Abstract
Study Objectives:
To investigate effectiveness, long-term compliance, and effects on subjective sleep of the Sleep Position Trainer (SPT) in patients with position-dependent obstructive sleep apnea syndrome (POSAS).
Design:
Prospective, multicenter cohort study.
Patients or Participants:
Adult patients with mild and moderate POSAS were included.
Interventions:
Patients asked to use the SPT for 6 mo. At baseline and after 1, 3, and 6 mo, questionnaires would be completed: Epworth Sleepiness Scale (ESS), Pittsburgh Sleep Quality Index (PSQI), Functional Outcomes of Sleep Questionnaire (FOSQ), and questions related to SPT use.
Measurements and Results:
One hundred forty-five patients were included. SPT use and SPT data could not be retrieved in 39 patients. In the remaining 106 patients, median percentage of supine sleep decreased rapidly during SPT's training phase (day 3 to 9) to near-total avoidance of supine sleep. This decrease was maintained during the following months of treatment (21% at baseline versus 3% at 6 mo). SPT compliance, defined as more than 4 h of nightly use, was 64.4%. Regular use, defined as more than 4 h of usage over 5 nights/w, was 71.2%. Subjective compliance and regular use were 59.8% and 74.4%, respectively. Median ESS (11 to 8), PSQI (8 to 6), and FOSQ (87 to 103) values significantly improved compared with baseline.
Conclusions:
Positional therapy using the Sleep Position Trainer (SPT) effectively diminished the percentage of supine sleep and subjective sleepiness and improved sleep related quality of life in patients with mild to moderate position-dependent obstructive sleep apnea syndrome. SPT treatment appeared to have sustained effects over 6 months. SPT compliance and regular use rate were relatively good. Subjective and objective compliance data corresponded well. The lack of a placebo-controlled group limited the efficacy of conclusions.
Citation:
van Maamen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the Sleep Position Trainer in the treatment of positional obstructive sleep apnea syndrome. SLEEP 2014;37(7):1209-1215.
Keywords: OSAS, position dependency, positional therapy, SPT, treatment
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is the most prevalent type of sleep disordered breathing. It affects 4% of middle-aged men and 2% of middle-aged women1 and is associated with an increased risk of traffic accidents,2 an increased cardiovascular disease risk and an increase in all-cause mortality.3
Positional obstructive sleep apnea syndrome (POSAS) occurs in approximately 56% of patients with OSAS4,5 and is defined as an apnea-hypopnea index (AHI) greater than 5 and an AHI in the supine position twice as high or more when compared with AHI in nonsupine positions together with subjective complaints.6 POSAS correlates negatively with body mass index (BMI) and OSAS severity.7 Methods to avoid the supine sleeping position, i.e., positional therapy (PT), in whichever form, have a substantial influence on OSAS severity.8 PT with a bulky mass placed against the patient's back has proved to be as effective as continuous positive airway pressure (CPAP) in reducing AHI in patients with mild (5 < AHI < 15) and moderate (15 < AHI < 30) POSAS.9 Recently, the Sleep Position Trainer (SPT), a new form of PT, has been introduced. It has been shown to reduce the average percentage of supine sleep time from 45.6% to 5.3% and to cure (defined by an AHI < 5.0) 48% of patients with mild and moderate POSAS. Furthermore, patients report that it is less bulky and more comfortable to wear.10
Most of the currently available prospective studies investigating PT in patients with POSAS have studied short-term effects on AHI and/or subjective sleep parameters during 1 day, week, or month of use.11–16 However, it is a clinical reality that most OSAS treatment options in which a device has to be applied (CPAP, mandibular advancement device (MAD), PT) suffer from long-term compliance problems and hence hamper therapeutic effectiveness.17–19
The aim of the current study was to investigate the effectiveness, long-term compliance, and effects on subjective sleep parameters in a group of patients with POSAS using the SPT for a period of 6 mo.
METHODS
Patients
From February through August 2012, participants were consecutively recruited from 18 major sleep clinics in the Netherlands for an implementation cohort study. Adult participants, in whom no longer than 3 mo earlier mild or moderate POSAS had been diagnosed by means of a polysomnography (PSG) and who could be followed up digitally and were computer literate, were included. Exclusion criteria included any prior (P)OSAS treatment, central sleep apnea, uncontrolled or serious illness (i.e., cancer, chronic heart failure (New York Heart Association (NYHA) class II and higher), chronic obstructive pulmonary disease (Global initiative for chronic Obstructive Lung Disease (GOLD) stage II and higher), any other comorbid sleep disorder (for example narcolepsy, parasomnia, periodic limb movement disorder, restless legs syndrome, or primary insomnia), seizure disorders, cardiac pacemaker, mental retardation, current psychiatric disorders (i.e. substance related disorders and psychotic, mood, anxiety, somatoform, factitious, impulse control and adjustment disorders), or physical problems causing inability to sleep on the side. The baseline visit consisted of a medical history and physical examination by the on-site physician investigator. Inclusion consisted of enrollment in an online database. A Dutch medical device distributor company contacted and visited the included patient to deliver the SPT and instruct the patient on its use. The patient was assigned an account online to upload SPT data after registration of his or her SPT in the online database. The online inclusion system digitally sent the following questionnaires to all registered subjects at baseline, and after the first, third, and sixth month of use: Epworth Sleepiness Scale (ESS, range 0-24),20 Pittsburgh Sleep Quality Index (PSQI, range 0-21),21 and the Functional Outcomes of Sleep Questionnaire (FOSQ, range 0-120).22 Patients would use the SPT for 6 mo and could keep their device after the study period ended. Patients could stop using the SPT at any time. This study was centrally approved by the Medical Ethics Committee (Amsterdam), followed by local committees. All participants signed informed consent prior to the initiation of any research activities.
Polysomnography
PSG recordings were carried out on-site using a digital poly-graph system. This system recorded the electroencephalogram, electrooculogram, electrocardiogram, and submental and anterior tibial electromyogram results. Nasal airflow was measured using a pressure sensor and arterial oxygen saturation by finger pulse oximetry. Thoracoabdominal motion was recorded by straps containing piezoelectric transducers. Snoring was recorded using a piezo snoring sensor. Body position was determined using a position sensor, which was attached to the midline of the abdominal wall. This sensor differentiated between the upright, left side, right side, prone, and supine positions. Data were downloaded to the computer and analyzed by dedicated sleep software and manually reviewed for analysis by an experienced on-site sleep investigator.
Sleep Position Trainer
The SPT has been described earlier10 and consisted of a small, lightweight device (72 × 35 × 10 mm, 25 g) that was worn across the chest with a neoprene strap (Figure 1). The device used a three-dimensional digital accelerometer to determine body position. A video-based validation study by the manufacturer showed that SPT has an accuracy of 96.3% in the measurement of body position over 167 turns.23 Treatment with the SPT was divided into three phases: a diagnostic phase, a training phase, and a therapy phase. The first 2 nights were defined as the diagnostic phase, during which the SPT monitored and recorded the sleeping position and no active feedback was given to the patient. The following 7 nights entailed the training phase, during which the SPT began to vibrate in an increasing amount of episodes of supine sleep. Night 10 onward comprised the therapy phase, during which the SPT vibrated every time a supine sleeping position was detected in order to urge the patient to change his or her sleeping position. The vibration stimulus was adapted automatically in strength, pattern, and duration, until a nonsupine position was detected. If the patient did not react, the vibrations were paused and reinitiated after 2 min. Furthermore, the SPT provided an internal memory to store data on sleeping posture and a USB port to recharge the integrated battery and to communicate data to an online self-monitoring system, which also enabled distance monitoring by the patient's physician.
Figure 1.

The Sleep Position Trainer.
Definitions
The criteria for OSAS included an AHI of 5 or more and evidence of daytime sleepiness. The AHI was defined as the mean number of apneas and hypopneas per hour during sleep, and an apnea as a period of 10 sec or more with a reduction of oronasal airflow of greater than 90%. Hypopnea was defined as an episode of greater than 30% reduced oronasal airflow for 10 sec or more accompanied by a decrease of 4% or more of the arterial oxyhemoglobin saturation. AHI thresholds were 5, 15, and 30 events per hour for mild, moderate, and severe levels of OSAS,24 respectively. POSAS was defined as an AHI of 5 or higher and an AHI in the supine position at least twice as high when compared to each of the AHI values found in the other positions.25 Effectiveness was defined in relation to percentage of supine sleep time. The SPT would be considered effective when the use of the device would provide a clinically significant reduction in percentage of supine sleep time. Compliance, in line with CPAP's compliance definition, was defined as the continuous use of the SPT for at least 4 h per night, as an average over all nights observed. Additionally, regular SPT use was defined as at least 4 h of SPT usage on 70% of the days monitored, in line with CPAP's criteria for regular use.26 Objective data on compliance were obtained through means of the SPT on a day-to-day basis. Subjective compliance was measured using online questionnaires after 1, 3, and 6 mo with the questions “How many hours do you use the SPT per night?” and “How many days per week do you use the SPT?”
Statistical Analysis
Changes in parameters before and after treatment were tested with the Wilcoxon signed rank test. A P value < 0.05 was considered to be significant. All statistical analyses were performed with SPSS statistics software (version 20, IBM Corporation, Chicago, Illinois, USA). To evaluate the effects of SPT, a per-protocol analysis was performed.
RESULTS
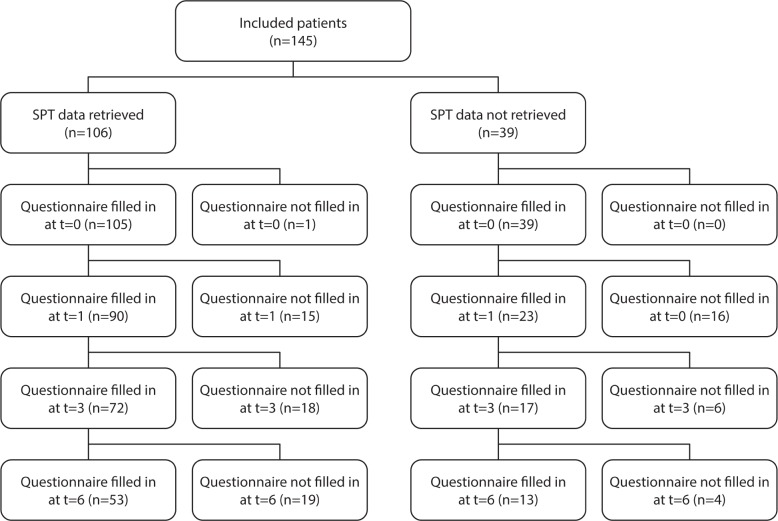
A total of 145 patients with mild and moderate POSAS, who met the inclusion criteria, were initially included in our study and entered into the online database. Baseline patient characteristics of those 145 patients are shown in Table 1. All patients filled in the questionnaires (t = 0), which resulted in the baseline questionnaire scores depicted in Table 2. A patient flow diagram, filtering out to the sample's full records list of 53 patients at t = 6, is depicted in Figure 2.
Table 1.
Patient characteristics at baseline inclusion polysomnography (n = 145)
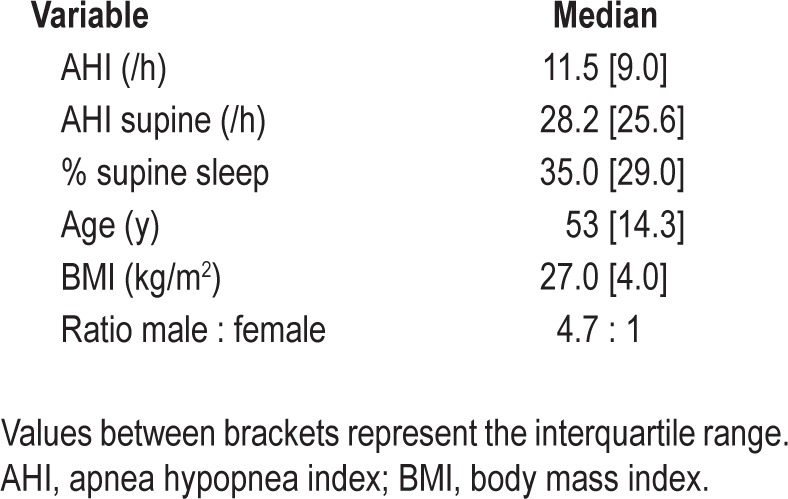
Table 2.
Questionnaire values and percentage of supine sleep time during 6 months of Sleep Position Trainer) treatment for all available Sleep Position Trainer users
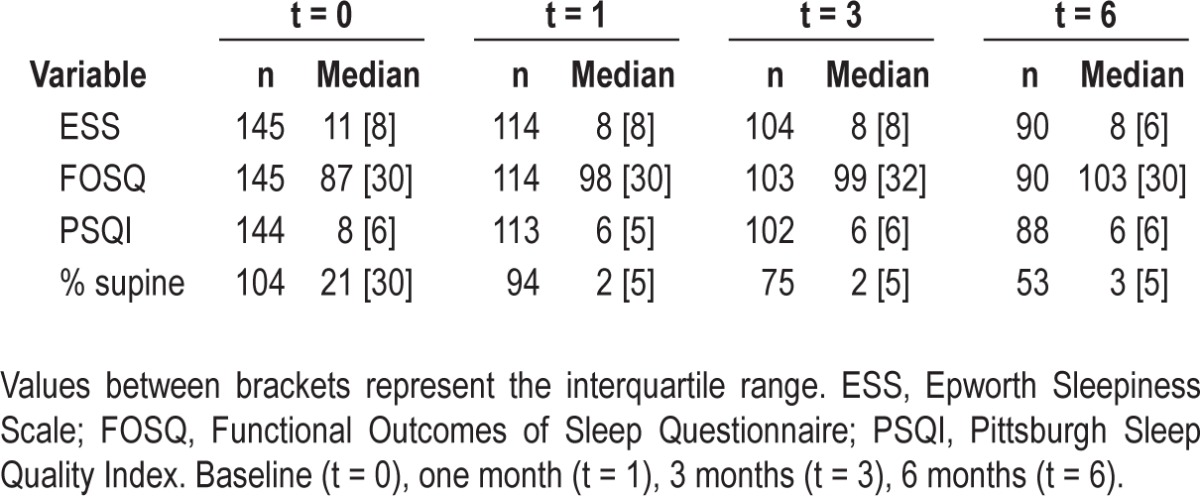
Figure 2.
Patient flow diagram. Baseline (t = 0), one month (t = 1), 3 months (t = 3), 6 months (t = 6).
Objective Compliance and Hours of Use
Of the initial 145 patients, neither SPT use nor SPT data could be retrieved in 39 of them because patients had not registered their SPT in the online database and were lost to follow-up. When reviewing inclusion data for these 39 patients, no significant differences could be found in terms of baseline patient characteristics when compared to the group of patients that registered their SPT. For the group of 106 patients that did upload their SPT data, the distribution of hours of SPT use is shown in Table 3. Median SPT use during 6 mo was 923 h (interquartile range [IQR] = 760], or 5.5 h on average per night for all nights. As shown in Table 4, 35 patients used the SPT during all 168 nights. Median SPT use for the 106 patients was 163 of the 168 days (IQR = 98). Figure 3 shows the gradual decrease in the number of patients from whom SPT data could be retrieved and shows the eventual number of patients who were using the SPT and uploading the data during the full study period. Objective SPT compliance in this group of 106 patients, defined as more than 4 h of usage per night as an average over 168 nights, was 64.4%. Regular SPT use was 71.2% over all nights observed.
Table 3.
Distribution of hours of Sleep Position Trainer use (n = 106).
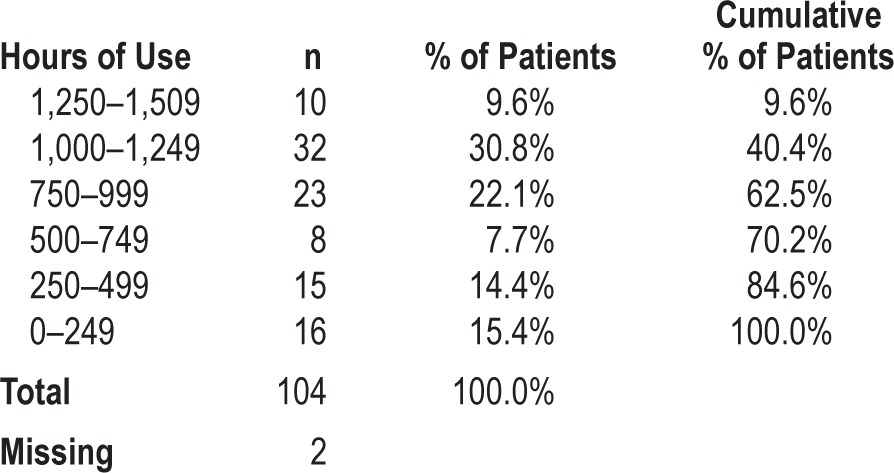
Table 4.
Distribution of days of Sleep Position Trainer use (n = 106)
Figure 3.
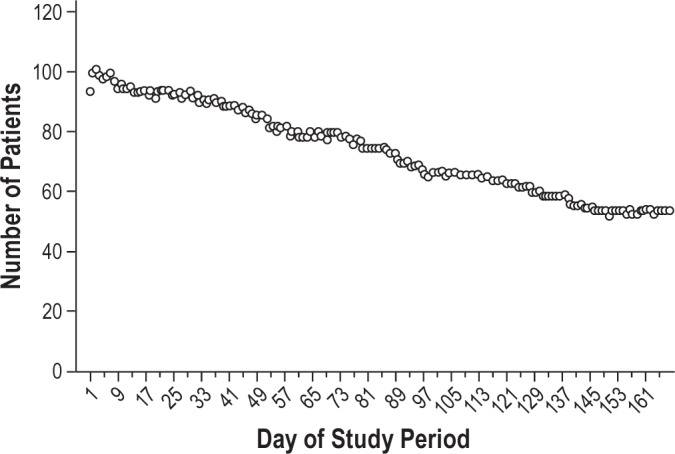
Patients who used the SPT nightly for 1 h or more during the study period.
Effectiveness and Effect on Subjective Sleep Parameters
Figure 4 illustrates that the median percentage (and IQR) of supine sleep time, as measured by the SPT, quickly decreases from baseline to day 9 and that this reduced percentage of supine sleep is maintained over time. The SPT's diagnostic and training (day 1 and 2, and 3 to 9, respectively) and therapeutic phase (from day 10 onward) can be clearly identified from Figure 4. Table 2 shows median questionnaire scores and percentage of supine sleep with IQR values for all available SPT users at the different time points. According to test-by-test exclusion approach on missing data, all parameters showed a significant decrease when compared to baseline. The median percentage of supine sleep time decreased significantly from 21% to 2% after 1 mo (Z = -8.015; P < 0.001), to 2% after 3 mo (Z = -7.473; P < 0.001) and to 3% after 6 mo (Z = -6.251; P < 0.001).
Figure 4.
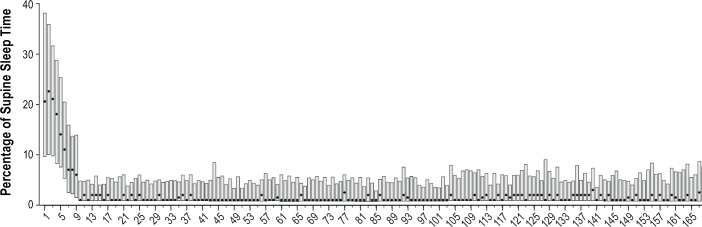
Median percentage of sleep time in the supine position per night (dots); the bars depict the interquartile range.
ESS values significantly decreased from 11 to 8 after 1 mo (Z = -6.291; P < 0.001), to 8 after 3 mo (Z = -6.647; P < 0.001), to 8 after 6 mo (Z = -6.749; P < 0.001). FOSQ significantly increased from 87 to 98 after 1 mo (Z = -5.874;P < 0.001) to 99 after 3 mo (Z = -5.865; P < 0.001) to 103 after 6 mo (Z = -6.063; P < 0.001). PSQI significantly decreased from 8 to 6 after 1 mo (Z = -3.922; P < 0.001), to 6 after 3 mo (Z = -4.329; P < 0.001) to 6 after 6 mo (Z = -4.410; P < 0.001).
Subjective Compliance
Data on self-reported continued use were obtained using the online questionnaires at three time points. After 1 mo of therapy, subjective compliance (> 4 h per night, 7 days per week) was 91.8% (n = 110). After 3 mo of therapy 74.3% of the patients were self-reportedly compliant (n = 101) and after 6 mo the subjective compliance was 59.8% (n = 87). Subjective regular SPT use (> 4 h per night, 5 days per week) was 96.4%, after 3 mo and 89.1% and 74.4% after 6 mo.
Follow-up Cohort
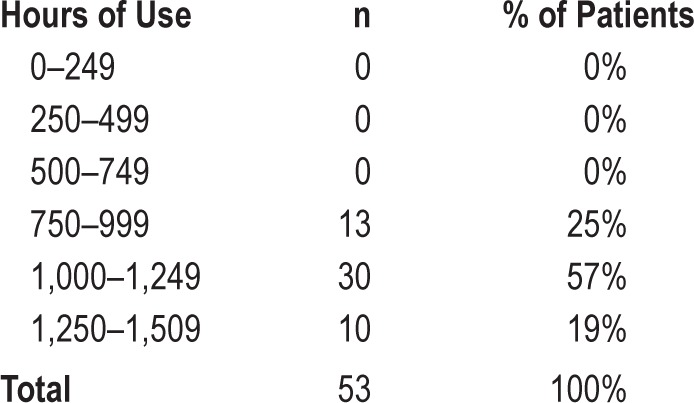
Of the 106 patients, we chose to further analyze all patients for whom complete records could be collected. Questionnaire scores and percentage of supine sleep time of these 53 patients are shown in Table 5. Distribution of hours of SPT use is shown in Table 6. Median SPT use during 6 mo was 1,127 h [IQR = 191], or 6.7 h on average per night for all nights. Objective SPT compliance in this group of 53 patients (> 4 h per night, 7 days per week) was 100%.
Table 5.
Questionnaire values and percentage of supine sleep time during 6 months of Sleep Position Trainer treatment (n = 53).
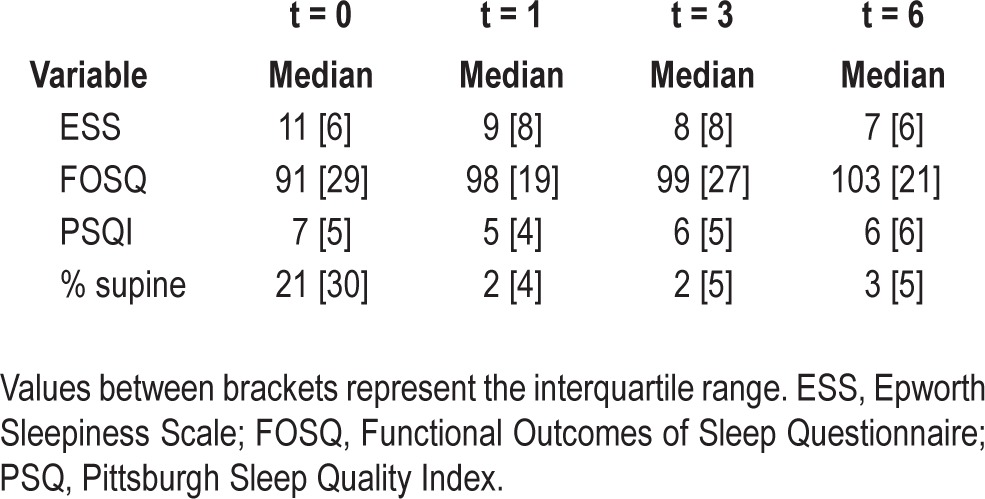
Table 6.
Distribution of hours of Sleep Position Trainer use (n = 53)
DISCUSSION
This is the first long-term follow-up study to evaluate a large group of patients with POSAS sleeping with the SPT during a period of 6 mo. The main finding is that selected patients with mild to moderate POSAS can be effectively treated with the SPT, reducing the percentage of supine sleep time persistently over the course of 6 mo. In concordance with our previous study,10 sleeping with the SPT diminishes subjective sleepiness and improves sleep related quality of life in patients with mild to moderate POSAS (Table 4). The long-term decrease in median ESS from 11 (considered sleepy) to 8 (considered normal) in our group of patients with mild and moderate POSAS seems comparable to subjective sleep results using the ESS in patients with mild and moderate OSAS using CPAP (mean ESS decrease from 10 to 8 in 6 mo).27 Effectiveness of PT and of PT using the SPT in terms of AHI decrease for patients with POSAS have been demonstrated before.9,10 Figure 4 illustrates that the median percentage of supine sleep quickly decreases from 21% at baseline to approximately 6% at day 9 (end of training phase), that this percentage of supine sleep further decreases during the therapy phase (starting on day 10) to 2–3%, and that this percentage of supine sleep is maintained over time. This is an interesting finding because the most studied form of PT, the tennis ball technique, has been shown to be minimally effective in the long term, because more than 80% of long-term users either do not use it nor avoid the supine position while asleep during tennis ball therapy.17
Long-term follow-up is important in any study that evaluates treatment with a detachable device, because the device only exerts its effects when in use. CPAP devices currently are equipped with built-in counters to enable assessment of the hours of use. Several studies have shown that 29–83% of CPAP users are noncompliant with therapy (using study periods of 3 mo to 1 y), when compliance is defined as at least 4 h of CPAP use per night.19 Objective usage data for MAD, until recently,28 have been difficult to collect and limited to subjective self-report. However, recently it was shown that objective and subjective long-term MAD compliance data show a high correspondence.29 Long-term subjective MAD compliance rates vary greatly between studies and have shown to be within a range of 4% to 82% after 1 y of treatment.30–33 Long-term PT compliance has been hampered by discomfort and reports on compliance so far have been limited to subjective measurements. One study evaluated the tennis ball technique (TBT) and used a follow-up questionnaire in 67 patients for whom the TBT was prescribed, with an average follow-up time of 2.5 ± 1.0 y, and found that long-term compliance was less than 10%.17 Another group studied 14 patients with POSAS for whom a supine sleeping position preventive vest was prescribed, and found subjective compliance at an average time of 24 mo to be less than 30%.34 To our knowledge, only one study has been conducted evaluating objective long-term use of PT. In this study, 16 patients used a somewhat bulky mass placed against the back for a period of 3 mo. Using a built-in actigraphic device they found that their device, on average, was used during 73.7% ± 29.3% of nights for 8.0 ± 2.0 h/night.35 The SPT is equipped with a built-in sensor enabling assessment of hours of use by both physician and patient. Compliance, using CPAP's compliance criteria,26 was 64.4% in our current study. Self-reported compliance after 6 mo of therapy seems to correspond well with the objective compliance rate and was 59.8%. Objective regular SPT use was 71.2%; subjective regular use was 74.4%. The high correspondence between subjective and objective compliance data is in line with a recent study focusing on subjective and objective MAD compliance data.29 This finding could suggest that patients are relatively accurate in their estimation and report of their mean use of more than 4 h per night. However, correlation between the self-reported and objective data on continuous use could not be calculated because of the differences in structure of the data. The subjective compliance rate was based on a single question at 1, 3, and 6 mo in which the mean use per night during the overall preceding period of use was questioned. The objective data on continuous use were measured on a day-to-day basis by the SPT. In addition, increased patient guidance and the use of educational and positive reinforcement programs might be used to even further increase SPT compliance because these tools have been shown to increase CPAP compliance.36–39 To our knowledge, 64.4% is the second highest long-term compliance rate of any positional therapy device studied so far. Only the study by Heinzer et al.35 reported on a higher PT compliance rate. However, the shorter study period (3 versus 6 mo), the smaller sample size (n = 16 compared to n = 106) and strict inclusion criteria used in that study (prior nontolerance of either CPAP or oral device therapy and a required < 10% of total sleep time spent in supine sleeping position during a test night with the device) might overestimate their reported compliance rate.
There are some limitations concerning our study setup and data that need to be addressed. One hundred forty-five patients were included in our study. Thirty-nine patients did not register their SPT in the online database. No SPT use or SPT data could be retrieved in these patients, despite implementation of protocolled safety nets; registered patients would receive an email reminding them to fill out the questionnaires in case they had not done so in time. When designing this trial, the possibility of patients not registering online was not fully taken into account. SPT instructions and delivery were taken care of by a Dutch medical device distributor company. The process of registering online was left to the patient, which was not ideal from a research perspective in hindsight. However, the results of this study, in terms of follow-up potential, are likely a good reflection of clinical reality. Of these 106 patients, only 53 patients uploaded their SPT data for the full study period and filled in the questionnaires at two or more time points. Patients did not receive any other incentives to fill out the questionnaires or upload their data. The data retrieved over the full 6 mo might therefore have resulted in a positive selection bias, showing merely the best SPT users. However, some patients reportedly stopped using the SPT because they felt better, no longer had any subjective complaints, and learned to avoid sleeping in the supine position. We were not able to collect their experiences in the questionnaires because most of these patients had already stopped using the SPT in the first weeks of use and were therefore lost to follow-up. Any potential learning effect13 of PT in general or in sleeping with the SPT remains to be investigated.
This study lacks objective measures on treatment efficacy by means of a repeat PSG in all patients to evaluate effects on AHI and snoring. Although effects of SPT use on AHI have been reported before,10 effects of the SPT on snoring remain to be investigated. Another limitation of our study is the lack of a control group. Our results and conclusions could have been stronger and more valuable had we compared the SPT users to a group of patients with POSAS with another treatment regimen.
A final limitation of the current study was the lack of an educational program or positive reinforcement program for the patients. Loss to follow-up would probably have been less and compliance would probably have been higher given the positive results in trials with CPAP users.36–39
It is our opinion that in patients with mild and moderate POSAS, PT could be the ideal method and maybe should be the initial treatment of POSAS. The treatment concept of SPT, which consists of a small apparatus that is able to register body position as well as provide active feedback effectively to its user during both night and day, seems to be the best currently available option for treating patients with POSAS. This cohort of patients with POSAS using the SPT for a 6-mo study period has shown that the SPT is capable of quickly reducing the percentage of supine sleep time within 10 days and maintaining this decreased percentage of supine sleep time over time. Furthermore, SPT use diminished subjective sleepiness and improved sleep-related quality of life. However, future prospective long-term research is necessary and should focus on objective PSG parameters in direct comparison with other generally accepted treatment modalities as well as objective measurements of continuous usage.
CONCLUSION
Over a period of 6 mo, sleeping with the SPT effectively and persistently decreases the percentage of supine sleep time, from 21% to 3% within 10 days and maintaining this 3% supine sleep time over 6 mo. The SPT significantly diminishes subjective sleepiness and improves sleep-related quality of life in patients with mild to moderate POSAS. Of the 106 patients studied, 64.4% using the SPT were considered compliant, defined as SPT use of more than 4 h per night during 7 days/w and 71.2% of patients used the SPT on a regular basis, defined as more than 4 h during 5 days/w. Subjective and objective compliance data corresponded well. Future research needs to focus on objective long-term treatment effects, particularly in relation to other already generally accepted POSAS treatment modalities.
DISCLOSURE STATEMENT
This study was supported by Achmea Holding, Mediq-Tefa and NightBalance. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Dr. de Vries is consultant to NigthtBalance, Philips and Olympus, and is a member of the Medical Advisory Board of NightBalance and ReVent. Dr. van Maanen has indicated no financial conflicts of interest. There was no off-label use.
ACKNOWLEDGMENTS
The authors acknowledge the advice and criticism of Herman Braat, consultant data analytics, and the following persons and the departments in which they work for actively being involved in patient enrollment:
R van Uffelen, MD, Albert Schweitzer Ziekenhuis;
RJAM van der Hulst, MD, PhD and JTh Schmidt MD, PhD, Amstelland Ziekenhuis;
WJA Wijnands, MD, PhD, Deventer Ziekenhuis; J Dolsma, MD, Diaconessenhuis;
F Brijker, MD, PhD, Diakonessenhuis;
MAC Klaaver, MD, Havenziekenhuis;
AF Kuipers, MD, Isala Klinieken;
H Janssen, MD, Kempenhaeghe;
LS Hoep, MD, Laurentius Ziekenhuis;
JQPJ Claessen, MD, PhD, Martini Ziekenhuis;
R Hilgerink, MSc, Medisch Spectrum Twente;
PJE Vos, MD, PhD, Rijnstate Ziekenhuis;
EFG Kapteijns, MD, Rode Kruis Ziekenhuis;
D Kox, MD, Sint Antonius Ziekenhuis;
WEJJ Hanselaar, MD, Sint Franciscus Gasthuis;
JGM Ribbert, MD, Zuwe Hofpoort Ziekenhuis.
ABBREVIATIONS
- AHI
apnea hypopnea index
- BMI
body mass index
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- OSAS
obstructive sleep apnea syndrome
- POSAS
positional obstructive sleep apnea syndrome
- PSQI
Pittsburgh Sleep Quality Index
- PT
positional therapy
- SPT
Sleep Position Trainer
REFERENCES
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apneahypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–77. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kendzerska T, Mollayeva T, Gershon AS, Leung RS, Hawker G, Tomlinson G. Untreated obstructive sleep apnea and the risk for serious long-term adverse outcomes: A systematic review. Sleep Med Rev. 2014;18:49–59. doi: 10.1016/j.smrv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Oksenberg A, Silverberg DS, Arons E, Radwan H. Positional vs nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest. 1997;112:629–39. doi: 10.1378/chest.112.3.629. [DOI] [PubMed] [Google Scholar]
- 5.Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263:946–50. doi: 10.1007/s00405-006-0090-2. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14:546–52. doi: 10.1093/sleep/14.6.546. [DOI] [PubMed] [Google Scholar]
- 7.Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. doi: 10.1378/chest.128.4.2130. [DOI] [PubMed] [Google Scholar]
- 8.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37:1000–28. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 9.Permut I, Diaz-Abad M, Chatila W, et al. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238–43. [PMC free article] [PubMed] [Google Scholar]
- 10.van Maanen JP, Meester KA, Dun LN, et al. The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath. 2013;17:771–9. doi: 10.1007/s11325-012-0764-5. [DOI] [PubMed] [Google Scholar]
- 11.Berger M, Oksenberg A, Silverberg DS, Arons E, Radwan H, Iaina A. Avoiding the supine position during sleep lowers 24 h blood pressure in obstructive sleep apnea (OSA) patients. J Hum Hypertens. 1997;11:657–64. doi: 10.1038/sj.jhh.1000510. [DOI] [PubMed] [Google Scholar]
- 12.Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG. Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med. 2011;7:376–83. doi: 10.5664/JCSM.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cartwright RD, Lloyd S, Lilie J, Kravitz H. Sleep position training as treatment for sleep apnea syndrome: a preliminary study. Sleep. 1985;8:87–94. doi: 10.1093/sleep/8.2.87. [DOI] [PubMed] [Google Scholar]
- 14.Maurer JT, Stuck BA, Hein G, Verse T, Hormann K. Schlafapnoetherapie mit einer neuartigen rückenlage-verhinderungs-weste. Dtsch Med Wochenschr. 2003;128:71–5. doi: 10.1055/s-2003-36658. [DOI] [PubMed] [Google Scholar]
- 15.van Maanen JP, Richard W, van Kesteren ER, et al. Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res. 2012;21:322–9. doi: 10.1111/j.1365-2869.2011.00974.x. [DOI] [PubMed] [Google Scholar]
- 16.Zuberi NA, Rekab K, Nguyen HV. Sleep apnea avoidance pillow effects on obstructive sleep apnea syndrome and snoring. Sleep Breath. 2004;8:201–7. doi: 10.1007/s11325-004-0201-5. [DOI] [PubMed] [Google Scholar]
- 17.Bignold JJ, Deans-Costi G, Goldsworthy MR, et al. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Clark GT. Mandibular advancement devices and sleep disordered breathing. Sleep Med Rev. 1998;2:163–74. doi: 10.1016/s1087-0792(98)90019-3. [DOI] [PubMed] [Google Scholar]
- 19.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5:173–8. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 21.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 22.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20:835–43. [PubMed] [Google Scholar]
- 23.van der Star A, Mekking S, van Riet M. The technical validation of the Sleep Position Trainer. Nightbalance Research and Development [Internet] 2012. Oct, [cited 2013 Dec 6]:1-12. Available from http://www.nightbalance.com/research.
- 24.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 25.Cartwright RD, Diaz F, Lloyd S. The effects of sleep posture and sleep stage on apnea frequency. Sleep. 1991;14:351–3. doi: 10.1093/sleep/14.4.351. [DOI] [PubMed] [Google Scholar]
- 26.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 27.Kushida CA, Nichols DA, Holmes TH, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: The Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2012;35:1593–602. doi: 10.5665/sleep.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68:91–6. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dieltjens M, Braem MJ, Vroegop AV, et al. Objectively measured vs. self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144:1495–502. doi: 10.1378/chest.13-0613. [DOI] [PubMed] [Google Scholar]
- 30.Nakazawa Y, Sakamoto T, Yasutake R, et al. Treatment of sleep apnea with prosthetic mandibular advancement (PMA) Sleep. 1992;15:499–504. doi: 10.1093/sleep/15.6.499. [DOI] [PubMed] [Google Scholar]
- 31.Walker-Engstrom ML, Wilhelmsson B, Tegelberg A, Dimenas E, Ringqvist I. Quality of life assessment of treatment with dental appliance or UPPP in patients with mild to moderate obstructive sleep apnoea. A prospective randomized 1-year follow-up study. J Sleep Res. 2000;9:303–8. doi: 10.1046/j.1365-2869.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- 32.Walker-Engstrom ML, Ringqvist I, Vestling O, Wilhelmsson B, Tegelberg A. A prospective randomized study comparing two different degrees of mandibular advancement with a dental appliance in treatment of severe obstructive sleep apnea. Sleep Breath. 2003;7:119–30. doi: 10.1007/s11325-003-0119-3. [DOI] [PubMed] [Google Scholar]
- 33.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel S, Smith E, Leiacker R, Fischer Y. Effektivität und Langzeit-Compliance der Therapie mit Rückenlage-Verhinderungsweste bei obstruktiver Schlafapnoe. Laryngorhinootologie. 2007;86:579–83. doi: 10.1055/s-2007-966179. [DOI] [PubMed] [Google Scholar]
- 35.Heinzer RC, Pellaton C, Rey V, et al. Positional therapy for obstructive sleep apnea: an objective measurement of patients' usage and efficacy at home. Sleep Med. 2012;13:425–8. doi: 10.1016/j.sleep.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 36.Leseux L, Rossin N, Sedkaoui K, et al. [Education of patients with sleep apnea syndrome: Feasibility of a phone coaching procedure. Phone-coaching and SAS] Rev Mal Respir. 2012;29:40–6. doi: 10.1016/j.rmr.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Willemin MC, Fry S, Peres S, Wallaert B, Mallart A. [Effects of an educational program in non-adherent apneic patients treated with continuous positive airway pressure] Rev Pneumol Clin. 2013;69:70–5. doi: 10.1016/j.pneumo.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs FS, Pittarelli A, Hahn EG, Ficker JH. Adherence to continuous positive airway pressure therapy for obstructive sleep apnea: impact of patient education after a longer treatment period. Respiration. 2010;80:32–7. doi: 10.1159/000243161. [DOI] [PubMed] [Google Scholar]
- 39.Ballard RD, Gay PC, Strollo PJ. Interventions to improve compliance in sleep apnea patients previously non-compliant with continuous positive airway pressure. J Clin Sleep Med. 2007;3:706–12. [PMC free article] [PubMed] [Google Scholar]