a black background. In the first block, two of the squares turned red one at a time, and the participants were required to replicate the sequence by tapping the corresponding squares in either the same or the reverse order. If the participants were correct in at least one of the trials, they were presented with the three-square block and so on. The maximum number of squares in a trial was 12. Performance in each test was indicated by the total number of trials in which participants provided the correct sequence.
Verbal Memory
Verbal Paired Associates Test2
Participants were required to learn the associations of eight unrelated word pairs (e.g., Pebble-Train). The word pairs were first read out to the participants with a 1-sec break between each word of the same pair and a 2-sec break between word pairs. Participants' verbal memory was assessed with an immediate cued recall test in which the experimenter read aloud the first word of each pair and the participants were required to say aloud the second word. Stimulus presentations and immediate cued recall tests were repeated for another three times. A delayed cued recall test was administered 25-30 min later. In each cued recall test, performance was indicated by the total number of correct responses.
Rey Auditory Verbal Learning Test4
A list of 15 unrelated words were read aloud to the participants at a speed of one word per sec. Participants' memory was assessed in an immediate recall test. The same list was repeated four more times, each followed by an immediate recall test. Afterwards, a distractor list of 15 words was read aloud to the participants, followed by an immediate recall test of these distractor words. Then, memory of the original list was tested for the sixth time. After a 25- to 30-min break during which participants performed other tasks in the neuropsychological battery, a delayed recall test of the original list was presented. In all the tests, there was no time limit for recall, and the order of words recalled did not matter. In each recall test, performance was indicated by the total number of correct responses.
Visuospatial Memory
Visual Reproduction Test in WMS-III2
Participants were shown designs one at a time from a booklet for 10 sec each, and were required to reproduce the designs on paper from memory in an immediate recall test. As the task progressed, the designs contained more features and hence, the task became more difficult. Participants' memory of all the designs were tested in a delayed recall test 25–30 min later during which they performed other tasks in the neuropsychological battery. There was no time limit for the participant to reproduce the designs. Answers were scored using the scoring manual provided in WMS-III test battery. Performance was indicated by the number of correct responses averaged across the immediate and the delayed recall tests.
Visual Paired Associates Test
Participants needed to learn the associations of eight pattern-location pairs. Two patterns were placed on each side of a computer screen against a black background. At the beginning of the task, each pattern was covered by a gray rectangle. The eight rectangles disappeared one after another in a random sequence, thereby revealing the pattern underneath for 1 sec each. Immediately after all the patterns had been shown, one of the patterns appeared in the middle of the screen, and participants were required to indicate where this pattern was previously located by clicking the rectangle that was covering it. If the response was correct, a tick appeared over the correct rectangle. If the response was incorrect, a cross appeared in the appropriate rectangle to indicate where the pattern actually was. After the memory of each of the eight pairs had been tested, the same pattern-location pairs were presented again and memory was immediately tested for another three cycles. Memory of the pattern-location associations was also tested in a delayed recall test after a 25- to 30-min period during which the participants performed other tasks in the neuropsychological battery. Performance was indicated by the average number of correct responses across all the four immediate and the delayed recall tests.
To limit the number of comparisons, for each cognitive domain, the participant's scores in the respective tests were first transformed to T scores (mean = 50 and SD = 10 at phase 3) and then averaged for the computation of the composite score (see reference 6 for details).
REFERENCES
- 1.Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 2.Wechsler D. San Antonio: The Psychological Corporation; 1997. Wechsler Memory Scale, Third Edition (WMS-III): Administration and Scoring Manual. [Google Scholar]
- 3.Reitan RM, Wolfson D. Tucson, AZ: Neuropsychology Press; 1985. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. [Google Scholar]
- 4.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. Oxford: Oxford University Press; 2004. [Google Scholar]
- 5.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 6.Chee MW, Chen KH, Zheng H, et al. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage. 2009;46:257–69. doi: 10.1016/j.neuroimage.2009.01.036. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Carlson NE, Moore MM, Dame A, et al. Trajectories of brain loss in aging and the development of cognitive impairment. Neurology. 2008;70:828–33. doi: 10.1212/01.wnl.0000280577.43413.d9. [DOI] [PubMed] [Google Scholar]
- 2.Henneman WJ, Sluimer JD, Barnes J, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]
- 4.Kronholm E, Sallinen M, Suutama T, Sulkava R, Era P, Partonen T. Self-reported sleep duration and cognitive functioning in the general population. J Sleep Res. 2009;18:436–46. doi: 10.1111/j.1365-2869.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 5.Ohayon MM, Vecchierini MF. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162:201–8. doi: 10.1001/archinte.162.2.201. [DOI] [PubMed] [Google Scholar]
- 6.Ohayon MM, Vecchierini MF. Normative sleep data, cognitive function and daily living activities in older adults in the community. Sleep. 2005;28:981–9. [PubMed] [Google Scholar]
- 7.Schmutte T, Harris S, Levin R, Zweig R, Katz M, Lipton R. The relation between cognitive functioning and self-reported sleep complaints in nondemented older adults: results from the Bronx aging study. Behav Sleep Med. 2007;5:39–56. doi: 10.1207/s15402010bsm0501_3. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg DA, Ballard K, Hardy JL, Katz B, Doraiswamy PM, Scanlon M. The largest human cognitive performance dataset reveals insights into the effects of lifestyle factors and aging. Front Hum Neurosci. 2013;7:292. doi: 10.3389/fnhum.2013.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tworoger SS, Lee S, Schernhammer ES, Grodstein F. The association of self-reported sleep duration, difficulty sleeping, and snoring with cognitive function in older women. Alzheimer Dis Assoc Disord. 2006;20:41–8. doi: 10.1097/01.wad.0000201850.52707.80. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Jiang CQ, Lam TH, et al. Short or long sleep duration is associated with memory impairment in older Chinese: The Guangzhou Biobank Cohort Study. Sleep. 2011;34:575–80. doi: 10.1093/sleep/34.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res. 2009;18:427–35. doi: 10.1111/j.1365-2869.2009.00759.x. [DOI] [PubMed] [Google Scholar]
- 12.Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the Mini-Mental Score: the Northern Manhattan Study. J Clin Sleep Med. 2013;9:669–73. doi: 10.5664/jcsm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrie JE, Shipley MJ, Akbaraly TN, Marmot MG, Kivimaki M, Singh-Manoux A. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34:565–73. doi: 10.1093/sleep/34.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loerbroks A, Debling D, Amelang M, Sturmer T. Nocturnal sleep duration and cognitive impairment in a population-based study of older adults. Int J Geriatr Psychiatry. 2010;25:100–9. doi: 10.1002/gps.2305. [DOI] [PubMed] [Google Scholar]
- 15.Bastien CH, Fortier-Brochu E, Rioux I, LeBlanc M, Daley M, Morin CM. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia: relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 16.Blackwell T, Yaffe K, Ancoli-Israel S, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 17.Cricco M, Simonsick EM, Foley DJ. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 18.Garcia S, Alosco ML, Spitznagel MB, et al. Poor sleep quality and reduced cognitive function in persons with heart failure. Int J Cardiol. 2012;156:248–9. doi: 10.1016/j.ijcard.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jelicic M, Bosma H, Ponds RW, Van Boxtel MP, Houx PJ, Jolles J. Subjective sleep problems in later life as predictors of cognitive decline. Report from the Maastricht Ageing Study (MAAS) Int J Geriatr Psychiatry. 2002;17:73–7. doi: 10.1002/gps.529. [DOI] [PubMed] [Google Scholar]
- 20.Nebes RD, Buysse DJ, Halligan EM, Houck PR, Monk TH. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J Gerontol B Psychol Sci Soc Sci. 2009;64:180–7. doi: 10.1093/geronb/gbn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Regestein QR, Friebely J, Shifren JL, et al. Self-reported sleep in postmenopausal women. Menopause. 2004;11:198–207. doi: 10.1097/01.gme.0000097741.18446.3e. [DOI] [PubMed] [Google Scholar]
- 22.Saint Martin M, Sforza E, Barthelemy JC, Thomas-Anterion C, Roche F. Does subjective sleep affect cognitive function in healthy elderly subjects? The Proof cohort. Sleep Med. 2012;13:1146–52. doi: 10.1016/j.sleep.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Sutter C, Zollig J, Allemand M, Martin M. Sleep quality and cognitive function in healthy old age: the moderating role of subclinical depression. Neuropsychology. 2012;26:768–75. doi: 10.1037/a0030033. [DOI] [PubMed] [Google Scholar]
- 24.Huang CQ, Dong BR, Zhou Y. Association Between Sleep Quality and Cognitive Impairment Among Chinese Nonagenarians/Centenarians. J Clin Neurophysiol. 2012;29:250–5. doi: 10.1097/WNP.0b013e3182570f2e. [DOI] [PubMed] [Google Scholar]
- 25.Virta JJ, Heikkila K, Perola M, et al. Midlife sleep characteristics associated with late life cognitive function. Sleep. 2013;36:1533–41. doi: 10.5665/sleep.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jack CR, Jr., Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62:591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB. Brain changes in older adults at very low risk for Alzheimer's disease. J Neurosci. 2013;33:8237–42. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz N. The aging brain observed in vivo: Differential changes and their modifiers. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2005. pp. 19–57. [Google Scholar]
- 29.Bigler ED, Maxwell WL. Neuroimaging and neuropathology of TBI. NeuroRehabilitation. 2011;28:63–74. doi: 10.3233/NRE-2011-0633. [DOI] [PubMed] [Google Scholar]
- 30.Frisoni GB, Fox NC, Jack CR, Jr., Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayo A, Jennings RG, Van Horn JD. Study factors influencing ventricular enlargement in schizophrenia: a 20 year follow-up meta-analysis. Neuroimage. 2012;59:154–67. doi: 10.1016/j.neuroimage.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry. 2003;54:338–52. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- 33.Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychol Bull. 2011;137:753–84. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nat Neurosci. 2001;4:567–8. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- 35.Taki Y, Hashizume H, Thyreau B, et al. Sleep duration during weekdays affects hippocampal gray matter volume in healthy children. Neuroimage. 2012;60:471–5. doi: 10.1016/j.neuroimage.2011.11.072. [DOI] [PubMed] [Google Scholar]
- 36.Meier-Ewert HK, Ridker PM, Rifai N, et al. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol. 2004;43:678–83. doi: 10.1016/j.jacc.2003.07.050. [DOI] [PubMed] [Google Scholar]
- 37.Mullington JM, Simpson NS, Meier-Ewert HK, Haack M. Sleep loss and inflammation. Best Pract Res Clin Endocrinol Metab. 2010;24:775–84. doi: 10.1016/j.beem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–29. doi: 10.1016/j.arr.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teunissen CE, van Boxtel MP, Bosma H, et al. Inflammation markers in relation to cognition in a healthy aging population. J Neuroimmunol. 2003;134:142–50. doi: 10.1016/s0165-5728(02)00398-3. [DOI] [PubMed] [Google Scholar]
- 40.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61:76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 41.Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78:720–7. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- 42.Taki Y, Thyreau B, Kinomura S, et al. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum Brain Mapp. 2013;34:2418–24. doi: 10.1002/hbm.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chee MW, Chen KH, Zheng H, et al. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage. 2009;46:257–69. doi: 10.1016/j.neuroimage.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 44.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 45.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: A guide to assessment and intervention. New York: The Haworth Press; 1986. pp. 165–73. [Google Scholar]
- 46.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 47.Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Smith A. Symbol Digit Modalities Test. Los Angeles: Western Psychological Services; 1991. [Google Scholar]
- 49.Wechsler D. San Antonio: The Psychological Corporation; 1997. Wechsler Memory Scale, Third Edition (WMS-III): Administration and Scoring Manual. [Google Scholar]
- 50.Reitan RM, Wolfson D. Tucson, AZ: Neuropsychology Press; 1985. The Halstead-Reitan neuropsychological test battery: Theory and clinical interpretation. [Google Scholar]
- 51.Lezak MD, Howieson DB, Loring DW. Neuropsychological assessment. Oxford: Oxford University Press; 2004. [Google Scholar]
- 52.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 53.Salthouse TA. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–60. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Driscoll I, Davatzikos C, An Y, et al. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–13. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jovicich J, Czanner S, Han X, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner upgrade, scanner vendors and field strengths. Neuroimage. 2009;46:177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol. 2009;16:990–7. doi: 10.1111/j.1468-1331.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 57.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer's Disease and cognitive decline in older persons. Sleep. 2013;36:1027–32. doi: 10.5665/sleep.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald CR, McEvoy LK, Gharapetian L, et al. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–65. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Symonds LL, Archibald SL, Grant I, Zisook S, Jernigan TL. Does an increase in sulcal or ventricular fluid predict where brain tissue is lost? J Neuroimaging. 1999;9:201–9. doi: 10.1111/jon199994201. [DOI] [PubMed] [Google Scholar]
- 61.Zahr NM, Mayer D, Rohlfing T, et al. A mechanism of rapidly reversible cerebral ventricular enlargement independent of tissue atrophy. Neuropsychopharmacology. 2013;38:1121–9. doi: 10.1038/npp.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keage HAD, Banks S, Yang KL, Morgan K, Brayne C, Matthews FE. What sleep characteristics predict cognitive decline in the elderly? Sleep Med. 2012;13:886–92. doi: 10.1016/j.sleep.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Hultsch DF, MacDonald SWS, Hunter MA, Maitland SB, Dixon RA. Sampling and generalisability in developmental research: Comparison of random and convenience samples of older adults. Int J Behav Dev. 2002;26:345–59. [Google Scholar]
- 64.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–73. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 65.Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: slow-wave activity and spindle frequency activity in young and middle-aged men. Brain Res. 1996;738:205–12. doi: 10.1016/s0006-8993(96)00770-6. [DOI] [PubMed] [Google Scholar]
- 66.Pace-Schott EF, Spencer RM. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- 67.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 68.Mander BA, Rao V, Lu B, et al. Prefrontal atrophy, disrupted NREM slow waves and impaired hippocampal-dependent memory in aging. Nat Neurosci. 2013;16:357–64. doi: 10.1038/nn.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pearson correlations between high-sensitivity C-reactive protein, sleep duration at baseline, and the annual percentage change in brain structures and cognitive performance (n = 46).